Abstract
Biomaterials with direct intramyocardial injection devices have been developed and are being investigated as a potential cardiac regenerative therapy for end-stage ischemic heart failure (IHF). Decellularized extracellular matrix (ECM) has been shown to improve cardiac function and attenuate or reverse pathologic remodeling cascades. CorMatrix Cardiovascular, Inc. has developed a porcine small intestinal submucosa (SIS)-derived particulate extracellular matrix (P-ECM) and ECM Delivery System to provide uniform and controlled intramyocardial delivery of the injectable P-ECM material into infarcted regions. The CorMatrix ECM Delivery System is comprised of a Multi-Needle P-ECM Syringe Assembly, Automated Injection Controller, and Tissue Depth Measurement System (TMDS, portable ultrasound). Feasibility of the P-ECM delivery system was tested intraoperatively in a chronic IHF bovine model (n=11), and demonstrated the ability to control injection volume (0.1–1.0 mL) and depth of penetration (3–5 mm) under regulated injection pressure (150 psi CO2) into the ischemic region. Targeted intramyocardial delivery of P-ECM may improve efficacy and enable development of novel patient-specific therapy.
Keywords: Heart failure, ECM, Injection Device, Intramyocardial, Cardiac Regeneration, Biomaterials
INTRODUCTION
The progression of ischemic heart failure (IHF) following myocardial infarction (MI) is characterized by cardiac remodeling in the form of ventricular dilation and wall thinning.1–3 Short-term response to MI signals the upregulation of matrix metalloproteinases (MMPs) from inflammatory cells and myofibroblasts, which results in ECM proteolysis, fibrosis, and scar formation.4, 5 Ultimately, these myocardial morphologies lead to reduction in functional myocardium and higher wall stresses, which exacerbates the HF condition. Optimal medical management (OMM) may attenuate or delay the progression of HF, but is frequently limited in its effectiveness at stopping or reversing the pathologic remodeling process. Subsequently, HF patients may require costly surgical interventions with extended clinical management following OMM therapy.
Current surgical treatments for end-stage IHF include heart transplantation (HT) or the use of mechanical circulatory support (MCS). HT is limited by the lack of available donor organs, while MCS is associated with potential adverse event risks, including stroke, bleeding, device failure, and infection.6, 7 HT and MCS therapy also require long-term pharmacologic treatment for immunosuppression or anticoagulation, which may contribute to the increasing costs of HF management.8 In some instances, MCS device therapy has demonstrated reverse remodeling of ventricular geometry and cellular function during left ventricular (LV) pressure and volume unloading.9 Myocardial recovery have been reported in a small cohort of patients receiving MCS therapy; however, these cases of myocardial recovery have been rare (less than 1%) in patients with IHF that have excessive fibrosis compared to congestive HF.9, 10
To overcome limitations associated with HT and MCS therapy, injectable biomaterials have been developed as a potential treatment option for MI and IHF.11, 12, 13 Decellularized materials offer the promise of being non-immunogenic while providing a natural microenvironment to promote ECM rearrangement and cellular proliferation.13, 14 Subsequently, decellularized biologic technologies, such as small intestinal submucosal (SIS)-derived ECM, are being developed for clinical applications. SIS-derived ECM has demonstrated recruitment of vascular growth factors and promotion of myocardial regeneration in cardiac defects15, 16 and acute MI models.1, 17, 18 CorMatrix particulate-ECM (P-ECM, CorMatrix Cardiovascular, Inc., Roswell, GA) is ECM that has been cryogenically ground into an injectable particulate, allowing for intramyocardial delivery. CorMatrix P-ECM has shown biologically-favorable effects in preclinical studies as evidenced by the recruitment of c-kit-positive cardiac progenitor cells, vascular endothelial growth factor (VEGF) protein, and reduction of fibrous tissue.17 Mechanically-favorable effects of P-ECM include increased wall thickness and preservation of systolic function during reperfusion.17
Techniques for delivery of biomaterials include surface (epicardial) patches15, 16, 19, 27 , and intracoronary or intramyocardial injection1, 17, 18, 20–23. Existing intra-myocardial delivery devices use catheter-based endocardial approach, single injection needle, and manually operated (syringe)24. Currently there are no commercial devices specific for intra-myocardial injection via epicardial approach. CorMatrix has developed a novel ECM Delivery System that provides a configurable platform for consistent, targeted, and regionally-distributed injection of P-ECM into infarcted myocardium.. In this study, we present the results of feasibility testing of the Delivery System in a bovine model of chronic, ischemic HF.
METHODS
Device Design
The CorMatrix ECM Delivery System consists of three main components: 1) Multi-Needle P-ECM Syringe Assembly, 2) Automated Injection Controller, and 3) Tissue Depth Measurement System (TDMS, CorMatrix portable ultrasound unit) (Fig 1). The Multi-Needle P-ECM Syringe is an injection molded nylon syringe containing seven individual tubes, each with a volume capacity of 1.2 mL, summing to a total syringe capacity of 8.4 mL P-ECM. The dispensing-end of each tube is fitted with a 25-gauge ultra-thin wall needle and set for 3mm or 5mm penetration lengths for intramyocardial injection of the P-ECM. The needles are arranged in a circular pattern, providing a symmetric 1.1 cm injection field. The filling-end of each tube is plugged with a high-density polyethylene (HDPE) wiper piston that physically dispenses the P-ECM. The multi-needle syringe is connected to a machined polycarbonate driver cylinder assembly comprised of seven piston rods that engage the HDPE wiper pistons and provide positive displacement for P-ECM ejection. The driver cylinder includes a P-ECM shot adjuster subassembly for controlling the volume of P-ECM delivery for each injection cycle (0.1–1.0 mL). The assembled Multi-Needle P-ECM Syringe is connected to the Automated Injection Controller by a high pressure connector tube (0.125” outer diameter, 0.093” inner diameter). The Automated Injection Controller is pressure-activated by an 800 psi CO2 disposable canister (Leland Gas Technologies), operated by a touch screen system, and powered by a rechargeable lithium iron phosphate battery. The Automated Injection Controller regulates the driver pressure at 150 psi CO2 output delivered to each piston rod (seven rods total) through the primary driver piston of the P-ECM Syringe. Actuation of the Automated Injection Controller is controlled by a pneumatic footswitch pedal that enables the clinician to deliver the prescribed P-ECM volume. The TDMS consists of a computer tablet, stylus, and ultrasound probe to acquire ultrasound images for determining wall thickness and identifying infarcted areas to facilitate targeted injection of P-ECM. The ECM Delivery System provides autonomous P-ECM delivery capability with the following features: multi-needle design for uniform delivery over a 1.1-cm injection field, 3- and 5-mm needle penetration depths, precise volume regulation (0.1–1.0 mL volumes), portable imaging capabilities for targeting injection sites, and a rechargeable pressure-injector controller.
Figure 1. ECM Delivery System Component Design.
Top, assembled ECM Delivery System loaded with 8.4 ml P-ECM; Middle, exploded view of Multi-Needle P-ECM Syringe with wiper pistons shown in inset; Bottom left, Automated Injection Controller with disposable CO2 canister and pneumatic footswitch pedal; Bottom right, Tissue Depth Measurement System tablet for real-time cardiac imaging for targeting delivery of P-ECM.
Device Operation
The Multi-Needle P-ECM Syringe Assembly is aseptically assembled by first filling the seven-tube syringe with 8.4 mL prepared P-ECM through a luer-lock conduit adapter (Fig 2). The conduit adapter is removed and each of the seven tubes is sealed with a HDPE wiper piston using the piston insertion tool. The piston rods are set against each piston wiper and the filled multi-needle injection syringe is secured onto the driver cylinder using a threaded sliding nut. The driver port on the Multi-Needle P-ECM Syringe is connected to the Automated Injection Controller via a high pressure connector tube. A footswitch pedal actuator and an 800 psi CO2 canister are attached to the Automated Injection Controller. Once an adequate surgical window of the patient is made, the ultrasound probe of the TDMS is placed in a sterile sheath along with electrode gel which is used to measure tissue depths of the infarcted regions (Fig 3). The P-ECM Syringe can be configured with 3 or 5 mm length needles, depending on the thickness of the infarcted tissue. The P-ECM injection volume is set between 0.1–1.0 mL by manual control of the shot adjuster. The injector needles are then placed into the epicardial surface at the target location. Once full penetration of needles is confirmed, the clinician triggers the footswitch actuator which activates the Automated Injection Controller to output regulated pressure to the Multi-Needle P-ECM Syringe Assembly. The seven piston rods are positively displaced, pushing the wiper pistons to eject the chosen volume of P-ECM material. The process may be repeated at various target sites and for multiple injections up to the 8.4 mL volume capacity per syringe. Additional filled P-ECM multi-needle syringes can be attached for further volume injections.
Figure 2. Step-by-step P-ECM Delivery Procedure.
P-ECM is loaded into the multi-needle syringe via a luer-lock conduit adapter (A). The luer-lock adapter is removed and wiper pistons are placed into each syringe chamber using an insertion tool (B). The Driver Piston is then secured to the multi-needle syringe to complete the injection assembly (C). Next, the tissue width is measured using the Tissue Depth Measurement system (D). The Automated Injection Controller is activated by pressing and holding Power button to 2 – 3 seconds, and acknowledging ‘System Ready’ prompt (E). The high-pressure connector hose is attached to the injection assembly (F) and the injection volume is set by rotating the shot adjuster (G). The injector is then inserted into the targeted site (H) and the footswitch pedal is pressed by the clinician to actuate injection.
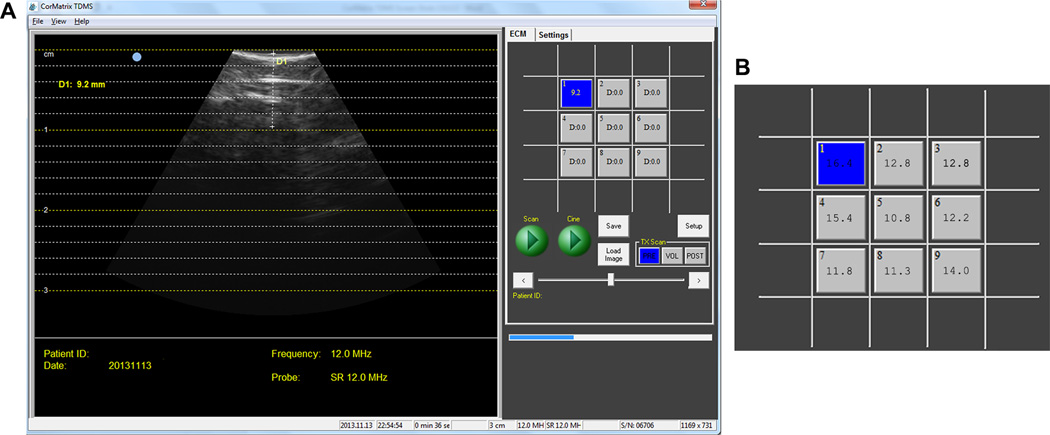
Figure 3. Ultrasound Images Taken by Tissue Depth Measurement System.
A. Tissue Depth Measurement System (TDMS) screenshot of ischemic myocardium with measured left ventricle wall thickness. B. Tissue thicknesses can be measured and recorded by the TDMS for defined locations of a target area, as shown by representative LV wall thickness measurements (in mm) over a 3×3 grid area of bovine ischemic myocardium.
Animal Models
Feasibility of the ECM Delivery System was tested in a chronic IHF (n=11) bovine model (male, Jersey or Holstein, 80–160 kg). The chronic IHF model was created using a coronary microembolization technique, as previously reported.25 Under general isoflurane anesthesia, a 6-French diagnostic catheter was advanced from the carotid artery to selectively engage the left main coronary artery of the calf. A suspension of 90 µm polystyrene microspheres (Polysciences, Inc., Warrington, PA) was injected into the left main coronary artery to induce chronic IHF. Real-time observation of hemodynamic and electrocardiographic changes demonstrated severe and irreversible myocardial ischemia. After the procedure, animals were recovered, and treated for progressive chronic IHF for up to 60 days, as previously reported.26 All animal procedures were performed in accordance and approval with the University of Louisville’s Institutional Animal Care and Use Committee.
Experimental Procedures
A left lateral thoracotomy was performed under general isoflurane anesthesia to gain access to the heart for P-ECM delivery. The pericardium was opened to expose the heart, and a target area was drawn over ischemic regions of myocardium located on the LV lateral surface. The TDMS was used to measure myocardial wall thicknesses within the targeted regions. Target regions were intramyocardially injected 10–11 times at different locations with 1.0±0.15 mL P-ECM volume, summing to a total volume of 10.8 ± 0.3 mL administered over the entire targeted region (note: single syringe volume limited to 8.4 mL, however, a second syringe injection was used in this study). Following therapeutic application of P-ECM, the chest was closed and the animals were recovered and continuously monitored until study endpoint.26
Histology
Necropsies were performed for all animals to assess effectiveness of P-ECM delivery into the ischemic myocardium. The hearts were excised and end-organs were stained with hematoxylin and eosin (H&E). Pathology reports were prepared by an independent pathologist (Mass Histology Service, Worcester, MA).
Delivery System Evaluation
The technical performance of the delivery system was assessed at the time of P-ECM injection. Any challenges or errors with the delivery system were noted and recorded. The P-ECM volume per injection and total volume were recorded. Direct visual observations at the injection sites pre-, intra-, and post-injection were performed. During in vivo injection, syringe positioning and P-ECM retention were characterized. P-ECM delivery was directly assessed visually at the time of injection while animal health was monitored during the post-operative period. Necropsy and histology evaluation were performed at study termination to identify any potential adverse responses to P-ECM delivery, such as the presence of emboli or inflammatory response.
RESULTS
Feasibility of the ECM Delivery System to consistently deliver P-ECM into the myocardium of a beating heart was demonstrated in chronic IHF bovine models. Surgeries and P-ECM delivery progressed without any technical complications. There were no ECM Delivery System device failures in any of the animal experiments. All of the injected P-ECM material was retained in the myocardial wall (Fig 4). The P-ECM Delivery Syringe shot adjuster (0.1–1.0 mL) controlled injection volume (±0.1 ml) and the Automated Injector Controller (150 psi) regulated driving pressure (±1 psi). The TDMS accurately measured LV wall thickness (±0.1 mm) in healthy and ischemic rmyocardium, leading to appropriate selection of needle length (3 or 5 mm) and delivery of P-ECM into ischemic region, while minimizing the risk of injecting P-ECM into the ventricular chamber. Average wall thickness measurements in the IHF bovine model was 13.2 ± 0.8 mm. The minimum thickness measured at an injection site was 8.6 mm, which satisfied the safety factor criterion (2-times thickness) for injection with 3 mm length needle. The P-ECM injections resulted in 1.6±0.1 mm wall thickness increase per mL P-ECM. Minimal blood loss was observed with multi-needle syringe penetration and injection. No arrhythmias were observed in any animals pre- and intra-operatively.
Figure 4. Particulate-Extracellular Matrix (P-ECM) Intramyocardial Injection Sites and Penetration Depth.
A. P-ECM injection sites (white arrows) can be observed in vivo in bovine LV lateral wall epicardium. Needle penetration and P-ECM injection resulted in minimal blood loss. B. Close-up photo of P-ECM material with measured penetration distance in the left ventricular wall of bovine myocardium.
The P-ECM was visually observed in the myocardial wall and characterized as white cylindrical depositions with the long axis perpendicular to LV epicardial surface at necropsy (Fig 4). The location and spacing of the P-ECM matched the injection pattern of the multi-needle syringe. There was no indication of transmyocardial penetration into the LV chamber. P-ECM delivery did not yield any adverse events attributable to P-ECM injection or the ECM Delivery System. One calf developed arrhythmias on post-operative day-3. These arrhythmias were were medically managed with antiarrhythmics and ceased by post-operative day-11. Another calf was euthanized at post-operative day-71 due to multi-organ failure, which was likely a result of advanced IHF, as necropsies did not reveal any emboli in cardiac tissue or end-organs, and inflammation markers did not exceed inflammatory response expected with cardiac thoracotomy procedures in any of the calves (Fig 5).
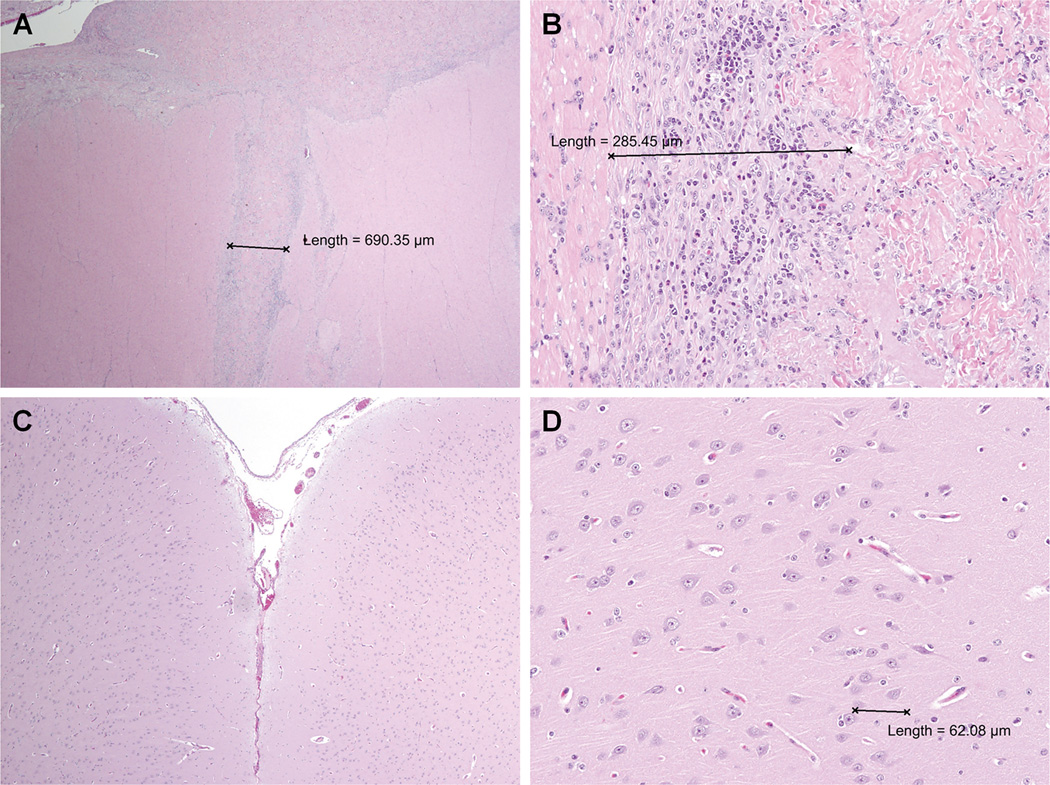
Figure 5. Hematoxylin and Eosin (H&E) Stain of Particulate-Extracellular Matrix (P-ECM) Integration.
A, B) H&E stains showing P-ECM integration in the lateral LV of an IHF calf after 7 days. An injection tract of P-ECM surrounded by mild inflammation and fibrosis is observed (purple nuclei). C, D) Gross and histopathologic examination of brain section revealed no evidence of infarction or pathologic process.
DISCUSSION
Initial techniques for delivery of biomaterials to the myocardium included cardiac patches 15, 16, 19,,27 and epicardial direct injections. 1, 17, 18 Cardiac patches require suturing to the myocardial surface, limiting therapy to the exterior surface at the location where it’s applied. Epicardial direct injections offer the benefit of delivery of materials intramyocardially into the infarcted tissue, but only a few studies have been reported and delivery techniques have largely been limited to manual, single needle-syringe injections. These rudimentary delivery tools can be imprecise (lack controllable injection volume and penetration depth) and inefficient (small injection field), which may limit therapy efficacy and present potential safety risk. Transcatheter approaches have been developed as an alternative to the invasive surgical procedures associated with cardiac patches and direct injections.28–31 To date, transcatheter delivery approaches have also used conjunctive biomaterials containing ECM with stem cell components. However, biomaterials delivered through transcatheter approaches must satisfy stringent gelation or liquid properties to avoid complications such as clogging or introduction of emboli.
Critical components toward achieving effective P-ECM therapy are targeting delivery to the infarcted myocardium and designating the injection profile.32, 33 Epicardial injections of ECM into ischemic regions may provide a structural and biomolecular microenvironment that is advantageous for cardiac cell viability or neovascularization.1, 17, 18 LV wall thickening was observed at the injection site (treatment area) in contrast to LV wall thinning in untreated (non-injection) myocardium, suggesting the potential benefit of efficient P-ECM injection that targets all of the ischemic tissue (surface area and intramyocardium). In this feasibility study, the ECM Delivery System effectively injected P-ECM into the infarcted myocardium. In preparation of P-ECM delivery, the TDMS enables clinicians to identify ischemic region boundaries and pre-select injection needle length (3 or 5 mm) to restrict P-ECM delivery to the treatment area and prevent injection into the ventricular chamber minimizing the risk of peripheral embolization. Additionally, the ECM delivery system provides a seven-needle 1.1-cm injection field to improve P-ECM injection efficiency over the entire targeted area (ischemic region), and enables precise control of the P-ECM delivery volume. These features, along with uniform pressure regulation (150 psi) by the Automated Injection Controller, facilitate P-ECM retention in the myocardial tissue (i.e. limits epicardial “leakage” as seen with single syringe-needle injections). Considering the recent emergence of injectable biomaterials, limited in vivo research has been conducted for the biological and mechanical benefits of intramyocardial injections. As a specific device for intramyocardial delivery, the ECM Delivery System may provide a useful tool to investigate the correlation of injection profiles with therapy outcomes, and to tailor potential therapeutic efficacy to individual patients.
Application of biomaterials as targeted therapy for the restoration of functional myocardium has generated great clinical interest as a promising reparative treatment for IHF. As development of biomaterials continues to advance, there is an unmet clinical need for a safe, effective, reliable, and reproducible delivery system for precise, targeted injection into infarcted myocardial tissue. The CorMatrix P-ECM Delivery System may enable better identification of ischemic injury while providing automated, controlled intramyocardial injection, which may enable strategic targeting of ischemic areas to cater the therapy to patient-specific needs. Coupling a biomaterial possessing potential myocardial regenerative properties to a specific delivery system may provide a platform for alternative or supplemental IHF treatments.
Acknowledgments
Funding for this project was provided, in part, by research support from CorMatrix Cardiovascular, Inc. (Roswell, GA).
Footnotes
Disclaimer: None
Disclosures: Authors Robert Matheny, MD, Beecher Lewis, and Michael Hennick are employees of CorMatrix Cardiovascular, Inc. No conflicts of interest were reported by other authors.
REFERENCES
- 1.Okada M, Payne TR, Oshima H, et al. Differential efficacy of gels derived from small intestinal submucosa as an injectable biomaterial for myocardial infarct repair. Biomaterials. 2010;31:7678–7683. doi: 10.1016/j.biomaterials.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 2.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: Pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 3.Anversa P, Li P, Zhang X, et al. Ischaemic myocardial injury and ventricular remodeling. Cardiovasc Res. 1993;27:145–157. doi: 10.1093/cvr/27.2.145. [DOI] [PubMed] [Google Scholar]
- 4.Spinale FG, Janicki JS, Zile MR. Membrane-associated matrix proteolysis and heart failure. Circ Res. 2013;112:195–208. doi: 10.1161/CIRCRESAHA.112.266882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindsey ML, Mann DL, Entman ML, et al. Extracellular matrix remodeling following myocardial injury. Ann Med. 2003;35:316–326. doi: 10.1080/07853890310001285. [DOI] [PubMed] [Google Scholar]
- 6.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: Risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Trans. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Soucy KG, Koenig SC, Giridharan GA, et al. Rotary pumps and diminished pulsatility: Do we need a pulse? ASAIO J. 2013;59:355–366. doi: 10.1097/MAT.0b013e31829f9bb3. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birks EJ. Molecular changed after left ventricular assist device support for heart failure. Circ Res. 2013;113:777–791. doi: 10.1161/CIRCRESAHA.113.301413. [DOI] [PubMed] [Google Scholar]
- 10.Drakos SG, Kfoury AG, Selzman CH, et al. Left ventricular assist device unloading effects on myocardial structure and function: Current status of the field and call for action. Curr Op Card. 2011;26:245–255. doi: 10.1097/HCO.0b013e328345af13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Nelson DM, Ma Z, Fujimoto K, et al. Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges. Acta Biomaterialia. 2011;7:1–15. doi: 10.1016/j.actbio.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singelyn JM, Christman KL. Injectable materials for the treatment of myocardial infarction and heart failure: The promise of decellularized matrices. J Cardiovasc Trans Res. 2010;3:478–486. doi: 10.1007/s12265-010-9202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel J, Abe K, McFetridge PS. Development of the human umbilical vein scaffold for cardiovascular tissue engineering applications. ASAIO J. 2005;51:252–261. doi: 10.1097/01.mat.0000160872.41871.7e. [DOI] [PubMed] [Google Scholar]
- 15.Badylak S, Obermiller J, Geddes L, et al. Extracellular matrix for myocardial repair. Heart Surg Forum. 2003;6:E20–E26. doi: 10.1532/hsf.917. [DOI] [PubMed] [Google Scholar]
- 16.Rosen M, Roselli EE, Faber C, et al. Small intestinal submucosa intracardiac patch: an experimental study. Surg Innov. 2005;12:227–231. doi: 10.1177/155335060501200307. [DOI] [PubMed] [Google Scholar]
- 17.Zhao ZQ, Puskas JD, Xu D, et al. Improvement in cardiac function with small intestine extracellular matrix is associated with recruitment of C-kit cells, myofibroblasts, and macrophages after myocardial infarction. Journal of the American College of Cardiology. 2010;55:1250–1261. doi: 10.1016/j.jacc.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 18.Toeg HD, Tiwari-Pandey R, Seymour R, et al. Injectable small intestine submucosal extracellular matrix in an acute myocardial infarction model. Ann Thor Surg. 2013;96:1686–1694. doi: 10.1016/j.athoracsur.2013.06.063. [DOI] [PubMed] [Google Scholar]
- 19.Kochupura PV, Azeloglu EU, Kelly DJ, Doronin SV, Badylak SF, Krukenkamp IB, Cohen IS, Gaudette GR. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation. 2005;112(9) suppl:I–144. doi: 10.1161/CIRCULATIONAHA.104.524355. [DOI] [PubMed] [Google Scholar]
- 20.Kornowski R, Leon MB, Fuchs S, Vodovotz Y, Flynn MA, Gordon DA, Pierre A, Kovesdi I, Keiser JA, Epstein SE. Electromagnetic guidance for catheter-based transendocardial injection: a platform for intramyocardial angiogenesis therapy: Results in normal and ischemic porcine models. Journal of the American College of Cardiology. 2000;35(4):1031–1039. doi: 10.1016/s0735-1097(99)00642-7. [DOI] [PubMed] [Google Scholar]
- 21.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. European heart journal. 2006;27(9):1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 22.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, et al. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. Journal of molecular and cellular cardiology. 2008;44(3):486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 23.McCall FC, Telukuntla KS, Karantalis V, Suncion VY, Heldman AW, Mushtaq M, Williams AR, Hare JM. Myocardial infarction and intramyocardial injection models in swine. Nature protocols. 2012;7(8):1479–1496. doi: 10.1038/nprot.2012.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheter-based delivery of cells to the heart. Nature Clinical Practice Cardiovascular Medicine. 2006;3:S57–S64. doi: 10.1038/ncpcardio0446. [DOI] [PubMed] [Google Scholar]
- 25.Bartoli CR, Sherwood LC, Giridharan GA, et al. Bovine model of chronic ischemic cardiomyopathy: Implications for ventricular assist device research. Artif Organs. 2013;37:E202–E214. doi: 10.1111/aor.12129. [DOI] [PubMed] [Google Scholar]
- 26.Sherwood LC, Sobieski MA, Koenig SC, et al. Benefits of aggressive medical management in a bovine model of chronic ischemic heart failure. ASAIO J. 2013;59:221–229. doi: 10.1097/MAT.0b013e3182894e66. [DOI] [PubMed] [Google Scholar]
- 27.Robinson KA, Li J, Mathison M, et al. Extracellular matrix scaffold for cardiac repair. Circulation. 2005;112:I135–I143. doi: 10.1161/CIRCULATIONAHA.104.525436. [DOI] [PubMed] [Google Scholar]
- 28.Perin EC, Dohmann HF, Borojevic R, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 29.Smits PC, van Geuns RJ, Poldermans D, et al. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: Clinical experience with six-month follow-up. J Am Coll Card. 2003;42:2063–2069. doi: 10.1016/j.jacc.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. NEJM. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 31.Leor J, Tuvia S, Guetta V, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in swine. J Am Coll Card. 2009;54:1014–1023. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Wall ST, Walker JC, Healy KE, et al. Theoretical impact of the injection of material into the myocardium: A finite element model simulation. Circulation. 2006;114:2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 33.Wenk JF, Wall ST, Peterson RC, et al. A method for automatically optimizing medical devices for treating heart failure: designing polymeric injection patterns. J Biomech Eng. 2009;131:121011. doi: 10.1115/1.4000165. [DOI] [PubMed] [Google Scholar]