Abstract
GSK2251052, a novel leucyl-tRNA synthetase (LeuRS) inhibitor, was in development for the treatment of infections caused by multidrug-resistant Gram-negative pathogens. In a phase II study (study LRS114688) evaluating the efficacy of GSK2251052 in complicated urinary tract infections, resistance developed very rapidly in 3 of 14 subjects enrolled, with ≥32-fold increases in the GSK2251052 MIC of the infecting pathogen being detected. A fourth subject did not exhibit the development of resistance in the baseline pathogen but posttherapy did present with a different pathogen resistant to GSK2251052. Whole-genome DNA sequencing of Escherichia coli isolates collected longitudinally from two study LRS114688 subjects confirmed that GSK2251052 resistance was due to specific mutations, selected on the first day of therapy, in the LeuRS editing domain. Phylogenetic analysis strongly suggested that resistant Escherichia coli isolates resulted from clonal expansion of baseline susceptible strains. This resistance development likely resulted from the confluence of multiple factors, of which only some can be assessed preclinically. Our study shows the challenges of developing antibiotics and the importance of clinical studies to evaluate their effect on disease pathogenesis. (These studies have been registered at ClinicalTrials.gov under registration no. NCT01381549 for the study of complicated urinary tract infections and registration no. NCT01381562 for the study of complicated intra-abdominal infections.)
INTRODUCTION
Approximately 5% of patients admitted to hospitals in the United States develop nosocomial infections that increase not only patient mortality (1) but also hospitalization time and cost of treatment (2, 3). In the United States, Gram-negative bacterial pathogens, such as Escherichia coli, Klebsiella pneumoniae/Klebsiella oxytoca, Pseudomonas aeruginosa, and Acinetobacter baumannii, are responsible for 35% of the most common hospital-acquired infections (HAIs) or conditions, including urinary tract infections (UTIs), pneumonia, and surgical site and bloodstream infections. Furthermore, >70% of the bacteria causing HAIs are resistant to at least one of the most commonly used antibiotics (4–6). The continuing emergence of resistance has compromised treatment options for Gram-negative bacterial pathogens and forced the use of polymyxins (7), an old antibiotic class with nephrotoxicity issues. Despite the clear need for alternatives to treat these multidrug-resistant life-threatening pathogens, a review of the antibacterial pipeline reveals a scarcity of new antibiotic candidates (8).
Aminoacyl-tRNA synthetases (AaRSs) play an essential role in protein synthesis (9) and have been clinically validated to be antibacterial targets of mupirocin (10), an inhibitor of isoleucyl-tRNA synthetase that has been successfully used since 1985 in the topical treatment of Gram-positive bacterial skin infections (11). Their ubiquitous nature, high degree of conservation within a broad spectrum of bacterial species, and considerable divergence between prokaryotic and eukaryotic enzymes have made AaRSs the subject of multiple antibacterial programs (12, 13), although no other AaRS inhibitor has reached the market to date.
The demonstration that the antifungal tavaborole could specifically inhibit yeast cytoplasmic leucyl-tRNA synthetase (LeuRS) (14) triggered the search for additional benzoxaboroles capable of inhibiting the editing domain of the bacterial counterpart. A structure-based design effort focused on the production of potent Gram-negative bacterial LeuRS inhibitors led to the discovery of GSK2251052 (AN3365) (15). This bacteriostatic compound demonstrates in vitro activity against a wide range of anaerobic (16) and Gram-negative bacteria, including multidrug-resistant pathogens, such as extended-spectrum-β-lactamase-, metallo-β-lactamase-, and carbapenemase-producing organisms (17), and has a low propensity to be effluxed (15). A phase I study in healthy volunteers showed that GSK2251052 had linear pharmacokinetics (18) and caused no serious or dose-limiting adverse events (15, 19). These data from the phase I study, combined with the impressive in vitro microbiology profile of GSK2251052 against multidrug-resistant Gram-negative organisms, led to the initiation of two prospective, multinational, randomized, double-blind phase IIa clinical trials. These studies were conducted to evaluate the safety, tolerability, and efficacy of two twice-daily (BID) doses (750 and 1,500 mg) of intravenous (i.v.) GSK2251052 in adult subjects with complicated urinary tract infections (cUTIs; study LRS114688, ClinicalTrials.gov registration no. NCT01381549) or complicated intra-abdominal infections (cIAIs; study LRS114689, ClinicalTrials.gov registration no. NCT01381562).
(Part of this work was presented at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, September 2013 [20].)
MATERIALS AND METHODS
Study design.
Study LRS114688 involved eight centers in three countries (the United States, Spain, and Greece) and was conducted between 28 June 2011 and 6 March 2012. Study LRS114689 involved five centers in four countries (the United States, Spain, France, and Russia) and was conducted between 3 October 2011 and 5 March 2012.
In both studies, 210 subjects at least 18 years old were to be treated for ≥5 to ≤14 days. Subjects in cUTI study LRS114688 were randomized (1:1:1) to receive GSK2251052 at 750 mg or 1,500 mg BID or imipenem-cilastatin at 500 mg every 6 h. Twenty subjects had been enrolled when this study was terminated, and 14 of them had received GSK2251052 at doses of 750 mg (n = 6) or 1,500 mg (n = 8) BID. Nineteen of the 20 subjects had a qualifying Gram-negative bacterial uropathogen at the baseline, with E. coli being the most frequently isolated pathogen (18 of 26 isolates). Subjects in cIAI study LRS114689 were randomized (1:1:1) to receive GSK2251052 at 750 mg or 1,500 mg BID or meropenem at 1 g three times a day. Fifteen subjects had been enrolled when this study was terminated, with nine of them having received GSK2251052 at doses of 750 mg (n = 5) or 1,500 mg (n = 4) BID. Ten of the 15 subjects had a confirmed Gram-negative bacterial pathogen at the baseline, with E. coli being the most frequently isolated pathogen (9 of 28 isolates).
The institutional review boards of the institutions involved in the clinical trials reviewed and approved the studies prior to subject enrollment. Written informed consent was obtained from each subject prior to the performance of any study-specific procedures.
Susceptibility testing.
All bacterial strains were cultured at 35°C on Trypticase soy agar (TSA) with 5% sheep blood or in cation-adjusted Mueller-Hinton broth (MHB). E. coli ATCC 25922, 1162222, and 1161435 were obtained from the GlaxoSmithKline Microbiology Culture Collection. E. coli, Proteus mirabilis, Enterococcus faecalis, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Staphylococcus epidermidis isolates were obtained from four subjects participating in the LRS114688 cUTI clinical trial.
MICs of antibacterial agents were determined using the broth microdilution methodology (21). Validated dry-form custom-made broth microdilution panels were used in cross-resistance studies with GSK2251052, amikacin, ampicillin, aztreonam, cefepime, ceftazidime, ceftriaxone, ciprofloxacin, doripenem, ertapenem, imipenem, levofloxacin, meropenem, nitrofurantoin, piperacillin-tazobactam, tigecycline, and trimethoprim-sulfamethoxazole.
The frequency of spontaneous resistance to GSK2251052 was determined by spreading bacterial suspensions of the different E. coli strains in MHB (∼2.5 × 108 CFU) onto TSA plates containing 4× or 10× MIC of GSK2251052 and a control plate with no compound. Colonies were counted after 48 h incubation at 35°C. To confirm the resistance phenotypes, the susceptibility to GSK2251052 of a representative number of colonies from each antibiotic concentration-strain combination was tested. Colonies were defined as resistant if their MIC was ≥4-fold that of the parent strain. The total number of resistant colonies was extrapolated from this representative set. The frequency of resistance was calculated by dividing the total number of resistant colonies by the total number of CFU plated.
Genetic characterization of isolates with reduced susceptibility to GSK2251052.
The PCR primers used for DNA amplification were designed from the appropriate regions of publically available genomic sequences. To prepare the PCR templates, cells from overnight culture plates were resuspended in 50 μl Tris-EDTA (TE) buffer, boiled for 10 min, and placed on ice for 5 min before a 2-min high-speed centrifugation. Supernatants (2 μl) were used as the templates in the PCRs, carried out on an Applied Biosystems GeneAmp PCR system 9700. The PCR products were sequenced using a BigDye Terminator cycle sequencing kit (v3.1; Applied Biosystems) and analyzed in a genetic analyzer (3730XL; Applied Biosystems).
Fragments of the leuS gene were amplified by PCR, sequenced, and aligned with the sequences from their corresponding parent organisms. The K. pneumoniae DNA sequence from subject 751 was aligned with sequences from the published genomes of strains MGH78578 and JH1 and the proprietary genome of strain 1161486. A well-validated PCR-based allelic replacement method utilizing lambda Red recombinase (22) was used to introduce a LeuRS T247I mutation in E. coli 1162222 and E. coli 1161435.
Genomic DNA from both strains was used as the template for the amplification of their leuS gene by a 3-step PCR protocol, which allowed the introduction of a nucleotide change (ACT to ATT) in the codon corresponding to LeuRS residue 247. Subsequently, these PCR fragments were introduced by electroporation into the corresponding strains expressing the lambda Red recombination system in plasmid pKD46 (22). Isolates carrying the LeuRS T247I mutation were selected on plates containing GSK2251052. Mutations were confirmed by PCR amplification and sequencing of the leuS gene.
Illumina library preparation and whole-genome sequencing.
DNA samples were prepared from monocultures of single bacterial colonies. Fragment libraries (mode length, 350 bp) were constructed using an Illumina paired-end DNA sample preparation kit (v2) according to the instructions of the manufacturer, with some modifications. Genomic DNA (1 μg in 50 μl TE buffer) was sheared for 180 s on a Covaris S220 sonicator (duty factor, 10; peak power, 175 W; 200 cycles per burst). For accurate quantification, quantitative PCR was performed in triplicate using a KAPA library quantification kit on a 7900HT real-time PCR instrument (Life Technologies). Samples (2 nmol) were normalized and pooled into 7 batches containing 2 samples each. A unique adapter index sequence was used for each sample to allow independent samples to be pooled for sequencing and subsequent bioinformatic segregation of the data output. Fragment libraries were separated and size selected (300- to 400-bp insert range), and primer dimers were removed using an Agilent high-sensitivity DNA kit on an Agilent 2100 bioanalyzer. The Illumina GAII sequencer instrument, reagents, and flow cell were prepared according to Illumina TruSeq SBS kit (v5) protocols. Libraries were diluted to a final 12-pmol dilution. The instructions of the TruSeq PE Cluster kit (v2) for clustering using the Illumina cBot system were followed.
Genomic analysis.
Sequencing was performed in paired-end multiplex 2× 72-bp mode. Base calling and demultiplexing were performed using the CASAVA program (v1.7) on default parameters. Sequence analyses were performed with the CLC Genomics Workbench (v5.1.5) sequencing module using default parameters. Raw reads were processed to remove adapter sequences and low-sequence-quality reads or ambiguous base calls.
The uropathogenic E. coli strain CFT073 (23) genome was selected as the assembly scaffold for the genomes from subjects 202 (baseline and blood samples) and subject 252 (baseline and urine samples) on the basis of the overall highest BLASTN (24) homology to selected conserved housekeeping genes. Whole-genome sequences of E. coli isolates recovered on days 2, 4, 8, and 13 and at follow-up (subject 252) or recovered from blood on days 2, 3, and 4 and urine on day 1 (subject 202) were mapped to the reference sequences of isolates recovered on day 1 to identify mutations relative to the sequences of those genomes. On average, all samples were sequenced to approximately 250-fold coverage.
Day 1 reference sequence assembly required at least a 20-fold coverage of the single nucleotide variants (SNVs) identified in the resistant isolates. Only those SNVs that occurred in protein-coding genes in 100% of the reads are reported.
Evolutionary relationships were determined from concatenated nucleotide alignments on the basis of (i) E. coli conserved multilocus sequence typing (MLST) genes (9,018 nucleotides [nt]) or (ii) complete genes with one or more SNVs (8,637 nt). Phylogenetic trees were reconstructed by neighbor-joining and Bayesian methods. A neighbor-joining tree with 1,000 bootstrap replicates was reconstructed using the programs DNADIST (default F84 matrix) and NEIGHBOR from the PHYLIP (v3.6) package (25). A Bayesian posterior probabilities phylogenetic tree method, as implemented by the software MrBayes (v3.0B4) (26, 27), was also used. A gamma-distributed rate (GTR) model with 6 discrete rate categories was chosen. Markov chains were run for 1 × 106 generations, burn-in values were set for 1 × 104 generations, and trees were sampled every 100 generations. All trees were visualized with the program TREEVIEW (28). Venn diagrams were generated using the VennDiagram R package (29).
Nucleotide sequence accession numbers.
This whole-genome shotgun project has been deposited at DDBJ/EMBL/GenBank under accession numbers AXOA00000000, AXOB00000000, AXOC00000000, AXOD00000000, AXOE00000000, JTDS00000000, JTDT00000000, JTDU00000000, JTDX00000000, JSYR00000000, and JSYS00000000.
RESULTS
Phase II clinical trials with GSK2251052.
Twenty subjects, 14 of whom received i.v. BID doses of GSK2251052, were enrolled in study LRS114688 when Gram-negative bacterial isolates with reduced susceptibility to GSK2251052 were recovered from four subjects (subjects 202, 203, 252, and 751) (Table 1), and, as a consequence, the study was terminated. The isolates from three of these four subjects demonstrated a significant increase (≥32-fold) in GSK2251052 MIC between the baseline and day 2. One subject (subject 202) received doses of 1,500 mg for 4 days, and two subjects (subjects 203 and 252) received doses of 750 mg for 6 and 14 days, respectively. The fourth subject (subject 751) had different pathogens isolated at the baseline and postbaseline and had received 1,500-mg doses for 6 days.
TABLE 1.
Characterization of isolates from clinical trial LRS114688, their susceptibility to GSK2251052, and mutations in LeuRS identified by Sanger sequencinga
| Subject no. (BID treatment), source of pathogen | Day 1 (baseline) |
Day 2 (on therapy) |
Day 3 (on therapy) |
Day 4 (on therapy) |
Day 5 (on therapy) |
Day 8 (on therapy) |
Day 13 (on therapy) |
End of therapy |
Test of curec,d |
Late follow-up |
Microbiological responsed | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate | MIC (μg/ml) | LeuRSb | Isolate | MIC (μg/ml) | LeuRS | Isolate | MIC (μg/ml) | LeuRS | Isolate | MIC (μg/ml) | LeuRS | Isolate | MIC (μg/ml) | LeuRS | Isolate | MIC (μg/ml) | LeuRS | Isolate | MIC (μg/ml) | LeuRS | Isolate | MIC (μg/ml) | LeuRS | Isolate | MIC (μg/ml) | LeuRS | Isolate | MIC (μg/ml) | LeuRS | ||
| 202 (1,500 mg) | |||||||||||||||||||||||||||||||
| Urine | E. coli | 0.25 | None | No growth | No growth | No growth | No growth | No growth | No growth | ND | ND | ND | NAe | NAe | NAe | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Failure |
| Blood | E. coli | 0.5 | None | E. coli | 16 | P339L | E. coli | 16 | P339L | E. coli | 16 | P339L | NAe | NAe | NAe | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Failure |
| 252 (750 mg), urine | E. coli | 0.25 | None | E. coli | 512 | T247I | E. coli | 512 | T247I | E. coli | 512 | T247I | E. coli | 512 | T247I | E. coli | 1,024 | T247I + A323V | E. coli | 1,024 | T247I + D251N | E. coli | 1,024 | T247I + A323V | NA | NA | NA | E. coli | 1,024 | R344H + D345A | Failure |
| 203 (750 mg), urine | P. mirabilis | 0.5 | None | P. mirabilis | 1,024 | T247I | ND | ND | ND | ND | ND | ND | ND | ND | ND | NAe | NAe | NAe | NA | NA | NA | P. mirabilis | 1,024 | T247I | NA | NA | NA | NA | NA | NA | Failure |
| 751 (1,500 mg) | |||||||||||||||||||||||||||||||
| Urine | P. aeruginosa | 2 | NT | No growth | No growth | No growth | No growth | No growth | No growth | No growth | No growth | No growth | ND | ND | ND | ND | ND | ND | ND | ND | ND | No growth | No growth | No growth | No growth | No growth | No growth | K. pneumoniae | 1,024 | R344S | Success |
| Blood | E. faecalis | 32 | NT | E. faecalis | 32 | NT | E. faecalis | 8, 32 | NT | E. faecalis | 32 | NT | E. faecalis | 16 | NT | ND | ND | ND | ND | ND | ND | E. faecalis | 16 | NT | E. faecalis | 32 | NT | S. epidermidis | 0.5 | NT | Success |
NT, not tested; ND, not done, sample not taken; NA, not applicable due to a change in antibacterial therapy. Isolates selected for whole-genome sequencing are in bold font.
leuS and promoter regions sequenced.
The primary efficacy objective was microbiological, and the clinical response was evaluated at the test-of-cure visit (5 to 9 days posttherapy).
The responses at the test of cure, end of therapy, and follow-up were the same.
Day that therapy was changed.
E. coli isolates with GSK2251052 MICs of 0.25 and 0.5 μg/ml were isolated, respectively, from baseline cultures of urine and blood samples from subject 202. While there was no growth from subsequent urine cultures on days 2 and 3, blood cultures continued to grow E. coli isolates with a GSK2251052 MIC of 16 μg/ml. Proteus mirabilis with a GSK2251052 MIC of 0.5 μg/ml was isolated from the baseline culture of the urine sample from subject 203, whereas there was no growth from cultures of blood samples. On day 2, the culture of the urine sample from subject 203 grew a P. mirabilis isolate with a GSK2251052 MIC of 1,024 μg/ml. An E. coli isolate with a GSK2251052 MIC of 0.25 μg/ml was isolated from the baseline culture of the urine sample from subject 252, whereas there was no growth from cultures of blood samples. All subsequent urine samples recovered up to the late follow-up visit from subject 252 grew E. coli isolates with GSK2251052 MICs of ≥512 μg/ml. The baseline culture of the urine sample from subject 751 grew a P. aeruginosa isolate with a GSK2251052 MIC of 2 μg/ml, but subsequent cultures of urine samples collected on days 2 to 4 showed no growth; however, the culture of the urine sample collected at the late follow-up visit grew a K. pneumoniae isolate with a GSK2251052 MIC of 1,024 μg/ml.
Due to the emergence of resistance in study LRS114688, study LRS114689, which had 15 subjects enrolled, 9 of whom had received GSK2251052, was terminated. In this cIAI study, for isolates from three subjects for whom postbaseline samples were available, no significant increase (>4-fold) in the GSK2251052 MIC between pathogens recovered at baseline and postbaseline visits was demonstrated.
Definitive conclusions regarding the safety and/or efficacy of GSK2251052 could not be made in either study due to the limited number of subjects enrolled at the time of early termination.
Characterization of clinical isolates with reduced GSK2251052 susceptibility.
Sequencing of the promoter and coding regions of the leuS gene, encoding LeuRS, from the E. coli, P. mirabilis, and K. pneumoniae isolates recovered from study LRS114688 subjects showed that while the sequences of the baseline isolates were identical to those of the published genomes, all organisms recovered postbaseline contained either single or double mutations in the editing domain of LeuRS (Table 1) that were presumably responsible for their reduced susceptibility to GSK2251052. Amino acid substitution T247I, the most commonly encountered mutation, conferred high-level resistance, with the MICs for the isolates being 512 or 1,024 μg/ml. Equivalent resistance levels were associated with changes in residue R344 (GSK2251052 MIC, 1,024 μg/ml), whereas mutation P339L increased GSK2251052 MICs to 16 μg/ml (Table 1).
In cross-resistance studies, no differences in the MICs of antibacterials typically used to treat Gram-negative bacterial infections were observed between baseline and postbaseline isolates. All E. coli isolates recovered from subjects 202 and 252 were susceptible to the comparator agents with activity against Gram-negative bacteria tested. The P. mirabilis isolates from subject 203 were susceptible to all comparators except imipenem (intermediate), ampicillin, nitrofurantoin, and trimethoprim-sulfamethoxazole. The K. pneumoniae isolate recovered from subject 751 was ampicillin resistant.
Susceptibility of E. coli LeuRS T247I isogenic mutants to GSK2251052.
In order to confirm that specific changes in the editing domain of LeuRS were solely responsible for the resistance phenotype, a mutation was introduced in the leuS gene of two E. coli strains, 1162222 and 1161435, to render LeuRS T247I. Susceptibility testing of the isogenic pairs demonstrated that this mutation causes high-level resistance to GSK2251052. In E. coli 1161435, MICs increased from 1 μg/ml (parent) to 512 μg/ml (mutant), and similarly, in strain 1162222, they increased from 0.5 μg/ml to 256 μg/ml.
Whole-genome DNA sequencing of E. coli isolates from subjects 202 and 252.
E. coli isolates from subjects 202 and 252 (Table 1) were selected for whole-genome DNA sequencing on the basis of their broad time course of sampling and the availability of day 1 baseline isolates to be used as reference genomes to identify SNVs.
All mutations previously identified in the LeuRS editing domain of these E. coli isolates by Sanger DNA sequencing were confirmed by whole-genome sequencing. The numbering of specific LeuRS amino acid residues differs slightly between methods, because a different reference E. coli strain was used in each analysis: E. coli K-12 (Sanger DNA sequencing) and CFT073, a known uropathogenic E. coli (UPEC) strain (Illumina DNA sequencing) (Tables 2 and 3).
TABLE 2.
Amino acid residue variations between GSK2251052-resistant E. coli urine isolates and the baseline urine isolate from subject 252 determined by whole-bacterial-genome DNA sequencing
| Time(s) of sample collection | Gene | Description | Mutation(s)a |
|---|---|---|---|
| Day 13 | leuS | Leucyl-tRNA synthetase | D298N (D251N) |
| Days 2, 4, 8, and 13 | leuS | Leucyl-tRNA synthetase | T294I (T247I) |
| Day 8 | leuS | Leucyl-tRNA synthetase | A370V (A323V) |
| Follow-up | leuS | Leucyl-tRNA synthetase | D392A, R391H (D345A, R344H) |
| Days 2, 4, 8, and 13 and follow-up | acnB | Bifunctional aconitate hydratase | V705I |
| Days 2, 4, and 8 and follow-up | c0279 | Hypothetical protein | T21A |
| Day 4 | c1464 | Tail fiber component K of prophage | G41b |
| Follow-up | c1472 | Hypothetical protein | T15A |
| Follow-up | c1473 | Hypothetical protein | R8G, 42Eb |
| Follow-up | c1474 | Hypothetical protein | K209Q |
| Day 2 | c3157 | Hypothetical protein | T152A |
| Days 2 and 4 and follow-up | c3157 | Hypothetical protein | A158V, W159S, P218L |
| Day 2 and follow-up | c3157 | Hypothetical protein | R136L, D142G |
| Day 8 | c3157 | Hypothetical protein | W159S |
| Days 8 and 13 | c3157 | Hypothetical protein | T152A, A158V, P218L |
| Days 2, 8, and 13 | c3158 | Tail component of prophage | Y85H |
| Follow-up | c3670 | Hypothetical protein | E148G |
| Follow-up | c3671 | RadC-like protein YeeS | L13P |
| Days 2, 4, 8, and 13 | c3674 | Hypothetical protein | A160P |
| Days 2 and 4 and follow-up | c3679 | Hypothetical protein | C13Y, L17S |
| Day 4 | c4492 | ShiA-like protein | K302Q, E305K |
| Day 2 and 8 | c4572 | Hypothetical protein | P98H |
| Days 2, 4, 8, and 13 and follow-up | c5158 | Hypothetical protein | S30,b I22V |
| Day 8 | c5213 | Transposase IS629 | 207K,b H203Y |
| Days 4, 8, and 13 and follow-up | ompR | Osmolarity response regulator | R207H |
| Days 2, 4, 8, and 13 follow-up | ycbS | Outer membrane usher protein YcbS | M315I |
| Follow-up | ydfR | Hypothetical protein | M59R |
Amino acid residue according to E. coli CFT073 numbering system, with E. coli K12 equivalents in parentheses.
Nonsense mutation.
TABLE 3.
Amino acid residue variations between GSK2251052-resistant E. coli blood and urine isolates from baseline blood isolate from subject 202 determined by whole-bacterial-genome DNA sequencing
| Time(s) of sample collection or sample | Gene | Description | Mutation(s)a |
|---|---|---|---|
| Days 2, 3, and 4 blood | leuS | Leucyl-tRNA synthetase | P386L (P339L) |
| Baseline urine | atpF | ATP synthase FoF1 subunit B | A5E |
| Days 2, 3, and 4 blood and baseline urine | c1471 | Hypothetical protein | D59V |
| Days 2, 3, and 4 blood and baseline urine | c1472 | Hypothetical protein | I75N |
| Baseline urine | c1527 | Hypothetical protein | T45S |
| Days 2, 3, and 4 blood and baseline urine | c1590 | Tail component of prophage | A803S |
| Baseline urine | c2021 | Electron transport complex protein RnfC | E624A, E624D |
| Days 2, 3, and 4 blood and baseline urine | c2295 | Hypothetical protein | 38Sb |
| Days 2, 3, and 4 blood and baseline urine | c2473 | Transposase | I113V |
| Day 3 blood | c4898 | Hypothetical protein | I45S |
Amino acid residue according to E. coli CFT073 numbering system. E. coli K12 equivalents in parentheses.
Nonsense mutation.
In both subjects, nonsynonymous (NS) codon changes mostly occurred by day 2 and were retained over subsequent time points (Fig. 1A and B), with the exception of those in E. coli LeuRS from subject 252, where mutations T247I (days 2, 4, 8, and 13), A323V (day 8), and D251N (day 13) were absent in the posttherapy follow-up isolate (Tables 2 and 3), which had, instead, two different mutations, D345A and R344H.
FIG 1.
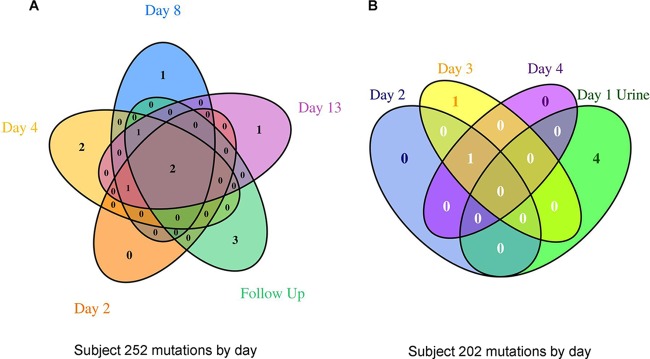
Venn diagram showing the variation in nonsynonymous SNVs in protein-coding genes relative to the sequence of the genome at baseline day 1 for subject 252 (A) and subject 202 (B). Variants in prophage, transposase, and hypothetical genes were excluded from the figure to focus on SNVs in genes with a functional annotation.
Isolates from subjects 202 and 252 did not share any NS mutations, and only a hypothetical protein-encoding gene (c1472) was mutated, at different positions, in some E. coli isolates from both subjects. Among isolates from subject 252, amino acid changes occurred in various hypothetical proteins and prophage elements, as well as in several known proteins, such as OmpR, a DNA-binding response regulator; YcbS, an outer membrane usher protein; and AcnB, a bifunctional aconitate hydratase 2/2-methylisocitrate dehydratase (Fig. 1A; Table 2).
Fewer amino acid changes in the sequences of isolates from subject 202 relative to the sequence of the baseline organism from blood were observed (Fig. 1B; Table 3). In addition to the NS changes in LeuRS, mutations were seen in several hypothetical genes, a transposase, and a prophage gene. Some changes, such as those in AtpF (ATP synthase FoF1 subunit B) and RnfC (an electron transport protein), were specific to the baseline urine isolate.
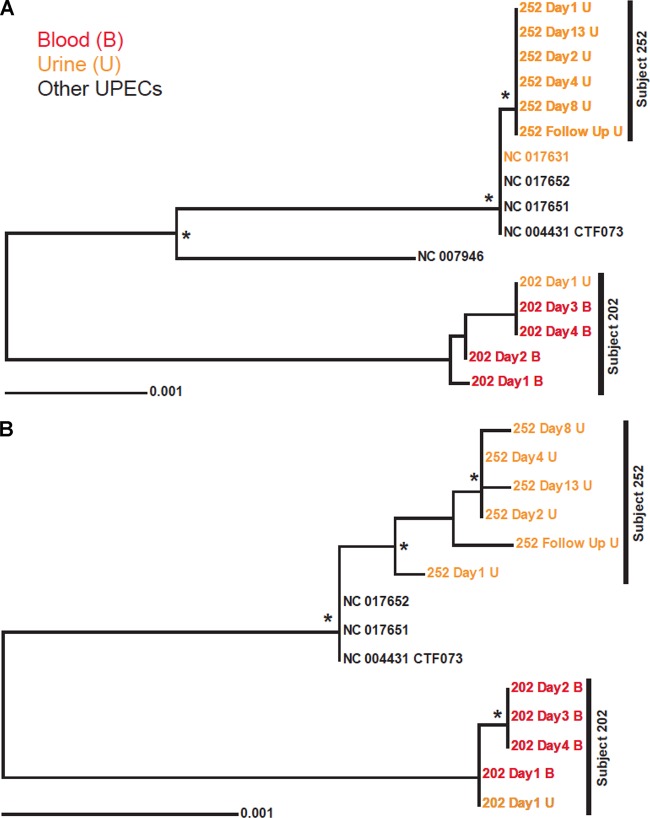
Phylogenetic trees based on the nucleotide sequences of either multilocus sequencing typing (MLST) or SNV genes showed that E. coli isolates were more similar within subjects than between subjects (Fig. 2A and B), suggesting that they were highly clonal and no new reinfections had occurred, at least over the course of the study period and for the bacterial genomes sequenced. Furthermore, the sequences of these genomes were similar but not identical to those of any published UPEC genomes.
FIG 2.
Phylogenetic trees of E. coli strains based on published concatenated nucleotide sequences of representative E. coli isolates causing urinary tract infection. (A) Phylogeny of MLST genes based on the alignment of 9,018 nt. (B) Phylogeny of loci with SNVs based on the alignment of 8,637 nt derived from whole-genome comparisons of day 1 isolates from subjects 202 and 252 to those genomes from the respective subsequent days. Trees were reconstructed using both bootstrap neighbor-joining (shown) and Bayesian methods. Branch points marked with an asterisk were supported in more than 80% of 1,000 bootstrap replications and by a Bayesian posterior probability of ≤0.01. The location of E. coli CFT073, used for gene annotation mapping, is also indicated.
Characterization of laboratory-isolated mutants resistant to GSK2251052.
To ascertain if these clinical isolates developed resistance at a higher rate than other E. coli strains, the in vitro frequency of spontaneous resistance to GSK2251052 was determined in two standard strains, E. coli ATCC 25922 and E. coli 1161435, and in the two baseline isolates from subjects 202 and 252, E. coli 998584 and 671925, respectively.
GSK2251052-resistant mutants appeared at similarly high rates, 1 × 10−7 to 4 × 10−7, in both sets of strains, E. coli laboratory standards and clinical isolates (see Table S1 in the supplemental material), and had no obvious in vitro growth defect in standard media. Equally similar was the percentage of mutants that showed high-level resistance to GSK2251052, as the majority of isolates of E. coli ATCC 25922 (83%), 1161435 (79%), 998584 (88%), and 671925 (78%) had GSK2251052 MIC increases of ≥32-fold with respect to those for their parent strains.
To identify the mutations responsible for this resistance phenotype, a fragment of leuS encoding the editing domain of LeuRS was sequenced from a set of ∼40 representative colonies of each of the different E. coli strains. In 119 mutants, 43 single base changes were identified in 20 different amino acids of the LeuRS editing domain (Table 4). One mutant with a 27-bp insertion in this region, one mutant with a 12-bp deletion in this region, and one mutant with double mutations in this region were also identified. The additional 36 isolates did not have any sequence change in the editing domain of LeuRS. Nearly 50% of the mutants carried mutations in one of three residues: G229 (n = 20), D345 (n = 20), and D251 (n = 18). Comparable numbers of isolates with mutations in a specific residue were obtained from the standard laboratory strains and from the two clinical isolates, except in one case. Mutations in D345 were more commonly encountered in the clinical strains, specifically, in E. coli 671925, where 14 of the 39 mutants analyzed carried substitutions in that residue (see Table S2 in the supplemental material).
TABLE 4.
Summary of characterization of laboratory-isolated mutants with reduced susceptibility to GSK2251052
| LeuRS amino acid residuea | Amino acid change(s) | No. of mutants |
Fold increase in MIC | |
|---|---|---|---|---|
| Standard strains | Clinical strains | |||
| S227 | F/Y | 1 | 1 | >256/16 |
| G229 | D/S/V | 12 | 8 | >256 |
| Y246 | C/H/N | 4 | 4 | 64–>256/>256/64–>256 |
| T247 | N | 0 | 1 | >256 |
| T248 | P | 4 | 0 | >256 |
| R249 | C/H/L/P | 1 | 3 | 16/>256/64/>256 |
| D251 | G/H/N/V/Y | 11 | 7 | 8–64 |
| C256 | R | 0 | 1 | 32 |
| A260 | P | 1 | 0 | 16 |
| W321 | WAANFVLME ×2 | 1 | 0 | >256 |
| V326 | G | 0 | 1 | 256 |
| Y330 | C/D/L/N | 0 | 4 | 8/>256/32/>256 |
| G331 | D | 2 | 3 | 64–128 |
| G333 | C/D/V | 5 | 2 | 8/128–256/256 |
| A334 | P/T/V | 3 | 3 | >256/4–16/8 |
| A337 | P | 1 | 0 | >256 |
| V338 | F/ΔVPGH | 2 | 0 | >256 |
| P339 | L/Q | 3 | 2 | 64/16–64 |
| D342 | G/Y | 4 | 1 | 8/64 |
| D342+R344 | G | 0 | 1 | >256 |
| R344 | H | 2 | 3 | >256 |
| D345 | G/N/Y | 3 | 17 | >256/16–32/>256 |
| No change in editing domain | 19 | 17 | 4–>128 | |
Amino acid numbers based on E. coli strain K-12 LeuRS.
Mutations in the single residues associated with clinical resistance, P339, T247, and R344, were also found among the laboratory-isolated mutants. Although some carried identical substitutions (P339L, R344H), mutation T247I, found in isolates from two of the four subjects in study LRS114688, was not detected. Substitutions in amino acids D251 and D345, which appeared as secondary mutations in isolates from subject 252, were also represented amid the in vitro-obtained mutants, but isolates with changes in A323 were not found.
DISCUSSION
Prior to initiating clinical trials, in vitro studies showed that resistance to the novel LeuRS inhibitor GSK2251052 developed in strains of E. coli, P. aeruginosa, and K. pneumoniae at a rate of 1 × 10−7 to 8 × 10−7, similar to that observed with other antibacterials, such as ceftazidime or ciprofloxacin (15). The mutants recovered from these studies were susceptible to norvaline and, therefore, likely to be editing deficient (15). Although we recognize the limitations of animal models when investigating resistance, given that total bacterial loads can be significantly below those in the clinic, multiple attempts were made in our laboratories to select resistant organisms in rodent models of infection with only a single mutant organism isolated. Despite this, resistance to GSK2251052 developed very rapidly during therapy in 3 of 14 subjects enrolled in the phase II cUTI study LRS114688. In fact, isolates with GSK2251052 MIC increases of ≥32-fold with respect to the MIC for the baseline pathogen were recovered from the infections on day 2, indicating that resistance either was present in the bacterial cell population at the start of therapy or emerged within the first 24 h of treatment. The rapid isolation of these mutants also suggests that there was no in vivo fitness cost associated with these mutations. Cultures of blood from subject 202, treated with 1,500 mg BID, and cultures of urine from subjects 203 and 252, each receiving 750 mg BID, were positive for GSK2251052-resistant Gram-negative bacterial pathogens. All cases were microbiological failures; however, subject 252 showed clinical resolution of signs and symptoms. Neither of the two dosing regimens used could yield the GSK2251052 blood levels necessary to prevent the growth of these resistant isolates, but in this very limited data set, the rapid emergence of resistance seemed to occur more frequently in subjects exposed to lower drug concentrations (2/6 in the 750-mg group versus 1/8 in the 1,500-mg group). Treatment of subject 751 with 1,500 mg GSK2251052 BID did not result in the development of resistance in the baseline isolate, but a different pathogen resistant to GSK2251052 was recovered from a urine sample taken at the late follow-up visit. A possible hypothesis is that for this subject the emergence of resistance occurred at an alternate infection site, as the MIC for this K. pneumoniae isolate was ≥512-fold higher than that for the expected wild-type distribution (17). Without a corresponding pretreatment isolate, the exact origin of resistance cannot be fully elucidated. Resistance was not observed in the additional subjects treated with GSK2251052, and at the end of therapy, most (3 of 4 receiving 750 mg and 5 of 6 receiving 1,500 mg) had both clinical and microbiological successes.
Development of resistance during antibacterial therapy has previously been documented for other agents (30). A review of 173 clinical trials involving ∼14,000 subjects and eight antibacterial classes found that resistance emerged in 4% of the organisms and was more frequent with penicillin (6.4%) and aminoglycoside (5.5%) monotherapies (30). The type of infection and causative agent and underlying diseases also seem to play a critical role in the development of resistance during therapy.
Analysis of leuS sequences from the study LRS114688 clinical isolates shows that GSK2251052 resistance is due to specific target-based mutations and not to the existence of strains with an additional refractory LeuRS, acquired by gene duplication or horizontal gene transfer, as has been previously described with resistance to other AaRS inhibitors (31–33). Cocrystallization studies have shown that benzoxaboroles make key interactions with LeuRS editing domain residues, including T247 (15), and, therefore, the mutations identified in these isolates, such as P339L, T247I, and R344S, might be expected to affect the binding of GSK2251052 and induce the resistance observed. In fact, E. coli isogenic mutants carrying LeuRS substitution T247I showed a 512-fold increase in the GSK2251052 MIC with respect to that of their parent strains.
In vitro frequency-of-resistance studies showed that baseline isolates from subjects 202 and 252 were not more prone to the development of resistance than standard laboratory strains. Rates of resistance were high in all cases, and 82% of the resistant isolates had GSK2251052 MIC increases of ≥32-fold. Sequence analysis of the LeuRS editing domains of 158 lab-generated resistant strains revealed 45 individual mutations at 22 different amino acid positions, which were very likely responsible for the resistance phenotype observed. The large variety of mutations associated with reduced susceptibility to GSK2251052 and the lack of any obvious fitness cost clearly contribute to the relatively high in vitro frequency of resistance observed with this compound.
To reveal new information about the evolution of pathogens at the level of individual patients and to confirm the resistance mechanisms, several E. coli isolates from subjects 202 and 252 were selected for next-generation DNA sequencing (34), a technology recently used to characterize whole genomes of bacterial pathogens isolated from clinical outbreaks (35, 36). Phylogenetic analysis showed that E. coli isolates from subjects 202 and 252 were very similar within subjects, suggesting that they resulted from clonal expansion of their corresponding baseline pathogen and were not the result of new infections. Mutations conferring resistance to GSK2251052 occurred by day 2 in both cases and were retained in subsequent days. Few amino acid substitutions were observed in isolates from subject 202, but changes in some interesting proteins were identified in isolates from subject 252 from days 2/4 to the follow-up visit. OmpR, the transcriptional regulator in the EnvZ/OmpR two-component signal transduction system, involved in osmoregulation, plays a pivotal role in UPEC survival and pathogenicity in the urinary tract (37, 38). Mutation R207H is located in an area of the protein that has been directly implicated in the expression of OmpF even in high-osmolarity medium (39). Amino acid changes also occur in YcbS, an outer membrane usher protein, and AcnB, an aconitase that acts as an iron and oxidative stress-responsive posttranscriptional regulator and modulates levels of the flagellum protein FliC, impacting E. coli motility and colonization in the urinary tract (40, 41). These mutations could play a role in the maintenance of UPEC in the urinary tract, as they appear early and are maintained throughout the infection but are not present in the blood isolates from subject 202.
This report demonstrates the importance of performing thorough preclinical in vitro resistance studies and the potential role of genome-wide sequencing of bacteria in the clinical development of novel antibiotics to better understand their efficacy in patients and evaluate drug resistance mechanisms.
The development of resistance to the LeuRS inhibitor GSK2251052 in subjects enrolled in study LRS114688 is likely the result of a perfect storm involving a high spontaneous frequency of resistance across bacterial species, high-level resistant mutants in which resistance has no obvious in vivo fitness cost, the high bacterial burden of the disease, the genetic plasticity of the target gene, and, even possibly, the bacteriostatic nature of GSK2251052. Although some of these factors can be assessed preclinically in in vitro and in vivo studies, the clinical relevance of such work can be unclear, as the clinical efficacy of an antibacterial agent is not solely dependent on its inherent properties and those of the specific pathogen involved in the infection. The pathogenesis of the disease also plays a critical role in the final outcome, which, ultimately, can be evaluated only in clinical studies. This work highlights the impressive adaptation of bacterial pathogens during human infection and further emphasizes the challenges of antibacterial drug discovery.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Quest Diagnostics for performing the cross-resistance studies. Editorial support in the form of development of the first draft of the manuscript, collation of author comments, assembly of the tables, and preparation of the final version for publication was provided by Magdalena Zalacain at Zala Drug Discovery Consulting and was funded by GSK.
We all worked for GlaxoSmithKline at the time that the work was performed.
This project has been funded in whole or in part with federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under contract no. HHSO100201000016C.
K.O., K.I., S.M., and C.J. designed and performed in vitro laboratory studies and analyzed the data; A.S. performed the computational biology analysis of the genome data; K.O., D.J.H., S.R., A.K., G.P.L., G.S., L.A.M., N.E.S.-O., and J.R.B. designed the studies and performed general data analysis; E.T. and S.V.H. participated in the design of the whole-genome sequencing project and performed the studies; M.T., J.T., M.D., M.C., and N.E.S.-O. contributed to the design, coordination, monitoring, and data analysis of the clinical studies; and K.O., D.J.H., S.R., L.A.M., M.T., J.T., M.D., M.C., N.E.S.-O., and J.R.B. contributed to the writing/editing of the manuscript. We all reviewed and approved the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03774-14.
REFERENCES
- 1.Klevens RM, Edwards JR, Richards CL Jr, Horan TC, Gaynes RP, Pollock DA, Cardo DM. 2007. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 122:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts RR, Hota B, Ahmad I, Scott RD, Foster SD, Abbasi F, Schabowski S, Kampe LM, Ciavarella GG, Supino M, Naples J, Cordell R, Levy SB, Weinstein RA. 2009. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 49:1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 3.Eber MR, Laxminarayan R, Perencevich EN, Malani A. 2010. Clinical and economic outcomes attributable to health care-associated sepsis and pneumonia. Arch Intern Med 170:347–353. doi: 10.1001/archinternmed.2009.509. [DOI] [PubMed] [Google Scholar]
- 4.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 5.Peleg AY, Hooper DC. 2010. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med 362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S. 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the reemerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 8.Livermore DM. 2011. Discovery research: the scientific challenge of finding new antibiotics. J Antimicrob Chemother 66:1941–1944. doi: 10.1093/jac/dkr262. [DOI] [PubMed] [Google Scholar]
- 9.Ibba M, Soll D. 2000. Aminoacyl-tRNA synthesis. Annu Rev Biochem 69:617–650. doi: 10.1146/annurev.biochem.69.1.617. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland R, Boon RJ, Griffin KE, Masters PJ, Slocombe B, White AR. 1985. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob Agents Chemother 27:495–498. doi: 10.1128/AAC.27.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudson IR. 1994. The efficacy of intranasal mupirocin in the prevention of staphylococcal infections: a review of recent experience. J Hosp Infect 27:81–98. doi: 10.1016/0195-6701(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 12.Hurdle JG, O'Neill AJ, Chopra I. 2005. Prospects for aminoacyl-tRNA synthetase inhibitors as new antimicrobial agents. Antimicrob Agents Chemother 49:4821–4833. doi: 10.1128/AAC.49.12.4821-4833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochsner UA, Sun X, Jarvis T, Critchley I, Janjic N. 2007. Aminoacyl-tRNA synthetases: essential and still promising targets for new anti-infective agents. Expert Opin Investig Drugs 16:573–593. doi: 10.1517/13543784.16.5.573. [DOI] [PubMed] [Google Scholar]
- 14.Rock FL, Mao W, Yaremchuk A, Tukalo M, Crepin T, Zhou H, Zhang YK, Hernandez V, Akama T, Baker SJ, Plattner JJ, Shapiro L, Martinis SA, Benkovic SJ, Cusack S, Alley MR. 2007. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science 316:1759–1761. doi: 10.1126/science.1142189. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez V, Crepin T, Palencia A, Cusack S, Akama T, Baker SJ, Bu W, Feng L, Freund YR, Liu L, Meewan M, Mohan M, Mao W, Rock FL, Sexton H, Sheoran A, Zhang Y, Zhang YK, Zhou Y, Nieman JA, Anugula MR, Keramane EM, Savariraj K, Reddy DS, Sharma R, Subedi R, Singh R, O'Leary A, Simon NL, De Marsh PL, Mushtaq S, Warner M, Livermore DM, Alley MR, Plattner JJ. 2013. Discovery of a novel class of boron-based antibacterials with activity against gram-negative bacteria. Antimicrob Agents Chemother 57:1394–1403. doi: 10.1128/AAC.02058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein EJ, Citron DM, Tyrrell KL, Merriam CV. 2013. Comparative in vitro activities of GSK2251052, a novel boron-containing leucyl-tRNA synthetase inhibitor, against 916 anaerobic organisms. Antimicrob Agents Chemother 57:2401–2404. doi: 10.1128/AAC.02580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendes RE, Alley MR, Sader HS, Biedenbach DJ, Jones RN. 2013. Potency and spectrum of activity of AN3365, a novel boron-containing protein synthesis inhibitor, tested against clinical isolates of Enterobacteriaceae and nonfermentative Gram-negative bacilli. Antimicrob Agents Chemother 57:2849–2857. doi: 10.1128/AAC.00160-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfaro B, Hernandez I. 2013. Evolution of the indigenous microbiota in modified atmosphere packaged Atlantic horse mackerel (Trachurus trachurus) identified by conventional and molecular methods. Int J Food Microbiol 167:117–123. doi: 10.1016/j.ijfoodmicro.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Tenero D, Bowers G, Rodvold KA, Patel A, Kurtinecz M, Dumont E, Tomayko J, Patel P. 2013. Intrapulmonary pharmacokinetics of GSK2251052 in healthy volunteers. Antimicrob Agents Chemother 57:3334–3339. doi: 10.1128/AAC.02483-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twynholm M, Dalessandro M, Barker K, Scangarella-Oman N, Li G, Min S, Ingraham K, Jakielaszek C, O'Dwyer K, Holmes D, Tomayko J. 2013. Termination of phase II program due to emergence of resistance (EOR) on-therapy, abstr G-1251. Abstr 53rd Intersci Conf Antimicrob Agents Chemother American Society for Microbiology, Washington, DC. [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 8th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welch RA, Burland V, Plunkett G III, Redford P, Roesch P, Rasko D, Buckles EL, Liou SR, Boutin A, Hackett J, Stroud D, Mayhew GF, Rose DJ, Zhou S, Schwartz DC, Perna NT, Mobley HL, Donnenberg MS, Blattner FR. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A 99:17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felsenstein J. 1989. PHYLIP–Phylogeny Inference Package (version 3.2). Cladistics 5:164–166. [Google Scholar]
- 26.Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 27.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 28.Page RD. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Boutros PC. 2011. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics 12:35. doi: 10.1186/1471-2105-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fish DN, Piscitelli SC, Danziger LH. 1995. Development of resistance during antimicrobial therapy: a review of antibiotic classes and patient characteristics in 173 studies. Pharmacotherapy 15:279–291. [PubMed] [Google Scholar]
- 31.Brown JR, Gentry D, Becker JA, Ingraham K, Holmes DJ, Stanhope MJ. 2003. Horizontal transfer of drug-resistant aminoacyl-transfer-RNA synthetases of anthrax and Gram-positive pathogens. EMBO Rep 4:692–698. doi: 10.1038/sj.embor.embor881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gentry DR, Ingraham KA, Stanhope MJ, Rittenhouse S, Jarvest RL, O'Hanlon PJ, Brown JR, Holmes DJ. 2003. Variable sensitivity to bacterial methionyl-tRNA synthetase inhibitors reveals subpopulations of Streptococcus pneumoniae with two distinct methionyl-tRNA synthetase genes. Antimicrob Agents Chemother 47:1784–1789. doi: 10.1128/AAC.47.6.1784-1789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown JR, Zhang J, Hodgson JE. 1998. A bacterial antibiotic resistance gene with eukaryotic origins. Curr Biol 8:R365–R367. doi: 10.1016/S0960-9822(98)70238-6. [DOI] [PubMed] [Google Scholar]
- 34.Didelot X, Bowden R, Wilson DJ, Peto TE, Crook DW. 2012. Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet 13:601–612. doi: 10.1038/nrg3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris SR, Clarke IN, Seth-Smith HM, Solomon AW, Cutcliffe LT, Marsh P, Skilton RJ, Holland MJ, Mabey D, Peeling RW, Lewis DA, Spratt BG, Unemo M, Persson K, Bjartling C, Brunham R, de Vries HJ, Morre SA, Speksnijder A, Bebear CM, Clerc M, de Barbeyrac B, Parkhill J, Thomson NR. 2012. Whole-genome analysis of diverse Chlamydia trachomatis strains identifies phylogenetic relationships masked by current clinical typing. Nat Genet 44:413–419, S1. doi: 10.1038/ng.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris SR, Feil EJ, Holden MT, Quail MA, Nickerson EK, Chantratita N, Gardete S, Tavares A, Day N, Lindsay JA, Edgeworth JD, de Lencastre H, Parkhill J, Peacock SJ, Bentley SD. 2010. Evolution of MRSA during hospital transmission and intercontinental spread. Science 327:469–474. doi: 10.1126/science.1182395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rentschler AE, Lovrich SD, Fitton R, Enos-Berlage J, Schwan WR. 2013. OmpR regulation of the uropathogenic Escherichia coli fimB gene in an acidic/high osmolality environment. Microbiology 159:316–327. doi: 10.1099/mic.0.059386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwan WR. 2009. Survival of uropathogenic Escherichia coli in the murine urinary tract is dependent on OmpR. Microbiology 155:1832–1839. doi: 10.1099/mic.0.026187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nara F, Matsuyama S, Mizuno T, Mizushima S. 1986. Molecular analysis of mutant ompR genes exhibiting different phenotypes as to osmoregulation of the ompF and ompC genes of Escherichia coli. Mol Gen Genet 202:194–199. doi: 10.1007/BF00331636. [DOI] [PubMed] [Google Scholar]
- 40.Haugen BJ, Pellett S, Redford P, Hamilton HL, Roesch PL, Welch RA. 2007. In vivo gene expression analysis identifies genes required for enhanced colonization of the mouse urinary tract by uropathogenic Escherichia coli strain CFT073 dsdA. Infect Immun 75:278–289. doi: 10.1128/IAI.01319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang Y, Guest JR, Artymiuk PJ, Read RC, Green J. 2004. Post-transcriptional regulation of bacterial motility by aconitase proteins. Mol Microbiol 51:1817–1826. doi: 10.1111/j.1365-2958.2003.03954.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.