Abstract
Background
There is growing interest in characterizing spatial distribution of glutamate (Glu) in brain disorders. Comparing differences in Glu concentration using magnetic resonance spectroscopy (MRS) is hampered by the confounding effects of different anatomical regions and tissue composition.
New Method
Effect of tissue composition on Glu concentrations was studied by selecting closely adjacent voxels within a designated cortical region. Glu regional differences were assessed using voxels comprising essentially the same tissue composition from different cortical regions.
Results
Using point-resolved-spectroscopy (PRESS)-based averaged echo time method, Glu concentration in the anterior cingulate cortex (ACC) was found to correlate strongly with tissue gray matter (GM) fraction (r = 0.87, p = 10−5). No significant regional difference in Glu concentration was found between frontal and occipital lobes (p = 0.23) when the two measured voxels had essentially the same tissue composition.
Comparison with Existing Methods
The method of the current study is aimed to circumvent the difficulties in differentiating anatomical region from tissue composition, given that both can lead to Glu variations in brain. Glu concentration versus tissue composition was measured in the same anatomical region, while the comparison of regional differences was performed with the two regions that had essentially the same tissue composition.
Conclusions
In brain cortices, Glu level is significantly higher in GM than in WM. Glu level difference between frontal lobe and occipital lobe is insignificant.
Keywords: human brain, glutamate, 1H MRS, averaged echo time, regional difference, tissue composition, metabolite quantification
1. INTRODUCTION
Glutamate (Glu) is the principal excitatory neurotransmitter in the central nervous system (CNS). It is involved in glutamatergic neurotransmission through the Glu-glutamine (Glu-Gln) cycle between neurons and surrounding astrocytes (Hertz, 2004). Glutamatergic pathways are involved in diverse processes and brain disorders such as depression, epilepsy, schizophrenia, and ischemic brain damage. In addition to functioning as a neurotransmitter, Glu also serves as a source of energy and ammonia, linking the metabolism of carbon and nitrogen (Erecinska and Silver, 1990). The dual roles of Glu in neurotransmission and metabolism are intricately related. Glu concentrations measured by 1H magnetic resonance spectroscopy (MRS) result from the interplay between Glu anabolism and catabolism. Differences in Glu concentration across different brain regions and tissues—or lack thereof—provide important insight into alterations in Glu metabolism and neurotransmission.
Glu is very similar to glutamine (Gln) in molecular structure, characterized by the coupled spins of C2–C4 hydrogen nuclei. At prevalent field strength of 1.5 or 3.0 Tesla (T), it is challenging to separate Glu and Gln by conventional one-dimensional spectroscopic methodologies due to the heavy overlap over all their resonance frequencies. The resonances are mostly assigned to a mixture of Glu and Gln, often referred to as Glx. The echo time (TE)-averaged spectroscopy showed the feasibility of measuring Glu without Gln interference (Hurd et al, 2004). Moreover, TE-averaged spectra exhibited simplified resonance patterns and relatively flat baselines, whereby mitigating the statistical bias in quantification of weekly represented metabolites such as Glu (Zhang et al, 2011).
Due to time constraints associated with most clinical studies, the weak Glu signal requires the use of large voxels encompassing both gray matter (GM) and white matter (WM), making it particularly difficult to separate regional differences from those originating from different GM and WM compositions (Kraguljac et al, 2012; Ono et al, 2014; van der Veen and Shen, 2013; Pollack et al, 2008).
In this study, the point-resolved-spectroscopy (PRESS)-based single-voxel TE-averaged spectroscopy method was employed to investigate Glu level differences in the cortices of the human brain. The study was aimed to circumvent the difficulty in differentiating the regional difference from the effect of tissue composition on Glu concentration, given that both could lead to Glu variations in brain. Three closely adjacent voxels in the anterior cingulate cortext (ACC) were used to measure the effect of tissue composition. Two different voxels with essentially the same GM and WM fractions were placed in median prefrontal cortex (MPFC) and occipital cortex (OCC) to assess the regional difference in Glu concentration or lack thereof, without confounding contributions from the tissue type difference.
2. MATERIALS AND METHODS
2.1 Subjects
Twenty healthy volunteers (12 male, eight female; ages 22–55; mean age = 32 years) were recruited for the current study as approved by local institutional review board.
2.2 Data Acquisition
The scans were performed on a 3 T GE whole body scanner (General Electric Medical Systems, Milwaukee,Wisconsin, USA) running the 15M4 software platform. A Medical Advances (Intermagnetics General Corporation, Milwaukee,Wisconsin, USA) RF quadrature transmit/receive coil was used with an inner diameter of 25 cm and a length of 20 cm. Scan sessions began with a T1 weighted anatomical scan conducted via three-dimensional spoiled gradient echo sequence (TR = 7.3 ms, TE = 2.7 ms, flip angle 12°; in plane resolution 0.9 mm × 0.9 mm; matrix size 192 × 256; field of view 240 mm × 240 mm; slice thickness 2 mm; total scan time 2 min 32 s). Voxel positioning is shown in Figure 1. The ACC WM voxel was placed in the right frontal WM, directly adjacent to the rostral ACC. The mixed voxel was placed between the GM and WM voxels. The MPFC voxel was placed over the midline in the frontal lobe just anterior to the ventricles. The OCC voxel was placed over the midline in the occipital lobe just posterior to the ventricles. The location of the OCC voxel was chosen to approximate the same amount of GM andWM as the MPFC voxel. All voxels in the ACC region had a volume of 2.0 × 2.0 × 4.5 cm3. The MPFC and OCC voxels had a volume of 3.0 × 3.0 × 2.0 cm3.
Figure 1.

(a): Locations of the two voxels in the median prefrontal cortex (MPFC) and occipital cortex (OCC). (b): locations of the gray matter (GM) voxel (red), the white matter (WM) voxel (yellow), and the mixed voxel (green) in anterior cingulate cortex (ACC).
TE-averaged spectra were acquired with 32 different echo times. The number of average (NA) is four. TE started at 35 ms (8.5 ms from the excitation pulse to the first refocusing pulse; 17.5 ms from the first refocusing pulse to the second refocusing pulse), and was step-wised increased by 6.0 ms after each echo. All spectra of different echo times were summed to yield a TE-averaged spectrum. Its time domain decay was then approximated by an effective TE of 105 ms. Reference unsuppressed water scans were collected with NA = 2 immediately after the acquisition of spectral data. Spectra were sampled with a repetition time (TR) of two seconds, bandwidth of 5 kHz, and 4096 complex data points.
2.3 Spectral Fitting
Spectral fitting was performed automatically with the software developed in-house using interactive data language (IDL; Research Systems, Inc., Boulder, CO). The number of data points (4096) was expanded to 8192 by zero-filling in the time domain, followed by Fourier transform. Glu and Gln are indistinguishable at the resonance of 3.7 ppm due to the nearly complete overlap. Spectral fitting over the region 1.6–3.4 ppm allows the using of a less complicated model to quantify Glu. As such, the spectral model in the current study consisted of Glu, N-acetylaspartate (NAA), N-acetyl-aspartyl-glutamate (NAAG), creatine (Cr), and choline (Cho)-containing compounds.
The model basis spectra were generated by spin density matrix calculation. The coupling constants were obtained from the literature (Govindaraju et al, 2000). The lineshape was described with the Voigt model (Bruce et al, 2000) in which the Gaussian decay factor was a common parameter shared by all components in the spectral model. The linewidth of Glu was constrained by the linewidth ratio of Glu-Cr. The frequency of NAAG was constrained to be 4.8 Hz up-field from the peak of NAA at 2.0 ppm, and its linewidth was constrained to the linewidth of NAA. All constraints were enforced in a soft manner (Provencher, 1982 and 1993; Zhang and Shen, 2013). The estimated concentrations of NAA and NAAG were combined to yield a total concentration (tNAA). Because it is essentially impossible to differentiate Cr from phosphocreatine (PCr) at 3 T, the value of total concentration (tCr) was reported. Similarly, choline-containing compounds gave a single concentration (tCho).
2.4 T1 and T2 Corrections
The contents of a cortical voxel generally comprise GM, WM, and cerebrospinal fluid (CSF). The anatomical images were segmented with SPM5 (Ashburner and Friston, 1997; Geramita et al, 2011) to determine the fractions of GM, WM, and CSF. An in-house written program in IDL was used to extract the coordinates of the spectroscopy data files and their respective tissue fractions from the segmentation results (Geramita et al, 2011).
The CSF fraction can also be determined by measuring T2 of unsuppressed water (Ernst et al, 1993; Piechnik et al, 2009). In the current study, the water signal decay with different TEs was fitted with a bi-exponential decay model. T2 of the longer decay component was fixed at 500 ms (Piechnik et al, 2009) and was ascribed to CSF. The shorter T2 component accounted for the composite MR-visible signal arising from GM and WM. T1 attenuation was corrected by the factor 1 − exp(TR/T1), assuming T1 = 1084 ms for WM, and 1820 ms for GM (Stanisz et al, 2005). The values for the water densities of pure GM (43300 mM) and WM (35880 mM) were obtained from the literature (Ernst et al, 1993). The reference water signal intensity after the removal of CSF was corrected by:
| [1] |
where Sref and Sref, corr, represent the reference water signal intensities before and after correction for T1 relaxation, respectively. Note that Sref was extrapolated to TE = 0 with the fitted T2 as mentioned above. fg and fw are the volume fractions for GM and WM within voxels, respectively. They are determined by tissue segmentation. Wg and Ww are the water densities for GM and WM.
The estimated metabolite concentration (denoted by Cm) is the composite concentration of GM (denoted by Cg) and WM (denoted by Cw):
| [2] |
where Cm was estimated from the ratio of the fitted metabolite intensity (denoted by Sm) to the reference water intensity:
| [3] |
where αm is the factor that accounts for the proton number of the metabolite molecule relative to that of the water molecule. Similar to the reference water signal Sref, Sm should also be corrected for both T1 and T2 relaxation decays. Because of T1 and T2 heterogeneity between GM and WM, the decay could only be approximately described by a mono-exponential decaying function with an apparent T1 or T2 factor. Based on the literature (Ethofer et al, 2003; Traber et al, 2004; Choi et al, 2006; Tsai et al, 2007; Kirov et al, 2010), an apparent T1 of 1400 ms and 1200 ms were used for NAA and the rest of the metabolite components, respectively, and the apparent T2 values (ms) were: 200, 260, 160, and 220 for Glu, NAA, Cr, and Cho, respectively.
3. RESULTS
In Figure 1, the anatomic images show the positioning of voxels. The MPFC voxel and the OCC voxel had similar tissue compositions. The GM fractions of the MPFC voxels ranged from 0.47 to 0.63 across all subjects, with an average of 0.53 and a standard deviation of 0.045. For the OCC voxels, the GM fractions ranged from 0.40 to 0.61 with an average of 0.53 and a standard deviation of 0.051. As summarized in Table 1, there were essentially no differences in tissue composition between the MPFC and OCC voxels, with p = 0.86 and 0.73 for GM and WM, respectively. Figure 2 shows the comparison of TE-averaged spectra between MPFC and OCC. In contrast, the ratio of GM to WM tissue varied significantly over the three ACC voxels (0.62, 0.26, and 0.41 for GM, WM, and mixed voxels, respectively). Figure 3 shows the three TE-averaged spectra that correspond to GM, WM, and mixed voxels; data were acquired from the three adjacent voxels in ACC (see Figure 1).
Table 1.
Fractions (± standard deviation) of gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) for the voxels of interest.
| Region | MPFC | OCC | ACC/GM | ACC/WM | ACC/Mixed |
|---|---|---|---|---|---|
| GM | 0.53(±0.045) | 0.53(±0.051) | 0.62(±0.052) | 0.26(±0.031) | 0.41(±0.041) |
| WM | 0.37(±0.053) | 0.38(±0.055) | 0.27(±0.045) | 0.71(±0.053) | 0.53(±0.034) |
| CSF | 0.08(±0.022) | 0.09(±0.037) | 0.11(±0.035) | 0.03(±0.028) | 0.06(±0.012) |
Figure 2.
Representative echo time (TE)-averaged spectra of the median prefrontal cortex (MPFC) (a) and occipital cortex (OCC) (b). Fitted spectra are displayed in red. Top and bottom traces are fit residuals and baselines, respectively.
Figure 3.
Representative echo time (TE)-averaged spectra with different compositions of gray matter (GM) and white matter (WM) in the anterior cingulate cortex (ACC). GM fractions were 0.62, 0.41, and 0.26, corresponding to (a), (b), and (c), respectively. The spectral data were acquired from the same subject. Fitted spectra are displayed in red. Top and bottom traces are fit residuals and baselines, respectively.
Table 2 compares the metabolite concentrations between MPFC and OCC in 15 subjects (aged from 22 to 41; mean age = 29). No difference was observed in Glu concentration between the two voxels (p = 0.49). In contrast, tCho levels were significantly higher in MPFC than in the OCC (p < 10−10). The differences in tNAA and tCr levels between MPFC and OCC did not reach statistical significance (p = 0.23 and 0.069, respectively). p-values were calculated using student t-statistics with unequal variances.
Table 2.
Metabolite concentrations (millimolar (coefficient of variation)*) for 15 subjects.
| Region | Glu | tNAA | tCr | tCho |
|---|---|---|---|---|
| MPFC | 10.1(9.2%) | 12.5(5.5%) | 8.14(6.6%) | 2.53(6.4%) |
| OCC | 9.72(5.4%) | 12.2(2.6%) | 7.67(3.3%) | 1.61(4.8%) |
the coefficients of variation were derived from Cramer-Rao Lower Bounds (CRLB).
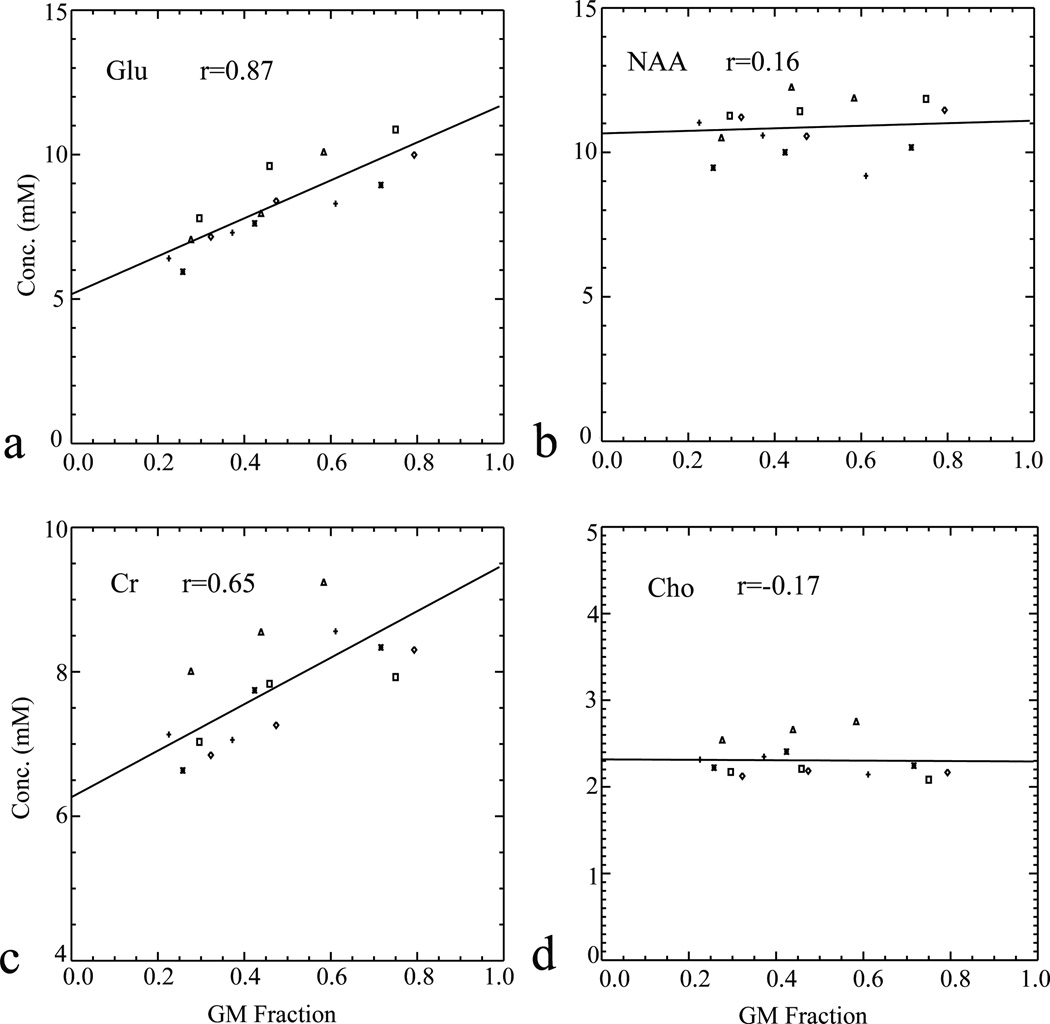
Figure 4 displays the linear regressions of metabolite concentrations against GM fractions. Data were acquired from three adjacent voxels in the ACC of five subjects (aged from 30 to 55; mean age = 35) with tissue characterized by GM, WM, and mixed composition, respectively. Glu and tCr levels depend significantly on tissue compositions (r = 0.87 and 0.65, p =10−5 and 0.004, respectively). No significant changes in tNAA or tCho levels were observed over the three different voxels (r = 0.16 and −0.17, p = 0.28 and 0.27, respectively).
Figure 4.
Pearson’s correlations of metabolite concentrations versus fractions of gray matter (GM) in the anterior cingulate cortex (ACC). Data of individual subjects are labeled by square, triangle, plus sign, times sign, and rhombus, respectively.
4. DISCUSSION
There are several aspects of the current study designed to improve assessing the differences in Glu concentration. First, the effect of tissue composition on Glu concentration was separated from the potential differences associated with region. Secondly, TE-averaged spectroscopy was utilized so that the Gln interference was minimized. Third, a new fitting method was used, which reduced variables and minimized the baseline influences on metabolite quantification. Notably, we found that Glu concentration varied significantly with tissue composition rather than brain region in the cortices investigated; higher Glu levels were in GM, and lower levels in the WM. In addition, tCr concentrations were also positively correlated with GM fractions. tCho concentrations showed a weakly negative correlation with GM fractions, though they demonstrated the largest regional difference. Cho levels in the frontal lobe were significantly higher than in the occipital lobe. Finally, no region or tissue composition variations were found for the combined concentration of NAA and NAAG.
Inconsistencies from the previous studies are noticeable. Given that both tissue composition and regional differences affect metabolite concentrations, the impetus for this study was born from the assumption that teasing apart these two factors should yield a more reliable assessment of the spatial distribution of metabolite concentrations.
While the intense signals from NAA, Cr, and Cho can be analyzed from a large number of voxels, measuring Glu concentrations is difficult because of the relatively low MRS detection sensitivity and the spectral overlapping from Gln. For these reasons, data in the current study were acquired with PRESS based single-voxel TE-averaged spectroscopy and with relatively larger voxel sizes than typically used in short TE spectroscopy. TE-averaged spectroscopy offers a way to cancel out the C4 signal of Gln (Hurd et al, 2004), whereby yielding unobstructed detection of the remaining Glu signal. In addition, it suppresses macromolecular signals and thus has a flatter baseline than short TE spectroscopy.
Incorporating a baseline into the fitting model is necessary in order to achieve a good fit for in vivo MRS including TE-averaged spectroscopy. However, a good fit of the spectral model, indicated by a minimized least squares difference between the model and data, does not necessarily indicate that metabolite and baseline signal contributions are correctly estimated. An under-smoothed baseline can lead to large errors even though the fitting residuals are minimized. Conversely, an over-smoothed baseline often results in poor fitting, characterized by large fit residuals, because background signals are not well represented. In this study, the spectral baselines were determined by a recently proposed method (Zhang and Shen, in press). This method minimizes the error of both the fit residuals and the uncertainties arising from the interaction between the baseline and metabolite peaks. In Table 2, the mean coefficients of variation were derived from Cramer-Rao Lower Bounds (CRLB) (Cavassila et al, 2001; Zhang and Shen, in press). Note that the MPFC spectra have larger CRLBs than the OCC spectra, echoing that the former have poorer spectral quality than the latter (see Figure 2).
In the present study, no significant differences were detected in Glu levels between the two regions of essentially the same tissue composition in the frontal lobe and the occipital lobe, though Glu in GM is significantly higher than in WM. Higher Glu and/or Glx levels in GM have also been consistently reported in the literature (Kassem and Bartha, 2003; Pan et al, 1996; Sailasuta et al, 2008 McLean et al, 2000; Srinivasan et al, 2006). To the best of our knowledge, this report is the first to control the GW and WM content when comparing the regional difference in Glu concentration. Our results highlight the importance of careful control of tissue composition when investigating the regional difference in Glu concentration since the difference caused by tissue composition may dominate.
In addition to Glu, we found that tCr concentrations were significantly increased in GM compared to WM (Figure 4c) in agreement with early studies (Pouwel and Frahm, 1998; Schuff et al, 2001; Wiedermann et al, 2001). Baker and colleagues (2008) reported a trend toward decreased tCr levels in WM from anterior to posterior, and that this trend was reversed in GM. The current study agrees with the general consensus established in previous studies that tCr levels are higher in GM than in WM. The present study observed slightly higher Cr concentrations in MPFC than OCC.
The weak association observed in the present study between tCho and GM fractions (r = − 0.17, p = 0.27; see Figure 4d) is in keeping with most previous findings (Schuff et al, 2001; McLean et al, 2000; Wiedermann et al, 2001). tCho levels in the OCC were significantly lower than in the MPFC (p < 10−10). Figure 2 clearly shows the difference in the two tCho peak heights. This observation echoes previous MRS studies (Pouwels and Frahm,1998; Baker et al, 2008), and is supported by a postmortem finding of lower Cho levels in the occipital cortex than in either the frontal or parietal cortices (Nitsch et al,1993).
As for tNAA, previous MRS studies reported small or no differences (Pouwels and Frahm,1998; Baker et al, 2008; Maudsley et al, 2009; McLean et al, 2000; Srinivasan et al, 2006). In the present study no significant differences in tNAA levels were observed, regardless of brain region or tissue composition.
A variety of 1H MRS methods have been proposed for in vivo studies of metabolites. Short TE spectroscopy and short TE-based magnetic resonance spectroscopic imaging (MRSI) are two of the most popular methods. In particular, MRSI can simultaneously detect the metabolites in different regions of interest and has attracted increasing attention (Posse et al, 2013). Separation of Glu from Gln, the aspartyl moiety of NAA and the macromolecule baseline is also feasible at 3 T using short-TE MRSI (Posse et al, 2013). TE-averaged spectroscopy has a long effective TE, and its spectra thus suffer larger signal loss than short TE spectra. Its long TE also makes metabolite quantification more sensitive to T2 uncertainties. Despite of these disadvantages of TE-averaged spectroscopy, it dramatically simplifies spectral pattern and hence reduces unknown factors. Strong baseline arising from macromolecule and/or lipid signals is known to significantly confound quantification of metabolite concentrations, especially for weekly represented metabolites such as Glu. Thanks to the reduced variables in the spectral model, Glu measured by TE-averaged spectroscopy is less dependent on the fidelity of the model including the baseline.
5. CONCLUSIONS
Glu concentrations were found to vary significantly with tissue composition rather than brain region in the cortices investigated. Higher Glu levels were observed in the GM, and lower levels in the WM. No significant regional differences in Glu levels were observed. As with Glu concentration, tCr concentrations were also positively correlated with GM fractions. tCho concentrations were weakly and negatively correlated with GM fraction, though they demonstrated the largest regional difference. Cho levels in the frontal lobe were significantly higher than in the occipital lobe. Finally, no region or tissue composition variations were found for the combined concentration of NAA and NAAG. The findings of this study provide reference for patient studies involving MRS of Glu. In particular, it points out the possibility of using OCC Glu as an internal control for monitoring therapies that targeting the frontal region.
HIGHLIGHTS.
Glutamate levels versus tissue compositions are measured in the same anatomical region of human brain.
Regional differences in glutamate levels are assessed with regions comprising essentially the same tissue composition.
Glutamate levels are found to correlate strongly with tissue composition.
No significant difference of glutamate concentration is found between frontal and occipital regions.
ACKNOWLEDGEMENTS
This study was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH). The authors thank Ms. Ioline Henter (NIMH) for her excellent editorial assistance.
ROLE OF FUNDING SOURCE
The NIMH had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Hertz L. Intercellular metabolic compartmentation in the brain: past, present and future. Neurochem Int. 2004;45:285–296. doi: 10.1016/j.neuint.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35:245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- Hurd R, Sailasuta N, Srinivasan R, Vigneron DB, Pelletier D, Nelson SJ. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magn Reson Med. 2004;51:435–440. doi: 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li S, Marenco S, Shen J. Quantitative measurement of N-Acetyl-aspartyl-glutamate at 3 T using TE-averaged PRESS spectroscopy and regularized lineshape deconvolution. Magn Reson Med. 2011;66:307–313. doi: 10.1002/mrm.23029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Reid MA, White DM, den Hollander J, Lahti AC. Regional decoupling of N-acetyl-aspartate and glutamate in schizophrenia. Neuropsychopharmacology. 2012;37:2635–2642. doi: 10.1038/npp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Kitagawa M, Ito D, Tanaka N, Watari T. Regional variations and age-related changes detected with magnetic resonance spectroscopy in the brain of healthy dogs. Am J Vet Res. 2014;75:179–186. doi: 10.2460/ajvr.75.2.179. [DOI] [PubMed] [Google Scholar]
- Pollack MH, Jensen JE, Simon NM, Kaufman RE, Renshaw PF. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: response to treatment with levetiracetam. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:739–743. doi: 10.1016/j.pnpbp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- van der Veen JW, Shen J. Regional difference in GABA levels between medial prefrontal and occipital cortices. J Magn Reson Imaging. 2013;38:745–750. doi: 10.1002/jmri.24009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Provencher SW. A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comput Phys Commun. 1982;27:213–227. [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shen J. Soft constraints in nonlinear spectral fitting with regularized lineshape deconvolution. Magn Reson Med. 2013;69:912–919. doi: 10.1002/mrm.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Multimodal image coregistration and partitioning - a unified framework. NeuroImage. 1997;6:209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- Geramita M, van der Veen JW, Barnett AS, Savostyanova AA, Shen J, Weinberger DR, Marenco S. Reproducibility of prefrontal γ-aminobutyric acid measurements with J-edited spectroscopy. NMR in Biomed. 2011;24:1089–1098. doi: 10.1002/nbm.1662. [DOI] [PubMed] [Google Scholar]
- Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in human brain. I. Compartments and water. J Magn Reson B. 1993;102:1–8. [Google Scholar]
- Piechnik SK, Evans J, Bary LH, Wise RG, Jezzard P. Functional changes in CSF volume estimated using measurement of water T2 relaxation. Magn Reson Med. 2009;61:579–586. doi: 10.1002/mrm.21897. [DOI] [PubMed] [Google Scholar]
- Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM. T1, T2 relaxation and magnetization transfer in tissue at 3 T. Magn Reson Med. 2005;54:507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- Traber F, Block W, Lamerichs R, Gieseke J, Schild HH. 1H Metabolite Relaxation Times at 3.0 Tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging. 2004;19:537–545. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Mader I, Seeger U, Helms G, Erb M, Grodd W, Ludolph A, Klose U. Comparison of longitudinal metabolite relaxation times in different regions of the human brain at 1.5 and 3 tesla. Magn Reson Med. 2003;50:1296–1301. doi: 10.1002/mrm.10640. [DOI] [PubMed] [Google Scholar]
- Choi C, Coupland NJ, Bhardwaj PP, Kalra S, Casault CA, Reid K, Allen PS. T2 measurement and quantification of glutamate in human brain in vivo. Magn Reson Med. 2006;56:971–977. doi: 10.1002/mrm.21055. [DOI] [PubMed] [Google Scholar]
- Tsai S, Posse S, Lin Y, Ko C, Otazo R, Chung H, Lin F. Fast mapping of the T2 relaxation time of cerebral metabolites using proton echo-planar spectroscopic imaging (PEPSI) Magn Reson Med. 2007;57:859–865. doi: 10.1002/mrm.21225. [DOI] [PubMed] [Google Scholar]
- Kirov II, Liu S, Fleysher R, Fleysher L, Babb JS, Herbert J, Gonen O. Brain metabolite proton T2 mapping at 3.0 T in relapsing-remitting multiple sclerosis. Radiology. 2010;254:858–866. doi: 10.1148/radiol.09091015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shen J. Smoothness of in vivo spectral baseline determined by mean-square error. Magn Reson Med. doi: 10.1002/mrm.25013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavassila S, Deval S, Huegen C, van Ormondt D, Graveron-Demilly D. Cramer-Rao bounds: an evaluation tool for quantitation. NMR Biomed. 2001;14:278–283. doi: 10.1002/nbm.701. [DOI] [PubMed] [Google Scholar]
- Posse S, Otazo R, Dager SR, Alger J. MR spectroscopic imaging: principles and recent advances. J Magn. Reson Imaging. 2013;37:1301–1325. doi: 10.1002/jmri.23945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem MN, Bartha R. Quantitative proton short-echo-time LASER spectroscopy of normal human white matter and hippocampus at 4 Tesla incorporating macromolecule subtraction. Magn Reson Med. 2003;49:918–927. doi: 10.1002/mrm.10443. [DOI] [PubMed] [Google Scholar]
- Pan JW, Mason GF, Pohost GM, Hetherington HP. Spectroscopic imaging of human brain glutamate by water-suppressed J-refocused coherence transfer at 4.1 T. Magn Reson Med. 1996;36:7–12. doi: 10.1002/mrm.1910360103. [DOI] [PubMed] [Google Scholar]
- McLean MA, Woermann FG, Barker GJ, Duncan JS. Quantitative analysis of short echo time 1H MRSI of cerebral gray and white matter. Magn Reson Med. 2000;44:401–411. doi: 10.1002/1522-2594(200009)44:3<401::aid-mrm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Sailasuta N, Ernst T, Chang L. Regional variations and the effects of age and gender on glutamate concentrations in the human brain. Magn Reson Imaging. 2008;26:667–675. doi: 10.1016/j.mri.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Cunningham C, Chen A, Vigneron D, Hurd R, Nelson S, Pelletier D. TE-averaged two dimensional proton spectroscopic imaging of glutamate at 3T. Neuroimage. 2006;30:1171–1178. doi: 10.1016/j.neuroimage.2005.10.048. [DOI] [PubMed] [Google Scholar]
- Pouwels PJ, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med. 1998;39:53–60. doi: 10.1002/mrm.1910390110. [DOI] [PubMed] [Google Scholar]
- Baker EH, Basso G, Barker PB, Smith MA, Bonekamp D, Horska A. Regional apparent metabolite concentrations in young adult brain measured by 1H MR spectroscopy at 3 Tesla. J Magn. Reson Imaging. 2008;27:489–499. doi: 10.1002/jmri.21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Ezekiel F, Gamst A, Amend D, Capizzano AA, Maudsley AA, Weiner MW. Region and tissue differences of metabolites in normally aged brain using 1H magnetic resonance spectroscopic imaging. Magn Reson Med. 2001;45:899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedermann D, Schuff N, Matson GB, Soher BJ, Du AT, Maudsley AA, Weiner MW. Short echo time multislice proton magnetic resonance spectroscopic imaging in human brain: metabolite distributions and reliability. Magn Reson Imaging. 2001;19:1073–1080. doi: 10.1016/s0730-725x(01)00441-6. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Blusztajn JK, Doyle FM, Robitaille Y, Wurtman RJ, Growdon JH, Kish SJ. Phospholipid metabolite levels are altered in cerebral cortex of patients with dominantly inherited olivopontocerebellar atrophy. Neurosci Lett. 1993;161:191–194. doi: 10.1016/0304-3940(93)90291-r. [DOI] [PubMed] [Google Scholar]
- Maudsley AA, Domenig C, Govind V, Darkazanli A, Studholme C, Arheart K, Bloomer C. Mapping of brain metabolite distributions by volumetric proton MR Spectroscopic Imaging (MRSI) Magn Reson Med. 2009;61:548–559. doi: 10.1002/mrm.21875. [DOI] [PMC free article] [PubMed] [Google Scholar]