Background: The mechanism of transglutaminase II-mediated allergic inflammation-promoted tumor metastasis remains unknown at the molecular level.
Results: The transglutaminase II/miR-218/-181a loop regulates allergic inflammation-promoted tumor metastasis.
Conclusion: Transglutaminase II mediates a positive feedback between allergic inflammation and tumor metastasis.
Significance: Transglutaminase II can be a target for the development of allergy and cancer therapeutics.
Keywords: Angiogenesis, Gene Regulation, Macrophage, Mast Cell, Tumor Metastasis, Allergic Inflammation, Transglutaminase II, MicroRNA-181a, MicroRNA-218
Abstract
The molecular mechanism of transglutaminase II (TGaseII)-mediated allergic inflammation remains largely unknown. TGaseII, induced by antigen stimulation, showed an interaction and co-localization with FcϵRI. TGaseII was necessary for in vivo allergic inflammation, such as triphasic cutaneous reaction, passive cutaneous anaphylaxis, and passive systemic anaphylaxis. TGaseII was necessary for the enhanced metastatic potential of B16F1 melanoma cells by passive systemic anaphylaxis. TGaseII was shown to be a secreted protein. Recombinant TGaseII protein increased the histamine release and β-hexosaminidase activity, and enhanced the metastatic potential of B16F1 mouse melanoma cells. Recombinant TGaseII protein induced the activation of EGF receptor and an interaction between EGF receptor and FcϵRI. Recombinant TGaseII protein displayed angiogenic potential accompanied by allergic inflammation. R2 peptide, an inhibitor of TGaseII, exerted negative effects on in vitro and in vivo allergic inflammation by regulating the expression of TGaseII and FcϵRI signaling. MicroRNA (miR)-218 and miR-181a, decreased during allergic inflammation, were predicted as negative regulators of TGaseII by microRNA array and TargetScan analysis. miR-218 and miR-181a formed a negative feedback loop with TGaseII and regulated the in vitro and in vivo allergic inflammation. TGaseII was necessary for the interaction between mast cells and macrophages during allergic inflammation. Mast cells and macrophages, activated during allergic inflammation, were responsible for the enhanced metastatic potential of tumor cells that are accompanied by allergic inflammation. In conclusion, the TGaseII/miR-218/-181a feedback loop can be employed for the development of anti-allergy therapeutics.
Introduction
Transglutaminase II (TGaseII)3 is a protein cross-linking enzyme with diverse functions (1–5). The activation of transglutaminase is associated with the release of histamine from the cells induced by an IgE-dependent mechanism (2). Transglutaminase-mediated cross-linking of a peptic fraction of ω-5 gliadin enhances IgE reactivity in wheat-dependent, exercise-induced anaphylaxis (3). TGaseII is overexpressed in asthmatic airways and functions to increase sPLA(2)-X enzymatic activity and acts as a key initiator of the airway inflammatory cascade in asthma (6). Group X-secreted phospholipase A2 mediates allergen-induced airway inflammation and remodeling in a mouse asthma model by regulating the production of eicosanoids (7). TGaseII is necessary for allergic skin inflammation induced by PMA (8). Pulmonary epithelial cells damaged by allergens triggers TGaseII-mediated IL-33 expression, leading to type 2 responses by recruiting both innate and adaptive arms of the immune system (9). 5-Hydroxytryptophan suppresses allergic inflammation by decreasing the expression of TGaseII (10). TGaseII regulates production of reactive oxygen species and secretion of Th2 cytokines and mediates in vitro and in vivo allergic inflammation (11). Tissue transglutaminase mediates airway inflammation of toluene diisocyanate-induced occupational asthma by regulating the production of reactive oxygen species (12). Epithelial TGaseII is a critical inducer of pulmonary inflammation in bleomycin-treated mice (13). TGaseII expressed in mast cells enhances IgE level in B cells by regulating CD40L (14). R2 peptide, an inhibitor of TGaseII, reduces allergic responses by regulating NF-κB/TGaseII activity in a mouse model of allergic asthma (15). Octapeptide R2 (KVLDGQDP), which has anti-transglutaminase (TGase) activity, decreases inflammation in an allergic conjunctivitis model in guinea pigs (16). TGaseII inhibitors reduce allergic conjunctivitis by inhibiting phospholipase A2 activity (17).
MicroRNAs (miRNAs) are small, single-stranded non-coding RNAs that play important roles in the post-transcriptional regulation of gene expression in mammalian cells by regulating translation. The silencing of Dicer, a key enzyme of miRNA biogenesis, attenuates degranulation, indicating that miRNAs are involved in mast cell degranulation (18). The overexpression of miR-142-3p enhances FcϵRI-mediated degranulation, and miR-142-3p rescues the reduction of degranulation by silencing Dicer (18). Many miRNA expressions were altered in allergic rhinitis, and differentially expressed miRNAs appear to be involved in the development of allergic rhinitis (19). miR-155 regulates allergic asthma by modulating TH2 response through the transcription factor PU.1 (20). miR-145 is necessary for allergic airway diseases resulting from the house dust mite (21). miR-21 mediates allergic airway inflammation by regulating the expression of IL-12, a molecule germane to the Th polarization (22). miR-126 is also necessary for allergic airway diseases (23). These reports suggest a role of miRNAs in allergic inflammation. To date, miRNAs that bind to and regulate the expression of TGaseII have not been identified.
In this study, we show that TGaseII constitutes the FcϵRI signaling network and interacts with FcϵRIβ. We show that TGaseII is necessary for in vitro and in vivo allergic inflammation. We show that TGaseII forms a negative feedback loop with miR-218 and miR-181a. We show that miR-218 and miR-181a exert negative effects on in vitro and in vivo allergic inflammation. We present evidence that TGaseII is responsible for angiogenesis and the enhanced metastatic potential of mouse melanoma cells accompanied by allergic inflammation. R2 peptide, an inhibitor of TGaseII, confirms the role of TGaseII in allergic inflammation. We show that the interaction between mast cells and macrophages occurs during allergic inflammation in a TGaseII-dependent manner. We present evidence that in vivo allergic inflammation promotes the metastatic potential of mouse melanoma cells and involves the interaction between tumor cells and stromal cells, such as mast cells and macrophages. Thus, the TGaseII/miR-218/-181a feedback loop would be a valuable target for the development of anti-allergic drugs.
EXPERIMENTAL PROCEDURES
β-Hexosaminidase Activity Assays
The β-hexosaminidase activity assay was performed according to standard procedures (24).
Histamine Release Assay
Serum histamine level was measured according to the manufacturer's instructions (SPI-Bio). For serum histamine levels, blood from each mouse was collected by cardiac puncture under anesthesia. To measure the cellular histamine level, culture supernatants were used.
Cell Lines and Cell Culture
RBL2H3 cells were obtained from the Korea Cell Line Bank (Seoul, Korea). Cells were grown in Dulbecco's modified Eagle's medium containing heat-inactivated fetal bovine serum, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Cultures were maintained in 5% CO2 at 37 °C. Bone marrow-derived mouse mast cells were isolated and cultured according to standard procedures (24). B16F1 melanoma cells were cultured in Dulbecco's modified minimal essential medium (DMEM; Invitrogen) supplemented with heat-inactivated 10% fetal bovine serum (FBS; Invitrogen) and antibiotics at 37 °C in a humidified incubator with a mixture of 95% air and 5% CO2. Ear/lung mast cells and lung macrophages were isolated according to standard procedures (25).
Histological Analyses
Harvested tissues (lung) were frozen in optimal cutting temperature compound by Tissue Tek (OCT; Allegiance, McGaw, IL). Frozen tissues were cryosectioned (6–10 μm) and placed on positively charged glass slides. Nonspecific binding of antibodies was blocked by incubation with 1% bovine serum albumin (BSA) for 1 h before incubation with primary antibodies. The following primary antibodies were used for single and double staining: anti-FcϵR1β (1:100; Santa Cruz Biotechnology, Inc.) and anti-TGaseII (1:100; Santa Cruz Biotechnology). The sections were incubated with primary antibodies overnight at 4 °C. After washing, secondary antibodies were applied at 1:100 or 1:200 dilutions for 1 h. We used goat anti-rabbit IgG-FITC (Santa Cruz Biotechnology) for TGaseII and rabbit anti-goat Alexa Fluor 546 for FcϵRIβ staining (Molecular Probes). DAPI (Molecular Probes) was added to stain nuclei. Confocal images were acquired using a confocal laser-scanning microscope (FV-1000, Olympus). Immunohistochemical staining of ear tissue or lung tissues was performed using an established avidin-biotin detection method (Vectastain ABC kit, Vector Laboratories Inc., Burlingame, CA). Briefly, 4–6-μm-thick sections of the paraffin-embedded tissue blocks were cut, mounted on positively charged glass slides, and dried in an oven at 56 °C for 30 min. The sections were deparaffinized in xylene and then rehydrated in graded ethanol and water. Endogenous peroxidase was blocked by incubation in 3% (v/v) hydrogen peroxide for 15 min. Antigen retrieval was accomplished by pretreatment of the sections with citrate buffer at pH 6.0 for 20 min at 56 °C in a microwave oven and then allowing the sections to cool for 30 min at room temperature. Nonspecific endogenous protein binding was blocked using 1% BSA. The sections were then incubated with primary antibodies overnight at 4 °C. The following primary antibodies were used for single and double staining: anti-c-Kit (1:100; Santa Cruz Biotechnology), anti-TGaseII (1:100, Santa Cruz Biotechnology), anti-tryptase (1:100, Santa Cruz Biotechnology), and anti-MCP1 (monocyte chemoattractant protein-1) (1:50; Abcam). After washing, biotinylated secondary antibodies were applied at 1:100 or 1:200 dilutions for 1 h. Color was developed with diaminobenzidine (Vector Laboratories Inc.). Sections were counterstained with Mayer's hematoxylin. Sections incubated without primary antibody served as controls.
Chemicals and Reagents
Oligonucleotides used in this study were commercially synthesized by Bionex Co. (Seoul, Korea). Chemicals used in this study were purchased from Sigma. DNP-HSA and DNP-specific IgE antibody were purchased from Sigma. TNP-BSA was purchased from Santa Cruz Biotechnology. TNP-specific IgE antibody was purchased from BioLegend Co. All other antibodies were purchased from Cell Signaling Co. (Beverly, MA). Anti-mouse and anti-rabbit IgG-horseradish peroxidase-conjugated antibody was purchased from Pierce. Lipofectamine and PlusTM reagent for transfection were purchased from Invitrogen. The microRNA array kit was purchased from Koma Biotech (Seoul, Korea). Recombinant TGaseII protein was purchased from R&D Systems, Inc. R2 peptide (KVLDGQDP) was commercially synthesized by Peptron Co. (Daejon, Korea). miR mimic and miR inhibitor were purchased from Bioneer Co. (Daejon, Korea).
Mice
Five-week-old female BALB/c mice were purchased from Nara Biotech (Seoul, Korea) and maintained in specific pathogen-free conditions. All animal experiments were approved by the Institutional Animal Care and Use Committee of Kangwon National University (KW-140707-1). For lung metastasis experiments, B16F1 melanoma cells (1 × 106 cells in 0.2 ml of PBS), after induction of passive systemic anaphylaxis, were injected into the tail vein of BALB/c mice. Fourteen days after IgE sensitization, the mice were sacrificed, and the tumor nodules on the surface of the lungs were counted under a dissecting microscope. H&E staining served to validate the identity of the malignant colonies in the lungs of mice that had received tumor cells intravenously. For H&E staining, lung tumor tissue samples were fixed in 10% (v/v) buffered formalin, embedded in paraffin, sectioned at 4 μm, and then stained with hematoxylin and eosin.
Transfection
Transfections were performed according to the manufacturer's instructions. Lipofectamine and Plus reagents (Invitrogen) were used. The construction of siRNA was carried out according to the instruction manual provided by the manufacturer (Ambion, Austin, TX). For miR-218 knockdown, cells were transfected with 50 nm oligonucleotide (inhibitor) with Lipofectamine 2000 (Invitrogen), according to the manufacturer's protocol. The sequences used were 5′-ACAUGGUUAGAUCAAGCACA-3′ (miR-218 inhibitor) and 5′-GCAUCUAUCUAUAUAUCUA-3′ (control inhibitor).
miR-218, miR-181a, and pGL3–3′UTR-TGaseII Construct
To generate miR-218 expression vector, a 621-bp genomic fragment encompassing primary miR-218 gene was PCR-amplified and cloned into the BamHI/XhoI site of the pcDNA3.1 vector. To generate miR-181a expression vector, a 467-bp genomic fragment encompassing the primary miR-181a gene was PCR-amplified and cloned into the HindIII/XhoI site of pcDNA3.1 vector. To generate the pGL3-3′UTR-TGaseII construct, a 1396-bp mouse TGaseII gene segment encompassing the 3′-UTR was PCR-amplified and subcloned into the XbaI/XbaI site of the pGL3 luciferase plasmid. The mutant pGL3–3′UTR-TGaseII construct was made with deletion of the miR-218- or mR-181a-responsive element. The luciferase activity assay was performed according to the instruction manual (Promega).
Immunofluorescence Staining
RBL2H3 cells were seeded onto glass coverslips in 24-well plates and were sensitized with DNP-specific IgE (100 ng/ml) for 16 h. After stimulation with DNP-HSA (100 ng/ml) for 1 h, cells were fixed with 4% paraformaldehyde (v/v) for 10 min and then permeabilized with 0.4% Triton X-100 for 10 min. Nonspecific antibody binding sites were blocked by incubation with 1% BSA in TBST for 30 min. Cells were then incubated with primary antibody specific to TGaseII (1:200; BD Biosciences) or FcϵRIβ (1:200; Santa Cruz Biotechnology) for 2 h, followed by washing with TBS-T three times. Anti-goat IgG-FITC (for detection of TGaseII) or anti-rabbit Alexa Fluor 586 (for detection of FcϵRIβ) secondary antibody (Molecular Probes) was added to cells and incubated for 1 h. Coverslips were then washed and mounted by applying Mount solution (Biomeda Corp., Foster City, CA). Fluorescence images were acquired using a confocal laser-scanning microscope and software (Fluoview version 2.0) with a ×60 objective (FV300, Olympus, Tokyo, Japan). To examine the effect of miR-218 on the co-localization of HDAC3 (histone deacetylase 3) with FcϵRIβ, RBL2H3 cells were transfected with control vector (1 μg) or miR-218 construct (1 μg). The next day, cells were sensitized with DNP-specific IgE (100 ng/ml) for 24 h, followed by stimulation with DNP-HSA (100 ng/ml) for 1 h. Immunofluorescence staining was performed.
RNA Extraction and Quantitative RT-PCR (qRT-PCR)
Total miRNA was isolated using the mirVana miRNA isolation kit (Ambion). miRNA was extended by a poly(A) tailing reaction using the A-Plus poly(A) polymerase tailing kit (Cell Script). cDNA was synthesized from miRNA with poly(A) tail using a poly(T) adaptor primer and qScriptTM reverse transcriptase (Quanta Biogenesis). Expression levels of miR-218 or miR-181a were quantified with the SYBR Green qRT-PCR kit (Ambion) using an miRNA-specific forward primer and a universal poly(T) adaptor reverse primer. The expression of miR-218 or miR-181a was defined based on the threshold (Ct), and relative expression levels were calculated as 2−((Ct of miR-218 or miR-181a) − (Ct of U6)) after normalization with reference to expression of U6 small nuclear RNA. For quantitative PCR, the SYBR PCR Master Mix (Applied Biosystems) was used in a CFX96 real-time system thermocycler (Bio-Rad). To determine the level of TGaseII mRNA, total RNA was isolated with TRIzol reagent (Invitrogen). RNA was then reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Invitrogen) and random primers (Roche Applied Science). The cDNA was amplified with specific primers and Power SYBR Green PCR Master Mix (Applied Biosystems).
Western Blot Analysis
Western blot and immunoprecipitation were performed according to the standard procedures (24). For analysis of proteins from tissues, frozen samples were ground to a fine powder using a mortar and pestle over liquid nitrogen. Proteins were solubilized in radioimmune precipitation assay buffer containing protease inhibitors, and insoluble material was removed by centrifugation.
Chromatin Immunoprecipitation (ChIP) Assay
Assays were performed according to the manufacturer's instructions (Upstate Biotechnology). The antibody immunoprecipitates were reverse cross-linked. PCR was done on the phenol/chloroform-extracted DNA with specific primers. To examine the binding of TGaseII to the miR-218 promoter sequences, specific primers of the miR-218 promoter-1 sequences (5′-TGCAGGAATTCACCATTTGGC-3′ (sense) and 5′-TACACCAACCACTAGGCCAG-3′ (antisense)), miR-218 promoter-2 sequences (5′-TGTCTCACACACAGTTCATCCA-3′ (sense) and 5′-ACTAGAAGAAGGTACATCGGGGA-3′ (antisense)), and miR-218 promoter-3 sequences (5′-ACATGCATTCAGTGAACCTAGT-3′ (sense) and 5′-GGCAATGCAACGGCCTAAAA-3′ (antisense)) were used. To examine the binding of TGaseII to the miR-181a promoter sequences, specific primers of the miR-181a promoter-1 sequences (5′-GCCAAAGTGAAACAAGTTGAGT-3′ (sense) and 5′-GAAGCAACCTGTGAGCCAGA-3′ (antisense)) and miR-181a promoter-2 sequences (5′-CTGAATGCATACCATCAGCAGA-3′ (sense) and 5′-ACCTATGCATACTAGCTGAGACT-3′ (antisense)) were used.
Preparation of siRNA Duplexes
Construction of siRNA was carried out according to the instruction manual provided by the manufacturer (Ambion).
IgE-dependent Triphasic Cutaneous Reaction (TpCR) in the Mouse Ear
To induce the IgE-dependent TpCR in the ear of female BALB/c mice, mice were sensitized by injecting DNP-specific IgE antibody (10 μg/kg) intravenously. Twenty-four hours later, a cutaneous reaction was evoked by painting with 25 μl of 0.15% DNFB acetone/olive oil (3:1) solution onto each surface of both ear lobes. Ear thickness was measured by using a digital gauge. In order to examine the effect of TGaseII on TpCR, scrambled (100 nm) or TGaseII siRNA (100 nm) was injected intravenously on the day of IgE sensitization.
Passive Cutaneous Anaphylaxis
BALB/c mice were passively sensitized with an intravenous injection of TNP-specific IgE (0.5 μg/kg) or DNP-specific IgE (0.5 μg/kg). The mice were challenged 24 h later with an intravenous injection of TNP-BSA (250 μg/kg) or DNP-HSA (250 μg/kg) plus 250 μl of PBS containing 2% (v/v) Evans blue solution. Thirty minutes after TNP-BSA challenge, the mice were euthanized, and the 2% (v/v) Evans blue dye was extracted from each dissected ear in 700 μl of acetone/water (7:3) at room temperature overnight. The absorbance of Evans blue in the extracts was measured with a spectrophotometer at 620 nm. To determine the effect of TGaseII on passive cutaneous anaphylaxis (PCA), BALB/c mice were given an intradermal injection of scrambled (100 nm) or TGaseII siRNA (100 nm) on the next day of the sensitization with DNP-specific IgE or TNP-specific IgE. One hour after the injection of siRNA, BALB/c mice were challenged with TNP-BSA (250 μg/kg) or DNP-HSA (250 μg/kg) plus 250 μl of PBS containing 2% (v/v) Evans blue solution for determining the extent of vascular permeability accompanied by PCA.
IgE-dependent Passive Systemic Anaphylaxis
For the induction of passive systemic anaphylaxis, BALB/c mice were sensitized by an intravenous injection of DNP-specific IgE (0.5 μg/kg). The next day, sensitized mice were challenged by an intravenous injection of DNP-HSA (250 μg/kg). To determine the effect of TGaseII on the metastatic potential of B16F1 melanoma cells, scrambled (100 nm) or TGaseII siRNA(100 nm) was injected intravenously into the tail vein of a BALB/c mouse on the day of antigen challenge. Five days after the injection of IgE, BALB/c mice were given an intravenous injection of B16F1 melanoma cells. To examine the direct effect of TGaseII on the metastatic potential of B16F1 melanoma cells, mouse recombinant TGaseII protein (5 μg/kg) was intravenously injected into the tail vein of a BALB/c mouse. The next day, B16F1 melanoma cells were injected intravenously into a BALB/c mouse. Fourteen days after the injection of recombinant TGaseII protein, the extent of lung metastasis was determined.
Whole Mount Staining
BALB/c mice were given an intravenous injection of mouse recombinant TGaseII protein (5 μg/kg) twice in a total of 5 days. Mouse ears were fixed in 4% (v/v) paraformaldehyde and blocked with TNB buffer (PerkinElmer Life Sciences) containing 0.3% Triton X-100. Primary antibody, rabbit anti-CD31 (PECAM-1), was diluted in TNB buffer, and ear samples were incubated with primary antibody overnight at 4 °C. The samples were then washed three times and subsequently incubated with the secondary antibody, anti-rabbit IgG Alex 488 (Molecular Probes, Inc.).
Intravital Microscopy
Male BALB/c mice (6–8 weeks old) were obtained from Daehan Biolink (Korea). In vivo angiogenesis was assessed as follows. The mice were anesthetized with 2.5% avertin (v/v) via intraperitoneal injection (Surgivet), and abdominal wall windows were implanted. Next, a titanium circular mount with eight holes on the edge was inserted between the skin and the abdominal wall. Growth factor-reduced Matrigel containing the conditioned medium was applied to the space between the windows, and a circular glass coverslip was placed on top and fixed with a snap ring. After 4 days, the animals were anesthetized and injected intravenously with 50 μl of 25 ng/ml fluorescein isothiocyanate-labeled dextran (Mr ∼2,000,000) via the tail vein. The mice were then placed on a Zeiss Axiovert 200 M microscope. The epi-illumination microscopy setup included a 100-watt mercury lamp and filter set for blue light. Fluorescence images were recorded at random locations of each window using an electron-multiplying charge-coupled device camera (Photo Max 512, Princeton Instruments) and digitalized for subsequent analysis using the Metamorph program (Universal Imaging). The assay was scored from 0 (negative) to 5 (most positive) in a double-blinded manner. To determine the effect of TGaseII on the passive systemic anaphylaxis (PSA)-promoted angiogenesis, BALB/c mice were sensitized to DNP-specific IgE (0.5 μg/kg) by an intravenous injection. The next day, BALB/c mice were given an intravenous injection of DNP-HSA (250 μg/kg) or PBS along with R2 peptide (9 mg/kg). One hour after stimulation with DNP-HSA (250 μg/kg), lung tissues were isolated. Lung mast cells were then isolated. The conditioned medium of lung mast cells (10 μl) was mixed with growth factor-reduced Matrigel. Intravital microscopy was performed as described.
Flow Cytometry Assay
Expression of CD163 on M2 Macrophages was examined by fluorescence-activated cell sorter (FACS) analysis. Cells were fixed using 1% paraformaldehyde for 10 min at 37 °C and then permeabilized with ice-methanol for 30 min. Nonspecific antibody-binding sites were blocked by incubation with 3% BSA in PBS for 30 min at 37 °C. Cells were then incubated with primary antibody specific to CD163 (1:100; Santa Cruz Biotechnology) for 1 h at 37 °C, followed by washing with PBS-T three times. Anti-goat Alexa Fluor 546 antibody (Invitrogen) was added to cells and incubated for 30 min at 37 °C. Samples were analyzed on a FACSCaliber cell analyzer (BD Biosciences). Data were analyzed using BD FACStation software (BD Biosciences).
Wound Migration
Cells were plated overnight to achieve a confluent layer in 24-well plates. A scratch was made on the cell layer with a micropipette tip, and cultures were washed twice with serum-free medium. Wound healing was visualized by comparing photographs taken at 0 and 48 h postscratch. To examine the effect of mast cells on the migration potential of B16F1 melanoma cells, the conditioned medium of lung mast cells obtained after PSA was added to B16F1 melanoma cells in serum-free medium in a 1:1 ratio.
miRNA Target Analysis
Genes that contain the miR-binding site(s) in the UTR were obtained using the TargetScan program.
Statistical Analysis
Data were analyzed and graphed using the GraphPad Prism statistics program (GraphPad Software). Results are presented as mean ± S.E. Statistical analysis was performed using Student's t tests with differences between means considered significant when p was <0.05.
RESULTS
TGaseII Is Induced by Antigen Stimulation and Shows an Interaction with FcϵRIβ
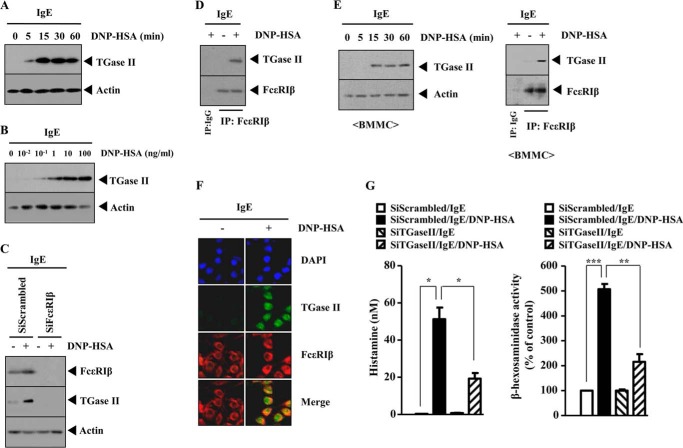
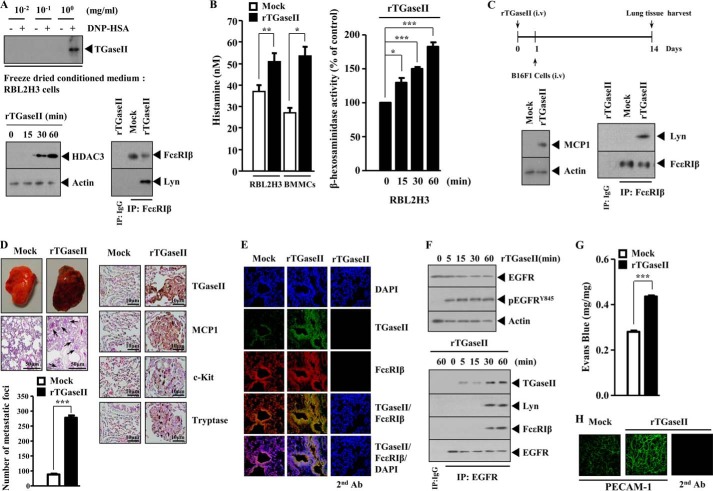
We previously reported the role of TGaseII in allergic inflammation (11). However, the mechanism of TGaseII-promoted allergic inflammation requires further studies. Antigen stimulation induced the expression of TGaseII in a time- and dose-dependent manner in RBL2H3 cells (Fig. 1, A and B). The down-regulation of FcϵRIβ prevented antigen from inducing the expression of TGaseII in RBL2H3 cells (Fig. 1C). Antigen stimulation induced an interaction between TGaseII and FcϵRIβ in RBL2H3 cells (Fig. 1D). Antigen stimulation induced the expression of TGaseII and an interaction between TGaseII and FcϵRIβ in bone marrow-derived mouse mast cells (BMMCs) (Fig. 1E). Immunofluorescence staining shows a co-localization of TGaseII with FcϵRIβ in antigen-stimulated RBL2H3 cells (Fig. 1F). The down-regulation of TGaseII prevented antigen from increasing the secretion of histamine and prevents antigen from increasing β-hexosaminidase activity (Fig. 1G). Taken together, these results suggest that TGaseII mediates allergic inflammation through interaction with FcϵRIβ.
FIGURE 1.
TGaseII is induced by antigen stimulation and interacts with FcϵRIβ. A, RBL2H3 cells were sensitized with DNP-specific IgE (100 ng/ml). The next day, cells were then stimulated with DNP-HSA (100 ng/ml) for various time intervals. Cell lysates prepared at each time point were subjected to Western blot analysis. B, the IgE-sensitized RBL2H3 cells were stimulated with various concentrations of DNP-HSA (100 ng/ml) for 1 h. Cell lysates prepared were subjected to Western blot analysis. C, RBL2H3 cells were transiently transfected with the indicated siRNA (10 nm each) prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA (100 ng/ml) for 1 h, followed by Western blot analysis. D, the IgE-sensitized RBL2H3 cells were stimulated with DNP-HSA (100 ng/ml) for 1 h. Cell lysates were immunoprecipitated with the indicated antibody (2 μg/ml), followed by Western blot analysis. E, the IgE-sensitized BMMCs were stimulated with DNP-HSA (100 ng/ml) for various time intervals. Cell lysates prepared at each time point were subjected to Western blot analysis (left). IgE-sensitized BMMCs were stimulated with DNP-HSA (100 ng/ml) for 1 h. Cell lysates were then immunoprecipitated with the indicated antibody (2 μg/ml), followed by Western blot analysis (right). F, the IgE-sensitized RBL2H3 cells were stimulated with DNP-HSA (100 ng/ml) for 1 h. Immunofluorescence staining employing the indicated antibody was performed as described. G, RBL2H3 cells were transiently transfected with the indicated siRNA (10 nm each) prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA (100 ng/ml) for 1 h. A histamine release assay and β-hexosaminidase activity assays employing cell lysates were performed as described. *, p < 0.05; **, p < 0.005; ***, p < 0.0005. Error bars, S.E.
TGaseII Mediates in Vivo Allergic Skin Inflammation
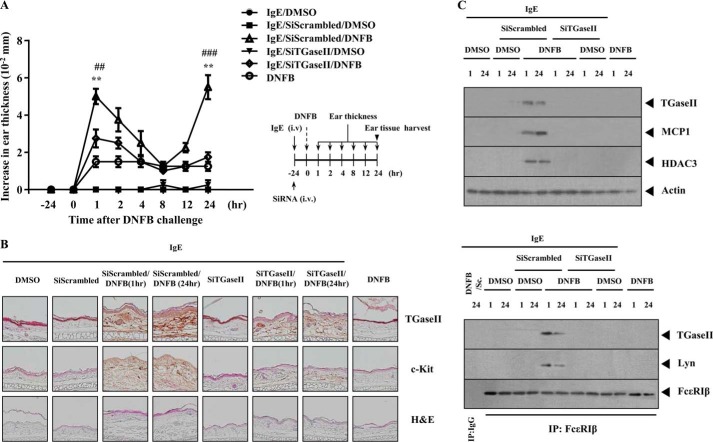
We next examined the role of TGaseII in an in vivo allergic inflammation reaction. For this, we employed a BALB/c mouse model of triphasic cutaneous reaction (TpCR). Ear swelling is seen at 1 and 24 h after 2,4-dinitrofluorobenzene (DNFB) stimulation on ears (Fig. 2A). The in vivo down-regulation of TGaseII by siRNA exerted a negative effect on the increased ear thickness by DNFB stimulation (Fig. 2A). Immunohistochemical staining (Fig. 2B) and Western blot analysis (Fig. 2C) of BALB/c mouse ear tissue lysates show the induction of TGaseII by antigen stimulation. Immunohistochemical staining shows that the in vivo down-regulation of TGaseII prevents antigen from inducing the expression of c-Kit, a marker protein of mast cell activation (Fig. 2B). TGaseII was necessary for the induction of HDAC3 and MCP1 in TpCR (Fig. 2C). The role of HDAC3 and MCP1 in allergic skin inflammation has been shown (24). Antigen stimulation induced an interaction between TGaseII and FcϵRIβ (Fig. 2C). TGaseII was necessary for an interaction between FcϵRIβ and Lyn, an essential molecule for FcϵRI signaling (Fig. 2C). Taken together, these results suggest that TGaseII mediates in vivo allergic skin inflammation through interaction with FcϵRIβ.
FIGURE 2.
TGaseII is necessary for TpCR. A, BALB/c mice were given an intravenous (i.v.) injection with DNP-specific IgE antibody (10 μg/kg) along with scrambled or TGaseII siRNA (each at 100 nm). The next day, both ears of mice were painted with DNFB or DMSO. At each time point after DNFB stimulation, ear thickness was measured. Each experimental group consisted of four BALB/c mice. Means ± S.E. (error bars) of three independent experiments are depicted. **, p < 0.005, compared with IgE/scrambled; ##, p < 0.005, compared with IgE/SiTGaseII/DNFB (1 h); ###, p < 0.0005, compared with IgE/SiTGaseII/DNFB (24 h). B, paraffin section of the ear tissue of the indicated BALB/c mouse was subjected to immunohistochemical staining employing anti-TGaseII or anti-c-Kit antibody. Representative images from four animals of each experimental group are shown (magnification, ×400; Olympus). H&E staining was also performed to determine the extent of leukocyte infiltration. C, ear tissue lysates prepared at the indicated time point were subjected to Western blot analysis (top). The same ear tissue lysates were immunoprecipitated (IP) with the indicated antibody (2 μg/ml), followed by Western blot analysis (bottom). Sc, SiScrambled. Ear tissue lysates were isolated from each mouse of each experimental group of mice.
TGaseII Is Necessary for Passive Cutaneous Anaphylaxis
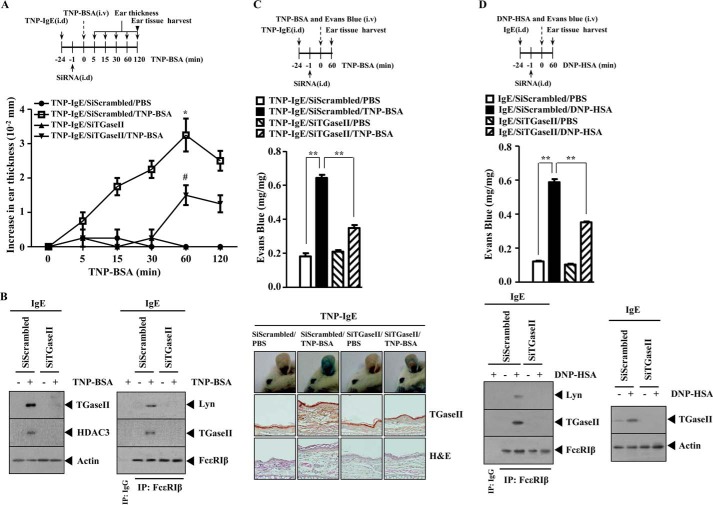
We further examined the role of TGaseII in in vivo allergic inflammation. PCA involves the increased ear thickness by antigen stimulation (24). To examine the role of TGaseII in PCA, BALB/c mice were given an intradermal injection of TNP-specific IgE. Twenty-four hours later, BALB/c mice were given an intravenous injection of TNP-BSA. Ear swelling was evident 15 min after injection of TNP-BSA and was decreased 120 min after TNP-BSA injection (Fig. 3A). The in vivo down-regulation of TGaseII exerts a negative effect on the increased ear thickness by TNP-BSA stimulation (Fig. 3A). Western blot of ear tissue lysates shows that PSA induces the expression of TGaseII and HDAC3 (Fig. 3A). We previously reported the role of HDAC3 in allergic skin inflammation (24). The in vivo down-regulation of TGaseII prevented an interaction between FcϵRIβ and Lyn induced by PCA (Fig. 3B). Immunohistochemical staining shows that PCA involved the increased expression of TGaseII and inflammation (Fig. 3C). The in vivo down-regulation of TGaseII exerted a negative effect on enhanced vascular permeability associated with PCA and prevented an interaction between FcϵRIβ and TGaseII and between FcϵRIβ and Lyn (Fig. 3D). PCA, induced by DNP-HSA, involved the increased ear thickness (data not shown). Taken together, these results further suggest the involvement of TGaseII in in vivo allergic inflammation.
FIGURE 3.
TGaseII is necessary for PCA. A, BALB/c mice were given an intravenous (i.v.) injection of TNP-specific IgE (0.5 μg/kg). The next day, BALB/c mice were given an intradermal (i.d.) injection of scrambled (100 nm) or TGaseII siRNA (100 nm). One hour after the injection of the siRNA, the mice were challenged with PBS or TNP-BSA (250 μg/kg). Ear thickness was measured as described. −24 and −1, 24 and 1 h before the challenge with TNP-BSA. B, ear tissue lysates prepared at 1 h after stimulation with TNP-BSA were subjected to Western blot analysis (left). The same ear tissue lysates were immunoprecipitated with the indicated antibody (2 μg/ml), followed by Western blot analysis (right). C, BALB/c mice were given an intravenous injection of TNP-specific IgE (0.5 μg/kg). The next day, BALB/c mice were given an intradermal injection of scrambled (100 nm) or TGaseII siRNA (100 nm). One hour after the injection of the siRNA, BALB/c mice were given an intravenous injection of PBS or TNP-BSA (250 μg/kg) along with 2% (v/v) Evans blue solution. One hour after the injection of Evans blue solution, the dye was eluted from the ear in 700 μl of formamide at 63 °C. The absorbance was measured at 620 nm (middle). **, p < 0.005. A paraffin section of the ear tissue of the indicated experimental group of BALB/c mice was subjected to immunohistochemical staining employing anti-TGaseII antibody (bottom). Representative images from four animals of each experimental group are shown (magnification, ×400; Olympus). H&E staining was also performed (bottom). −24 and −1, 24 and 1 h before the challenge with TNP-BSA. D, BALB/c mice were given an intravenous injection of DNP-specific IgE (0.5 μg/kg). The next day, BALB/c mice were given an intradermal injection of scrambled (100 nm) or TGaseII siRNA (100 nm). One hour after the injection of siRNA, BALB/c mice were given an intravenous injection of PBS or DNP-HSA (250 μg/kg) along with 2% (v/v) Evans blue solution. One hour after the injection of Evans blue solution, the dye was eluted from the ear in 700 μl of formamide at 63 °C. The absorbance was measured at 620 nm (middle). **, p < 0.005. Ear tissue lysates were isolated from each mouse of each experimental group of mice and were immunoprecipitated with the indicated antibody (2 μg/ml), followed by Western blot analysis (bottom). Ear tissue lysate were also subjected to Western blot analysis (bottom). −24 and −1, 24 and 1 h before the challenge with DNP-HSA. Error bars, S.E.
TGaseII Is Necessary for the Enhanced Metastatic Potential of Mouse Melanoma Cell by PSA
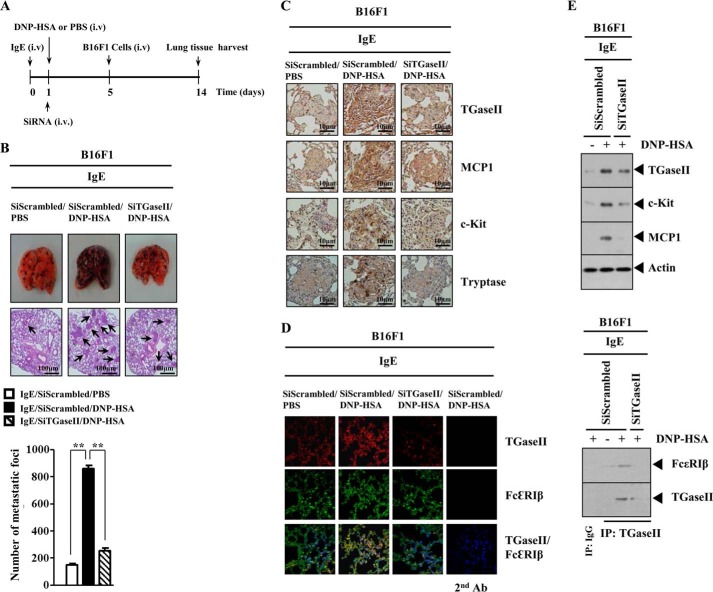
Allergic inflammation promotes tumor metastasis (25). Mast cells play a protumorigenic role in primary cutaneous lymphoma (26). Because TGaseII was necessary for allergic inflammation, we examined the role of TGaseII in allergic inflammation-promoted enhanced metastatic potential of tumor cells. For this, we employed a mouse model of PSA. PSA involves the activation of FcϵRI signaling (25). Systemic anaphylaxis is an immediate hyperacute reaction that is mediated by bioactive mediators, mostly from mast cells (27). These mediators cause severe hypotension, a decrease in body temperature, and an increase in β-hexosaminidase activity (27). Anaphylaxis requires activation of mast cells and basophils (28). After the induction of PSA, B16F1 melanoma cells were injected intravenously into the tail vein of a BALB/c mouse (Fig. 4A). PSA enhanced the metastatic potential of B16F1 melanoma cells in a TGaseII-dependent manner (Fig. 4B). Immunohistochemical staining of the lung tumor tissue shows that the induction of TGaseII, MCP1, C-Kit, and tryptase by PSA occurred in a TGaseII-dependent manner (Fig. 4C). Immunofluorescence staining of lung tumor tissue shows a co-localization of TGaseII with FcϵRIβ by PSA (Fig. 4D). Western blot analysis of lung tumor tissue lysates shows the induction of TGaseII, c-Kit, and MCP1 by PSA (Fig. 4E). Immunoprecipitation of lung tumor tissue shows an interaction between TGaseII and FcϵRIβ by PSA (Fig. 4E). The above results suggest that mast cell activation is responsible for PSA-promoted enhanced metastatic potential of B16F1 melanoma cells. Taken together, these results suggest that TGaseII is necessary for allergic inflammation-promoted enhanced metastatic potential of mouse melanoma cells.
FIGURE 4.
TGaseII mediates enhanced metastatic potential of B16F1 melanoma cells by PSA. A, BALB/c mice were sensitized with DNP-specific IgE (0.5 μg/kg) by an intravenous (i.v.) injection. The next day, BALB/c mice were given an intravenous injection of DNP-HSA (250 μg/kg) along with scrambled (100 nm) or TGaseII siRNA (100 nm). Each mouse received an intravenous injection of B16F1 melanoma cells (2 × 105) on day 5 of the time line. On day 14 of the time line, lung tissues were harvested. B, formalin-fixed lung sections were stained with H&E. Black arrows, lung metastatic foci (scale bar, 100 μm). The extent of lung metastasis was determined as described. **, p < 0.005. Each group consisted of four mice. C, imunonohistochemical staining of lung tumor tissue employing the indicated antibody was performed as described. Each group consisted of four mice. D, cryosections of lung tumor tissues were prepared, and immunofluorescence staining employing the indicated antibody was performed. DAPI staining was also performed for nuclear staining. E, lung tumor tissue lysates were isolated from each mouse of each experimental group of mice and were subjected to Western blot analysis (top). Lung tumor lysates were isolated from each mouse of each experimental group of mice and were immunoprecipitated (IP) with the indicated antibody (2 μg/ml), followed by Western blot analysis (bottom). Error bars, S.E.
Recombinant TGaseII Protein Enhances the Metastatic Potential of Mouse Melanoma Cells and Activates EGFR and FcϵRI
Because TGaseII was necessary for the enhanced metastatic potential of mouse melanoma cells, we examined whether TGaseII would directly enhance the metastatic potential of B16F1 melanoma cells. Several reports suggest TGaseII as a secreted protein (29, 30). We examined the possibility of TGaseII as a secreted protein. The conditioned medium of antigen-stimulated RBL2H3 cells was obtained and freeze-dried. Freeze-dried conditioned medium was subjected to Western blot analysis. The conditioned medium obtained from antigen-stimulated RBL2H3 cells showed the expression of TGaseII (Fig. 5A). The recombinant TGaseII protein, when added to RBL2H3 cells, induced the expression of HDAC3 and an interaction between FcϵRIβ and Lyn (Fig. 5A). The recombinant TGaseII protein increased the secretion of histamine in RBL2H3 cells and BMMCs (Fig. 5B). The recombinant TGaseII protein also increased β-hexosaminidase activity in RBL2H3 cells in a time-dependent manner (Fig. 5B). We examined the effect of recombinant TGaseII on the metastatic potential of B16F1 melanoma cells (Fig. 5C). Western blot analysis of lung tumor lysates shows the induction of MCP1 and FcϵRIβ-Lyn interaction by recombinant TGaseII protein (Fig. 5C). Recombinant TGaseII protein enhanced the metastatic potential of B16F1 melanoma cells (Fig. 5D). Immunohistochemical staining of lung tumor section shows that the recombinant TGaseII protein induces the expression of MCP1, c-Kit, and tryptase (Fig. 5D). This indicates that the enhanced metastatic potential of B16F1 melanoma cells results from the activation of mast cells. MCP1, through interaction with CCR2 (a receptor for MCP1), mediates allergic inflammation-promoted enhanced metastatic potential of mouse melanoma cells (25). This suggests that MCP1-CCR2 interaction, induced by TGaseII, is responsible for the enhanced metastatic potential of B16F1 melanoma cells. Immunofluorescence staining of cryosection of lung tumor tissue shows a co-localization of TGaseII with FcϵRIβ by recombinant TGaseII protein (Fig. 5E). Allergic inflammation involves the activation of EGFR (31). We therefore examined whether TGaseII would induce the activation of EGFR. The recombinant TGaseII protein increased the phosphorylation of EGFR and induced an interaction between EGFR and FcϵRIβ in RBL2H3 cells (Fig. 5F). This implies that cross-talk between EGFR and FcϵRI, induced by TGaseII, may mediate allergic inflammation. The metastatic potential of tumor cells is closely related with angiogenesis (24). The inhibition of EGFR by cetuximab prevents angiogenesis associated with allergic inflammation (31). PCA involved the increased vascular permeability (Fig. 3, C and D). We therefore hypothesized that PSA-promoted enhanced metastatic potential of B16F1 melanoma cells would be correlated with the angiogenic potential. The recombinant TGaseII protein enhances vascular permeability of BALB/c mouse (Fig. 5G) and increases the expression of PECAM-1, a marker of angiogenesis, based on immunofluorescence staining (Fig. 5H). Taken together, these results suggest that TGaseII mediates allergic inflammation-promoted enhanced metastatic potential of tumor cells and angiogenesis.
FIGURE 5.
Recombinant TGaseII protein enhances the metastatic potential of B16F1 melanoma cells and activates EGFR and FcϵRIβ. A, the conditioned medium of RBL2H3 cells stimulated with DNP-HSA (100 ng/ml) for 1 h was freeze-dried. The indicated amount of freeze-dried conditioned medium was subjected to Western blot analysis (top). RBL2H3 cells were treated with recombinant TGaseII (1 ng/ml) for various time intervals. Cell lysates prepared at each time point were subjected to Western blot analysis (bottom). RBL2H3 cells were treated with recombinant TGaseII (1 ng/ml) for 1 h. Cell lysates were immunoprecipitated (IP) with the indicated antibody (2 μg/ml), followed by Western blot analysis (bottom). B, RBL2H3 cells or BMMCs were treated with recombinant TGaseII protein (1 ng/ml) for 1 h. Histamine release assay and β-hexosaminidase activity assays were performed as described. *, p < 0.05; **, p < 0.005; ***, p < 0.005. C, BALB/c mice were given an intravenous injection of recombinant TGaseII protein (100 ng) or PBS. The next day, BALB/c mice were given an intravenous injection of B16F1 melanoma cells. On day of 14 of the time line, lung tumor tissues were harvested. Lung tumor tissue lysates were isolated from each mouse of each experimental group of mice and were subjected to Western blot analysis (left). Lung tumor tissue lysates were isolated from each mouse of each experimental group of mice and were immunoprecipitated with the indicated antibody (2 μg/ml), followed by Western blot analysis (right). D, the extent of lung metastasis was determined. Black arrows, lung metastatic foci (scale bar, 50 μm). Imunonohistochemical staining of lung tumor tissue employing the indicated antibody was performed as described (scale bar, 10 μm, right). ***, p < 0.0005. Representative images from four animals of each experimental group are shown. E, cryosections of lung tumor tissues were prepared, and immunofluorescence staining employing the indicated antibody was performed. F, RBL2H3 cells were treated with mouse recombinant TGaseII protein (1 ng/ml) for various time intervals. Cell lysates prepared at each time point were subjected to Western blot analysis (top). Cell lysates were also immunoprecipitated with the indicated antibody (2 μg/ml), followed by Western blot analysis (bottom). G, BALB/c mice were given an intravenous injection of mouse recombinant TGaseII protein (5 μg/kg) or PBS. One hour or 24 h after injection, BALB/c mice were given an intravenous injection of 2% (v/v) Evans blue solution. Representative images from four animals of each experimental group are shown. ***, p < 0.0005. H, BALB/c mice were given an intravenous injection of mouse recombinant TGaseII protein (5 μg/kg) or PBS. Five days after the injection of recombinant TGaseII protein, ears of BALB/c mice were excised and subjected to whole mount staining employing anti-PECAM-1 antibody. Representative images from four animals of each experimental group are shown (magnification, ×100; Olympus). Error bars, S.E.
EGFR Is Necessary for TGaseII-promoted Allergic Inflammation
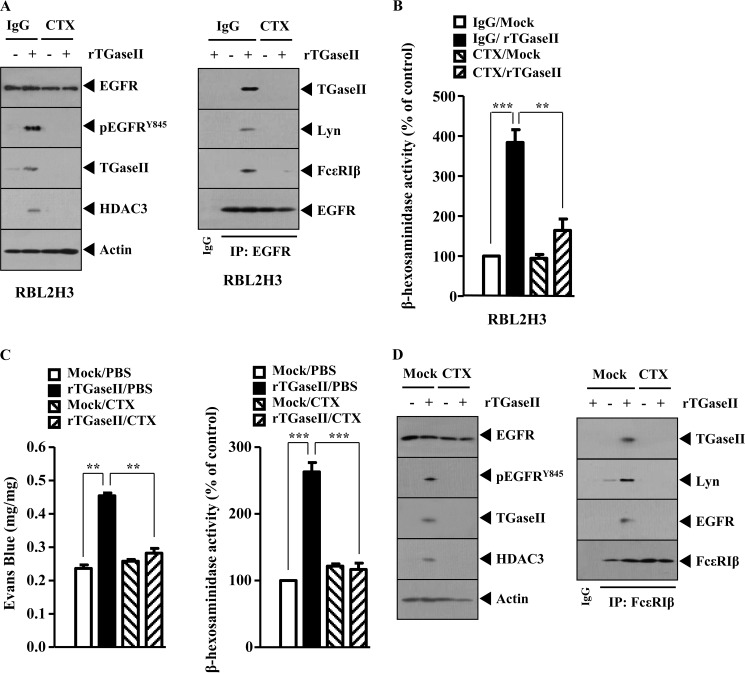
We further examined the mechanism of TGaseII-mediated allergic inflammation. The recombinant TGaseII protein increases the phosphorylation of EGFR and the expression of TGaseII and HDAC3 (Fig. 6A). Cetuximab (CTX), an inhibitor of EGFR, prevented recombinant TGaseII protein from increasing the phosphorylation of EGFR, the expression of TGaseII, and HDAC3 (Fig. 6A). CTX prevented recombinant TGaseII protein from inducing an interaction of EGFR with FcϵRI, Lyn, and TGaseII (Fig. 6A). Recombinant TGaseII protein increased β-hexosaminidase activity (Fig. 6B). The recombinant TGaseII protein, when injected into BALB/c mouse, induced vascular permeability and β-hexosaminidase activity in an EGFR-dependent manner (Fig. 6C). The recombinant TGaseII protein, when injected into a BALB/c mouse, increased the phosphorylation of EGFR, increased the expression of TGaseII and HDAC3, and induced an interaction of FcϵRI with EGFR, Lyn, and TGaseII in an EGFR-dependent manner (Fig. 6D). Taken together, these results suggest that EGFR is necessary for TGaseII-promoted allergic inflammation.
FIGURE 6.
EGFR is necessary for TGaseII-promoted allergic inflammation. A, RBL2H3 cells were pretreated with IgG (2 μg/ml) or CTX (0.5 μg/ml) for 30 min, followed by treatment with recombinant TGaseII protein (1 ng/ml) for 1 h. Cell lysates were subjected to Western blot (left) or immunoprecipitation employing the indicated antibody (right). B, same as A except that a β-hexosaminidase activity assay was performed. C, BALB/c mice were given an intradermal injection of recombinant TGaseII protein (5 μg/kg). The next day, BALB/c mice were given an intravenous injection of CTX (2 μg/kg). One hour after the injection of CTX, BALB/c mice were given an intravenous injection of 2% (v/v) Evans blue solution. One hour after the injection of Evans blue solution, the dye was eluted from the ear in 700 μl of formamide at 63 °C. The absorbance was measured at 620 nm (left). Two hours after the injection of CTX, ear tissue lysates were prepared and subjected to a β-hexosaminidase activity assay (right). D, ear tissue lysates isolated after the injection of CTX were subjected to Western blot analysis (left) or immunoprecipitation (right). Error bars, S.E.
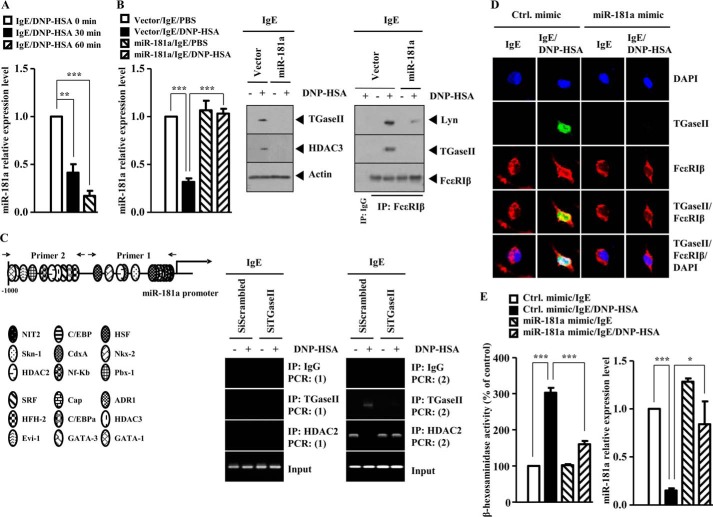
miR-218 Negatively Regulates the Expression of TGaseII and Allergic Inflammation
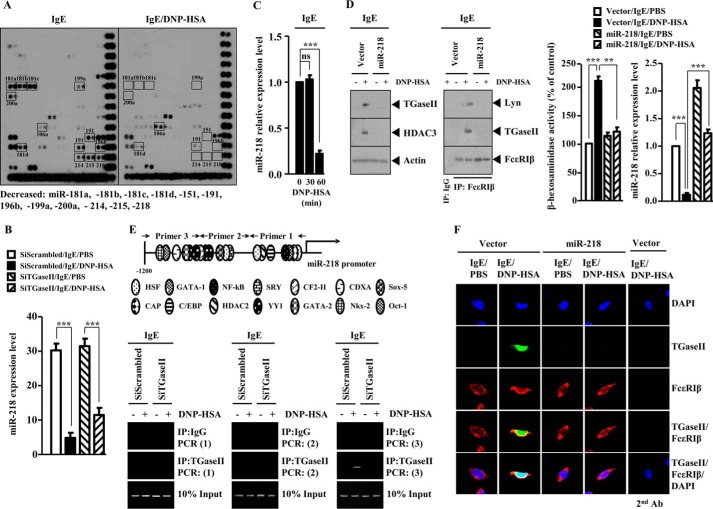
Because TGaseII mediated allergic inflammation, we wanted to identify miRNA genes that regulate the expression of TGaseII. For this, we performed miRNA array analysis. Antigen stimulation in RBL2H3 decreased the expression of various miRNA genes, including miR-218, miR-181a, miR-181b, miR-181d, miR-199a, miR-151, miR-191, miR-214, miR-215, miR-200a, and miR-196b (Fig. 7A). It is probable that these miRNAs may act as negative regulators of allergic inflammation. The expression level of miR-106a was increased in antigen-stimulated RBL2H3 cells (Fig. 7A). The down-regulation of TGaseII by siRNA decreased the expression of miR-106a in antigen-stimulated RBL2H3 cells (data not shown). It is probable that miR-106a mediates allergic inflammation. miR-218 is encoded by an intron of the Slit genes. miR-218 directly represses the expression of Robo1, Robo2, and glucuronyl C5-epimerase, and an intact miR-218-Slit-Robo regulatory network is essential for normal vascularization of the retina (32). Because PCA involves the enhanced vascular permeability and angiogenesis (24), it is probable that miR-218 may regulate in vitro and in vivo allergic inflammation. We examined the possibility of miR-218 as a negative regulator of allergic inflammation. The down-regulation of TGaseII restored the expression of miR-218 in antigen-stimulated RBL2H3 cells (Fig. 7B). qRT-PCR analysis shows that antigen stimulation decreases the expression of miR-218 in RBL2H3 cells in a time-dependent manner (Fig. 7C). The overexpression of miR-218 prevented antigen from increasing the expression of TGaseII and HDAC3 and prevented antigen from inducing an interaction between FcϵRIβ and TGaseII and an interaction between FcϵRIβ and Lyn in antigen-stimulated RBL2H3 cells (Fig. 7D). miR-218 exerted a negative effect on the increased β-hexosaminidase activity in antigen-stimulated RBL2H3 cells (Fig. 7D). ChIP assays show the binding of TGaseII to the promoter sequences of miR-218 (Fig. 7E). These results suggest a feedback regulatory loop between TGaseII and miR-218. Immunofluorescence staining shows that the overexpression of miR-218 prevents a co-localization of TGaseII with FcϵRIβ in antigen-stimulated RBL2H3 cells (Fig. 7F). Taken together, these results suggest that miR-218 negatively regulates allergic inflammation by forming a negative feedback loop with TGaseII.
FIGURE 7.
miR-218 regulates the expression of TGaseII. A, RBL2H3 cells were transiently transfected with the indicated siRNA (10 nm each) prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA (100 ng/ml) for 1 h. miRNA array analysis was performed as described. Below are shown miRNAs that are decreased by antigen stimulation in RBL2H3 cells. B, same as A except that qRT-PCR was performed to determine the expression level of miR-218. ***, p < 0.0005. C, the IgE-sensitized RBL2H3 cells were stimulated with DNP-HSA (100 ng/ml) for various time intervals. At each time point, the expression level of miR-218 was determined by qRT-PCR analysis. ***, p < 0.0005. ns, not significant. D, RBL2H3 cells were transiently transfected with control vector (1 μg) or miR-218 construct (1 μg) prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA (100 ng/ml) for 1 h. Cell lysates prepared were subjected to Western blot analysis (left). The prepared cell lysates were immunoprecipitated with the indicated antibody (2 μg/ml), followed by Western blot analysis (middle). The prepared cell lysates were subjected to β-hexosaminidase activity assays (right). qRT-PCR analysis was performed to determine the expression level of miR-218 (right). **, p < 0.005; ***, p < 0.0005. E, promoter sequences of TGaseII (top). RBL2H3 cells were transiently transfected with the indicated siRNA (10 nm each) prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA (100 ng/ml) for 1 h. ChIP assays employing the indicated antibody were performed as described. Numbers in parentheses denote primer binding sites. F, same as D except that immunofluorescence staining employing the indicated antibody was performed. Error bars, S.E.
The Inhibition of miR-218 Induces the Features of Allergic Inflammation
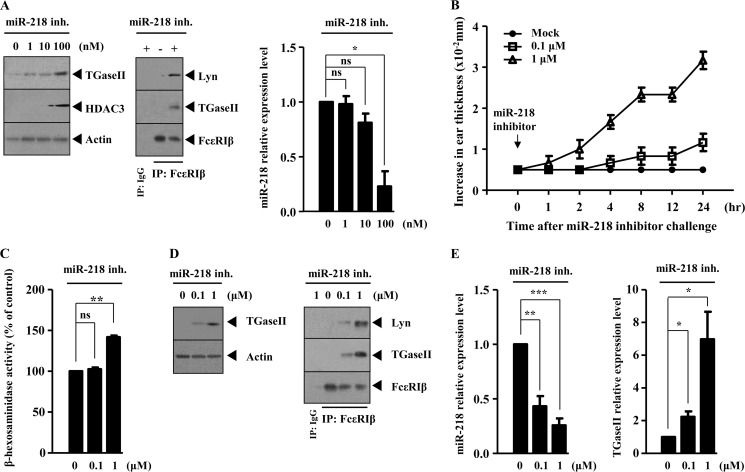
In an effort to confirm the role of miR-218 as a negative regulator of allergic inflammation, the effect of miR-218 inhibitor on the features of allergic inflammation was examined. miR-218 inhibitor increased the expression of TGaseII in a dose-dependent manner in RBL2H3 cells (Fig. 8A) and induced an interaction between FcϵRIβ and TGaseII and an interaction between FcϵRIβ and Lyn in RBL2H3 cells (Fig. 8A). miR-218 inhibitor, when injected into the ear of a BALB/c mouse, increased ear thickness, typical of in vivo allergic skin inflammation (Fig. 8B). miR-218 inhibitor increased β-hexosaminidase activity in the BALB/c mouse (Fig. 8C). Western blot analysis of ear tissue lysates shows the increased expression of TGaseII by miR-218 inhibitor (Fig. 8D). The immunoprecipitation of ear tissue lysates shows that miR-218 inhibitor induces an interaction between FcϵRIβ and TGaseII and an interaction between FcϵRIβ and Lyn (Fig. 8D). qRT-PCR analysis shows that miR-218 inhibitor increases the expression of TGaseII at the transcriptional level (Fig. 8E). Taken together, these results further suggest that miR-218 negatively regulates allergic inflammation.
FIGURE 8.
The inhibition of miR-218 induces the features of allergic inflammation. A, RBL2H3 cells were transiently transfected with various concentrations of miR-218 inhibitor. At 24 h after transfection, the prepared cell lysates were subjected to Western blot analysis (left). RBL2H3 cells were transiently transfected with miR-218 inhibitor (100 nm). At 24 h after transfection, cell lysates were immunoprecipitated with the indicated antibody (2 μg/ml), followed by Western blot analysis (middle). The expression level of miR-218 at each time point was determined by qRT-PCR (right). *, p < 0.05. ns, not significant. B, various concentrations of miR-218 inhibitor were injected into ears of BALB/c mice. At each time point after the injection of miR-218 inhibitor, ear thickness was measured as described. C, ear tissue lysates isolated from each mouse of each experimental group of mice injected with miR-218 inhibitor at the indicated concentration were subjected to β-hexosaminidase activity assays. **, p < 0.005. ns, not significant. D, same as C except that Western blot analysis (left) or immunoprecipitation (right) was performed. E, same as C except that qRT-PCR analysis was performed. *, p < 0.05; **, p < 0.005; ***, p < 0.0005. Error bars, S.E.
miR-181a Negatively Regulates in Vitro Allergic Inflammation by Forming a Negative Feedback Loop with TGaseII
The expression level of miR-181a is decreased in antigen-stimulated RBL2H3 cells (Fig. 7A). The down-regulation of TGaseII by siRNA restores the expression of miR-181a in antigen-stimulated RBL2H3 cells in miRNA array analysis (data not shown). This implies that TGaseII and miR-181a may form a negative feedback loop. miR-181a exerts anti-inflammatory effects on monocytes and macrophages by down-regulating IL-1a (33). miR-181a targets Prox1 and has implications for the control of Prox1 expression during vascular development and neo-lymphangiogenesis (34). These reports suggest a role of miR-181a in allergic inflammation. qRT-PCR analysis shows that antigen stimulation decreases the expression of miR-181a in RBL2H3 cells in a time-dependent manner (Fig. 9A). miR-181a prevented antigen from increasing the expression of TGaseII, and HDAC3 and prevented antigen from inducing an interaction between FcϵRIβ and TGaseII and an interaction between FcϵRIβ and Lyn in RBL2H3 cells (Fig. 9B). ChIP assays show the binding of TGaseII to the promoter sequences of miR-181a in antigen-stimulated RBL2H3 cells (Fig. 9C). This suggests a direct regulation of miR-181a by TGaseII. HDAC2, which is decreased in antigen-stimulated RBL2H3 cells (24), bound to the promoter sequences of miR-181a in RBL2H3 cells (Fig. 9C). The down-regulation of TGaseII by siRNA restored the binding of HDAC2 to the promoter sequences of miR-181a (Fig. 9C). Immunofluorescence staining shows that miR-181a mimic exerts a negative effect on the co-localization of TGaseII with FcϵRIβ in antigen-stimulated RBL2H3 cells (Fig. 9D). miR-181a mimic exerted a negative effect on the increased β-hexosaminidase activity and restored the expression of miR-181a in antigen-stimulated RBL2H3 cells (Fig. 9E). Taken together, these results suggest that miR-181a negatively regulates allergic inflammation by forming a negative feedback loop with TGaseII.
FIGURE 9.
miR-181a negatively regulates the in vitro allergic inflammation. A, the IgE-sensitized RBL2H3 cells were stimulated with DNP-HSA (100 ng/ml) for various time intervals. The expression level of miR-181a was determined by qRT-PCR. **, p < 0.005; ***p < 0.0005. B, RBL2H3 cells were transiently transfected with the indicated construct (1 μg) prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA (100 ng/ml) for 1 h. The expression level of miR-181a was determined by qRT-PCR (left). Cell lysates were subjected to Western blot analysis (middle) or were immunoprecipitated with the indicated antibody (2 μg/ml), followed by Western blot analysis (right). ***, p < 0.0005. C, promoter sequences of miR-181a. RBL2H3 cells were transiently transfected with the indicated siRNA (10 nm each) prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA (100 ng/ml) for 1 h, followed by ChIP assays employing the indicated antibody (2 μg/ml). Numbers in parentheses denote primer binding sites. D, RBL2H3 cells were transiently transfected with the indicated mimic (10 nm) prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA (100 ng/ml) for 1 h. Immunofluorescence staining employing the indicated antibody was performed as described. E, same as D except that β-hexosaminidase activity assays and qRT-PCR analysis were performed. *, p < 0.05; ***, p < 0.0005. IP, immunoprecipitation. Error bars, S.E.
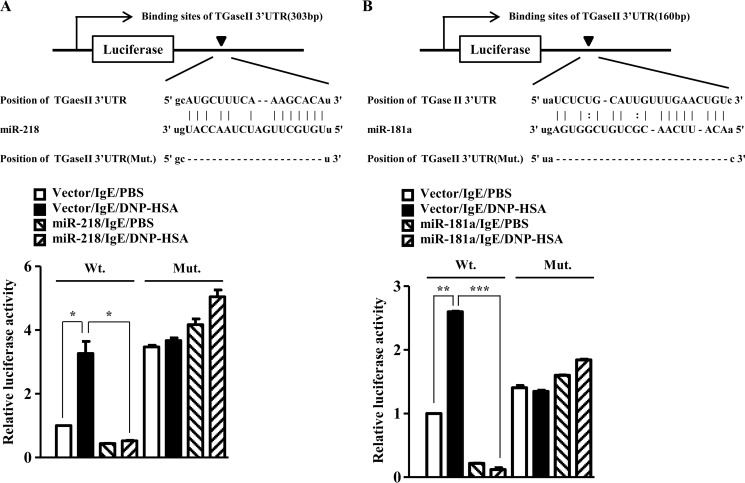
miR-218 and miR-181a Target TGaseII
Because mi-218 and miR-181a regulate the expression of TGaseII, we examined the possibility of the direct regulation of TGaseII by miR-218 and miR-181a. The 3′-UTR of TGaseII contains binding sites for miR-218 and miR-181a (Fig. 10, A and B). The overexpression of miR-218 decreases the luciferase activity associated with the wild type TGaseII 3′-UTR-Luciferase (Fig. 10A). However, miR-218 did not affect the luciferase activity associated with the mutant TGaseII 3′-UTR-Luciferase (Fig. 10A). The overexpression of miR-181a decreased the luciferase activity associated with the wild type TGaseII 3′-UTR-Luciferase (Fig. 10B). However, miR-181a did not affect the luciferase activity associated with the mutant TGaseII 3′-UTR-Luciferase (Fig. 10B). Taken together, these results suggest that miR-218 and miR-181a directly regulate the expression of TGaseII.
FIGURE 10.
miR-218 and miR-181a target TGaseII. A, potential binding of miR-218 to the 3′-UTR of TGaseII (top). RBL2H3 cells were transiently transfected with control vector (1 μg) or miR-218 construct (1 μg) along with wild type 3′-UTR-luciferase construct (Wt) or mutant 3′-UTR-luciferase construct (Mt), prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA (100 ng/ml) for 1 h. The luciferase activity assay was performed as described (bottom). *, p < 0.05. B, potential binding of miR-181a to the 3′-UTR of TGaseII (top). RBL2H3 cells were transiently transfected with control vector or miR-218 construct along with wild type 3′-UTR-luciferase construct or mutant 3′-UTR-luciferase construct prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA (100 ng/ml) for 1 h. The luciferase activity assay was performed as described (bottom). **, p < 0.005; ***, p < 0.0005. Error bars, S.E.
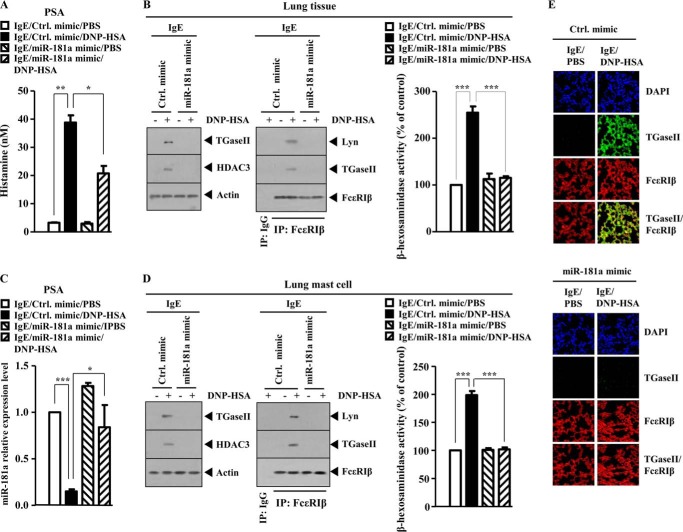
miR-181a Negatively Regulates PSA
We next examined the effect of miR-181a on in vivo allergic inflammation. miR-181a mimic exerted a negative effect on the increased secretion of histamine in a mouse model of PSA (Fig. 11A). The miR-181a mimic prevented antigen from increasing the expression of TGaseII and HDAC3 and prevented antigen from inducing an interaction between FcϵRIβ and TGaseII and an interaction between FcϵRIβ and Lyn in mouse model of PSA (Fig. 11B). miR-181a mimic exerts a negative effect on the increased β-hexosaminidase activity and restores the expression of miR-181a in mouse model of PSA (Fig. 11C). Western blot analysis of lung mast cells isolated from lung tissue shows that miR-181a mimic prevents antigen from increasing the expression of TGaseII, and HDAC3 prevents antigen from inducing an interaction between FcϵRIβ and TGaseII and an interaction between FcϵRIβ and Lyn (Fig. 11D). The miR-181a mimic exerted a negative effect on the increased β-hexosaminidase activity in lung mast cells isolated from lung tissue in a mouse model of PSA (Fig. 11D). Immunofluorescence staining of lung tissue shows that the miR-181a mimic inhibits a co-localization of TGaseII with FcϵRIβ in a mouse model of PSA (Fig. 11E). Taken together, these results suggest that miR-181a negatively regulates in vivo allergic inflammation by regulating the expression of TGaseII and FcϵRI signaling.
FIGURE 11.
miR-181a negatively regulates PSA. A, BALB/c mice were sensitized to DNP-specific IgE (0.5 μg/kg) by an intravenous injection. The next day, BALB/c mice were also given an intravenous injection of control mimic (100 nm) or miR-181a mimic (100 nm) along with DNP-HSA (250 μg/kg). Twenty-four hours after stimulation with DNP-HSA, a histamine release assay employing the sera of BALB/c mice was performed. *, p < 0.05; **, p < 0.005. B, same as A except that Western blot, immunoprecipitation, and β-hexosaminidase activity assays were performed by employing lung tissue obtained after PSA. ***, p < 0.0005. Lung tissue lysates were isolated from each mouse of each experimental group of mice. C, same as A except that the expression level of miR-181a was determined by quantitative real-time PCR employing lung tissue obtained after PSA. *, p < 0.05; ***, p < 0.005. D, same as B except that mast cells isolated from lung tissue were employed. ***, p < 0.005. E, same as A except that immunofluorescence staining employing cryosection of lung tissue obtained after PSA was performed. Error bars, S.E.
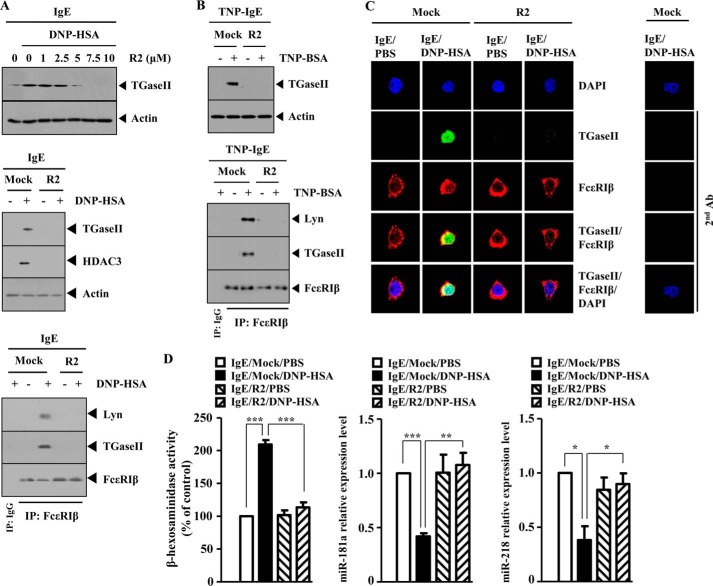
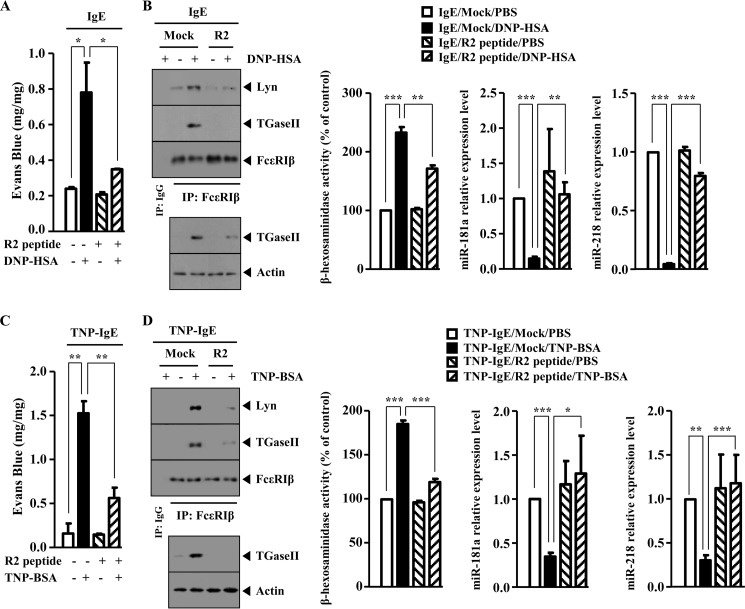
R2 Peptide, an Inhibitor of TGaseII, Negatively Regulates in Vitro Allergic Inflammation
To further confirm the role of TGaseII in allergic inflammation, the effect of the R2 peptide, an inhibitor of TGaseII, on the allergic inflammation was examined. R2 peptide decreased the expression of TGaseII in a dose-dependent manner and prevented antigen from increasing the expression of TGaseII and HDAC3 in antigen (DNP-HSA)-stimulated RBL2H3 cells (Fig. 12A). R2 peptide prevented antigen from inducing an interaction between FcϵRIβ and TGaseII and an interaction between FcϵRIβ and Lyn in RBL2H3 cells (Fig. 12A). R2 peptide prevented antigen from increasing the expression of TGaseII and prevented antigen from inducing an interaction between FcϵRIβ and TGaseII and an interaction between FcϵRIβ and Lyn in antigen (TNP-BSA)-stimulated RBL2H3 cells (Fig. 12B). R2 peptide prevented a co-localization of FcϵRIβ with TGaseII in antigen-stimulated RBL2H3 cells (Fig. 12C). R2 peptide exerted a negative effect on the increased β-hexosaminidase activity and restored the expression of miR-218 and miR-181a in antigen-stimulated RBL2H3 cells (Fig. 12D). Taken together, these results further suggest the involvement of TGaseII in allergic inflammation.
FIGURE 12.
R2 peptide, an inhibitor of TGaseII, negatively regulates in vitro allergic inflammation. A, the IgE-sensitized RBL2H3 cells were pretreated with various concentrations of R2 peptide for 1 h, followed by stimulation with DNP-HSA (100 ng/ml) for 1 h. Cell lysates isolated were subjected to Western blot analysis (top). The IgE-sensitized RBL2H3 cells were pretreated with R2 peptide (5 μm), followed by stimulation with DNP-HSA (100 ng/ml) for 1 h. Cell lysates were subjected to Western blot analysis (middle) or immunoprecipitated (IP) with the indicated antibody (2 μg/ml) followed by Western blot analysis (bottom). B, the IgE-sensitized RBL2H3 cells were pretreated with R2 peptide (5 μm), followed by stimulation with TNP-BSA (100 ng/ml) for 1 h. Cell lysates were then subjected to Western blot analysis (top). Cell lysates were also immunoprecipitated with the indicated antibody (2 μg/ml), followed by Western blot analysis (bottom). C, the IgE-sensitized RBL2H3 cells were pretreated with R2 peptide (5 μm) for 1 h, followed by stimulation with DNP-HSA (100 ng/ml) for 1 h. Immunofluorescence staining employing the indicated antibodies (2 μg/ml) was performed. D, same as C except that a β-hexosaminidase activity assay and qRT-PCR analysis to determine the expression of miR-218 and miR-181a were performed. *, p < 0.05; **, p < 0.005; ***, p < 0.0005. Error bars, S.E.
R2 Peptide Negatively Regulates PCA
Because R2 peptide negatively regulated in vitro allergic inflammation, the effect of R2 peptide on the in vivo allergic inflammation was examined. R2 peptide exerts a native effect on the increased ear thickness by PCA (data not shown). R2 peptide exerted a negative effect on the increased vascular permeability in a mouse model of PCA (Fig. 13A). R2 peptide prevented antigen from increasing the expression of TGaseII and HDAC3 and prevented antigen from inducing an interaction between FcϵRIβ and TGaseII and an interaction between FcϵRIβ and Lyn in a mouse model of PCA (Fig. 13B). R2 peptide exerted a negative effect on the increased β-hexosaminidase activity and prevented antigen from decreasing the expression of miR-218 and miR-181a in the mouse model of PCA (Fig. 13B). The mouse model of PCA employing TNP-specific IgE was also employed. R2 peptide exerted a negative effect on the increased vascular permeability in a mouse model of PCA (Fig. 13C). R2 peptide prevented antigen (TNP-BSA) from increasing the expression of TGaseII and prevented antigen from inducing an interaction between FcϵRIβ and TGaseII and an interaction between FcϵRIβ and Lyn (Fig. 13D). R2 peptide exerted a negative effect on the increased β-hexosaminidase activity and prevented antigen from decreasing the expression of miR-218 and miR-181a in a mouse model of PCA (Fig. 13D). Taken together, these results show the role of TGaseII in PCA.
FIGURE 13.
R2 peptide negatively regulates PCA. A, BALB/c mice were given an intradermal injection of DNP-specific IgE antibody (0.5 μg/kg) or DNP-specific IgG (0.5 μg/kg). The next day, BALB/c mice were given an intravenous injection of PBS or DNP-HSA (250 μg/kg) and R2 peptide (9 mg/kg) along with 2% (v/v) Evans blue solution. One hour after the injection, the extent of vascular permeability was determined as described. Means ± S.E. of three independent experiments are depicted. *, p < 0.05. Each experimental group consisted of four mice. B, ear tissue lysates were isolated from each mouse of each experimental group of mice and were subjected to Western blot analysis, immunoprecipitation, and β-hexosaminidase activity assays. Ear tissue lysates were also subjected to qRT-PCR analysis to determine the expression of miR-181a and miR-218. **, p < 0.005; ***, p < 0.0005. C, BALB/c mice were given an intradermal injection of TNP-specific IgE (0.5 μg/kg) or DNP-specific IgG (0.5 μg/kg). The next day, BALB/c mice were given an intravenous injection of PBS or TNP-BSA (250 μg/kg) along with R2 peptide (9 mg/kg). The extent of vascular permeability was determined as described. **, p < 0.005. Each experimental group consisted of four mice. D, ear tissue lysates were isolated from each mouse of each experimental group of mice and were subjected to Western blot analysis, immunoprecipitation (IP), and β-hexosaminidase activity assays. Ear tissue lysates were also subjected to qRT-PCR analysis to determine the expression of miR-181a and miR-218. *, p < 0.05; **, p < 0.005; ***, p < 0.0005. Error bars, S.E.
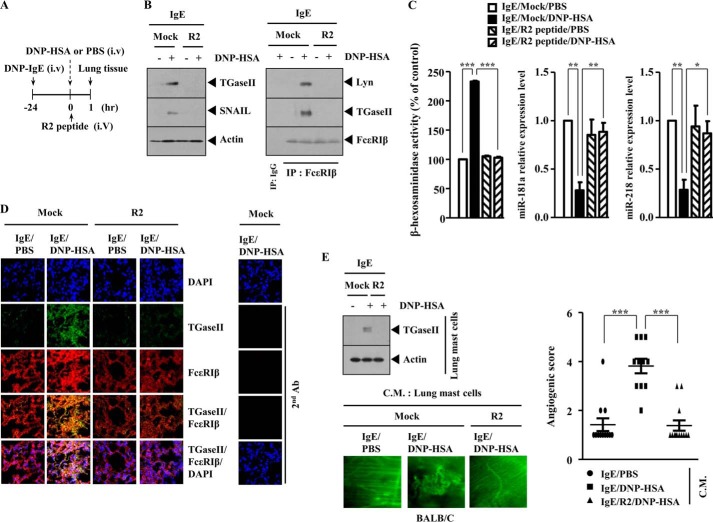
R2 Peptide Negatively Regulates PSA and Angiogenesis Accompanied by PSA
We next examined the effect of R2 peptide on PSA. R2 peptide was injected intravenously into the tail vein of a BALB/c mouse on the same day of antigen (DNP-HSA) injection (Fig. 14A). R2 peptide prevented antigen from increasing the expression of TGaseII and SNAIL and prevented antigen from inducing an interaction between FcϵRIβ and TGaseII and an interaction between FcϵRIβ and Lyn in a mouse model of PSA (Fig. 14B). The expression of SNAIL is known to be increased in a mouse model of PSA (25). R2 peptide exerted a negative effect on the increased β-hexosaminidase activity and restored the expression of miR-218 and miR-181a in a mouse model of PSA (Fig. 14C). Immunofluorescence staining of lung tissue shows that R2 peptide prevents a co-localization of TGaseII with FcϵRIβ (Fig. 14D). We reported that PSA was accompanied by an enhanced angiogenesis (24). Lung mast cells isolated after PSA show the increased expression of TGaseII by antigen stimulation (Fig. 14E). Intravital microscopy employing the conditioned medium of lung mast cells shows that the R2 peptide exerts a negative effect on the enhanced angiogenesis by PSA (Fig. 14E). Taken together, these results suggest that TGaseII is necessary for PSA and angiogenesis accompanied by PSA.
FIGURE 14.
R2 peptide negatively regulates PSA and angiogenesis associated with PSA. A, BALB/c mice were sensitized to DNP-specific IgE (0.5 μg/kg) by an intravenous injection (i.v.) into the tail vein. The next day, BALB/c mice were given an intravenous injection of DNP-HSA (250 μg/kg) along with R2 peptide (9 mg/kg). One hour after stimulation with DNP-HSA (250 μg/kg), lung tissues were isolated. B, lung tissue lysates were isolated from each mouse of each experimental group of mice and were subjected to Western blot analysis (left) or immunoprecipitated with the indicated antibody (2 μg/ml), followed by Western blot analysis (right). C, lung tissue lysates were subjected to β-hexosaminidase activity assays. Lung tissue lysates isolated were also subjected to qRT-PCR analysis to determine the expression level of miR-218 or miR-181a. *, p < 0.05; **, p < 0.005; ***, p < 0.0005. D, immunofluorescence staining employing cryosection of lung tissue was performed as described. E, the conditioned medium of lung mast cells obtained after PSA was mixed with Matrigel. Intravital microscopy was performed as described. ***, p < 0.0005. Western blot of lung mast cells obtained after PSA was also performed. Error bars, S.E.
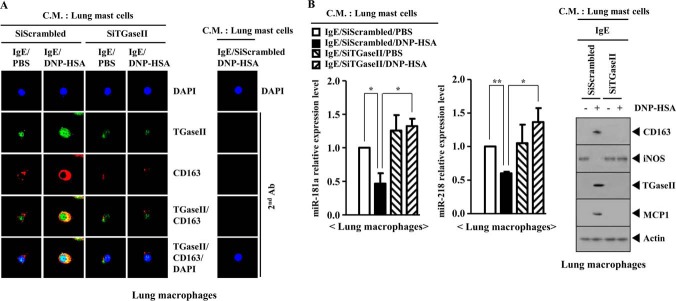
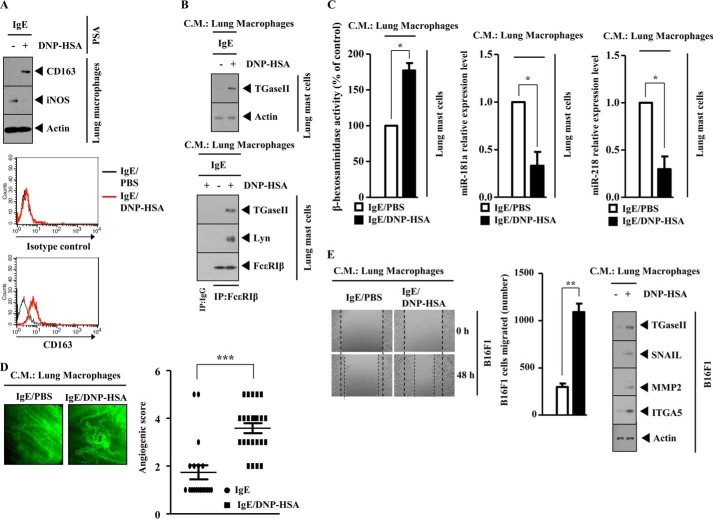
Mast Cells Activate Macrophage in a TGaseII-dependent Manner
PSA-promoted enhanced metastatic potential of mouse melanoma cells involves the activation of macrophages (25). House dust mite-driven allergic inflammation involves the activation of lung macrophages, and the activated lung macrophages show the increased expression of HIF-1α (35). Macrophages have been known to be associated with tumor metastasis (36). The expression of miR-181a is higher in M1 macrophages (tumor-suppressive macrophages) than in tumor-activating M2 macrophages (37). PSA promotes the metastatic potential of B16F1 melanoma cells in a TGaseII-dependent manner (Fig. 4B). We hypothesized that mast cells activated by PSA would activate macrophages. The conditioned medium of lung mast cells obtained after PSA, when added to lung macrophages, increased the expression of CD163, a marker of the activated macrophages, and induced a co-localization of TGaseII with CD163 (Fig. 15A). The conditioned medium of lung mast cells obtained after PSA, when added to lung macrophages, regulated the expression of miR-218, miR-181, CD163, inducible nitric-oxide synthase (a marker of the inhibitory macrophages), and MCP1 in a TGaseII-dependent manner (Fig. 15B). These results suggest that macrophages, activated by mast cells, mediate the allergic inflammation-promoted enhanced metastatic potential of tumor cells.
FIGURE 15.
Mast cells activate macrophages during allergic inflammation in a TGaseII-dependent manner. A, BALB/c mouse model of PSA was performed as described. Lung mast cell and lung macrophages were isolated as described. The conditioned medium of lung mast cells was prepared and added to lung macrophages in serum-free medium in a 1:1 ratio. Twenty-four hours after the addition of the conditioned medium, immunofluorescence staining employing the indicated antibody was performed. C.M., conditioned medium. B, same as A except that qRT-PCR and Western blot analysis were performed. *, p < 0.05; **, p < 0.005. Error bars, S.E.
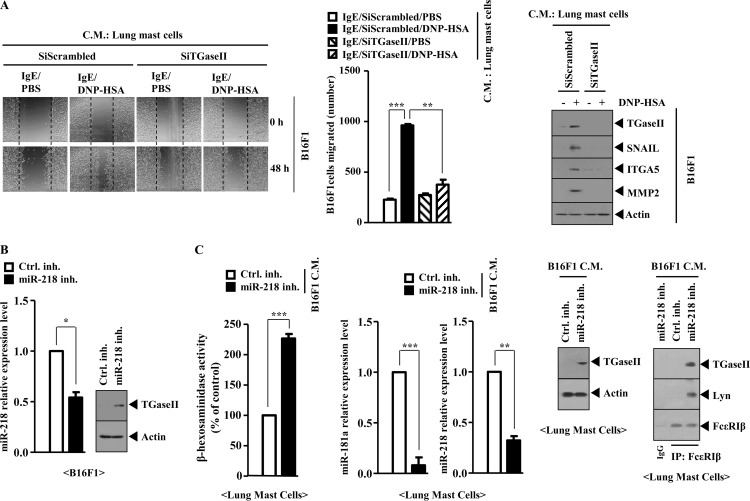
Mast Cells and Tumor Cells Form a Positive Feedback Loop
Because mast cells activated macrophages, we examined the effect of mast cells on the migration potential of tumor cells. The conditioned medium of lung mast cells isolated after PSA, when added to B16F1 melanoma cells, enhanced the migration potential of B16F1 melanoma cells and increased the expression of TGaseII, SNAIL, MMP-2 (matrix metalloproteinase-2), and integrin α5 in a TGaseII-dependent manner (Fig. 16A). Integrin α5 mediated the interaction between T cells and fibroblasts in airway inflammation (38). We examined whether mast cells and tumor cells would form a positive feedback loop. The inhibition of miR-218 increases the expression of TGaseII in B16F1 cells (Fig. 16B). The conditioned medium of B16F1 cells transfected with miR-218 inhibitor, when added to lung mast cells, increased β-hexosaminidase activity and the expression of TGaseII and induced an interaction between FcϵRI and TGaseII while decreasing the expression of miR-181a and miR-218 (Fig. 16C). Taken together, these results suggest that a positive feedback loop between mast cells and tumor cells is necessary for the allergic inflammation-promoted enhanced metastatic potential of tumor cells.
FIGURE 16.
Mast cells and tumor cells form a positive feedback loop. A, conditioned medium (C.M.) of lung mast cells after PSA was prepared and added to B16F1 melanoma cells in serum-free medium in a 1:1 ratio. Movement of cells into the wound was shown for the indicated cancer cell line at 0 and 48 h postscratch (magnification, ×40). Data were the means of three independent experiments. Error bars, S.D. Broken lines, boundary lines of scratch. The conditioned medium of lung mast cells was prepared and added to lung macrophages in serum-free medium in a 1:1 ratio. Twenty-four hours after the addition of the conditioned medium, cell lysates were prepared and subjected to Western blot analysis (right). **, p < 0.005; ***, p < 0.0005. B, B16F1 cells were transfected with the indicated inhibitor (50 nm each). At 48 h after transfection, qRT-PCR analysis and Western blot analysis were performed. C, conditioned medium of B16F1 cells obtained after transfection with the indicated inhibitor was added to lung mast cells. At 24 h after the addition of the conditioned medium, a β-hexosaminidase activity assay, qRT-PCR analysis, Western blot analysis, and immunoprecipitation were performed.
Macrophages, Activated by PSA, Activate Mast Cells, Display Angiogenic Potential, and Enhance the Migration Potential of B16F1 Melanoma Cells
Because mast cells activated macrophages in a TGaseII-dependent manner (Fig. 15, A and B), we examined whether mast cells and macrophages would form a positive feedback loop. PSA induced the expression of CD163 while decreasing the expression of inducible nitric-oxide synthase in lung macrophages (Fig. 17A). Flow cytometry also shows that PSA induces the surface expression of CD163 in lung macrophages (Fig. 17A). The conditioned medium of lung macrophages isolated after PSA, when added to lung mast cells, increased the expression of TGaseII and induced an interaction between FcϵRIβ and TGaseII and an interaction between FcϵRIβ and Lyn (Fig. 17B). The conditioned medium of lung macrophages isolated after PSA, when added to lung mast cells, increased β-hexosaminidase activity while decreasing the expression of miR-218 and miR-181a (Fig. 17C). The conditioned medium of lung macrophages isolated after PSA, when mixed with Matrigel, shows angiogenic potential (Fig. 17D). This suggests that macrophages, activated by PSA, are necessary for the enhanced angiogenic potential associated with PSA. The conditioned medium of lung macrophages isolated after PSA, when added to B16F1 melanoma cells, enhanced the migration potential of B16F1 melanoma cells and increased the expression of TGaseII, SNAIL, MMP-2, and integrin α5 (Fig. 17E). Taken together, these results indicate that macrophages form a positive feedback loop with mast cells and enhance the migration potential of B16F1 melanoma cells.
FIGURE 17.
Macrophages activate mast cells, are necessary for angiogenesis associated with PSA, and enhance the migration potential of B16F1 melanoma cells. A, BALB/c mouse model of PSA was performed as described. Lysates isolated from lung macrophages after PSA were subjected to Western blot analysis (top). Flow cytometry analysis employing anti-CD163 antibody was performed as described (bottom). B, the conditioned medium of lung macrophages isolated after PSA was prepared and added to lung mast cells in serum-free medium in a 1:1 ratio. Twenty-four hours after the addition of the conditioned medium, Western blot analysis (top) and immunoprecipitation (IP) (bottom) were performed. C, same as B except that that the β-hexosaminidase activity assay and qRT-PCR were performed. *, p < 0.05. D, the conditioned medium of lung macrophages obtained after PSA was mixed with Matrigel. Intravital microscopy was performed as described. ***, p < 0.0005. E, the conditioned medium of lung macrophages isolated after PSA was prepared and added to B16F1 melanoma cells in serum-free medium in a 1:1 ratio. Movement of cells into the wound was shown for the indicated cancer cell line at 0 and 48 h postscratch (magnification, ×40). Data are the means of three independent experiments. Error bars, S.D. Broken lines, boundary lines of scratch. **, p < 0.005. The conditioned medium of lung macrophages was prepared and added to lung macrophages in serum-free medium in a 1:1 ratio. At 24 h after the addition of the conditioned medium, cell lysates were prepared and subjected to Western blot analysis.
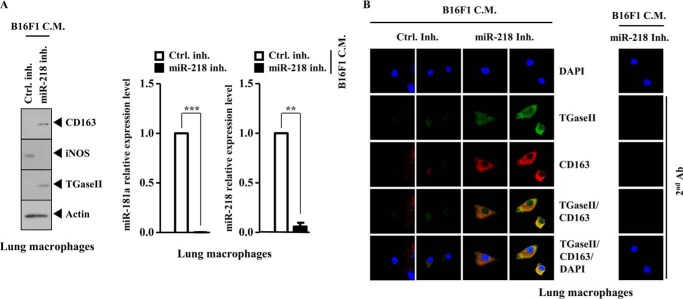
Macrophages Form a Positive Feedback Loop with Tumor Cells
Because macrophages enhanced the migration potential of B16F1 melanoma cells (Fig. 17E), the possibility of a positive feedback loop between macrophages and tumor cells was examined. The conditioned medium of B16F1 cells transfected with miR-218 inhibitor, when added to lung macrophages, increased the expression of CD163 and TGaseII while decreasing the expression of inducible nitric-oxide synthase, miR-218, and miR-181a (Fig. 18A). The conditioned medium of B16F1 melanoma cells transfected with miR-218 inhibitor, when added to lung macrophages, increased the expression of CD163 and TGaseII and induced a co-localization of TGaseII with CD163 (Fig. 18B). Taken together, these results suggest that macrophages and tumor cells form a positive feedback loop. This positive feedback loop may be necessary for the allergic inflammation-promoted enhanced metastatic potential of tumor cells.
FIGURE 18.
Macrophages form a positive feedback loop with tumor cells. A, the conditioned medium (C.M.) of B16F1 cells obtained after miR-218 inhibitor transfection was added to lung macrophages in serum-free medium in a 1:1 ratio. At 24 h after the addition of the conditioned medium, cell lysates were subjected to Western blot analysis (left). qRT-PCR analysis to determine the expression of miR-218 and miR-181a was also performed (right). B, at 24 h after the addition of the conditioned medium, immunofluorescence staining was performed. Error bars, S.E.
DISCUSSION
In this study, we investigated molecular mechanisms of TGaseII-mediated allergic inflammation. We first investigated the mechanism of the expression regulation of TGaseII. The TGaseII promoter contains putative binding sites for SP1, HDAC2, and NF-κB.4 Antigen stimulation increases the expression of SP1 in RBL2H3 cells.4 ChIP assays show the binding of SP1 to the promoter sequences of TGaseII.4 This shows the direct regulation of TGaseII by SP1. SP1, through interaction with GATA-2, increases the expression of c-Kit, which is essential for mast cell development (39). The down-regulation of SP1 prevents antigen from increasing β-hexosaminidase activity in RBL2H3 cells.4 HDAC3, through interaction with SP1, mediates allergic skin inflammation by regulating the expression of MCP1 (24). It is probable that HDAC3, through interaction with SP1, increases the expression of TGaseII in allergic inflammation. It is reasonable that SP1 may regulate in vivo allergic inflammation. It will be interesting to examine the effect of SP1 on the expression of miR-218 and miR-181a. For better understanding of the role of SP1 in allergic inflammation, it will be necessary to identify miRNAs that form a feedback loop with SP1.
Unlike HDAC3, the expression of HDAC2 is decreased in antigen-stimulated RBL2H3 cells (24). The TGaseII promoter contains a binding site for HDAC2. It is probable that HDAC2 binds to the promoter sequences of TGaseII to repress the expression of TGaseII. It is probable that HDAC2 acts as a positive regulator of miR-218 and miR-181a. It will be interesting to examine the binding of HDAC2 to the promoter sequences of miR-218 and miR-181a. The role of HDAC2 in allergic inflammation has not been reported.
Integrin α5 interacts with EGFR and is necessary for the activation of FcϵRI signaling (31). Integrin α4 interacts with FcϵRIβ in antigen-stimulated RBL2H3 cells (31). EGFR signaling is activated by asthma-related cytokines and inflammation (40). House dust mite allergen (Der p) increases the secretion of thymus and activation-regulated chemokine in an EGFR-dependent manner, resulting in allergic inflammation (41). It is probable that cross-talk between EGFR and FcϵRI is necessary for allergic inflammation. Reportedly, TGaseII interacts with integrin α5 (42). This led us to hypothesize that TGaseII might be involved in the activation of FcϵRIβ signaling. In this study, we show an interaction between FcϵRIβ and TGaseII (Fig. 1D). Mouse recombinant TGaseII protein increases the phosphorylation of EGFR and induces an interaction between EGFR and FcϵRIβ (Fig. 5F). This suggests that TGaseII is responsible for the interaction between EGFR and FcϵRI accompanied by allergic inflammation. It would be necessary to examine the effect of miR-218 and miR-181a on the cross-talk between EGFR and FcϵRI in allergic inflammation.
Tissue transglutaminase promotes RhoA activation via integrin clustering (43). A plasma transglutaminase promotes angiogenesis by binding to the αvβ3 integrin on the endothelial cell surface, followed by cross-linking the αvβ3 integrin with VEGFR-2, thus leading to VEGF-independent activation of VEGFR-2 (44). CXCR2 is critical for both endothelial progenitor cell recruitment and the angiogenic response in this model of allergic inflammation of the airways (45). Human mast cells are both a source and a target of angiogenic factors and therefore might play a role in inflammatory and neoplastic angiogenesis through the expression of several forms of VEGFs and their receptors (46). These reports suggest that in vivo allergic inflammation involves angiogenesis. We show that recombinant TGaseII protein enhances vascular permeability (Fig. 5G) and angiogenesis (Fig. 5H) in the BALB/c mouse. We also show that TGaseII is necessary for the enhanced angiogenic potential accompanied by PSA (Fig. 14E). It would be interesting to examine the effect of miR-218 and miR-181a on the angiogenesis accompanied by allergic inflammation. It is probable that TGaseII, miR-218, and miR-181a negatively regulate VEGF receptor signaling in mouse endothelial cells. It would be necessary to identify angiogenic factors and miRNAs that are regulated by TGaseII, miR-218, and miR-181a.
miRNAs have the potential to repress transcription factors, which repress the same miRNAs. Therefore, miRNAs are suited to take part in a feedback loop (25). TargetScan analysis predicts that TGaseII, YY1, SP1, integrin α1, Integrin α3, and integrin α4 serve as potential targets of mR-218.4 In this study, we found increased expression of SP1, integrin α1, integrin α3, and integrin α4 in antigen-stimulated RBL2H3 cells.4 TargetScan analysis predicts that TGaseII, YY1, SP1, integrin α1, integrin α2, integrin α3, integrin α4, SMAD7, and guanine nucleotide exchange factor serve as targets of mR-181a. The inhibition of TGFβ signaling by SMAD7 exerts a negative effect on allergic asthma (47). The increased level of TGFβ1 is observed in chronic allergic asthma induced by house dust mites (48). Asthma, induced by ovalbumin, involves the increased expression of TGFβ1 (49). TGaseII regulates the expression of TGFβ1 (50). Human mast cells show the increased expression of TGFβ1 in response to histamine (51). These reports suggest a role of TGFβ signaling in allergic inflammation. Overexpression of miR-181a decreases the expression of activin receptor IIA, a receptor of the TGFβ superfamily, cyclin D2, and proliferating cell nuclear antigen (52). This implies that miR-181a negatively regulates TGFβ signaling. It would be necessary to examine the effect of miR-218 and miR-181a on TGFβ receptor signaling in relation to the animal model of PCA and PSA employed in this study.
miRNA array analysis shows that miR-214 is decreased in RBL2H3 cells by antigen stimulation (Fig. 7A). In vivo down-regulation of miR-214 enhances the formation of a perfused vascular network in implanted Matrigel plugs and retinal developmental angiogenesis in mice (53). This implies that allergic inflammation is closely associated with angiogenesis. TargetScan analysis predicts that miR-214 regulates the expression of TGaseII and SP1. It will be necessary to examine whether miR-214 would form a negative feedback loop with TGaseII. It will also be necessary to examine the role of miR-214 in PCA and PSA. miR-200a, decreased by antigen stimulation in RBL2H3 cells, represses the expression of TGFβ1 and TGFβ2 and regulates TGFβ-dependent epithelial-mesenchymal transition and fibrosis (54). Because TGFβ signaling is associated with allergic inflammation, it is probable that miR-200a acts as a negative regulator of allergic inflammation. The expression level of miR-106a is increased by antigen stimulation in RBL2H3 cells (Fig. 7A). miRNA array analysis shows that the down-regulation of TGaseII by siRNA decreases the expression of miR-106a in antigen-stimulated RBL2H3 cells.4 The knockdown of miR-106a alleviates most of the features of asthma, such as airway hyperresponsiveness, airway inflammation, increased Th2 response, goblet cell metaplasia, and subepithelial fibrosis along with an increase in IL-10 levels in lungs (55). This implies the role of miR-106a in allergic inflammation. ChIP assay shows the binding of TGaseII to the promoter sequences of miR-106a in antigen-stimulated RBL2H3 cells.4 It is probable that miR-106a and TGaseII form a positive feedback loop to mediate allergic inflammation. It will be necessary to examine the effect of the miR-106 inhibitor on the expression of TGaseII. The miR-106a promoter contains the putative binding sites for HDAC2, YY1, NF-κB, and AP1.4 HDAC2 shows the binding to the promoter sequences of miR-106a in the absence of antigen stimulation in RBL2H3 cells.4 This suggests that HDAC2 may act as a negative regulator of miR-106a. The down-regulation of TGaseII induces the binding of HDAC2 to the promoter sequences of miR-106a in antigen-stimulated RBL2H3 cells.4 These results suggest that the TGaseII/HDAC2/miR-106a feedback loop regulates allergic inflammation.
In this study, we show that PSA induces a positive feedback between mast cells and macrophages in a TGaseII-dependent manner (Figs. 15, A and B). It is probable that miR-218 and miR-181a are necessary for cellular interaction accompanied by allergic inflammation. It would be necessary to identify more molecules regulated by miR-218 and/or miR-181a for a better understanding of cellular interaction during allergic inflammation.
In this study, we show the regulatory role of TGaseII/miR-218/-181a feedback loop in allergic inflammation. The TGaseII/miRNA feedback loop will be valuable for the development of anti-allergy therapeutics. The expression regulation of TGaseII by peptide or miRNAs, such as miR-218 and miR-181a, may offer a strategy for the development of anti-allergic drugs.
This work was supported by National Research Foundation Grants 2012H1B8A2025495, 2014R1A2A2A01002448, and 2012R1A1A3009993; a grant from the BK21 Plus program; National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea, Grant 1320160; and Kangwon National University Grant 120140305.
S. Eom, Y. Kim, M. Kim, D. Park, H. Lee, Y. S. Lee, J. Choe, Y. M. Kim, and D. Jeoung, unpublished observations.
- TGaseII
- transglutaminase II
- BMMC
- bone marrow-derived mouse mast cell
- DNFB
- 2,4-dinitrofluorobenzene
- DNP-HSA
- dinitrophenly-human serum albumin
- EGFR
- epidermal growth factor receptor
- miR
- microRNA
- PCA
- passive cutaneous anaphylaxis
- PSA
- passive systemic anaphylaxis
- qRT-PCR
- quantitative RT-PCR
- RBL2H3
- rat basophilic leukemia cells
- TNP
- trinitrophenyl
- TpCR
- triphasic cutaneous reaction
- TGaseII
- transglutaminase II
- CTX
- cetuximab.
REFERENCES
- 1. Lorand L., Weissmann L. B., Epel D. L., Bruner-Lorand J. (1976) Role of the intrinsic transglutaminase in the Ca2+-mediated crosslinking of erythrocyte proteins. Proc. Natl. Acad. Sci. U.S.A. 73, 4479–4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fesus L., Szucs E. F., Barrett K. E., Metcalfe D. D., Folk J. E. (1985) Activation of transglutaminase and production of protein-bound γ-glutamylhistamine in stimulated mouse mast cells. J. Biol. Chem. 260, 13771–13778 [PubMed] [Google Scholar]
- 3. Palosuo K., Varjonen E., Nurkkala J., Kalkkinen N., Harvima R., Reunala T., Alenius H. (2003) Transglutaminase-mediated cross-linking of a peptic fraction of ω-5 gliadin enhances IgE reactivity in wheat-dependent, exercise-induced anaphylaxis. J. Allergy Clin. Immunol. 111, 1386–1392 [DOI] [PubMed] [Google Scholar]
- 4. Lai T. S., Greenberg C. S. (2013) Histaminylation of fibrinogen by tissue transglutaminase-2 (TGM-2): potential role in modulating inflammation. Amino Acids 45, 857–864 [DOI] [PubMed] [Google Scholar]
- 5. Molberg O., Mcadam S. N., Körner R., Quarsten H., Kristiansen C., Madsen L., Fugger L., Scott H., Norén O., Roepstorff P., Lundin K. E., Sjöström H., Sollid L. M. (1998) Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 4, 713–717 [DOI] [PubMed] [Google Scholar]
- 6. Hallstrand T. S., Wurfel M. M., Lai Y., Ni Z., Gelb M. H., Altemeier W. A., Beyer R. P., Aitken M. L., Henderson W. R. (2010) Transglutaminase 2, a novel regulator of eicosanoid production in asthma revealed by genome-wide expression profiling of distinct asthma phenotypes. PLoS One 5, e8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henderson W. R., Jr., Chi E. Y., Bollinger J. G., Tien Y. T., Ye X., Castelli L., Rubtsov Y. P., Singer A. G., Chiang G. K., Nevalainen T., Rudensky A. Y., Gelb M. H. (2007) Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J. Exp. Med. 204, 865–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park M. K., Cho S. A., Lee H. J., Lee E. J., Kang J. H., Kim Y. L., Kim H. J., Oh S. H., Choi C., Lee H., Kim S. Y. (2012) Suppression of transglutaminase-2 is involved in anti-inflammatory actions of glucosamine in 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation. Biomol. Ther. (Seoul) 20, 380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oh K., Seo M. W., Lee G. Y., Byoun O. J., Kang H. R., Cho S. H., Lee D. S. (2013) Airway epithelial cells initiate the allergen response through transglutaminase 2 by inducing IL-33 expression and a subsequent Th2 response. Respir. Res. 14, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abdala-Valencia H., Berdnikovs S., McCary C. A., Urick D., Mahadevia R., Marchese M. E., Swartz K., Wright L., Mutlu G. M., Cook-Mills J. M. (2012) Inhibition of allergic inflammation by supplementation with 5-hydroxytryptophan. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L642–L660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim Y., Eom S., Kim K., Lee Y. S., Choe J., Hahn J. H., Lee H., Kim Y. M., Ha K. S., Ro J. Y., Jeoung D. (2010) Transglutaminase II interacts with rac1, regulates production of reactive oxygen species, expression of snail, secretion of Th2 cytokines and mediates in vitro and in vivo allergic inflammation. Mol. Immunol. 47, 1010–1022 [DOI] [PubMed] [Google Scholar]
- 12. Hur G. Y., Kim S. H., Park S. M., Ye Y. M., Kim C. W., Jang A. S., Park C. S., Hong C. S., Park H. S. (2009) Tissue transglutaminase can be involved in airway inflammation of toluene diisocyanate-induced occupational asthma. J. Clin. Immunol. 29, 786–794 [DOI] [PubMed] [Google Scholar]
- 13. Oh K., Park H. B., Byoun O. J., Shin D. M., Jeong E. M., Kim Y. W., Kim Y. S., Melino G., Kim I. G., Lee D. S. (2011) Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J. Exp. Med. 208, 1707–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hong G. U., Park B. S., Park J. W., Kim S. Y., Ro J. Y. (2013) IgE production in CD40/CD40L cross-talk of B and mast cells and mediator release via TGase 2 in mouse allergic asthma. Cell. Signal. 25, 1514–1525 [DOI] [PubMed] [Google Scholar]
- 15. Kim D. Y., Park B. S., Hong G. U., Lee B. J., Park J. W., Kim S. Y., Ro J. Y. (2011) Anti-inflammatory effects of the R2 peptide, an inhibitor of transglutaminase 2, in a mouse model of allergic asthma, induced by ovalbumin. Br. J. Pharmacol. 162, 210–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suh G. Y., Ham H. S., Lee S. H., Choi J. C., Koh W. J., Kim S. Y., Lee J., Han J., Kim H. P., Choi A. M., Kwon O. J. (2006) A peptide with anti-transglutaminase activity decreases lipopolysaccharide-induced lung inflammation in mice. Exp. Lung Res. 32, 43–53 [DOI] [PubMed] [Google Scholar]
- 17. Sohn J., Kim T. I., Yoon Y. H., Kim J. Y., Kim S. Y. (2003) Novel transglutaminase inhibitors reverse the inflammation of allergic conjunctivitis. J. Clin. Invest. 111, 121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamada Y., Kosaka K., Miyazawa T., Kurata-Miura K., Yoshida T. (2014) miR-142-3p enhances FcϵRI-mediated degranulation in mast cells. Biochem. Biophys. Res. Commun. 443, 980–986 [DOI] [PubMed] [Google Scholar]
- 19. Shaoqing Y., Ruxin Z., Guojun L., Zhiqiang Y., Hua H., Shudong Y., Jie Z. (2011) Microarray analysis of differentially expressed microRNAs in allergic rhinitis. Am. J. Rhinol. Allergy 25, e242–e246 [DOI] [PubMed] [Google Scholar]
- 20. Malmhäll C., Alawieh S., Lu Y., Sjöstrand M., Bossios A., Eldh M., Rådinger M. (2014) MicroRNA-155 is essential for TH2-mediated allergen-induced eosinophilic inflammation in the lung. J. Allergy Clin. Immunol. 133, 1429–1438, 1438.e1–1438.e7 [DOI] [PubMed] [Google Scholar]
- 21. Collison A., Mattes J., Plank M., Foster P. S. (2011) Inhibition of house dust mite-induced allergic airways disease by antagonism of microRNA-145 is comparable to glucocorticoid treatment. J. Allergy Clin. Immunol. 128, 160–167.e4 [DOI] [PubMed] [Google Scholar]
- 22. Lu T. X., Munitz A., Rothenberg M. E. (2009) MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J. Immunol. 182, 4994–5002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mattes J., Collison A., Plank M., Phipps S., Foster P. S. (2009) Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc. Natl. Acad. Sci. U.S.A. 106, 18704–18709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim Y., Kim K., Park D., Lee E., Lee H., Lee Y. S., Choe J., Jeoung D. (2012) Histone deacetylase 3 mediates allergic skin inflammation by regulating expression of MCP1 protein. J. Biol. Chem. 287, 25844–25859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eom S., Kim Y., Park D., Lee H., Lee Y. S., Choe J., Kim Y. M., Jeoung D. (2014) Histone deacetylase-3 mediates positive feedback relationship between anaphylaxis and tumor metastasis. J. Biol. Chem. 289, 12126–12144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rabenhorst A., Schlaak M., Heukamp L. C., Förster A., Theurich S., von Bergwelt-Baildon M., Büttner R., Kurschat P., Mauch C., Roers A., Hartmann K. (2012) Mast cells play a protumorigenic role in primary cutaneous lymphoma. Blood 120, 2042–2054 [DOI] [PubMed] [Google Scholar]
- 27. Olivera A., Dillahunt S. E., Rivera J. (2013) Interrogation of sphingosine-1-phosphate receptor 2 function in vivo reveals a prominent role in the recovery from IgE- and IgG-mediated anaphylaxis with minimal effect on its onset. Immunol. Lett. 150, 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song Y., Qu C., Srivastava K., Yang N., Busse P., Zhao W., Li X. M. (2010) Food allergy herbal formula 2 protection against peanut anaphylactic reaction is via inhibition of mast cells and basophils. J. Allergy Clin. Immunol. 126, 1208–1217.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akimov S. S., Belkin A. M. (2001) Cell surface tissue transglutaminase is involved in adhesion and migration of monocytic cells on fibronectin. Blood 98, 1567–1576 [DOI] [PubMed] [Google Scholar]
- 30. Wang Z., Griffin M. (2012) TG2, a novel extracellular protein with multiple functions. Amino Acids 42, 939–949 [DOI] [PubMed] [Google Scholar]
- 31. Kim Y., Kim K., Park D., Eom S., Park H., Lee H., Lee Y. S., Choe J., Hahn J. H., Kim Y. M., Ro J. Y., Jeoung D. (2011) Integrin α5 interacts with EGFR, is necessary for FcϵRI signaling and is necessary for allergic inflammation in relation with angiogenesis. Mol. Immunol. 48, 1035–1045 [DOI] [PubMed] [Google Scholar]
- 32. Small E. M., Sutherland L. B., Rajagopalan K. N., Wang S., Olson E. N. (2010) MicroRNA-218 regulates vascular patterning by modulation of Slit-Robo signaling. Circ. Res. 107, 1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xie W., Li M., Xu N., Lv Q., Huang N., He J., Zhang Y. (2013) MiR-181a regulates inflammation responses in monocytes and macrophages. PLoS One 8, e58639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kazenwadel J., Michael M. Z., Harvey N. L. (2010) Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood 116, 2395–2401 [DOI] [PubMed] [Google Scholar]
- 35. Byrne A. J., Jones C. P., Gowers K., Rankin S. M., Lloyd C. M. (2013) Lung macrophagescontribute to house dust mite driven airway remodeling via HIF-1α. PLoS One 8, e69246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Na Y. R., Yoon Y. N., Son D. I., Seok S. H. (2013) Cyclooxygenase-2 inhibition blocks M2 macrophage differentiation and suppresses metastasisin murine breast cancer model. PLoS One 8, e63451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y., Zhang M., Zhong M., Suo Q., Lv K. (2013) Expression profiles of miRNAs in polarized macrophages. Int. J. Mol. Med. 31, 797–802 [DOI] [PubMed] [Google Scholar]
- 38. Loubaki L., Semlali A., Boisvert M., Jacques E., Plante S., Aoudjit F., Mourad W., Chakir J. (2010) Crosstalk between T cells and bronchial fibroblasts obtained from asthmatic subjects involves CD40L/α5β1 interaction. Mol. Immunol. 47, 2112–2118 [DOI] [PubMed] [Google Scholar]
- 39. Maeda K., Nishiyama C., Ogawa H., Okumura K. (2010) GATA2 and Sp1 positively regulate the c-kit promoter in mast cells. J. Immunol. 185, 4252–4260 [DOI] [PubMed] [Google Scholar]
- 40. Le Cras T. D., Acciani T. H., Mushaben E. M., Kramer E. L., Pastura P. A., Hardie W. D., Korfhagen T. R., Sivaprasad U., Ericksen M., Gibson A. M., Holtzman M. J., Whitsett J. A., Hershey G. K. (2011) Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am. J. Physiol. Lung Cell Mol. Physiol. 300, L414–L421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heijink I. H., Marcel Kies P., van Oosterhout A. J., Postma D. S., Kauffman H. F., Vellenga E. (2007) Der p, IL-4, and TGF-β cooperatively induce EGFR-dependent TARC expression in airway epithelium. Am. J. Respir. Cell Mol. Biol. 36, 351–359 [DOI] [PubMed] [Google Scholar]
- 42. Kang S. K., Yi K. S., Kwon N. S., Park K. H., Kim U. H., Baek K. J., Im M. J. (2004) α1B-adrenoceptor signaling and cell motility: GTPase function of Gh/transglutaminase 2 inhibits cell migration through interaction with cytoplasmic tail of integrin α subunits. J. Biol. Chem. 279, 36593–36600 [DOI] [PubMed] [Google Scholar]
- 43. Janiak A., Zemskov E. A., Belkin A. M. (2006) Cell surface transglutaminase promotes RhoA activation via integrin clustering and suppression of the Src-p190RhoGAP signaling pathway. Mol. Biol. Cell 17, 1606–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dardik R., Loscalzo J., Eskaraev R., Inbal A. (2005) Molecular mechanisms underlying the proangiogenic effect of factor XIII. Arterioscler. Thromb. Vasc. Biol. 25, 526–532 [DOI] [PubMed] [Google Scholar]
- 45. Jones C. P., Pitchford S. C., Lloyd C. M., Rankin S. M. (2009) CXCR2 mediates the recruitment of endothelial progenitor cells during allergic airways remodeling. Stem Cells 27, 3074–3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Detoraki A., Staiano R. I., Granata F., Giannattasio G., Prevete N., de Paulis A., Ribatti D., Genovese A., Triggiani M., Marone G. (2009) Vascularendothelialgrowth factors synthesized by human lung mast cells exert angiogenic effects. J. Allergy Clin. Immunol. 123, 1142–1149, 1149.e1–1149.e5 [DOI] [PubMed] [Google Scholar]
- 47. Luo X., Ding Q., Wang M., Li Z., Mao K., Sun B., Pan Y., Wang Z., Zang Y. Q., Chen Y. (2010) In vivo disruption of TGF-β signaling by Smad7 in airway epithelium alleviates allergicasthma but aggravates lung carcinogenesis in mouse. PLoS One 5, e10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen Z. G., Zhang T. T., Li H. T., Chen F. H., Zou X. L., Ji J. Z., Chen H. (2013) Neutralization of TSLP inhibits airway remodeling in a murine model of allergic asthma induced by chronic exposure to house dust mite. PLoS One 8, e51268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stumm C. L., Halcsik E., Landgraf R. G., Camara N. O., Sogayar M. C., Jancar S. (2014) Lung remodeling in a mouse model of asthma involves a balance between TGF-β1 and BMP-7. PLoS One 9, e95959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Telci D., Collighan R. J., Basaga H., Griffin M. (2009) Increased TG2 expression can result in induction of transforming growth factor β1, causing increased synthesis and deposition of matrix proteins, which can be regulated by nitric oxide. J. Biol. Chem. 284, 29547–29558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jemima E. A., Prema A., Thangam E. B. (2014) Functional characterization of histamine H4 receptor on human mast cells. Mol. Immunol. 62, 19–28 [DOI] [PubMed] [Google Scholar]
- 52. Zhang Q., Sun H., Jiang Y., Ding L., Wu S., Fang T., Yan G., Hu Y. (2013) MicroRNA-181a suppresses mouse granulosa cell proliferation by targeting activin receptor IIA. PLoS One 8, e59667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van Mil A., Grundmann S., Goumans M. J., Lei Z., Oerlemans M. I., Jaksani S., Doevendans P. A., Sluijter J. P. (2012) MicroRNA-214 inhibits angiogenesis by targeting Quaking and reducing angiogenic growth factor release. Cardiovasc. Res. 93, 655–665 [DOI] [PubMed] [Google Scholar]
- 54. Wang B., Koh P., Winbanks C., Coughlan M. T., McClelland A., Watson A., Jandeleit-Dahm K., Burns W. C., Thomas M. C., Cooper M. E., Kantharidis P. (2011) miR-200a prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes 60, 280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sharma A., Kumar M., Ahmad T., Mabalirajan U., Aich J., Agrawal A., Ghosh B. (2012) Antagonism of mmu-mir-106a attenuates asthma features in allergic murine model. J. Appl. Physiol. 113, 459–464 [DOI] [PubMed] [Google Scholar]