Abstract
Background
Infants with complex congenital heart disease requiring surgical intervention within the first days or weeks of life may be the most seriously ill infants needing intensive nursing and medical care immediately after birth. Skin to skin contact (SSC) is well-accepted and practiced as a positive therapeutic intervention in premature infants, but is not routinely offered to infants in cardiac intensive care units. Physiologic effects of SSC in the congenital heart disease population must be examined before recommending incorporation of SSC into standard care routines.
Objective
The purpose of this case study was to describe the physiologic response to a single session of SSC in an 18-day-old infant with hypoplastic left heart syndrome.
Methods
Repeated measures of heart rate, respiratory rate, oxygen saturation, blood pressure, and temperature were recorded 30 minutes prior to SSC, during SSC (including interruptions for bottle and breast feedings), and 10 minutes after SSC was completed.
Results
All physiologic parameters were clinically acceptable throughout the 135-minute observation.
Conclusion
This case study provides beginning evidence that SSC is safe in full-term infants following surgery for complex congenital heart disease. Further research with a larger sample is needed to examine effects of SSC on infant physiology before surgery and earlier in the postoperative time period as well as on additional outcomes such as length of stay, maternal-infant interaction, and neurodevelopment.
Keywords: skin to skin contact, kangaroo care, congenital heart disease, infant physiology
Skin-to-skin contact (SSC; also known as kangaroo care) is a commonly used intervention in neonatal intensive care units in which the mother holds her infant, clothed only in a diaper, against her bare chest between her breasts. Numerous benefits for premature infants have been documented, including enhanced physiologic stability,1,2,3 neurobehavioral maturation,4,5 and adaptive stress responses.4,6,7 Infants with complex congenital heart disease (CCHD) requiring corrective or palliative surgery within the first days or weeks of life share many characteristics with premature infants, including physiologic instability,8 immature brain development,9 and exposure to an intensive care environment. However, infants with CCHD have the additional challenges of surgery and impaired cardiac function. Only one study has been published in which the use of SSC was examined in infants with CCHD. A small sample of five infants in the cardiothoracic intensive care unit recovering from surgery for CCHD received three cycles of two hours of SSC followed by two hours in an incubator over a 12-hour time period.10 Cardiorespiratory stability was significantly improved during SSC in comparison to non-SSC periods.
Infants with CCHD, as a group, are perhaps the most seriously ill infants needing intensive nursing and medical care and commonly experience hemodynamic instability in response to various stimuli.11 For these reasons, physiologic effects of SSC in the CCHD population must be examined before recommending incorporation of SSC into standard care routines. We began our investigations by conducting a case study to document physiologic responses while assessing feasibility and safety in the CCHD population. The purpose of the case study was to describe the physiologic response (heart rate, respiratory rate, oxygenation, blood pressure, temperature) to a single session of SSC in an 18-day-old infant in the pediatric intensive care unit following a hybrid procedure for treatment of hypoplastic left heart syndrome. Observations were made before SSC, during transfers into and out of SSC, during SSC, during bottle and breast feeding, and after SSC.
Case Study
The case study reported here was part of a larger, IRB-approved study of maternally provided SSC for which the mother provided written informed consent. The male infant was born at 39 3/7 weeks, weighed 3050 grams, and had APGARS of 7 and 7 following vaginal delivery to a 40 year old, G7 P7 married mother. The infant was diagnosed at 27 weeks gestation with hypoplastic left heart syndrome (HLHS). HLHS is one of the most serious types of congenital heart defects in which the aorta and the left side of the heart are underdeveloped with a tiny aorta and a minimally functional left ventricle. In this single ventricle physiology, the right ventricle pumps blood to both the pulmonary and systemic circulation. Palliation of the HLHS defect involves two major surgeries within the first months of life followed by a third surgery at age two or three years. The first surgical intervention for this infant was the hybrid stage I procedure. There are two parts to the hybrid procedure. First, a stent is placed in the ductus arteriosus to maintain mixing of blood between the aorta and the pulmonary artery and bands are placed around bilateral branch pulmonary arteries to reduce pressure on the pulmonary vasculature.12,13 Second, an atrial septostomy is performed in the cardiac catheterization suite several days after the initial procedure to increase the mixing of pulmonary and systemic blood. The hybrid procedure allows palliation and improved oxygenation without requiring cardio-pulmonary bypass.12,13 The infant had completed both parts of the hybrid procedure six days before SSC was observed for this case study.
At delivery, the infant was vigorous with heart rate in the 160s and peripheral oxygen saturation of 100% on room air. The infant’s diagnosis of HLHS was confirmed, and he underwent part 1 of the hybrid procedure at seven days of age. The evening of this initial hybrid procedure, the infant experienced two episodes of significant bradycardia requiring compressions for five and two minutes respectively. At 12 days of age, part 2 of the hybrid procedure (an atrial septostomy) was performed without incident. The infant was diagnosed with a gram negative pneumonia at 13 days of age and was treated with intravenous piperacillin-tazobactam for seven days. The infant experienced eight days of mechanical ventilation, was in the intensive care unit for a total of 19 days, and was discharged home at 21 days of age. The SSC reported here was administered on the 18th day of life. At this time, the infant had been extubated for three days and was receiving IV antibiotics through a peripherally inserted central catheter (PICC). Nasogastric feedings had been started nine days prior and oral feedings with a bottle one day prior to the SSC observation. Although the mother planned to breastfeed, no breast feedings had yet occurred.
Measurement and Procedure
Observations during one session of SSC were conducted in a 4-bed intensive care room in the pediatric intensive care unit and began 30 minutes following a4-minute afternoon bottle feeding of 11 milliliters of expressed breast milk. Privacy curtains were pulled around the infant’s bedside. Heart rate (HR), respiratory rate (RR), and peripheral oxygen saturations (SpO2) were recorded manually by a trained research assistant every 30 seconds from data displayed on the hospital bedside monitor (Spacelabs Healthcare, OSI Systems, Inc.) based on a 5-lead ECG and pulse oximeter sensor wrapped around the right mid-foot. Blood pressure (BP) was recorded from data displayed on the bedside monitor after the bedside nurse inflated the BP cuff on the infant’s left upper arm once before SSC and three times during SSC. Axillary temperature was taken once by the bedside nurse using a digital thermometer (Welch Allyn, Inc.) 17 minutes after beginning SSC. Behavioral state was observed following documentation of the physiologic data every 30 seconds, and the predominant infant state during each 30-second epoch was recorded. The Brazelton Scale14 was used for scoring infant state and included six possible states: Quiet Sleep (regular respiratory pattern throughout an 8 second screen displaying the wave pattern), Active Sleep (irregular respiratory pattern), Sleep-Wake Transition (drowsy, eyes opening and closing), Quiet Alert (eyes open), Active Alert (body movements), and Crying.
Though bottle fed just 30 minutes before, the infant displayed hunger cues shortly after initial placement in SSC, so the infant was transferred out of SSC and bottle fed. Following placement back in SSC, the infant continued to display hunger cues, and was breastfed while continuing SSC. At the end of feeding at the breast, SSC was completed without further interruptions. Therefore, observations were made for the following periods:
Pre-SSC
For 30 minutes, the mothers at relatively still, holding her infant while seated upright (80 degree) in a padded glider rocker at the infant’s bedside. The infant was lying in his mother’s lap on his side facing his mother, wearing a cotton shirt and a diaper and was wrapped in a single receiving blanket folded in half.
Transfer into Initial SSC (Transfer 1)
The mother unwrapped and undressed the infant down to his diaper with the infant in her lap and remained in a seated position in the recliner. The mother then lifted the infant into an upright position so the infant’s ventral surface was in direct contact with her chest between her breasts. The mother then covered the infant with a double-folded blanket and wrapped her robe around the infant. The mother elevated her legs on a padded ottoman and leaned back in the rocker at a 60 degree angle. The 2.5 minute transfer process was complete when the mother stated she was comfortable.
Initial SSC (SSC-1)
Observations began immediately after transfer and continued for 3 minutes during which the infant was restless and then cried, resulting in the mother’s decision to bottle feed the infant again.
Transfer out of SSC-1 for bottle feeding (Transfer 2)
The mother moved the infant to a cradled position and ventral-to-ventral skin contact was absent. Transfer 2 took less than one minute.
Bottle feeding
The mother fed her infant expressed breast milk by bottle. During the bottle feeding, the infant choked and gasped several times while trying to coordinate sucking, swallowing, and breathing. After 4 minutes, the infant refused further attempts to feed by bottle.
Transfer into SSC (Transfer 3)
The mother moved the infant back into an upright position on her chest, covering his back with a single folded blanket and wrapping her robe around him. Transfer 3 was completed in less than one minute.
Resumption of SSC (SSC-2)
Once again positioned chest-to-chest between the mother’s breasts, the infant was initially wakeful, fell asleep briefly (about 1.5 minutes), then awoke exhibiting rooting behaviors, bobbing his head across mother’s chest, and crying. After alerting the nurse and receiving approval to breastfeed, the mother slid the infant down to a cradled position, maintaining ventral-to-ventral skin contact, and helped the infant initiate breast feeding.
SSC with breast feeding (SSC-BF)
The infant latched on and regulated sucking, swallowing, and breathing without difficulty for 4.5 minutes, then began falling asleep. Even though SSC-2 was maintained during this episode of breast feeding, we considered this feeding a separate time period for recording infant physiology so the infant’s response to breast feeding could be captured.
Sustained SSC (SSC-3)
Maintaining ventral-to-ventral contact, the mothers lid the infant back into an upright, prone position between her breasts, covered the infant with a double-folded receiving blanket, and wrapped her robe around the infant. The mother and infant remained in this position for 65 minutes. SSC-3 was the primary time period used for comparative analyses as the infant and mother maintained their respective positions for a sustained period of time.
Transfer out of SSC-3
When mother indicated she was ready to complete SSC, she moved the infant from her chest to a supine position on her lap, transferring him out of SSC in less than one minute.
Post-SSC
After transfer, the mother dressed the infant in his cotton shirt and covered him in a blanket while he lay supine on her lap. The bedside nurse administered oral medications to the infant at the 4th minute and during the final 2.5 minutes of post-SSC.
Data Analysis
Descriptive statistics were used to describe physiologic values for each period of observation. Regression discontinuity (RD) analysis15 was used to examine differences in means, and slopes of heart rate, respiratory rate, and oxygen saturation between the pre-SSC period and sustained SSC (SSC-3). In RD, comparisons are made between two series of measures on either side of a cut-off point. The cut-off point in our analysis was set at the end of the pre-SSC period of observation and the comparison was made with the SSC-3 period. RD takes into account the dependency of the two series of measures and provides an unbiased estimate of intervention effects. For greater precision, we used a parametric/global strategy of RD in which every observation in the rating period was used to model the outcome of each physiologic variable, i.e. the data were used to fit the appropriate model. Regression lines were estimated separately for each side of the cut-off point, and zero slope RD models were built to test mean differences between the two series of values. The resulting parameter estimates are interpreted as a weighted average treatment effect. A two-tailed Type I error rate of 0.05 was used. Because unavoidable patient care interventions occurred during post-SSC, comparisons between post-SSC and other periods were not made.
Findings
Descriptive statistics
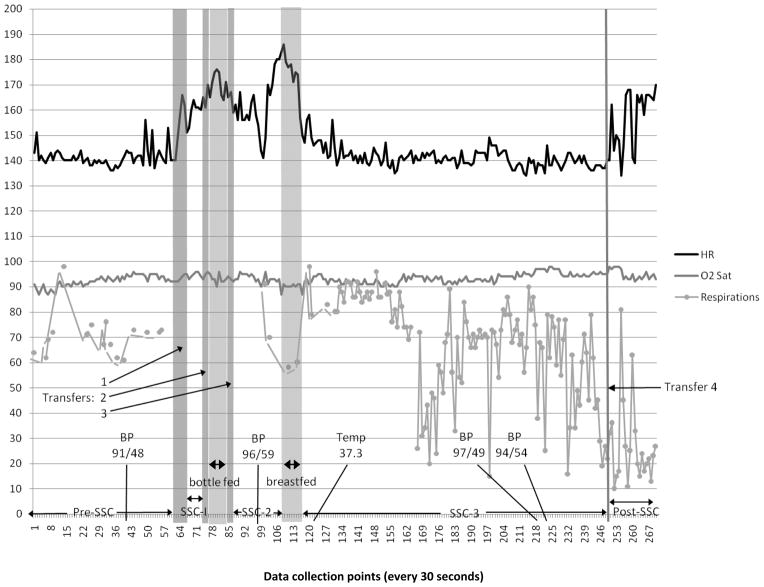
Figure 1 illustrates the trajectory of physiologic variables across periods, and includes location and timing of feedings and transfers. In Table 1, infant state, blood pressure, and temperature, as well as means, standard deviations (SD), and ranges of heart rate, respiratory rate, and oxygen saturations, are reported for all periods. Mean heart rates were essentially the same between pre-SSC (141.18 bpm) and SSC-3(141.11 bpm) and were similar during bottle (170.00 bpm) and breast(169.40 bpm) feedings. However, standard deviations were about 66% lower during bottle feeding (4.66) than breast feeding (13.28). Average respiratory rates appeared slightly lower during SSC-3(66.82 breaths/minute) when compared with pre-SSC rates (70.88 breaths/minute). Mean oxygen saturation values appeared similar between pre-SSC (92.13%) and SSC-3(93.38%) periods.
Figure 1.
Trajectories of heart rate (HR), oxygen saturations (O2 Sat), and respirations across skin-to-skin contact (SSC) observations: pre-SSC, initiation of SSC (SSC-1), resumption of SSC (SSC-2), feedings, sustained SSC (SSC-3), post-SSC, and all transfers. Intermittent values of blood pressure (BP) and axillary temperature (Temp) are marked at time of measurement.
Infant heart rates following the hybrid procedure are expected to be within the range of those of healthy term infants,16 i.e., 90–164 bpm.17 Recorded heart rates (measured every 30 seconds) were higher than 164 bpm 89% of the time during bottle feeding, 70% of the time during breast feeding, and 45% of the time during post-SSC. Heart rates also exceeded 164 bpm during the transfer out of SSC-1 in order to bottle feed. No heart rate values were less than 90 bpm.
Respiratory rates for healthy infants typically range from 25–66 breaths per minute.17 Respiratory rates in infants with this type of cardiac defect, in which there is increased pulmonary blood flow, are commonly higher than 66 even without observable infant distress, with expected rates in the 60s – 90s, rates described as “peaceful tachypnea.”16 (p1810). Respirations were not displayed on the monitor during transfers, SSC-1, and bottle feeding and, therefore, could not be recorded. Respiratory rates were greater than 66 for the majority of time in all periods in which it was recorded except during breast feeding (no elevations in respiratory rate) and post-SSC in which 1 of 20 observations (5%) was higher than 66. Respiratory rates were lower than 25 during SSC-3 (5.1%) and post SSC (60%).
Oxygen saturation for full term infants in open air is expected to be between 88% and 100%. For an infant following hybrid palliation, acceptable room-air oxygen saturation is 75–90%, reflecting the mixed venous and arterial blood in circulation.16 In this case study, oxygen saturations were between 87 and 98 across all observation periods (see Table 1).
Table 1.
Physiologic Variables and Infant State Before, During, and After SSC
| Pre-SSC | Transfer 1 | SSC-1 | Transfer 2 | Bottle fed | Transfer 3 | SSC-2 | SSC-BF | SSC-3 | Transfer 4 | Post SSC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Minute | 1–31 | 31.5–34 | 34.5–37.5 | 38.0–38.5 | 39.0–43.0 | 43.5–44 | 44.5–54 | 54.5–59 | 59.5–124.5 | 125 | 125.5–135 |
| HR | n = 62 | n=6 | n=7 | n=2 | n=9 | n=2 | n=20 | n = 10 | n = 131 | n=1 | n=20 |
| mean | 141.18 | 156.50 | 161.71 | 167.50 | 170.00 | 160.50 | 162.55 | 169.40 | 141.11 | 140.00 | 157.50 |
| sd | 3.74 | 6.66 | 1.98 | 3.54 | 4.66 | 2.12 | 11.92 | 13.28 | 3.99 | 11.51 | |
| range | 136–156 | 149–166 | 160–165 | 165–170 | 164–176 | 159–162 | 141–183 | 147–186 | 134–158 | 134–170 | |
|
| |||||||||||
| RR | n = 16 | n=0 | n=0 | n=0 | n=0 | n=0 | n=2 | n = 2 | n = 118 | n=1 | n=20 |
| mean | 70.88 | 80.50 | 59.00 | 66.72 | 32.00 | 27.20 | |||||
| sd | 8.67 | 14.85 | 1.41 | 20.42 | 17.82 | ||||||
| range | 61–98 | 70–91 | 58–60 | 15–98 | 10–81 | ||||||
|
| |||||||||||
| SpO2 | n = 62 | n=6 | n=7 | n=2 | n=9 | n=2 | n=20 | n = 10 | n = 131 | n=1 | n=20 |
| mean | 92.13 | 93.67 | 94.71 | 95.50 | 92.89 | 92.50 | 93.10 | 90.30 | 93.38 | 98.00 | 94.65 |
| sd | 2.21 | 1.21 | 1.11 | 0.71 | 1.62 | 0.71 | 2.15 | 1.34 | 1.86 | 1.98 | |
| range | 87–96 | 92–95 | 93–96 | 95–96 | 90–96 | 92–93 | 87–96 | 87–92 | 90–98 | 92–98 | |
|
| |||||||||||
| BP | Minute 20.5 | Minute 51 | Minute 109 | ||||||||
| 91/48 | 96/59 | 97/49 | |||||||||
| Minute 111 | |||||||||||
| 94/54 | |||||||||||
|
| |||||||||||
| Temperature | Minute 62 | ||||||||||
| 99.2 | |||||||||||
|
| |||||||||||
| Infant state | Quiet sleep | Quiet awake | Active awake | Active awake | Active awake | Active awake | Active awake | Quiet awake | Quiet sleep | Quiet sleep | Quiet sleep to active awake |
Note. SSC = skin-to-skin contact, SSC-1 = initial SSC, SSC-2 = resumption of SSC after bottle feeding, SSC-BF = breast feeding within SSC-2, SSC-3 = sustained SSC, HR = heart rate, RR = respiratory rate, SpO2 = peripheral oxygen saturation, BP = blood pressure
Blood pressure for this population of infants with CCHD is expected to be similar to that of healthy newborn infants. For infants of approximately 2 weeks of age, the 95th percentile for systolic BP is 98 mmHg and diastolic BP is 70mm Hg.18 Systolic BP in the study infant ranged between 91 and 97 mm Hg and diastolic BP between 48 and 54 mm Hg. BP was slightly higher during SSC-3 (97/49 and 94/54) when compared with pre-SSC (91/48). Axillary temperature (37.3 C) was at the upper end of the normal range for a newborn infant(35.6–37.2).19 The infant was in a quiet sleep state pre-SSC.
The infant awoke when transferring into SSC and remained predominantly in an active alert state through bottle feeding and transfers, moving into a quiet alert state during SSC with breast feeding. During SSC-3, the infant moved almost immediately into quiet sleep and did not rouse for an axillary temperature recording after 2.5 minutes in SSC-3, or blood pressure examinations after 49.5 and 51.5 minutes in SSC-3. Brief startles were noted after 14.5 and 53.5 minutes in SSC-3, but these did not awaken the infant. The infant remained in a quiet sleep state throughout SSC-3, transfer out of SSC-3, and for the first 4 minutes post SSC. Then medications needed to be administered, and the infant was aroused to an active alert state which continued to the end of the 10 minute post-SSC observation.
Comparisons of physiologic measures between pre-SSC and sustained SSC
Zero slope and linear slope regression discontinuity (RD) analyses are displayed in Tables 2 and 3. Figures 2, 3, and 4 depict trajectories of physiologic values as well as means and linear regression lines for the pre-SSC and SSC-3 periods.
Table 2.
Zero Slope Regression Discontinuity Analysis of Heart Rate, Respiratory Rate, and Oxygen Saturation on Period of Skin-to-Skin Contact
| Period | # Observations | Mean | SE | SD | Mean Difference | SE | df | t | CI (95%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| HR | Pre-SSC | 62 | 141.18 | 0.47 | 3.74 | 0.07 | 0.60 | 191 | 0.12 | −1.12 to 1.26 |
| SSC-3 | 131 | 141.11 | 0.35 | 3.99 | ||||||
| RR | Pre-SSC | 16 | 70.88 | 2.17 | 8.67 | 4.15 | 5.18 | 132 | 0.80 | −6.09 to 14.40 |
| SSC-3 | 118 | 66.72 | 1.88 | 20.42 | ||||||
| SpO2 | Pre-SSC | 62 | 92.13 | 0.28 | 2.21 | −1.25 | 0.31 | 191 | −4.10*** | −1.86 to −0.65 |
| SSC-3 | 131 | 93.38 | 0.16 | 1.86 |
Note. HR = heart rate, RR = respiratory rate, SpO2= peripheral oxygen saturation, Pre-SSC = before skin-to-skin contact, SSC-3 = sustained skin-to-skin contact.
= p < .05,
= p < .01,
= p < .001
Table 3.
Linear Slope Regression Discontinuity Analysis of Heart Rate, Respiratory Rate, and Oxygen Saturation on Period of Skin-to-Skin Contact
| Period | Test of slope | Test of differences in slope | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Coefficient | SE | t | CI (95%) | Coefficient | SE | t | CI (95%) | ||
| HR | Pre-SSC | 0.02 | 0.03 | 0.60 | −0.04 to 0.07 | −0.06 | 0.03 | −2.35* | −0.12 to −0.01 |
| SSC-3 | −0.05 | 0.01 | −5.92*** | −0.07 to −0.03 | |||||
| RR | Pre-SSC | −0.01 | 0.25 | −0.05 | −0.50 to 0.48 | −0.28 | 0.25 | −1.11 | −0.77 to 0.22 |
| SSC-3 | −0.29 | 0.04 | −6.56*** | −0.38 to −0.20 | |||||
| SpO2 | Pre-SSC | 0.10 | 0.01 | 9.37*** | 0.08 to 0.12 | −0.07 | 0.01 | −6.16*** | −0.09 to −0.05 |
| SSC-3 | 0.03 | 0.00 | 9.28*** | 0.02 to 0.04 | |||||
Note. HR = heart rate, RR = respiratory rate, SpO2= peripheral oxygen saturation, Pre-SSC = before skin-to-skin contact, SSC-3 = sustained skin-to-skin contact.
= p < .05,
= p < .01,
p < .001
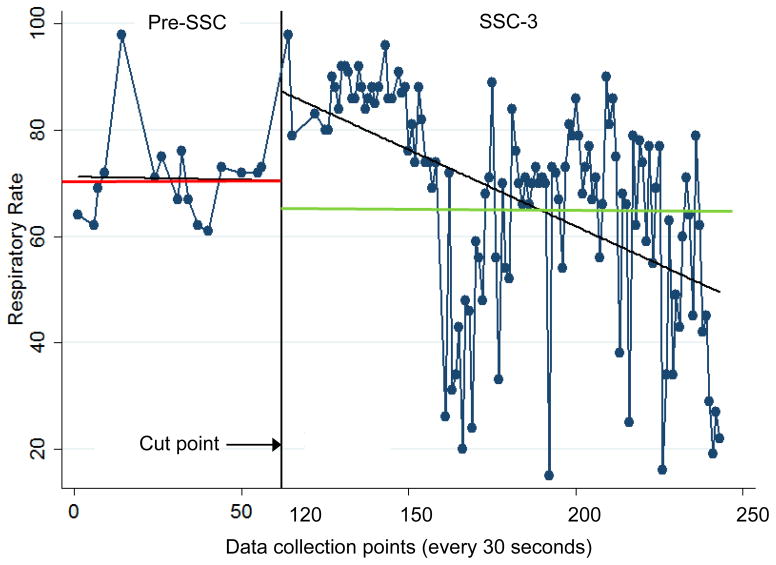
Figure 2.
Series of heart rate measures during 2 periods: pre-SSC (skin-to-skin contact; data collection points 0 to 62) and SSC-3 (data collection points 120 to 249). Comparisons are made between the series of measures on either side of the cut point. Angled lines indicate slope; horizontal lines indicate mean heart rate for respective observation periods.
Figure 3.
Series of respiratory rate measures during 2 periods: pre-SSC (skin-to-skin contact; data collection points 0 to 62) and SSC-3 (data collection points 120 to 249). Comparisons are made between the series of measures on either side of the cut point. Angled lines indicate slope; horizontal lines indicate mean respiratory rate for respective observation periods.
Figure 4.
Series of oxygen saturation (SpO2) measures during 2 periods: pre-SSC (skin-to-skin contact; data collection points 0 to 62) and SSC-3 (data collection points 120 to 249). Comparisons are made between the series of measures on either side of the cut point. Angled lines indicate slope; horizontal lines indicate mean oxygen saturation for respective observation periods.
Heart rate
Zero slope RD analysis showed no mean heart rate differences between pre-SSC and SSC-3. However, the linear slope during SSC-3 was significantly different from pre-SSC, i.e. when compared with pre-SSC, SSC-3 showed a significant negative linear slope toward a reduction in heart rate across SSC (p = 0.020; see Table 3).
Respiratory rates
Zero slope RD analysis showed no mean differences in respiratory rate between pre-SSC and SSC-3 and no significant differences in linear slope between these periods.
Oxygen saturation
Zero slope RD showed SpO2 during SSC-3 was significantly higher than pre-SSC values (p = .0001; see Table 2). However, linear slope of SpO2 during SSC-3 was significantly less steep than the linear slope pre-SSC (p = 0.000; see Table 3).
Discussion
Physiologic responses
Physiologic responses to being held on mother’s lap (pre-SSC), fed by bottle and breast, held in SSC, held on mother’s lap (post-SSC), and during transfers were measured in a full-term newborn male with hypoplastic left heart syndrome who had received a hybrid palliation. The infant demonstrated heart rate, respiratory rate, oxygen saturation, blood pressure, and temperature values predominantly within clinically acceptable ranges throughout our observations.
Heart rate
Similar to observations in premature infants,20,21 heart rates in this case study infant during sustained SSC (SSC-3) were essentially unchanged when compared with pre-SSC values and the standard deviation was small (3.99). However, there was a significant difference in slope of heart rate when comparing the pre-SSC and SSC-3 periods. Pre-SSC heart rate was fairly consistent with a slight increase in slope; heart rate during SSC-3 showed a consistent slope in the negative direction, demonstrating gradual reductions in heart rate as SSC-3 continued. This reduction in heart rate suggests predominantly parasympathetic activity during SSC, as has been reported in premature infants.22 Our results are consistent with the one published study examining SSC in infants with CCHD in which significantly lower heart rates were observed during SSC when compared with times out of SSC.10 Heart rates higher than are normally seen in infants were observed when the infant was exhibiting hunger cues and during bottle and breast feedings, as well as during post-SSC upon awakening and administration of medication. These increases in HR were likely due to sympathetic activation from hunger, feeding, arousal from sleep, and active body movements.
Respiratory rate
Respiratory rates were slightly lower in SSC-3, but were not significantly different compared with pre-SSC values. The unequal number of respiratory observations between pre-SSC (16 values) and sustained SSC (118 values) may have limited our ability to detect significant differences. Improved regulation of respiration has been reported in premature infants during SSC. In one randomized controlled trial comparing premature infants shortly before discharge home, infants receiving one episode of SSC for the approximately three hour interval between feedings demonstrated significantly less periodic breathing during SSC when compared with infants in open cribs.20 Other studies of premature infants have noted reduced respiratory rates,23,24 although respirations may be less regular depending on airway positioning during SSC.25 In one study examining SSC in infants with CCHD, significantly lower respiratory rates were observed during SSC when compared with times out of SSC.10 Lower than expected respiratory rates (less than 25 breaths per minute) were observed in this infant in 5.1% (6 of 118)of the observations during SSC-3. These low respiratory rates may be associated with central oxytocin release during SSC which downregulates sympathetic activation and accentuates parasympathetic function, calming the infant26 and lowering respiratory rates. Given that the slope of SpO2 increased throughout SSC-3, these lower rates did not compromise oxygen saturations in this infant.
Oxygen saturation
Oxygen saturations were significantly higher during SSC-3 when compared with pre-SSC. There was a significant difference in oxygen saturation slopes between pre-SSC and SSC-3. Prior to our pre-SSC observations, this infant had finished a four minute bottle feeding which may have reduced oxygen saturations. Our pre-SSC observations may have captured recovery from lower SpO2 which occurred before we began recording. Indeed, our observed oxygen saturations appeared lower during the bottle and breast feedings that occurred prior to SSC-3, accounting for the lower saturations at the start of SSC-3. In spite of these changes in oxygenation with feedings, all values remained within clinically acceptable ranges.
Reports of oxygen saturation changes during SSC have been mixed: arterial oxygen saturation (SaO2) has increased,21,24,27 decreased,20,28 and not changed at all.25,29,30 The most common finding in previous SSC studies is that SaO2 remains relatively unchanged from baseline during SSC31 and metanalyses have confirmed stability during SSC.28,32 Overall, oxygen saturation changes during SSC have been slight and are accompanied by increased stability with values remaining within clinically acceptable ranges. Significantly higher oxygen saturations during SSC coupled with gradual reductions in heart rates in this infant suggest a minimization of oxygen consumption and, thus, reduced oxygen requirement and less stress on the heart.
Blood pressure
This case study provides seminal data about infant blood pressure during SSC that has not previously been described. Because blood pressure is affected by position, a prolonged tilted position as is done in SSC may be a concern in infants with potential or actual hemodynamic instability. BP in this infant was obtained (1) mid-way through the pre-SSC observation when the infant was in a supine position, (2) when the infant was held in SSC, but displaying hunger cues, and (3) when the infant had been in SSC-3 for about 50 minutes. Blood pressure was slightly higher in SSC-3 when compared with pre-SSC, but remained within a clinically acceptable range for healthy infants. Clinically insignificant increases in mean arterial BP with SSC have been observed in premature infants2,24 Change from a reclining to an upright position stimulates baroreceptors, resulting in sympathetic activation, increased peripheral vascular resistance, and increased BP.33 Once the infant has adapted to the position change, BP stabilizes.33 Previous research has shown that during SSC, sympathetic activation is reduced and parasympathetic function is enhanced,22 cortisol levels are reduced,34 and behavioral manifestations of relaxation are exhibited.35 Our findings of stable hemodynamics and quiet sleep with SSC are consistent with enhanced parasympathetic activity and low infant distress.
Temperature
The axillary temperature for this infant was taken early in the SSC-3 period and found to be 37.3 C, which is at the upper limits of a normal range of axillary temperature for infants.19 Previous research has shown that temperatures during SSC can be either higher or lower than those of an infant in an incubator or open crib. Higher temperatures that are still within normal ranges are seen during SSC in premature infants.36 Extremely premature infants (25–28 weeks gestation) and premature infants of a higher gestational age (29–32 weeks) are able to regulate temperature during SSC,36 although lower temperatures may be observed during transfers.37 Healthy term infants held in SSC shortly after birth also demonstrate the ability to maintain temperatures within normal ranges.38 This infant did not have difficulty regulating temperature during SSC. However, our single measure did not allow us to compare stability of temperature across SSC periods.
The data reported here are encouraging and supplement the limited evidence-base of SSC effects on infants with CCHD. Only one previous study with CCHD infants has been published.10 Five infants recovering in the cardiothoracic intensive care unit from surgery for CCHD were followed to document physiologic responses to SSC during immediate postoperative recovery. Three cycles of two hours of SSC followed by two hours in an incubator were conducted over 12-hours immediately following extubation. During SSC, significant increases in oxygen saturations and reductions in transcutaneous carbon dioxide, heart rate, and respiratory rates were observed. Although the use of the SSC intervention is considered standard of care in neonatal intensive care units, SSC is not a common intervention in cardiothoracic intensive care units. Infants with heart disease are considered particularly vulnerable to cardiorespiratory instability,8 but both Gazzolo’s and our findings suggest that SSC may contribute to cardiorespiratory stability. Consistent with findings of enhanced physiologic regulation in high-risk premature infants and with infants with CCHD early in the post-operative recovery period, this infant with serious heart disease demonstrated maintenance of infant physiologic stability during SSC, as well as during transfers into and out of SSC.
Elements of SSC are known to enhance cardiorespiratory stability. First, infants in SSC are inclined at approximately 45–60 degree angle which is known to improve oxygenation.39,40 Second, in ventilated preterm infants, prone positioning is associated with higher oxygen saturations and reduced physical activity, thus reducing energy expenditure,41 as well as more quiet sleep and fewer stress behaviors.42 Third, SSC stimulates C tactile afferent nerves which cause central oxytocin release,43,44 likely via direct stimulation of vagal nerve efferents.32,45 Central release of oxytocin reduces sympathetic and increases parasympathetic activity, thus promoting cardiorespiratory stability and changing infant psychophysiologic state from stress to calmness.26 Finally, the close physical presence of the mother provides cardiorespiratory stability through regulation of infant autonomic, neuroendocrine, and behavioral responses.46 Maternal regulation of infant physiologic processes may occur through the calming effect of SSC on mothers,47 likely mediated by enhanced maternal oxytocin release with SSC.26
Additional observations
In addition to enhancing cardiorespiratory regulation, other benefits of SSC have been suggested by the case study reported here. Feeding problems are frequent in infants with CCHD.48 Although we were not specifically measuring markers of feeding skill, we observed both feeding by bottle out of SSC and feeding at the breast during SSC. Both feedings were physiologically challenging to the infant as demonstrated by physiologic values exceeding clinically acceptable ranges, but the breast feeding was much less disruptive than the feeding by bottle. The single time the infant appeared dysregulated during our entire observation was during the feeding by bottle, in which the infant clearly was unable to coordinate sucking, swallowing, and breathing. In contrast, the breast feeding that occurred during SSC was accomplished with no apparent infant distress and with skill even though this was the infant’s first breast feeding experience. The SSC intervention in premature infants is closely associated with increased breast feeding initiation, duration, exclusivity, and maternal milk volume.38,49,50 Researchers have demonstrated in numerous studies of hospitalized preterm infants that infants are better able to coordinate sucking, swallowing, and breathing during breast feeding than they are during bottle feeding, resulting in enhanced oxygen saturations.51,52 Similar to preterm infants, infants with cardiac disease often experience desaturations with bottle feeding, but not with breast feeding.53 No desaturations with feeding were observed in the case study infant whose oxygen saturations were actually higher than expected for an infant with this type of heart disease. Breast feeding rates in the CCHD population are low-- less than 25% are breast feeding at time of discharge after neonatal surgery and less than 10% are breast feeding at time of admission for second stage palliative procedure.54 By enhancing infant physiologic regulation, SSC may be able to improve the CCHD infant’s ability to regulate feeding and improve breast feeding in this high risk population.
SSC has also been found to lead to better quality of quiet sleep.5,55 Indeed, at the end of our observations, this mother commented that her infant had slept better and more deeply during SSC than he had slept since birth when in an incubator, crib, or on her lap. Improved quiet sleep is consistent with studies demonstrating more mature sleep patterns with SSC than without SSC in preterm infants.5,56
Limitations
This case study reported on the physiologic responses to SSC in one infant following surgical intervention for CCHD, and, as such cannot be used to draw general conclusions about responses to the SSC intervention in this population. Although the pre-SSC time period was adequate, interruptions for nursing care shortened the post-SSC observation period, limiting assessment of potential continuing effects. In addition, SSC immediately following the pre-SSC observation was interrupted by infant hunger cues and subsequent feedings. However, these interruptions provided additional unplanned, but important, observations of infant physiologic response to feeding. Finally, measurements of temperature pre- and post-SSC and of blood pressure post-SSC would have enabled a closer examination of possible changes in these parameters.
Conclusions and Future Research
This case study provides beginning evidence that heart rate, respiratory rate, oxygen saturation, blood pressure, and temperature are clinically acceptable during SSC in full-term infants following surgery for complex congenital heart disease. Well-accepted and practiced as a therapeutic intervention in the premature infant population, the rich evidence-base of positive SSC effects in preterm and full term infants31,57 suggests that similar benefits of SSC may be experienced by CCHD infants. However, the science of SSC with CCHD infants is only just emerging and further research is needed. This case study measured infant physiologic responses during SSC when the infant had begun feedings post-operatively. Immediate physiologic responses during SSC must be examined in a larger sample of infants with various cardiac defects and observed pre-operatively as well as earlier in the post-operative period. Associations of SSC with short-term outcomes known to be positively impacted in premature infants need study in infants with CCHD, including feeding,58 sleep,55 length of stay,49 and maternal-infant interactions.49 In addition, SSC is known to enhance both autonomic nervous system function and neurodevelopment in premature infants.4,59,60 Since infants with CCHD are known to have deficits in both of these areas,61–65 testing effects of SSC on these long-term outcomes is crucial. Increasing our knowledge of effects of this low-cost, parent-delivered intervention in infants with CCHD has the potential to improve the care provided to, and ultimately the outcomes experienced by, these vulnerable infants.
What’s New.
An 18-day-old infant remained physiologically stable during a 65-minute skin-to-skin intervention following surgery for hypoplastic left heart syndrome.
Additional research is needed to confirm the safety and feasibility of skin to skin contact in cardiothoracic intensive care units before the routine inclusion of this therapeutic intervention in the care of infants with complex congenital heart disease.
Acknowledgments
This study was supported by the National Institute of Nursing Research Grant #P20 NR008992; Center for Health Trajectory Research, University of Minnesota School of Nursing. The authors would like to give special acknowledgement to Roger Brown, PhD, for providing statistical services.
Contributor Information
Tondi M. Harrison, Assistant Professor, University of Minnesota School of Nursing, Minneapolis, Minnesota.
Susan Ludington-Hoe, Carl W. and Margaret Davis Walter Professor of Pediatric Nursing, Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, Ohio.
References
- 1.Carbasse A, Kracher S, Hausser M, et al. Safety and effectiveness of skin-to-skin contact in the NICU to support neurodevelopment in vulnerable preterm infants. J Perinat Neonat Nurs. 2013;27(3):255–262. doi: 10.1097/JPN.0b013e31829dc349. [DOI] [PubMed] [Google Scholar]
- 2.De Oliveira Azevedo VMG, Xavier CC, de Oliveira Gontijo F. Safety of kangaroo mother care in intubated neonates under 1500g. J Trop Pediatr. 2012;58:38–42. doi: 10.1093/tropej/fmr033. [DOI] [PubMed] [Google Scholar]
- 3.Maastrup R, Greisen G. Extremely preterm infants tolerate skin-to-skin contact during the first weeks of life. Acta Paediat. 2010;99:115–1149. doi: 10.1111/j.1651-2227.2010.01806.x. [DOI] [PubMed] [Google Scholar]
- 4.Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol Psychiatry. 2014;75:56–64. doi: 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Scher M, Ludington-Hoe S, Kaffashi F, Johnson MW, Holditch-Davis D, Lopara KA. Neurophysiologic assessment of brain maturation after an eight week trial of skin-to-skin contact on preterm infants. Clin Neurophysiol. 2009;120:1812–1818. doi: 10.1016/j.clinph.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cong X, Cusson RM, Hussain N, Zhang D, Kelly SP. Kangaroo care and behavioral and physiologic pain responses in very low birth weight twins: A case study. Pain Manag Nurs. 2012;13(3):127–138. doi: 10.1016/j.pmn.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Porges SW, Furman SA. The early development of the autonomic nervous system provides a neural platform for social behavior: A polyvagal perspective. Infant Child Dev. 2011;20:106–118. doi: 10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman JIE. The natural and unnatural history of congenital heart disease. West Sussex UK: Wiley-Blackwell; 2009. [Google Scholar]
- 9.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 10.Gazzalo D, Masetti P, Meli M. Kangaroo care improves post-extubation cardiorespiratory parameters in infants after open heart surgery. Acta Paediatr. 2000;89:728–729. doi: 10.1080/080352500750044098. [DOI] [PubMed] [Google Scholar]
- 11.Sadowski SL. Congenital cardiac disease in the newborn infant: past, present, and future. Crit Care Nurs Clin North Am. 2009;21:37–48. doi: 10.1016/j.ccell.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Galantowicz M, Cheatham J. Lessons learned from the development of a new hybrid strategy for the management of hypoplastic left heart syndrome. Pediatr Cardiol. 2005;26:190–199. doi: 10.1007/s00246-005-8962-6. [DOI] [PubMed] [Google Scholar]
- 13.Galantowicz M, Cheatham J, Phillips A, et al. Hybrid approach for hypoplastic left heart syndrome: Intermediate results after the learning curve. Ann Thorac Surg. 2008;85:2063–2071. doi: 10.1016/j.athoracsur.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Brazelton TB. Clinics in Developmental Medicine. 50. Spastics International Medical Publications; London: Wm Heinemann medical Books Ltd; Philadelphia: J. P. Lippincott; 1973. Neonatal Behavioral assessment Scale. [Google Scholar]
- 15.Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. Boston, MA: Houghton Mifflin Company; 2002. [Google Scholar]
- 16.Galantowicz M, Yates A, Cua C, Naguib A, Simsic J, Cheatham JP. Peri-operative and interstage considerations for the hybrid approach for hypoplastic left heart syndrome. In: da Cruz EM, Ivy D, Jaggers J, editors. Pediatric and Congenital Cardiology, Cardiac Surgery, and Intensive Care. London: Springer-Verlag; 2014. pp. 1809–1824. [Google Scholar]
- 17.Fleming S, Thompson M, Steens R, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377:1011–1018. doi: 10.1016/S0140-6736(10)62226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Report of the second task force on blood pressure control in children—1987. Pediatrics. 1987;79:1–25. [PubMed] [Google Scholar]
- 19.Morley CJ, Hewson PH, Thornton AJ, Cole TJ. Axillary and rectal temperature measurements in infants. Arch Dis Child. 1992;67:122–125. doi: 10.1136/adc.67.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludington-Hoe SM, Anderson GC, Swinth JY, Thompson C, Hadeed AJ. Randomized controlled trial of Kangaroo Care: Cardiorespiratory and thermal effects on healthy preterm infants. Neonatal Netw. 2004;23:39–48. doi: 10.1891/0730-0832.23.3.39. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell AJ, Yates C, Williams K, Hall RW. Effects of daily kangaroo care on cardiorespiratory parameters in preterm infants. J Neonatal Perinatal Med. 2013;6:243–249. doi: 10.3233/NPM-1370513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCain GC, Ludington-Hoe SM, Swinth JY, Hadeed AJ. Heart rate variability responses of a preterm infant to kangaroo care. J Obstet Gynecol Neonatal Nurs. 2005;34:689–694. doi: 10.1177/0884217505281857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson GC, Moore E, Hepworth J, Bergman N. Early skin –to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2003;(2):CD003519.806. doi: 10.1002/14651858.CD003519. [DOI] [PubMed] [Google Scholar]
- 24.Van Zanten HA, Havenaar AJ, Stigt HJH, Ligthart PAH, Walther FJ. The kangaroo method is safe for premature infants under 30 weeks of gestation during ventilator support. J Neonatal Nurs. 2007;13:186–190. [Google Scholar]
- 25.Bohnhorst B, Gill D, Dordelmann M, Peter CS, Poets CF. Bradycardia and desaturation during skin-to-skin care: No relationship to hyperthermia. J Pediatr. 2004;145:499–502. doi: 10.1016/j.jpeds.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Uvnäs-Moberg K, Arn I, Magnusson D. The psychobiology of emotion: The role of the oxytocinergic system. Int J Behav Med. 2005;12:59–65. doi: 10.1207/s15327558ijbm1202_3. [DOI] [PubMed] [Google Scholar]
- 27.Kadam S, Binoy S, Kanbur W, Mondkar JA, Fernandez A. Feasibility of Kangaroo mother care in Mumbai. Indian J Pediatr. 2005;72(1):35–38. doi: 10.1007/BF02760578. [DOI] [PubMed] [Google Scholar]
- 28.Mori R, Rajesh K, Pledge D, Nakayama T. Meta-analysis of physiological effects of skin-to-skin contact for newborns and mothers. Pediatr Int. 2010;52:161–170. doi: 10.1111/j.1442-200X.2009.02909.x. [DOI] [PubMed] [Google Scholar]
- 29.Begum EA, Bonno M, Ohtani N, et al. Cerebral oxygenation responses during kangaroo care in low birth weight infants. Neonatal Intensive Care. 2008;2:2–25. doi: 10.1186/1471-2431-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludington-Hoe SM, Hosseini R, Torowicz DL. Skin-to-skin contact (Kangaroo Care) analgesia for preterm infant heel stick. AACN Clin Issues. 2005;16:373–387. doi: 10.1097/00044067-200507000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludington-Hoe SM. Thirty years of kangaroo care science and practice. Neonatal Netw. 2011;30:357–362. doi: 10.1891/0730-0832.30.5.357. [DOI] [PubMed] [Google Scholar]
- 32.Moore ER, Anderson GC, Bergman N, Dowswell T. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database of Syst Rev. 2012;(5):CD003519. doi: 10.1002/14651858.CD003519.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrod L, Walter J. Effect of head-up body tilt position on autonomic function and cerebral oxygenation in preterm infants. Biology of the Neonate. 2002;81:255–259. doi: 10.1159/000056756. [DOI] [PubMed] [Google Scholar]
- 34.Neu M, Hazel N, Robinson J, Schmiege S, Laudenslager M. Effect of holding on co-regulation in preterm infants: a randomized controlled trial. Early Hum Dev. 2014;90:141–147. doi: 10.1016/j.earlhumdev.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Widstrom A-M, Lilja G, Aaltomaa-Michalias P, Dahllof A, Lintula M, Nissen E. Newborn behavior to locate the breast when skin-to-skin: a possible method for enabling early self-regulation. Acta Paediat. 2011;100:79–85. doi: 10.1111/j.1651-2227.2010.01983.x. [DOI] [PubMed] [Google Scholar]
- 36.Park HK, Choi BS, Lee SJ, Son IA, Seol IJ, Lee HJ. Practical application of kangaroo mother care in preterm infants: clinical characteristics and safety of kangaroo mother care. J Perinat Med. 2013 doi: 10.1515/jpm-2013-0066. [DOI] [PubMed] [Google Scholar]
- 37.Karlsson V, Heinemann AB, Sjörs G, Nykvist KH, Ågren J. Early skin-to-skin care in extremely preterm infants: Thermal balance and care environment. J Pediatr. 2012;161:422–426. doi: 10.1016/j.jpeds.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 38.Moore ER, Anderson GC, Bergman N, Dowswell T. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database of Syst Rev. 2012;(5):CD003519. doi: 10.1002/14651858.CD003519.pub3. [DOI] [PMC free article] [PubMed]
- 39.Dellagrammaticas HD, Kapetanakis J, Papadimitriou M, Kourakis G. Effect of body tilting on physiological functions in stable very low birthweight neonates. Arch Dis Child. 1991;66:429–432. doi: 10.1136/adc.66.4_spec_no.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thoresen M, Cowan F, Whitelaw A. Effect of tilting on oxygenation in newborn infants. Arch Dis Child. 1998;63:315–317. doi: 10.1136/adc.63.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang YJ, Anderson GC, Dowling D, Lin CH. Decreased activity and oxygen desaturation in prone ventilated preterm infants during the first postnatal week. Heart Lung. 2002;31:34–42. doi: 10.1067/mhl.2002.120241. [DOI] [PubMed] [Google Scholar]
- 42.Chang YJ, Anderson GC, Lin CH. Effects of prone and supine positions on sleep state and stress responses in mechanically ventilated preterm infants during the first postnatal week. J Adv Nurs. 2002;40:161–169. doi: 10.1046/j.1365-2648.2002.02358.x. [DOI] [PubMed] [Google Scholar]
- 43.Bystrova K. Novel mechanism of human fetal growth regulation: A potential role of lanugo, vernixcaseosa and a second tactile system of unmyelinated low-threshold C-afferents. Med Hypotheses. 2009;72:143–146. doi: 10.1016/j.mehy.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 44.Olausson H, Wessberg J, Morrison I, McGlone F, Vallbo A. The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev. 2010;34:185–191. doi: 10.1016/j.neubiorev.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Kojima S, Stewart RA, Demas GE, Alberts JR. Maternal contact differentially modulates central and peripheral oxytocin in rat pups during a brief regime of mother-pup interaction that induces a filial huddling preference. J Neuroendocrinol. 2012;24:831–840. doi: 10.1111/j.1365-2826.2012.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatr. 1994;397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- 47.Morelius E, Theodorsson E, Nelson N. Salivary cortisol and mood and pain profiles during skin-to-skin care for an unselected group of mothers and infants in neonatal intensive care. Pediatrics. 2005;116:1105–1113. doi: 10.1542/peds.2004-2440. [DOI] [PubMed] [Google Scholar]
- 48.Medoff-Cooper B, Ravishankar C. Nutrition and growth in congenital heart disease: a challenge in children. Curr Opin Cardiol. 2013;28:122–129. doi: 10.1097/HCO.0b013e32835dd005. [DOI] [PubMed] [Google Scholar]
- 49.Conde-Agudelo A, Diaz-Rossello JL, Belizam JM. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2011;(3):CD002771. doi: 10.1002/14651858.CD002771.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Nyqvist KH, Haggkvist An-P, Hansen MN, et al. Expansion of the baby-friendly hospital initiative ten steps to successful breast feeding into neonatal intensive care: Expert group recommendations. J Hum Lact. 2013;29:300–309. doi: 10.1177/0890334413489775. [DOI] [PubMed] [Google Scholar]
- 51.Meier P. Bottle and breast-feeding: effects on transcutaneous oxygen pressure and temperature in preterm infants. Nurs Res. 1988;37:36–41. [PubMed] [Google Scholar]
- 52.Chen CH, Wang TM, Chang HM, Chi CS. The effect of breast- and bottle-feeding on oxygen saturation and body temperature in preterm infants. J Hum Lact. 2000;18(1):21–27. doi: 10.1177/089033440001600105. [DOI] [PubMed] [Google Scholar]
- 53.Marino BL, O’Brien P, LoRe H. Oxygen saturations during breast and bottle feedings in infants with congenital heart disease. J Pediatr Nurs. 1995;10:360–364. doi: 10.1016/S0882-5963(05)80033-8. [DOI] [PubMed] [Google Scholar]
- 54.Anderson JB, Beekman RH, Border WL, et al. Lower weight-for-age z score adversely affects hospital length of stay after the bidirectional Glenn procedure in 100 infants with a single ventricle. J Thorac Cardiovasc Surg. 2009;138:397–404. doi: 10.1016/j.jtcvs.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 55.Ludington-Hoe SM, Johnson MW, Morgan K, Lewis T, Gutman P, Wilson D, Scher MS. Neurophysiologic assessment of neonatal sleep organization: preliminary results of a randomized controlled trial of skin contact with preterm infants. Pediatrics. 2006;117:e909–e923. doi: 10.1542/peds.2004-1422. [DOI] [PubMed] [Google Scholar]
- 56.Feldman R, Weller A, Sirota L, Eidelman A. Skin-to-skin contact (kangaroo care) promotes self-regulation in premature infants: sleep-wake cyclicity, arousal modulation, and sustained exploration. Dev Psychol. 2002;38:194–207. doi: 10.1037//0012-1649.38.2.194. [DOI] [PubMed] [Google Scholar]
- 57.Ludington-Hoe SM. Kangaroo care is developmental care. In: Kenner C, McGrath JM, editors. Developmental Care of Newborns and Infants. 2. Glenview IL: National Association of Neonatal Nurses; 2010. pp. 349–388. [Google Scholar]
- 58.Renfrew MJ, Craig D, Dyson LO, et al. Breast feeding promotion for infants in neonatal units: a systematic review and economic analysis. Health Technol Assess. 2009;13:1–146. doi: 10.3310/hta13400. [DOI] [PubMed] [Google Scholar]
- 59.Feldman R, Eidelman AI. Skin-to-skin contact (kangaroo care) accelerates autonomic and neurobehavioral maturation in preterm infants. Dev Med Child Neurol. 2003;45:274–281. doi: 10.1017/s0012162203000525. [DOI] [PubMed] [Google Scholar]
- 60.Feldman R, Weller A, Sirota L, Eidelman AI. Skin-to-skin contact (kangaroocare) promotes self-regulation in premature infants: Sleep-wake cyclicity, arousal modulation, and sustained exploration. Developmental Psychology. 2002;38:194–207. doi: 10.1037//0012-1649.38.2.194. [DOI] [PubMed] [Google Scholar]
- 61.Harrison TM, Brown R. Autonomic nervous system function in infants with transposition of the great arteries. Biological Research for Nursing. 2012;14:257–268. doi: 10.1177/1099800411407687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaltman JR, Hanna BD, Gallagher PR, et al. Heart rate variability following neonatal heart surgery for complex congenital heart disease. PACE. 2006;29:471–478. doi: 10.1111/j.1540-8159.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 63.McGlone L, Patel N, Young D, Danton MD. Impaired cardiac autonomic nervous control after cardiac bypass surgery for congenital heart disease. Interactive Cardiovascular and Thoracic Surgery. 2009;9:218–222. doi: 10.1510/icvts.2008.194555. [DOI] [PubMed] [Google Scholar]
- 64.Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 65.Massaro AN, El-Dib M, Glass P, Aly H. Factors associated with adverse neurodevelopmental outcomes in infants with congenital heart disease. Brain & Development. 2008;30:437–446. doi: 10.1016/j.braindev.2007.12.013. [DOI] [PubMed] [Google Scholar]