ABSTRACT
Reverse transcriptase (RT) of human immunodeficiency virus type 1 (HIV-1) is synthesized and packaged into the virion as a part of the GagPol polyprotein. Mature RT is released by the action of viral protease. However, unlike other viral proteins, RT is subject to an internal cleavage event leading to the formation of two subunits in the virion: a p66 subunit and a p51 subunit that lacks the RNase H domain. We have previously identified RNase H to be an HIV-1 protein that has the potential to be a substrate for the N-end rule pathway, which is an ubiquitin-dependent proteolytic system in which the identity of the N-terminal amino acid determines the half-life of a protein. Here we examined the importance of the N-terminal amino acid residue of RNase H in the early life cycle of HIV-1. We show that changing this residue to an amino acid structurally different from the conserved residue leads to the degradation of RT and, in some cases, integrase in the virus particle and this abolishes infectivity. Using intravirion complementation and in vitro protease cleavage assays, we show that degradation of RT in RNase H N-terminal mutants occurs in the absence of active viral protease in the virion. Our results also indicate the importance of the RNase H N-terminal residue in the dimerization of RT subunits.
IMPORTANCE HIV-1 proteins are initially made as part of a polyprotein that is cleaved by the viral protease into the proteins that form the virus particle. We were interested in one particular protein, RNase H, that is cleaved from reverse transcriptase. In particular, we found that the first amino acid of RNase H never varied in over 1,850 isolates of HIV-1 that we compared. When we changed the first amino acid, we found that the reverse transcriptase in the virus was degraded. While other studies have implied that the viral protease can degrade mutant RT proteins, we show here that this may not be the case for our mutants. Our results suggest that the presence of active viral protease is not required for the degradation of RT in RNase H N-terminal mutants, suggesting a role for a cellular protease in this process.
INTRODUCTION
Like all retroviruses, human immunodeficiency virus type 1 (HIV-1), the causative agent of AIDS, synthesizes and packages its main structural and enzymatic proteins as precursor polyproteins. For HIV-1, these polyproteins are p55 (Gag) and p160 (GagPol). Gag is the most abundant polyprotein and is translated from a genome-length mRNA that contains the Gag and GagPol open reading frames. The synthesis of GagPol requires a ribosomal frameshift leading to a Gag/GagPol ratio of about 20:1 in the virus particle (1). Individual mature viral proteins are generated following viral assembly as a result of a series of proteolytic cleavage events at specific positions catalyzed by the viral protease, which is synthesized as a part of GagPol (2).
One protein that is released as a result of proteolytic processing of GagPol is reverse transcriptase (RT). RT catalyzes the reaction for the conversion of viral RNA to double-stranded DNA (3). In contrast to the other viral enzymes encoded by the pol gene, RT functions as a heterodimer of two subunits, p66 and p51 (4–6). Formation of this heterodimer requires the proteolytic cleavage of the RNase H domain from one of the p66 subunits, resulting in p51, which is associated with p66 to form the heterodimer (4). The RNA-dependent DNA polymerase and RNase H activities of HIV-1 RT are mainly carried out by the p66 subunit, while p51 was thought to be enzymatically inactive and serve only a structural role (5, 7–10). However, recent structural and biochemical evidence suggests that the C-terminal end of the p51 subunit is involved in hydrolysis and positioning of the RNA/DNA hybrid formed during the reverse transcription process (11–13).
Retroviral RNase H is a member of a family of enzymes that are found in all domains of life (14). It functions as an endonuclease that degrades RNA from the RNA/DNA hybrid formed during the first phase of reverse transcription. This function is crucial for the processing and completion of reverse transcription, as it creates an RNA primer for plus strand DNA synthesis and as it facilitates the first and second jumps by removing the 5′ end of viral RNA and tRNA, respectively (8, 15, 16). In the virus particle, RNase H is found both as a part of p66 and as a free protein (4). However, it is not definitively established whether the RNase H species that is generated by the viral protease has any specific function.
The N-end rule pathway is an ubiquitin-dependent proteolytic system in which the identity of the N-terminal amino acid determines the half-life of a protein. Since proteolytic cleavage of viral polyproteins can result in N-terminal residues that dictate a short half-life for the cleaved protein, we have recently examined the involvement of the N-end rule pathway in the retroviral life cycle. Using N-end rule mutant cells and N-terminal amino acid substitution mutants, we studied the effects of the N-end rule pathway on the mature integrase (IN) protein, which bears a highly conserved destabilizing residue (17). Our results showed an impact of the N-end rule pathway on HIV-1 but not on the life cycle of murine leukemia virus (MLV). However, the interaction of the N-end rule pathway machinery on the HIV-1 pathway was not at the level of the integrase N-terminal residue (17). One of the differences in the protein composition of HIV-1 and MLV is that, unlike the heterodimeric RT of HIV-1, MLV RT functions as a monomer protein of about 75 kDa and, hence, does not require a proteolytic cleavage to remove the RNase H domain (18, 19). Due to this difference between HIV-1 and MLV and the fact that HIV-1 RNase H also has the potential to be a substrate for the N-end rule pathway, we turned our attention to this protein.
In this study, we investigated the role of the N-terminal amino acid residue of HIV-1 RNase H. Here we show that the N-terminal residue of RNase H is highly conserved and changing this residue to an amino acid that is structurally different from the wild-type (WT) residue leads to the degradation of RT and, in some cases, integrase in the virus particle. Notably, this degradation in the virus particle is independent of the N-end rule pathway that would be manifest in the target cell. We demonstrate that this degradation is processive and does not extend to WT RT species that are added into the viral particle in trans. We observed that the liberation of RT from GagPol or a Vpr fusion polyprotein is required for the degradation of RNase H N-terminal mutants. Moreover, we show that an RT molecule that is degraded in the viral particle of RNase H N-terminal mutants is not degraded in vitro in the presence of excess viral protease. We also present evidence that some RNase H N-terminal mutants have a defect in forming an RT heterodimer. Finally, we show that RT in the RNase H N-terminal mutant viruses is degraded even if the viral protease is inactivated. Taken together, these results suggest a possible role for one or more cellular proteases in the degradation of mutant RT in the virus.
MATERIALS AND METHODS
Reagents and cell culture.
293T and Jurkat cells were obtained from the American Type Culture Collection (ATCC). 293T cells were maintained in Dulbecco's modified Eagle medium (Cellgro) supplemented with 10% fetal bovine serum (FBS; Gemini Bioproducts). Jurkat cells were maintained in Iscove's modified Dulbecco's medium (ATCC) supplemented with 20% FBS.
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: p24 monoclonal antibody (183-H12-5C) from Bruce Chesebro and Kathy Wehrly, HIV-1 HXB2 integrase antiserum (amino acids 23 to 34) from Duane P. Grandgenett, HIV-1 RT polyclonal antibody, pEGFP-Vpr (catalog number 11386) from Warner C. Greene, and ritonavir. Monoclonal antibody for RT (1.149 B6) was previously isolated and characterized (20). Mouse monoclonal (IgG2a) antibody against HIV-1 Vpr was obtained from Cosmo Bio USA (Carlsbad, CA). Secondary p24 antibody for enzyme-linked immunosorbent assay (ELISA) was collected from a hybridoma cell line obtained from ATCC (antibody HB-9725). Isolation of antibody from the hybridoma cell line was performed using standard protocols, as described previously (17). Secondary antibody for ELISA, goat anti-mouse-horseradish peroxidase (HRP) IgG2A, was obtained from Southern Biotech (Birmingham, AL). Goat anti-mouse-HRP and goat anti-rabbit-HRP secondary antibodies and West Femto enhanced chemiluminescent (ECL) HRP substrate were obtained from Thermo Scientific (Rockford, IL).
Plasmid constructs and mutagenesis.
The plasmids used for vesicular stomatitis virus glycoprotein (VSVg)-pseudotyped HIV-1 production were CSII-EGFP, an HIV-1-based vector encoding enhanced green fluorescent protein (EGFP) driven by the EF-1a promoter; ΔNRF, which encodes gag, pol, rev, tat, and vpu of HIV-1; and pMDg, which encodes vesicular stomatitis virus glycoprotein. RNase H N-terminal mutations were introduced into ΔNRF by PCR mutagenesis (PCR primers are available upon request). PCR products which contained the specific mutations were cut with KpnI and ligated back into KpnI-digested plasmid ΔNRF. The viral protease-inactivating D25A mutation was introduced into ΔNRF by overlap PCR (primers are available upon request). The final PCR product was cut with SacII and SbfI and cloned into ΔNRF. For the vpr complementation assays, a mammalian expression plasmid, pRK5, was used to clone the Vpr fusion proteins. Vpr was amplified from pEGFP-Vpr using the following primers: vpr forward primer 5′-CTC GGA TTC ACC GCC ATG GAA CAA GCC CCA GAA GAC-3′ and vpr reverse primer 5′-CGC GAA GCT TCA GTT CCA GAT CTG AGT AGG ATC TAC TGG CTC CAT TTC TT-3′. The PCR products were digested with BamHI and HindIII and cloned into pRK5. This construct, pRK5-Vpr, was used for cloning WT or RNase H mutant RT or RT-integrase. pRK5-Vpr-RT constructs were generated by amplifying the RT sequence, including the protease cleavage site between protease and RT, from WT or RNase H mutant ΔNRF constructs using the following primers: forward primer 5′-GCT CAA GCT TAC TTT AAA TTT TCC CAT TAG TCC-3′ and reverse primer 5′-GCT CAA GCT TTT ATA GTA CTT TCC TGA TTC-3′. The PCR products were then digested with HindIII and cloned into pRK5-Vpr. The same strategy was used for generating pRK5-Vpr-RT-IN constructs. The following reverse primer together with the forward primer described above was used for generating the PCR product including RT and integrase: 5′-GCT CAA GCT TTT AAT CCT CAT CCT GTC TAC-3′. pLR2P-Vpr-p51-IRES-p66 was previously described and was a kind gift from John Kappes (21). RNase H mutants were cloned into this plasmid by first amplifying the ΔNRF construct with the corresponding mutation with the following primers: 5′-CAG TAA ATT TAA AGC CCG GGA TGG ATG G-3′ and 5′-GGA TCT CGA GTT ATA GTA CTT TCC TGA T-3′. PCR products were digested with XmaI and XhoI and cloned into pLR2P-Vpr-p51-IRES-p66. pLR2P-Vpr-pro50-p51-IRES-p66 was generated by first amplifying a region of ΔNRF containing 150 nucleotides of viral protease and the p51 subunit of RT using the following primers: 5′-TAG ATC AGA TCT AAT TGG AGG TTT TAT CAA AGT AG-3′ and 5′-ATC TAC ACG CGT TTA GAA AGT TTC TGC TCC TAT-3′. The PCR products were digested with BglII and MluI and ligated into pLR2P-Vpr-p51-IRES-p66 that had been cut with the same enzymes. pLR2P-Vpr-p51Δp66 was generated by hydrolyzing pLR2P-Vpr-p51-IRES-p66 with XmaI and XhoI and then religating the plasmid after generating blunt ends of the 5′ overhangs with T4 DNA polymerase.
pRK5-GagPol was generated by first PCR amplifying GagPol from ΔNRF using the following primers: 5′-TCG ATT GAA TTC GCC ATG GGT GCG AGA GCG TCG G-3′ and 5′-GCT CCT GTC GAC TTA ATC CTC ATC CTG TCT-3′. The PCR products were digested with EcoRI and SalI and cloned into pRK5. pRK5-GagPol-FS was generated by overlap PCR using the following primers: primers 5′-GGC AAA GAA GGG CAC ACA GCC-3′ and 5′-CCC TGA GGA AGT TAG CCT GTC TCT CAG TAC-3′ for the left pair and primers 5′-GGC TAA CTT CCT CAG GGA AGA TCT GGC CTT CC-3′ and 5′-GTT GAC AGG TGT AGG TCC TAC-3′ for the right pair. The final product was digested with ApaI and BclI and cloned into pRK5-GagPol. RNase H mutants were cloned into pRK5-GagPol-FS from the ΔNRF plasmid containing the corresponding mutation using BsrGI digestion.
Virus production and infectivity assays.
HIV-1 vectors were generated by transiently transfecting three plasmids into 293T cells as described previously (22, 23). Fifteen micrograms of CSII-EGFP, 10 μg of ΔNRF, and 5 μg of pMDG were transfected using the method of Chen and Okayama (24). For vpr complementation assays, 10 μg of the corresponding Vpr fusion protein-expressing plasmids was used for the transfections. At 72 h after transfection, virus was collected and filtered through a 0.45-μm-pore-size membrane. Filtered virus was concentrated by ultracentrifugation (100,000 × g, 2 h at 4°C). The viral pellet was resuspended in phosphate-buffered saline (PBS), and aliquots were stored at −80°C. The amounts of concentrated virions were normalized to the amount of WT virus using a p24 ELISA as described previously (17). The multiplicity of infection (MOI) for the WT was determined by infecting 1 × 105 Jurkat cells with 10-fold dilutions of the viral preparation. Infections for mutant or vpr-complemented viruses were performed using p24 levels equated with p24 levels (and corresponding MOI) of WT virus. At 72 h after the infections, enhanced green fluorescent protein (EGFP) expression was quantified by flow cytometry on a Becton Dickinson FACSCalibur flow cytometer.
In vitro transcription and translation assays.
Transcription and translation of WT and RNase H mutant pRK5-GagPol-FS were performed using a TNT coupled reticulocyte lysate system with SP6 polymerase (Promega) according to the manufacturer's instructions. Four microliters of the translation product was used for Western blotting. For the external HIV-1 protease-processing reactions, 4 μl of the translation products was incubated with 1 μg of HIV-1 protease (Abcam) in phosphate buffer (25 mM NaCl, 25 mM Na2HPO4, 1 mM dithiothreitol, pH 7.0) in 20-μl reaction volumes at 30°C for 2 h. The entire reaction volume was used for Western blotting.
Analysis of viral proteins by immunoblotting.
Viral pellets and in vitro transcription/translation products were dissolved in loading buffer (0.25 M Tris-HCl, pH 6.8, 15% SDS, 50% glycerol, 25% β-mercaptoethanol, 0.01% bromophenol blue), and proteins were separated by SDS-PAGE on a 12% polyacrylamide gel and subjected to immunoblotting using the antibodies indicated below.
For the analysis of protein expression from the Vpr-pro50-p51-IRES-p66 construct, 5 × 105 293T cells which were cotransfected with this plasmid together with ΔNRF D25A were lysed in cell lysis buffer (20 mM Tris-HCl [pH 8.0], 1 mM CaCl2, 150 mM NaCl, 1% Triton X-100), and proteins were separated by SDS-PAGE on a 12% polyacrylamide gel and analyzed by immunoblotting, as described above.
Coimmunoprecipitation (co-IP) assay.
293T cells were cotransfected with ΔNRF D25A and pLR2P-Vpr-p51-IRES-p66wt, pLR2P-Vpr-p51-IRES-p66pro, pLR2P-Vpr-p51-IRES-p66leu, pLR2P-Vpr-p51-IRES-p66met, pLR2P-Vpr-p51-IRES-p66gly, pLR2P-Vpr-p51-IRES-p66ala, or pLR2P-Vpr-p51Δp66, as described above. At 24 h after transfection, 5 × 106 cells were lysed in immunoprecipitation buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate). Five percent of this lysate was used as an input control for the assay. The remaining lysate was incubated with the Vpr antibody for 1 h at 4°C. Protein G beads (BioWorld) were added, and the mixture was incubated for 2 h at 4°C. The lysate-bead mixture was concentrated in a microcentrifuge and washed with the immunoprecipitation buffer 3 times. Immunoprecipitated proteins were eluted from the beads with loading buffer (0.25 M Tris-HCl, pH 6.8, 15% SDS, 50% glycerol, 25% β-mercaptoethanol, 0.01% bromophenol blue), separated by SDS-PAGE, and immunoblotted as described above.
RESULTS
The N-terminal amino acid residue of HIV-1 RNase H is highly conserved.
In our previous study we reported that 7 HIV-1 mature proteins have primary destabilizing residues, as defined by the N-end rule (17). Further analysis indicated that only 4 of these proteins (p1, transframe octapeptide, RNase H, and integrase) have a conserved residue at the N terminus. Here we investigated the role of the N-terminal residue of RNase H during HIV-1 infection. Comparison of the sequence of the cleavage site between the p51 subunit of RT and RNase H in HIV-1 isolates present in the Los Alamos HIV sequence database revealed that the sequence for the N-terminal amino acid residue of RNase H is absolutely conserved for all the isolates found in this database (Fig. 1). This suggests a hypothesis that changing this residue would have deleterious effects on viral infectivity.
FIG 1.
The N-terminal residue of HIV-1 RNase H is highly conserved. The sequence of the protease cleavage site between RT (p51) and RNase H was analyzed for 1,850 isolates of HIV-1 and chimpanzee simian immunodeficiency virus present in the Los Alamos HIV sequence database (http://www.hiv.lanl.gov) using the web alignment tool. The amino acid that corresponds to the conserved sequence is shown at the bottom. Arrows indicate the protease cleavage site between RT and RNase H. The sequence logo at the top was generated using WebLogo (http://weblogo.berkeley.edu).
RNase H N-terminal mutations impact intravirion protein levels and viral infectivity.
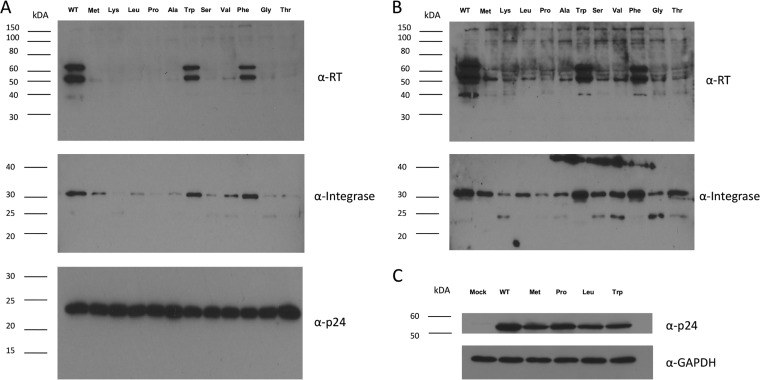
To test the role of the N-terminal residue of RNase H in the life cycle of HIV-1, we changed this residue to several different amino acids. Selection of mutants was based on the N-end rule designation or the structure of the specific amino acid compared to that of the wild-type (WT) residue of tyrosine (Table 1). We first analyzed the effects of these N-terminal RNase H mutations on viral protein packaging and maturation by immunoblotting. We observed that viral pellets of most of the RNase H mutants contained drastically reduced levels of RT compared to WT viral pellets (Fig. 2A). Extended exposure of the immunoblot (using enhanced chemiluminescence for detection) revealed that some mutant virions (i.e., virions with proline [Pro] and lysine [Lys]) contained barely detectable levels of RT, while others contained diminished amounts of p51 (Fig. 2B). In contrast, two of the mutants (mutants with tryptophan [Trp] and phenylalanine [Phe]) containing amino acids structurally similar to the WT tyrosine residue had slightly reduced RT levels compared to the WT (Fig. 2A, top). We further probed our RNase H mutants for the presence of other HIV-1 proteins, and this analysis revealed that some of the mutants also had diminished levels of integrase (Fig. 2A, middle). Mutants with mutations that had the most effect on the RT levels (i.e., those with Pro and Lys) showed the most pronounced decrease in virion integrase levels as well. We further probed the RNase H mutant virions for viral capsid protein (p24) to determine whether the mutations that we introduced caused any gross aberrations in assembly or processing. As shown in Fig. 2A (bottom), a product of Gag processing, p24, was found in all mutants, suggesting WT levels of protease activity on the Gag polyprotein in virions. This was reflected in the analysis of p24 expression in producer cells, where stable and unstable mutants showed similar levels of expression (Fig. 2C). GagPol incorporation was also detected at similar levels in virions (see Fig. 4).
TABLE 1.
HIV-1 RNase H N-terminal mutants
| Cleavage sitea (RT/RNase H) | N-end rule designation | Amino acid at P-1′ | Reason for selection |
|---|---|---|---|
| AETF/YVD (WT) | Destabilizing | Tyrosine | WT |
| AETF/MVD | Stabilizing | Methionine | N-end rule |
| AETF/FVD | Destabilizing | Phenylalanine | Structurally conserved |
| AETF/WVD | Destabilizing | Tryptophan | Structurally conserved |
| AETF/TVD | Stabilizing | Threonine | N-end rule |
| AETF/LVD | Destabilizing | Leucine | N-end rule |
| AETF/KVD | Destabilizing | Lysine | Long basic side chain |
| AETF/AVD | Stabilizing | Alanine | N-end rule |
| AETF/PVD | Stabilizing | Proline | Drastic structural change |
| AETF/GVD | Stabilizing | Glycine | Small side chain |
| AETF/SVD | Stabilizing | Serine | N-end rule |
| AETF/VVD | Stabilizing | Valine | Small hydrophobic side chain |
Mutated amino acids are underlined.
FIG 2.
Changing the N-terminal residue of HIV-1 RNase H leads to the instability of RT and integrase in the virus particle. (A) Equivalent amounts of WT and RNase H N-terminal mutant virions (according to normalization of the amount of p24) were analyzed by Western blotting following production in 293T cells using helper plasmids and concentration by ultracentrifugation. Particles were probed with RT (top), integrase (middle), and p24 (bottom) antibodies. (B) Extended exposure of the immunoblots from panel A. (C) 293T cells transfected with helper plasmids producing WT or RNase H mutant virions were lysed 48 h after transfection, and the protein contents of equivalent amounts of cells were analyzed by immunoblotting with the indicated antibodies. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
FIG 4.
Intravirion processing of Gag and Gag-Pol polyproteins in HIV-1 RNase H N-terminal mutants. The WT and two representatives of RNase H mutant HIV-1 virion particles were produced in the presence of various concentrations of the HIV-1 protease inhibitor ritonavir, and the cleavage patterns of the Gag and GagPol polypeptides were analyzed by immunoblot analysis. The WT, as well as methionine (Met) and leucine (Leu) RNase H mutants, were probed with antibodies to RT (top) or p24 (bottom).
Next, we tested the infectivity of the N-terminal RNase H mutants of HIV-1. As shown in Fig. 3, infectivity was slightly decreased (by a factor of 0.75) for Trp and Phe mutants that were not compromised for RT levels in the virions. Infectivity was undetectable in all the other mutants tested except the Met mutant, which showed a 100-fold decrease in infectivity compared to the WT.
FIG 3.
Effect of N-terminal RNase H mutations on HIV-1 infectivity. Jurkat cells were infected with equivalent amounts (according to normalization of the amount of p24) of VSVg-pseudotyped WT or mutant RNase H mutant HIV-1 vectors. Infectivity was measured by flow cytometry at 3 days postinfection. The MOI for the WT was measured as indicated in the Materials and Methods section.
Packaging and processing of viral polyproteins with RNase H N-terminal mutations.
Despite the correct processing of Gag to p24 in all of the N-terminal RNase H mutants tested (Fig. 2A, bottom), diminished levels of RT and integrase in some mutants indicate the possibility of aberrant packaging or aberrant processing of GagPol. To better assess this, we analyzed the mutant virions for the presence of GagPol, Gag, and other proteolytic processing products. We produced WT and RNase H mutant virions (mutants with Met and Leu, which were typical for the residual RT of the other mutants) in the presence of various amounts of ritonavir, an HIV-1 protease inhibitor. Following concentration, virions were analyzed by immunoblotting. As shown in Fig. 4 (bottom), WT and mutant virions showed similar patterns of cleavage intermediates when probed for p24. As the concentration of ritonavir increased, the levels of processed p24 decreased, with a corresponding increase in the amounts of higher-molecular-mass products, such as Gag and GagPol, being found (Fig. 4, bottom). Notably, at the highest concentration of ritonavir, the Gag and GagPol levels were similar in all virions, suggesting that the mutants are not compromised in the assembly of Gag and GagPol into virion particles. When probed with RT antibody, the cleavage patterns of the WT and mutant virions were comparable only at the higher ritonavir concentrations (Fig. 4, top). For virions produced in the presence of 1 or 0.5 μM ritonavir, WT virus showed the predicted processing intermediates, consistent with previous reports (25, 26). There is a loss of immunoreactive epitopes in the two mutants examined (Fig. 4, top). From this analysis, we cannot conclude whether a higher-molecular-mass intermediate or the RT p66 species is mainly susceptible to the degradation observed in the mutants. However, the appearance of similar levels of GagPol and Gag in both mutants and the WT at the highest ritonavir level indicates correct packaging of these polyproteins in RNase H N-terminal mutants.
Delivery of RT independently of GagPol into HIV-1 virions.
Our analysis of proteolytic processing in RNase H mutants did not allow us to conclude whether this degradation requires only the RT p66 species or a higher-molecular-mass processing intermediate. In addition, we wondered if this degradation could affect WT RT p66 species that may dimerize with a mutant RT if both were present in the virion. To examine these questions, we delivered p66 synthesized independently of GagPol into virions containing WT or RNase H mutant GagPol. This was accomplished by using fusions to the HIV-1 accessory protein Vpr, which is packaged into the virion by binding to the p6 domain of Gag (27). We generated constructs by fusing Vpr to WT or RNase H mutant RT-integrase (Fig. 5A and B). The protease cleavage site (PC) between protease and RT was retained in the Vpr fusion to enable cleavage and release of RT-integrase in the virus particle. We generated WT or N-terminal RNase H mutant viral particles in the presence or absence of Vpr fusion constructs. Concentrated virions were analyzed by immunoblotting following p24 ELISA to enable equivalent loading on the basis of the viral capsid concentration. As shown in Fig. 6, top, when probed with a Vpr antibody, WT or mutant viruses complemented with Vpr-RT-IN WT fusion (Vpr-RT-INwt) constructs contained the predicted 4 bands as a result of protease cleavage at 3 different sites: between Vpr and RT, p51 and RNase H, and RNase H and integrase (Fig. 6, top, lanes +Vpr-RT-INwt). When we probed Vpr-RT-IN-complemented mutant virions with an RT antibody, we observed the almost complete rescue of both RT subunits only when the Vpr constructs contained WT or Trp mutant RNase H fusions (Fig. 6A and C, middle). RNase H N-terminal mutants that are structurally different from the WT showed either diminished levels of RT subunits (Fig. 6C) or the appearance of only low levels of p51 (Fig. 6A). Notably, the Vpr fusion polyproteins appeared to be present at similar levels in virion particles (Fig. 6, top and middle) and only the RT subunits were subject to degradation, suggesting that these are the substrates for proteolysis. Similar to the results obtained with the Vpr-RT-integrase fusions, we observed the appearance of both RT subunits only in the virions complemented with the Vpr-RT WT or the Vpr-RT Trp mutant when we delivered Vpr-RT-fused RNase H mutants (data not shown). These results support the idea that the mutant RT p66 is the substrate for proteolysis, once it is liberated from the polyprotein (from GagPol or from the Vpr fusion).
FIG 5.
Constructs used for Vpr complementation and in vitro studies. The position and identity of RNase H N-terminal substitutions are indicated. The frameshift causing a mutation in pRK5-GagPol-FS is shown. PC, protease cleavage site between viral protease and RT; RH, RNase H.
FIG 6.
Analysis of Vpr-RT-integrase-complemented HIV-1 virions. HIV-1 virions with GagPol containing WT (B) and RNase H N-terminal Leu (A) or Pro (C) mutants were produced in 293T cells with helper plasmids in the presence or absence of expression plasmids encoding Vpr-RT-integrase fusion proteins with WT or N-terminal RNase H mutant RT. Concentrated virions were normalized for their p24 contents by ELISA and analyzed by Western blotting using Vpr (top), RT (middle), and p24 (bottom) antibodies.
Infectivity of Vpr-RT-integrase-complemented viruses.
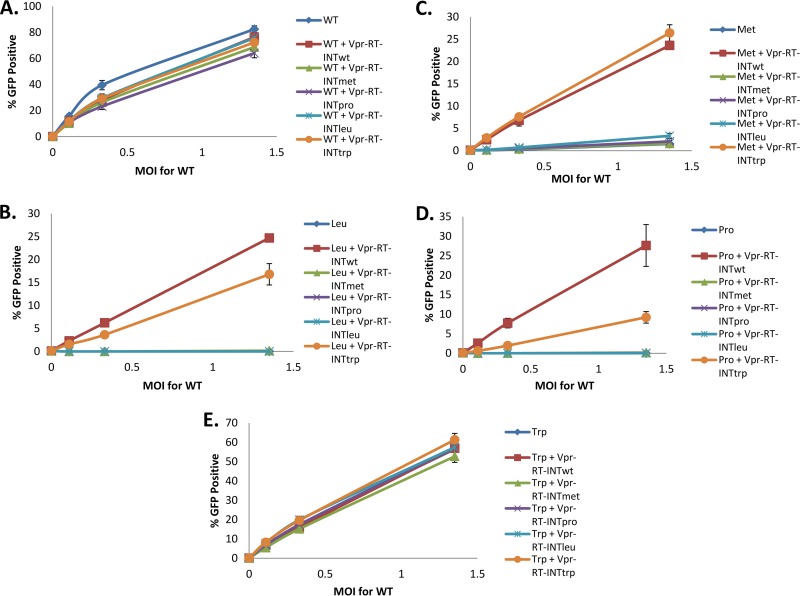
Since the Vpr-RT-IN-complemented RNase H N-terminal mutants showed the rescue of intravirion RT when complemented with the WT or Trp mutant, we tested if this molecular rescue also extends to the infectivity of the RNase H mutants. We infected Jurkat cells with WT or mutant Vpr-RT-IN-complemented viruses (Fig. 7). As predicted by the immunoblotting data, the infectivity of the Leu, Met, and Pro mutants was rescued to similar levels by complementation with Vpr-RT-INwt (Fig. 7B to D). We also observed the rescue of infectivity by the Vpr-RT-IN construct containing a Trp mutation (Vpr-RT-INtrp), albeit at different levels for each mutant (Fig. 7B to D). In contrast, complementation with Vpr-RT-IN constructs containing RNase H mutants that had diminished levels of RT in the virion did not rescue infectivity in any of our mutants (Fig. 7B to D). Of note, there was a general slight decrease in infectivity in WT virions that were complemented with different Vpr constructs.
FIG 7.
Infectivity of Vpr-RT-integrase-complemented WT or RNase H N-terminal mutant viruses. Jurkat cells were infected with equivalent amounts (according to normalization of the amount of p24) of WT (A) or N-terminal RNase H Leu (B), Met (C), Pro (D), or Trp (E) mutant VSVg-pseudotyped HIV-EGFP containing WT or RNase H mutant Vpr-RT-IN fusion proteins. Infectivity was determined by flow cytometry at 3 days postinfection. The MOI for the WT was measured as described in Materials and Methods.
RT with an RNase H N-terminal mutation is stable in vitro.
Our observations revealed that the RT of the majority of N-terminal RNase H mutants is degraded in virions and that this degradation is dependent on the release of the mutant p66 from the polypeptide in which it is embedded (Fig. 4 for GagPol; Fig. 6 for Vpr-RT-integrase). However, it is not clear whether this degradation is caused by the viral protease (for example, by the mutation uncovering cryptic target sites) or a cellular protease that is packaged into the virion. We first investigated this by using a rabbit reticulocyte lysate (RRL) in vitro transcription/translation system. Since Gag is the dominant protein species translated from full-length viral mRNA, this would interfere with our ability to detect RT that is made from the frameshifted GagPol polyprotein (1). Hence, we used a GagPol transcript with an engineered frameshift that would produce only full-length GagPol polyprotein (Fig. 5C). We further introduced an N-terminal RNase H mutation (Pro) into this construct since this mutation had the most drastic effect on RT stability in the virion. Previous studies with the in vitro-translated GagPol polyprotein showed that the encoded protease cannot completely process the GagPol precursor to mature viral proteins (26, 28, 29). A strong initial cleavage event between p2 and the nucleocapsid (NC) was observed, followed by minor cleavages at other sites (26). However, addition of HIV-1 protease to the reaction mixture has been shown to generate mature viral proteins (28). In our assay system, we first translated GagPol in vitro and then incubated the final product with exogenous HIV-1 protease and used immunoblot analysis to probe for RT release and stability. As shown in Fig. 8, in the absence of exogenous protease, we observed the predicted proteins that result from a single dominant cleavage of GagPol by the intrinsic protease (a 40-kDa protein and an approximately 120-kDa protein). We also observed lower-molecular-mass peptides using this RT monoclonal antibody, consistent with the several minor cleavage products observed previously (26, 29). Consistent with these previous findings, addition of HIV-1 protease resulted in the processing of GagPol with the appearance of p24 for both the WT and the RNase H proline mutant, when probed with a p24 antibody (Fig. 8, top). We also observed the appearance of the p51 and p66 subunits of RT from WT GagPol following incubation with the viral protease (Fig. 8, bottom). In contrast to the intravirion degradation of RT in the RNase H proline mutant, the in vitro maturation of GagPol containing the RNase H proline mutant resulted in stable RT subunits (p51 and p66) at levels similar to those in the WT (Fig. 8, bottom). Addition of greater concentrations of viral protease or longer incubation times did not reveal any additional cleavages of the final products (data not shown). These results suggest that HIV-1 protease that is active and site specific for cleavage (i.e., competent to release p24 and the RT p51 and p66 subunits) does not degrade RNase H mutant RT.
FIG 8.
In vitro analysis of the role of viral protease in the degradation of RNase H mutants. pRK5-GagPol-FS was used for in vitro transcription translation by use of an RRL system. Translation products were incubated at 30°C for 2 h with HIV-1 protease or phosphate buffer and analyzed via immunoblotting. Arrows, the identity of the fragments detected with RT and p24 antibodies. The antibodies used for each blot are indicated in bold with an α prefix.
Some RNase H N-terminal mutations lead to impairment of dimerization of RT subunits.
While the findings of the in vitro experiments presented above suggest that viral protease may not be responsible for the degradation of intravirion RT of some RNase H N-terminal mutants, there remains the possibility that in the context of the viral milieu, specific concentrations of viral protease or specific interactions between the viral proteins inside the virion are required for the mutant RT to be degraded by the HIV-1 protease. To properly investigate these possibilities, we needed to package the p66 RT subunit into virions without fusing it to any protein. This would enable a test of the intravirion stability of RT in the absence of an active viral protease. To this end, we utilized a previously described construct, pLR2P-Vpr-p51-IRES-p66 (Vpr-p51/p66) (21). In this construct, a Vpr-fused p51 subunit is packaged into the virion through the interaction of Vpr with the p6 domain of Gag, while the p66 subunit is packaged via its interaction with the p51 subunit in the Vpr-p51 fusion (21). Since the Vpr-p51/p66 construct makes it difficult to distinguish between the Vpr-p51 fusion protein and p66 on immunoblots probed with an RT antibody (30), we generated a construct that contains 50 amino acids from the C terminus of HIV-1 protease between Vpr and p51 (Vpr-pro50-p51) (Fig. 5D). This enables differentiation between Vpr-pro50-p51 and p66 on immunoblots. To test for the involvement of viral protease in the degradation of RT in RNase H mutants, we inactivated the viral protease by introducing a D25A mutation into the protease gene in our viral production helper plasmid ΔNRF. Since the packaging of p66 into the virus particle requires binding to the p51 subunit in the Vpr-pro50-p51 fusion protein, we tested the dimerization of RT subunits harboring the RNase H proline mutant using a co-IP assay. For this we incubated extracts of 293T cells cotransfected with ΔNRF D25A (protease inactive) and Vpr-pro50-p51Δp66, Vpr-pro50-p51/p66wt, or Vpr-p51/p66pro with a Vpr antibody. Bound protein complexes were pulled down with protein G-conjugated beads and analyzed by immunoblotting. As shown in Fig. 9A, top, both Vpr-pro50-p51 and p66 can be detected from the cells expressing WT p66 (lanes under pLR2P WT) in the input and immunoprecipitated extracts. In contrast, p66 was present only in the lysate and not in the immunoprecipitates of the cells expressing RNase H proline mutant p66 (Fig. 9A, top, lanes under pLR2P Pro). These results indicate that dimerization of RT p66 with p51 is impaired when the N-terminal residue of RNase H is changed to proline. To further examine the importance of the RNase H N-terminal residue in the dimerization of RT subunits, we tested the association of RT subunits in other RNase H mutants, i.e., those with glycine, leucine, alanine, and methionine substitutions. As shown in Fig. 9B, top, we detected both Vpr-pro50-p51 and p66 in both the lysate (the input [lanes Inp]) and the immunoprecipitate (lanes IP) from the cells expressing RNase H glycine and leucine mutant p66 (lanes under pLR2P Gly and pLR2P Leu) at ratios similar to those for the wild type. In contrast, p66 was present only in the lysate (lanes Inp) of RNase H mutants with alanine and methionine (lanes under pLR2P Ala and pLR2P Met). These results highlight the importance of the N-terminal RNase H residue in the dimerization of RT subunits.
FIG 9.
The RNase H N-terminal residue is critical for RT subunit association. (A) 293T cells were cotransfected with Vpr-pro50-p51-IRES-p66 plasmids encoding WT or proline RNase H mutant p66 and ΔNRF D25A. Vpr-p51Δp66 was used as a control. Co-IP was performed on cell extracts using Vpr antibody and protein G beads. (B) 293T cells were cotransfected with Vpr-pro50-p51-IRES-p66 plasmids encoding WT or different RNase H mutant p66 proteins and ΔNRF D25A. Vpr-p51Δp66 was used as a control. Co-IP was performed on cell extracts using Vpr antibody and protein G beads. The antibodies used for each blot are indicated in bold with an α prefix. Lanes IP, immunoprecipitated proteins; lanes Inp, input proteins.
RT with an RNase H N-terminal mutation is still degraded in the absence of active viral protease.
We next examined the stability of RNase H N-terminal mutants in the presence and absence of viral protease. We used the glycine and leucine RNase H mutants since we have determined that association of the subunits is not impaired for these mutants. We produced virions with active or inactive viral protease complemented with WT or RNase H mutant Vpr-pro50-p51-IRES-p66 and analyzed them by immunoblotting. To avoid confusion over the RT subunit bands contributed by GagPol and the Vpr-pro50-p51-IRES-p66 constructs in the immunoblot, we chose GagPol with the RNase H proline mutation to produce virions with active viral protease. This mutant completely lacks RT subunits inside the virion (Fig. 2), so the only RT subunits observed are derived from the complementing Vpr-pro50-p51-IRES-p66 construct. GagPol containing the D25A mutation in the viral protease was used to produce virions with inactive protease. We observed that both protease-active and -inactive virions with WT Vpr-pro50-p51-IRES-p66 contained the expected peptides for Vpr-pro50-p51 and p66 (Fig. 10, top, lanes with pLR2P WT in the lane labels). In contrast, for both glycine and leucine mutants, both Vpr-pro50-p51 and p66 were almost completely absent with or without active HIV-1 protease (Fig. 10, top, lanes with pLR2P Leu and pLR2P Gly in the lane labels). For these mutants, the formation of the heterodimer independently of the HIV-1 protease (i.e., by expression of the subunits from the Vpr-pro50-p51 and internal ribosome entry site [IRES] expression of p66) leads to degradation of the heterodimer. These results suggest that the presence of active viral protease may not be required for the intravirion degradation of RT in RNase H N-terminal mutants.
FIG 10.
Intravirion analysis of the role of viral protease in the RNase H degradation mutants. HIV-1 virions with either protease-inactivating D25A mutant GagPol or RNase H N-terminal proline mutant GagPol (labeled GagPol Pro) were produced in 293T cells in the presence or absence of Vpr-pro50-p51-IRES-p66 plasmids carrying WT or RNase H N-terminal mutant p66. Concentrated virions were analyzed by Western blotting using the indicated antibodies following normalization of their amounts via p24 ELISA. The antibodies used for each blot are indicated in bold with an α prefix. Numbers on the left are molecular masses (in kDa).
DISCUSSION
RNase H of HIV-1 is excised from half of the viral p66 RT species that are synthesized, and this results in the association of heterodimeric subunits of RT, p51, and p66 (4). This proteolytic cleavage releases RNase H into the virion, and this mature form of RNase H bears a conserved N-terminal amino acid residue that makes it a potential substrate for the cellular N-end rule pathway (Fig. 1). In this study, we set out to examine the role of the N-terminal residue of RNase H on the life cycle of HIV-1. To this end we generated virions containing mutations of the N-terminal RNase H tyrosine residue. We observed that most of the amino acid substitutions led to a complete or almost complete absence of RT in the virion (Fig. 2). The nature of the amino acid substitution that leads to the absence of detectable RT in the virion was important, since mutants that contained amino acids structurally similar to the WT amino acid (i.e., Trp and Phe) at the N terminus of RNase H had both subunits of RT at levels similar to those in the WT. Similar observations were reported by another group following the introduction of multiple mutations in the p51/RNase H cleavage site region (31). Since that study introduced multiple mutations that led to the degradation of RT in the virus particle, it was not possible to determine which mutation was responsible or what type of amino acid was tolerated at any specific residue. In this study, we report on the mutagenesis of only the N-terminal residue, and our results point to the importance of the nature of the amino acid at the N-terminal amino acid residue of the RNase H for the stability of RT in the virus particle. This was also corroborated by the high degree of conservation of the Tyr residue across HIV-1 isolates (Fig. 1).
In addition to the absence of RT, some RNase H mutants also contained diminished amounts of intravirion integrase (Fig. 2A). There was also a concomitant appearance of a peptide at about 25 kDa (Fig. 2B). It is probable that a subset of RT mutations leads to a structural change in the RT-integrase polyprotein that may expose an alternative protease cleavage site in integrase. It is important to note that some of the mutant virions did not contain significant amounts of this possible alternative cleavage product, including the proline mutant, which had the most drastic effect on RT stability. This suggests a different or additional mechanism for integrase degradation for these mutants. It is important to note that the relative amounts of integrase correlated with the relative amounts of RT found in the RNase H mutants. This suggests a mechanism of degradation of a higher-molecular-mass intermediate presumably induced by some structural change in the polyprotein.
The fact that we observed proteolytic processing of Gag to p24 by viral protease and the presence of at least some amount of integrase in each mutant, combined with the observation that GagPol and Gag are expressed in the RNase H mutants, leads us to conclude that a deficiency in GagPol production and packaging cannot explain the drastically low levels of RT found in some of the RNase H N-terminal mutants.
The infectivity of the N-terminal RNase H mutants correlated with the RT content in the virion. Viruses containing amino acids structurally similar to the WT Tyr amino acid at the RNase H N terminus (Trp and Phe) had only a slight decrease in infectivity, while the other mutants had a complete or almost complete lack of infectivity (Fig. 3). Even though some mutants retained a limited amount of p51 in the virus particle, the presence of p66 is required for the reverse transcription process (8). One of our mutants (the mutant with Met) showed limited levels of infectivity. Longer exposures of the immunoblots probed with monoclonal or polyclonal RT antibody revealed that the Met mutant contained limited amounts of p66 in the virion, which correlated with the residual level of infectivity observed for this mutant (Fig. 2B).
Despite the degradation of both RT and integrase, we did not observe a deficiency in intravirion incorporation of GagPol in our RNase H mutants. Moreover, the appearance of identical proteolytic cleavage patterns in Gag processing indicates that the activity of viral protease is not impacted by mutations at the N-terminal amino acid residue of HIV-1 RNase H (Fig. 4).
The mechanism of formation of the RT p51/p66 heterodimer is not clearly established, and two working models have been proposed. In the concerted model, p66 and p51 subunits are separately cleaved out of different GagPol molecules and come together to form the heterodimer. In contrast, the sequential model posits that following the formation of an initial homodimer by two p66 subunits, the RNase H domain of one of the subunits is cleaved to form the heterodimer of p66/p51. Even though most of the crystallographic and functional evidence points to the sequential model, definitive evidence is still missing. Since the RT in the virus particles of some of our RNase H N-terminal mutants was degraded, we explored the possibility that this degradation affected a nonmutant RT p66 species by delivering the RT as a GagPol-independent Vpr fusion protein into the virus. While these results do not clearly distinguish between the two models of RT heterodimer formation, they indicate that degradation of RT caused by the RNase H N-terminal substitutions is confined to the specific p66 species that contains the mutation. Hence, we observed that trans-complementation with the WT RT-integrase Vpr fusion protein substantially rescued infectivity in our RNase H mutants.
Proteolytic processing of the precursor polyproteins of HIV-1 is an ordered series of events carried out by the viral protease following the assembly of viral components. A conformational change due to a mutation in the polyprotein may expose alternative cleavage sites, leading to additional cleavages of the viral polyprotein and resulting in a loss of infectivity. We tested the possibility that the RNase H N-terminal mutants were degraded by the HIV-1 protease using both an in vitro assay and an intravirion assay. In vitro experiments relied on the ability of externally added viral protease to complete the processing of the in vitro-translated GagPol precursor leading to the appearance of mature viral proteins (28). With the caveat that in vitro conditions may not exactly represent the conditions inside the virus particle, we did not observe any degradation of RT for the proline RNase H mutant (Fig. 8). This led us to question the role of HIV-1 protease in the degradation of RT in N-terminal RNase H mutants. An intravirion assay showed the absence of RT p66 species bearing the proline RNase H mutant in the virions with inactivated viral protease (data not shown), although its expression in the producer cell was unaffected (Fig. 9A). However, further analysis using co-IP showed that the absence of RT p66 in these virions was caused by the impaired interaction between Vpr-pro50-p51 and RNase H proline mutant p66 (Fig. 9A). This indicates that dimerization of RT subunits is impaired in this RNase H mutant. To properly test the role of viral protease in the intravirion degradation of RNase H N-terminal mutants, we sought to find an RNase H mutant that is not defective in the association of RT subunits. This search led to the identification of two RT mutants (with glycine and leucine) that are not defective in RT subunit binding. Using these mutants, we showed that both p66 and Vpr-pro50-p51 were degraded in the virus particle (Fig. 10). While we did not anticipate that degradation of RNase H mutant p66 would be accompanied by the degradation of Vpr-pro50-p51, we hypothesize that delivery of these proteins to the virus particle as a heterodimerized complex leads to degradation of both subunits. Collectively, these results indicate that viral protease may not be required for the degradation of RT containing RNase H N-terminal mutants in the virus particle. Moreover, our results suggest that one or more cellular proteases may be packaged into virus particles, resulting in the degradation of RT in RNase H N-terminal mutants.
Previous studies have shown that dimerization of RT subunits is a prerequisite for the activity of the enzyme (32–34). Moreover, mutations in the tryptophan repeat motif of RT have been shown to cause defects in dimerization (33, 34), and these results were later confirmed using the same pLR2p constructs utilized in this study (35). While some of these tryptophan motif mutants also showed decreased levels of RT in the virus particle, the degradation was not as pronounced as the degradation that we observed in RNase H N-terminal mutants. This suggests that the lack of dimerization per se may not be the initiating event for degradation by the extent of the structural change caused by the mutation. Moreover, since some of our unstable RNase H mutants showed a successful association of p66 and Vpr-pro50-p51, degradation of RT in RNase H N-terminal mutants cannot be linked to the defect in RT subunit association. Others have speculated that the RT-RNase H cleavage site is not involved in RT dimer formation (36). Here we provide evidence that at least one of the amino acids in this cleavage site can impact the association of RT subunits.
It is notable that while the results from the in vitro and intravirion assays indicate that viral protease may not be responsible for the degradation of the RNase H proline mutant of RT p66, our Vpr complementation and protease inhibitor experiments clearly indicate that the release of the mutant RT p66 species from the polyproteins by viral protease cleavage is required, since GagPol and Vpr fused polyproteins are stable even when harboring the unstable RNase H mutations. This observation suggests the need for caution in ascribing the degradation of mutant RT to the action of HIV-1 protease only on the basis of data from the inhibition of HIV-1 protease; i.e., the action of HIV-1 protease is a prerequisite for degradation that may be mediated by another protease.
Recent advances in proteomic technology have enabled more detailed analysis of the protein content of HIV-1 particles. Indeed, multiple studies to date have identified cellular proteins packaged into HIV-1 virions (37–40). Relevant to this study, the presence of multiple cellular proteases has been reported. However, there is no significant overlap in the identity of the proteases identified in these studies. Indeed, the only protease that is common to two of these studies is carboxypeptidase D (37, 38). In addition, Santos et al. found this protease in viral particles generated from only one of the cell lines that they used (38). While this indicates the importance of the specific cell line and the methodology used for these studies, we believe that degradation of RT mutants is caused by a protease that is broadly expressed, since the same phenomenon (of degradation of RT mutants as result of mutations in this region) was observed when monkey kidney cells (COS-7 cells) and human T cells (MT-2 cells) were used for virus production (31).
Due to its importance as an antiviral target, reverse transcriptase of HIV-1 is one of the most highly studied proteins. Several groups have introduced amino acid substitutions at different subdomains of RT (11, 31, 33, 41–45). Many of these mutations led to destabilization of the RT protein in the virus particle as well as a loss of infectivity. Some studies have concluded that the viral protease is responsible for the degradation of the mutant RT. Our results indicate that liberation of the mutant subunit from the fusion polypeptide is a prerequisite for degradation, and results presented previously should be reevaluated in this light. This study could have implications for the development of small molecules that distort the structure of the HIV-1 polyprotein in the Pol polypeptide that signals destruction of essential viral enzymes.
ACKNOWLEDGMENTS
We acknowledge the Flow Cytometry Core Facility of the University of Minnesota Cancer Center, a comprehensive cancer center designated by the National Cancer Institute, supported in part by grant P30 CA77598. This work was supported by an NIH grant (R21 AI087466) to N.V.S.
REFERENCES
- 1.Jacks T, Power MD, Masiarz FR, Luciw PA, Barr PJ, Varmus HE. 1988. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature 331:280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- 2.Louis JM, Nashed NT, Parris KD, Kimmel AR, Jerina DM. 1994. Kinetics and mechanism of autoprocessing of human immunodeficiency virus type 1 protease from an analog of the Gag-Pol polyprotein. Proc Natl Acad Sci U S A 91:7970–7974. doi: 10.1073/pnas.91.17.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.di Marzo Veronese F, Copeland TD, DeVico AL, Rahman R, Oroszlan S, Gallo RC, Sarngadharan MG. 1986. Characterization of highly immunogenic p66/p51 as the reverse transcriptase of HTLV-III/LAV. Science 231:1289–1291. doi: 10.1126/science.2418504. [DOI] [PubMed] [Google Scholar]
- 4.Schulze T, Nawrath M, Moelling K. 1991. Cleavage of the HIV-1 p66 reverse transcriptase/RNase H by the p9 protease in vitro generates active p15 RNase H. Arch Virol 118:179–188. doi: 10.1007/BF01314028. [DOI] [PubMed] [Google Scholar]
- 5.Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA. 1992. Crystal structure at 3.5 Å resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science 256:1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- 6.Szilvay AM, Nornes S, Kannapiran A, Haukanes BI, Endresen C, Helland DE. 1993. Characterization of HIV-1 reverse transcriptase with antibodies indicates conformational differences between the RNAse H domains of p 66 and p 15. Arch Virol 131:393–403. doi: 10.1007/BF01378640. [DOI] [PubMed] [Google Scholar]
- 7.Le Grice SF, Naas T, Wohlgensinger B, Schatz O. 1991. Subunit-selective mutagenesis indicates minimal polymerase activity in heterodimer-associated p51 HIV-1 reverse transcriptase. EMBO J 10:3905–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hostomsky Z, Hostomska Z, Fu TB, Taylor J. 1992. Reverse transcriptase of human immunodeficiency virus type 1: functionality of subunits of the heterodimer in DNA synthesis. J Virol 66:3179–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris D, Lee R, Misra HS, Pandey PK, Pandey VN. 1998. The p51 subunit of human immunodeficiency virus type 1 reverse transcriptase is essential in loading the p66 subunit on the template primer. Biochemistry 37:5903–5908. doi: 10.1021/bi9728452. [DOI] [PubMed] [Google Scholar]
- 10.Tasara T, Amacker M, Hübscher U. 1999. Intramolecular chimeras of the p51 subunit between HIV-1 and FIV reverse transcriptases suggest a stabilizing function for the p66 subunit in the heterodimeric enzyme. Biochemistry 38:1633–1642. doi: 10.1021/bi9821162. [DOI] [PubMed] [Google Scholar]
- 11.Chung S, Miller JT, Johnson BC, Hughes SH, Le Grice SF. 2012. Mutagenesis of human immunodeficiency virus reverse transcriptase p51 subunit defines residues contributing to vinylogous urea inhibition of ribonuclease H activity. J Biol Chem 287:4066–4075. doi: 10.1074/jbc.M111.314781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung S, Miller JT, Lapkouski M, Tian L, Yang W, Le Grice SF. 2013. Examining the role of the HIV-1 reverse transcriptase p51 subunit in positioning and hydrolysis of RNA/DNA hybrids. J Biol Chem 288:16177–16184. doi: 10.1074/jbc.M113.465641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lapkouski M, Tian L, Miller JT, Le Grice SF, Yang W. 2013. Complexes of HIV-1 RT, NNRTI and RNA/DNA hybrid reveal a structure compatible with RNA degradation. Nat Struct Mol Biol 20:230–236. doi: 10.1038/nsmb.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerritelli SM, Crouch RJ. 2009. Ribonuclease H: the enzymes in eukaryotes. FEBS J 276:1494–1505. doi: 10.1111/j.1742-4658.2009.06908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Repaske R, Hartley JW, Kavlick MF, O'Neill RR, Austin JB. 1989. Inhibition of RNase H activity and viral replication by single mutations in the 3′ region of Moloney murine leukemia virus reverse transcriptase. J Virol 63:1460–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanese N, Telesnitsky A, Goff SP. 1991. Abortive reverse transcription by mutants of Moloney murine leukemia virus deficient in the reverse transcriptase-associated RNase H function. J Virol 65:4387–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boso G, Tasaki T, Kwon YT, Somia NV. 2013. The N-end rule and retroviral infection: no effect on integrase. Virol J 10:233. doi: 10.1186/1743-422X-10-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth MJ, Tanese N, Goff SP. 1985. Purification and characterization of murine retroviral reverse transcriptase expressed in Escherichia coli. J Biol Chem 260:9326–9335. [PubMed] [Google Scholar]
- 19.Tanese N, Goff SP. 1988. Domain structure of the Moloney murine leukemia virus reverse transcriptase: mutational analysis and separate expression of the DNA polymerase and RNase H activities. Proc Natl Acad Sci U S A 85:1777–1781. doi: 10.1073/pnas.85.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orvell C, Unge T, Bhikhabhai R, Bäckbro K, Rudén U, Strandberg B, Wahren B, Fenyö EM. 1991. Immunological characterization of the human immunodeficiency virus type 1 reverse transcriptase protein by the use of monoclonal antibodies. J Gen Virol 72(Pt 8):1913–1918. doi: 10.1099/0022-1317-72-8-1913. [DOI] [PubMed] [Google Scholar]
- 21.Mulky A, Sarafianos SG, Arnold E, Wu X, Kappes JC. 2004. Subunit-specific analysis of the human immunodeficiency virus type 1 reverse transcriptase in vivo. J Virol 78:7089–7096. doi: 10.1128/JVI.78.13.7089-7096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 23.Somia NV, Schmitt MJ, Vetter DE, Van Antwerp D, Heinemann SF, Verma IM. 1999. LFG: an anti-apoptotic gene that provides protection from Fas-mediated cell death. Proc Natl Acad Sci U S A 96:12667–12672. doi: 10.1073/pnas.96.22.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C, Okayama H. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7:2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pettit SC, Sheng N, Tritch R, Erickson-Viitanen S, Swanstrom R. 1998. The regulation of sequential processing of HIV-1 Gag by the viral protease. Adv Exp Med Biol 436:15–25. doi: 10.1007/978-1-4615-5373-1_2. [DOI] [PubMed] [Google Scholar]
- 26.Pettit SC, Everitt LE, Choudhury S, Dunn BM, Kaplan AH. 2004. Initial cleavage of the human immunodeficiency virus type 1 GagPol precursor by its activated protease occurs by an intramolecular mechanism. J Virol 78:8477–8485. doi: 10.1128/JVI.78.16.8477-8485.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, Liu H, Xiao H, Conway JA, Hunter E, Kappes JC. 1997. Functional RT and IN incorporated into HIV-1 particles independently of the Gag/Pol precursor protein. EMBO J 16:5113–5122. doi: 10.1093/emboj/16.16.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettit SC, Lindquist JN, Kaplan AH, Swanstrom R. 2005. Processing sites in the human immunodeficiency virus type 1 (HIV-1) Gag-Pro-Pol precursor are cleaved by the viral protease at different rates. Retrovirology 2:66. doi: 10.1186/1742-4690-2-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettit SC, Gulnik S, Everitt L, Kaplan AH. 2003. The dimer interfaces of protease and extra-protease domains influence the activation of protease and the specificity of GagPol cleavage. J Virol 77:366–374. doi: 10.1128/JVI.77.1.366-374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulky A, Kappes JC. 2005. Analysis of human immunodeficiency virus type 1 reverse transcriptase subunit structure/function in the context of infectious virions and human target cells. Antimicrob Agents Chemother 49:3762–3769. doi: 10.1128/AAC.49.9.3762-3769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abram ME, Parniak MA. 2005. Virion instability of human immunodeficiency virus type 1 reverse transcriptase (RT) mutated in the protease cleavage site between RT p51 and the RT RNase H domain. J Virol 79:11952–11961. doi: 10.1128/JVI.79.18.11952-11961.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tachedjian G, Radzio J, Sluis-Cremer N. 2005. Relationship between enzyme activity and dimeric structure of recombinant HIV-1 reverse transcriptase. Proteins 60:5–13. doi: 10.1002/prot.20480. [DOI] [PubMed] [Google Scholar]
- 33.Wapling J, Moore KL, Sonza S, Mak J, Tachedjian G. 2005. Mutations that abrogate human immunodeficiency virus type 1 reverse transcriptase dimerization affect maturation of the reverse transcriptase heterodimer. J Virol 79:10247–10257. doi: 10.1128/JVI.79.16.10247-10257.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tachedjian G, Aronson HE, de los Santos M, Seehra J, McCoy JM, Goff SP. 2003. Role of residues in the tryptophan repeat motif for HIV-1 reverse transcriptase dimerization. J Mol Biol 326:381–396. doi: 10.1016/S0022-2836(02)01433-X. [DOI] [PubMed] [Google Scholar]
- 35.Mulky A, Sarafianos SG, Jia Y, Arnold E, Kappes JC. 2005. Identification of amino acid residues in the human immunodeficiency virus type-1 reverse transcriptase tryptophan-repeat motif that are required for subunit interaction using infectious virions. J Mol Biol 349:673–684. doi: 10.1016/j.jmb.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 36.Abram ME, Sarafianos SG, Parniak MA. 2010. The mutation T477A in HIV-1 reverse transcriptase (RT) restores normal proteolytic processing of RT in virus with Gag-Pol mutated in the p51-RNH cleavage site. Retrovirology 7:6. doi: 10.1186/1742-4690-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brégnard C, Zamborlini A, Leduc M, Chafey P, Camoin L, Saïb A, Benichou S, Danos O, Basmaciogullari S. 2013. Comparative proteomic analysis of HIV-1 particles reveals a role for Ezrin and EHD4 in the Nef-dependent increase of virus infectivity. J Virol 87:3729–3740. doi: 10.1128/JVI.02477-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos S, Obukhov Y, Nekhai S, Bukrinsky M, Iordanskiy S. 2012. Virus-producing cells determine the host protein profiles of HIV-1 virion cores. Retrovirology 9:65. doi: 10.1186/1742-4690-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chertova E, Chertov O, Coren LV, Roser JD, Trubey CM, Bess JW, Sowder RC, Barsov E, Hood BL, Fisher RJ, Nagashima K, Conrads TP, Veenstra TD, Lifson JD, Ott DE. 2006. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J Virol 80:9039–9052. doi: 10.1128/JVI.01013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saphire AC, Gallay PA, Bark SJ. 2006. Proteomic analysis of human immunodeficiency virus using liquid chromatography/tandem mass spectrometry effectively distinguishes specific incorporated host proteins. J Proteome Res 5:530–538. doi: 10.1021/pr050276b. [DOI] [PubMed] [Google Scholar]
- 41.Olivares I, Mulky A, Boross PI, Tözsér J, Kappes JC, López-Galíndez C, Menéndez-Arias L. 2007. HIV-1 protease dimer interface mutations that compensate for viral reverse transcriptase instability in infectious virions. J Mol Biol 372:369–381. doi: 10.1016/j.jmb.2007.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn LL, McWilliams MJ, Das K, Arnold E, Hughes SH. 2009. Mutations in the thumb allow human immunodeficiency virus type 1 reverse transcriptase to be cleaved by protease in virions. J Virol 83:12336–12344. doi: 10.1128/JVI.00676-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang HJ, Wang YX, Wu H, Jin DY, Wen YM, Zheng BJ. 2009. The y271 and i274 amino acids in reverse transcriptase of human immunodeficiency virus-1 are critical to protein stability. PLoS One 4:e6108. doi: 10.1371/journal.pone.0006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiang CC, Wang SM, Pan YY, Huang KJ, Wang CT. 2010. A single amino acid substitution in HIV-1 reverse transcriptase significantly reduces virion release. J Virol 84:976–982. doi: 10.1128/JVI.01532-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishitsuji H, Yokoyama M, Sato H, Yamauchi S, Takaku H. 2011. Identification of amino acid residues in HIV-1 reverse transcriptase that are critical for the proteolytic processing of Gag-Pol precursors. FEBS Lett 585:3372–3377. doi: 10.1016/j.febslet.2011.09.034. [DOI] [PubMed] [Google Scholar]