Abstract
Human hepatocellular carcinoma (HCC) mostly develops as a complication of fibrosis or cirrhosis. While most human studies of HCC provide crucial insights into the molecular signatures of HCC, seldom they address the etiology of HCC. Mouse models are essential tools for investigating pathogenesis of HCC; however, the overwhelming majority of cancer models in rodents do not feature liver fibrosis. This unit details a protocol for an experimental model of HCC in the mouse that arises in conjunction with advanced liver fibrosis. A single injection of N-nitrosodiethylamine (DEN) is followed by repeat dosing with carbon tetrachloride (CCl4). A dramatic potentiation of the liver tumor incidence can be observed following treatment with DEN and CCl4 where 100% of mice develop liver tumors at 5 months of age. This model can be utilized in studies of the molecular mechanisms of fibrogenesis and HCC development, and in cancer hazard/chemotherapy testing of novel agents.
Keywords: liver, fibrosis, cancer, mechanisms, genotoxic
INTRODUCTION
Hepatocellular carcinoma (HCC) is a prevalent human cancer with high mortality rate (Center and Jemal, 2011). Overall cancer incidence and death rates are steadily declining over the past decade (AACR Cancer Progress Report Writing Committee et al., 2013); however, the incidence of HCC continues to increase. HCC is a multistep pathological process characterized by the progressive, sequential evolution of liver disease stages from chronic liver injury, to inflammation, hepatocellular degeneration and necrosis, hepatocellular regeneration and small cell dysplasia, followed by the appearance of low- and high-grade dysplastic nodules, which eventually manifest as HCC (Aravalli et al., 2013; Farazi and DePinho, 2006).
The etiology of HCC in humans is complex and many causative factors were identified. These include viral infections, consumption of alcoholic beverages, environmental chemicals, hemochromatosis, and metabolic diseases related to insulin resistance collectively identified as non-alcoholic steatohepatitis (Della Corte and Colombo, 2012; El Serag and Rudolph, 2007). Because the diagnosis of HCC in humans most often occurs at the late stages of the disease, rodent models are invaluable for the understanding of both causality and pathogenesis of HCC (Fausto and Campbell, 2010; Vucur et al., 2010). An excellent summary of chemical-induced, xenograft and genetically-induced experimental mouse liver tumor models, and their relevance to human HCC has been published (Heindryckx et al., 2009). Importantly, while in humans, 70–90% of clinical HCC cases are associated with advanced liver fibrosis or cirrhosis (Alkofer et al., 2011), the overwhelming majority of chemical-induced liver adenomas and carcinomas in rodents arise in fibrosis-free liver. Thus, among many limitations, chronic rodent cancer bioassays do not address the key feature of human HCC, chronic liver inflammation and fibrosis/cirrhosis.
This protocol aims to address this major limitation of rodent models of HCC and describes a two-stage application of chemicals to the liver for the initiation and promotion of hepatocellular tumors. As an initiator, this protocol utilizes a single injection of a low dose of DEN (1 mg/kg, i.p.) into 14 day-old male mice, a well-known model genotoxic carcinogen that induces liver carcinogenesis (Druckrey et al., 1964; Vesselinovitch and Mihailovich, 1983). As a promoter, repeat dosing with the low dose of a pro-fibrogenic agent CCl4 (0.2 ml/kg, i.p.) is performed starting at 8 weeks of age for up to 14 consecutive weeks. It is well established that continuous exposure to CCl4 leads to the development of hepatic fibrosis and compensatory cell proliferation (Stowell et al., 1951). In addition advanced liver fibrosis, repeated dosing of CCl4 leads to progressively worsening anisonucleosis, a morphological manifestation of nuclear injury characterized by variation in the size of the hepatocyte nuclei, an observation that has been associated with hepatic oxidative stress (Guzman et al., 2011). In this model of co-treatment of mice with DEN and CCl4, as opposed to either agent alone, the resultant chronic liver fibrosis is accompanied by a dramatic increase in the liver tumor incidence where 100% of the animals in the co-treatment group developed liver tumors at 5 months of age (Uehara et al., 2013).
The molecular pathways (Uehara et al., 2013) and epigenetic alterations (Chappell et al., 2014) observed in fibrosis-associated liver tumors in this mouse model indicate important features involved in the development of human liver tumors that arise from fibrosis and cirrhosis, a common progression according to human clinico-pathological evidence. This two-stage model of chemical liver carcinogenesis provides an opportunity to study the molecular events associated with early and late events in HCC development and progression, with a particular focus on a combination of etiological factors such as genotoxicity and advanced liver fibrosis. In addition, because human HCC requires liver cirrhosis, the model described here is more likely, as compared with traditional 2-year cancer bioassays or studies in mouse transgenic models (Bucher, 1998), to be able to recapitulate fibrotic phenotype seen in humans and used to test for cancer hazard of chemicals and drugs.
BASIC PROTOCOL: DEN and CCl4-induced Mouse Model of HCC
Synopsis
First, male B6C3F1 mice are administered DEN (single i.p. injection of 1 mg/kg at 14 days of age). Second, CCl4 (0.2 ml/kg, 2 times per week i.p. starting at 8 weeks of age) is administered for up to 14 weeks, at which point 100% incidence of liver adenomas is expected (Uehara et al., 2013). Time-course evaluation of histopathological features of underlying liver disease (single cell necrosis, ballooning degeneration and hypertrophy of hepatocytes, and fibrosis with inflammatory cells infiltration), as well as the incidence of pre-cancerous lesions (foci) and tumors (adenomas and carcinomas) is described in detail Uehara et al. (2013). The tumors are determined at sacrifice by examining the liver macroscopically and microscopically. The degree of liver fibrosis is evaluated by Masson’s trichrome stain.
NOTE: This protocol uses live animals; thus, all experiments must first be reviewed and approved by an Institutional Animal Care and Use Committee and must conform to all governmental regulations regarding the care and use of laboratory animals. This protocol uses chemicals (N-nitrosodiethylamine and carbon tetrachloride) that are regarded as reasonably anticipated to be human carcinogens (National Toxicology Program, 2011); thus, these experiments must be approved by the local environmental health and safety authority and adhere to appropriate best laboratory practices for handling and disposal of all contaminated materials (National Research Council, 2005).
Materials
Female pregnant B6C3F1/J mice (Jackson Laboratory, Bar Harbor, ME) arrived about one week before delivery.
Diethylnitrosamine (CAS 55-18-5, N-nitrosodiethylamine, DEN; Sigma, St. Louis, MO), see recipe.
Sterile phosphate buffered saline (PBS; vehicle).
Carbon tetrachloride (CAS 56-23-5, CCl4; Sigma, St. Louis, MO), see recipe.
5-Bromo-2′-deoxyuridine (CAS 59-14-3, BrDU; Sigma, St. Louis, MO), see recipe.
Olive oil (vehicle).
Nembutal (Oak Pharmaceutical, Lake Forest, Illinois) for anesthesia.
Neutral buffered formalin (10%).
Disposable plastic syringes (1.0 mL) with needles for i.p. injection.
0.9% NaCl–moistened filter paper.
Sharp dissecting scissors.
Razor blades.
Heparin-containing Serum gel Z/1.1 ml centrifuge tubes (Sardstedt, Nümbrecht, Germany).
Prepare the animals
-
1
Acclimate timed pregnant B6C3F1/J mice (Jackson) for about one week before delivery of offspring.
Animals are acclimated under standard lighting and temperature conditions to eliminate the effect of stress. Food and water are available ad libitum. Other strains may be used; however, both spontaneous and chemical-induced liver cancer incidence varies greatly among mouse strains (Bannasch, 1983).
-
2
After birth, randomly assign litters of male pups to treatment groups.
Male mice are selected because male gender is a risk factor for human HCC (Jepsen et al., 2007); however, females may also be used should the research question require the use of females. From one pregnant B6C3F1/J mouse, 3–8 male pups can be expected in a litter; however, the number of pups in a litter and their gender may vary greatly and mainly depends on the lot of animals.
-
3
At 14 days of age, weigh each pup to determine the administered dose. Administer either (i) DEN (1 mg/kg i.p. in PBS), or (ii) PBS (vehicle) alone. Dosing volume is 15 ml/kg body weight.
To minimize contamination of each treatment during the lactation period, pups in each litter should be assigned to the same treatment group and treated in the same manner. Because of its carcinogenicity, DEN should be handled using the “basic prudent practices” and precautions for work with compounds of high chronic toxicity (National Research Council, 2005).
-
4
At 3–4 weeks of age, wean animals from their mothers.
At 8 weeks of age (6 weeks after a single injection of DEN or vehicle as detailed above), begin administration of CCl4 (0.2 ml/kg i.p. in olive oil), or olive oil alone (vehicle) twice a week for up to 14 additional weeks (dose volume: 15 ml/kg body weight). Treatment for 9 weeks is expected to yield 100% incidence of pre-neoplastic liver foci and up to 40% incidence of liver adenomas and 20% for carcinomas, while treatment for 14 weeks is expected to yield 100% incidence of liver adenomas and 50% for carcinomas (Uehara et al., 2013). Tumors observed in this model have not been found to metastasize. No tumors or pre-neoplastic lesions are expected in vehicle-treated mice. Tumors and pre-neoplastic lesions are not detectable by means other than visual and light microscopy examination of the liver after sacrifice.
Because of its carcinogenicity, CCl4 should be handled using the “basic prudent practices” and precautions for work with compounds of high chronic toxicity (National Research Council, 2005). Application of a different amount (up to 0.5 ml/kg has been used by others) may affect tumor numbers and size yet will result in more pronounced liver inflammation and thus may affect the molecular pathogenesis of disease (Dapito et al., 2012).
If animals die or appear sick and need to be euthanized during the experiment, necropsy should be performed to establish the cause of death. A frequent cause of premature death is intestinal perforation as a result of error (e.g., accidental puncture of intestine and resultant peritonitis) in administering repeated i.p. injections.
-
5
On the day of the last injection, change the water that animals have access to from regular animal facility-approved source to drinking water containing bromodeoxyuridine (5-bromo-2′-deoxyuridine, BrDU, 0.2 g/l). Allow animals to have free access to this solution for three days. Sacrifice animals at the end of BrDU treatment.
This process can be omitted if cellular proliferation labeling index is not needed. [*Author: If this is not done what else would this procedure be used for? Straightforward histopath?]
Necropsy
-
6
Record body weights of each animal.
-
7
Inject animals with Nembutal (120 mg/kg, i.p.) or other anesthetic agent that is approved for humane euthanasia by the Institutional Animal Care and Use Committee.
-
8
Upon verifying that the animal expired, place the animal on its back and open the abdomen through vertical incision with a pair of sharp dissecting scissors. Collect blood from the inferior vena cava or a common iliac vein into appropriate anticoagulant (e.g., heparin)-containing syringes.
Avoid injuring major vessels or internal organs to prevent premature bleeding by lifting the abdominal skin with forceps while performing the incision.
-
9
With a pair of sharp dissecting scissors, remove the liver.
-
10
Record the wet liver weight.
-
11
Place the liver out on 0.9% NaCl–moistened filter paper.
-
12
Perform step-sectioning of the liver at 5–7 mm by using sharp razor blades to reveal macroscopically-visible tumors.
-
13
Record the number and size of tumors on macroscopic examination.
-
14
Excise liver sections (including grossly visible tumors, Figure 1) of a desirable size and place them in 10% neutral buffered formalin.
-
15
Embed the tissue in paraffin, and cut it into 3–5 μm sections as needed.
Grossly visible large tumors can be separated from non-cancerous liver tissue by careful excision from the liver. If needed, frozen tissue samples can also be obtained. See step 4 for expected tumor burden.
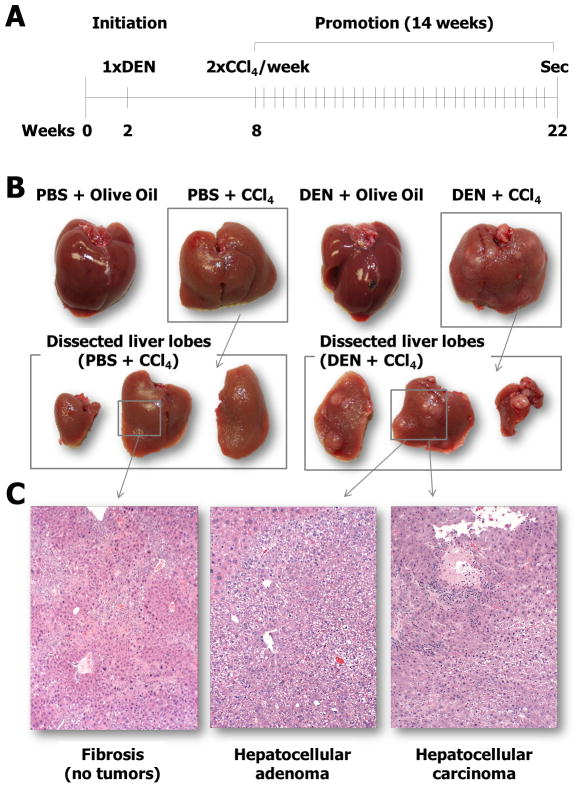
Fig. 1. Experimental design, gross pathology, and histopathology of the liver tumors.
(A) A single injection of a mutagenic carcinogenic agent N-nitrosodiethylamine (DEN) is followed by repeat dosing with carbon tetrachloride (CCl4) for up to 14 consecutive weeks. For more detail, see the BASIC PROTOCOL section. (B) Representative photographs of the livers from the animals in each dosing group. (C) Representative photographs of the histopathology of the livers including fibrosis, hepatocellular adenoma and carcinoma.
Histopathological examination
-
16
Stain the sections with hematoxylin and eosin, and examine tumors and liver tissues histopathologically under the light microscope (see Table 1).
-
17
Stain the sections with Masson’s trichrome procedure, and examine liver fibrosis histopathologically under the light microscope (see Table 2).
Table 1.
Major diagnostic criteria for (pre)neoplastic lesions in the mouse liver.
| Types of (pre)neoplastic lesions | Characteristics of histopathology |
|---|---|
| Preneoplastic foci of altered hepatocytes | Normal or minimal compression of the surrounding parenchyma Foci of hepatocytes with increased basophilic staining (basophilic type) Glycogen and/or some clear cells may be present |
| Hepatocellular adenoma | Relatively uniform hepatocytes accompanied with loss of normal lobular architecture Compression of the surrounding parenchyma |
| HCC | Broad trabecular growth pattern of atypical hepatocytes With hemorrhage and ischemic necrosis in the central of tumors Vascular and stromal invasions occasionally |
Table 2.
Scoring system for liver fibrosis in mice
| Score | Characteristics of histopathology |
|---|---|
| 0 | No fibrotic changes |
| 1 | Slight fibrotic changes around the central vein and occasionally with thin bridging fibrosis |
| 2 | Thick bridging fibrosis and pseudo-lobule formation with dissecting nodules |
REAGENTS AND SOLUTIONS
DEN
Dissolve 1 mg DEN in 15 mL PBS (0.67%, w/v final) on the day of dosing.
CCl4
Dissolve 1 ml CCl4 in olive oil at the final volume of 10 ml (0.01%, v/v). Protect the prepared dosing solution from light, and prepare it fresh at least once a week.
BrDU
Prepare stock by mixing 1 g BrDU with 100 ml distilled water; place in brown glass water bottle (or wrap in foil) and store at 4°C. Prepare fresh solution for administering to animals every 24–48 hrs from the stock as follows. Mix 20 ml of stock solution with 980 ml of tap water, place into brown water bottles and place in animal cages in place of regular animal facility water. Notify the animal facility staff not to change water bottles.
COMMENTARY
Background Information
While the incidence and mortality from all types of cancer have been declining in the past decade, the incidence of liver cancer, including HCC, continues to increase worldwide (Siegel et al., 2013). The etiological factors contributing to HCC are believed to be only a part of such an increase as the metabolic diseases related to insulin resistance collectively identified as non-alcoholic steatohepatitis, have been recognized as additional important factors in the increase in prevalence of HCC (Della Corte and Colombo, 2012). Notably, most HCC cases are diagnosed in subjects with fibrotic or cirrhotic livers (Alkofer et al., 2011), pathological states that are a consequence of chronic liver injury (Fattovich et al., 2004). However, the underlying molecular mechanisms that are responsible for the high rate of HCC development in the chronically injured and fibrotic liver remain largely unknown and the understanding of HCC pathogenesis is incomplete (Luedde and Schwabe, 2011).
There is growing evidence that chronic inflammatory processes are involved in triggering the molecular and cellular events leading from chronic liver injury to liver fibrosis and ultimately to HCC. Activation of resident and systemic circulation-derived macrophages and neutrophils, as well as the resultant release of pro-inflammatory cytokines and chemokines and other damage-associated molecular patterns, is thought to play an important role in the pathogenesis of many chronic liver diseases, including HCC (Grivennikov et al., 2010). In the last few years some of these pathological processes have been recapitulated in different mouse models (Fausto and Campbell, 2010; Vucur et al., 2010). However, it is still unclear whether and how these mouse models reflect the clinical realities of human HCC (Heindryckx et al., 2009). Animal models, most often rat or mouse, are commonly used to test for the cancer hazard potential of chemicals and drugs (Wells and Williams, 2009) and liver is a common tissue target for tumor development in experimental rodent studies of chronic exposure to xenobiotics (Hoenerhoff et al., 2009). However, the overwhelming majority of the positive (i.e., significant increases in the incidence of liver adenomas and carcinomas) chronic rodent cancer bioassays fail to produce liver fibrosis or cirrhosis (Huff et al., 1991).
Lack of fibrosis and cirrhosis in most positive rodent 2-year cancer bioassays is in stark contrast to human HCC where liver cirrhosis is both the most common histopathological feature observed in subjects with HCC, and an important mechanism of hepatocarcinogenesis (Farazi and DePinho, 2006). In a few cases when cirrhotic changes were observed in rodent liver in association with chemical-induced hepatocellular neoplasms (e.g., thioacetamide and N-nitrosomorpholine), high necrogenic doses were applied (Becker, 1983; Oh et al., 2002). Thus, among many limitations, chronic rodent cancer bioassays do not address this key feature of human HCC. Given the importance of liver cirrhosis in the development of human HCC, integrative studies that evaluate the mechanisms of fibrogenesis, and how they relate to hepatocyte transformation and modulation of oncogenic signaling, are most relevant to better understanding of the pathogenesis of human disease.
The protocol detailed herein describes an animal model that is designed to mimic pathophysiological features of liver disease leading to HCC development in humans, where a combination of etiological factors such as genotoxic injury and advanced fibrosis are likely to be in play (Fausto and Campbell, 2010). Accordingly, this protocol selected a well-known model of liver carcinogenesis induced by a single injection of a low dose genotoxic agent DEN into 14 day-old male mice (Druckrey et al., 1964; Vesselinovitch and Mihailovich, 1983). Then, repeat dosing of the pro-fibrogenic agent carbon tetrachloride (CCl4) is performed starting at 8 weeks of age for up to 14 consecutive weeks.
Hepatocellular adenomas that develop in this model display proliferation of relatively uniform hepatocytes accompanied with a loss of normal lobular architecture and compression of the surrounding parenchyma. The histopathological features of hepatocellular carcinomas consist of a broad trabecular growth pattern of atypical hepatocytes with hemorrhaging and ischemic necrosis in the center of the tumors. Avascular and stromal invasions are also occasionally present in the hepatocellular carcinomas. While the histopathological features of the liver tumors observed in this model are similar to human HCC, the latter is a particularly heterogeneous tumor type with a wide variety of morphologic features and complex histogenesis (Mitchell, 2013). Thus, most recent classifications of HCC mainly rely on the clinical and molecular features, rather than the histopathology findings (Zucman-Rossi, 2010). Additional research to determine what are the molecular similarities of his mouse model and human HCC is needed.
This experimental design, incorporating chronic exposure to a relatively low dose of CCl4, 2.5–5 times lower than that used in other studies of CCl4-induced fibrosis or hepatocarcinogenesis (Dapito et al., 2012; Dragani et al., 1986; Fujii et al., 2010), with a low dose of environmental chemical carcinogen DEN, as much as 25 times lower than that used in other studies of co-morbidity (Dapito et al., 2012), may provide a useful model to further investigate the contribution of inflammation and fibrosis to liver cancer. There is much debate about the relative role of inflammation, growth signals and cytokines in progression of HCC and recent studies have presented conflicting evidence with respect to the contribution of these factors in murine models of HCC (Dapito et al., 2012; Luedde and Schwabe, 2011; Maeda et al., 2005). One reason for the somewhat conflicting results may be the dose of a genotoxic agent and the severity of inflammation caused by a pro-fibrogenic agent. In this regard, our model shows minimal necrogenic changes and shows that activation of cancer stem cells is the primary mechanism for a dramatic elevation in the incidence of HCC when both genotoxic and fibrogenic factors are present (Uehara et al., 2013). In this model we found that that epigenetic events, rather than mutations in known cancer-related genes, play a prominent role in increased incidence of liver tumors in this mouse model of fibrosis-associated liver cancer (Chappell et al., 2014). The epigenetic changes include demethylation of genomic DNA and repetitive elements, a decrease in histone 3 lysine 9 trimethylation, promoter hypermethylation and functional down regulation of Riz1, a histone lysine methyltransferase tumor suppressor gene, and increased expression of long interspersed nucleotide elements 1 and short interspersed nucleotide elements B2, which indicate genomic instability.
Such “fibrotic” liver murine model may not only be more human-relevant, but is also less time-consuming (hepatocellular carcinomas and adenomas develop in as little as 17 weeks of age) than a traditional 2-year rodent cancer bioassay used widely to ascribe cancer hazard to chemicals and drugs. As both research and regulatory needs are directed at the development of short-term transgenic mouse assays to supplant the need for most 2-year bioassays (Jacobson-Kram, 2010), the model characterized in our protocol may represent a more clinically relevant scenario for testing of drugs and environmental chemicals for their potential to pose human cancer hazard. In addition, as it is well recognized that most of the human HCC are a result of multiple etiologies and require liver cirrhosis, the model described here is more likely, as compared to several transgenic models currently being evaluated (Bucher, 1998), to recapitulate the fibrotic phenotype of HCC seen in humans. These features may thus be exploited in oncology drug discovery and/or the evaluation of oncological therapeutic agents directed at human HCC. While the tumor models described herein can be used in a chemo preventive or chemo therapeutic study design, this application has not yet been explored.
Critical Parameters/Troubleshooting
Pregnant mice are more sensitive to noise and smells. To decrease the incidence of cannibalism, move the cage and pups as a unit by using rubber gloved hands when changing cages with very young litters in them. In addition, avoid taking the cage off the shelf repeatedly to look at the mice.
It is important to remember that all pups before weaning are still fragile and sensitive. Pups should be weaned at 3–4 weeks of age. If pups are weaned too early, they will not survive, so it is critical that pups are weaned at an appropriate time.
Anticipated Results
In animals that receive only DEN (1 mg/kg), a single non-necrogenic dose to study the initiation phase of carcinogenesis, no evidence of injury or gross liver pathology will be observed; however, a marked increase in the incidence of liver foci with or without the occurrence of hepatocellular adenomas will be detected at 22 weeks of age (Goldsworthy and Fransson-Steen, 2002).
In CCl4 (0.2 ml/kg) only-treated animals, a significant increase in liver-body weight ratio and progressively worsening (with time) changes in liver histopathology will be detected. Specifically, single cell necrosis, ballooning degeneration of hepatocytes and steatosis will be observed in central and midlobular regions, often associated with fibrosis and inflammatory cell infiltration. Non-necrotic hepatocytes will exhibit hypertrophy with anisonucleosis. Liver foci and adenomas are to be expected in B6C3F1/J mice to occur in 12.5% of mice at 22 weeks of age, while the incidence of carcinomas should be around 25%. At earlier time point, no or limited number of foci, adenomas or carcinomas may be observed.
In DEN and CCl4-treated B6C3F1/J mice, all animals will exhibit increases in relative liver weight and marked elevation of liver injury at 22 weeks of age. All animals will develop liver adenomas and 50% should also exhibit HCC. It is of note that in CCl4-treated animals the severity of liver injury (single cell necrosis, ballooning degeneration and hypertrophy of hepatocytes, and fibrosis with inflammatory cells infiltration) in non-cancerous tissue will be of similar grade regardless of whether the animals were initiated with DEN or not.
No liver injury, pre- or neoplastic lesions should be found in the vehicle group at 22 weeks of age.
Time Considerations
Each individual mouse must be weighed at least once a week before each dosing and be administered twice weekly during the promotion period. Depending on the number of animals studied, this procedure should take 1 to 2 hr. On the day of necropsy, a significant amount of time is needed for preparation, sampling, and gross examination of liver tumors in the individual animals.
Acknowledgments
These studies were supported, in part, by the National Institutes of Health grants P42 ES005948 and R01 ES015241.
LITERATURE CITED
- AACR Cancer Progress Report Writing Committee et al. AACR Cancer Progress Report 2013. Clin Cancer Res. 2013;19:S4–98. doi: 10.1158/1078-0432.CCR-13-2107. [DOI] [PubMed] [Google Scholar]
- Alkofer B, Lepennec V, Chiche L. Hepatocellular cancer in the non-cirrhotic liver. J Visc Surg. 2011;148:3–11. doi: 10.1016/j.jviscsurg.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Aravalli RN, Cressman EN, Steer CJ. Cellular and molecular mechanisms of hepatocellular carcinoma: an update. Arch Toxicol. 2013;87:227–247. doi: 10.1007/s00204-012-0931-2. [DOI] [PubMed] [Google Scholar]
- Bannasch P. Strain and species differences in susceptibility to liver tumour induction. IARC Sci. Publ; 1983. pp. 9–38. [PubMed] [Google Scholar]
- Becker FF. Thioacetamide hepatocarcinogenesis. J Natl Cancer Inst. 1983;71:553–558. [PubMed] [Google Scholar]
- Bucher JR. Update on national toxicology program (NTP) assays with genetically altered or “transgenic” mice. Environ Health Perspect. 1998;106:619–621. doi: 10.1289/ehp.98106619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011;20:2362–2368. doi: 10.1158/1055-9965.EPI-11-0643. [DOI] [PubMed] [Google Scholar]
- Chappell G, et al. Genetic and epigenetic changes in fibrosis-associated hepatocarcinogenesis in mice. Int J Cancer. 2014 doi: 10.1002/ijc.28610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapito DH, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Corte C, Colombo M. Surveillance for hepatocellular carcinoma. Semin Oncol. 2012;39:384–398. doi: 10.1053/j.seminoncol.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Dragani TA, Manenti G, Della PG. Enhancing effects of carbon tetrachloride in mouse hepatocarcinogenesis. Cancer Lett. 1986;31:171–179. doi: 10.1016/0304-3835(86)90008-x. [DOI] [PubMed] [Google Scholar]
- Druckrey H, Steinhoff D, Preussmann R, Ivankovic S. Induction of Cancer by a Single Dose of Methylnitroso-Urea and Various Dialkylnitrosamines in Rats. Z Krebsforsch. 1964;66:1–10. [PubMed] [Google Scholar]
- El Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Fausto N, Campbell JS. Mouse models of hepatocellular carcinoma. Semin Liver Dis. 2010;30:87–98. doi: 10.1055/s-0030-1247135. [DOI] [PubMed] [Google Scholar]
- Fujii T, et al. Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. 2010;10:79. doi: 10.1186/1471-230X-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy TL, Fransson-Steen R. Quantitation of the cancer process in C57BL/6J, B6C3F1 and C3H/HeJ mice. Toxicol Pathol. 2002;30:97–105. doi: 10.1080/01926230252824770. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman G, et al. Nucleometric study of anisonucleosis, diabetes and oxidative damage in liver biopsies of orthotopic liver transplant recipients with chronic hepatitis C virus infection. Pathol Oncol Res. 2011;17:191–199. doi: 10.1007/s12253-010-9296-0. [DOI] [PubMed] [Google Scholar]
- Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90:367–386. doi: 10.1111/j.1365-2613.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenerhoff MJ, Hong HH, Ton TV, Lahousse SA, Sills RC. A review of the molecular mechanisms of chemically induced neoplasia in rat and mouse models in National Toxicology Program bioassays and their relevance to human cancer. Toxicol Pathol. 2009;37:835–848. doi: 10.1177/0192623309351726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff J, Cirvello J, Haseman J, Bucher J. Chemicals associated with site-specific neoplasia in 1394 long-term carcinogenesis experiments in laboratory rodents. Environmental Health Perspectives. 1991;93:247–270. doi: 10.1289/ehp.9193247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson-Kram D. Cancer risk assessment approaches at the FDA/CDER: Is the era of the 2-year bioassay drawing to a close? Toxicol Pathol. 2010;38:169–170. doi: 10.1177/0192623309351892. [DOI] [PubMed] [Google Scholar]
- Jepsen P, Vilstrup H, Tarone RE, Friis S, Sorensen HT. Incidence rates of hepatocellular carcinoma in the U.S. and Denmark: recent trends. Int J Cancer. 2007;121:1624–1626. doi: 10.1002/ijc.22860. [DOI] [PubMed] [Google Scholar]
- Luedde T, Schwabe RF. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Mitchell KA. Hepatocellular carcinoma: histologic considerations: pure, mixed, and motley. J Clin Gastroenterol. 2013;47(Suppl):S20–6. doi: 10.1097/MCG.0b013e318291f237. [DOI] [PubMed] [Google Scholar]
- National Research Council. Prudent practices in the laboratory: handling and disposal of chemicals. The National Academies Press; Washington, DC: 2005. [Google Scholar]
- National Toxicology Program. 12th Report on Carcinogens. Department of Health and Human Services, RTP, NC; 2011. [Google Scholar]
- Oh JY, et al. Ultrasonographic evidence of phenotypic instability during hepatocarcinogenesis in N-nitrosomorpholine-treated rats. Exp Mol Pathol. 2002;73:67–73. doi: 10.1006/exmp.2002.2434. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Stowell RE, Lee CS, Tsuboi KK, Villasana A. Histochemical and microchemical changes in experimental cirrhosis and hepatoma formation in mice by carbon tetrachloride. Cancer Res. 1951;11:345–354. [PubMed] [Google Scholar]
- Uehara T, et al. Molecular mechanisms of fibrosis-associated promotion of liver carcinogenesis. Toxicol Sci. 2013;132:53–63. doi: 10.1093/toxsci/kfs342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesselinovitch SD, Mihailovich N. Kinetics of diethylnitrosamine hepatocarcinogenesis in the infant mouse. Cancer Res. 1983;43:4253–4259. [PubMed] [Google Scholar]
- Vucur M, et al. Mouse models of hepatocarcinogenesis: what can we learn for the prevention of human hepatocellular carcinoma? Oncotarget. 2010;1:373–378. doi: 10.18632/oncotarget.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MY, Williams ES. The transgenic mouse assay as an alternative test method for regulatory carcinogenicity studies--implications for REACH. Regul Toxicol Pharmacol. 2009;53:150–155. doi: 10.1016/j.yrtph.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Zucman-Rossi J. Molecular classification of hepatocellular carcinoma. Dig Liver Dis. 2010;42(Suppl 3):S235–241. doi: 10.1016/S1590-8658(10)60511-7. [DOI] [PubMed] [Google Scholar]