Abstract
Background
Efficient development of atopic diseases requires interaction between allergen and adjuvant to initiate and amplify underlying inflammatory responses. Substance P (SP) and hemokinin-1 (HK-1) are neuropeptides that signal via the neurokinin-1 receptor (NK1R) to promote inflammation. Mast cells initiate the symptoms and tissue effects of atopic disorders, secreting TNF and IL-6 following FcεRI crosslinking by Ag-IgE complexes, (FcεRI-MCs). Additionally, MCs express the NK1R suggesting an adjuvant role of NK1R agonists for FcεRI-MC mediated pathologies, however in depth research addressing this relevant aspect of MC biology is lacking.
Objective
To investigate the effect of NK1R-signaling and the individual roles of SP and HK-1 as potential adjuvants for FcεRI-MC mediated allergic disorders.
Methods
Bone marrow (BM) MCs derived from C57BL/6-wild type (WT) or NK1R−/− mice were used to investigate the effects of NK1R signaling of FcεRI-activated MCs. BMMCs generated from Tac1−/− mice or following culture with Tac4 siRNA were used to address the adjuvancy of SP and HK-1. WT, NK1R−/− and c-KitW-sh/W-sh mice reconstituted with WT or NK1R−/− BMMCs were utilized to evaluate NK1R signaling on FcεRI-MC-mediated passive local and systemic anaphylaxis and airway inflammation.
Results
FcεRI-activated MCs up-regulated NK1R and HK-1 transcripts and protein synthesis, without modifying SP. In a positive signaling loop, HK-1 promoted TNF and IL6 secretion by MC degranulation and protein synthesis the later via the PI3K/Akt/NFκB pathways. In vivo, NK1R signaling was necessary for development of passive local and systemic anaphylaxis and chronic airway inflammation.
Conclusions
FcεRI-stimulation of MCs promotes autocrine secretion of HK-1 which signals via NK1R to provide adjuvancy for efficient development of FcεRI-MC-mediated disorders.
Keywords: Hemokinin-1, Substance P, NK1R, Mast cells, IgE, FcεRI, TNF, IL-6, Passive Anaphylaxis, Airway Inflammation
INTRODUCTION
The immune system is designed to eliminate foreign Ag while maintaining tissue integrity. To accomplish these functions, innate and adaptive immune responses should effectively be resolved following the neutralization of foreign Ag. Adjuvant mediated increases in intensity or prolongation of inflammatory reactions result in the fatal outcome in the case of anaphylaxis, and irreversible tissue damage with function loss. Inflammatory responses are amplified by neuropeptides from the tachykinin family such as, substance P (SP) and hemokinin-1 (HK-1)1. Both neuropeptides exert their effects by signaling via the neurokinin-1 receptor (NK1R), a seven trans-membrane domain G-protein coupled receptor (GPCR)2. NK1R signaling mediates pain, inflammation and immune function, the later by dendritic cell and monocyte activation3–6, chemotaxis and cytotoxicity of natural killer cells7, 8, and differentiation and survival of pro-T and -B lymphocytes9–12. Previous reports on SP and HK-1 pro-inflammatory function and the observation that SP is increased in autoimmune disorders13–15 highly suggest an adjuvant role for NK1R agonists.
Peripheral tissue resident mast cells (MCs) express the NK1R16 and are ideally positioned to respond to foreign antigens with innate and adaptive immune functions17, 18. Activation of MCs via the high affinity IgE receptor (FcεRI-MCs) is central to their pathologic inflammatory function. Crosslinking of surface FcεRI in MCs initiates a biphasic inflammatory response comprising immediate degranulation with release of stored pro-inflammatory mediators, and delayed secretion of de novo synthesized pro-inflammatory cytokines. FcεRI-activated MCs release TNF and IL-6 that trigger anaphylaxis and mediate the symptoms and tissue effects of chronic atopic disorders17, 18.
Mechanistically, FcεRI activation recruits Src family kinases18 to signal via phosphatidylinositide3-kinase (PI3K) and phospholipase C cascades that interconnect with intracellular signaling pathways initiated by GPCR2, 18. Accordingly, interactions between FcεRI and NK1R signaling may regulate MC inflammatory functions. While scarce reports have associated SP with IgE-independent MC functions19–21, the mechanisms and individual roles of NK1R agonists in the biology and function FcεRIMCs remain unknown. Furthermore, to our knowledge, information regarding the contribution of HK-1 to MC inflammatory functions is lacking.
In the present work, we demonstrate that signaling murine MCs via FcεRI up-regulates, i) the expression of the NK1R, ii) transcription of the HK-1 gene (Tac4) and, iii) synthesis of HK-1, without modifying transcription of the SP gene (Tac1) or secretion of SP peptide. In an autocrine/paracrine positive signaling loop, binding of the NK1R by HK-1 is critical for FcεRI-mediated MC secretion of granule-stored and the novo synthesized TNF leading to the in vivo initiation of local and systemic anaphylaxis as well as development or maintenance of airway inflammation.
MATERIALS AND METHODS
Supplemental methods can be found in the Online Repository for this manuscript.
Mice
Female wild type (WT) C57BL/6 and B.Cg Tac1tm1Bm/j (Tac1−/−) mice (8–12 week old) purchased from The Jackson Laboratory and rested for 1 week before use. C57BL/6-KitW-sh/W-sh mice were initially purchased from Jackson Laboratories and bred at the University of Pittsburgh's Animal Facility. NK1R−/− mice, initially provided by Dr. Christopher Paige, University of Toronto, have been back-crossed to homozygosity by breeding 8 generations before use. Studies were performed following IACUC approval of protocols and procedures (University of Pittsburgh).
Statistical Analysis
Data were analyzed by 1- or 2-way ANOVA with Bonferroni post-hoc analysis using GraphPad Prism v5.0 (GraphPad Software). When only two groups were compared, significant differences were determined by Student's two-tailed t-test. A p-value <0.05 was considered significant.
RESULTS
NK1R signaling enhances FcεRI-initiated MC functions
We analyzed the presence of the functional NK1R by detection of its intracellular C-terminus motif 2 in IL-3-derived wild type (WT) C57BL/6 BMMCs. The basal level of NK1R expression was low in non-activated BMMCs, as reported previously16. However, FcεRI activation of BMMCs significantly increased NK1R mRNA and protein expression (Fig 1, A and B). It has been shown that differentiation of BMMCs with high concentrations of IL-4 increases NK1R expression16. Since FcεRI activation causes release of IL-4 from BMMCs17 (Fig. E1, A in the Online Repository), we hypothesized that autocrine IL-4 may play a role in the regulation of NK1R expression. Inhibition of autocrine IL-4 with neutralizing anti-IL-4 antibody inhibited FcεRI-driven NK1R expression (Fig. E1, B in the Online Repository). In contrast, BMMCs cultured with exogenous IL-4 but without FcεRI activation were unable to further increase NK1R expression (Fig. E1, C in the Online Repository). Together, these results indicate that in our working conditions NK1R expression in BMMCs is regulated by autocrine IL-4 secretion initiated by FcεRI signaling.
FIG 1. Role of NK1R in FcεRI-BMMCs.
A, Expression of NK1R in control (filled histogram) and FcεRI-BMMCs (open histogram) [loaded with IgE (SPE-07, 1.0 μg/ml, 1 h then cross-linked with Ag (DNP-HSA, 200 ng/ml) for 18 h]. Mean ± 1 SD of the percent positive from 3 experiments. B, Signaling pathways involved in NK1R transcript synthesis in FcεRI-BMMCs 2 hours after FcεRI ligation with Ag in the presence of inhibitors specific for the indicated pathways. Means + 1 SD from 3 experiments. C, Calcium flux in WT (black line) or NK1R−/− (gray line) BMMCs loaded with Ig and pulsed with Ag or ionomycin at the indicated time. One representative of 3 experiments. D–E, Degranulation of WT and NK1R−/− FcεRI-BMMCs, 90 min following FcεRI ligation with Ag. D, A representative flow plot from three experiments; values are means ± 1 SD of the percentage of degranulating BMMCs either loaded with IgE (1.0 μg/ml, 1 h) then cross-linked with Ag (200 ng/ml, 90 min) or activated with compound (c) 48/80 (1.0 μg/ml). E, Data points depict the mean ± 1 SD of the percentage of degranulating BMMCs from three experiments. F, TNF and IL-6 release by FcεRI-BMMCs, 18h Ag. Mean + 1 SD of duplicate values from a representative of three experiments. *p<0.05 **p<0.01, ***p<0.001, ****p<0.0001.
Then, we investigated IL-4 independent signaling pathways regulating NK1R expression in FcεRI-BMMCs including, NFκB, JNK and ERK, which regulate NK1R expression in dendritic cells3, 4 and other cell types22, 23. Inhibition of NFκB and JNK, but not ERK, reduced NK1R mRNA induced by FcεRI ligation (Fig 1, B) demonstrating that FcεRI-ligation of MCs promotes NK1R expression through common intracellular pathways described in other cell types3, 4, 22, 23.
Ca+2 dependent FcεRI-MC-degranulation is potentiated by certain GPCR agonists17, 18. Since the NK1R is a GPCR, we analyzed whether it was involved in Ca+2 flux and MC degranulation by comparing these two functions in FcεRI-BMMCs generated from WT or NK1R−/− mice. Both BMMC strains had comparable differentiation and maturation stages according to their identical expression of c-Kit and FcεRI, (Fig E2 in the Online Repository) and they displayed similar changes in intracellular Ca+2 levels (Fig 1, C). However, fewer NK1R−/− BMMCs degranulated compared to WT BMMCs (Fig 1, D), irrespective of Ag concentration (Fig 1, E). Conversely, degranulation of MCs induced with the Compound (c) 48/80, an independent GPCR signaling stimulus24, triggered robust degranulation in both, WT and NK1R−/− BMMCs. These data demonstrate that the NK1R belongs to the group of GPCR family members that potentiate FcεRI mediated MCs degranulation.
In line with deficient degranulation, NK1R−/− FcεRI-BMMCs had reduced TNF and IL-6 secretion compared to WT FcεRI-BMMCs (Fig 1, F). This effect was receptor specific since it was prevented by pretreatment with the NK1R antagonists, RP67,580 (Fig E3 A and B in the Online Repository) or L733,060 (Fig E3 C and D in the Online Repository). Collectively, these data demonstrate that NK1R stimulation represents a downstream component of the proinfammatory signaling cascade initiated by FcεRI activation of MCs.
MCs secrete HK-1
The previous results, obtained with a highly pure MC population and without addition of exogenous NK1R agonists suggested that MCs are the source of NK1R agonists that increase their inflammatory functions in a highly regulated fashion. Therefore, we investigated the capacity of FcεRI-MCs to synthesize and secrete HK-1 and SP. We analyzed the regulation of Tac4 and Tac1 transcripts (encoding HK-1 and SP respectively), and secretion of HK-1 and SP peptides in WT untreated (control) and FcεRI-BMMCs. Tac4 and Tac1 mRNAs were detected in untreated BMMCs but only Tac4 transcripts increased in FcεRI-MCs (Fig 2, A). Accordingly, Tac4 mRNA and HK-1 secretion were induced to significantly higher levels than Tac1 mRNA and SP in FcεRI-BMMCs (Fig 2, A, B). The analysis of the signaling pathways regulating Tac4 transcription in BMMCs demonstrated a role for NFκB and JNK, but not ERK following FcεRI ligation (data not shown). These pathways which have been previously demonstrated to regulate Tac4 levels in other cell types25, 26 also regulated NK1R transcription in FcεRI-BMMCs (Fig 1, B).
FIG 2. Hemokinin-1 is required for maximal TNF-α secretion from FcεRI BMMCs.
A, Quantification of Tac4 or Tac1 mRNAs in WT FcεRI-BMMCs. Means + 1 SD from two experiments. B, HK-1 and SP levels secreted by WT FcεRI-BMMCs 18 h after Ag cross-linking. Means + 1 SD from three experiments. C, TNF and IL-6 secretion by Tac4-silenced WT FceRI-BMMCs, or D, Tac1−/− BMMCs. Means + 1 SD from two experiments. E, TNF and IL-6 secreted by WT or NK1R−/− FcεRI-BMMCs in which exogenous HK-1 was added at the time of Ag. Means + 1 SD from three independent experiments. Cytokines were measured in supernatants 18h after Ag cross-linking. *p<0.05, **p<0.01, ***p<0.001.
Next, we analyzed the role of autocrine/paracrine HK-1 and SP on MC-proinflammatory functions. To address this question we compared TNF and IL-6 secretion in culture supernatants of WT FcεRI-BMMCs transfected with Tac4 siRNA, which reduced Tac4 mRNA expression by 30.5 ± 3.5%), or in Tac1−/− FcεRI-BMMCs, which lack substance P and neurokinin A, a low affinity for NK1R ligand. Inhibition of HK-1 significantly reduced TNF and showed a consistent reduction of IL-6 secretion, an effect that was not observed in the absence of SP (Fig 2, C, D).
Since autocrine HK-1 amplified the function of FcεRI-BMMCs, we also investigated whether exogenous HK-1 at concentrations within the range of that produced by MCs (Fig. 2, B) could modify the pro-inflammatory effect of autocrine HK-1. However, exogenous HK-1 did not have any significant effect on degranulation (not shown) or in TNF or IL-6 secretion by FcεRI-BMMCs (Fig 2, E). Moreover, exogenous HK-1 was unable to affect the threshold for IgE-Ag-activation in BMMCs (data not shown). Additionally, secretion of TNF and IL-6 from NK1R−/− FcεRI-BMMCs were not modified. This later observation shows that the proinflammatory effects of HK-1 require signaling via the functional NK1R variant. Together, these results demonstrate that autocrine HK-1 is the MC-derived neuropeptide that signals via functional NK1R to provide adjuvancy to the pro-inflammatory functions of FcεRI-BMMCs.
NK1R autocrine signaling promotes TNF and IL-6 synthesis in FcεRI-BMMCs
The previous data indicate that autocrine HK-1/NK1R positive loop, increases the pro-inflammatory potential of FcεRI-BMMCs by promoting secretion of TNF and IL-6 through degranulation and de novo synthesis. To answer these questions we analyzed cytokine secretion in culture supernatants of WT and NK1R−/− BMMCs harvested 0–2 h (degranulation) or 2–18 h (de novo synthesis) after FcεRI crosslinking. WT FcεRIBMMCs secreted higher levels of both cytokines than NK1R−/− FcεRI-BMMCs at both time points (Fig 3, A). These results demonstrate that NK1R signaling by autocrine secreted agonists enhances TNF and IL-6 release by FcεRI-BMMCs during both, immediate and delayed phases of the MC-inflammatory response.
FIG 3. NK1R signaling potentiates PI3K/Akt/NFκB activation in FcεRI-BMMCs.
A, TNF and IL-6 released by BMMCs. Mean + 1 SD from three experiments. B, Left, histograms comparing intracellular phospho-(p)Ser473Akt in WT FcεRI- (black line) and NK1R−/− BMMCs (gray line) loaded with IgE and stimulated with Ag for 10 min as detected by flow cytometry. Filled histograms are Ig isotype controls. Right, depicts the mean ± 1 SD of the MFI for the kinetics of pSer473-Akt detected by intracellular flow cytometry in WT or NK1R−/− FcεRI-BMMCs. from 3 independent experiments. C, Left, IκB-α expression in FcεRI-BMMCs, 2h after Ag cross-linking. Right, kinetics of IκB-α degradation in FcεRI-BMMCs expressed as the percentage of the MFI of untreated controls. Means ± 1 SD from 3 independent experiments. D, Reporter assays of NFκB activity in FcεRI-BMMCs transfected with pLuc-NFκB, measured after 18h of FcεRI ligation. Mean + 1 SD of quadruplicates from a representative of 3 experiments. *p<0.05, **p<0.01.
To investigate the mechanisms integrating NK1R signaling pathways2, 4 related to TNF and IL-6 synthesis in FcεRI-BMMCs27, 28, we focused on PI3K/Akt and IκB-α/NFκB pathways. In BMMCs, PI3K-dependent phosphorylation of Ser473-Akt occurs within 10 min of FcεRI activation and Akt is restored to its inactive, unphosphorylated state within 30 mins27, 28. Accordingly, Ser473-Akt phosphorylation increased in WT FcεRI-BMMCs (Fig 3, B) 10 min following activation and returned to basal levels by 30 min. Ser473-Akt phosphorylation was reduced in NK1R−/− BMMCs and this effect was inhibited by culture with the NK1R antagonists RP67,580 and L733,060 (not shown). The levels of IκB-α expression, analyzed by flow cytometry29, significantly decreased 2h after Ag cross-linking (Fig 3, C) and NFκB nuclear translocation, assessed in luciferase reporter assays (Fig 3, D) and ELISA (not shown) significantly increased in WT-compared to NK1R−/− FcεRI-BMMCs. Together, these findings demonstrate that the NK1R signals via the PI3K/Akt/NFκB axis to regulate transcription and synthesis of TNF and IL-6 in FcεRI-BMMCs.
NK1R signaling is relevant for MC-dependent local and systemic anaphylaxis
The relevance of NK1R signaling in type I hypersensitivity reactions was analyzed in the context of FcεRI-MC mediated models of passive cutaneous and systemic anaphylaxis (PCA and PSA, respectively). PCA, in response to IgE sensitization, occurs in two phases. During the acute phase of PCA, dermal MCs release histamine leading to increased vascular permeability and edema. During the late phase, cutaneous MCs secrete TNF to recruit polymorphonuclear (PMN) leukocytes30. We assessed the role of NK1R signaling in PCA by comparing changes in ear thickness, edema, and leukocyte infiltrate in IgE-sensitized versus non-sensitized (vehicle control) ears of WT and NK1R−/− mice. During the acute phase of PCA, the sensitized ears of WT but not NK1R−/− mice showed significant thickness increase caused by edema (Fig 4, A–C). To specifically confirm the role of NK1R signaling in MCs in this model, we reconstituted the ears of c-KitW-sh/W-sh mice with WT or NK1R−/− BMMCs prior to the induction of PCA. Reconstitution with WT but not NK1R−/− BMMCs induced an early PCA response in c-KitW-sh/W-sh mice (Fig 4, D, E).
FIG 4. NK1R signaling amplifies the early phase of PCA.
PCA was induced by injecting IgE (20 ng/ear, i.d.). 24h later, Ag (DNP-HSA, 100 μg, i.v.). was administered. A, Increases in ear thickness following Ag injection. Means + 1 SEM of 13 mice from three independent experiments. B, Histology of skin sections comparing edema (arrows) in IgE-sensitized or control ears of WT and NK1R−/− mice, 2h after Ag challenge. 200x. C, Evan's Blue extravasion from ear tissue, 2h after Ag challenge. Means + 1 SEM from 2 experiments. D–E, c-KitW-sh/W-sh mice were reconstituted with WT or NK1R−/− BMMCs, (1 × 106/ear, i.d) 8 weeks prior to the PCA induction. D, Depicts increases in ear thickness following induction of PCA. Mean + 1 SEM from 4–5 mice per group. E, Histology of skin sections comparing edema, 2h after sensitization. 200x. **p<0.01, ***p<0.005, ****p<0.001.
During late PCA, ear thickness increase in IgE-sensitized and Ag challenged WT mice was caused by PMN leukocyte infiltration, and this response was negligible in equally treated ears of NK1R−/− mice (Fig 5 A, B). Given the effect of NK1R signaling on increasing TNF release (Fig 1, E), we hypothesized that the reduced late PCA response in NK1R−/− mice was caused by deficient TNF secretion. Accordingly, TNF levels increased in IgE-sensitized WT mouse ears compared to vehicle treated controls. Importantly, TNF levels from IgE-sensitized NK1R−/− mouse ears, were consistently lower than those observed in equally treated WT mice (Fig 5, C). By fluorescence microscopy, we further confirmed that cytoplasmic TNF was present in dermal c-Kit+ MCs of ear sections from sensitized WT mice but not in dermal MCs from equally treated NK1R−/− mouse ears (Fig 5, D).
Fig. 5. NK1R signaling is required for the TNF dependent development of late phase PCA.
A, Increase in ear thickness and B, histology of mouse ears 24h following Ag challenge in late PCA. C, TNF levels from the IgE-sensitized ears depicted as percent control ears. Means ± 1 SEM of two experiments. C, Immunofluorescence microscopy of ears of WT mice 24 h following the induction of PCA, showing c-Kit+ MCs (green) containing TNF-α (red). 200x. D, Increase in ear thickness in IgE sensitized WT or NK1R−/− mice injected with TNF-α (40 U/ear). Means + 1 SEM of 5 mice/experimental group. E, Histology illustrating the severity of the inflammatory infiltrate in ear dermis and epidermis. H&E, 200x. Inset shows PMN leukocytes, 1000x. G–H, Late phase PCA was evaluated in c-KitW-sh/W-sh mice reconstituted (i.d) with either WT or NK1R−/− BMMCs. G, Increases in ear thickness or H, histology of skin sections, 24h after PCA induction. 200x and inset 500x. *p<0.05, **p<0.01 ***p<0.001.
Deficient recruitment of PMN leukocytes depends on TNF dependent30 and independent31 mechanisms. To test if under our experimental conditions the lack of PMN leukocytes in NK1R−/− mouse ears was associated with TNF deficit we pre-sensitized WT and NK1R−/− mice ears with IgE. After Ag challenge we injected i.d. mouse TNF to one ear. The ear skin of NK1R−/− mice locally treated with TNF evidenced infiltrating PMN leukocytes to a same extent to that observed in WT mice (Fig 5, E and F).
Then, we investigated the role of NK1R signaling in MCs during late phase PCA using c-KitW-sh/W-sh mice reconstituted with WT or NK1R−/− BMMCs. Reconstitution with WT BMMCs, but not NK1R−/− BMMCs restored late phase PCA induction in c-KitW-sh/W-sh mice as demonstrated by increases in ear thickness and significant inflammatory infiltrate (Fig 5, G and H). These results demonstrate that NK1R signaling is required for the elicitation of the late phase of PCA by a mechanism mediated by TNF secretion from cutaneous FcεRI-MCs.
Next, we analyzed the relevance of NK1R signaling in the onset and progression of FcεRI-MC-mediated PSA. We evaluated hypothermia, changes in pulmonary vascular permeability, and serum concentrations of TNF and IL-6. At early time points (10–20 min after Ag challenge), WT and NK1R−/− mice developed hypothermia to a similar extent (Fig E4 in the Online Repository, A). However, NK1R−/− mice recovered faster and displayed signs of well-being (i.e. alertness, normal activity) not observed in WT mice and increased edema was significantly higher in the lungs of WT compared to NK1R−/− mice (Fig E4 in the Online Repository, B and C). Accelerated recovery from PSA is associated with low TNF concentrations in mouse serum32. Likewise, in our working conditions, TNF and IL-6 levels were significantly lower in NK1R−/− mice compared to WT mice (Fig E4 in the Online Repository, D). The role of NK1R expressing MC in PSA was investigated using c-KitW-sh/W-sh mice (which do not develop PSA) reconstituted with WT or NK1R−/− BMMCs. Reconstitution with WT BMMCs, but not NK1R−/− BMMCs restored PSA in c-KitW-sh/W-sh mice demonstrated by induction of significant hypothermia (Fig E4 in the Online Repository, E). Collectively, these findings demonstrate that NK1R signaling is required for FcεRI-MC-dependent immediate and delayed responses of local and systemic anaphylaxis due to a mechanism requiring TNF.
NK1R signaling by HK-1 is necessary for FcεRI-MC-dependent experimental airway inflammation
The role of NK1R signaling in chronic atopic disorders was investigated in a FcεRI-MC-dependent model of chronic airway inflammation induced in WT and NK1R−/− mice with OVA33. This protocol requires MC-produced TNF to initiate Th2-bias and to recruit PMN leukocyte and eosinophil infiltration in airways and lungs. We evaluated (i) leukocyte infiltration; (ii) myeloperoxidase (MPO) and eosinophil peroxidase (EPO) activities and, (iii) cytokine concentrations in bronchioalveolar lavage fluids (BALs) in the lungs of OVA-sensitized and -challenged mice compared to vehicle treated controls.
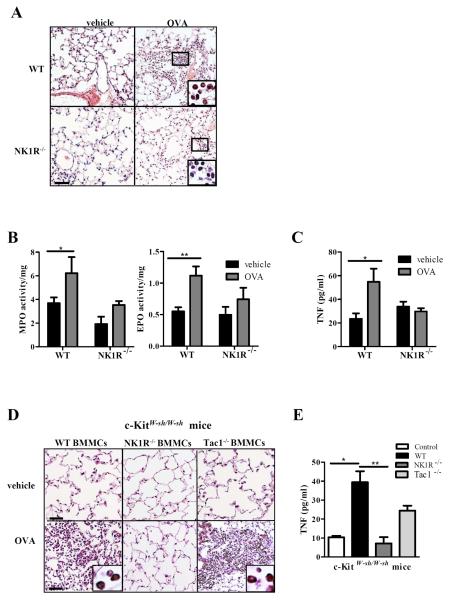
The lungs of OVA-challenged WT mice exhibited severe PMN and eosinophil infiltration distributed in foci throughout the parenchyma and airways (Fig 6, A). The activities of MPO and EPO increased in the lungs of OVA-challenged WT mice compared to the activities of MPO and EPO in lungs of vehicle-treated WT mice (Fig 6, B). Conversely, lungs from OVA-challenged NK1R−/− mice exhibited negligible infiltration by lymphocytes and eosinophils (Fig 6, A) and lower increases in MPO and EPO activity than the lungs of equally treated WT mice (Fig 6, B). Likewise, concentrations of TNF (Fig 6, C) and Th2 cytokines, IL-4, IL-5 and IL-13, in the BALs increased significantly in OVA-challenged WT mice, but not in NK1R−/− mice, whereas the low levels of IFNγ detected remained similar between WT and NK1R−/− mice (Fig E5 in the Online Repository). The impaired MC-dependent inflammatory and immune responses of NK1R−/− mice were not due to deficient IgE secretion, since both groups had similar concentrations of serum total and OVA-specific IgE (Fig E6 in the Online Repository).
FIG 6. The NK1R is required for onset of MC-dependent airway inflammation.
To induce MC-dependent chronic asthma, mice were treated with either vehicle or OVA (10 μg, i.p.) on days 0, 2, 4, 6, 8, 10, and 12 then challenged (200 μg, i.n.) on days 40, 43, and 46. A, Inflammatory infiltrate in OVA-sensitized/challenged lungs or vehicle controls mice at day 47. H&E, 200x. Insets show PMN leukocytes including eosinophils and neutrophils. 500x. Bar=20 μm. B, Quantification of EPO and MPO in lungs. Means + 1 SEM of 5 mice/group are illustrated. C, TNF concentration in BAL from WT or NK1R−/− mice. D, Histology of KitW-sh/W-sh lungs from mice reconstituted with WT, NK1R−/− or Tac1−/− BMMCs 8 weeks prior to induction of airway inflammation. H&E, 200x. Inset shows eosinophils in the lung of KitW-sh/W-sh reconstituted with WT BMMCs, 1000x. E, TNF in BALs of c-KitW-sh/W-sh mice reconstituted with WT, NK1R−/−, or Tac1−/− BMMCs prior to induction of experimental airway inflammation. Means ± SEM of 3 mice/group. *p<0.05, **p<0.01.
The pathophysiologic role of NK1R signaling by HK-1 in the pro-inflammatory function of MCs relevant for chronic airway inflammation was further confirmed by comparing the levels of TNF in the BALs and the inflammatory infiltrate in the lungs of MC-deficient (KitW-sh/W-sh) mice reconstituted with WT, NK1R−/− or Tac1−/− BMMCs prior to OVA-sensitization. Following OVA-challenge, KitW-sh/W-sh mice reconstituted with WT or Tac1−/− BMMCs had severe eosinophil infiltrate in the lungs whereas equally treated KitW-sh/W-sh mice reconstituted with NK1R−/− BMMCs did not develop inflammatory responses or other lung immune-pathology (Fig 6, D). Following OVA-challenge, BALs from KitW-sh/W-sh mice engrafted with WT or Tac1−/− BMMCs had significantly higher TNF concentration than those from mice reconstituted with NK1R−/− BMMCs (Fig 6, E), correlating with eosinophilic infiltration. Collectively, these results demonstrate that NK1R signaling by autocrine HK-1 is critical for the pro-inflammatory function of FcεRI-MCs accounting for the onset and maintenance of chronic airway inflammation.
DISCUSSION
Given the role of neuroinflammation and MCs in type 1-hypersensitivity and Th2 mediated chronic allergic diseases there has been an interest in understanding the regulation of MC functions by neuropeptides. Although historical studies addressed the relevance of SP in MC function, these reports were not focused on the activation of MC by IgE-Ag complexes19–21. Moreover, the use of high concentrations of SP, and technical limitations in these studies, preclude the understanding of the physiological function of the NK1R in MC biology in vivo34. More recently, the description of HK-1 as a potent agonist of the NK1R secreted mainly by leukocytes has changed, in part, the way we regard at neuropeptides as relevant regulators of immune functions. In the present study, we focused on understanding the function of the neuropeptides, SP and HK-1, as preferential agonists of the NK1R and their potential endogenous adjuvant effect in the context of MC activation by IgE.
It has been reported that in the absence of FcεRI activation, up-regulation of the NK1R surface expression by mouse BMMCs required exogenous IL-416. Similarly, we found dim expression of the functional intracellular form of the NK1R in BMMCs. Expression of the NK1R significantly increased in Fc;RI-BMMCs, which was due, in part, to autocrine IL-4 secretion following FcεRI ligation.
Under our experimental conditions, HK-1, not SP, favored MC pro-inflammatory functions. In fact, we demonstrate that following FcεRI ligation and up-regulation of the NK1R, MCs significantly increased Tac4 transcription and HK-1 secretion, whereas the levels of Tac1 mRNA and SP remained low. Utilizing NK1R−/−, Tac1−/− BMMCs, Tac4 silencing and NK1R antagonists, we demonstrate that the HK-1 / finctional NK1R signaling pathway provides adjuvancy to FcεRI-BMMC pro-inflammatory functions. Different from SP, that is stored in neuronal bodies and secreted predominantly by sensory nerve fibers, HK-1 is synthetized mainly by leukocytes, indicating that HK-1's pro-inflammatory function is highly regulated in and by leukocytes. In fact, previous works have demonstrated the roles of HK-1 in leukocyte differentiation, survival and immune functions. Accordingly, HK-1 promotes B and T cell differentiation, proliferation and activation9–12 and the pro-inflammatory function of monocytes6. Here, we analyzed the relationship between the NK1R and FcεRI signaling in MCs and demonstrated that autocrine HK-1 amplifies MC inflammatory responses. Based on our finding and published works, we could speculate that in vivo, activation of MC via FcεRI involves a highly regulated mechanism requiring: i) circulating Ag-specific IgE synthesis by activated plasma cells, ii) IgE-Ag complexes to crosslink the FcεRI, and iii) downstream activation of intracellular pathways resulting in upregulation of NK1R and MC synthesis and autocrine secretion of HK-1. In this model, HK-1 provides an adjuvant effect and is required for maximal MC activation and efficient development of IgE-mediated atopic disorders. Interestingly, exogenous HK-1 was unable to modify the effects of autocrine HK-1 even when added a high non-physiological concentrations. We speculate that this may be caused by autocrine HK-1 binding the NK1R and NK1R internalization, a mechanism previously described as necessary to terminate NK1R signaling, ultimately making the NK1R unavailable.
Certain GPCRs potentiate degranulation18, 34, 35, potentially through interactions mediating cytoskeletal changes36. Here, we found that fewer NK1R−/− BMMCs degranulated in vitro in response to FcεRI crosslinking and that edema associated with FcεRI-mediated degranulation was reduced in NK1R−/− mice. These findings demonstrate a role for NK1R signaling in MC degranulation, as described for other GPCRs34.
We have previously reported in dendritic cells that NK1R signaling activates the PI3K/Akt pathway4. In MCs, the PI3K/Akt pathway activates NFκB. Here, we demonstrated that NK1R signaling in FcεRI-BMMC triggers PI3K/Akt activation downstream NFκB nuclear translocation, necessary for the synthesis of TNF and IL-627, 28 during the late phase of the MC inflammatory response.
Connective tissue and mucosa resident MCs are master initiators of acute hypersensitivity reactions and chronic atopic diseases linked to neuromediators. Our data demonstrate that HK-1 signaling via the NK1R of MCs is crucial for the onset and maintenance of these disorders and that HK-1/NK1R interaction is required for every step in the development of PCA and PSA by inflammatory MCs. Previous publications have shown that MC-dependent recruitment of PMN cells in IgE-mediated PCA relies on changes in IL-3337 or IL-638. Under our working conditions, the absence of PMN cells in infiltrating the skin of NK1R−/− mice during late PCA was completely restored following TNF administration, clearly indicating the importance of TNF in this model.
The adjuvant function of the HK-1/NK1R interaction in MCs was further demonstrated in a model of chronic atopic airway inflammation in which, NK1R signaling was necessary for the onset and maintenance of the disease and proved to be critical for Th2-polarization of the adaptive immune response and recruitment of inflammatory cells to the lungs. Using a MC independent model of airway hyper-reactivity, a recent study has shown that NK1R antagonists reduced levels of Ag-IgE concentrations39. Under our working conditions, using a MC dependent-model of chronic airway inflammation abrogation of the pathology did not affect Ag-IgE secretion, confirming that efficient development of the disease requires NK1R signaling that provides with adjuvancy to FcεRI-MC mediated disorders.
In summary, we present a comprehensive analysis of the function of the NK1R in MCs in vitro and in vivo. To our knowledge, this is the first report addressing the key role that NK1R signaling of FcεRI-MCs has in the pathogenesis of anaphylaxis and Th2-dependent atopic disorders. Specifically our findings demonstrate that HK-1 and NK1R interaction is a critical component of FcεRI-MCs mediated inflammatory and immune functions and provide insight into the mechanisms underlying MC-mediated disorders. Our data are immunological, physiological and clinically relevant as current therapeutic strategies targeting MC functions have shown limited efficacy40 in part due to the inability to down-regulate TNF and IL-6 secretion initiated through the Fc–RI. The data presented here supports the development of alternative immune therapies based on neutralization of endogenously secreted pro-inflammatory NK1R agonists.
Supplementary Material
KEY MESSAGES.
In vitro stimulation of MCs via FcεRI by Ag-IgE complexes initiates the synthesis and autocrine secretion of HK-1 that signals the NK1R to promote the secretion of the pro-inflammatory cytokines TNF and IL-6.
In vivo expression and agonistic signaling of NK1R in MCs is required to initiate local and systemic passive anaphylaxis and to sustain airway inflammation.
FcεRI signaling of MCs triggers an autocrine/paracrine positive loop mediated by agonistic HK-1/NK1R interaction, wich provides with endogenous adjuvancy for efficient development of FcεRI -MC-dependent allergic reactions functions.
The data presented here demonstrate previously unknown aspects of MC-neuropeptide interactions and infer a physiologically oriented therapeutic alternative for IgE-mediated atopic diseases.
Acknowledgments
Sources of Funding: This study was supported by the National Institutes of Health grant: AI077511 to A.T. Larregina and the T.E. Starzl Postdoctoral Fellowship in Transplantation Biology, to D.M. Rojas-Canales.
ABBREVIATIONS
- Ag
Antigen
- BAL
Bronchioalveolar lavage
- BM
Bone marrow
- BMMC
bone marrow derived mast cells
- c48/80
Compound 48/80
- c-KitW-sh/W-sh
MCKO mice
- EPO
eosinophil peroxidase
- FcεRI-MC
FcεRI activated mast cells
- GPCR
G protein coupled receptor
- HK-1
Hemokinin-1
- JNK
c-Jun N-terminal kinase
- MC
mast cell
- NK1R
Neurokinin-1 receptor
- MPO
Myeloperoxidase
- PCA
Passive cutaneous anaphylaxis
- PSA
Passive systemic anaphylaxis
- PI3K
Phosphoinositide 3-kinase
- PMN
Polymorphonuclear cells
- OVA
ovalbumin
- SP
Substance P
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neuro. 2012;15:1063–7. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douglas SD, Leeman SE. Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Ann N Y Acad Sci. 2011;1217:83–95. doi: 10.1111/j.1749-6632.2010.05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathers AR, Tckacheva OA, Janelsins BM, Shufesky WJ, Morelli AE, Larregina AT. In vivo signaling through the neurokinin 1 receptor favors transgene expression by Langerhans cells and promotes the generation of Th1- and Tc1-biased immune responses. J Immunol. 2007;178:7006–17. doi: 10.4049/jimmunol.178.11.7006. [DOI] [PubMed] [Google Scholar]
- 4.Janelsins BM, Mathers AR, Tkacheva OA, Erdos G, Shufesky WJ, Morelli AE, et al. Proinflammatory tachykinins that signal through the neurokinin 1 receptor promote survival of dendritic cells and potent cellular immunity. Blood. 2009;113:3017–26. doi: 10.1182/blood-2008-06-163121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janelsins BM, Sumpter TL, Tkacheva OA, Rojas-Canales DM, Erdos G, Mathers AR, et al. Neurokinin-1 receptor agonists bias therapeutic dendritic cells to induce type-1 immunity by licensing host dendritic cells to produce IL-12. Blood. 2013 doi: 10.1182/blood-2012-07-446054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunin P, Caillon A, Corvaisier M, Garo E, Scotet M, Blanchard S, et al. The tachykinins substance P and hemokinin-1 favor the generation of human memory Th17 cells by inducing IL-1beta, IL-23, and TNF-like 1A expression by monocytes. J Immunol. 2011;186:4175–82. doi: 10.4049/jimmunol.1002535. [DOI] [PubMed] [Google Scholar]
- 7.Feistritzer C, Clausen J, Sturn DH, Djanani A, Gunsilius E, Wiedermann CJ, et al. Natural killer cell functions mediated by the neuropeptide substance P. Regul Pept. 2003;116:119–26. doi: 10.1016/s0167-0115(03)00193-9. [DOI] [PubMed] [Google Scholar]
- 8.Monaco-Shawver L, Schwartz L, Tuluc F, Guo CJ, Lai JP, Gunnam SM, et al. Substance P inhibits natural killer cell cytotoxicity through the neurokinin-1 receptor. J Leukoc Biol. 2011;89:113–25. doi: 10.1189/jlb.0410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Li Q, Zhang J, Wu H, Yin Y, Ge Q, et al. Hemokinin-1 activates the MAPK pathway and enhances B cell proliferation and antibody production. J Immunol. 2010;184:3590–7. doi: 10.4049/jimmunol.0901278. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Lu L, Furlonger C, Wu GE, Paige CJ. Hemokinin is a hematopoietic-specific tachykinin that regulates B lymphopoiesis. Nat Immunol. 2000;1:392–7. doi: 10.1038/80826. [DOI] [PubMed] [Google Scholar]
- 11.Beinborn M, Blum A, Hang L, Setiawan T, Schroeder JC, Stoyanoff K, et al. TGF-beta regulates T-cell neurokinin-1 receptor internalization and function. Proc Natl Acad Sci U S A. 2010;107:4293–8. doi: 10.1073/pnas.0905877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Paige CJ. T-cell developmental blockage by tachykinin antagonists and the role of hemokinin 1 in T lymphopoiesis. Blood. 2003;102:2165–72. doi: 10.1182/blood-2002-11-3572. [DOI] [PubMed] [Google Scholar]
- 13.Green PG. Gastrin-releasing peptide, substance P and cytokines in rheumatoid arthritis. Arthritis Res Ther. 2005;7:111–3. doi: 10.1186/ar1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolis KG, Gershon MD. Neuropeptides and inflammatory bowel disease. Cur Opin Gastroenterol. 2009;25:503–11. doi: 10.1097/MOG.0b013e328331b69e. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Markus I, Saghire HE, Perera DS, King DW, Burcher E. Distinct differences in tachykinin gene expression in ulcerative colitis, Crohn's disease and diverticular disease: a role for hemokinin-1? Neurogastroenterol Motil. 2011;23:475–83. e179–80. doi: 10.1111/j.1365-2982.2011.01685.x. [DOI] [PubMed] [Google Scholar]
- 16.van der Kleij HP, Ma D, Redegeld FA, Kraneveld AD, Nijkamp FP, Bienenstock J. Functional expression of neurokinin 1 receptors on mast cells induced by IL-4 and stem cell factor. J Immunol. 2003;171:2074–9. doi: 10.4049/jimmunol.171.4.2074. [DOI] [PubMed] [Google Scholar]
- 17.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–30. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 19.Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol. 1993;150:4478–85. [PubMed] [Google Scholar]
- 20.Matsuda H, Kawakita K, Kiso Y, Nakano T, Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989;142:927–31. [PubMed] [Google Scholar]
- 21.Yano H, Wershil BK, Arizono N, Galli SJ. Substance P-induced augmentation of cutaneous vascular permeability and granulocyte infiltration in mice is mast cell dependent. J Clin Invest. 1989;84:1276–86. doi: 10.1172/JCI114295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simeonidis S, Castagliuolo I, Pan A, Liu J, Wang CC, Mykoniatis A, et al. Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-kappa B site on its promoter. Proc Natl Acad Sci U S A. 2003;100:2957–62. doi: 10.1073/pnas.0530112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh YH, Tamizhselvi R, Bhatia M. Extracellular signal-regulated kinase 1/2 and c-Jun NH2-terminal kinase, through nuclear factor-kappaB and activator protein-1, contribute to caerulein-induced expression of substance P and neurokinin-1 receptors in pancreatic acinar cells. J Pharmacol Exp Ther. 2010;332:940–8. doi: 10.1124/jpet.109.160416. [DOI] [PubMed] [Google Scholar]
- 24.Palomaki VA, Laitinen JT. The basic secretagogue compound 48/80 activates G proteins indirectly via stimulation of phospholipase D-lysophosphatidic acid receptor axis and 5-HT1A receptors in rat brain sections. Br J Pharmacol. 2006;147:596–606. doi: 10.1038/sj.bjp.0706671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran AH, Berger A, Wu GE, Paige CJ. Regulatory mechanisms in the differential expression of Hemokinin-1. Neuropeptides. 2009;43:1–12. doi: 10.1016/j.npep.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Sakai A, Takasu K, Sawada M, Suzuki H. Hemokinin-1 gene expression is upregulated in microglia activated by lipopolysaccharide through NF-kappaB and p38 MAPK signaling pathways. PLoS One. 2012;7:e32268. doi: 10.1371/journal.pone.0032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitaura J, Asai K, Maeda-Yamamoto M, Kawakami Y, Kikkawa U, Kawakami T. Akt-dependent cytokine production in mast cells. J Exp Med. 2000;192:729–40. doi: 10.1084/jem.192.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, et al. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity. 2002;16:441–51. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 29.Kingeter LM, Paul S, Maynard SK, Cartwright NG, Schaefer BC. Cutting edge: TCR ligation triggers digital activation of NF-kappaB. J Immunol. 2010;185:4520–4. doi: 10.4049/jimmunol.1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991;87:446–53. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32:461–9. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oskeritzian CA, Price MM, Hait NC, Kapitonov D, Falanga YT, Morales JK, et al. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J Exp Med. 2010;207:465–74. doi: 10.1084/jem.20091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, et al. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120:48–55. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 34.Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcepsilonRI-mediated mast cell activation. Immunol Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Crit Rev Immunol. 2011;31:475–529. doi: 10.1615/critrevimmunol.v31.i6.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vibhuti A, Gupta K, Subramanian H, Guo Q, Ali H. Distinct and shared roles of beta-arrestin-1 and beta-arrestin-2 on the regulation of C3a receptor signaling in human mast cells. PLoS One. 2011;6:e19585. doi: 10.1371/journal.pone.0019585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS One. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mican JA, Arora N, Burd PR, Metcalfe DD. Passive cutaneous anaphylaxis in mouse skin is associated with local accumulation of interleukin-6 mRNA and immunoreactive interleukin-6 protein. J Allergy Clin Immunol. 1992;90:815–24. doi: 10.1016/0091-6749(92)90107-d. [DOI] [PubMed] [Google Scholar]
- 39.Ramalho R, Almeida J, Beltrao M, Pirraco A, Costa R, Sokhatska O, et al. Substance P antagonist improves both obesity and asthma in a mouse model. Allergy. 2013;68:48–54. doi: 10.1111/all.12052. [DOI] [PubMed] [Google Scholar]
- 40.MacGlashan DW., Jr. IgE-dependent signaling as a therapeutic target for allergies. Trends Pharmacol Sci. 2012;33:502–9. doi: 10.1016/j.tips.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.