Abstract
Histone methylations have been implicated to play important roles in diverse cellular processes. Of particular interest is the methylation of histone H3K79, which is catalyzed by an evolutionarily conserved methyltransferase, disruptor of telomeric silencing (Dot1)-like (Dot1L). To investigate the role of Dot1L during vertebrate development, we have generated a Dot1L-specific transcription activator-like effector nuclease (TALEN) nuclease to knockdown endogenous Dot1L in Xenopus tropicalis, a diploid species highly related to the well-known developmental model Xenopus laevis, a pseudotetraploid amphibian. We show that the TALEN was extremely efficient in mutating Dot1L when expressed in fertilized eggs, creating essentially Dot1L knockout embryos with little H3K79 methylation. Importantly, we observed that Dot1L knockdown had no apparent effect on embryogenesis because normally feeding tadpoles were formed, consistent with the lack of maternal Dot1L expression. On the other hand, Dot1L knockdown severely retarded the growth of the tadpoles and led to tadpole lethality prior to metamorphosis. These findings suggest that Dot1L and H3K79 methylation play an important role for tadpole growth and development prior to metamorphosis into a frog. Our findings further reveal interesting similarities and differences between Xenopus and mouse development and suggest the existence of 2 separate phases of vertebrate development with distinct requirements for epigenetic modifications.—Wen, L., Fu, L., Guo, X., Chen, Y., Shi, Y.-B. Histone methyltransferase Dot1L plays a role in postembryonic development in Xenopus tropicalis.
Keywords: epigenetics, histone modification, activation mark, organogenesis
Eukaryotic DNA is present in chromatin, which is made of nucleosomes and a linker histone. Within each nucleosome, the DNA is wrapped around a histone octamer consisting of a [3H]/H4 heterotetramer and 2 H2A/H2B heterodimers. The histones, particularly their N- and C-terminal tails, are subjected to a large number of post-translational modifications, including acetylation, methylation, phosphorylation, and ubiquitylation (1–4). These histone modifications seem to have a substantial influence on chromatin structure and a number of cellular processes including transcription, DNA repair, and cell cycle regulation (1, 2, 4–6).
Various studies in cell culture have revealed the association of different histone modifications (histone marks) with either high levels of gene expression or gene silencing, and these histone marks are thus referred to as activation or repression marks, respectively (1, 2, 6–11). Among them, the methylation of histone [3H] lysine 79 (H3K79) is associated with high levels of gene expression and thus are known as an activation histone mark. Unlike the vast majority of histone modifications that occur at the N- or C-terminal tails of the core histones, H3K79 methylation takes place in a loop within the globular domain of histone [3H] on the surface of the nucleosome and is carried out by only a single known methyltransferase (5). This enzyme, Dot1L, was originally identified as Dot1 in Saccharomyces cerevisiae (12) and is a member of the lysine methyltransferase family (KMTs) (5, 13–15). Interestingly, other than Dot1L, all KMTs contain a SET {Su(var)3-9, Enhancer of Zeste [E(Z)], and Trithorax (trx)} domain. Dot1L is the only known non-SET domain-containing KMT and is the only known KMT that possesses histone methyltransferase activity toward histone [3H] lysine (K) 79 in vitro (5, 13, 16). In agreement with this, Dot1 deletion in yeast, Drosophila, and mice leads to a complete loss of H3K79 methylation (17–19).
We have been studying the role of Dot1L during vertebrate development by using X. tropicalis as a model. We previously identified Dot1L as a direct target gene of thyroid hormone (TH) receptor (TR) during TH-dependent metamorphosis in X. tropicalis (20), a process that resembles the so-called “postembryonic development” around birth in mammals when TH levels are also high in the plasma (21, 22). Dot1L was highly up-regulated by TH in different organs during metamorphosis. Interestingly, H3K79 methylation levels are increased at TR target genes upon TH treatment or during natural metamorphosis when endogenous TH levels are high (23), suggesting that Dot1L may be transcriptionally activated by liganded TR, and Dot1L, in turn, enhances chromatin remodeling and gene activation by TR during metamorphosis.
To investigate the role of Dot1L during Xenopus development, we generated a Dot1L-specific TALEN (24, 25). We show that microinjecting mRNAs for both the left and right arm of the Dot1L TALEN, but not the mRNAs for individual arms or a nonspecific TALEN, into fertilized egg leads to efficient mutations of the endogenous Dot1L gene. More importantly, this knockdown of the endogenous Dot1L also results in the loss of H3K79 methylation in the injected embryos. Surprisingly, the knockdown of Dot1L has no observed effect on embryogenesis as the injected eggs develop normally into feeding tadpoles. On the other hand, such tadpoles subsequently experience severe growth retardation and die prior to the onset of metamorphosis, suggesting an important role of Dot1L for tadpole growth and development.
MATERIALS AND METHODS
Animal rearing and staging
All animal care and treatment were done as approved by Animal Use and Care Committee of Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), U.S. National Institutes of Health (NIH). Adult X. tropicalis were purchased from NASCO (Fort Atkinson, WI, USA). Embryos were staged according to the description for X. laevis (26). Embryos were reared in 0.1 × Marc's modified Ringers in agar-coated petri dish for 4 d at 25°C, and then transferred to a large volume (≥1 L) container. Different groups of tadpoles were reared at a similar density.
For the tadpole survival studies, some tadpoles were taken out for experiments or adjusting tadpole density. Thus, to account for these changes, the tadpole survival rate was calculated by dividing the number of live tadpoles at a given day by the number of live tadpoles on the day earlier and then multiplying the resulting value by the survive rate for the day earlier.
TALEN assembly and TALEN mRNA preparation
A TALEN pair targeting X. tropicalis Dot1L were assembled as described (25). The Dot1L TALEN left (TALEN-L) arm recognizes the sequence GAAAAACTCAACAA and the TALEN right (TALEN-R) arm recognizes the sequence TCTCCATAGACCTCA in the Dot1L coding region. A control TALEN pair consisted of the TALEN-L arm recognizing ACATCCCCAGCTATCT and TALEN-R arm recognizing ATCACTGCACACCACACAC in an unrelated gene. To generate the TALEN mRNA in vitro, the individual TALEN plasmid was linearized with NotI. Capped RNA was produced by using the linearized DNA and the Ambion (Grand Island, NY, USA) in vitro transcription kit. After removing the DNA template by DNaseI digestion, capped RNA was purified by RNAeasy kit (Qiagen, Valencia, CA, USA).
Embryo injection
Mature adult X. tropicalis frogs, a few females and a male, were primed 1 d before the experiment with 20 U of human chorionic gonadotropin (hCG; Novarel; Ferring Pharmaceuticals Inc. Parsippany, NJ, USA). The injected frogs were boosted with 200 U of hCG on the second day. Just before the females started to lay eggs, the male was sacrificed to obtain testes. One testis was smashed to prepare a sperm suspension in 300 μl 1× MMR. For in vitro fertilization, freshly squeezed eggs from an hCG-injected female were mixed with the sperm suspension for about 2 min. The sperm in the mixture was then activated by diluting it to 0.1× MMR. The fertilized eggs were dejelled in 3% cysteine solution pH 8.0. After washing with 0.1× MMR several times, the fertilized eggs were placed on an agar-coated plate. For TALEN mRNA injection, equal amounts of the TALEN-L and -R arm mRNAs were mixed and injected into the fertilized egg at 400 pg for each mRNA/egg.
Detection and screening for TALEN-induced mutations
Genomic DNA was isolated from TALEN mRNA-injected embryos at indicated ages/stages. A DNA fragment encompassing the 2 TALEN-recognized regions of the TALEN pair was amplified by using primer F (5′-GAAAGCCACATTGAAGTGGCAC-3′) and R (5′-TGCAAAAAGGAGCACCAGCCTG-3′) and cloned into pCR-TOPO T vector. Preliminary screening of mutant colonies was carried out by colony PCR with primers f (5′-CCCCACTAATACAACAACCGGC-3′), r (5′-AATGGGGGTGCCTAACCACT-3′), and r1 (5′-CATAGACCTCAGGAGAAAACG-3′), whose 3′ end lies in the middle of the region targeted for cleavage by the TALEN. Mutant colonies were verified by sequencing. As a control for specificity of the mutations, we carried out PCR cloning and sequencing of the Dot1L region from the animals injected with the control TALEN and failed to detect any mutations (not shown).
RNA extraction and quantitative RT-PCR
RNA was extracted by using Trizol (Invitrogen, Grand Island, NY, USA). Reverse transcription was carried out with the Applied Biosystems high-capacity cDNA reverse-transcription kit (Applied Biosystms, Foster City, CA, USA). Quantitative RT-PCR were carried out by using the SYBR green method with F1 5′-AGCAGCAAGACGCAGACAAC-3′ and R1 5′-GCGACCTGCCATTTTTCTGCC-3′ for Dot1L. The expression of ornithine decarboxylase (ODC) was analyzed as a control with forward primer (5′-CCTGCCTGGTTATTTGTGGCCTC-3′) and reverse primer (5′-CTCTGCGCTGTTCTGCTGTTTG-3′) (27). To compare the expression of Dot1L in the wild-type and TALEN-injected animals, total RNA was isolated from tadpoles 10 d postinjection. Quantitative RT-PCR was carried out with primer f1 5′-AGCAGCAAGACGCAGACAAC-3′ and mutation screening primer r1 for Dot1L, which would have reduced PCR amplification if Dot1L is mutated by the TALEN, or with F1 and R1, which would not be affected by TALEN-induced mutations. The Dot1L expression levels were normalized to that of elongation factor 1α, which was analyzed with 5′-AAGAGGGATCTGGCAGCGG-3′ and 5′-AAGGACACCAGTCTCCACAC-3′.
Protein extraction and Western blot
Xenopus tropicalis embryos at indicated age/stage were pooled together and homogenized into 10 μl per embryo of RIPA solution (2.5 mM Tris pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate) with proteinase inhibitor cocktail (Roche, Basel, Switzerland). Lysates were centrifuged at 4°C, 12,000 g for 20 min. The supernatants were mixed with equal volume of 2× loading buffer and boiled for 5 min. Western blot was carried out as previously described (28) with histone [3H] antibody (diluted 1:5000; Abcam, Cambridge, United Kingdom) or H3K79me2 antibody (diluted 1:500; Abcam).
Immunofluorescence
Tadpoles were fixed in 4% MOPS/EGTA/magnesium sulfate/formaldehyde buffer at 4°C overnight and then dehydrated in a tissue processor (Thermo Scientific). The dehydrated tissues were embedded in paraffin and the embedded blocks were sectioned with a microtome (MICROM HM315; Thermo Scientific). Tissue sections (5 μm) were mounted on polylysine-coated slides (Fisher Scientific, Pittsburgh, PA, USA). For immunofluorescence, the slides were washed 3 times in xylene for 5 min each, and after rehydration in a series of different concentrations of ethanol, sections were boiled in the antigen retrieval buffer (1 mM Tris, 1 mM EDTA, and 0.05% Tween-20) for 3 min. The slides were then washed in 1× TBST (Tris-buffered saline plus 0.1% Tween-20) and incubated in the blocking buffer (10% normal goat serum) for 1 h at room temperature. Anti-proliferating cell nuclear antigen (PCNA) antibody (Novocastra, dilution 1:100; Leica Microsystems, Buffalo Grove, IL, USA) binding was carried out at 4°C overnight. After washing in 1× TBST several times, the sections were incubated with FITC-labeled secondary antibody (Millipore, Billerica, MA, USA) for 1 h at room temperature. Slides were mounted with DAPI-containing mounting medium (Vector, Burlingame, CA, USA). The fluorescence pictures for different colors and/or different sections were taken under the same settings.
RESULTS
Expression of Dot1L TALEN leads to efficient mutation of the Dot1L gene in X. tropicalis embryos
To knock down the endogenous Dot1L gene, we generated a Dot1L-specific TALEN made of 2 arms targeting exon 5 of the X. tropicalis Dot1L gene (Fig. 1A, C) as described (24, 25). The plasmid DNA containing each arm was transcribed in vitro to prepare capped mRNAs for Dot1L TALEN arms (TALEN Dot1L-L or -R, for the left and right arm, respectively). In addition, control mRNAs for the left and right arm (TALEN-L and -R) of an unrelated gene were also prepared similarly. The mRNAs were injected in various combinations, both TALEN-Dot1L-R and -L mRNAs, or both control TALEN-L and -R mRNAs, or 1 TALEN-Dot1L arm and 1 control TALEN arm, into fertilized X. tropicalis eggs. After 3 d of development, fertilized eggs developed into feeding tadpoles (around stage 45, Fig. 1), and there was no observable difference among animals without or with the injection of different mRNA combinations (data not shown and see below).
Figure 1.
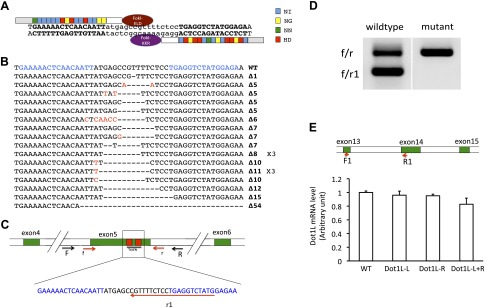
Expression of Dot1L TALEN leads to mutations in Dot1L gene in X. tropicalis embryos. A) Schematic representation of Dot1L TALEN and the targeted DNA sequence in the Dot1L gene in X. tropicalis. The 4 types of RVDs recognizing nucleotide A, G, T, or C are depicted in different colors, respectively. The left arm contained the FokI-ELD nuclease and the right arm contained the FokI-KKR nuclease, which, when both arms bind to their respective binding sites, heterodimerize to form a functional nuclease to make a double-stranded break in the intervening sequence. B) Dot1L TALEN induces mutations in Dot1L gene. TALEN-injected embryos were used for PCR cloning of the target region in Dot1L gene and individual clones were sequenced. The sequences from mutant clones were aligned with wild-type sequence. Characters in red indicate nucleotide changes from wild-type sequence. Dashes represent nucleotide deletions. The TALEN-recognized DNA sequences were shown in blue. The ×3 shown on the right of 2 mutant sequences indicated 3 independent clones having the corresponding sequences. The number after Δ on the right indicated the number of nucleotides that were deleted in the mutant compared to the wild type. C) Schematic diagram of the Dot1L gene showing the TALEN target site and the primers used for mutant screening. The exons were shown as numbered, colored boxes. The TALEN-recognized sequences were shown as red boxes (top) or blue letters (below). Arrows represent primers. F and R were used for amplification of genomic DNA. Primers of f, r, and r1 were used for colony PCR screening for mutations. Mutations in the target region between the 2 TALEN-recognized sequences would reduce the PCR efficiency when f/r1 primers were used but would not affect the PCR by f/r primers. D) Representative PCR results from 1 wild-type and 1 mutant colony. The bacterial colonies were directly PCR amplified with primers f/r1/r. The wild-type colony produced 2 bands due to PCR amplification by f/r and f/r1, respectively. However, for the mutant, only the large band (i.e., from f/r amplification) was seen, suggesting the presence of a mutation(s) that affected the amplification by f/r1. E) Dot1L TALEN-injection does not alter total Dot1L mRNA level. Upper panel: schematic diagram of the Dot1L gene showing the locations of the primers used for RT-PCR analysis of Dot1L expression. The exons were shown as numbered, colored boxes. Lower panel: relative expression levels of Dot1L in 10-d-old tadpoles with various TALEN mRNA injections. Dot1L-L + R: injection of the mRNAs for Dot1L TALEN left and right arm. Dot1L-L: injection of TALEN Dot1L left arm mRNA plus the TALEN right arm mRNA for a control gene. Dot1L-R: injection of TALEN Dot1L right arm mRNA plus the TALEN left arm mRNA for a control gene. WT, uninjected wild-type animals.
To determine whether the expression of the Dot1L TALEN caused any mutation of the endogenous Dot1L gene, a DNA fragment containing the region targeted by the Dot1L TALEN was amplified by PCR from genomic DNA isolated from whole tadpoles with or without the injection of various TALEN mRNAs. The PCR fragment was cloned into a plasmid and individual bacterial colonies were analyzed by PCR with 2 primer pairs nested inside the primer pair used for the initial cloning (Fig. 1C). One of the primer pairs (f/r) was located outside the TALEN targeted region and the second one (f/r1) had a primer (r1) whose 3′-end was located within the targeted region. Mutations/deletions in the targeted region thus would likely affect the PCR amplification efficiency, resulting in reduced amount of PCR product with the f/r1 primer set for the mutant colonies compared with the wild-type one, and the amplification with the f/r primer set would be independent of TALEN-generated mutations/deletions (Fig. 1D). The putative mutant colonies were subsequently sequenced and were found to contain mutations/deletions in the TALEN-targeted region of Dot1L gene (Fig. 1B). By using this method, we found that 1 d after the mRNA injection when embryos reached around stage 26, about 70% of the colonies from the PCR-amplified Dot1L region were mutants, and after 3 d (stage 45 tadpoles), about 90% of the colonies were mutants (data not shown, i.e., about 90% of the Dot1L locus had mutations in Dot1L TALEN injected animals). In contrast, no mutant colonies were obtained when the Dot1L region was PCR amplified from genomic DNA of animals injected with mRNAs encoding only one Dot1L TALEN arm and a nonspecific TALEN arm or both arms of the nonspecific TALEN (data not shown). Thus, the Dot1L TALEN was very effective and specific in causing mutations/deletions in the endogenous Dot1L gene during embryogenesis.
To determine if Dot1L TALEN affected the expression of Dot1L, we analyzed the expression of Dot1L mRNA (we could not analyzed Dot1L protein level due to the lack of a good antibody recognizing Xenopus Dot1L). First, we used a pair of primers located away from the TALEN-targeted region (F1/R1, Fig. 1E) to carry out RT-PCR analysis on tadpoles with or without various injections of TALEN mRNAs. The results showed that 4 different groups of animals had similar levels of Dot1L expression, indicating that the TALEN-induced mutations did not affect the transcription from the Dot1L gene (Fig. 1E). However, when we used primers f1 and r1, of which the r1 primer was used for mutation screening described previously, we found that Dot1L expression was reduced drastically in animals injected with both Dot1L TALEN arms when compared with the wild-type or control TALEN-injected animals (data not shown). The result was consistent with the result of the genomic DNA analyses above and reflected the effect of the TALEN-induced mutations on PCR involving the r1 primer. Thus, Dot1L TALEN caused mutation of Dot1L gene, leading to the expression of mutant Dot1L mRNA with little wild-type Dot1L mRNA expressed.
Endogenous Dot1L expression is absent in the egg but is activated after midblastula transition
The effective knockdown of Dot1L in early embryos but apparent normal embryogenesis to form feeding tadpoles prompted us to investigate the expression of Dot1L during development. RT-PCR analysis on RNA isolated from total embryos and tadpoles revealed that Dot1L mRNA was absent in fertilized eggs and early embryos. Dot1L mRNA was first detected around stage 8, concurrent with the activation of zygotic transcription at midblastula transition (MBT). Dot1L mRNA remained low until stage 30, the tail bud stage, and rose to high levels by stage 46, when tadpole feeding begins (Fig. 2A).
Figure 2.
There is no maternal Dot1L expression or H3K79 methylation but both are up-regulated during embryogenesis in Xenopus tropicalis. A) qRT-PCR analysis of Dot1L mRNA level during X. tropicalis development. Total RNA was isolated from embryos/tadpoles at indicated stages, and the Dot1L mRNA levels were normalized with those of the control gene ornithine decarboxylase. The expression at the early stages was magnified in the insert, showing the up-regulation of the Dot1L expression at stage 8, the midblastula stage when zygotic transcription begins. B) Western blot analysis of H3K79 dimethylation during X. tropicalis development. Note, like the lack of Dot1L mRNA at the early stages, no dimethylated H3K79 (H3K79me2) was detected until around stage 22, after the activation of zygotic Dot1L transcription (A).
To investigate the activity of Dot1L during development, we analyzed the methylation of H3K79 because Dot1L is the only methyltransferase capable of methylating this residue in vitro. Figure 2B showed that H3K79 dimethylation was absent in eggs or early embryos but was up-regulated around stage 22 and reached high levels after stage 30, correlating with the activation of Dot1L expression during embryogenesis. Thus, Dot1L appears to the only enzyme responsible for methylating H3K79 during Xenopus development.
Knocking down Dot1 expression reduces H3K79 methylation during development
As indicated previously, Dot1L TALEN expression in embryos appeared not to affect embryogenesis. To investigate this further, we analyzed a large number of embryos injected with different combinations of TALEN mRNAs from a few different batches. By 3 d, free-living, feeding tadpoles around stage 45 were formed apparently normally under all mRNA injection conditions, and they grew normally even by day 6 (around stage 47; Fig. 3A, B). When the H3K79 methylation was analyzed in the tadpoles, we observed that dimethylation (Fig. 3C) levels at H3K79 were drastically reduced in tadpoles injected with mRNAs for both the left and right arms of the Dot1L TALEN. In contrast, uninjected animals and animals expressing only 1 of the Dot1L TALEN arm or expressing the 2 control TALEN arms had similar levels of H3K79 methylation. These results suggest that Dot1L mediates H3K79 methylation during Xenopus development.
Figure 3.

Dot1L knockdown significantly suppressed H3K79 methylation during development. A, B) Dot1L knockdown had no observable effect on embryo morphology at 3 d (around stage 45) (A) or 6 d (stage 47) (B) postinjection. The first column is lateral view and the second is dorsal view. Numbers in the first column indicated the number of tadpoles for the sample group at 3 d. Dot1L-L + R, injection of the mRNAs for Dot1L TALEN left and right arm; Dot1L-L, injection of TALEN Dot1L left arm mRNA plus the TALEN right arm mRNA for a control gene; Dot1L-R, injection of TALEN Dot1L right arm mRNA plus the TALEN left arm mRNA for a control gene; WT, uninjected wild-type animals. C) Western blot analysis of H3K79 methylation and total [3H] in the tadpoles in (A) and (B). Note the reduction in H3K79 methylation only when the mRNAs for both Dot1L TALEN arms were injected.
Dot1L knockdown reduces cell proliferation and causes lethality during tadpole growth prior to metamorphosis
When the tadpoles from eggs injected with various combinations of TALEN mRNAs were allowed to develop further, we noticed growth retardation for tadpoles injected with mRNAs for both Dot1L TALEN arms at fertilized egg stage by 10 d of development (around stage 47–49; Fig. 4A, B). Furthermore, we observed that some of the Dot1L knockdown tadpoles began to die around 8 d. By 20 d of age (around stage 50), only about 10% of the Dot1L knockdown tadpoles were alive, and the surviving ones were much smaller than the wild-type (Fig. 4B). In contrast, the animals injected with mRNAs for only 1 of the Dot1L TALEN arms and a control TALEN arm survived and had similar growth rates as the wild-type animals (Fig. 4A–C).
Figure 4.
Dot1L knockdown caused tadpole developmental retardation and lethality. A) Representative photos of tadpoles at 10 (around stage 47–49) or 16 (around stage 49) d postinjection. Note that compared to wild-type, the tadpoles were much smaller by 10 d when the mRNAs for both Dot1L TALEN arms were injected. At 10 d, the total number of animals in the TALEN injected L + R group (see Fig. 3 for more details) was 70 with a survival rate 73.0%, L group was 55 with a survival rate 93.1%, R group was 53 with a survival rate 96.4%, and WT group was 90 with a survival rate 95.7%. At 16 d postinjection, the total number of animals in the L + R group was 50 with a survival rate 55.3%, L group was 51 with a survival rate 91.3%, R group was 49 with a survival rate 94.4%, and WT group was 49 with a survival rate 93.8%. B) Developmental retardation caused by Dot1L TALEN knockdown. Tadpoles were classified into 4 categories as large (L), medium (M), small (S), and extremely small (ES) according to their sizes (see photos) at 20 d postinjection, around stage 50. The total numbers for TALEN L + R, L, R, and WT groups were 16 with a survival rate 17.1%, 45 with a survival rate 85.7%, 46 with a survival rate 94.4%, and 43 with a survival rate 89.6%, respectively. C) Tadpole survival rate over time for different TALEN injected groups. D) PCNA-immunofluorescence analysis revealed reduced cell proliferation when the mRNAs for both Dot1L TALEN arms were injected. Sections were made from the brain regions of tadpoles 17 d postinjection (around stage 49) and PCNA immunohistochemistry (green) was carried out to detect cell proliferation. The sections were also stained with DAPI (blue) for DNA. WT, uninjected wild-type animals.
To investigate the underlying mechanism of the growth retardation, we analyzed the expression of the PCNA, a cell proliferation marker, in the brain where cell proliferation is rapid. The results showed that knockdown of Dot1L drastically reduced PCNA expression in the brain compared with the control TALEN-injected or wild-type animals at 17 d of age. Thus, Dot1L knockdown reduced cell proliferation, at least in the brain, and thus led to growth retardation.
DISCUSSION
A number of post-translational modifications of histones have been defined as activation or repression marks based on their association with gene expression levels in yeast or cultured cells (2–4, 6–11). Among them is the activation histone mark H3K79 methylation. Unlike most of the modified histone residues that are located at N- or C-terminus, H3K79 is located in the globular domain of histone [3H]. In addition, only a single known methyltransferase, Dot1L, has been shown to be capable of methylation H3K79. These and the highly conserved nature of Dot1L across diverse eukaryotes from yeast to human suggest that Dot1L play critical roles in cellular processes, such as transcription and replication. Interestingly, our in vivo studies here suggest that Dot1L is important for H3K79 methylation and tadpole growth and development prior to metamorphosis.
Dot1L is the only methyltransferase for H3K79 methylation during Xenopus development
In vitro studies have shown that Dot1L is the only known methyltransferase capable of H3K79 methylation (5, 13, 16). Our analyses here indicate that Dot1L is also the only enzyme for H3K79 methylation in vivo during frog development. First, there is a strong correlation between Dot1L expression and H3K79 methylation during embryogenesis. Both Dot1L mRNA and H3K79 are absent in fertilized eggs, indicating that Dot1L is not a maternal gene. The Dot1L gene is activated after stage 8, the MBT stage when zygotic transcription begins, and the levels of H3K79 methylation correlated tightly with Dot1L mRNA levels. Second and more importantly, knocking down the endogenous Dot1L by injecting into fertilized eggs the mRNA encoding Dot1L TALEN led to a parallel reduction in H3K79 methylation in the resulting tadpoles (e.g., about 90% mutations in Dot1L gene in 3-d-old tadpoles and more than 80% reduction in H3K79 dimethylation; Fig. 3C), indicating that during frog development, Dot11L is the only or the predominant methyltransferase for H3K79.
Zygotic transcription of Dot1L is essential for growth and development of premetamorphic tadpoles
Our functional studies with the Dot1L TALEN indicate that Dot1L knockdown does not appear to affect Xenopus embryogenesis. This seems to differ from the observation in mouse where a total knockout leads to an embryonic lethal phenotype (17). One possibility for the different findings between frog and mouse may be that TALEN-induced mutation was incomplete. Although there may be some Dot1L loci not mutated in the embryos/tadpoles or with mutations that still produce a functional protein (e.g., in frame deletion/insertion, or point mutations), this is unlikely to be responsible for the normal embryogenesis observed in the Dot1L TALEN-injected animals. First, our PCR screening and RT-PCR expression analysis might miss some of the mutations (e.g., when mutations occurred upstream of the primer r1 or point insertions/deletions/mutations occurred near the 5′-end of the primer r1), consequently having little or no effect on the PCR with the r1 primer (Fig. 1C). Thus, the actual mutation rate would likely be higher than the 90% observed. Second, all the mutant genomic colonies that we obtained had deletions, suggesting that most of the mutations induced by the Dot1L TALEN might be deletions. In theory, about two-thirds of the insertions or deletions would lead to frame-shift, leading to truncated proteins that would likely be nonfunctional. The remaining one-third of the insertion or deletion mutants would be in-frame. However, the TALEN-targeted region is essential for Dot1L enzymatic activity (29). It is likely that even in such cases, the mutation might affect Dot1L function. Thus, the TALEN-injected animals would have significantly reduced Dot1L function. Consistently, we observed that Dot1L TALEN injection led to a significant reduction in H3K79 methylation in the resulting tadpoles. This indicates that the Dot1L mutants cause a reduction of H3K79 methylation, but if the Dot1L mutants are degraded or lack critical residues remains to be shown. Finally, there is no maternal Dot1L or H3K79 methylation. All these argue against a critical role of Dot1L expression and H3K79 methylation for early embryogenesis, although we cannot rule out the possibility that the residual Dot1L activity in the knockdown animals was responsible for the apparent normal embryogenesis.
More importantly, a careful examination suggests a similar role of Dot1L in mouse and Xenopus development. Like in Xenopus, H3K79 methylation is also absent from mouse zygotes, and its level increases only after blastocyst stage (5, 30). Although Dot1L knockout mice are embryonic lethal (17), the animals die quite late, around 10.5 d postcoitum or TS17 (Theiler stage 17), in the middle of organogenesis (http://php.med.unsw.edu.au/embryology/index.php?title=Mouse_Stages). This temporally resembles the lethal phenotype that we observed for Dot1L knockdown tadpoles. Xenopus development takes place in 2 phases. Its embryogenesis produces a free-living tadpole, and then after a growth period, the tadpole undergoes metamorphosis to form a frog. Extensive studies have shown that thyroid hormone (TH)-dependent amphibian metamorphosis mimics the so-called “postembryonic development” in mammals, which is the period around birth when TH levels are also high (21, 22, 31, 32). The tadpole growth period prior to metamorphosis would temporally lie in the middle of mouse organogenesis, suggesting that Dot1L plays a temporally similar role in vertebrate development. Thus, our findings here reveal interesting similarities and differences between Xenopus and mouse development and further suggest that an important role of Dot1L and H3K79 methylation was evolved for late development in vertebrates. The compression of vertebrate development from a biphasic process (embryogenesis and subsequent metamorphosis) in amphibians into a single phase in mammals masks these 2 separate phases of vertebrate development with distinct requirements for epigenetic modifications.
How Dot1L affects vertebrate development (premetamorphic tadpole growth in Xenopus and organogenesis in mouse) remains to be investigated. Dot1L has been shown to be involved in diverse cellular processes. Dot1L was originally identified from a yeast genetic screen for genes that affect telomeric silencing (5, 12). Dot1L knockout mouse embryonic stem cells have growth defect, aberrant telomere elongation, and the loss of heterochromatin marks at the telomeres and centromeres (17). In addition, Dot1L has also been implicated to play important roles in DNA repair and cell cycle progression (5). However, such effects are unlikely responsible for the observed lethal phenotype in either mouse or Xenopus because early development, which includes rapid cell proliferation shortly after fertilization, is apparently normal.
Dot1L is best known as the methyltransferase for H3K79, and methylated H3K79 is associated with high levels of gene expression and gene activation by diverse transcription factors (2–6, 20, 23, 33). Thus, it is more likely that Dot1L functions as a critical coactivator for 1 or more transcription factors important for organogenesis in mouse or tadpole growth during premetamorphosis. Clearly, identifying the transcription factors utilizing Dot1L and the corresponding target genes during this developmental period will be key to understanding how Dot1L knockdown causes growth retardation and tadpole lethality in frog and embryonic lethality during organogenesis in mouse.
Acknowledgments
This research was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, U.S. National Institutes of Health. The authors declare no conflicts of interest.
Glossary
- Dot1L
Dot1 (disruptor of telomeric silencing)-like
- hCG
human chorionic gonadotropin
- KMTs
lysine methyltransferase family
- MBT
midblastula transition
- MMR
Marc's modified Ringers
- PCNA
proliferating cell nuclear antigen
- RVD
repeat variable diresidue
- TALEN
transcription activator-like effector nuclease
- TH
thyroid hormone
- TR
TH receptor
REFERENCES
- 1.Kouzarides T. (2007) Chromatin modifications and their function. Cell 128, 693–705 [DOI] [PubMed] [Google Scholar]
- 2.Li B., Carey M., Workman J. L. (2007) The role of chromatin during transcription. Cell 128, 707–719 [DOI] [PubMed] [Google Scholar]
- 3.Wang Z., Schones D. E., Zhao K. (2009) Characterization of human epigenomes. Curr. Opin. Genet. Dev. 19, 127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barth T. K., Imhof A. (2010) Fast signals and slow marks: the dynamics of histone modifications. Trends Biochem. Sci. 35, 618–626 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen A. T., Zhang Y. (2011) The diverse functions of Dot1 and H3K79 methylation. Genes Dev. 25, 1345–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maunakea A. K., Chepelev I., Zhao K. (2010) Epigenome mapping in normal and disease States. Circ. Res. 107, 327–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H., Tempst P., Jones R. S., Zhang Y. (2002) Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039–1043 [DOI] [PubMed] [Google Scholar]
- 8.Cao R., Zhang Y. (2004) The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr. Opin. Genet. Dev. 14, 155–164 [DOI] [PubMed] [Google Scholar]
- 9.Wang Z., Zang C., Rosenfeld J. A., Schones D. E., Barski A., Cuddapah S., Cui K., Roh T. Y., Peng W., Zhang M. Q., Zhao K. (2008) Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 40, 897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roh T. Y., Cuddapah S., Cui K., Zhao K. (2006) The genomic landscape of histone modifications in human T cells. Proc. Natl. Acad. Sci. USA 103, 15782–15787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barski A., Cuddapah S., Cui K., Roh T. Y., Schones D. E., Wang Z., Wei G., Chepelev I., Zhao K. (2007) High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837 [DOI] [PubMed] [Google Scholar]
- 12.Singer M. S., Kahana A., Wolf A. J., Meisinger L. L., Peterson S. E., Goggin C., Mahowald M., Gottschling D. E. (1998) Identification of high-copy disruptors of telomeric silencing in Saccharomyces cerevisiae. Genetics 150, 613–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greer E. L., Shi Y. (2012) Histone methylation: a dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 13, 343–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dillon S. C., Zhang X., Trievel R. C., Cheng X. (2005) The SET-domain protein superfamily: protein lysine methyltransferases. Genome Biol. 6, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barry E. R., Corry G. N., Rasmussen T. P. (2010) Targeting DOT1L action and interactions in leukemia: the role of DOT1L in transformation and development. Expert Opin. Ther. Targets 14, 405–418 [DOI] [PubMed] [Google Scholar]
- 16.Feng Q., Wang H., Ng H. H., Erdjument-Bromage H., Tempst P., Struhl K., Zhang Y. (2002) Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr. Biol. 12, 1052–1058 [DOI] [PubMed] [Google Scholar]
- 17.Jones B., Su H., Bhat A., Lei H., Bajko J., Hevi S., Baltus G. A., Kadam S., Zhai H., Valdez R., Gonzalo, S., Zhang, Y., Li, E., Chen, T.(2008) The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure. PLoS Genet. 4, e1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shanower G. A., Muller M., Blanton J. L., Honti V., Gyurkovics H., Schedl P. (2005) Characterization of the grappa gene, the Drosophila histone H3 lysine 79 methyltransferase. Genetics 169, 173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Leeuwen F., Gafken P. R., Gottschling D. E. (2002) Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell 109, 745–756 [DOI] [PubMed] [Google Scholar]
- 20.Matsuura K., Fujimoto K., Das B., Fu L., Lu C. D., Shi Y. B. (2012) Histone H3K79 methyltransferase Dot1L is directly activated by thyroid hormone receptor during Xenopus metamorphosis. Cell Biosci 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y.-B. (1999) Amphibian Metamorphosis: From Morphology to Molecular Biology, John Wiley & Sons, Inc., New York [Google Scholar]
- 22.Tata J. R. (1993) Gene expression during metamorphosis: an ideal model for post-embryonic development. BioEssays 15, 239–248 [DOI] [PubMed] [Google Scholar]
- 23.Matsuura K., Fujimoto K., Fu L., Shi Y.-B. (2012) Liganded thyroid hormone receptor induces nucleosome removal and histone modifications to activate transcription during larval intestinal cell death and adult stem cell development. Endocrinology 153, 961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei Y., Guo X., Liu Y., Cao Y., Deng Y., Chen X., Cheng C. H., Dawid I. B., Chen Y., Zhao H. (2012) Efficient targeted gene disruption in Xenopus embryos using engineered transcription activator-like effector nucleases (TALENs). Proc. Natl. Acad. Sci. USA 109, 17484–17489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei Y., Guo X., Deng Y., Chen Y., Zhao H. (2013) Generation of gene disruptions by transcription activator-like effector nucleases (TALENs) in Xenopus tropicalis embryos. Cell Biosci 3, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieuwkoop P. D., Faber J. (1956) Normal Table of Xenopus laevis, North Holland Publishing, Amsterdam [Google Scholar]
- 27.Cao Y., Zhao H., Hollemann T., Chen Y., Grunz H. (2001) Tissue-specific expression of an ornithine decarboxylase paralogue, XODC2, in Xenopus laevis. Mech. Dev. 102, 243–246 [DOI] [PubMed] [Google Scholar]
- 28.Wen L., Yang Y., Wang Y., Xu A., Wu D., Chen Y. (2010) Appl1 is essential for the survival of Xenopus pancreas, duodenum, and stomach progenitor cells. Dev. Dyn. 239, 2198–2207 [DOI] [PubMed] [Google Scholar]
- 29.Min J., Feng Q., Li Z., Zhang Y., Xu R. M. (2003) Structure of the catalytic domain of human DOT1L, a non-SET domain nucleosomal histone methyltransferase. Cell 112, 711–723 [DOI] [PubMed] [Google Scholar]
- 30.Ooga M., Inoue A., Kageyama S., Akiyama T., Nagata M., Aoki F. (2008) Changes in H3K79 methylation during preimplantation development in mice. Biol. Reprod. 78, 413–424 [DOI] [PubMed] [Google Scholar]
- 31.Hasebe T., Fu L., Miller T. C., Zhang Y., Shi Y. B., Ishizuya-Oka A. (2013) Thyroid hormone-induced cell-cell interactions are required for the development of adult intestinal stem cells. Cell Biosci 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishizuya-Oka A., Shi Y. B. (2011) Evolutionary insights into postembryonic development of adult intestinal stem cells. Cell Biosci 1, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y. B., Matsuura K., Fujimoto K., Wen L., Fu L. (2012) Thyroid hormone receptor actions on transcription in amphibia: The roles of histone modification and chromatin disruption. Cell Biosci 2, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]




