Abstract
BACKGROUND
Approximately18-25% of patients with alcohol use disorders admitted to the hospital develop alcohol withdrawal syndrome (AWS). Symptom-triggered dosing of BZDs appears to lead to shorter courses of treatment, lower cumulative BZD dose and more rapid control of symptoms in non-critically ill patients. This study compares the outcomes of critically ill patients with AWS when treated using a protocolized, symptom-triggered, dose-escalation approach versus a non-protocolized approach.
METHODS
This is a retrospective pre-post study of patients ≥18 years with AWS admitted to an ICU. The PRE cohort (PRE) was admitted between 2-2008 and 2-2010. The Post-intervention cohort (POST) was admitted between 2-2012 and 1-2013. PRE were treated by physician preference and compared to POST that were given escalating doses of BZDs and/or phenobarbital according to an AWS protocol, titrating to light sedations (RASS of 0 to −2).
RESULTS
There were 135 episodes of AWS in 132 critically ill patients. POST (n=75) were younger (50.7±13.8 vs 55.7±8.7 years, p=0.03) than PRE (n=60). SOFA scores were higher in PRE (6.1±3.7 vs 3.9±2.9, p=0.0004). There was a significant decrease in mean ICU LOS from 9.6±10.5 to 5.2±6.4 days (p=0.0004) in the POST group. The POST group also had significantly fewer ventilator days (5.6 ± 13.9 vs 1.31±5.6 days, p<0.0001) as well as a significant decrease in BZD usage (319±1084 vs 93±171 mg, p=0.002). There were significant differences between the two cohorts with respect to need for continuous sedation (p<0.001), duration of sedation (p<0.001), and intubation secondary to AWS (p<0.001). In all of these outcomes, the POST cohort had a notably lower frequency of occurrence.
CONCLUSION
A protocolized treatment approach of AWS in critically ill patients involving symptom-triggered, dose escalations of diazepam and phenobarbital may lead to a decreased ICU LOS, decreased time spent on mechanical ventilation, and decreased BZD requirements.
Keywords: Alcohol Withdrawal Syndrome, benzodiazepines, sedation, ICU
Background
Alcohol use is common in the United States. More than half of all Americans over the age of 12 self-report to be current drinkers and 18.6 million (7.4%) admit dependence on alcohol.1 Approximately 18-25% of patients with alcohol use disorders admitted to the hospital develop alcohol withdrawal syndrome (AWS).2 Alcoholism is associated with increased risk of nosocomial infection, sepsis, length of stay, and mortality in critically ill patients. 2
Although no formal guidelines are published, benzodiazepines (BZDs) are widely accepted as first line treatment for AWS.3 Historically, BZDs have been administered in a continuous or scheduled fashion and gradually tapered following a 5- to 7- day course. However, two studies in non-critically ill hospitalized patients found that those who received symptom-triggered dosing of BZDs had similar outcomes to those who received scheduled BZDs, but required shorter courses of treatment and lower cumulative BZD doses.4,5 In patients admitted to an ICU solely for treatment of AWS, a symptom-triggered strategy of escalating doses of BZDs in combination with phenobarbital resulted in a significant decrease in need for mechanical ventilation and a trend toward decreased ICU length of stay and rate of nosocomial pneumonia.6 Most studies of AWS only included critically ill patients admitted for AWS and explicitly excluded patients with other acute problems or major comorbidities that are common in the ICU.2,6,7
These evidence-based elements of AWS management were incorporated into a protocol that applied a symptom-triggered, dose-escalation approach using BZDs and phenobarbital. An agitation sedation scoring system was used to direct bedside dosing and monitoring. The purpose of this study was to compare patient outcomes in critically ill patients with AWS, regardless of their admission ICU diagnosis, that were treated with this protocolized approach versus a non-protocolized approach.
Methods
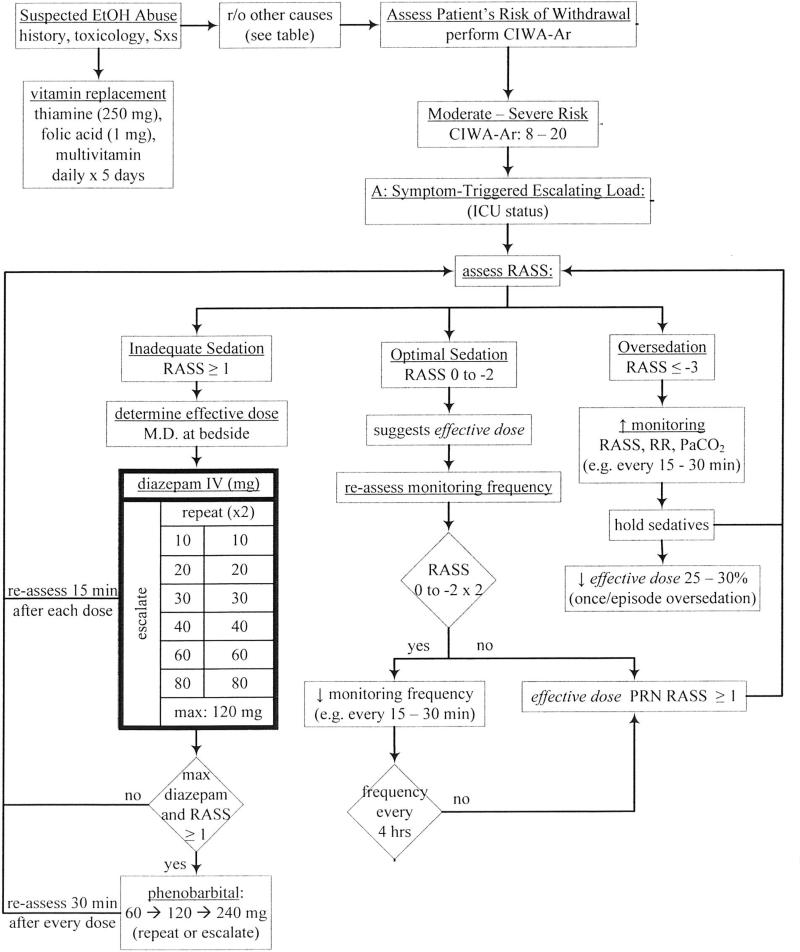
This single-center, pre-post study was conducted in adult patients with AWS admitted to the ICU at an urban, academic tertiary referral center. The institutional review board approved the study and waived the need for informed consent. Patients in the Pre-intervention group (PRE) were treated in a non-protocolized fashion and typically received continuous infusions or scheduled doses of BZDs per physician preference. Patients in the Post-intervention group (POST) were given escalating doses of diazepam and phenobarbital according to an AWS protocol (Figure 1) which was available via an electronic order set. Patients received symptom-triggered doses of diazepam every 15 to 30 minutes until target sedation level was achieved. Target sedation level was defined as a Richmond Agitation Sedation Scale (RASS) of 0 to -2 which corresponds to light sedation.8 Nurses were directed to continue escalating diazepam doses up to a maximum of 120mg. Phenobarbital was included in the protocol as an adjunct to be given every 30 minutes in a similar dose escalation fashion up to a maximum of 240mg.
Figure 1.
Alcohol withdrawal protocol based on a symptom-triggered, dose-escalation approach using BZDs and phenobarbital. RASS is used for monitoring and administering sedation. BZD: benzodiazepine; RASS: Richmond Agitation Sedation Scale
Subjects in the PRE group were identified from electronic medical records by automated reports capturing patients by ICD-9 code—alcohol withdrawal (291.81), unspecified alcohol dependence (303.90) and alcohol dependence with continuous drinking behavior (303.91). Subjects for the POST group were consecutively admitted patients that received orders for the AWS order set. A search of the electronic medical record for concurrent use of thiamine and folate was used to supplement and capture potential patients in both PRE and POST groups. Patients were included if AWS was identified as a diagnosis in the history and physical or a progress note.
Patients were included that were 18 years of age or older and admitted to an ICU between February 2008 and February 2010 (PRE) or between February 2012 and January 2013 (POST). A primary diagnosis of AWS was not required for study inclusion. However, patients were characterized according to the primary reason for ICU admission, ie AWS or another diagnosis. Patients with all other comorbidities were deliberately included with the exception of severe brain injury—defined as persistent Glasgow Coma Score < 8—due to the inability to fully assess withdrawal symptoms and sedation levels in these patients.
The primary outcome measure was ICU length of stay. Secondary outcomes included mean and median BZD use, mean and median phenobarbital use, duration of sedation, requirement for mechanical ventilation (MV), ventilator-free days, and requirement for MV due to AWS. BZD usage was measured in lorazepam equivalents using the following formula: 5 mg diazepam = 1 mg lorazepam equivalents, 2 mg midazolam = 1 mg lorazepam equivalents.9 Baseline patient characteristics were collected to determine severity of illness and severity of AWS in the study population. Ventilator-free days were defined as the number of days of unassisted breathing to day 28. Patients that died were considered to have zero ventilator-free days.
To avoid over-fitting while at the same time using care to control for confounding variables, we used a two-phase model selection process. First we performed a series of univariate tests to see how various patient characteristics and risk factors differed between the PRE and POST patients. Any variables which were significant at the 0.1 level or lower were included for further testing. Next, for the continuous response variables, time on ventilator, ICU length of stay, and ventilator-free days, a multiple linear regression was fit using a SAS® software backward selection procedure GLMSELECT using the variables from the previous phase. The full model included the explanatory variables admission secondary to AWS, phenobarbital usage, diazepam usage, age, duration of sedation, and SOFA score. The selection criterion used was the optimized Schwarz Bayesian Information Criterion. For the binary variables, death, requiring intubation, seizures while admitted, requiring continuous sedation, multiple logistic regression was fit using a SAS® software backward selection procedure LOGISTIC. The selection criterion used was a p-value of less than 0.05.
Results
There were 135 episodes of AWS in 132 critically ill patients. The majority of these patients were treated by the medical ICU (~50%) or trauma surgery services (~30%). The remaining patients were treated by orthopedic surgery, ENT, burn, transplant, cardiology, neurosurgery, and general medicine services.
Demographic information is found in Table 1. There were significant differences between PRE and POST groups in age, and SOFA score. Compared to the PRE group, the POST group was significantly younger with a mean age of 50.7 ± 13.8 versus 55.7 ± 8.7 years, respectively (p=0.03). SOFA scores upon ICU admission for the PRE versus POST group were 6.1 ± 3.7 and 3.9 ± 2.9, respectively (p=0.0004).
Table 1.
Baseline characteristics of both PRE and POST groups.
| PRE group (n=60) | POST group (n=75) | P-value | |
|---|---|---|---|
| Mean Age | 55.7 ± 8.7 yrs | 50.7 ± 13.8 yrs | 0.03 |
| Male | 81.6% | 81.3% | 1.0 |
| History of Alcohol Withdrawal | 40% | 30.6% | 0.28 |
| History of Psychosis | 10% | 12% | 0.78 |
| History of Delirium Tremens | 10% | 4% | 0.19 |
| History of Seizure | 18.3% | 21% | 0.83 |
| Mean SOFA score on admit | 6.1 ± 3.7 | 3.9 ± 2.9 | 0.0004 |
| Mean blood alcohol level on admit (mg/dL) | 135 ± 156 | 134 ± 140 | 0.56 |
Means are presented as Mean ± SD
Table 2 shows both primary and secondary outcomes. There was a significant difference between the two groups in the primary outcome of ICU LOS, 9.6 ± 10.5 days in the PRE group versus 5.2 ± 6.4 days in the POST group (p=0.0004). There was insufficient evidence of an effect due to any of the variables other than duration of sedation and the use of the protocol. There was weak evidence of an effect due to the use of the protocol (p=0.096). There was overwhelming evidence of an effect due to duration of sedation. The ICU LOS increased 0.7 days for each additional day of sedation (p<0.0001).
Table 2.
Primary and Secondary Outcomes
| PRE group (n=60) | POST group (n=75) | P-value | |
|---|---|---|---|
| ICU LOS | 9.6 ± 10.5 | 5.2 ± 6.4 | 0.0004 |
| Time on Ventilator (days) | 5.6 ± 13.9 | 1.31 ± 5.6 | <0.0001 |
| Ventilator-free days | 21.3 ± 9.5 | 26.3 ± 5.6 | 0.0004 |
| Intubation due to AWS | 13 (22%) | 4 (5%) | <0.001 |
| Need for continuous sedation | 33 (55%) | 18 (24%) | <0.001 |
| Duration of sedation (days) | 10.8 ± 8.9 | 3.5 ± 3.5 | <0.001 |
| Death | 7 (12%) | 2 (3%) | 0.07 |
Means are presented as Mean ± SD
ICU LOS: intensive care unit length of stay; AWS: alcohol withdrawal syndrome
Mean sedative use is shown in Figure 2. There was a substantial decrease in mean BZD usage between the two groups with POST group patients requiring less than a third of total BZD compared to the PRE group (p=0.0002). The medians showed a trend towards less BZD use between PRE and POST groups (33 mg vs 23 mg, p=0.06). Mean phenobarbital usage increased in the POST group (p=0.04). Very few patients required phenobarbital in either PRE or POST groups and the medians were zero for each. The POST group had significantly less need for continuous sedation and duration of sedation. See Table 2. There was a strong link between SOFA score and the need for continuous sedation based on multi-variate regression analysis. For every unit increase in the SOFA score, the odds of requiring continuous sedation increased by about 36% (p<0.0001).
Figure 2.

Mean BZD and PB use in both PRE and POST groups. Means are presented as Mean ± SD BZD: benzodiazepine; PB: phenobarbital
The PRE group required almost 5 days more in time on the ventilator than the POST group (5.6 ± 13.9 versus 1.31 ± 5.6 days, p<0.0001). This is also reflected in a significant increase in the number of ventilator-free days in the POST group (p= 0.0004). The need for intubation due to AWS was significantly reduced in the POST group (22% vs 5%, p <0.001). After controlling for severity of illness with multi-variate regression analysis, every additional day of sedation was associated with an additional 0.53 days on the ventilator (p<0.0001). Similarly, after controlling for duration of sedation, the average time on the ventilator increased 0.61 days for each unit increase in SOFA score (p=0.01). There was very strong evidence of an effect due to BZD on the number of ventilator-free days. There was also strong evidence that patients admitted primarily for AWS were at higher risk for intubation--due to AWS--than patients admitted for another critical illness (p=0.04). Patients in the PRE group were at a greater risk for intubation than POST group patients (p=0.02). Increased SOFA scores were associated with an increased risk for intubation (p<0.0001).
Seven patients (11.6%) in the PRE group died during their hospitalization, while two patients in the POST group (2%) died (p=0.07). None of these deaths were directly attributable to AWS. Increasing SOFA scores was directly associated with an increased risk of death (p=0.0002). For every unit increase in SOFA score the odds of death increased by 61%. There was insufficient evidence of an effect due to any of the other variables tested.
Discussion
This study compared protocolized treatment of AWS to non-protocolized care. An alcohol withdrawal order set developed for the electronic medical record facilitated high level of concordance with the protocol. The key elements of the protocol were based on Gold's symptom-triggered, dose-escalation approach using BZDs and phenobarbital.6 There were a number of unique features in the design and execution of this study. In contrast to most studies of the treatment of AWS,6,7,10,11,12 this study included all patients with AWS regardless of primary diagnosis upon admission. The improvement in patient outcomes seen supports the use of this management strategy in all types of ICU patients, not just those admitted for AWS.
A critically ill patient with AWS can strain ICU resources. They often require one-to-one nursing and sedation requirements can be enormous. This protocol was developed to equip the bedside nurse to monitor the patient and administer sedation using a sedation assessment tool and a symptom-triggered approach. The Clinical Institute Withdrawal Assessment (CIWA-Ar)13 is not useful in most ICU patients for a number of reasons that include the following. It requires cooperation and communication on the part of the patient which eliminates those patients that are delirious or intubated. Parameters are subjective and very labor intensive, taking at least 5-15 minutes to complete. CIWA-Ar scores may be confounded by comorbidities such as trauma or critical illness. CIWA-Ar is not an effective treatment monitor for ICU patients as it was essentially developed to triage patients based on severity of alcohol withdrawal. In the study protocol, CIWA-Ar is used for determining the patient's risk of developing severe AWS and need for ICU management. Previous studies have demonstrated the difficulty of serial assessment using CIWA-Ar.14,15 RASS was selected for treatment monitoring because our institution already uses it for sedation assessment. It can be performed easily and quickly, and is similar to other sedation assessment tools, eg Ramsey, Riker Sedation-Agitation Scale. The main advantage of basing therapy on a sedation assessment tool is that the bedside nurse can seamlessly assess the symptom and response with the same tool.
There were two key differences between the PRE and POST patients. The PRE group was older, which may signal the probability of more comorbidities, and their SOFA score on admission was higher. Although the SOFA score indicates that the PRE group was sicker, the difference may be due in part to the differences in management between the PRE and POST groups. In the PRE group, the increased need for intubation for AWS and consequent use of sedation may have driven the SOFA score higher. Intubation and change in Glasgow Coma Score from sedation could easily account for a two-point difference in SOFA score. Despite this possibility, there were still differences in the clinical outcomes favoring the POST group after controlling for age and severity of illness with regression analysis.
The substantial reduction in ICU LOS and duration of sedation between the two groups suggests that early, aggressive, symptom-triggered dosing of diazepam and phenobarbital can obtain rapid relief of symptoms, lead to shorter courses of therapy and reduce the need for intubation. These benefits were noted previously in critically ill patients with AWS.6,10 but to our knowledge they have never been shown simultaneously in a single study. Gold et al also noted a statistically significant reduction in the need for intubation following the implementation of, “guidelines emphasizing escalating doses of diazepam in combination with phenobarbital”. Additionally, a trend in reduction in ICU LOS was noted between groups.6 However, in our study, ICU LOS was significantly decreased in POST patients which may be related to significantly less time on the ventilator. It is well established that intubation is associated with longer hospital stay and higher rates of nosocomial pneumonia. Therefore, it is logical to suggest that decreasing the use of mechanical ventilation by using symptom-triggered bolus dosing of BZDs and phenobarbital will lead to decreased LOS and decreased nosocomial pneumonia in previous studies.6,11 Additionally, there was a trend towards decreased mortality in the POST group which may be related to the decreased need for intubation and time on the ventilator or it could be related to the difference in SOFA score on admission.
Another factor that may have played a role in the decrease in ICU LOS is the decreased use of continuous infusions in the POST group. Limited data suggest that intermittent-bolus dosing of medications may be superior to continuous infusion for AWS,11 an assertion supported by our study. Benzodiazepine exposure was notably lower in the POST group relative to the PRE group. Providing escalating BZD doses, guided by RASS scores, allows nurses to target patient-specific benzodiazepine needs. The ability to quickly and effectively target an individual patient's required sedative dose allows for a shorter duration of sedation, as demonstrated by previous studies.4-7,11 Patients with severe AWS that receive escalating, intermittent doses of BZD do not experience escalation of symptoms when controlled early and effectively in contrast to those treated with continuous sedation. On the other hand, patients with mild AWS symptoms are spared a scheduled dosing regimen when they potentially require minimal BZDs to reach a targeted sedation level. In our study, a third of both PRE and POST groups required 10 mg or less of BZD. The increase seen in phenobarbital usage in the POST group is explained by its presence on the protocol which led to more wide spread use (3% versus 13%).
BZDs are the currently accepted treatment of choice for AWS, although some have advocated for bolus doses of phenobarbital as first-line treatment.16-18 However, phenobarbital is more often added as an adjunct to BZDs for those patients requiring large doses of BZD and who remain agitated.6,19 In our protocol, BZDs were chosen over other available therapeutic agents due to their low cost. Although reserved for refractory cases of AWS, dexmedetomidine may also serve as an another useful, adjunctive agent for AWS.20-22 Other adjunctive agents such as beta blockers or clonidine may be employed for AWS, but there is insufficient evidence to support their general use.
The limitations of this study include its retrospective, single-center design. Although there are disadvantages of a pre-post study design when compared to a randomized control trial, it does demonstrate this strategy may be executed using resources commonly available in the ICU such as a sedation assessment tool and BZDs. Because of its retrospective nature, potential differences between groups may not have been identified or controlled for. Potential confounders included varied AWS management strategies in the PRE group, withdrawal from other illicit drugs, and differences in ICU management. Age and severity of illness were significantly different between the two groups. To control for these explanatory variables, regression analysis was performed for the primary and secondary outcomes.
Conclusions
This study suggests that a protocolized treatment approach using early, aggressive, symptom-triggered dosing of diazepam and phenobarbital is associated with decreased need for intubation, less time on mechanical ventilation, ICU LOS, reduced BZD exposure and possibly decreased mortality.
Acknowledgments
Funding Sources: The statistical analysis was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through grant #UL1 TR000002.
Footnotes
Partial results were presented at SCCM Annual Congress, Houston, Tx (Feb 4-8th, 2012) Complete study presented at Western Trauma Association, Steamboat Springs, CO; March 2-7, 2014
Author Contribution: All authors were involved with the literature search, study design, data collection, data analysis, data interpretation, writing and critical revision
This manuscript includes the unlabeled use of benzodiazepines, phenobarbital and dexmedetomidine for alcohol withdrawal syndrome.
Contributor Information
Jeremiah J. Duby, University of California Davis Medical Center, 2315 Stockton Blvd, Sacramento, CA 95817, UCSF College of Pharmacy, UC Davis School of Medicine dubyjj@gmail.com.
Andrew J. Berry, Cardiology Banner Good Samaritan Medical Center, Phoenix, AZ, Andrew.berry@bannerhealth.com.
Paricheh Ghayyem, UC San Diego Thornton Medical Center, La Jolla, CA Pghayyem@gmail.com.
Machelle D. Wilson, Clinical & Translational Science Center, Department of Public Health Sciences, University of California Davis, 2921 Stockton Blvd, Suite 1400, Sacrmaento, CA 95817, Office: (916) 703-9106, mdwilson@phs.ucdavis.edu.
Christine S. Cocanour, University of California Davis Medical Center, 2315 Stockton Blvd, Sacramento, CA 95817, Professor, Department of Surgery, Division of Trauma and Emergency Surgery Services.
References
- 1.Substance Abuse and Mental Health Services Administration . Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Rockville, MD.: 2010. (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10-4586 Findings) [Google Scholar]
- 2.De Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest. 2010;138(4):994–1003. doi: 10.1378/chest.09-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649–55. [PMC free article] [PubMed] [Google Scholar]
- 4.Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal. JAMA. 1994;272(7):519–23. [PubMed] [Google Scholar]
- 5.Daeppen JB, Gache P, Landry U, Sekera E, Schweizer V, Gloor S, Yersin B. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117–21. doi: 10.1001/archinte.162.10.1117. [DOI] [PubMed] [Google Scholar]
- 6.Gold JA, Rimal B, Nolan A, Nelson LS. A strategy of escalating doses of benzodiazepines and phenobarbital administration reduces the need for mechanical ventilation in delirium tremens. Crit Care Med. 2007;35(3):724–30. doi: 10.1097/01.CCM.0000256841.28351.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mueller SW, Preslaski CR, Kiser TH, Fish DN, Lavelle JC, Malkoski SP, MacLaren R. A randomized, double-blind, placebo-controlled dose range study of dexmedetomidine as adjunctive therapy for alcohol withdrawal. Crit Care Med. 2013 Dec 17; doi: 10.1097/CCM.0000000000000141. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 9.Dundee JW, McGowan WAW, Lilburn JK, McKay AC, Hegarty JE. Comparison of the actions of diazepam and lorazepam. Br J Anaesth. 1979;51:439–445. doi: 10.1093/bja/51.5.439. [DOI] [PubMed] [Google Scholar]
- 10.DeCarolis DD, Rice KL, Ho L, Willenbring ML, Cassaro S. Symptom-driven lorazepam protocol for treatment of severe alcohol withdrawal delirium in the intensive care unit. Pharmacotherapy. 2007;27(4):510–8. doi: 10.1592/phco.27.4.510. [DOI] [PubMed] [Google Scholar]
- 11.Spies CD, Otter HE, Hüske B, Sinha P, Neumann T, Rettig J, Lenzenhuber E, Kox WJ. Alcohol withdrawal severity is decreased by symptom-oriented adjusted bolus therapy in the ICU. Intensive Care Med. 2003;29(12):2230–8. doi: 10.1007/s00134-003-2033-3. [DOI] [PubMed] [Google Scholar]
- 12.Ng K, Dahri K, Chow I, Legal M. Evaluation of an alcohol withdrawal protocol and a preprinted order set at a tertiary care hospital. Can J Hosp Pharm. 2011;64(6):436–445. doi: 10.4212/cjhp.v64i6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 14.Hecksel KA, Bostwick JM, Jaeger TM, Cha SS. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274–9. doi: 10.4065/83.3.274. [DOI] [PubMed] [Google Scholar]
- 15.Bostwick JM, Lapid MI. False positives on the Clinical Institute Withdrawal Assessment for Alcohol—Revised: is this scale appropriate for use in the medically ill? Psychosomatics. 2004;45(3):256–261. doi: 10.1176/appi.psy.45.3.256. [DOI] [PubMed] [Google Scholar]
- 16.Young GP, Rores C, Murphy C, Dailey RH. Intravenous phenobarbital for alcohol withdrawal and convulsions. Ann Emerg Med. 1987;16(8):847–50. doi: 10.1016/s0196-0644(87)80520-6. [DOI] [PubMed] [Google Scholar]
- 17.Hendey GW, Dery RA, Barnes RL, Snowden B, Mentler P. A prospective, randomized, trial of phenobarbital versus benzodiazepines for acute alcohol withdrawal. Am J Emerg Med. May. 2011;29(4):382–5. doi: 10.1016/j.ajem.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Michaelsen IH, Anderson JE, Fink-Jensen A, Allerup P, Ulrichsen J. Phenobarbital versus diazepam for delirium tremens - a retrospective study. Dan Med Bul. 2010;57(8):A4169. [PubMed] [Google Scholar]
- 19.Hayner CE, Wuestefeld NL, Bolton PJ. Phenobarbital treatment in a patient with resistant alcohol withdrawal syndrome. Pharmacotherapy. 2009;29(7):875–8. doi: 10.1592/phco.29.7.875. [DOI] [PubMed] [Google Scholar]
- 20.Maccioli GA. Dexmedetomidine to facilitate drug withdrawal. Anesthesiology. 2003;98(2):575–7. doi: 10.1097/00000542-200302000-00041. [DOI] [PubMed] [Google Scholar]
- 21.Rovasalo A, Tohmo H, Aantaa R, Kettunen E, Palojoki R. Dexmedetomidine as an adjuvant in the treatment of alcohol withdrawal delirium: a case report. Gen Hosp Psychiatry. 2006;28(4):362–3. doi: 10.1016/j.genhosppsych.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Darrouj J, Puri N, Prince E, Lomonaco A, Spevetz A, Gerber DR. Dexmedetomidine infusion as adjunctive therapy to benzodiazepines for acute alcohol withdrawal. Ann Pharmacother. 2008;42(11):1703–5. doi: 10.1345/aph.1K678. [DOI] [PubMed] [Google Scholar]