Abstract
Antibiotic combinations are often used for treating Pseudomonas aeruginosa infections but their efficacy toward intracellular bacteria has not been investigated so far. We have studied combinations of representatives of the main antipseudomonal classes (ciprofloxacin, meropenem, tobramycin, and colistin) against intracellular P. aeruginosa in a model of THP-1 monocytes in comparison with bacteria growing in broth, using the reference strain PAO1 and two clinical isolates (resistant to ciprofloxacin and meropenem, respectively). Interaction between drugs was assessed by checkerboard titration (extracellular model only), by kill curves, and by using the fractional maximal effect (FME) method, which allows studying the effects of combinations when dose-effect relationships are not linear. For drugs used alone, simple sigmoidal functions could be fitted to all concentration-effect relationships (extracellular and intracellular bacteria), with static concentrations close to (ciprofloxacin, colistin, and meropenem) or slightly higher than (tobramycin) the MIC and with maximal efficacy reaching the limit of detection in broth but only a 1 to 1.5 (colistin, meropenem, and tobramycin) to 2 to 3 (ciprofloxacin) log10 CFU decrease intracellularly. Extracellularly, all combinations proved additive by checkerboard titration but synergistic using the FME method and more bactericidal in kill curve assays. Intracellularly, all combinations proved additive only based on both FME and kill curve assays. Thus, although combinations appeared to modestly improve antibiotic activity against intracellular P. aeruginosa, they do not allow eradication of these persistent forms of infections. Combinations including ciprofloxacin were the most active (even against the ciprofloxacin-resistant strain), which is probably related to the fact this drug was the most effective alone intracellularly.
INTRODUCTION
Antibiotic combination is widely recognized as a useful strategy not only for increasing the chances to effectively cover the offending organism(s) upon initiation of an empirical therapy but also to accelerate the reduction of the inoculum at the early stage of infection and to avoid the selection of resistance (1–4). This may be particularly critical when dealing with infections caused by organisms like Pseudomonas aeruginosa, which is often multiresistant and causes severe diseases. Accordingly, antibiotic combinations have been widely studied in vitro using both static (5–10) and dynamic (11–14) models. Most of these studies concluded that there was synergy but to an extent that proved to be highly strain dependent. While the exact mechanism of this synergy often remains uncertain, a number of pharmacological reasons have been proposed, such as the enhancement of the uptake of the companion antibiotics by colistin through its destabilizing effects on the outer membrane (15) or the increased diffusion of entry of aminoglycosides through the peptidoglycan when altered by β-lactams (16).
There is, however, increasing evidence that specific lifestyles, such as intracellular survival, may contribute to persistence of recurrence of the infection by creating a niche refractory to antibiotic action. Thus, many in vitro studies with P. aeruginosa document its ability to invade and survive within eukaryotic cells (references 17 and 18 and references cited therein). Moreover, intracellular reservoirs have been evidenced in vivo and appear to be associated with the maintenance and persistence of the infection due to insufficient intracellular killing (19, 20; see reference 18 for a review). These issues are not taken into account when assessing antibiotic combinations in vitro using classical approaches such as checkerboard titration, kill curves, or disk diffusion assays.
We showed previously that the intracellular forms of P. aeruginosa are poorly receptive to the action of most antibiotics, with ciprofloxacin being the only one with a definite intracellular bactericidal activity (17). Antibiotic combination may, therefore, be an appealing strategy to increase intracellular efficacy. Past studies made with the intracellular forms of small-colony variants of Staphylococcus aureus (another type of organism that is poorly susceptible to antibiotics) indeed showed that the combination of bactericidal antibiotics can be highly synergistic (21).
In the present study, we compared the activities of combinations of antibiotics, each representative of one class of well-known antipseudomonal drugs (meropenem [carbapenems], tobramycin [aminoglycosides], ciprofloxacin [fluoroquinolones], and colistin [polymyxins]), against the extracellular and intracellular forms of infection by P. aeruginosa using the reference strain PAO1 and two resistant clinical isolates. We evaluated interactions between drugs by use of concentration- and time-kill curves and fractional maximal effect (FME) methods. While extracellular synergy could easily be observed, only additivity was seen against intracellular bacteria for all combinations studies. Our work further documents the difficulty of eradicating intracellular bacteria and calls for further developments in this area to help in treating the infections caused by P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains, susceptibility testing, and extracellular kill curves.
We used P. aeruginosa strain ATCC PAO1 as a reference and two selected clinical strains phenotypically resistant to ciprofloxacin (PA50 [bacteremia]) or to meropenem (PA291 [expectoration]), which were obtained from collaborating clinical microbiology laboratories. Bacteria were grown in Mueller-Hinton broth, and CFU counting was performed by plating on tryptic soy agar. MICs were measured by serial 2-fold microdilution according to CLSI guidelines (inoculum of approximately 106 CFU/ml and reading after 20 to 24 h of incubation) in cation-adjusted Mueller-Hinton broth (22). Full concentration-kill curves were performed as previously described (17), with a starting inoculum of 106 CFU/ml in the Muller-Hinton broth. A bactericidal effect was defined as a decrease of the inoculum of ≥3 log10 CFU, and the limit of detection was a decrease of >5 log10 CFU, both compared to the original inoculum.
Cells, cell culture, and intracellular infection.
Human THP-1 cells were cultivated in RPMI 1640 medium supplemented with 10% fetal calf serum as described previously (23). Intracellular infection was performed as previously described (17). In brief, bacteria were opsonized by 1 h of incubation with 10% human serum in RPMI 1640 medium without fetal calf serum. Phagocytosis was allowed for 2 h at a bacterium/cell ratio of 10:1, after which noninternalized bacteria were eliminated by incubation for 1 h with 100 mg/liter gentamicin. After 3 washes in phosphate-buffered saline (PBS) and resuspension in culture medium, the phagocytized inoculum (determined by plating on agar and normalized with respect to sample protein content) was typically 6.7 × 105 ± 3.8 × 104 CFU/mg of protein for PAO1, 6.0 × 105 ± 3.2 × 104 CFU/mg of protein for PA50, and 3.6 × 105 ± 4.3 × 104 CFU/mg of protein for PA 291.
Intracellular activity of antibiotics.
Antibiotics were added to the medium of the infected cells at extracellular concentrations ranging from 0.01 to 200 mg/liter to obtain a complete description of the concentration-response effects (17). After 24 h of incubation, cells were collected by centrifugation, resuspended in PBS, centrifuged again to eliminate extracellular bacteria, and collected in distilled water. Complete cell lysis was achieved by sonication (10 s), and lysates were used for determination of CFU after plating on agar of appropriate dilutions and of protein content by Lowry's assay using a commercially available detection kit (Bio-Rad DCTM protein assay; Bio-Rad Laboratories, Hercules, CA). Activity was expressed as the change from the initial inoculum after 24 h of incubation. A Hill function (slope factor = 1) was fitted to the data to calculate 4 key pertinent pharmacodynamic parameters, namely, Emin (maximal increase in inoculum [in log10 units] as extrapolated for an infinitely low antibiotic concentration), Emax (maximal decrease in inoculum [in log10 units] as extrapolated for an infinitely large antibiotic concentration), EC50 (extracellular antibiotic concentration [in mg/liter] causing a reduction of the inoculum halfway between Emin and Emax), and Cs (static concentration, i.e., the extracellular concentration resulting in no apparent bacterial growth).
Checkerboard titration.
A two-dimensional, two-agent broth microdilution checkerboard titration method with 96-well plates was used to study the interaction between antibiotics (24). The first antibiotic of the combination was serially diluted along the ordinate and the second one along the abscissa, after which a bacterial suspension (final inoculum, 0.5 × 106 to 1 × 106 CFU/ml) was added to all wells. After 20 h of incubation at 37°C, the MIC of each antibiotic was determined as the lowest concentration that completely inhibited the growth of the organism as detected with the naked eye. Interactions between antibiotics were then evaluated using the FIC indices, calculated as the sum of the fractional inhibitory concentrations (FICs) as follows: ΣFIC = FIC A + FIC B, where FIC A is MIC of drug A in the combination/MIC of drug A alone and FIC B is MIC of drug B in the combination/MIC of drug B alone (25). The combination was considered synergistic for ΣFIC ≤ 0.5, additive for 0.5 < ΣFIC ≤1, indifferent for 1 < ΣFIC <4, and antagonistic for ΣFIC ≥ 4, according to EUCAST definition (26).
Assessment of antibiotic combination activity.
Two different methods were used to evaluate the activities of combinations against extracellular or intracellular bacteria. First, antibiotics were combined at fixed concentrations corresponding either to their respective MICs in broth or to the maximal serum concentration (total drug) observed in patients after administration of conventional doses of the corresponding antibiotic in humans (Cmax) (see references and values in reference 17). Second, interactions between drugs within the combination were evaluated using the fractional maximal effect (FME) approach (27, 28), as previously done for determining interaction between antibiotics against intracellular S. aureus (21). The concentrations of each antibiotic to be tested (Cxp) were calculated from EC50 and Emax (defined as an FME of 1) to obtain FMEs of 0.1, 0.3, 0.5, 0.7, and 0.9, as follows: Cxp = (FME × EC50)/(1 − FME). Antibiotics were combined at different concentration ratios in order to obtained an expected FME equal to 1 (i.e., concentrations of antibiotic A [Cxp A] yielding FMEs of 0.1, 0.3, 0.5, 0.7, and 0.9, combined with concentrations of antibiotic B [Cxp B] yielding FMEs of 0.9, 0.7, 0.5, 0.3, and 0.1). The effect observed experimentally (Eobs) was then compared to the effect expected for an additive effect (Exp additive) as calculated from Katzper's formula (27), Exp additive = [(Emax A × Cxp A/EC50 A) + (Emax B × Cxp B/EC50 B)]/(1 + Cxp A/EC50 A + Cxp B/EC50 B), which allowed us to calculate the FME of the combination [FMEobs(A + B)] as Eobs/Exp additive.
Values of FMEobs(A + B) were then plotted as a function of the concentration ratios of the two drugs expected to give an FME of 1 (from 0.9:0.1 to 0.1:0.9), together with the values of the FME of each antibiotic alone using its actual concentration. Thus, a synergistic effect will yield an ordinate value larger than 1, an additive effect a value of 1, an indifferent effect a value lower than 1 but higher than the values of the FME of the corresponding antibiotics alone, and an antagonistic effect a value lower than those of the FME of the corresponding antibiotics alone (27).
Materials.
Colistin (sulfate salt; catalog no. C4461; potency, 67.50%) was purchased from Sigma-Aldrich (St. Louis, MO). The other antibiotics were obtained as microbiological standards from their corresponding manufacturers (ciprofloxacin [chlorhydrate; potency, 85%] from Bayer AG, Wuppertal, Germany, and tobramycin [potency, 100%] from SMB-Galephar, Marche-en-Famenne, Belgium) or as the commercial product registered in Belgium for parenteral use in humans from their respective marketing authorization holders or resellers (gentamicin [Gentalline; potency, 100%] from Schering-Plough, Brussels, Belgium, and meropenem [Meronem, potency, 87.7%] from Astra Zeneca, Brussels, Belgium). Unless stated otherwise, all other reagents were of analytical grade and were purchased from Sigma-Aldrich-Fluka. Cell culture or microbiology media were from Invitrogen (Paisley, Scotland) and Difco (Sparks, MD).
Curve fittings and statistical analyses.
Curve fittings were performed with GraphPad Prism (version 6.05) and statistical analyses with GraphPad InStat 3.10, both for Windows (GraphPad Prism Software, San Diego, CA).
RESULTS
Activity of antibiotics alone.
The MICs of the selected antibiotics for the 3 strains under study are shown in Table 1. Based on both EUCAST and CLSI interpretive criteria, all strains were clinically susceptible to tobramycin, while PA50 was clinically resistant to ciprofloxacin and clinically intermediate to colistin (CLSI only) and PA291 was clinically resistant to meropenem.
TABLE 1.
Pertinent regression parameters of concentration-response curves for extracellular (broth) and intracellular (THP-1 cells) activity of antibiotics against P. aeruginosa strainsa
| Antibiotic and strain | MICb (mg/liter) | Extracellular activity |
Intracellular activity |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Eminc | Emaxd | Cse | EC50f | Emin | Emax | Cs | EC50 | ||
| CIP | |||||||||
| PAO1 | 0.25 | 3.9 (2.8 to 4.9) | >−4.5 | 0.6 (0.4 to 0.9) | 0.2 (0.1 to 0.4) | 2.8 (2.0 to 3.5) | −2.7*# (−3.1 to −2.3) | 1.5 (0.9 to 2.4) | 0.4 (0.2 to 0.8) |
| PA50 | 16 | 4.0 (3.3 to 4.7) | >−4.5 | 1.3 (0.9 to 1.7) | 38.8*# (21.7 to 69.1) | 3.3 (2.89 to 3.7) | −2.3*# (−3.1 to −1.5) | 1.9 (1.4 to 2.5) | 21.0*# (12.1 to 36.4) |
| PA291 | 0.5 | 3.3 (2.7 to 3.9) | >−4.5 | 1.9 (1.5 to 2.5) | 2.4 (1.7 to 3.4) | 2.0* (1.5 to 2.6) | −3.1# (−3.5 to −2.8) | 1.2 (0.8 to 1.6) | 0.9 (0.5 to 1.4) |
| CST | |||||||||
| PAO1 | 1 | 3.6 (2.5 to 4.7) | >−4.5 | 1.2 (0.8 to 1.8) | 1.8 (1.0 to 3.4) | 2.9 (2.5 to 3.3) | −0.9 (−1.1 to −0.7) | 2.1 (1.5 to 3.0) | 0.7 (0.4 to 1.2) |
| PA50 | 4 | 3.9 (3.3 to 4.5) | >−4.5 | 1.4 (1.2 to 1.6) | 6.7 (4.8 to 9.5) | 3.5* (2.9 to 4.0) | −0.35# (−0.6 to −0.1) | 0.7 (0.3 to 1.7) | 0.2 (0.1 to 0.5) |
| PA291 | 1 | 3.1 (2.7 to 3.6) | >−4.5 | 2.3 (1.8 to 3.0) | 3.2 (2.0 to 5.1) | 2.7 (2.1 to 3.2) | −1.7* (−2.0 to −1.5) | 0.8 (0.5 to 1.3) | 0.6 (0.3 to 1.3) |
| MEM | |||||||||
| PAO1 | 1 | 3.8 (3.0 to 4.3) | >−4.5 | 0.8 (0.7 to 1.0) | 1.2 (0.9 to 1.6) | 2.7 (2.2 to 3.2) | −1.7# (−2.0 to −1.4) | 1.4 (1.0 to 2.1) | 0.5 (0.3 to 0.8) |
| PA50 | 2 | 4.0 (3.3 to 4.7) | >−4.5 | 1.4 (1.1 to 1.7) | 3.8 (2.6 to 5.5) | 2.9 (2.5 to 3.4) | −1.4 (−1.7 to −1.1) | 1.2 (0.8 to 1.7) | 1.1 (0.7 to 1.9) |
| PA291 | 64 | 3.3 (2.9 to 3.8) | >−4.5 | 0.6 (0.5 to 0.8) | 119.9*# (59.6 to 241.6) | 2.1* (1.9 to 2.4) | ND | 1.2 (1.0 to 1.6) | 222.1*# (62.7 to 786.8) |
| TOB | |||||||||
| PAO1 | 0.5 | 3.9 (3.1 to 4.8) | >−4.5 | 0.6 (0.4 to 1.0) | 0.4 (0.2 to 0.7) | 3.2 (2.9 to 3.6) | −0.9 (−1.2 to −0.6) | 10.3# (7.2 to 14.7) | 2.8# (1.8 to 4.4) |
| PA50 | 1 | 4.1 (2.8 to 5.4) | >−4.5 | 1.2 (0.9 to 1.6) | 1.8 (1.2 to 2.9) | 3.4 (2.9 to 3.8) | −0.9# (−1.3 to −0.6) | 11.5# (7.6 to 17.3) | 3.0 (1.8 to 5.0) |
| PA291 | 1 | 3.5 (3.3 to 3.8) | >−4.5 | 0.9 (0.8 to 1.0) | 1.2 (1.0 to 1.4) | 2.3* (1.8 to 2.9) | −2.0* (−2.4 to −1.5) | 5.4*# (4.3 to 6.7) | 2.5 (1.3 to 4.7) |
After 24 h of incubation with extracellular concentrations ranging from 0.01 to 100 to 200 mg/liter. CIP, ciprofloxacin; CST, colistin; MEM, meropenem; TOB, tobramycin. r2 > 0.91; values are given as means with 95% confidence intervals in parentheses. Statistical analysis was done by one-way analysis of variance with Tukey's test for multiple comparisons of each parameter. *, denotes significant difference among strains for the same antibiotic; #, significant difference between antibiotics for the same strain (P ≤ 0.05). ND, not determined (plateau not reached at the highest concentration tested).
EUCAST/CLSI susceptibility breakpoints (susceptible): CIP, ≤0.5/1; CST, 4/2; MEM, 2/2; TOB, 4/4.
CFU increase (in log10 units) from the corresponding initial inoculum at 24 h as extrapolated for an infinitely low antibiotic concentration.
CFU decrease (in log10 units) from the corresponding initial inoculum at 24 h as extrapolated from an infinitely large antibiotic concentration.
Static concentration, i.e., the extracellular concentration (total drug, in multiples of the MIC) resulting in no apparent bacterial growth (number of CFU identical to the initial inoculum), as calculated from the Hill equation of the concentration-response curve.
Extracellular antibiotic concentration (mg/liter) causing a reduction of the inoculum halfway between the number of CFU extrapolated for Emin and Emax.
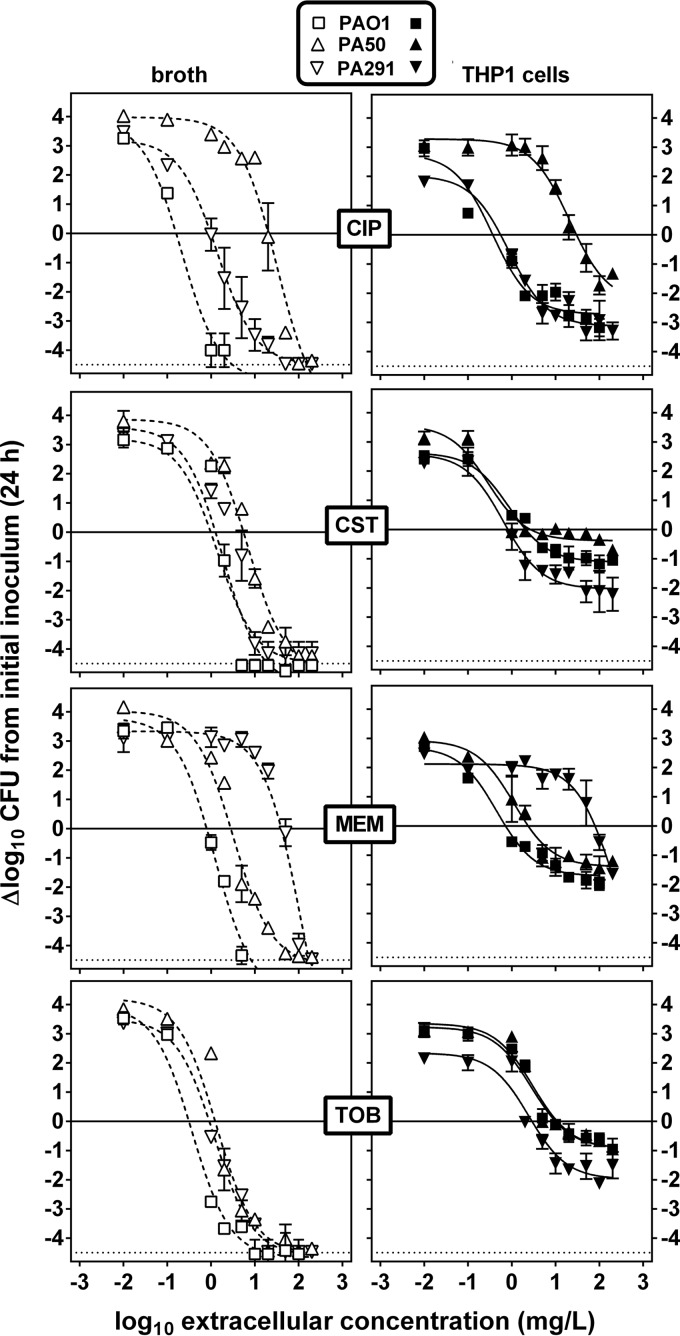
Bacteria in broth (extracellular forms) or in THP-1 cells having phagocytized bacteria (intracellular forms) were then exposed for 24 h to a broad range of antibiotic concentrations in order to obtain full concentration-response curves (Fig. 1) and to calculate the pertinent pharmacodynamic parameters of antibiotic activity (Emax, Emin, Cs, and EC50) (Table 1). As previously described for PAO1 (17), monophasic sigmoidal functions (with a Hill factor of 1) could be fitted to the experimental values. Against extracellular bacteria, an apparent static effect was observed for each antibiotic at a concentration close to its MIC, and Emax was always below the limit of detection. Against intracellular bacteria, Emin was systematically lower for PA291, indicating a slower intracellular growth. As for extracellular forms, static concentrations (Cs) against the intracellular forms were in the range of the corresponding MIC in broth, except for tobramycin, for which Cs values were systematically 5-fold (PA291) to 10-fold (PAO1 and PA50) higher, as previously observed for aminoglycosides against the PAO1 strain (17). Compared to those for bacteria in broth, Emax values for intracellular bacteria were considerably lower (less negative) for all 4 drugs whatever the strain considered, with only a 0.5 to 2 log10 CFU decrease from the original, postphagocytosis inoculum for colistin, tobramycin, and meropenem and about a 3 log10 CFU decrease for ciprofloxacin. Also of interest, Emax values were systematically larger (more negative) against PA291 (except for meropenem, to which the strain was resistant).
FIG 1.
Concentration-response curves of selected antibiotics against extracellular (left panels) and intracellular (THP-1 cells; right panels) P. aeruginosa PAO1, PA50, and PA291. The graphs show the change in the number of CFU (Δlog10 CFU from the initial inoculum) per ml of broth (extracellular, open symbols, dotted lines) or per mg of cell protein (intracellular, closed symbols, solid plain lines) after 24 h of incubation at increasing extracellular concentrations expressed in mg/liter (total drug). The solid horizontal line corresponds to a bacteriostatic effect (no change from the initial inoculum), and the dotted horizontal line shows the limit of detection (4.5 log CFU decrease). Values are means ± standard errors of the means (SEM) (2 experiments performed in triplicate); when not visible, the error bars are smaller than the symbols. CIP, ciprofloxacin; CST, colistin; MEM, meropenem; TOB, tobramycin.
Checkerboard titration of antibiotic combinations.
We first examined combinations between antibiotics using the checkerboard titration assay (Table 2). The results are presented as the lowest and highest ΣFIC values obtained for all combinations tested. Most combinations were additive based on the calculated ΣFIC (0.5 < ΣFIC ≤1), with synergy observed only for the colistin-meropenem combination against PAO1 and for the colistin-tobramycin combination against PA50.
TABLE 2.
FIC indices from checkerboard titration synergy testing
| Strain | Range of calculated FIC indices for drug combinationa (interpretation) |
|||||
|---|---|---|---|---|---|---|
| CIP-CST | CIP-TOB | CIP-MEM | CST-TOB | CST-MEM | TOB-MEM | |
| PAO1 | 0.56–1 (additivity) | 0.75–1 (additivity) | 0.63–1 (additivity) | 0.63–1 (additivity) | 0.26–1 (synergy/additivity) | 0.56–1 (additivity) |
| PA50 | 0.56–1 (additivity) | 1 (additivity) | 1 (additivity) | 0.26–1 (synergy/additivity) | 0.56–1 (additivity) | 0.63–1 (additivity) |
| PA291 | 1 (additivity) | 0.53–1 (additivity) | 0.63–1 (additivity) | 0.75–1 (additivity) | 1 (additivity) | 0.75–1 (additivity) |
CIP, ciprofloxacin; CST, colistin; MEM, meropenem; TOB, tobramycin. FICs were determined by checkerboard titration; the values correspond the minimal and maximal FIC values calculated for the different combinations of concentrations tested.
Combinations at fixed concentrations.
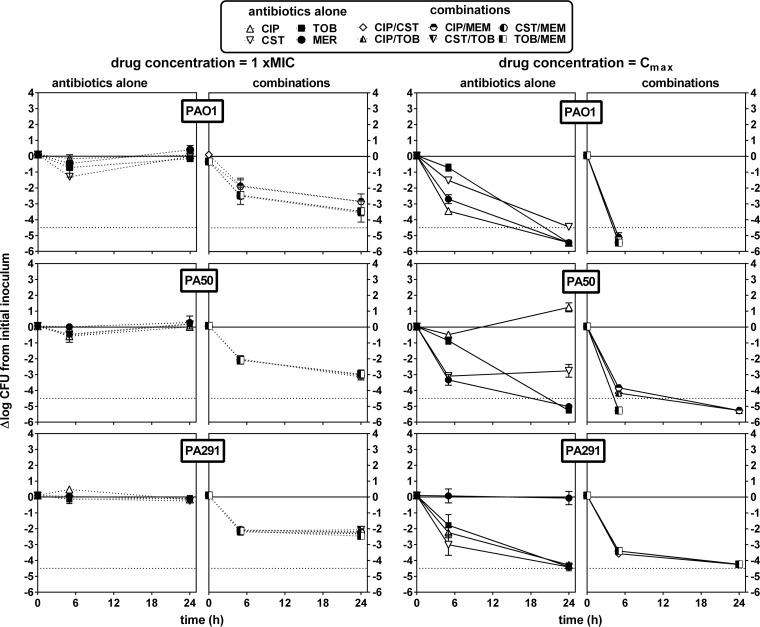
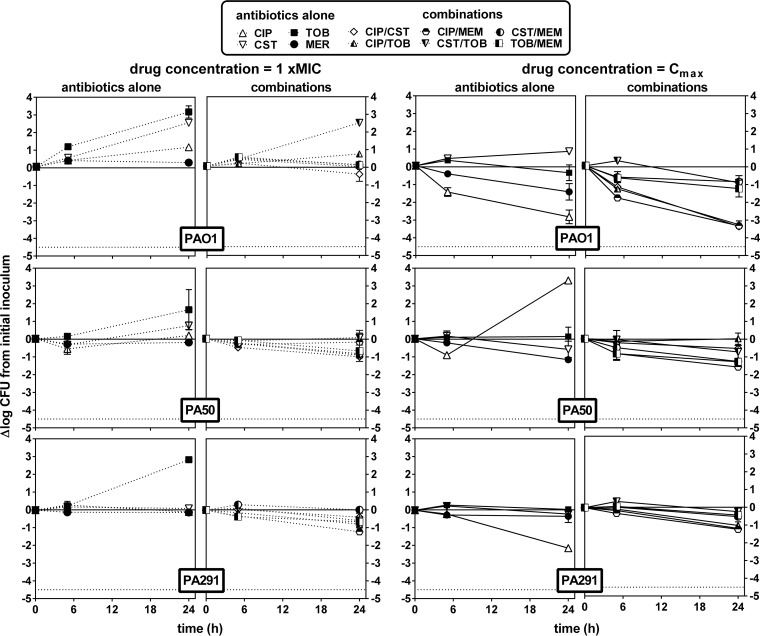
Antibiotics were then combined at two fixed concentrations, namely, (i) their respective MICs and (ii) a concentration corresponding to their human Cmax (total drug), and the decrease in CFU was evaluated after 6 or 24 h of incubation. Against extracellular bacteria (Fig. 2), antibiotics were essentially static when tested at their MIC in broth, whereas they were all bactericidal at 24 h at their Cmax, except for ciprofloxacin and meropenem when tested against the corresponding resistant strains (PA50 and PA291, respectively). Combining antibiotics at their MICs allowed a 1 to 2 log10 decrease in bacterial counts at 6 h and reductions of 2 to 3 log10 for PAO1 and PA50 and of 2 log10 for PA291 at 24 h. Combining antibiotics at their Cmax resulted in bactericidal effects at 6 h for all antibiotic combinations tested, with the limit of detection being reached at 6 h for PAO1 and at 24 h for PA50 and being almost reached at 24 h for PA291. Of interest, combinations including ciprofloxacin showed a slower effect against the fluoroquinolone-resistant strain PA50 than other combinations. Very contrasting results were observed against intracellular bacteria (Fig. 3). For PAO1, combinations were globally not more effective than the antibiotics used alone. For PA50 and PA291, all antibiotic combinations were essentially static even when tested at an extracellular concentration corresponding to their human Cmax (total drug).
FIG 2.
Influence of time on the rate and extent of killing of P. aeruginosa PAO1, PA50, and PA291 in broth by antibiotics alone or in combination used at concentrations corresponding to their respective MIC (dotted lines) or their human Cmax (solid lines) (human Cmax values are as follows: CIP, 4.6 mg/liter; CST, 5 mg/liter; MER, 57 mg/liter; and TOB, 6 mg/liter [see reference in reference 17]). The ordinate shows the change in the number of CFU (log10 scale) per ml of broth. The solid horizontal line corresponds to a bacteriostatic effect (no change from the initial inoculum), and the dotted horizontal line shows the limit of detection (−4.5 log CFU decrease).Values are means ± SEM (2 experiments performed in triplicate); when not visible, error bars are smaller than the symbols. CIP, ciprofloxacin; CST, colistin; MEM, meropenem; TOB, tobramycin.
FIG 3.
Influence of time on the rate and extent of killing of intracellular P. aeruginosa PAO1, PA50, and PA291 by antibiotics alone or in combination used at concentrations corresponding to their respective MIC (dotted lines) or their human Cmax (solid lines) (human Cmax values are as follows: CIP, 4.6 mg/liter; CST, 5 mg/liter; MER, 57 mg/liter; TOB, 6 mg/liter [see reference in reference 17]). The ordinate shows the change in the number of CFU (log10 scale) per mg of cell protein. The solid horizontal line corresponds to a bacteriostatic effect (no change from the initial inoculum), and the dotted horizontal line shows the limit of detection (−4.5 log CFU decrease). Values are means ± SEM (2 experiments performed in triplicate); when not visible, error bars are smaller than the symbols. CIP, ciprofloxacin; CST, colistin; MEM, meropenem; TOB, tobramycin.
Interactions in combinations by determination of FME.
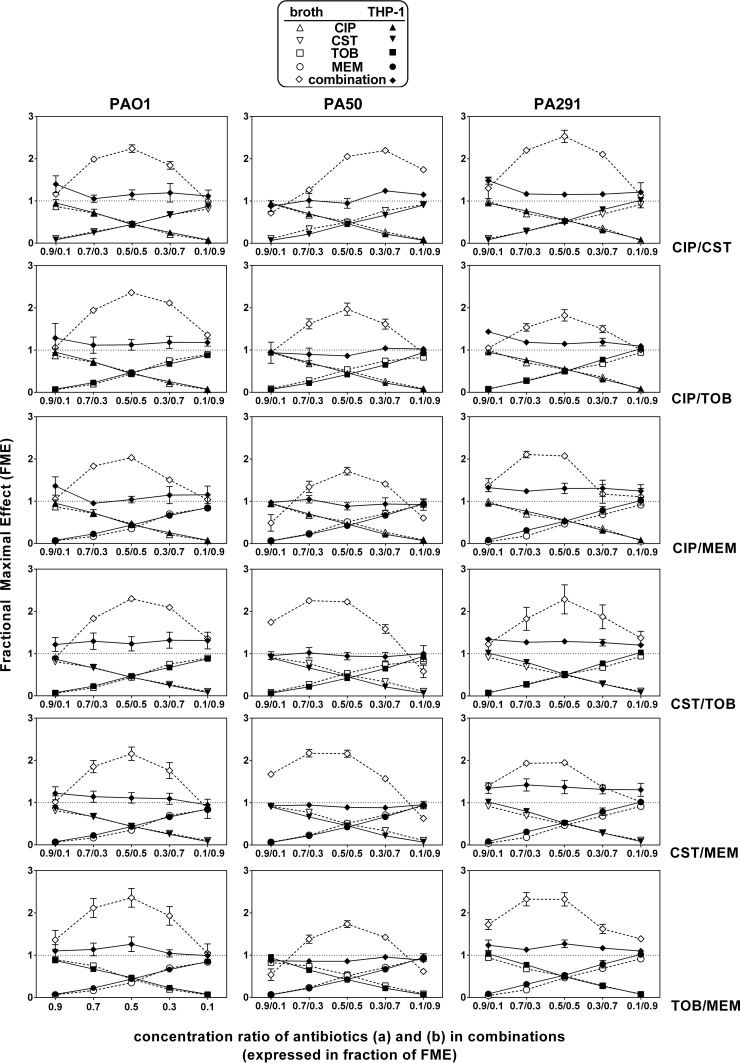
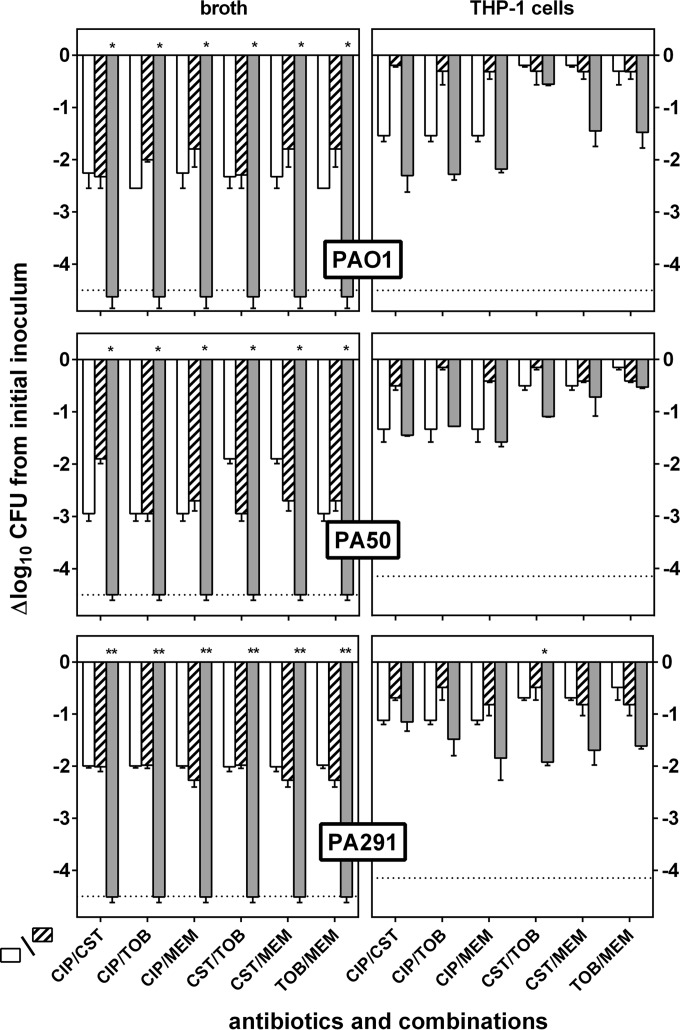
In these experiments, antibiotics were combined at different concentration ratios calculated based on the equations of the concentration-response curves (Table 1) in order to cover a whole range of combinations, starting from a proportion denoting mainly the activity of antibiotic A (fractional maximal effect [FME] of 0.9 for antibiotic A and of 0.1 for antibiotic B) to a proportion corresponding to the reverse situation (FME of 0.1 for antibiotic A and of 0.9 for antibiotic B) (the actual concentrations tested are shown in Table 3). The experimentally observed FMEs were then compared to the corresponding calculated values, and the results are shown in Fig. 4. Against extracellular bacteria, all combinations tested appeared to be synergistic, with bell-shaped curves indicative of a maximal synergy when both antibiotics were combined at concentrations generating an FME of 0.5. Against intracellular bacteria, only additive effects were observed, whatever the ratio of antibiotic concentrations used. Figure 5 shows in a synoptic fashion the gains in decreases of CFU (more negative values) observed when antibiotics were combined at concentrations allowing an FME of 0.5 to be obtained compared to the values observed for each antibiotic when used alone at the same concentration (the corresponding concentrations are shown in Table 3). For bacteria in broth, antibiotics alone caused only a 2 log10 reduction in inoculum against PAO1 or PA291 and a 3 log10 decrease against PA50 (except colistin, for which only a 2 log10 decrease was observed), while all combinations allowed the limit of detection to be reached. For bacteria in THP-1 cells, the intracellular inoculum was reduced by at most 1.5 log10 by ciprofloxacin for all strains and by only 0.2 to 1 log10 by all other antibiotics given alone. While all combinations had an additive effect globally, the gains in decreases of CFU never reached statistical significance over antibiotics alone except for the colistin-tobramycin combination against PA291.
TABLE 3.
Concentrations of antibiotics (Cxp) used for the FME experiments illustrated in Fig. 4 and for the comparative activities of the most effective combinations in Fig. 5
| Antibiotic | Target FME |
Cxp (mg/liter)a for strain and model: |
|||||
|---|---|---|---|---|---|---|---|
| PAO1 |
PA50 |
PA291 |
|||||
| Extracellular | Intracellular | Extracellular | Intracellular | Extracellular | Intracellular | ||
| Ciprofloxacin | 0.1 | 0.20 | 0.45 | 26.52 | 35.43 | 1.16 | 0.71 |
| 0.3 | 0.32 | 0.69 | 45.22 | 51.52 | 1.84 | 1.15 | |
| 0.5 | 0.53 | 1.11 | 78.86 | 80.48 | 3.06 | 1.96 | |
| 0.7 | 1.02 | 2.08 | 157.38 | 148.07 | 5.89 | 3.83 | |
| 0.9 | 3.47 | 6.98 | 549.93 | 485.99 | 20.09 | 13.18 | |
| Colistin | 0.1 | 1.66 | 2.39 | 6.50 | 2.98 | 2.97 | 0.98 |
| 0.3 | 2.65 | 3.32 | 9.86 | 3.91 | 4.73 | 1.45 | |
| 0.5 | 4.44 | 5.00 | 15.91 | 5.59 | 7.88 | 2.29 | |
| 0.7 | 8.61 | 8.91 | 30.01 | 9.71 | 15.25 | 4.24 | |
| 0.9 | 29.48 | 28.48 | 100.52 | 29.09 | 52.06 | 14.01 | |
| Meropenem | 0.1 | 1.13 | 0.86 | 3.75 | 6.82 | 57.84 | 113.80 |
| 0.3 | 1.79 | 1.23 | 5.9 | 9.44 | 108.72 | 210.28 | |
| 0.5 | 2.99 | 1.91 | 9.78 | 14.15 | 200.30 | 383.28 | |
| 0.7 | 5.79 | 3.48 | 18.84 | 25.14 | 413.98 | 789.15 | |
| 0.9 | 10.77 | 11.36 | 64.12 | 80.12 | 1482.39 | 2815.20 | |
| Tobramycin | 0.1 | 0.39 | 11.31 | 1.75 | 12.42 | 1.15 | 3.50 |
| 0.3 | 0.61 | 15.34 | 2.77 | 16.83 | 1.82 | 5.20 | |
| 0.5 | 1.00 | 22.61 | 4.6 | 24.78 | 3.03 | 8.26 | |
| 0.7 | 1.91 | 39.56 | 8.89 | 43.31 | 5.85 | 15.40 | |
| 0.9 | 6.50 | 124.32 | 30.30 | 135.96 | 19.97 | 51.10 | |
Concentration of each individual antibiotic in the combination calculated to reach an FME ranging from 0.1 to 0.9, using the EC50s from the sigmoid equation of the concentration-effect experiments presented in Fig. 1 (see values in Table 1) and based on the following equation: Cxp = (FME × EC50)/(1 − FME). Concentration values in bold are those used in Fig. 5, corresponding to a FME of 0.5 for each drug in the combination.
FIG 4.
FME plots of antibiotics against extracellular (open symbols, dashed lines) and intracellular (closed symbols, solid lines) P. aeruginosa strains. In each graph, the abscissa shows the FMEs value calculated for antibiotics A and B based on concentration effects shown in Fig. 1 (see Table 3 for the corresponding concentrations), and the ordinate shows the value of the FMEobs for each antibiotic alone or for the combination. FMEobs values for the combination that are >1 denote a synergistic effect, values equal to 1 an additive effect, values <1 but higher an indifferent effect, and values <1 and lower than FMEobs for each individual antibiotic in the combination an antagonistic effect.
FIG 5.
Comparative activities of antibiotics at a fixed concentration giving rise to an expected FME of 0.5 and tested alone (open and hatched bars) (see Table 3 for the corresponding concentrations) or combined (gray bars) against P. aeruginosa strains in broth (left) or infecting THP-1 cells (right). Data are expressed as the decrease in CFU per ml of broth (extracellular) or per mg cell protein (intracellular) compared to the initial inoculum. Values are the means ± SEM (2 experiments performed in triplicate). The horizontal dotted line corresponds to the limit of detection. Statistical analysis: P < 0.05 (*) or P < 0.01 (**) for the combination compared to each of the two antibiotics alone (t test).
DISCUSSION
To the best of our knowledge, this study is the first one to examine the potential of antibiotic combinations for improving antibiotic activity against intracellular forms of P. aeruginosa. Starting from a previously developed intracellular model of infection of THP-1 cells by the reference strain PAO1, we expanded our studies to clinical isolates, including strains resistant to two commonly recommended antipseudomonal antibiotics. Differences in internalization and intracellular growth were noticed, with PA291 being somewhat less phagocytized and displaying slower growth than the reference strain PAO1 or the other clinical isolate, PA50. The reasons for these differences may be related to variations in the expression of virulence factors, such as the type III secretion system (29), flagellin (30), pili (31), quorum sensing (32), multidrug efflux systems (33), or rhamnolipids (34), and will need to be studied in details. However, they did not prevent us from performing the pharmacological studies described here. Moreover, the demonstration of the ability of these clinical isolates to survive within the cells without killing them supports the suggested importance of intracellular survival in the persistence of pseudomonal infection (19, 35).
Considering first the intracellular activity of antibiotics when tested alone, we extend here our previous observation that the relative potency of most drugs against intracellular P. aeruginosa is directly related to their corresponding MICs as determined in broth, even for drugs that accumulate within cells, such as ciprofloxacin (17). This reinforces our conclusion that antibiotic accumulation per se is not the only factor to consider when assessing intracellular activity but that other parameters, such as intracellular bioavailability, need to be taken into account (see reference 36 for a recent example with fluoroquinolones and THP-1 cells infected with Staphylococcus aureus or Listeria monocytogenes [typical phagolysosomal and cytosolic organisms]). In the specific case of aminoglycosides, which accumulate to much lower levels and are restricted to phagolysosomes, static concentrations are higher than their MICs in broth, possibly due to the defeating effect of the acidic pH of phagolysosomes on their activity (37). We also confirm that a fluoroquinolone such as ciprofloxacin is more effective than the other classes of antipseudomonal drugs whatever the resistance phenotype of the strains, indicating that the level of intracellular efficacy of an antibiotic may also depend on its mode of action (17). Of note, slightly higher maximal reductions in the intracellular inoculum were achieved for PA291 for all drugs. This is possibly related to its lower internalization, as most antibiotics show inoculum effects.
Moving to antibiotic combinations, several methods have been proposed in the literature to assess their use in vitro against extracellular bacteria, such as checkerboard titration, time-kill curves, disk diffusion, Etest (with crossed or closely apposed strips), or pharmacodynamic models (24, 38), but none have been proposed for intracellular bacteria. Concentrating first on extracellular bacteria, checkerboard titration is probably a most useful way for assessing synergy in vitro, because it allows testing of a wide range of concentrations (39). In our study, however, this method failed to evidence any synergy between antibiotics for extracellular bacteria, which seems contradictory with the results obtained with the other methods. Discordances between time-kill and checkerboard titration methods have been previously reported (5, 25, 40, 41), with agreement rates between the two methods as low as 50% (40). This may be due to the methodology itself. FIC indices are highly dependent on the applied dilution series (42), and synergy is usually considered significant only if the FIC is lower than 0.5, which means a 4-fold reduction in the MIC measured for the combination versus each drug alone (43). Yet, more minor synergies (with FICs between 0.5 and 1, such as those observed in the present study) could be of clinical importance as well (44). This method should therefore be combined with others in order to better define interactions between drugs (45). In addition to checkerboard titration, time-kill curves represent another reference method whose results may better correlate with cure rates in animal models (46, 47). Its main limitation, however, is in the number of combinations (time × concentration) that can be simultaneously tested (48). Using this approach and focusing on two concentrations (MIC and human Cmax), we showed here that combinations allow restoration of bactericidal activity against resistant strains extracellularly, though the limit of detection was sometimes reached more slowly than for the reference strain, as also observed by others (49, 50). FME methodology is claimed to circumvent this limitation by exploring broader ranges of concentrations and taking into account the nonlinear concentration responses that are usually observed in pharmacology (28). Under these conditions, all the combinations tested proved synergistic, confirming the widely accepted concept of using bi-or tritherapies for difficult-to-treat infections (1–4).
Moving to intracellular bacteria, the main message from our experiments is that no synergy was observed, whatever the testing approach used. Thus, killing curves failed to evidence any increase in killing rates, and the FME method revealed only additive effects. In the context of the present study, we focused on the two key and most meaningful pharmacodynamic parameters derived from concentration effect-kill curves, namely, Emax and EC50 (27, 28), to select concentrations to be tested. This method has been applied successfully in other models of intracellular infection to demonstrate synergy (21), which means that the negative results observed here need to be taken at face value. Also, the fact that our FME experiments parallel the kill curve experiments for the intracellular model lends more credence to our results. We now have to understand why synergy is lost intracellularly, which may be related, among many possibilities, to modulations of bacterial metabolism in the intracellular milieu (making them less susceptible to antibiotic action) or to intracellular sequestration of the bulk of the antibiotics in distinct subcellular compartments (reducing their effective concentration at the site of infection). This contrasts with our previous observations using the same approach with intracellular small-colony variants of S. aureus, where we showed that combining highly bactericidal antibiotics (oritavancin, moxifloxacin, or rifampin) can provide considerable gains in reduction of the intracellular bacterial load (21). Elucidating the reasons why intracellular P. aeruginosa is acted upon poorly by antibiotics, such as exploring how the cellular environment may affect its responsiveness, could help in defining novel strategies to better act upon these persistent forms of infection.
Although leading to coherent observations, this study suffers from limitations inherent to the model. First, we could not include strains resistant to aminoglycosides, because we did not find any efficient alternative to a short incubation with gentamicin to eliminate extracellular bacteria. Colistin-resistant strains could not be studied either, since all strains we had access to were also resistant to aminoglycosides. Second, the number of strains that could be analyzed was small due to the complexity of experiments dealing with combinations of multiple concentrations against intracellular bacteria. Taking these caveats into account, we can nevertheless draw some pharmacologically and possibly also clinically meaningful conclusions. In a nutshell, we found that antibiotic combinations provided no more than an additive effect against intracellular P. aeruginosa, even when combining drugs at their human Cmax, which may represent the maximal exposure in humans. This may explain why eradication of P. aeruginosa seems such a difficult-to-achieve goal, as intracellular bacteria may represent a protected storage site from which unaffected organisms can be liberated even during or after conventional antibiotic treatments.
ACKNOWLEDGMENTS
We are grateful to O. Denis (Hôpital Erasme) and D. Pierard (Universitair Ziekenhuis Brussel), Brussels, for providing us with the clinical isolates and to K. Santos and M. C. Cambier for expert technical assistance. We thank all the manufacturers for the kind gifts of the corresponding antibiotics.
J.M.B. was a postdoctoral fellow of the BioWin program (Région Wallonne, Belgium), and F.V.B. is Maître de Recherches of the Belgian Fonds National de la Recherche Scientifique (FRS-FNRS). This work was supported by the BioWin program, the Fonds de la Recherche Scientifique (grants 3.4639.09 and 3.4530.12), the Interuniversity Attraction Poles Programme initiated by the Belgian Science Policy Office (program IAP P7/28), and a grant from the Fonds Alphonse et Jean Forton.
REFERENCES
- 1.Sun HY, Fujitani S, Quintiliani R, Yu VL. 2011. Pneumonia due to Pseudomonas aeruginosa. II. Antimicrobial resistance, pharmacodynamic concepts, and antibiotic therapy. Chest 139:1172–1185. doi: 10.1378/chest.10-0167. [DOI] [PubMed] [Google Scholar]
- 2.Boyd N, Nailor MD. 2011. Combination antibiotic therapy for empiric and definitive treatment of gram-negative infections: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 31:1073–1084. doi: 10.1592/phco.31.11.1073. [DOI] [PubMed] [Google Scholar]
- 3.Tamma PD, Cosgrove SE, Maragakis LL. 2012. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev 25:450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traugott KA, Echevarria K, Maxwell P, Green K, Lewis JS. 2011. Monotherapy or combination therapy? The Pseudomonas aeruginosa conundrum. Pharmacotherapy 31:598–608. doi: 10.1592/phco.31.6.598. [DOI] [PubMed] [Google Scholar]
- 5.Cappelletty DM, Rybak MJ. 1996. Comparison of methodologies for synergism testing of drug combinations against resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother 40:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer RD, Liu S. 1988. In vitro synergy studies with ciprofloxacin and selected beta-lactam agents and aminoglycosides against multidrug-resistant Pseudomonas aeruginosa. Diagn Microbiol Infect Dis 11:151–157. doi: 10.1016/0732-8893(88)90017-X. [DOI] [PubMed] [Google Scholar]
- 7.He W, Kaniga K, Lynch AS, Flamm RK, Davies TA. 2012. In vitro Etest synergy of doripenem with amikacin, colistin, and levofloxacin against Pseudomonas aeruginosa with defined carbapenem resistance mechanisms as determined by the Etest method. Diagn Microbiol Infect Dis 74:417–419. doi: 10.1016/j.diagmicrobio.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Pankuch GA, Lin G, Seifert H, Appelbaum PC. 2008. Activity of meropenem with and without ciprofloxacin and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob Agents Chemother 52:333–336. doi: 10.1128/AAC.00689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess DS, Nathisuwan S. 2002. Cefepime, piperacillin/tazobactam, gentamicin, ciprofloxacin, and levofloxacin alone and in combination against Pseudomonas aeruginosa. Diagn Microbiol Infect Dis 44:35–41. doi: 10.1016/S0732-8893(02)00420-0. [DOI] [PubMed] [Google Scholar]
- 10.Fish DN, Choi MK, Jung R. 2002. Synergic activity of cephalosporins plus fluoroquinolones against Pseudomonas aeruginosa with resistance to one or both drugs. J Antimicrob Chemother 50:1045–1049. doi: 10.1093/jac/dkf211. [DOI] [PubMed] [Google Scholar]
- 11.McGrath BJ, Lamp KC, Rybak MJ. 1993. Pharmacodynamic effects of extended dosing intervals of imipenem alone and in combination with amikacin against Pseudomonas aeruginosa in an in vitro model. Antimicrob Agents Chemother 37:1931–1937. doi: 10.1128/AAC.37.9.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappelletty DM, Kang SL, Palmer SM, Rybak MJ. 1995. Pharmacodynamics of ceftazidime administered as continuous infusion or intermittent bolus alone and in combination with single daily-dose amikacin against Pseudomonas aeruginosa in an in vitro infection model. Antimicrob Agents Chemother 39:1797–1801. doi: 10.1128/AAC.39.8.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergen PJ, Tsuji BT, Bulitta JB, Forrest A, Jacob J, Sidjabat HE, Paterson DL, Nation RL, Li J. 2011. Synergistic killing of multidrug-resistant Pseudomonas aeruginosa at multiple inocula by colistin combined with doripenem in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 55:5685–5695. doi: 10.1128/AAC.05298-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergen PJ, Forrest A, Bulitta JB, Tsuji BT, Sidjabat HE, Paterson DL, Li J, Nation RL. 2011. Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula. Antimicrob Agents Chemother 55:5134–5142. doi: 10.1128/AAC.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon NC, Png K, Wareham DW. 2010. Potent synergy and sustained bactericidal activity of a vancomycin-colistin combination versus multidrug-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother 54:5316–5322. doi: 10.1128/AAC.00922-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MH, Feinstein SA, Chow RT. 1987. Early effects of beta-lactams on aminoglycoside uptake, bactericidal rates, and turbidimetrically measured growth inhibition in Pseudomonas aeruginosa. Antimicrob Agents Chemother 31:108–110. doi: 10.1128/AAC.31.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buyck JM, Tulkens PM, Van Bambeke F. 2013. Pharmacodynamic evaluation of the intracellular activity of antibiotics towards Pseudomonas aeruginosa PAO1 in a model of THP-1 human monocytes. Antimicrob Agents Chemother. 57:2310–2318. doi: 10.1128/AAC.02609-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovewell RR, Patankar YR, Berwin B. 2014. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 306:L591–L603. doi: 10.1152/ajplung.00335.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmiedl A, Kerber-Momot T, Munder A, Pabst R, Tschernig T. 2010. Bacterial distribution in lung parenchyma early after pulmonary infection with Pseudomonas aeruginosa. Cell Tissue Res 342:67–73. doi: 10.1007/s00441-010-1036-y. [DOI] [PubMed] [Google Scholar]
- 20.Engel J, Eran Y. 2011. Subversion of mucosal barrier polarity by Pseudomonas aeruginosa. Front Microbiol 2:114. doi: 10.3389/fmicb.2011.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen HA, Denis O, Vergison A, Tulkens PM, Struelens MJ, Van Bambeke F. 2009. Intracellular activity of antibiotics in a model of human THP-1 macrophages infected by a Staphylococcus aureus small-colony variant strain isolated from a cystic fibrosis patient: study of antibiotic combinations. Antimicrob Agents Chemother 53:1443–1449. doi: 10.1128/AAC.01146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standard Institute. 2013. Performance standards for antimicrobial susceptibility testing; 23th informational supplement. CLSI document MS100-S23. CLSI, Wayne PA. [Google Scholar]
- 23.Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother 50:841–851. doi: 10.1128/AAC.50.3.841-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eliopoulos GM, Moellering RCJ. 1996. Antimicrobial combinations, p 330–396. In Lorian V. (ed), Antibiotics in laboratory medicine. The Williams & Wilkins Co., Baltimore, MD. [Google Scholar]
- 25.Bonapace CR, Bosso JA, Friedrich LV, White RL. 2002. Comparison of methods of interpretation of checkerboard synergy testing. Diagn Microbiol Infect Dis 44:363–366. doi: 10.1016/S0732-8893(02)00473-X. [DOI] [PubMed] [Google Scholar]
- 26.European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). 2000. EUCAST definitive document E. Def 1.2, May 2000: terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin Microbiol Infect 6:503–508. doi: 10.1046/j.1469-0691.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- 27.Desbiolles N, Piroth L, Lequeu C, Neuwirth C, Portier H, Chavanet P. 2001. Fractional maximal effect method for in vitro synergy between amoxicillin and ceftriaxone and between vancomycin and ceftriaxone against Enterococcus faecalis and penicillin-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother 45:3328–3333. doi: 10.1128/AAC.45.12.3328-3333.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li RC, Schentag JJ, Nix DE. 1993. The fractional maximal effect method: a new way to characterize the effect of antibiotic combinations and other nonlinear pharmacodynamic interactions. Antimicrob Agents Chemother 37:523–531. doi: 10.1128/AAC.37.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauser AR, Fleiszig S, Kang PJ, Mostov K, Engel JN. 1998. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect Immun 66:1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleiszig SM, Arora SK, Van R, Ramphal R. 2001. FlhA, a component of the flagellum assembly apparatus of Pseudomonas aeruginosa, plays a role in internalization by corneal epithelial cells. Infect Immun 69:4931–4937. doi: 10.1128/IAI.69.8.4931-4937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plotkowski MC, Saliba AM, Pereira SH, Cervante MP, Bajolet-Laudinat O. 1994. Pseudomonas aeruginosa selective adherence to and entry into human endothelial cells. Infect Immun 62:5456–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kondo A, Hirakata Y, Gotoh N, Fukushima K, Yanagihara K, Ohno H, Higashiyama Y, Miyazaki Y, Nishide K, Node M, Yamada Y, Kohno S, Kamihira S. 2006. Quorum sensing system lactones do not increase invasiveness of a MexAB-OprM efflux mutant but do play a partial role in Pseudomonas aeruginosa invasion. Microbiol Immunol 50:395–401. doi: 10.1111/j.1348-0421.2006.tb03806.x. [DOI] [PubMed] [Google Scholar]
- 33.Hirakata Y, Srikumar R, Poole K, Gotoh N, Suematsu T, Kohno S, Kamihira S, Hancock RE, Speert DP. 2002. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J Exp Med 196:109–118. doi: 10.1084/jem.20020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClure CD, Schiller NL. 1996. Inhibition of macrophage phagocytosis by Pseudomonas aeruginosa rhamnolipids in vitro and in vivo. Curr Microbiol 33:109–117. doi: 10.1007/s002849900084. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Medina R, Dunne WM, Singh PK, Brody SL. 2005. Pseudomonas aeruginosa acquires biofilm-like properties within airway epithelial cells. Infect Immun 73:8298–8305. doi: 10.1128/IAI.73.12.8298-8305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallet CM, Marquez B, Ngabirano E, Lemaire S, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. 2011. Cellular accumulation of fluoroquinolones is not predictive of their intracellular activity: studies with gemifloxacin, moxifloxacin and ciprofloxacin in a pharmacokinetic/pharmacodynamic model of uninfected and infected macrophages. Int J Antimicrob Agents 38:249–256. doi: 10.1016/j.ijantimicag.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Baudoux P, Bles N, Lemaire S, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. 2007. Combined effect of pH and concentration on the activities of gentamicin and oxacillin against Staphylococcus aureus in pharmacodynamic models of extracellular and intracellular infections. J Antimicrob Chemother 59:246–253. doi: 10.1093/jac/dkl489. [DOI] [PubMed] [Google Scholar]
- 38.Michael J, Barth A, Kloft C, Derendorf H. 2014. Pharmacodynamic in vitro models to determine the effect of antibiotics, p 81–112. In Vincks AA, Derendorf H, Mouton JW (ed), Fundamentals of antimicrobial pharmacokinetics and pharmacodynamics. Springer, New York, NY. [Google Scholar]
- 39.Hsieh MH, Yu CM, Yu VL, Chow JW. 1993. Synergy assessed by checkerboard. A critical analysis. Diagn Microbiol Infect Dis 16:343–349. doi: 10.1016/0732-8893(93)90087-N. [DOI] [PubMed] [Google Scholar]
- 40.Bonapace CR, White RL, Friedrich LV, Bosso JA. 2000. Evaluation of antibiotic synergy against Acinetobacter baumannii: a comparison with Etest, time-kill, and checkerboard methods. Diagn Microbiol Infect Dis 38:43–50. doi: 10.1016/S0732-8893(00)00163-2. [DOI] [PubMed] [Google Scholar]
- 41.White RL, Burgess DS, Manduru M, Bosso JA. 1996. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob Agents Chemother 40:1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horrevorts AM, de Ridder CM, Poot MC, de Jonge MJ, Degener JE, Dzoljic-Danilovic G, Michel MF, Kerrebijn KF. 1987. Chequerboard titrations: the influence of the composition of serial dilutions of antibiotics on the fractional inhibitory concentration index and fractional bactericidal concentration index. J Antimicrob Chemother 19:119–125. doi: 10.1093/jac/19.1.119. [DOI] [PubMed] [Google Scholar]
- 43.Saiman L. 2007. Clinical utility of synergy testing for multidrug-resistant Pseudomonas aeruginosa isolated from patients with cystic fibrosis: ‘the motion for'. Paediatr. Respir Rev 8:249–255. doi: 10.1016/j.prrv.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Berenbaum MC. 1987. Minor synergy and antagonism may be clinically important. J Antimicrob Chemother 19:271–273. doi: 10.1093/jac/19.2.271. [DOI] [PubMed] [Google Scholar]
- 45.Mackay ML, Milne K, Gould IM. 2000. Comparison of methods for assessing synergic antibiotic interactions. Int J Antimicrob Agents 15:125–129. doi: 10.1016/S0924-8579(00)00149-7. [DOI] [PubMed] [Google Scholar]
- 46.Chandrasekar PH, Crane LR, Bailey EJ. 1987. Comparison of the activity of antibiotic combinations in vitro with clinical outcome and resistance emergence in serious infection by Pseudomonas aeruginosa in non-neutropenic patients. J Antimicrob Chemother 19:321–329. doi: 10.1093/jac/19.3.321. [DOI] [PubMed] [Google Scholar]
- 47.Visalli MA, Jacobs MR, Appelbaum PC. 1998. Determination of activities of levofloxacin, alone and combined with gentamicin, ceftazidime, cefpirome, and meropenem, against 124 strains of Pseudomonas aeruginosa by checkerboard and time-kill methodology. Antimicrob Agents Chemother 42:953–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eliopoulos GM, Eliopoulos CT. 1988. Antibiotic combinations: should they be tested? Clin Microbiol Rev 1:139–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidaillac C, Benichou L, Duval RE. 2012. In vitro synergy of colistin combinations against colistin-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae isolates. Antimicrob Agents Chemother 56:4856–4861. doi: 10.1128/AAC.05996-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kresken M, Korber-Irrgang B, Lauffer J, Decker-Burgard S, Davies T. 2011. In vitro activities of ceftobiprole combined with amikacin or levofloxacin against Pseudomonas aeruginosa: evidence of a synergistic effect using time-kill methodology. Int J Antimicrob Agents 38:70–75. doi: 10.1016/j.ijantimicag.2011.01.028. [DOI] [PubMed] [Google Scholar]