Abstract
Introduction
Globally, alcohol abuse and dependence are significant contributors to chronic disease and injury and are responsible for nearly 4% of all deaths annually. Acamprosate (Campral), one of only three pharmacological treatments approved for the treatment of alcohol dependence, has shown mixed efficacy in clinical trials in maintaining abstinence of detoxified alcoholics since studies began in the 1980’s. Yielding inconsistent results, these studies have prompted skepticism.
Areas Covered
Herein, the authors review the preclinical studies which have assessed the efficacy of acamprosate in various animal models of alcohol dependence and discuss the disparate findings from the major clinical trials. Moreover, the authors discuss the major limitations of these preclinical and clinical studies and offer explanations for the often contradictory findings. The article also looks at the importance of the calcium moiety that accompanies the salt form of acamprosate and its relevance to its activity.
Expert opinion
The recent discovery that large doses of calcium largely duplicate the effects of acamprosate in animal models has introduced a serious challenge to the widely-held functional association between this drug and the glutamate neurotransmission system. Future research on acamprosate or newer pharmacotherapeutics should consider assessing plasma and/or brain levels of calcium as a correlate or mediating factor in anti-relapse efficacy. Furthermore, preclinical research on acamprosate has thus far lacked animal models of chemical dependence on alcohol, and the testing of rodents with histories of alcohol intoxication and withdrawal is suggested.
Keywords: acamprosate, alcoholism, craving, relapse, glutamate, calcium
1. Introduction
Harmful use of alcohol is a significant health burden in most parts of the world, due to its intoxicating effects and capability to create a state of dependence, contributing to approximately 2.5 million deaths per year [1]. Alcohol abuse commonly results in harm not only to the drinker but also to family, coworkers and others in society [2]. Alcoholism, a disease present in an estimated 2.3% of the worldwide population, is characterized by the persistence of compulsive alcohol consumption despite serious adverse consequences [1]. In addition, intermittent and destructive patterns of excessive and compulsive drinking often proceed from strong feelings of craving [3]. An important trigger of craving in alcoholics is exposure to environmental cues previously conditioned to drinking experiences [4,5]. The means by which cue-triggered craving induces relapse after extended periods of abstinence is a matter of continuing debate [6,7], but ongoing research of treatment strategies have focused on developing methods for controlling craving and extending the duration of abstinence or number of drinking-free days [8,9].
The current selection of clinically available pharmacotherapeutic treatments for alcohol use disorders is limited. Disulfiram is an alcohol deterrent that inhibits the activity of acetaldehyde dehydrogenase resulting accumulation of acetaldehyde that produces nausea, tachycardia, and vomiting in the presence of alcohol t [10]. The opiate receptor antagonist naltrexone (ReVia) counteracts some of the mood-enhancing and pleasurable aspects of drinking, reducing the reinforcing qualities of alcohol[11]. However, both disulfiram and naltrexone treatment suffer from poor rates of patient compliance, although a recent development of a once-monthly, extended-release injectable version of naltrexone (Vivitrol) is expected to improve this metric [12]. Acamprosate (Campral) targets the craving feelings that arise in recently detoxified alcoholics, aiding the patient’s efforts to remain in abstinence [13,14]. Unlike naltrexone, the identities of primary sites of action of acamprosate have been under considerable debate since its discovery, with the predominant theories centering around GABA, taurine, opioid and glutamate systems.
This article is an overview of key preclinical studies and clinical trials in the development of acamprosate as a treatment of alcohol relapse. Acamprosate was first introduced to the scientific community in reports of positive clinical trials and a report that it reduced voluntary alcohol drinking in laboratory rats [15]. The primary directives of subsequent preclinical research have therefore been to understand the neurobiological mechanisms underlying the therapeutic effects of the drug, and to construct valid animal models of alcohol craving and relapse, the addictive behaviors targeted by this treatment. Many clinical studies have followed up the promising initial data with varying degrees of success, and have led to the testing of acamprosate/naltrexone combination therapies to improve patient outcomes [16–20].
2. Discovery and preclinical development
Acamprosate (calcium-bis-acetyl-homotaurinate) is one of a small collection of first-in-class drugs that were discovered by the process of modification of synthesized versions of natural compounds [30]. It is the calcium salt of N-acetyl-homotaurine, a synthetic analog of the endogenous amino acid taurine, but with extra carboxyl and methyl groups in its chain [31]. It bears a chemical resemblance to the neurotransmitter gamma-aminobutyric acid (GABA) and was therefore initially believed to exert its drug actions via GABAergic mechanisms [32]. However, N-acetyl-homotaurine also has similarities to several other amino acids, including glutamate, aspartate and glycine, and its actions therefore have been associated with a multitude of neurotransmitter systems [33].
2.1. Pharmacokinetics
Acamprosate has poor bioavailability from the gastrointestinal tract in humans (11%) and rodents (20%), and as a result relatively large doses are needed. In the U.S., acamprosate is marketed as Campral in 333 mg tablets of acamprosate calcium, equivalent to 300 mg acamprosate [34,35]. It is absorbed through the paracellular pathway, being transported through the intercellular space in gastrointestinal epithelia [36]. Acamprosate is excreted unmetabolized after glomerular filtration [37]. The absorption of acamprosate is also slower than its elimination, becoming the limiting factor of clearance and following a flip-flop pharmacokinetic model [38]. The presence of the drug in the body is therefore strongly dependent on route of administration, where oral and intravenous dosing result in half-lives of 13.0 and 3.2 hours, respectively [37]. This is in contrast to naltrexone, whose more rapid absorption allowed the formulation and eventual marketing of an extended release version. Importantly, acamprosate does not pharmacokinetically interact with other drugs commonly prescribed to abstinent alcoholics such as imipramine, desipramine, disulfiram, diazepam, nordiazepam or naltrexone, and it does not appear to be contraindicated with any other medications metabolized by the liver [39,40]. Additionally, the absorption and disposition of acamprosate is not affected by patient gender, hepatic function or alcoholism [40], and apparently does not interfere with the absorption of alcohol in patients [37]. However, due to its primary excretion via the kidneys, acamprosate is counterindicated in patients with renal insufficiency.
In addition to a slightly higher bioavailability in rodents, acamprosate also has an elimination half-life of only 30 minutes in rodent species [41]. This dramatic difference from humans has imposed complications in the design of animal models that attempt to mimic the schedule of chronic treatments with multiple within-day dosing, as well as patterns of alcohol abstinence and relapse.
2.2. Initial behavioral findings
After its discovery by chemical screening procedures, acamprosate was tested at the University of Rouen in France in groups of alcohol-drinking rats [15]. Long-Evans rats were subjected to 14 days of forced alcohol consumption via replacement of the drinking water with a solution of 12% (w/v) ethanol. Rats were then given a choice to consume either water or 12% ethanol, using a version of the two-bottle choice procedure (Figure 2). Pretreatment of 100–200 mg/kg calcium acamprosate (Ca-AOTA) resulted in decreased voluntary drinking of the ethanol solution, but a similar preparation of sodium acamprosate (Na-AOTA) had no effects. The effect of Ca-AOTA also appeared to be specific to ethanol consumption, as it exerted no effect on total fluid intake, or on the animals’ preference for a saccharin solution (evaluated in the same subset of ethanol-drinking rats). Later investigations revealed no substantial differences in the bioavailability of Ca-AOTA and Na-AOTA, showing that the different behavioral effects of these two formulations were not the result of pharmacokinetic factors [42].
Figure 2. Elements of the two-bottle choice paradigm.

(A) In order to induce a preference for alcohol or overt withdrawal symptoms, several early experiments included an initiation phase where rats were housed for approximately one week with 10 – 20% (w/v) alcohol (EtOH) as the sole drinking solution. Due to complications in maintaining normal body mass in the subjects, this practice is less common in current experiments. (B) In the standard two-bottle choice test, rats are housed with two drinking bottles, where one contains ordinary water and the other contains 10 – 20% (w/v) alcohol dissolved in tap water. The presentation of the solutions is alternated between the left and right bottles on a regular basis to avoid the development of side-bias. (C) Several experiments introduce alternative alcohol solutions with additional drinking bottles to measure the evolution of the rat’s preference for alcohol.
The initial result was confirmed by an experiment with outbred Wistar rats with a brief history of forced alcohol intoxication [45]. . Rats subjected to intoxication consumed more alcohol than previously alcohol-naïve rats over the 9-day testing period, and their consumption was significantly reduced following daily treatment of 200 – 450 mg/kg Ca-AOTA (also administered intragastrically). In contrast, intragastric saline had no effect on alcohol consumption, and only 450 mg/kg Ca-AOTA reduced alcohol intake in the alcohol-naïve rats. The high (450 mg/kg) dose of Ca-AOTA also resulted in a temporary reduction of water intake in all rats. The results suggested that calcium acamprosate was effective in reducing alcohol drinking in animals with a history of chronic intoxication, but the nature of the testing procedure, particularly the denial of drinking water prior to and during the test of alcohol consumption, makes these conclusions difficult to register with subsequent studies.
Another early experiment reported the capability of acamprosate to reduce alcohol consumption in rats with prior exposure to intoxication, in this case by housing the animals in sealed air-tight cages and replacing the air inside with a controlled alcohol/air mixture [46]. Acamprosate (200 to 400 mg/kg daily) was supplied to the rats throughout the intoxication period by dissolving the drug in the drinking water, and was reported to decrease consequent alcohol drinking during the subsequent two-bottle choice test. However, it is unclear what (if any) statistical analysis was used to process the behavioral data.
Taken together, these initial reports demonstrated a potential use for animal models in evaluating the potential efficacy of acamprosate in reducing alcohol consumption. However, the use of prolonged periods of water restriction in all of these studies suggests that the motivation to consume alcohol was difficult to measure without substantial manipulation. In all three cases, acamprosate may have been interacting with the complex effects of thirst on the animals’ behavior [47], in addition to altering their preference for the alcohol containing solution.
2.3. Effects on increased drinking following abstinence
In order to provide a model that more closely resembles the experience of alcohol relapse, several research laboratories have developed a procedure where rodents are first provided with alcohol as an optional drinking solution in the home cage over a span of several months, followed by removal of the alcohol containing solution (but never restricted from water) for at least two weeks, and then the alcohol solution is re-introduced and animals are tested for alcohol preference versus water in a two-bottle choice procedure [48]. Animals with an extended history of alcohol availability have been shown to exhibit a transient increase in their consumption of alcohol during this test, drinking at rates significantly above the levels they exhibited prior to the period of forced abstinence. This phenomenon, termed the alcohol deprivation effect (ADE), has been demonstrated in studies involving mice, rats, monkeys and humans [49–52]. Clinical descriptions of alcoholics who relapse from abstinence with bouts of heavy drinking impart a degree of face validity for the ADE model [53]. However, the magnitude of the ADE in alcohol-drinking animals has been found to be subject to a variety of factors, including strain, age, duration of abstinence and testing environment [54].
The interpretation of the ADE as evidence of alcohol “craving” in animals is also not universally accepted. The amount of alcohol consumed following deprivation has not been proven to correspond to the degree of craving [55], and is affected by the pharmacological effects of alcohol during the test. Even though a valid model of relapse in alcoholics would arguably include drinking following deprivation, the presence of intoxication obfuscates the ADE measurement as an alcohol-motivated versus alcohol-driven quantity. Nonetheless, one of the critical clinical therapeutic effects of acamprosate and naltrexone has been identified as reduced drinking following intervention, and the ADE model has been a valuable means by which relapse-like behavior following a theoretical intervention (deprivation) could be investigated.
The ADE was first used to evaluate the effects of acamprosate treatment on relapse-like drinking [56]. After three days of withdrawal from alcohol, the rats demonstrated an ADE as well as attenuation of drinking as a result of acamprosate pretreatment. The effective doses of acamprosate ranged between 100 and 200 mg/kg, injected intraperitoneally (i.p.) twice during the first 48 hours of the three-day ADE test. Unlike previous experiments, alcohol preference was established without water restriction or significant selection of the subject population. Instead, the long-term availability of alcohol in a drinking bottle gradually induced an incentive value that was observable as an ADE, even when a small amount of quinine was introduced to the alcohol bottle or when saccharin was dissolved in the water bottle during the test sessions. However, the means by which acamprosate reduced drinking remained unclear; the treatment effect could have been an indication for attenuated motivation for alcohol or an increased sensitivity to its intoxicating effects.
In general agreement with some of the initial preclinical findings, another study showed that ADE-enhanced drinking was more sensitive to acamprosate treatment than drinking levels prior to forced abstinence [57]. In this experiment, rats were trained to self-administer alcohol during daily sessions in operant conditioning chambers (Figure 4). During the initial phase of training, responses on one lever resulted in delivery of saccharin dissolved in water and responses on the other lever resulted in delivery of water only. After acquisition of responding for saccharin, the saccharin concentration was gradually reduced in subsequent sessions and replaced by 10% (w/v) ethanol. Following establishment of a baseline responding level for alcohol, rats were injected with 0-200 mg/kg acamprosate and tested for operant responding daily for five days. No effects on baseline responding were observed, but a matching regimen of acamprosate injections during the subsequent 5-day withdrawal period resulted in reduced responding for alcohol, attenuating or abolishing the ADE in a dose-dependent fashion. The effect of acamprosate on the ADE was therefore extended from 24-hour tests in two-bottle choice procedures to more limited daily (30 min) sessions where the timing and context of drinking behavior could be more tightly manipulated. However, the interpretation of changes in responding on the levers, even when reinforced by alcohol, does not exactly correspond to the previous observations of reduced consumption in the two-bottle choice model, which more directly models alcohol drinking in humans.
Figure 4. Self-administration apparatus with cues and controlled delivery of alcohol reinforcers.

In a typical operant conditioning chamber for the self-administration training, the front panel is equipped with two retractable levers (A, B). One lever is determined to be the active lever, where responding is reinforced with delivery of alcohol, and the other lever usually results in no programmed consequences. Above each lever is a stimulus light (C, D) that temporarily illuminates upon the successful schedule completion of the operant task, usually a single response on the active lever, producing a conditioned stimulus (CS+). The active and inactive levers may be alternated between sessions or groups of subjects. Alcohol is delivered in from a computer-controlled syringe pump into a receptacle installed in the rear wall (E). Sessions where alcohol reinforcement is available may be signaled by olfactory stimuli (S+) provided by drops baking extract in the bedding below the floor bars (F). An alternative odor would be used in sessions where schedule completions result in delivery of water or no reinforcement (S−). The chamber is also equipped with a tone generator that can be activated to signal a successful schedule completion in these sessions (CS−). Chambers are frequently equipped with a house light (G) and white noise generator (H) to provide additional means to generate discriminative cues (S+/CS+ and S−/CS−) during training and testing.
The influence of timing on the effectiveness of acamprosate treatment was investigated in an experiment utilizing operant training [58]. Rats were subjected to five months of continuous access to water and alcohol drinking bottles (5%, 10% and 20% v/v) in the home cage, and then moved into operant chambers for weekly 23-hour training sessions. Responding on one lever resulted in delivery of 20% alcohol and responding on the other lever resulted in delivery of water. After establishment of operant responding, rats were divided into one group where access to alcohol continued in their home cages between tests, and another group where only water was available at these times. Prior to subsequent operant sessions, the rats were treated with acamprosate (0 – 400 mg/kg, i.p.), resulting in a dose-dependent decrease in responding for alcohol, regardless of access to alcohol between test days. However, acamprosate was more potent at reducing behavior during the first hour of the test session in rats with no home-cage access to alcohol, suggesting that the influence of alcohol deprivation was apparent in the timing of the treatment effects.
Another operant self-administration experiment utilized a different training procedure to separate the efforts of obtaining alcohol reinforcement (i.e. appetitive behavior) from the consumption process of drinking [59]. After an initiation step making alcohol the only drinking solution in home cage for 3 days, rats were trained in daily sessions to press a lever 30 times in order to gain access to a sipper tube containing alcohol for 20 minutes. Half of the rats were trained to work for access to alcohol, and the other half were trained to work for a sucrose solution. Once stable responding was attained, rats were given 0 – 200 mg/kg acamprosate (i.p.) prior to weekly test sessions. This treatment did not affect the responding in either group, but reduced drinking in the alcohol group at all doses (50 – 200 mg/kg). Despite the small size of the experimental groups, this finding demonstrated that the effect of acamprosate on drinking was distinct from the level of motivation to initiate a drinking session.
2.4. Identification of neurotransmitter targets of action
While the behavioral effects of acamprosate were being established, many attempts were made at identifying its mechanisms of action. The initial study reported that the attenuation of drinking by acamprosate was partially reversed by bicuculline, an antagonist of GABAA receptors [15]. Given its structural similarity to GABA, acamprosate was hypothesized to exert its effects on the receptors for this neurotransmitter [15]. Other early suggested sites of action included opioid receptors, because of their role in addictive behaviors [45], as well as the binding sites of the endogenous amino acid neuromodulator taurine [60].
Several experiments utilized microdialysis to examine the neurochemical outcomes of systemic acamprosate administration in intact, behaving animals. One report found that the acamprosate pretreatment interfered with the effect of injected alcohol on release of dopamine in the nucleus accumbens [61]. However, the largest accumulation of behavioral and analytical reports has most consistently suggested that acamprosate exerts its effects via the glutamate neurotransmitter system. Untreated withdrawal from moderate alcohol intoxication results in elevated extracellular concentrations of glutamate throughout the brain [62], and this state has been hypothesized to be a critical physiological driver of alcohol consumption in dependent individuals [63]. Rats simultaneously treated with high amounts of acamprosate (400 mg/kg per day, via the drinking water) during alcohol vapor inhalation did not develop this hyperglutamatergic condition during subsequent withdrawal [64]. A smaller dose (200 mg/kg per day, i.p. during withdrawal) was required to attenuated withdrawal-induced hyperlocomotion in rats that had been drinking 20% (w/v) alcohol as their sole drinking solution for one week [65]. Per2 knockout mice, a strain characterized by a hyperactive glutamatergic system, were treated with acamprosate and subsequently exhibited wild-type levels of extracellular glutamate as well as an attenuated responding for alcohol [66].
Alcohol is known to inhibit the activity of NMDA receptors, resulting in well-known effects such as compromised cognitive acuity, impaired synaptic plasticity, and loss of memory. Experiments in cell cultures and harvested brain tissue led some investigators to identify NMDA receptors as a primary target for acamprosate [67–70]. Additionally, local applications of acamprosate to neurons appeared to increase the NMDA component of excitatory neurotransmission responses, as recorded by electrophysiological procedures [71]. Further current-clamp and voltage-clamp experiments determined that acamprosate simultaneously interacts with both NMDA and GABAA receptors to exert its neurochemical effects [72]. Other in vitro studies reported that metabotropic glutamate receptor 5 (mGluR5) was also a binding site for acamprosate [73,74]. Predominantly located on the postsynaptic neuron of glutamate synapses, mGluR5 has been associated with a variety of alcohol-related neurochemical effects [75]. Metabotropic glutamate receptors have a modulatory role on excitatory neurotransmission, plausibly lending acamprosate the means to act primarily against the hyperglutamatergic state of alcohol withdrawal and leaving physiologically normal glutamatergic states largely unaffected [33,76,77]. Similar dose-dependent reductions of alcohol related behaviors have been reported in mice treated with acamprosate and ligands of mGluR5 [78]. However, an investigation of the effects of acamprosate treatment on the expression of mGluR5 in Xenopus oocytes revealed no evidence of direct interactions [79], and to our knowledge, such effects have never been firmly established.
2.5. Effects on reinstatement of alcohol-seeking operant behavior
Acamprosate has also been investigated in rodents using a version of the extinction/reinstatement model for alcohol [80]. Rats were trained to self-administer an alcohol solution daily sessions in the presence of a combination of olfactory, visual and/or auditory cues (Figure 5). One set of cues (S+/CS+) was conditioned to alcohol reinforcement and another set of cues (S−/CS−) was conditioned to water reinforcement [81]. After a preference for alcohol versus water was established, the rats were subjected to daily sessions of extinction training inside the same operant chambers, where the levers were presented without cues and responding on either lever resulted in no reinforcement delivery. This training eventually extinguished responding to a low, nonspecific and stable baseline. The rats were then tested for the reinstatement of unreinforced operant behavior by reintroducing them to the chambers in the presence of S+/CS+ and S−/CS− cues in separate sessions. Their responding on the active lever in the presence of S+/CS+ cues was therefore labeled alcohol-seeking behavior, an analog of the craving phenomenon that precedes drinking in relapsing alcoholics.
Figure 5. Schematic of the extinction/reinstatement model using discriminative cues.
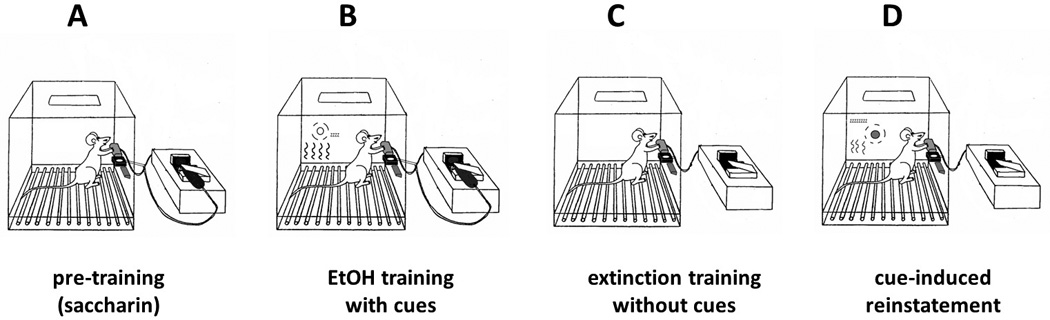
(A) Self-administration training is initiated by a multi-week procedure where the rat is trained to respond on the active lever for delivery of a sweetened solution (sucrose or saccharin), which is gradually replaced by alcohol (8-10%, w/v) dissolved in tap water. (B) Alcohol self-administration training continues in the same operant chamber, but two reinforcement contingencies are used in separate daily sessions. In the alcohol reinforcement condition, signaled by the combination of ambient and lever-contingent cues (S+/CS+), responses on the active lever are reinforced with delivery of the alcohol solution. In the control condition signaled by a distinct set of cues (S−/CS−), responses on the active lever result in delivery of water or no programmed consequences. (C) Following the establishment of stable self-administration behavior, rats undergo extinction training in the same chambers, where responding on either lever results in no delivery of reinforcement or presentation of cues. Extinction training continues in daily sessions until rats no longer respond on either lever above a low (20% of baseline self-administration levels) criterion. (D) In test sessions for reinstatement of alcohol-seeking behavior elicited by S+ cues, active lever responses result in presentation of the lever-contingent cues (CS+), but not alcohol reinforcement. These tests alternate with S−/CS− sessions to establish the specificity of reinstated operant behavior.
Prior to the reinstatement tests, a dose of acamprosate (0, 100 or 200 mg/kg, i.p.) was administered to evaluate the capability of this drug to attenuate cue-triggered alcohol responding [80]. The 200 mg/kg dose of acamprosate significantly reduced responding in the S+/CS+ condition, but had no effect on the level of responding in the S−/CS− condition. While this implicated the effect of treatment as specific to alcohol, responding during the S−/CS− tests did not elevate above extinction baseline levels, meaning that there was no reinstatement of water-seeking behavior available as a control.
The extinction/reinstatement model described above [80] holds important advantages and disadvantages compared to the ADE model used earlier [56]. The inclusion of S−/CS− tests allow for relatively frequent S+/CS+ tests without the possibility of spontaneous recovery (i.e., non-goal oriented responding on the levers) clouding the interpretation of results. In contrast, repeated measurements of ADE must bracket a sufficient span of alcohol withdrawal, often resulting in once-monthly tests. Alcohol consumption is also limited by feelings of intoxication and satiation in animals, and measurements of acamprosate treatment on drinking in the ADE must account for these factors. Testing of alcohol-motivated behavior in the presence of cues conditioned to alcohol allows the analysis of behavior under the influence of the treatment only, and fits with the strategies for investigating craving favored by researchers of many other drugs of abuse [82,83]. However, reinstatement in the presence of cues is not considered as valid a model of alcohol relapse as the ADE model, since the clinical definition of relapse includes resumed consumption of alcohol [48].
Conditioned effects resulting from long-term alcohol experience have been associated by multiple studies with glutamate neurotransmission [75,84]. Cue-triggered reinstatement of alcohol seeking has been shown to be sensitive to interference by various mGluR ligands [85,86]. Transient increases in glutamate and taurine concentrations were measured in the basolateral amygdala following presentation of an olfactory cue previously paired with injected alcohol [87]. Acamprosate was shown to reduce the expression of alcohol-conditioned performance in a maze task [88]. The interaction of acamprosate with glutamate neurotransmission was therefore thought to extend beyond reversing the physiological symptoms of acute withdrawal and into relevant behaviors important for long-term prevention of relapse [84].
3. Clinical development
The primary clinical verification of acamprosate as a viable treatment for alcohol dependence was performed in France and published in 1985 [13]. After three months of treatment, a greater proportion of patients given acamprosate (20 out of 33) remained in abstinence than the proportion of patients treated with placebo (12 out of 37). This effect was later corroborated by a large-scale, multicenter study involving over 560 alcohol patients after detoxification [89,90]. Acamprosate treatment resulted in a lower average level of plasma gamma-glutamyl transferase (GGT), a physiological biomarker of recent alcohol consumption, than placebo treatment over a test period of three months.
3.1. Successful clinical studies in Europe and FDA approval
The randomized placebo-controlled clinical studies of acamprosate utilized some common measures of treatment success [19]. Percent complete abstinence is the proportion of subjects who remained completely free of alcohol from randomization to the end of the study. Despite its simple binary nature of success versus failure, the method by which abstinence was measured has varied between studies. Continuous abstinence duration (CAD), also known as the time to first drink, was the number of days during or after treatment that a subject completely abstained from alcohol. The cumulative abstinence proportion measured the number of days of complete abstinence as a percentage of total, and days in treatment, and provided a more realistic assessment of subject’s activity during the study. Utilizing a combination of these measurements, acamprosate treatment outperformed placebo in clinical studies of over 500 patients treated for 12 months [91], over 450 patients treated for 12 months [92], 300 patients treated for 6 months [93], 300 patients treated for 12 months [94], 270 patients treated for 48 weeks and evaluated for 2 years [95], 250 patients treated for 6 months and evaluated for 1 year [96], 240 patients treated for 6 months and evaluated for 1 year [97], and in 120 patients treated for 3 months [98]. However, acamprosate failed to outperform placebo treatment in similar, but smaller studies [99,100]. The negative results found in Korea also involved a treatment period of two months, shorter than the other studies described above [100]. Another large study (over 580 subjects, treated for 6 months) in the United Kingdom failed to find a difference between the treatment effects of acamprosate and placebo [101], but intervention commenced 25 days after recovery from physical withdrawal symptoms, a substantially large latency compared to the other studies [19]. A meta-analysis of these studies and others [18,102–105] concluded a significant benefit for acamprosate treatment as assessed 6 months after the start of treatment [106]. A more recent analysis of the clinical data, where the effect of treatment in male and female subjects were analyzed separately, confirmed this conclusion and reported no sex differences in the beneficial effects of acamprosate [107].
A recent Cochrane meta-analysis of the various clinical studies conducted in Europe plus two in the United States evaluated the use of acamprosate versus placebo in the treatment of alcohol dependence [108]. The review found that, when combined with psychosocial treatment, the use of acamprosate reduced the risk of relapsing after detoxification by 86%, compared to placebo treatment, and increased the CAD by 11%. The margins of difference in these outcomes between acamprosate and placebo treatment regimens were narrow but statistically significant, and sufficient to encourage the use of acamprosate for treating alcoholism. Data from three European studies which had been instrumental in the U.S. FDA approval of acamprosate were reevaluated with more stringent standards for abstinence outcomes, reaching the conclusion that acamprosate treatment outperformed placebo [109].
One smaller clinical experiment (40 alcohol-dependent subjects) explored the effects of administering acamprosate treatment during the detoxification process [110]. While preclinical results have demonstrated that acamprosate alleviated glutamate dysregulation during acute withdrawal in rodents [64], no improvement in treatment outcomes were seen in patients receiving acamprosate during withdrawal. Moreover, patients who had been given acamprosate during detoxification drank more alcohol in the subsequent 10-week observation period than those who had been administered placebo. This result was not anticipated by the prevailing theories of acamprosate action on glutamate receptors at the time, and illustrated, along with a large British study [101], how the outcome of acamprosate treatment depends on the timing of intervention.
3.2. Failure to outperform naltrexone or placebo in multicenter clinical trials
Despite the promise shown by the initial European clinical studies and subsequent FDA approval in the U.S., the efficacy of acamprosate as a long-term strategy for the prevention of relapse has fallen under scrutiny [14]. An Australian multicenter study compared 12-week treatment regimens of acamprosate, naltrexone and placebo in detoxified alcoholic subjects, finding modest benefits for naltrexone but not acamprosate treatment [111]. Another clinical trial combined acamprosate and placebo treatment with counseling in a family setting, revealing benefits (measuring cumulative abstinence proportion) for the counseling but no differences between the pharmacological interventions [112]. A limited study of abstinent alcoholics also diagnosed with bipolar disorder also failed to reveal statistically significant effects of acamprosate treatment on drinking outcomes [113].
In an effort to improve on the modest benefits associated with acamprosate and naltrexone treatment against alcohol relapse, several investigators explored the results of combining the two treatments in alcoholic patients. Co-administration of acamprosate and naltrexone was shown to significantly raise peak plasma concentrations of acamprosate by 33% as well as reduce the time needed to reach peak plasma levels (33%), without affecting the pharmacokinetic properties of naltrexone [114]. By targeting (as was understood at the time) different primary neurochemical targets and therefore distinct aspects of alcohol craving and consumption behaviors, the favorable effect on acamprosate absorption by co-administered naltrexone motivated a large-scale investigation of a combined treatment regimen.
The initial study of a naltrexone/acamprosate combined treatment recruited 160 alcoholic patients into a double-blind, placebo controlled trial where naltrexone and acamprosate were tested separately and in combination [18]. Both drugs, and their combination, demonstrated positive treatment effects relative to placebo throughout the 12-week testing period, and the naltrexone/acamprosate combination was more effective than acamprosate alone (but not significantly better than naltrexone alone). Another clinical study reported similar results, where the naltrexone/acamprosate combination improved the outcomes of cognitive behavioral therapy, beyond the effects of either naltrexone or acamprosate alone [115].
The National Institute on Alcohol Abuse and Alcoholism Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) trial tested naltrexone, acamprosate and placebo treatments on 1383 alcoholic patients in multiple centers throughout the United States [16]. Naltrexone and acamprosate treatments were combined with each other and with one of two styles of behavioral therapy (brief sessions of medical management by physicians, with or without longer intensive counseling) during a four-month trial. The primary outcome measures were time to first drink and cumulative abstinence proportion. Surprisingly, all groups of subjects demonstrated improved outcomes, but acamprosate treatment failed to perform significantly better than placebo, alone or with naltrexone. Naltrexone treatment resulted in significant improvement in the time to first drink outcome, but not cumulative abstinence proportion. The COMBINE experiment concluded that naltrexone combined with intensive counseling was the recommended treatment against alcohol relapse.
The negative result from the COMBINE trial was particularly surprising given the size of the study and the record of success seen by acamprosate treatment in European trials. One potential key difference was arguably the detoxification procedure that preceded randomization and treatment: COMBINE study subjects were given four days for detoxification in an outpatient program, whereas European subject required seven or more days in an inpatient facility. This could have had a critical difference in the efficacy of acamprosate treatment, which was shown to underperform against placebo when initiated in patients before full detoxification was completed in a designed study [110] as well as a recent meta-analysis [116].
The conflicting results between the COMBINE study and earlier trials of acamprosate/naltrexone combination therapy motivated another large-scale clinical trial in Europe. The PREDICT trial was designed to closely resemble the single-treatment groups (acamprosate, naltrexone or placebo only) of the COMBINE study, matching inclusion and exclusion criteria of subjects, treatment doses and counseling techniques (translated from English into German) [117]. The PREDICT trial recruited 426 alcoholic subjects from alcohol treatment centers at German universities and received one of the three treatments with low intensity psychotherapy for 3 months, followed by a 3-month observation period without medication. The PREDICT study found no significant treatment effects for either acamprosate or naltrexone versus placebo for either of the abstinence duration or alcohol consumption outcome variables [118].
Despite the record of success in clinical trials leading up to its FDA approval for alcoholism in 2004, acamprosate treatment has been met with generally disappointing results in recent large-scale studies [16,115,118]. Meta-analyses incorporating the ongoing history of clinical experiments have continued to support the use of acamprosate [119], but the inability of clinical researchers to identify the ideal candidates for acamprosate reflects a general uncertainty about the nature of its neurobiological effects [120,121]. The molecular targets of acamprosate also continue to be an area of evolving debate [122]. However, acamprosate is generally regarded as well-tolerated and safe [14], permitting future clinical testing of combination treatments with antidepressants and other drugs targeting disorders comorbid with alcohol abuse [123].
4. Post-launch
By far the most important recent development in the preclinical investigation of acamprosate is the publication of a series of experiments that compellingly identified calcium as its key active moiety, rather than N-acetyl-homotaurine [42]. This study was inspired by analysis provided in the U.S. Patent for N-acetyl-homotaurine, which recommend its calcium salt for systemic pharmacological uses and its sodium salt for local applications [31]. As noted earlier, Ca-AOTA was shown to attenuate ADE in rats in a duplication of an earlier experiment [56], but the sodium salt Na-AOTA failed to have any effect. Furthermore, an equimolar treatment of calcium (administered as calcium chloride) attenuated ADE to the same degree as Ca-AOTA, implying that calcium was the critical component of acamprosate in this keystone behavioral effect. Ca-AOTA but not Na-AOTA was also shown to extinguish cue-triggered reinstatement of alcohol seeking in a replication of the extinction/reinstatement experiment described earlier [80]. Once again, an equimolar treatment of calcium chloride also attenuated alcohol seeking, identifying calcium as the critical chemical factor in the anti-craving capabilities of acamprosate. These results were further reinforced by a blinded treatment on Ca-AOTA, Na-AOTA and calcium chloride in another laboratory. Rats from an alcohol-preferring strain were trained to self-administer alcohol in a higher schedule of reinforcement (fixed ratio 3), and their responding was attenuated following Ca-AOTA treatment, replicating an earlier experiment [124]. However, calcium chloride but not Na-AOTA also produced a reduction of alcohol self-administration in these animals.
In an analysis of biological samples collected in a German clinical trial [18], Spanagel and colleagues found a correlation between the plasma concentration of calcium in acamprosate-treated alcoholics and the number of days before their first serious relapse episode [42]. These results point to a possible benefit for making calcium supplements available to alcoholic patients currently in behavioral or pharmacotherapy treatment. Interestingly, in the 1950s a Milwaukee physician developed a strategy of treating alcoholics with supplements of intravenous calcium to alleviate acute physical withdrawal symptoms [125]. Termed intensive calcium therapy, this intervention resulted in significantly fewer days of hospitalization and patients were better prepared for psychological therapy [126]. However, this research objective was discontinued for unreported reasons and has remained obscure for several decades. The potential benefits of intensive calcium therapy in combating feelings of craving and relapse beyond the initial stages of alcohol withdrawal have largely been uninvestigated.
5. Summary
After a large number of preclinical studies, the primary mechanisms underlying the attenuation of alcohol-motivated behaviors by acamprosate treatment remain disputed. Calcium acamprosate effectively reduces voluntary drinking, as well as operant responding in the presence of alcohol-paired cues, in trained rodents subjected to several days of abstinence from alcohol. These effects have been countered by manipulations of glutamate receptors, leading to a consensus that acamprosate exerts its effects through NMDA, mGluR or other components of the glutamate neurotransmission system. However, this evolving consensus has been challenged by the recent discovery that many of the behavioral effects of acamprosate can be duplicated by treatment of calcium alone. If these recent findings are confirmed, and strengthen the notion that the only biologically active component of acamprosate treatment has been the calcium required for practical administration, an intensive reevaluation of the techniques used to develop this drug must be considered. Additionally, the body of preclinical acamprosate research suffers from the notable lack of experiments investigating the effects of gender, age and chemical dependence on treatment efficacy.
The initial clinical reports of acamprosate treatment against alcohol relapse were positive and generally consistent throughout a variety of European medical centers, eventually resulting in approval by the FDA for clinical use. However, questions remained as to the drug’s relative efficacy against naltrexone and placebo, especially when the pharmaceutical therapies were combined with medical management or counseling sessions. The large-scale American COMBINE trial found that acamprosate was not significantly beneficial against relapse or post-treatment consumption, alone or combined with naltrexone. The PREDICT trial, designed to replicated the conditions of the COMBINE trial in Europe, also concluded that acamprosate was not more effective than placebo treatment, when combined with medical management. Interestingly, plasma samples retained from the PREDICT trial demonstrated a correlation between calcium concentration and the ability of acamprosate-treated subjects to remain abstinent. This finding supported the notion that the active component of acamprosate treatment had been widely misunderstood, but also suggested a novel path for improving this therapeutic.
6. Expert Opinion
While their experiments need to be verified and expanded with other animal models, the seminal work of Spanagel and colleagues has introduced credible doubt toward the existence of a direct neurotransmitter target for acamprosate [42]. These investigators performed calcium flux assays and electrophysiological experiments to demonstrate that Ca-AOTA produced neurologically significant increases in calcium concentrations, and the Ca-AOTA did not counteract effects of mGluR1/5 ligands on neuronal firing patterns, supporting their overall conclusions that acamprosate is less of a glutamatergic drug than a calcium supplement [42]. While acamprosate has been shown to counteract elevated glutamate levels in withdrawn alcoholics [127], the importance of this interaction toward lasting abstinence, already brought into question by the unsupportive results from recent large-scale clinical trials [121], has now been further marginalized by the possibility that homotaurine is not a biologically active compound [42,120]. Thus, future studies on acamprosate or novel pharmacotherapeutics for alcoholism should potentially consider alterations in plasma and/or brain levels of calcium as a possible correlate or mechanism of efficacy.
Acamprosate has been shown in preclinical experiments to exert greater potency in reducing consumption in animals that had previously experienced withdrawal or forced abstinence [57,58]. Acamprosate also has a well-known effect in alleviating the hyperglutamatergic symptoms of alcohol withdrawal, indicating a role for this treatment in the advanced stage of alcohol dependence [64,65]. Naltrexone has also been shown to extinguish S+/CS+ cue-elicited reinstatement in rats trained in the extinction/reinstatement procedure [128]. However, this effect appeared to weaken and disappear in rats subjected to multiple episodes of alcohol vapor intoxication and withdrawal [129]. The inclusion of repeated intervals of intoxication and withdrawal is arguably an important component in improving the design of preclinical experiments to more closely resemble the life experiences of addicted individuals [86, 130]. If acamprosate was shown to be equally, if not more, effective in attenuating cue-triggered reinstatement in animals with histories of alcohol intoxication versus those without such a history, the complementary roles could be exploited into a single combination treatment whose anti-craving potency is robust across different degrees of dependency.
Statistical evaluation of the clinical measurements in the COMBINE and PREDICT study included significant contributions of the so-called “placebo effect,” wherein all treated groups of subjects demonstrated improvements in primary outcomes [16,118]. The apparent enhancement of the placebo effect by intensive counseling sessions in these trials supports the importance of treating compulsive alcohol drinking by interpersonal exchanges and encouraging comprehensive lifestyle changes in the patient, concepts that date to the American Temperance Movement, if not earlier [22,23]. While the underpinnings of placebo effects in clinical trials are unclear, it could be argued that its large presence precluded any statistically conclusive judgment on the relative efficacy of acamprosate, naltrexone and their combination [131,132].
As it is, the greater incidence for successful outcomes in the PREDICT study versus the COMBINE study, despite their very similar designs, indicate the presence of a basic difference between the subject populations sampled in these studies. Acamprosate has in general been more efficacious in clinical trials conducted in Europe, which recruit alcoholic subjects from hospital treatment programs. In contrast, clinical trials in the United States usually depend on public advertisements to recruit self-identified candidates for study, which may result in a population with less severe forms of alcoholism [120]. The severity of alcohol dependence has been shown in multiple studies to be an important factor in subjects’ tendency to experience feelings of craving when exposed to alcohol cues, with heavy drinkers reporting more intense and persistent craving than moderate drinkers or healthy controls [133–135]. The context of recruitment and initiation of treatment therefore appear to be critical factors in the success of both acamprosate and other pharmacotherapies in maintaining abstinence in alcoholics. Future clinical studies that classify subjects into moderately and severely addicted individuals may serve to clarify the limits of efficacy of acamprosate treatment beyond placebo.
Figure 1.

Chemical structures for acamprosate (N-acetyl-homotaurine), taurine, GABA and glutamate.
Figure 3. The alcohol deprivation effect (ADE) paradigm.

(A) In a typical ADE experiment, rats are given access to both an alcohol (10 – 20%, w/v) solution and ordinary water in separate drinking bottles in their home cages over a span of several weeks. (B) After the establishment of regular daily consumption of the alcohol solution (which may only occur in a subset of rats, particularly if they are not bred to be alcohol-preferring), the alcohol solution is replaced with another ordinary water bottle for several (3 to 7 or more) days. (C) The alcohol solution is returned for one or more days of assessment. The side of the alcohol bottle is randomly determined and alternated between days in multiday tests. An increase in alcohol consumption relative to the pre-abstinence baseline is reported as evidence of ADE.
Article Highlights.
Acamprosate was initially discovered to reduce drinking in abstinent alcoholics
Acamprosate was found to reduce abstinence induced increases in drinking and cue-triggered reinstatement of alcohol-seeking behavior in different rodent models
Efforts to establish a primary site of pharmacological action have been inconclusive, but the most evidence indicated important glutamate-mediate effects
Recent clinical trials comparing acamprosate, naltrexone and placebo treatments have yielded mixed or negative results
A recent series of experiments indicates that the active moiety of acamprosate may be the calcium salt used to improve solubility
Acknowledgments
The authors are supported by a National Institutes of Health Public Health Service Grant (AA013852).
List of Abbreviations
- ADE
alcohol deprivation effect
- Ca-AOTA
calcium acamprosate: the calcium salt of N-acetyl-homotaurine
- CAD
continuous abstinence duration
- COMBINE
Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence
- CS+/ CS−
conditioned stimuli signaling availability or non-availability of reinforcement
- FDA
Food and Drug Administration
- GABA
gamma-aminobutyric acid
- GGT
gamma-glutamyl transferase
- i.p.
intraperitoneal
- i.v.
intravenous
- mGluR
metabotropic glutamate receptor
- Na-AOTA
the sodium salt of N-acetyl-homotaurine
- NMDA
N-methyl-D-aspartate receptor
- S+/ S−
discriminative stimuli signaling availability or non-availability of reinforcement
- v/v
volume-to-volume
- w/v
weight-to-volume
Footnotes
Financial and Competing Interests Disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.World Health Organization. Global status report on alcohol and health. Switzerland: Geneva; 2014. [Google Scholar]
- 2.Gmel G, Rehm J. Harmful alcohol use. Alcohol Res Health. 2003;27:52–62. [PMC free article] [PubMed] [Google Scholar]
- 3.O'Connor PG, Gottlieb LD, Kraus ML, et al. Social and clinical features as predictors of outcome in outpatient alcohol withdrawal. J Gen Int Med. 1991;6:312–316. doi: 10.1007/BF02597427. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig AM, Wikler A, Stark LH. The first drink: psychobiological aspects of craving. Arch Gen Psychiatry. 1974;30:539–547. doi: 10.1001/archpsyc.1974.01760100093015. [DOI] [PubMed] [Google Scholar]
- 5.McCusker CG, Brown K. Alcohol-predictive cues enhance tolerance to and precipitate "craving" for alcohol in social drinkers. J Stud Alcohol. 1990;51:494–499. doi: 10.15288/jsa.1990.51.494. [DOI] [PubMed] [Google Scholar]
- 6.McCusker CG, Brown K. Cue-exposure to alcohol-associated stimuli reduces autonomic reactivity, but not craving and anxiety, in dependent drinkers. Alcohol Alcohol. 1995;30:319–327. [PubMed] [Google Scholar]
- 7.Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;(95 Suppl 2):S145–S153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien CP, Childress AR, Ehrman R, et al. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 9.Litten RZ, Egli M, Heilig M, et al. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol. 2012;17:513–527. doi: 10.1111/j.1369-1600.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swift RM. Drug therapy for alcohol dependence. New Eng J Med. 1999;340:1482–1490. doi: 10.1056/NEJM199905133401907. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien CP. Treatment of alcoholism as a chronic disorder. In: Jansson B, Jonvall H, Rydberg U, Terenius L, Vallee BL, editors. Toward a molecular basis of alcohol use and abuse. Switzerland: Birkhauser, Basel; 1994. pp. 349–359. [Google Scholar]
- 12.Johnson BA. Naltrexone long-acting formulation in the treatment of alcohol dependence. Ther Clin Risk Management. 2007;3:741–749. [PMC free article] [PubMed] [Google Scholar]
- 13.Lhuintre JP, Daoust M, Moore ND, et al. Ability of calcium bis acetyl homotaurine, a GABA agonist, to prevent relapse in weaned alcoholics. Lancet. 1985;1(8436):1014–1016. doi: 10.1016/s0140-6736(85)91615-0. [DOI] [PubMed] [Google Scholar]
- 14. Yahn SL, Watterson LR, Olive MF. Safety and efficacy of acamprosate for the treatment of alcohol dependence. Subst Abuse Res Treat. 2013;6:1–12. doi: 10.4137/SART.S9345. * This recent review describes many of the clinical studies that have supported the safety and efficacy of acamprosate treatment leading up to FDA approval for alcohol use disorders.
- 15. Boismare F, Daoust M, Moore N, et al. A homotaurine derivative reduces the voluntary intake of ethanol by rats: are cerebral GABA receptors involved? Pharmacol Biochem Behav. 1984;21:787–789. doi: 10.1016/s0091-3057(84)80020-9. * This paper describes the initial preclinical finding that acamprosate treatment attenuated drinking in rats previously trained to drink alcohol in a two-bottle choice task. The authors hypothesized that the compound may have exerted its behavioral effects via GABA receptors, one of several neurotransmitter systems to be associated with acamprosate action.
- 16. Anton RF, O'Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study. A randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. * This paper describes the results of the COMBINE trial, a large-scale clinical evaluation of acamprosate, naltrexone and their combination against relapse behaviors in alcoholic patients. While naltrexone monotherapy was found to outperform placebo, acamprosate was not shown to be significantly effective alone or in combination with naltrexone.
- 17.Feeney GF, Connor JP. Acamprosate and naltrexone are safe and effective but have low compliance rates for people with alcohol dependence. Evid Based Mental Health. 2005;8:14. doi: 10.1136/ebmh.8.1.14. [DOI] [PubMed] [Google Scholar]
- 18.Kiefer F, Jahn H, Tarnaske T, et al. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60:92–99. doi: 10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- 19.Mann KF. Acamprosate: clinical data. In: Spanagel R, Mann K, editors. Drugs for relapse prevention of alcoholism. Switzerland: Birkhauser, Basel; 2005. pp. 85–93. [Google Scholar]
- 20.Mason BJ. Acamprosate and naltrexone treatment for alcohol dependence: an evidence-based risk-benefits assessment. Eur Neuropsychopharmacol. 2003;13:469–475. doi: 10.1016/j.euroneuro.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov. 2011;10:507–519. doi: 10.1038/nrd3480. [DOI] [PubMed] [Google Scholar]
- 22.Durlach J. Novel derivatives of 3-aminopropanesulfonic acid having a reinforced activity on membrane. United States Patent Office US4355043. 1982 [Google Scholar]
- 23.Andrews PR, Johnston GA. GABA agonists and antagonists. Biochem Pharmacol. 1979;28:2697–2702. doi: 10.1016/0006-2952(79)90549-5. [DOI] [PubMed] [Google Scholar]
- 24.Mann K, Kiefer F, Spanagel R, et al. Acamprosate: recent findings and future research directions. Alcohol Clin Exp Res. 2008;32:1105–1110. doi: 10.1111/j.1530-0277.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 25.Zornoza T, Cano MJ, Polache A, et al. Pharmacology of acamprosate: an overview. CNS Drug Rev. 2003;9:359–374. doi: 10.1111/j.1527-3458.2003.tb00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forest Laboratories. Campral prescribing information. 2004 [Google Scholar]
- 27.Zornoza T, Cano-Cebrian MJ, Nalda-Molina R, et al. Assessment and modulation of acamprosate intestinal absorption: comparative studies using in situ, in vitro (caco-2 cell monolayers) and in vivo models. Eur J Pharmaceut Sci. 2004;22:347–356. doi: 10.1016/j.ejps.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Durbin P, Hulot T, Chabac S. Pharmacodynamics and pharmacokinetics of acamprosate: an overview. In: Soyka M, editor. Acamprosate in relapse prevention of alcoholism. Berlin: Springer; 1996. pp. 47–64. [Google Scholar]
- 29.Zornoza T, Cano-Cebrian MJ, Hipolito L, et al. Evidence of a flip-flop phenomenon in acamprosate pharmacokinetics: an in vivo study in rats. Biopharmaceut Drug Dispos. 2006;27:305–311. doi: 10.1002/bdd.513. [DOI] [PubMed] [Google Scholar]
- 30.Mason BJ. Treatment of alcohol-dependent outpatients with acamprosate: a clinical review. J Clin Psychiatry. 2001;(62 Suppl 20):42–48. [PubMed] [Google Scholar]
- 31.Saivin S, Hulot T, Chabac S, et al. Clinical pharmacokinetics of acamprosate. Clin Pharmacokin. 1998;35:331–345. doi: 10.2165/00003088-199835050-00001. [DOI] [PubMed] [Google Scholar]
- 32.Chabenat C, Chretien P, Daoust M, et al. Physicochemical, pharmacological and pharmacokinetic study of a new GABAergic compound, calcium acetylhomotaurinate. Meth Find Exp Clin Pharmacol. 1988;10:311–317. [PubMed] [Google Scholar]
- 33. Spanagel R, Vengeliene V, Jandeleit B, et al. Acamprosate produces its anti-relapse effects via calcium. Neuropsychopharmacology. 2014;39:783–791. doi: 10.1038/npp.2013.264. ** The series of rigorous experiments described in this paper led to the surprising conclusion that the actions of acamprosate may be attributed to its calcium salt. Preclinical experiments that had been key to the development of the glutamate hypothesis of the actions of acamprosate were duplicated with calcium carbonate, and plasma samples from the PREDICT trial were analyzed to reveal a correlation between calcium levels and number of drink-free days after treatment.
- 34.Khanna JM, Kalant H, Shah G, et al. Comparison of sensitivity and alcohol consumption in four outbred strains of rats. Alcohol. 1990;7:429–434. doi: 10.1016/0741-8329(90)90027-a. [DOI] [PubMed] [Google Scholar]
- 35.Gauvin DV, Moore KR, Holloway FA. Do rat strain differences in ethanol consumption reflect differences in ethanol sensitivity or the preparedness to learn? Alcohol. 1993;10:37–43. doi: 10.1016/0741-8329(93)90051-o. [DOI] [PubMed] [Google Scholar]
- 36.Le Magnen J, Tran G, Durlach J, et al. Dose-dependent suppression of the high alcohol intake of chronically intoxicated rats by Ca-acetyl homotaurinate. Alcohol. 1987;4:97–102. doi: 10.1016/0741-8329(87)90005-x. [DOI] [PubMed] [Google Scholar]
- 37.Gewiss M, Heidbreder C, Opsomer L, et al. Acamprosate and diazepam differentially modulate alcohol-induced behavioural and cortical alterations in rats following chronic inhalation of ethanol vapour. Alcohol Alcohol. 1991;26:129–137. doi: 10.1093/oxfordjournals.alcalc.a045093. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery KC. The effect of the hunger and thirst drives upon exploratory behavior. J Comp Physiol Psych. 1953;46:315–319. doi: 10.1037/h0053497. [DOI] [PubMed] [Google Scholar]
- 39.Spanagel R. How to measure relapse in animals. In: Spanagel R, Mann K, editors. Drugs for relapse prevention of alcoholism. Switzerland: Birkhauser, Basel; 2005. pp. 13–21. [Google Scholar]
- 40.Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- 41.Salimov RM, Salimova NB. The alcohol-deprivation effect in hybrid mice. Drug Alcohol Depend. 1993;32:187–191. doi: 10.1016/0376-8716(93)80012-4. [DOI] [PubMed] [Google Scholar]
- 42.Sinclair AJ. The alcohol-deprivation effect in monkeys. Psychonom Sci. 1971;25:21–22. [Google Scholar]
- 43.Burish TG, Maisto SA, Cooper AM, et al. Effects of voluntary short-term abstinence from alcohol on subsequent drinking patterns of college students. J Stud Alcohol. 1981;42:1013–1020. doi: 10.15288/jsa.1981.42.1013. [DOI] [PubMed] [Google Scholar]
- 44.Larimer ME, Palmer RS, Marlatt GA. Relapse prevention: an overview of Marlatt's cognitive-behavioral model. Alcohol Res & Health. 1999;23:151–160. [PMC free article] [PubMed] [Google Scholar]
- 45.Spanagel R. Recent animal models of alcoholism. Alcohol Res & Health. 2000;24:124–131. [PMC free article] [PubMed] [Google Scholar]
- 46.Markou A, Weiss F, Gold LH, et al. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- 47.Spanagel R, Holter SM, Allingham K, et al. Acamprosate, alcohol: I .Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol. 1996;305:39–44. doi: 10.1016/0014-2999(96)00174-4. [DOI] [PubMed] [Google Scholar]
- 48.Heyser CJ, Schulteis G, Durbin P, et al. Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology. 1998;18:125–133. doi: 10.1016/S0893-133X(97)00130-9. [DOI] [PubMed] [Google Scholar]
- 49.Holter SM, Landgraf R, Zieglgansberger W, et al. Time course of camprosate action on operant ethanol self-administration after ethanol deprivation. Alcohol Clin Exp Res. 1997;21:862–868. [PubMed] [Google Scholar]
- 50.Czachowski CL, Legg BH, Samson HH. Effects of acamprosate on ethanol-seeking and self-administration in the rat. Alcohol Clin Exp Res. 2001;25:344–350. [PubMed] [Google Scholar]
- 51.De Witte P, Bachteler D, Spanagel R. Acamprosate: preclinical data. In: Spanagel R, Mann K, editors. Drugs for relapse prevention of alcoholism. Switzerland: Birkhauser, Basel; 2005. pp. 73–83. [Google Scholar]
- 52.Olive MF, Nannini MA, Ou CJ, Koenig HN, Hodge CW. Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release. Eur J Pharmacol. 2002;437:55–61. doi: 10.1016/s0014-2999(02)01272-4. [DOI] [PubMed] [Google Scholar]
- 53.Dahchour A, De Witte P. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur J Pharmacol. 2003;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- 54.Tsai G, Gastfriend DR, Coyle JT. The glutamatergic basis of human alcoholism. Am J Psychiatry. 1995;152:332–340. doi: 10.1176/ajp.152.3.332. [DOI] [PubMed] [Google Scholar]
- 55.Dahchour A, De Witte P. Ethanol and amino acids in the central nervous system: assessment of the pharmacological actions of acamprosate. Prog Neurobiol. 2000;60:343–362. doi: 10.1016/s0301-0082(99)00031-3. [DOI] [PubMed] [Google Scholar]
- 56.Spanagel R, Putzke J, Stefferl A, et al. Acamprosate, alcohol: II. Effects on alcohol withdrawal in the rat. Eur J Pharmacol. 1996;305:45–50. doi: 10.1016/0014-2999(96)00175-6. [DOI] [PubMed] [Google Scholar]
- 57.Spanagel R, Pendyala G, Abarca C, et al. The clock gene per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 58.Pierrefiche O, Daoust M, Naassila M. Biphasic effect of acamprosate on NMDA but not on GABAA receptors in spontaneous rhythmic activity from the isolated neonatal rat respiratory network. Neuropharmacology. 2004;47:35–45. doi: 10.1016/j.neuropharm.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Naassila M, Hammoumi S, Legrand E, et al. Mechanism of action of acamprosate. Part I. Characterization of spermidine-sensitive acamprosate binding site in rat brain. Alcohol Clin Exp Res. 1998;22:802–809. [PubMed] [Google Scholar]
- 60.Popp RL, Lovinger DM. Interaction of acamprosate with ethanol and spermine on NMDA receptors in primary cultured neurons. Eur J Pharmacol. 2000;394:221–231. doi: 10.1016/s0014-2999(00)00195-3. [DOI] [PubMed] [Google Scholar]
- 61.al Qatari M, Bouchenafa O, Littleton J. Mechanism of action of acamprosate. Part II. Ethanol dependence modifies effects of acamprosate on NMDA receptor binding in membranes from rat cerebral cortex. Alcohol Clin Exp Res. 1998;22:810–814. [PubMed] [Google Scholar]
- 62.Madamba SG, Schweitzer P, Zieglgansberger W, et al. Acamprosate (calcium acetylhomotaurinate) enhances the N-methyl-D-aspartate component of excitatory neurotransmission in rat hippocampal CA1 neurons in vitro. Alcohol Clin Exp Res. 1996;20:651–658. doi: 10.1111/j.1530-0277.1996.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 63.Berton F, Francesconi WG, Madamba SG, et al. Acamprosate enhances N-methyl-D-apartate receptor-mediated neurotransmission but inhibits presynaptic GABAB receptors in nucleus accumbens neurons. Alcohol Clin Exp Res. 1998;22:183–191. [PubMed] [Google Scholar]
- 64.Harris BR, Gibson DA, Prendergast MA, et al. The neurotoxicity induced by ethanol withdrawal in mature organotypic hippocampal slices might involve cross-talk between metabotropic glutamate type 5 receptors and N-methyl-D-aspartate receptors. Alcohol Clin Exp Res. 2003;27:1724–1735. doi: 10.1097/01.ALC.0000093601.33119.E3. [DOI] [PubMed] [Google Scholar]
- 65. Harris BR, Prendergast MA, Gibson DA, et al. Acamprosate inhibits the binding and neurotoxic effects of trans-ACPD, suggesting a novel site of action at metabotropic glutamate receptors. Alcohol Clin Exp Res. 2002;26:1779–1793. doi: 10.1097/01.ALC.0000042011.99580.98. * This study was one of the first to suggest that acamprosate may directly interact with metabotropic glutamate receptors. However, other investigators (see [70]) failed to confirm these findings.
- 66.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koob GF, Mason BJ, De Witte P, et al. Potential neuroprotective effects of acamprosate. Alcohol Clin Exp Res. 2002;26:586–592. [PubMed] [Google Scholar]
- 68.al Qatari M, Khan S, Harris B, et al. Acamprosate is neuroprotective against glutamate-induced excitotoxicity when enhanced by ethanol withdrawal in neocortical cultures of fetal rat brain. Alcohol Clin Exp Res. 2001;25:1276–1283. doi: 10.1097/00000374-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Blednov YA, Harris RA. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008;11:775–793. doi: 10.1017/S1461145708008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reilly MT, Lobo IA, McCracken LM, et al. Effects of acamprosate on neuronal receptors and ion channels expressed in Xenopus oocytes. Alcohol Clin Exp Res. 2008;32:188–196. doi: 10.1111/j.1530-0277.2007.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bachteler D, Economidou D, Danysz W, et al. The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology. 2005;30:1104–1110. doi: 10.1038/sj.npp.1300657. [DOI] [PubMed] [Google Scholar]
- 72.Katner SN, Magalong JG, Weiss F. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology. 1999;20:471–479. doi: 10.1016/S0893-133X(98)00084-0. [DOI] [PubMed] [Google Scholar]
- 73.Epstein DH, Preston KL, Stewart J, et al. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shaham Y, Shalev U, Lu L, et al. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 75.Olive MF, Cleva RM, Kalivas PW, et al. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–810. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- 77.Kufahl PR, Martin-Fardon R, Weiss F. Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR2/3 agonist LY379268 and increased functional activity of mGluR2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology. 2011;36:2762–2773. doi: 10.1038/npp.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quertemont E, de Neuville J, De Witte P. Changes in the amygdala amino acid microdialysate after conditioning with a cue associated with ethanol. Psychopharmacology. 1998;139:71–78. doi: 10.1007/s002130050691. [DOI] [PubMed] [Google Scholar]
- 79.Cole JC, Littleton JM, Little HJ. Acamprosate, but not naltrexone, inhibits conditioned abstinence behaviour associated with repeated ethanol administration and exposure to a plus-maze. Psychopharmacology. 2000;147:403–411. doi: 10.1007/s002130050009. [DOI] [PubMed] [Google Scholar]
- 80.Hillemand B, Lhuintre JP, Steru L, et al. Multicenter trial of calcium bis (acetylhomotaurinate) in treatment of alcohol dependence. Rev Alcohol. 1989;34:9–28. [Google Scholar]
- 81.Lhuintre JP, Moore N, Tran G, et al. Acamprosate appears to decrease alcohol intake in weaned alcoholics. Alcohol Alcohol. 1990;25:613–622. doi: 10.1093/oxfordjournals.alcalc.a045057. [DOI] [PubMed] [Google Scholar]
- 82.Paille FM, Guelfi JD, Perkins AC, et al. Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol. 1995;30:239–247. [PubMed] [Google Scholar]
- 83.Whitworth AB, Fischer F, Lesch OM, et al. Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet. 1996;347:1438–1442. doi: 10.1016/s0140-6736(96)91682-7. [DOI] [PubMed] [Google Scholar]
- 84.Tempesta E, Janiri L, Bignamini A, et al. Acamprosate and relapse prevention in the treatment of alcohol dependence: a placebo-controlled study. Alcohol Alcohol. 2000;35:202–209. doi: 10.1093/alcalc/35.2.202. [DOI] [PubMed] [Google Scholar]
- 85.Barrias J, Chabac S, Ferreria L, et al. Acamprosate: multicenter portuguese efficacy and tolerance evaluation study. Psiquiatria Clin. 1997;18:149–160. [Google Scholar]
- 86.Sass H, Soyka M, Mann K, et al. Relapse prevention by acamprosate: results from a placebo-controlled study on alcohol dependence. Arch Gen Psychiatry. 1996;53:673–680. doi: 10.1001/archpsyc.1996.01830080023006. [DOI] [PubMed] [Google Scholar]
- 87.Geerlings PJ, Ansoms C, van den Brink W. Acamprosate and prevention of relapse in alcoholics. Eur Addict Res. 1997;3:129–137. [Google Scholar]
- 88.Poldrugo F. Acamprosate treatment in a long-term community-based alcohol rehabilitation programme. Addiction. 1997;92:1537–1546. [PubMed] [Google Scholar]
- 89.Pelc I, Verbanck P, Le Bon O, et al. Efficacy and safety of acamprosate in the treatment of detoxified alcohol-dependent patients: a 90-day placebo-controlled dose-finding study. Br J Psychiatry. 1997;171:73–77. doi: 10.1192/bjp.171.1.73. [DOI] [PubMed] [Google Scholar]
- 90.Roussaeux JP, Hers D, Ferauge M. Does acamprosate influence alcohol consumption of weaned alcoholics? J Pharm Belg. 1996;51:65–68. [PubMed] [Google Scholar]
- 91.Namkoong K, Lee BO, Lee PG, et al. Acamprosate in korean alcohol-dependent patients: a multi-centre, randomized, double-blind, placebo-controlled study. Alcohol Alcohol. 2003;38:135–141. doi: 10.1093/alcalc/agg038. [DOI] [PubMed] [Google Scholar]
- 92.Chick J, Howlett H, Morgan MY, et al. United kingdom multicentre acamprosate study (UKMAS): a 6-month prospective study of acamprosate versus placebo in preventing relapse after withdrawal from alcohol. Alcohol Alcohol. 2000;35:176–187. doi: 10.1093/alcalc/35.2.176. [DOI] [PubMed] [Google Scholar]
- 93.Pelc I, Le Bon O, Verbanck P, et al. Calcium acetyl homotaurinate for maintaining abstinence in weaned alcoholic patients: a placebo-controlled double-blind multi-centre study. In: Naranjo C, Sellers E, editors. Novel pharmacological interventions for alcoholism. New York: Springer-Verlag; 1992. pp. 348–352. [Google Scholar]
- 94.Ladewig D, Knecht T, Leher P, et al. Acamprosate - a stabilizing factor in long-term withdrawal of alcoholic patients. Ther Umschau. 1993;50:182–188. [PubMed] [Google Scholar]
- 95.Besson J, Aeby F, Kasas A, et al. Combined efficacy of acamprosate and disulfiram in the treatment of alcoholism: a controlled study. Alcohol Clin Exp Res. 1998;22:573–579. doi: 10.1111/j.1530-0277.1998.tb04295.x. [DOI] [PubMed] [Google Scholar]
- 96.Gual A, Lehert P. Acamprosate during and after acute alcohol withdrawal: a double-blind placebo-controlled study in Spain. Alcohol Alcohol. 2001;36:413–418. doi: 10.1093/alcalc/36.5.413. [DOI] [PubMed] [Google Scholar]
- 97.Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51–63. doi: 10.1097/01.ALC.0000108656.81563.05. [DOI] [PubMed] [Google Scholar]
- 98.Mason BJ, Lehert P. Acamprosate for alcohol dependence: a sex-specific meta-analysis based on individual patient data. Alcohol Clin Exp Res. 2012;36:497–508. doi: 10.1111/j.1530-0277.2011.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rosner S, Hackl-Herrwerth A, Leucht S, et al. Acamprosate for alcohol dependence. Cochrane Database Sys Rev. 2010;9:CD004332. doi: 10.1002/14651858.CD004332.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kranzler HR, Gage A. Acamprosate efficacy in alcohol-dependent patients: summary of results from three pivotal trials. Am J Addict. 2008;17:70–76. doi: 10.1080/10550490701756120. [DOI] [PubMed] [Google Scholar]
- 101.Kampman KM, Pettinati HM, Lynch KG, et al. Initiating acamprosate within-detoxification versus post-detoxification in the treatment of alcohol dependence. Addict Behav. 2009;34:581–586. doi: 10.1016/j.addbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morley KC, Teesson M, Reid SC, et al. Naltrexone versus acamprosate in the treatment of alcohol dependence: a multi-centre, randomized, double-blind, placebo-controlled trial. Addiction. 2006;101:1451–1462. doi: 10.1111/j.1360-0443.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- 103.Berger L, Fisher M, Brondino M, et al. Efficacy of acamprosate for alcohol dependence in a family medicine setting in the united states: a randomized, double-blind, placebo-controlled study. Alcohol Clin Exp Res. 2013;37:668–674. doi: 10.1111/acer.12010. [DOI] [PubMed] [Google Scholar]
- 104.Tolliver BK, Desantis SM, Brown DG, et al. A randomized, double-blind, placebo-controlled clinical trial of acamprosate in alcohol-dependent individuals with bipolar disorder: a preliminary report. Bipolar Disord. 2012;14:54–63. doi: 10.1111/j.1399-5618.2011.00973.x. [DOI] [PubMed] [Google Scholar]
- 105.Mason BJ, Goodman AM, Dixon RM, et al. A pharmacokinetic and pharmacodynamic drug interaction study of acamprosate and naltrexone. Neuropsychopharmacology. 2002;27:596–606. doi: 10.1016/S0893-133X(02)00368-8. [DOI] [PubMed] [Google Scholar]
- 106.Feeney GF, Connor JP, Young RM, et al. Combined acamprosate and naltrexone, with cognitive behavioural therapy is superior to either medication alone for alcohol abstinence: a single centres' experience with pharmacotherapy. Alcohol Alcohol. 2006;41:321–327. doi: 10.1093/alcalc/agl007. [DOI] [PubMed] [Google Scholar]
- 107.Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275–293. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mann K, Kiefer F, Smolka M, et al. Searching for responders to acamprosate and naltrexone in alcoholism treatment: rationale and design of the predict study. Alcohol Clin Exp Res. 2009;33:674–683. doi: 10.1111/j.1530-0277.2008.00884.x. [DOI] [PubMed] [Google Scholar]
- 109. Mann K, Lemenager T, Hoffmann S, et al. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict Biol. 2013;18:937–946. doi: 10.1111/adb.12012. ** This paper describes the results of the PREDICT study, which was designed to duplicate the experimental circumstances of the COMBINE trial. The primary findings were that neither acamprosate or naltrexone treatments were beneficial against alcohol relapse than placebo
- 110.Dranitsaris G, Selby P, Negrete JC. Meta-analyses of placebo-controlled trials of acamprosate for the treatment of alcohol dependence: impact of the combined pharmacotherapies and behavior interventions study. J Addict Med. 2009;3:74–82. doi: 10.1097/ADM.0b013e318182d890. [DOI] [PubMed] [Google Scholar]
- 111.Heilig M. Acamprosate: an alcoholism treatment that may not be what we thought. Neuropsychopharmacology. 2014;39:781–782. doi: 10.1038/npp.2013.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kiefer F, Mann K. Acamprosate: how, where, and for whom does it work? Mechanism of action, treatment targets, and individualized therapy. Curr Pharmaceut Design. 2010;16:2098–2102. doi: 10.2174/138161210791516341. [DOI] [PubMed] [Google Scholar]
- 113.Tomek SE, Lacrosse AL, Nemirovsky NE, et al. NMDA receptor modulators in the treatment of drug addiction. Pharmaceuticals (Basel) 2013;6:251–268. doi: 10.3390/ph6020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kufahl PR, Barabas P, Halstengard C, et al. Potential use of antidepressants as therapies for drug use disorders. InFrontiers in clinical drug research - cns and neurological disorders. In: Atta-ur-Rahman, editor. Frontiers in Clinical Drug Research - CNS and Neurological Disorders. United Arab Emirates: Bentham Science Publishers; 2012. pp. 3–37. [Google Scholar]
- 115.Cowen MS, Adams C, Kraehenbuehl T, et al. The acute anti-craving effect of acamprosate in alcohol-preferring rats is associated with modulation of the mesolimbic dopamine system. Addict Biol. 2005;10:233–242. doi: 10.1080/13556210500223132. [DOI] [PubMed] [Google Scholar]
- 116.O'Brien CC. Experimental evidence in the treatment of alcoholism by intensive calcium therapy. J Am Osteopath Assoc. 1952;51:393–394. [PubMed] [Google Scholar]
- 117.O'Brien CC. Intensive calcium therapy as an initial approach to the psychotherapeutic relationship in the rehabilitation of the complsive drinker. J Psychol. 1964;57:125–129. doi: 10.1080/00223980.1964.9916681. [DOI] [PubMed] [Google Scholar]
- 118.Umhau JC, Momenan R, Schwandt ML, et al. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: A randomized controlled experimental medicine study. Arch Gen Psychiatry. 2010;67:1069–1077. doi: 10.1001/archgenpsychiatry.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dayas CV, Liu X, Simms JA, et al. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ciccocioppo R, Lin D, Martin-Fardon R, et al. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology. 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- 121.Rimondini R, Arlinde C, Sommer W, et al. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- 122.Edwards G. History of prevention of relapse. In: Spanagel R, Mann K, editors. Drugs for relapse prevention of alcoholism. Berlin: Birkhauser; 2005. pp. 1–13. [Google Scholar]
- 123.Levine HG. The discovery of addiction: changing conceptions of habitual drunkenness in America. J Stud Alcohol. 1978;39:143–174. doi: 10.15288/jsa.1978.39.143. [DOI] [PubMed] [Google Scholar]
- 124.Mason BJ, Heyser CJ. The neurobiology, clinical efficacy and safety of acamprosate in the treatment of alcohol dependence. Expert Opin Drug Safety. 2010;9:177–188. doi: 10.1517/14740330903512943. [DOI] [PubMed] [Google Scholar]
- 125.Weiss RD, O'Malley SS, Hosking JD, et al. Do patients with alcohol dependence respond to placebo? Results from the COMBINE study. J Stud Alcohol Drugs. 2008;69:878–884. doi: 10.15288/jsad.2008.69.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chaplin TM, Hong K, Fox HC, et al. Behavioral arousal in response to stress and drug cue in alcohol and cocaine addicted individuals versus healthy controls. Hum Psychopharmacol. 2010;25:368–376. doi: 10.1002/hup.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Greeley JD, Swift W, Prescott J, et al. Reactivity to alcohol-related cues in heavy and light drinkers. J Stud Alcohol. 1993;54:359–368. doi: 10.15288/jsa.1993.54.359. * This study reported that following exposure to the sight, smell, and taste of alcoholic drinks, self-reported feelings of craving were higher in heavy drinkers as compared to light drinkers. The findings are of significance as they suggest that studies of the ability of acamprosate to reduce alcohol craving should consider the severity of alcohol dependence in subjects.
- 128.Laberg JC. Alcohol and expectancy: subjective, psychophysiological and behavioral responses to alcohol stimuli in severely, moderately and non-dependent drinkers. Br J Addict. 1986;81:797–808. doi: 10.1111/j.1360-0443.1986.tb00407.x. [DOI] [PubMed] [Google Scholar]


