Abstract
Although peroxisome proliferator-activated receptor-gamma (PPARγ) is thought to play a protective role in the vasculature, its cell-specific impact, particularly in resistance vessels is poorly defined. Nitric oxide (NO) plays a major role in vascular biology in the brain. We examined the hypothesis that selective interference with PPARγ in vascular muscle would impair NO-dependent responses as well as augmenting vasoconstrictor responses in the cerebral circulation. We studied mice expressing a dominant negative mutation in human PPARγ (P467L) under the control of the smooth muscle myosin heavy chain promoter (S-P467L). In S-P467L mice, dilator responses to exogenously applied or endogenously produced NO were greatly impaired in cerebral arteries in vitro and in small cerebral arterioles in vivo. Select NO-independent responses, including vasodilation to low concentrations of potassium, were also impaired in S-P467L mice. In contrast, increased expression of wild-type PPARγ in smooth muscle had little effect on vasomotor responses. Mechanisms underlying impairment of both NO-dependent and NO-independent vasodilator responses following interference with PPARγ involved Rho kinase with no apparent contribution by oxidative stress-related mechanisms. These findings support the concept that via effects on Rho kinase-dependent signaling, PPARγ in vascular muscle is a major determinant of vascular tone in resistance vessels, and in particular NO-mediated signaling in cerebral arteries and brain microvessels. Considering the importance of NO and Rho kinase, these findings have implications for regulation of cerebral blood flow as well as the pathogenesis of large and small vessel disease in brain.
Keywords: resistance vessels, microcirculation, cerebral arteries, nitric oxide, small vessel disease
Introduction
The nuclear receptor PPARγ is widely expressed including in vascular cells. Through several mechanisms, PPARγ is thought to exert protective effects on the vasculature in models of disease and in patients with diabetes and atherosclerosis.1–4 Concepts regarding the biological impact of PPARγ are based predominately on studies using high affinity agonists [thiazolidinediones (TZDs)] for the ligand-binding domain of the molecule. While this approach has value experimentally, TZDs also exhibit off-target effects, lack cell specificity, and do not provide insight into the impact of PPARγ when activated by endogenous ligands.1, 5
Genetic approaches offer an alternative to the use of TZDs to define the importance of PPARγ. Mutations in PPARγ have been described, some of which alter the transcriptional activity of wild type PPARγ.6 Patients with dominant negative mutations in the molecule (eg, P467L) exhibit early-onset hypertension 7, 8 consistent with possible vascular effects. The use of transgenic mice expressing these same dominant negative molecules,9, 10 provides a novel approach to interfere with wild-type PPARγ, allowing insight into the importance of PPARγ without TZD treatment. Importantly, these models mimic reductions in expresson or activity of PPARγ described in disease and in the presence of select genetic variants.6, 7, 11
Although PPARγ regulates transcription of target genes, resulting effects are often cell-specific. We initially found that cell-specific interference with PPARγ in smooth muscle impaired responses to endothelium-derived nitric oxide (NO) in the aorta in vitro.10 Because the importance of PPARγ in smaller resistance vessels was unclear, we next examined the impact of PPARγ in smooth muscle in small mesenteric arteries. We found that PPARγ normally inhibits myogenic tone in these arteries via effects on regulator of G protein signaling 5 and Ca2+-activated potassium channels.12
NO plays a fundamental role in cerebrovascular biology influencing vascular structure, local blood flow, and protecting against thrombosis.13–15 Loss of NO-mediated signaling is thought to contribute to the pathogenesis of both large and small vessel disease, with resulting hypoperfusion, cognitive impairment, and stroke.13, 16 Because the impact of NO is prominent in the cerebral circulation,13, 15 we focused the current study on cerebral blood vessels. We hypothesized that interference with PPARγ in smooth muscle would impair NO-dependent responses as well as augmenting select vasoconstrictor responses. Our primary finding was that genetic interference with PPARγ in vascular muscle affects NO-dependent and select NO-independent responses in resistance vessels in brain via effects on Rho kinase-dependent signaling.
MATERIAL AND METHODS
Details regarding the experimental procedures are presented in the online Data Supplement. In brief, we studied transgenic mice expressing either a dominant negative mutation in human PPARγ (P467L) or human wild-type PPARγ under the control of the smooth muscle myosin heavy chain promoter (S-P467L or S-WT, respectively). We quantified 1) changes in diameter of cannulated, pressurized cerebral (basilar) and small mesenteric arteries in vitro; 2) changes in isometric tension in rings of carotid arteries; and 3) changes in diameter of arterioles in the pial microcirculation in vivo using anesthestized mice.
RESULTS
Interference with PPARγ in vascular muscle impairs vasodilator responses to NO
We first verified that the transgene of interest was expressed in cerebral arteries of S-P467L mice using quantitative real-time PCR. Similar to results in mesenteric arteries,12 we detected human PPARγ mRNA in cerebral arteries in transgenic mice (Ct values ranging from 21–25) but not non-transgenic (non-Tg) littermates (Ct was undetectable)(data not shown).
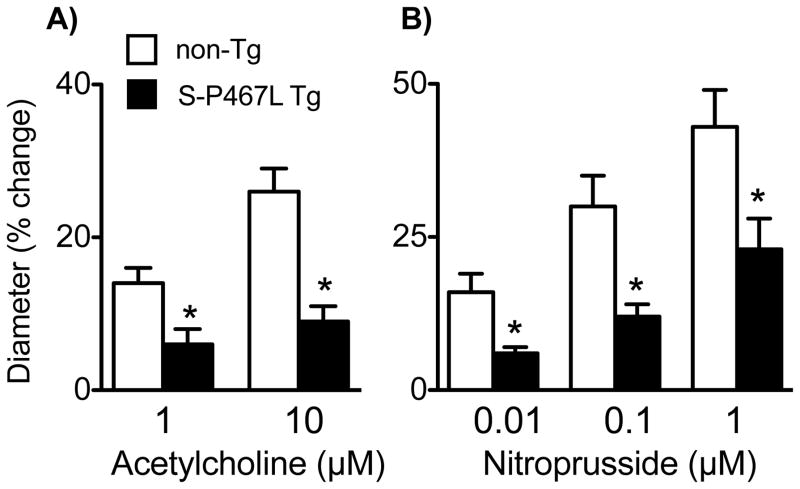
Baseline diameter of the basilar artery was similar in non-Tg and S-P467L mice, respectively (Figure 1A). Dilation of the basilar artery to the NO donor nitroprusside was greatly reduced in S-P467L mice (Figure 1B). For example, increases in vessel diameter in response to 100 μmol/L nitroprusside in control and S-P467L mice were 78±5 and 32±5% (P<0.01 vs non-Tg), respectively.
Figure 1.

Baseline diameter (A) of basilar arteries from non-Tg (n=25), S-P467L (n=21), and S-WT mice (n=6). Dilation of the basilar artery to nitroprusside (B)(n=9–11), acetylcholine (C)(n=21–25), and Ang 1-7 (D)(n=12–19) in non-Tg, S-P467L, and S-WT mice. * P<0.01 vs non-Tg, # P<0.05 vs non-Tg.
To test whether vascular phenotypes in S-P467L mice were due to dominant negative effects of the P467L mutation as opposed to overexpression of PPARγ, we examined responses in transgenic mice expressing wild-type human PPARγ specifically targeted to smooth muscle (S-WT).10 Baseline diameter of the basilar artery was similar in S-WT compared to non-Tg (Figure 1A). In contrast to the reduced effects of nitroprusside in S-P467L mice, effects of nitroprusside were similar in S-WT and controls (Figure 1B).
We next determined if responses to endogenously-produced NO are also affected in S-P467L mice using endothelium-dependent agonists. Dilation of the basilar artery to acetylcholine is prevented by inhibition of NO synthase.17 In the current experiments, vasodilation to angiotensin 1-7 (Ang 1-7) was greatly reduced by a mas receptor antagonist (A779, 1 μmol/L) and was completely inhibited by NG-nitro-L-arginine (100 μmol/L) (Figure S1) demonstrating this response was also mediated by NO. Dilation of the basilar artery to both acetylcholine and Ang 1-7 was reduced substantially in S-P467L mice (Figure 1C and 1D). In both strains of mice, responses to acetylcholine were similar in male and female mice (Figure S2). Responses to Ang 1-7 in arteries from S-WT were not impaired and were increased moderately at the highest concentration tested (Figure 1D).
Interference with PPARγ in smooth muscle impairs cerebral microvascular responses to NO
Considering the importance of microvascular NO and small vessel disease in brain, we examined the impact of PPARγ in the microcirculation in vivo. Baseline diameters of small cerebral (pial) arterioles in non-Tg and S-P467L mice were similar (34±1 and 35±2 μm, respectively, P>0.05). Vasodilation to acetylcholine (which is mediated by NO in brain microvessels18 and responses to nitroprusside were reduced by ~50–65% in S-P467L mice compared to non-Tg controls (Figure 2A and B).
Figure 2.
Changes in diameter of cerebral arterioles in response to acetylcholine (A) and nitroprusside (B) in non-Tg (n=15) and S-P467L (n=12) mice. *P<0.05 vs non-Tg.
Regional differences in the impact of PPARγ in vascular muscle
Our initial study found that vasodilation to NO is impaired in aorta from S-P467L mice.10 To determine if impaired responses to NO in S-P467L mice in the current study were unique to the cerebral circulation, we studied carotid arteries and small mesenteric arteries. In contrast to the marked reduction in responses in cerebral arteries in S-P467L mice, vasodilation to nitroprusside was impaired modestly in carotid arteries and was not impaired in small mesenteric arteries (Figure S3).
Interference with PPARγ in vascular muscle impairs select NO-independent responses in the cerebral circulation
To determine if other vasodilator mechanisms were affected in cerebral arteries in S-P467L mice, we examined responses to stimuli that act independent of NO. Depending on the concentration, changes in extracellular potassium (K+) can produce vasodilation or vasoconstriction. By activating inward rectifier K+ channels (KIR) in vascular muscle, modest elevations in extracellular K+ is a potent vasodilator stimulus.19, 20 In control mice, low concentrations of K+ produced vasodilation, a response that was significantly reduced by barium (30 μmol/L), an inhibitor of KIR (Figure 3A). Dilation of the basilar artery to K+ was greatly impaired in S-P467L mice (Figure 3B), but was not altered in S-WT compared to non-Tg controls (Figure 3B). To determine if the vasodilator effect of other K+ channels were affected, we tested responses to cromakalim, which activates ATP-sensitive K+ channels, and arachidonic acid, which activates calcium-dependent K+ channels in cerebral vessels.14 Dilation of the basilar artery to both agonists was impaired in S-P467L mice (Figure 3C and D). In contrast to NO and these select vasodilators, cerebral arteries dilated normally to the calcium channel blocker nifedipine and the endothelium-independent agonist papaverine (Figure 3E and F). Constriction of the basilar artery to Bay K 8644, a L-type calcium channel activator was similar in both groups of mice (data not shown).
Figure 3.
Dilation of the basilar artery to KCl in non-Tg mice in the absence or presence of barium (A)(n=3–6). Effects of Y-27632 on dilation of the basilar artery to KCl (B)(n=6–9) and responses to KCl in S-WT mice (C)(n=6). Responses of the basilar artery to arachidonic acid (D)(n=6), cromakalim (E)(n=5–8), and nifedipine (F)(n=5–10) in non-Tg and S-P467L mice, along with responses to papaverine (F) in non-Tg (n=25), S-P467L (n=21), and S-WT (n=6) mice. * P<0.01 vs non-Tg.
Vascular dysfunction in S-P467L mice is mediated by Rho-kinase
We next examined mechanisms that account for impaired responses in S-P467L mice. The primary molecular target of NO is soluble guanylate cyclase (sGC), which synthesizes cGMP following activation. Most vascular effects of NO require activation of sGC.13, 18 Consistent with this concept, dilation of the basilar artery to nitroprusside in both non-Tg and S-P467L mice were completely prevented by ODQ (10 μmol/L) (Figure S4), an inhibitor of sGC. Oxidative stress contributes to vascular disease through several mechanisms including impairment of NO-mediated effects.13, 16 We examined the possible role of oxidative stress using two approaches. Tempol (1 mmol/L), a superoxide dismutase mimetic, did not improve vasodilator responses to Ang 1-7 in S-P467L mice (Figure S5). A consequence of oxidative stress is activation of poly-ADP-ribose polymerase (PARP), a nuclear protein that plays a role in vascular dysfunction in some disease states. A selective inhibitor of PARP (PJ34, 3 μmol/L) did not alter responses to Ang 1-7 (Figure S5).
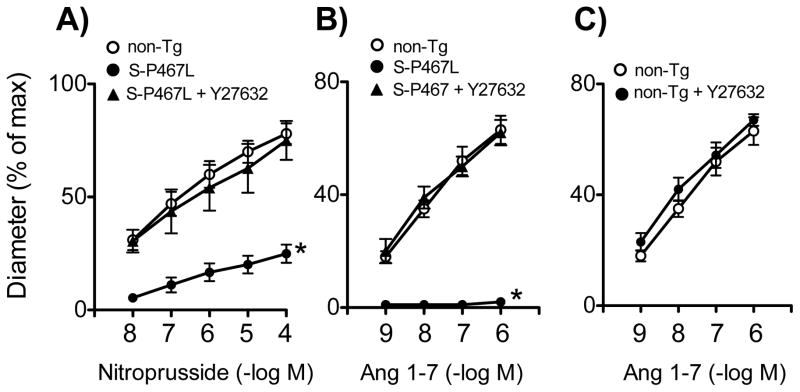
We next considered other potential targets of PPARγ that could impair regulation of vascular tone. Rho kinase, a target of the GTPase Rho,21, 22 is a major determinant of vascular tone and may be inhibited by TZD treatment.1, 10, 23 We previously obtained evidence that Rho kinase was the mediator of increased contractile responses in aorta to endothelin-1 (ET-1) in S-P467L mice.10 Treatment of cerebral arteries with an inhibitor of Rho kinase (Y-27632, 3 μmol/L) restored responses to nitroprusside, K+, and Ang 1-7 in S-P467L mice (Figures 3B, 4A, 4B). In time control experiments, treatment with Y-27632 alone did not alter U46619-induced tone (n=4)(data not shown). In addition, Y-27632 did not alter vasodilator responses to Ang 1-7 when tested in non-Tg mice (Figure 4C). These findings suggest that impaired vasodilator responses in S-P467L mice are mediated by Rho kinase. Expression of Rho kinase isoforms (ROCK1 and ROCK2) in cerebral arteries was similar in non-Tg and S-P467L mice although mRNA levels of ROCK2 tended to be increased (Figure S6).
Figure 4.
Effects of Y-27632 on dilation of the basilar artery to nitroprusside (A)(n=5) and Ang 1-7 (B)(n=6) in S-P467L mice and effects of Y-27632 on Ang 1-7 responses in non-Tg (n=5). * P<0.01 vs non-Tg.
Interference with PPARγ in vascular muscle augments cerebral vasoconstrictor responses
To determine if vasoconstrictor responses were affected in S-P467L mice, we examined effects of serotonin and ET-1, agonists implicated in vascular disease and stroke. Constriction of the basilar artery to both ET-1 and serotonin were increased in S-P467L mice (Figure 5A and B). In contrast, vasoconstriction to KCl (50 mmol/L) was not altered in S-P467L or S-WT mice compared to littermate controls (Figure 5C). Enhanced vasoconstriction to both ET-1 and serotonin in S-P467L mice was prevented by Y-27632 (Figure 5A and B).
Figure 5.
Responses of the basilar artery to serotonin (A)(n=11–15) and endothelin-1 (B) (n=9) in non-Tg and S-P467L mice in the absence and presence of Y-27632 (n=6–8). Responses to KCl in non-Tg (n=25), S-P467L (n=21), and S-WT (n=6) mice are also shown (C). * P<0.05 vs non-Tg.
DISCUSSION
There are several major new findings in this study. First, genetic interference with PPARγ in smooth muscle impaired responses to both exogenously applied and endogenously produced NO in cerebral arteries in vitro and small cerebral microvessels in vivo. These changes in resistance vessels in brain were selective, as responses of small mesenteric arteries to NO were not affected. Second, the impact of PPARγ in vascular muscle extended to select NO-independent mechanisms of vasodilation and some vasoconstrictor responses. Third, in contrast to the dominant negative form of PPARγ, increased expression of wild-type PPARγ in vascular muscle did not reduce responses to several stimuli including NO. Fourth, mechanisms underlying impairment of both NO-dependent and NO-independent vasodilator responses following interference with PPARγ involved Rho kinase with no apparent contribution by oxidative stress-related mechanisms. These findings support the concept that PPARγ in vascular muscle is a major determinant of vascular tone, particularly NO-mediated signaling, in cerebral arteries and microvessels. Considering the importance of NO and Rho kinase in resistance vessels, these findings have implications for mechanisms that regulate cerebral blood flow as well as the pathogenesis of large and small vessel disease (Figure S7).
PPARγ and the vasculature
PPARγ was initially thought to predominantly influence adipocytes function in addition to having effects on glucose and lipid metabolism.12, 24 More recent findings mostly based on studies using TZD treatment suggest PPARγ can exert effects in multiple cell types. PPARγ is expressed in vascular cells.2, 11, 12, 25 Using TZDs as an exogenous ligand, effects of PPARγ activation on vascular structure, vasomotor tone, and vascular permeability have been described.1, 2, 26–28 An underlying assumption of most TZD-based studies is that the effects observed are mediated by PPARγ acting within vascular cells. Because systemic administration of TZDs affects all cells, distinguishing vascular-specific effects is very difficult. In addition to activating PPARγ, TZDs can also exert off-target or PPARγ-independent effects.3, 5
Patients with select mutations in the ligand-binding domain of PPARγ (eg, P467L) exhibit early-onset hypertension and insulin resistance.7 Heterozygous knockin mice expressing this mutation in all cells share features of human disease including abnormal fat distribution.8 To avoid such systemic effects and to study the impact of cell-specific manipulation in vivo, we used S-P467L mice.10, 29 Global knockout of PPARγ is lethal and cell-specific knockout of PPARγ would be extremely rare.6, 12 A major advantage of using a model with specific alterations in PPARγ in smooth muscle is that it avoids metabolic effects seen with global genetic manipulation of PPARγ or whole body TZD treatment. In S-P467L mice, there is no change in body weight or adipose tissue depots as well as no effect on plasma glucose, insulin, or leptin.10, 29 S-P467L mice exhibit a modest elevation in systolic blood pressure (~12 mmHg).
PPARγ in vascular muscle exerts prominent effects on NO-mediated vasodilation in brain
Baseline diameter of cerebral arteries and responses to high concentrations of KCl or papaverine were not affected by transgenic expression of either human P467L or wild-type PPARγ in smooth muscle. Thus, these genetic alterations did not produce non-selective changes in vasoconstrictor or vasodilator capacity.
Although our previous study demonstrated that genetic interference with PPARγ in smooth muscle inhibited NO-mediated relaxation in aorta,10, 29 the importance of PPARγ in resistance vessels and the microcirculation in vivo was not defined. In brain, cerebral arteries and arterioles are important resistance vessels.13 Because of its prominent role in both large and small vessels in brain, we performed the present study with a focus on NO. We found that dilation of basilar arteries to an NO donor was markedly reduced in S-P467L mice. Similarly, responses to agonists that produce NO-mediated vasodilation (acetylcholine and Ang 1-7) were also greatly impaired.
Consistent with the results for the isolated basilar artery, we found that responses of small cerebral arterioles to NO were substantially reduced in S-P467L mice in vivo. Thus, the impact of PPARγ in smooth muscle extends along the vascular tree in brain and is functionally important in vivo. A recent study found that genetic deletion of PPARγ in smooth muscle did not affect responses to NO in small mesenteric arteries.30 This finding is consistent with our results in S-P467L mice, where responses to an NO donor were not altered in these same arteries. Overall, these findings suggest that in the mesenteric vasculature, PPARγ does not influence NO-mediated signaling in vascular muscle. The finding that the impact of PPARγ in vascular muscle exhibits such regional heterogeneity in relation to NO was unexpected but highlights the importance of this molecule in the cerebrovasculature.
Influence of PPARγ on NO-independent vasodilation
To determine if mechanisms other than those involving NO were affected by interference with PPARγ, we examined effects of stimuli that act independently from NO in vascular muscle. Modest elevations in extracellular K+ activate inward rectifier K+ channels in vascular muscle, producing marked vasodilation.19, 20 We found that vasodilation to K+ was greatly impaired in S-P467L mice. Because K+-induced vasodilation may contribute to neurovascular coupling,19 these finding implicate PPARγ as a potential determinant of functional hyperemia. To evaluate if the impact of vascular PPARγ extended to other K+ channels, we examined effects of cromkalim and arachidonic acid. Responses to these agonists were substantially reduced in S-P467L mice. In contrast, vasodilation to both papaverine and nifedipine were unaffected. These findings suggest that interference with PPARγ in smooth muscle has effects on both NO-dependent and select NO-independent mechanisms that regulate cerebrovascular tone.
Effects of PPARγ interference in vascular muscle: Role of Rho-kinase
Because interference with PPARγ in smooth muscle affected several vasodilator responses, it seemed likely that underlying mechanisms might involve pathways that more broadly influence vasomotor tone. In this context, we considered the possibility that oxidative stress was involved. However, neither a superoxide dismutase mimetic or a PARP inhibitor affected responses to NO in S-P467L mice.
A second candidate to explain reduced vasodilation in S-P467L mice was Rho kinase.21, 22 Due to effects on calcium sensitivity and the phosphorylation state of the regulatory myosin light chain, Rho kinase in vascular muscle is a major determinant of vascular tone.21, 22 Our initial study in aorta provided evidence that Rho kinase signaling is altered following interference with PPARγ in vascular muscle.10 Rho kinase plays a key role in the vasculature but its impact varies regionally and is cell specific.21, 22 Thus, the impact within resistance vessels was hard to predict. We observed a similar role for Rho kinase in resistance vessels in the current experiments. We found that inhibition of Rho-kinase restored responses to NO and low concentrations of K+ while preventing hyperresponsiveness to ET-1 and serotonin. In endothelium, NO-mediated signaling can be regulated via effects of Rho kinase on eNOS phosphorylation.31 Because the model used in the current study expressed a dominant negative form of PPARγ specifically in smooth muscle, we assume effects on responses to NO are mediated within vascular muscle. In addition, we found previously that level of total and phospho-Ser1177 eNOS protein was similar in aortic tissue from control and S-P467L mice.29
Perspectives
NO has diverse effects within the cerebral vasculature, mediating responses to neurotransmitters, shear forces, metabolic factors, and therapeutic agents while inhibiting vascular hypertrophy and metabolism of β-amyloid precursor protein.13, 15 Thus, loss of NO-mediated signaling is thought to be an important contributor to cerebrovascular disease. The present study suggests that PPARγ in smooth muscle is part of previously unrecognized mechanism that influences NO-mediated signaling and regulation of tone in resistance vessels in brain. Rho kinase contributes to vascular disease,21, 22 but mechanisms that regulate this pathway and interactions with NO are poorly defined, particularly at the cell-specific level. In addition to effects on vascular tone, Rho kinase has been implicated in atherosclerosis, small vessel disease, development of cerebral cavernous malformations and aneurysms, as well as vasospasm following subarachnoid hemorrhage (eg, 21, 32). Activity of Rho kinase is positively associated with cardiovascular events including stroke.33 Thus, the finding that PPARγ in vascular muscle is an important regulator of NO- and Rho kinase-dependent effects has diverse implications. Therapeutic approaches or other strategies to target PPARγ or its downstream effectors in vascular muscle may be beneficial in preventing or slowing the progression of large and small vessel disease in brain.
Supplementary Material
Novelty and Significance.
1. What Is New?
Our findings suggest that PPARγ in vascular muscle is a major determinant of tone in resistance vessels in brain, particularly in relation to NO-mediated signaling. This influence was apparent under normal conditions, in the absence of treatment with pharmacological activators of PPARγ.
Interference with PPARγ in smooth mucle had prominent effects in cerebral vessels, but no detectable effect in relation to NO in small mesenteric arteries.
These findings support the concept that PPARγ in vascular muscle is part of a previously unrecognized mechanism that inhibits Rho kinase-dependent signaling, thus affecting vasodilator responses.
2. What Is Relevant?
While NO is considered to be a key molecule in relation to its influence on cerebrovascular structure and function, mechanisms that regulate these influences at the cell-specific level are poorly defined.
Considering the importance of NO and Rho kinase in cerebral vessels, these findings have implications for mechanisms that regulate brain perfusion as well as the pathogenesis of large and small vessel disease.
3. Summary
This study provides genetic evidence that interference with PPARγ in smooth muscle impairs cerebrovascular responses to NO both in vitro and in vivo. Similar effects were were not seen in small mesenteric arteries. The impact of PPARγ in vascular muscle extended to select NO-independent mechanisms of vasodilation and some vasoconstrictor responses. Mechanisms underlying these effects involved Rho kinase. These findings support the concept that PPARγ in vascular muscle is a major determinant of vascular tone, and in particular NO-mediated signaling in cerebral arteries and brain microvessels. The results further suggest that PPARγ in vascular muscle normally protects against excessive Rho kinase-dependent signaling in resistance vessels.
Acknowledgments
GRANT SUPPORT
This work was supported by the National Institutes of Health (NS-24621, HL-62984, HL-48058, HL-61446, and HL-113863), the Department of Veterans Affairs (BX001399), and the Fondation Leducq (Transatlantic Network of Excellence). Post-doctoral Fellowship support was provided by the American Heart Association (12POST9150027 and 11POST5720021) and the National Health and Medical Research Council of Australia (1053786). The authors acknowledge the generous research support of the Roy J. Carver Trust.
The authors thank Dale Kinzenbaw for technical assistance.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Ketsawatsomkron P, Pelham CJ, Groh S, Keen HL, Faraci FM, Sigmund CD. Does peroxisome proliferator-activated receptor-γ (PPARγ) protect from hypertension directly through effects in the vasculature? J Biol Chem. 2010;285:9311–9316. doi: 10.1074/jbc.R109.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchesi C, Schiffrin EL. Peroxisome proliferator-activated receptors and the vascular system: beyond their metabolic effects. J Am Soc Hypertens. 2008;2:227–238. doi: 10.1016/j.jash.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Plutzky J. The PPAR-RXR transcriptional complex in the vasculature: energy in the balance. Circ Res. 2011;108:1002–1016. doi: 10.1161/CIRCRESAHA.110.226860. [DOI] [PubMed] [Google Scholar]
- 4.Davidson M, Meyer PM, Haffner S, Feinstein S, D’Agostino R, Sr, Kondos GT, Perez A, Chen Z, Mazzone T. Increased high-density lipoprotein cholesterol predicts the pioglitazone-mediated reduction of carotid intima-media thickness progression in patients with type 2 diabetes mellitus. Circulation. 2008;117:2123–2130. doi: 10.1161/CIRCULATIONAHA.107.746610. [DOI] [PubMed] [Google Scholar]
- 5.Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR) Toxicol Sci. 2006;90:269–295. doi: 10.1093/toxsci/kfj062. [DOI] [PubMed] [Google Scholar]
- 6.Heikkinen S, Auwerx J, Argmann CA. PPARγ in human and mouse physiology. Biochim Biophys Acta. 2007;1771:999–1013. doi: 10.1016/j.bbalip.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O’Rahilly S. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 8.Tsai YS, Kim HJ, Takahashi N, Kim HS, Hagaman JR, Kim JK, Maeda N. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARγ. J Clin Invest. 2004;114:240–249. doi: 10.1172/JCI20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beyer AM, Baumbach GL, Halabi CM, Modrick ML, Lynch CM, Gerhold TD, Ghoneim SM, De Lange WJ, Keen HL, Tsai YS, Maeda N, Sigmund CD, Faraci FM. Interference with PPARγ signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension. 2008;51:867–871. doi: 10.1161/HYPERTENSIONAHA.107.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPARγ function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexis JD, Wang N, Che W, Lerner-Marmarosh N, Sahni A, Korshunov VA, Zou Y, Ding B, Yan D, Berk BC, Abe J. Bcr kinase activation by angiotensin II inhibits peroxisome-proliferator-activated receptor γ transcriptional activity in vascular smooth muscle cells. Circ Res. 2009;104:69–78. doi: 10.1161/CIRCRESAHA.108.188409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ketsawatsomkron P, Lorca RA, Keen HL, Weatherford ET, Liu X, Pelham CJ, Grobe JL, Faraci FM, England SK, Sigmund CD. PPARγ regulates resistance vessel tone through a mechanism involving RGS5-mediated control of protein kinase C and BKCa channel activity. Circ Res. 2012;111:1446–1458. doi: 10.1161/CIRCRESAHA.112.271577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faraci FM. Protecting against vascular disease in brain. Am J Physiol. 2011;300:H1566–1582. doi: 10.1152/ajpheart.01310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faraci FM, Heistad DD. Regulation of the cerebral circulation: Role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 15.Katusic ZS, Austin SA. Endothelial nitric oxide: Protector of a healthy mind. Eur Heart J. 2014;35:888–894. doi: 10.1093/eurheartj/eht544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faraci FM, Modrick ML, Lynch CM, Didion LA, Fegan PE, Didion SP. Selective cerebral vascular dysfunction in Mn-SOD-deficient mice. J Appl Physiol. 2006;100:2089–2093. doi: 10.1152/japplphysiol.00939.2005. [DOI] [PubMed] [Google Scholar]
- 18.Sobey CG, Faraci FM. Effects of a novel inhibitor of guanylyl cyclase on dilator responses of mouse cerebral arterioles. Stroke. 1997;28:837–842. doi: 10.1161/01.str.28.4.837. [DOI] [PubMed] [Google Scholar]
- 19.Dunn KM, Nelson MT. Potassium channels and neurovascular coupling. Circ J. 2010;74:608–616. doi: 10.1253/circj.cj-10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knot HJ, Zimmermann PA, Nelson MT. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J Physiol. 1996;492:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budzyn K, Marley PD, Sobey CG. Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci. 2006;27:97–104. doi: 10.1016/j.tips.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Sawada N, Liao JK. Rho/Rho-associated coiled-coil forming kinase pathway as therapeutic targets for statins in atherosclerosis. Antioxid Redox Signal. 2014;20:1251–1267. doi: 10.1089/ars.2013.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakino S, Hayashi K, Kanda T, Tatematsu S, Homma K, Yoshioka K, Takamatsu I, Saruta T. Peroxisome proliferator-activated receptor γ ligands inhibit Rho/Rho kinase pathway by inducing protein tyrosine phosphatase SHP-2. Circ Res. 2004;95:e45–55. doi: 10.1161/01.RES.0000142313.68389.92. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ. J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 25.Marx N, Bourcier T, Sukhova GK, Libby P, Plutzky J. PPARγ activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPARγ as a potential mediator in vascular disease. Arterioscler Thromb Vasc Biol. 1999;19:546–551. doi: 10.1161/01.atv.19.3.546. [DOI] [PubMed] [Google Scholar]
- 26.Cipolla MJ, Bishop N, Vinke RS, Godfrey JA. PPARγ activation prevents hypertensive remodeling of cerebral arteries and improves vascular function in female rats. Stroke. 2010;41:1266–1270. doi: 10.1161/STROKEAHA.109.576942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts TJ, Chapman AC, Cipolla MJ. PPARγ agonist rosiglitazone reverses increased cerebral venous hydraulic conductivity during hypertension. Am J Physiol. 2009;297:H1347–1353. doi: 10.1152/ajpheart.00630.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPARγ agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension. 2004;43:661–666. doi: 10.1161/01.HYP.0000116303.71408.c2. [DOI] [PubMed] [Google Scholar]
- 29.Pelham CJ, Ketsawatsomkron P, Groh S, Grobe JL, de Lange WJ, Ibeawuchi SR, Keel HL, Weatherford ET, Faraci FM, Sigmund CD. Cullin-3 regulates vascular smooth muscle function and arterial blood pressure via PPARγ and RhoA/Rho-kinase. Cell Metabol. 2012;16:462–472. doi: 10.1016/j.cmet.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang L, Villacorta L, Zhang J, Garcia-Barrio MT, Yang K, Hamblin M, Whitesall ET, D’Alecy LG, Chen YE. Vascular smooth muscle cell-selective peroxisome proliferator-activated receptor-γ deletion leads to hypotension. Circulation. 2009;119:2161–2169. doi: 10.1161/CIRCULATIONAHA.108.815803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faraco G, Moraga A, Moore J, Anrather J, Pickel VM, Iadecola C. Circulating endothelin-1 alters critical mechanisms regulating cerebral microcirculation. Hypertension. 2013;62:759–766. doi: 10.1161/HYPERTENSIONAHA.113.01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson BT, Dibble CF, Borikova AL, Johnson GL. Cerebral cavernous malformation is a vascular disease associated with activated RhoA signaling. Biol Chem. 2013;394:35–42. doi: 10.1515/hsz-2012-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kajikawa M, Noma K, Maruhashi T, Mikami S, Iwamoto Y, Iwamoto A, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Nakashima A, Goto C, Liao JK, Higashi Y. Rho-associated kinase activity Is a predictor of cardiovascular outcomes. Hypertension. 2014;63:856–864. doi: 10.1161/HYPERTENSIONAHA.113.02296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.