Abstract
Background
Children exposed to social adversity carry a greater risk of poor physical and mental health into adulthood. This increased risk is thought to be due, in part, to inflammatory processes associated with early adversity that contribute to the etiology of many adult illnesses. The current study asks whether aspects of the prenatal social environment are associated with levels of inflammation in adulthood, and whether prenatal and childhood adversity both contribute to adult inflammation.
Methods
We examined associations of prenatal and childhood adversity assessed through direct interviews of participants in the Collaborative Perinatal Project between 1959–1974 with blood levels of C-reactive protein in 355 offspring interviewed in adulthood (mean age=42.2 years). Linear and quantile regression models were used to estimate the effects of prenatal adversity and childhood adversity on adult inflammation, adjusting for age, sex, and race and other potential confounders.
Results
In separate linear regression models, high levels of prenatal and childhood adversity were associated with higher CRP in adulthood. When prenatal and childhood adversity were analyzed together, our results support the presence of an effect of prenatal adversity on (log) CRP level in adulthood (β=0.73, 95% CI: 0.26, 1.20) that is independent of childhood adversity and potential confounding factors including maternal health conditions reported during pregnancy. Supplemental analyses revealed similar findings using quantile regression models and logistic regression models that used a clinically-relevant CRP threshold (>3 mg/L). In a fully-adjusted model that included childhood adversity, high prenatal adversity was associated with a 3-fold elevated odds (95% CI: 1.15, 8.02) of having a CRP level in adulthood that indicates high risk of cardiovascular disease.
Conclusions
Social adversity during the prenatal period is a risk factor for elevated inflammation in adulthood independent of adversities during childhood. This evidence is consistent with studies demonstrating that adverse exposures in the maternal environment during gestation have lasting effects on development of the immune system. If these results reflect causal associations, they suggest that interventions to improve the social and environmental conditions of pregnancy would promote health over the life course. It remains necessary to identify the mechanisms that link maternal conditions during pregnancy to the development of fetal immune and other systems involved in adaptation to environmental stressors.
Keywords: C-reactive protein, inflammation, prenatal exposure, childhood adversity, adult, stress, cohort study, longitudinal
Children exposed to adversity carry a greater risk of poor physical and mental health into adulthood; in part this increased risk is due to up-regulated inflammatory processes associated with early adversity that contribute to the etiology of many adult illnesses (Johnson et al., 2013; Miller et al., 2011). The current study asks: is adversity during the prenatal associated with inflammation in adulthood, and independent of that association, does adversity during childhood contribute additional risk for adult inflammation? Identifying developmental periods when adversity influences the development of stress response systems would provide information regarding the timing of interventions designed to mitigate the long-term harms of early adversity (Bornstein, 1989; Fox et al., 2010).
Most studies that have examined early life adversity in relation to adult inflammation have considered adversity occurring only during a single period. One small retrospective study (n=62) found an association between maternal prenatal stress (i.e., major negative life events during pregnancy) and altered cytokine production in response to antigen stimulation in their offspring in adulthood, adjusting for childhood stressors (Entringer et al., 2008a). Several larger studies show that abuse during childhood (e.g., between ages of 0 to 10 years (Danese et al., 2007)) and adolescence (e.g.,, between 11 to 17 years (Bertone-Johnson et al., 2012)) is associated with increased circulating inflammatory markers in adulthood. However, prior prospective studies have not examined the independent associations of prenatal and childhood social adversity on levels of inflammation in adulthood.
Inflammation is a common pathway to multiple diseases (Bosch et al., 2012; Mathilda Chiu et al., 2012; Ziol-Guest et al., 2009), and the acute phase reactant C-reactive protein (CRP) functions as a marker of systemic inflammation (Ridker et al., 2002). CRP is mainly produced in the liver (Libby et al., 2002), and rises in response to inflammatory cytokines that that are typically modulated by infection or injury (Ridker et al., 2002). Serum concentration of CRP is an independent predictor of future cardiovascular, metabolic, and psychiatric diseases (Danesh et al., 2004; Howren et al., 2009; Ridker, 2003; Ridker, 2007). The long-term stability of CRP levels in adulthood is similar to that of blood pressure and total serum cholesterol (Danesh et al., 2004).
Several mechanisms are thought to link prenatal adversity to inflammation later in life. First, maternal experiences of adversity could contribute to obstetric complications that are associated with elevated inflammation in the mother during pregnancy (Coussons-Read et al., 2007); these biological alterations can affect the developing immune and cardiovascular systems in the fetus in a number of ways (e.g., epigenetic changes, preterm birth) that put the fetus at risk for heightened inflammation later in life (Rogers and Velten, 2011). Second, maternal hyper-secretion of cortisol can influence development of the fetal hypothalamic-pituitary-adrenal (HPA) axis and immune functioning (Entringer et al., 2009; Entringer et al., 2008a; O’Donnell et al., 2009), particularly because glucocorticoids can cross the placenta (Gitau et al., 1998; Seckl and Meaney, 2004). The resulting increased activation of the HPA axis in the offspring induces a cascade of elevated levels of cortisol, followed by decreased responsiveness of immune cells to glucocorticoid signaling (that typically functions to down-regulate inflammatory processes), ultimately resulting in an increase in circulating markers of inflammation (Miller et al., 2009; Miller et al., 2011).
A number of mechanisms could explain why childhood adversity might lead to inflammation in adulthood, including dysregulated HPA-axis activity (as described above), emotional (Appleton et al., 2011) or behavioral (Slopen et al., 2013) problems in childhood, psychosocial functioning in adulthood (Taylor et al., 2006), increased body mass index (Slopen et al., 2011), greater susceptibility to infection (Dowd et al., 2009), and poorer health behaviors including poor diet, cigarette smoking, low physical activity, and excess alcohol consumption (O'Connor et al., 2009). Because of the plausibility of both of these sets of mechanisms, we hypothesize that adversity during the prenatal and childhood periods have independent effects on inflammation in adulthood. We use data from the New England Family Study, a multi-generation cohort study, to test this hypothesis.
METHODS
Sample
Participants were offspring of pregnant women enrolled between 1959 and 1966 in the Collaborative Perinatal Project (CPP) (Broman et al., 1975; Niswander and Gordon, 1972). At the time of enrollment, expectant mothers provided comprehensive data on health, behavior, and demographic characteristics; subsequent information on mothers and their offspring was collected at birth and periodically through the child’s first 7 years. The New England Family Study (NEFS) was initiated to locate and interview samples of the adult offspring at the Providence, Rhode Island, and Boston, Massachusetts sites (N=17,921 live births).
The sample for the present analysis comes from a study conducted between 2005 to 2007 on the pathways linking educational attainment and health. As reported previously, 618 NEFS participants (Gilman et al., 2008) were selected for a re-interview study that covered socioeconomic, psychological, and health histories as well as a clinical assessment (Loucks et al., 2012), including a blood draw in which 430 individuals participated (Appleton et al., 2011; Appleton et al., 2012). Respondents with CRP levels above 10 mg/L were removed from the sample (n=16) given that values greater than 10 can indicate infection or acute illness (Pearson et al., 2003). After we removed individuals with CRP values above 10 mg/L or with missing information on other covariates (n=59), there were 355 individuals in our analytic sample. Institutional review boards at Harvard and Brown Universities approved the study protocol, and written informed consent was obtained from participants at the time of interview.
Measures
Social Adversity
We created composite scores of social adversity prenatally and during early childhood (Evans et al., 2013; Sameroff, 1998). This approach accounts for the clustering of risk factors together, and allows for the examination of the inflammatory consequences of exposure to multiple forms of adversity at each time point. Consistent with prior research that has compared advantaged and disadvantaged groups of children into adulthood, we collapsed the prenatal and childhood adversity scores into 3-category variables (corresponding to low, medium, and high adversity) with roughly similar distributions for the prenatal and childhood scores. This allowed us to examine levels of inflammation across the spectrum of exposure to childhood adversity, making comparisons between the low and medium categories to the highest category, in a way that balances efficiency without imposing an assumption of linearity.
Prenatal adversity was measured using a composite score based on information collected during prenatal visits about family structure, parental education, parental occupation, and family income. Parental education was categorized as less than high school, high school, and above high school; parental occupation was categorized as unemployed (including a small number of full-time students), manual, and non-manual; economic risk was categorized as high (income-to-poverty ratio < 1), medium (income-to-poverty ratio of 1–1.5), and low (income-to-poverty ratio ≥ 1.5) (consistent with commonly-used thresholds used to define “poor” and “nearly poor” (Freeman and Corey, 1993)); and family structure was categorized as divorced/separated/widowed, single parent, or both parents present in the household. Our categorization of family structure was based on literature showing that conflict (related to divorce/separation) or tragedy (widowhood) would be more adverse than single status. Each component of adversity was scored from 0 to 2, with higher values reflecting greater disadvantage. The values for each component were then summed to create the prenatal adversity score: a score of 0 to 1 was categorized as low adversity, 2 to 3 was categorized as medium adversity, and 4 or above was categorized as high adversity.
Childhood adversity was measured using a composite score created from information on 8 characteristics of the respondents’ social environment recorded at the age 7 follow-up assessment. Categorical variables were constructed, and higher scores reflect greater disadvantage. Economic risk was categorized as high (income-to-poverty ratio < 1), medium (income-to-poverty ratio of 1–1.5), and low (income-to-poverty ratio > 1.5). Parental occupation was categorized as non-manual and manual. Changes in parental marital status between birth and age 7 were categorized as no marital status changes, 1 marital status change, and 2 or more marital status changes. Residential moves were categorized as 0 or 1 move, 2 moves, and 3 or more moves. Major shifts in the family environment (i.e., changes lasting more than 1 month in the persons responsible for the child) between birth and age 7 were categorized as none and one or more. Death of a sibling between birth and age 7 was categorized as no death of a sibling and death of a sibling. Father’s unemployment in the past year at age 7 assessment was categorized as father employed, father unemployed 1–12 weeks, and father unemployed 13+ weeks. Crowded housing at age 7 was categorized as housing density < 1 person per room, 1–1.5 persons per room, and >1.5 persons per room. The indicators of each type of childhood adversity were then summed to create the childhood adversity score: a score of 0 to 2 was categorized as low adversity, a score of 3 to 6 was categorized as medium adversity, and a score of 7 or above was categorized as high adversity.
C-Reactive Protein (CRP)
Serum concentration of CRP was analyzed using a high-sensitivity immunoturbidimetric assay on a Hitachi 917 analyzer (Roche Diagnostics, Indianapolis, IN), with reagents and calibrators from DiaSorin (Stillwater, MN). The assay had a sensitivity of 0.03 mg/L, and day-today variabilities of the assays at concentrations of 0.91, 3.07, and 13.38 mg/L were 2.81%, 1.61%, and 1.1%, respectively. Our primary analyses treated CRP as a continuous variable; this variable was log10-tranformed due to the skewed distribution. To evaluate clinical relevance, we also examined CRP as a dichotomous variable according to the Centers for Disease Control and Prevention /American Heart Association threshold for cardiovascular risk (>3 mg/L) (Pearson et al., 2003).
Covariates
Confounders of the association between prenatal and childhood adversity and inflammation in adulthood include maternal conditions that could act as prior common causes. Binary indicators for the following maternal self-reported conditions were assessed in the CPP upon mother’s enrollment into the study and were adjusted for in all of the analyses: A) lifetime history of psychiatric disorders (e.g. mental retardation, psychosis and neurosis, alcoholism, drug addiction); B) neurologic disorders (e.g. epilepsy, central nervous system infection and tumor, neurologic surgery, cerebral palsy); C) cardiovascular disorders (e.g. rheumatic fever, thrombophlebitis, anemia, cardiovascular surgery, pericarditis); D) pulmonary disorders (e.g. tuberculosis, pneumonia, bronchial asthma, pulmonary surgery, pulmonary embolism, sarcoidosis); and E) metabolic disorders (e.g. diabetes, thyroid disorders, endocrine surgery). In addition we included the following factors as control variables: maternal age at child’s birth, offspring age at follow-up interview (years), sex (male/female), and self-reported race (white/non-white).
Analyses
Regression analyses were used to examine associations of prenatal and childhood adversity with CRP in adulthood. First, we conducted a series of linear regression models that evaluated associations between prenatal and childhood adversity and CRP, with scores for prenatal and childhood adversity included individually and together in the same model. If both prenatal and childhood adversity are associated with CRP independent of each other, coefficients for both prenatal and childhood adversity should maintain significant associations with CRP in the model that includes both adversity scores (Baron and Kenny, 1986; Ben-Shlomo and Kuh, 2002). We present models with and without adjustment for child and maternal characteristics (child sex, race/ethnicity, and age; mother’s age at child’s birth, and pre-existing maternal health conditions reported during pregnancy).
Second, we assessed whether associations of prenatal and childhood adversity with CRP are constant across the distribution of CRP by fitting quantile regression models. Quantile regression can provide insights into possible variations in the associations between adversity and inflammation across segments of the distribution of CRP; this is more likely to be the case for non-normal dependent variables such as CRP, the distributions of which are often not fully captured by shifts in the mean. Quantile regression coefficients contrast the qth quantile (e.g., the 50th percentile, or median) of CRP across levels of childhood adversity (Glymour et al., 2012; Koenker and Bassett, 1978; Liu et al., 2012; Rehkopf, 2012). If adversity affects one part of the CRP distribution more than another part of the distribution, this will be reflected by systematic differences in quantile regression estimates across the distribution of CRP.
Third, we examined associations between prenatal and childhood adversity and clinically significant CRP values (> 3 mg/L) using logistic regression. In supplementary analyses, we examined the four component indicators of the prenatal adversity score separately, and we tested for a potential interaction between prenatal- and childhood adversity. Analyses were performed using SAS v. 9.2. Variance estimates were adjusted for the presence of sibling sets in the analysis sample using generalized estimating equations.
RESULTS
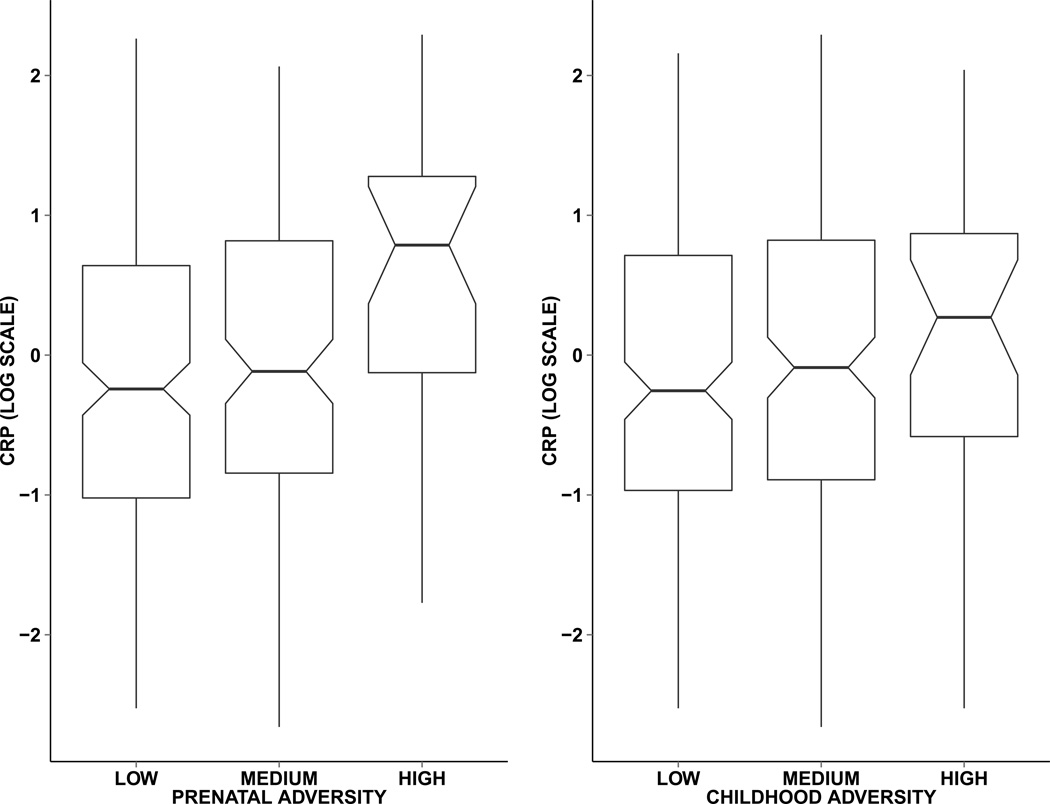
Characteristics of the sample are presented in Table 1. The sample had more females (57.75%) than males, and was predominantly white (80.66%). Ages at the adult follow-up ranged from 39 to 47 (mean ± SD, 42.07 ± 1.70). Approximately 37 percent of the sample was in the medium prenatal adversity category, and 8 percent of the sample was in the high prenatal adversity category. Considering childhood adversity, 44 percent of the sample was in the medium adversity category, and 9 percent was in the high adversity category. The prenatal and childhood adversity scores were moderately correlated with one another (r for the continuous adversity scores= 0.47). In the supplementary materials we present frequency tables of the individual components of the prenatal (Supplemental Table 1) and childhood (Supplemental Table 2) adversity measures, and the cross-tabulation of the prenatal score against the childhood score (Supplemental Table 3), which indicates the extent of transitions across prenatal and childhood adversity categories. CRP values ranged from 0.07 to 9.89 (mean ± SD, 1.69 ± 1.98), and 16.62 percent of the sample had CRP values above the threshold for clinical risk. Figure 1 presents the distributions of CRP by prenatal and childhood adversity categories.
Table 1.
Sample characteristics (N=355)
| N | % | Mean (SD) | |
|---|---|---|---|
| Prenatal adversity | |||
| Low | 196 | 55.21 | |
| Medium | 131 | 36.90 | |
| High | 28 | 7.89 | |
| Childhood adversity (%) | |||
| Low | 168 | 47.32 | |
| Medium | 156 | 43.94 | |
| High | 31 | 8.73 | |
| CRP (mean) | 355 | 1.69 (1.98) | |
| CRP > 3 | 59 | 16.62 | |
| Child’s sex (female) | 205 | 57.75 | |
| Child’s ethnicity (non-white) | 69 | 19.44 | |
| Child’s age | 42.07 (1.70) | ||
| Maternal age at child’s birth | 355 | 25.33 (5.31) | |
| Pre-existing maternal health conditions at pregnancy |
|||
| Psychiatric | 36 | 10.14 | |
| Neurologic | 20 | 5.63 | |
| Cardiovascular | 12 | 3.38 | |
| Pulmonary | 21 | 5.92 | |
| Metabolic | 18 | 5.07 | |
Abbreviations: SD=standard deviation; CRP=C-reactive protein.
Figure 1.
Box plots of C-reactive protein (CRP, log scale) by prenatal and childhood adversity categories. Central line: median; boxes: 25th to 75th percentiles; diagonal notches: 95% confidence intervals around the median. Prevalence of clinically relevant high CRP (>3 mg/L) follows the same patterning. Percent of respondents with high CRP across prenatal adversity categories: low, 13.27% (26/196 respondents); medium, 18.32% (24/131 respondents); high, 32.14% (9/28 respondents). Percent of respondents with high CRP across childhood adversity categories: low, 14.29% (24/168 respondents); medium, 18.59% (29/156 respondents); high, 19.35% (6/31 respondents).
As shown in Table 2, high prenatal adversity (Model 1) and high childhood adversity (Model 2) were associated with elevated CRP in bivariate associations (β=0.79, 95% confidence interval (CI): 0.35, 1.23, and β=0.43, 95% CI: 0.002, 0.85, respectively), and these associations were maintained in models that adjusted for participant sex, age, race, and pre-existing maternal health conditions (Models 3 and 4). In a model that included both prenatal and childhood adversity (Model 5), the regression coefficients for the associations of prenatal and childhood adversity with adult CRP were not substantially changed, but only the coefficient for high prenatal adversity was significantly different from zero (β=0.73, 95% CI: 0.26, 1.20).
Table 2.
Associations between prenatal and childhood adversity and log CRP (mg/L) (N=355)
| Model 1 β (95% CI) |
Model 2 β (95% CI) |
Model 3 β (95% CI) |
Model 4 β (95% CI) |
Model 5 β (95% CI) |
|
|---|---|---|---|---|---|
| Prenatal adversity | |||||
| High | 0.79 (0.35, 1.23) | 0.77 (0.30, 1.23) | 0.73 (0.26, 1.20) | ||
| Medium | 0.16 (−0.10, 0.41) | 0.16 (−0.11, 0.42) | 0.08 (−0.19, 0.36) | ||
| Low (reference) | Reference | Reference | Reference | ||
| Childhood adversity | |||||
| High | 0.43 (0.002, 0.85) | 0.45 (0.003, 0.90) | 0.40 (−0.05, 0.85) | ||
| Medium | 0.17 (−0.07, 0.41) | 0.15 (−0.09, 0.39) | 0.07 (−0.18, 0.32) | ||
| Low (reference) | Reference | Reference | Reference | ||
| Child covariates | |||||
| Male | 0.19 (−0.05, 0.43) | 0.22 (−0.03, 0.46) | 0.20 (−0.04, 0.44) | ||
| White | −0.13 (−0.44, 0.19) | −0.21 (−0.53, 0.11) | −0.11 (−0.43, 0.22) | ||
| Age | 0.04 (−0.04, 0.11) | 0.03 (−0.05, 0.10) | 0.04 (−0.03, 0.12) | ||
| Maternal covariates | |||||
| Age at child’s birth |
−0.01 (−0.04, 0.01) | −0.01 (−0.03, 0.01) | −0.01 (−0.03, 0.01) | ||
| Pre-existing maternal health conditions |
|||||
| Psychiatric | −0.02 (−0.42, 0.39) | 0.03 (−0.39, 0.44) | −0.05 (−0.46, 0.35) | ||
| Neurologic | −0.08 (−0.64, 0.47) | −0.02 (−0.59, 0.55) | −0.07 (−0.63, 0.49) | ||
| Cardiovascular | 0.56 (−0.03, 1.14) | 0.61 (−0.01, 1.23) | 0.59 (0.002, 1.19) | ||
| Pulmonary | −0.12 (−0.59, 0.35) | −0.21 (−0.68, 0.26) | −0.18 (−0.66, 0.31) | ||
| Metabolic | −0.06 (−0.63, 0.51) | −0.07 (−0.62, 0.48) | −0.11 (−0.65, 0.44) | ||
Note: coefficients were estimated using linear regression models that account for clustering within families; CI=confidence interval.
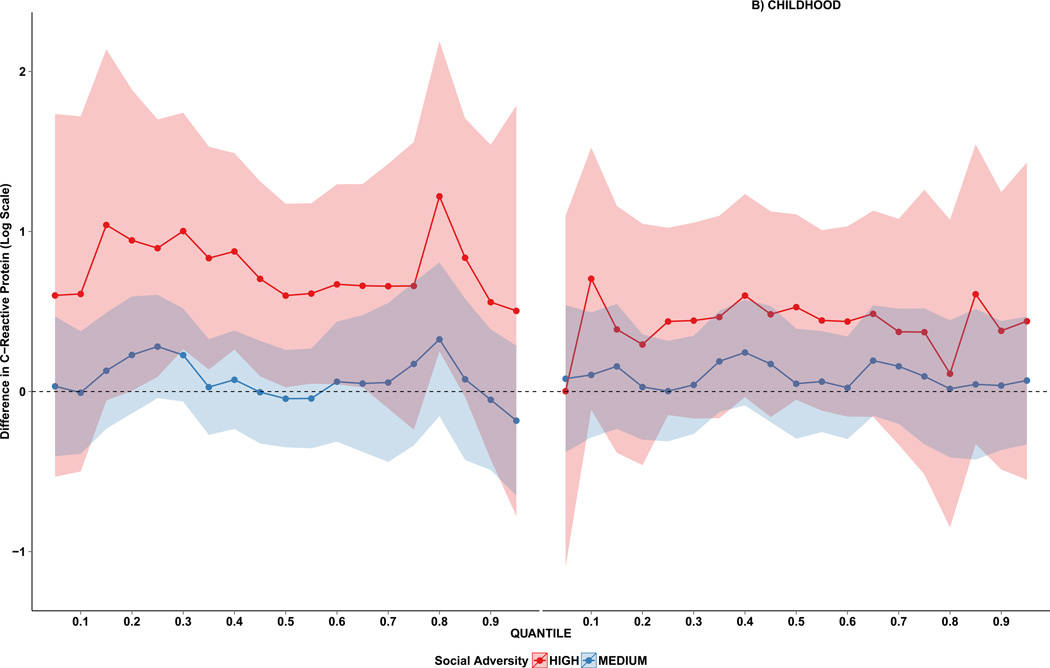
Table 3 presents quantile regression coefficients for deciles between the 10th to the 90th quantiles, adjusting for all covariates. The plotted values in Figure 2 contain the full set of quantile regression coefficients for the effects of medium and high prenatal (Panel A) and childhood (Panel B) adversity on CRP. Panel A illustrates that high prenatal adversity shifted the entire distribution of CRP upwards, particularly in the center of the distribution of CRP; the coefficients did not vary significantly across quantiles (χ2=41.3, df=36, p=0.25). Consistent with the linear regression analyses, there was no significant association between childhood adversity and CRP (Panel B).
Table 3.
Quantile regression coefficients for the associations between prenatal and childhood adversity and log CRP (mg/L) (N=355)
| Quantiles | |||||||
|---|---|---|---|---|---|---|---|
| 10th | 30th | 40th | 50th | 60th | 70th | 90th | |
|
Prenatal Adversity |
β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) |
| High | 0.61 (−0.50, 1.72) | 1.00 (0.26, 1.74) | 0.89 (0.26, 1.49) | 0.60 (0.03, 1.17) | 0.67 (0.05, 1.30) | 0.66 (−0.11, 1.42) | 0.56 (−0.42, 1.54) |
| Medium | −0.01 (−0.39, 0.38) | 0.23 (−0.06, 0.52) | 0.07 (−0.23, 0.38) | −0.04 (−0.35, 0.26) | 0.06 (−0.31, 0.44) | 0.06 (−0.44, 0.55) | −0.05 (−0.49, 0.39) |
| Low | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
|
Childhood Adversity |
|||||||
| High | 0.71 (−0.11, 1.52) | 0.44 (−0.17, 1.06) | 0.60 (−0.03, 1.23) | 0.53 (−0.05, 1.11) | 0.44 (−0.16, 1.03) | 0.37 (−0.33, 1.08) | 0.38 (−0.49, 1.25) |
| Medium | 0.10 (−0.29, 0.49) | 0.04 (−0.26, 0.35) | 0.24 (−0.09, 0.57) | 0.05 (0.29, 0.39) | 0.02 (−0.30, 0.34) | 0.16 (−0.20, 0.52) | 0.04 (−0.37, 0.44) |
| Low | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
Note: coefficients were estimated using quantile regression models that account for clustering within families; SE=standard error. Models were adjusted for participant sex, race, age, and mother’s age at child’s birth and pre-existing health conditions at time of pregnancy (psychiatric, neurological, cardiovascular, pulmonary, and metabolic). CI=confidence interval. Chi-square for difference in coefficients across quantiles=41.29 (df=36), p=0.25.
Figure 2.
Prenatal and childhood adversity quantile regression coefficients from the 10th to 90th percentiles for CRP. The red dotted lines and shaded area (95% confidence intervals) show differences in log CRP (mg/L) between high and low adversity; the blue dotted lines and shaded area show differences in log CRP (mg/L) between medium and low adversity. The lower quantiles correspond to the lower CRP values. Regression coefficients were generated from a single model that included variables for prenatal and childhood adversity, as well as participant age, sex, and race, and maternal age at child’s birth and pre-existing health conditions reported during pregnancy.
Analyses examining associations of prenatal and childhood adversity with clinically elevated CRP values (Table 4) showed that high prenatal adversity was associated with high-risk CRP in bivariate (Model 1) and adjusted (Model 3) models (adjusted odds ratio (OR)=3.18, 95% CI=1.22, 8.27). In contrast, high childhood adversity was not significantly associated with high-risk CRP in bivariate (Model 2) or adjusted (Model 4) models, although the odds ratios for medium and high childhood adversity were >1 (e.g., adjusted OR for high childhood adversity=1.50, 95% CI=0.51, 4.45). When both prenatal and childhood adversity were modeled together (Model 5), high prenatal adversity continued to be significantly associated with high-risk CRP (OR=3.03, 95% CI: 1.15, 8.02), and the effect estimate for high childhood adversity was somewhat attenuated (OR=1.34, 95% CI: 0.45, 4.06).
Table 4.
Associations between prenatal and childhood adversity and CRP > 3 mg/L (N=355)
| Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
Model 4 OR (95% CI) |
Model 5 OR (95% CI) |
|
|---|---|---|---|---|---|
| Prenatal adversity | |||||
| High | 3.10 (1.25, 7.68) | 3.18 (1.22, 8.27) | 3.03 (1.15, 8.02) | ||
| Medium | 1.47 (0.79, 2.73) | 1.36 (0.71, 2.64) | 1.28 (0.64, 2.53) | ||
| Low | 1.00 | 1.00 | 1.00 | ||
| Childhood adversity | |||||
| High | 1.44 (0.53, 3.93) | 1.50 (0.51, 4.45) | 1.34 (0.45, 4.06) | ||
| Medium | 1.37 (0.76, 2.46) | 1.32 (0.72, 2.42) | 1.12 (0.59, 2.14) | ||
| Low | 1.00 | 1.00 | 1.00 | ||
| Child covariates | |||||
| Male | 0.79 (0.43, 1.44) | 0.82 (0.45, 1.50) | 0.79 (0.43, 1.45) | ||
| White | 0.63 (0.31, 1.25) | 0.53 (0.27, 1.03) | 0.64 (0.32, 1.27) | ||
| Age | 1.04 (0.87, 1.25) | 1.01 (0.84, 1.22) | 1.05 (0.87, 1.26) | ||
| Maternal covariates | |||||
| Age at child’s birth | 1.02 (0.97, 1.07) | 1.02 (0.97, 1.08) | 1.02 (0.97, 1.08) | ||
| Pre-existing maternal health conditions |
|||||
| Psychiatric | 0.63 (0.23, 1.70) | 0.71 (0.25, 1.98) | 0.61 (0.22, 1.66) | ||
| Neurologic | 0.75 (0.16, 3.50) | 0.80 (0.18, 3.50) | 0.76 (0.16, 3.56) | ||
| Cardiovascular | 1.59 (0.43, 5.83) | 1.68 (0.45, 6.24) | 1.64 (0.44, 6.10) | ||
| Pulmonary | 0.82 (0.22, 3.03) | 0.73 (0.19, 2.89) | 0.78 (0.20, 3.07) | ||
| Metabolic | 0.56 (0.10, 3.17) | 0.58 (0.13, 2.48) | 0.53 (0.10, 2.77) | ||
Note: coefficients were estimated using logistic regression models that account for clustering within families. OR=odds ratio; CI=confidence interval.
In supplemental analyses, we fitted 4 linear regression models that analyzed the components of the prenatal adversity score separately. The strongest associations with CRP levels involved highest-risk categories of parental education and family structure, and to a lesser degree, parental occupation (Supplemental Table 4). However, the magnitudes of the coefficients for each adversity indicator individually were smaller than the estimate for the composite adversity score. Finally, using a linear regression model that tested for interactive effects of prenatal and childhood adversity, we did not find evidence for an interaction (p=0.35).
DISCUSSION
We sought to investigate the independent associations of prenatal and childhood adversity with adulthood inflammation, consistent with a life course accumulation model. When prenatal and childhood adversity were considered separately, each was associated with higher levels of inflammation in adulthood after adjustment for individual characteristics (age, sex, race) and other potential confounders including pre-existing maternal health conditions. When we analyzed the effects of prenatal and childhood adversity together, prenatal adversity predicted inflammation in adulthood independently from childhood adversity. Moreover, elevation of inflammatory risk was clinically significant: prenatal adversity was associated with over a 3-fold elevated odds of having CRP concentrations at or above the CDC/American Heart Association’s cut point for being at high-risk of cardiovascular disease. Finally, quantile regressions revealed that prenatal adversity shifted virtually the entire CRP distribution upwards. Exposure to adversity during gestation may therefore have long-term effects on immune function in adulthood, effects which are not contingent on social adversity in childhood.
The following limitations are important to consider. First, the composite scores of social adversity that we analyzed did not cover extreme forms of adversity (e.g., domestic violence, child maltreatment or neglect) that have been previously shown to predict elevated inflammation (Danese et al., 2007; Entringer et al., 2008a); as a result, our analyses may have underestimated the relative importance of prenatal or childhood as a sensitive periods for exposure to social adversity. That said, it is well documented that extreme forms of adversity are more likely to occur in the presence of characteristics such as those included in our composite measures (Brown et al., 1998; Sidebotham et al., 2002). Second, the prenatal adversities that we consider are not discrete time-limited events to which exposure is bounded by the prenatal period; rather, they reflect social and economic conditions that have the potential to change or endure. This limitation has several implications. From the developing child’s perspective, social adversity may not be defined in the same way across all periods of development. This poses a challenge for evaluating sensitive period effects, because differences in the associations of prenatal and childhood adversity with adult inflammation could also be due to measurement differences. Third, the current models may not be fully adequate to capture the pathophysiologic consequences of adverse environments if such consequences depend on the timing of exposure, magnitude of exposure, as well as the duration of exposure. Fourth, we assessed only a single marker of inflammation in adulthood. We cannot determine if elevations in CRP among adults exposed to childhood adversity reflect effects on a specific immune pathway, immune pathways with which CRP is correlated, or more generalized effects on immune functioning.
This study contributes to increasing empirical evidence from animal and human studies that suggest intrauterine exposures influence susceptibility for a variety of common chronic diseases. Stress-related maternal-placental-fetal endocrine and immune processes may mediate the effects of social adversity during gestation on offspring health, because affected hormones and cytokines are integral to key developmental events including cellular growth, replication, hand differentiation, and thus can alter structure and function of the brain and peripheral biology (Entringer, 2013; Entringer et al., 2010). Obstetric complications (e.g., hyperglycemia, pregnancy-induced hypertension, gestational diabetes, infection, reduced utero-placental blood flow), under- or over-nutrition, diet composition, and unhealthy behaviors during pregnancy (e.g., cigarette smoking) (Entringer et al., 2010), which are more common in disadvantaged pregnancies, may also influence the immune system of the fetus in a manner that promotes susceptibility to inflammation later in life.
Although the results of this study support the importance of social conditions during the prenatal period (and that may endure through childhood) as relevant to inflammation in adulthood, they do not exclude the role that other—potentially related—postnatal experiences have on inflammatory pathways. In this regard, Appleton et al. examined the role of children’s emotional functioning on adult CRP levels in the NEFS cohort, in combination with childhood socioeconomic disadvantage, and reported that socioeconomically deprived children exhibiting poor emotional functioning had the highest levels of CRP in adulthood (Appleton et al., 2012). Viewed together, these studies suggest that the socioeconomic conditions of infancy studied here have direct, lasting effects on inflammatory pathways, and may also influence the psychological or behavioral factors that activate mechanisms underlying inflammation. Other post-natal mechanisms that could explain our results and should be explored in future studies include metabolic function and obesity in childhood, and health status (e.g., body mass index, blood pressure) and mental health in adulthood.
Our findings support elevated CRP as a potential biological pathway for documented associations between prenatal environment and later risk for chronic diseases (Gluckman et al., 2007; Roseboom et al., 2006). However, this is unlikely to be the only pathway to elevated chronic disease risk. For example, several small retrospective studies show that prenatal stress exposure was a significant predictor of telomere length (Entringer et al., 2011) and alterations in glucose-insulin metabolic function (Entringer et al., 2008b) in young adult offspring, independent of childhood events and concurrent stress. Other research from a large population-based cohort shows that maternal bereavement one year before conception until birth is associated risk factors for adult chronic diseases, including overweight (Li et al., 2010) and type-2 diabetes (Li et al., 2012) in children.
Our findings align with the results of prior studies showing that adverse exposures during the prenatal period and early infancy have effects independent of subsequent adversity, in samples of children (Mathilda Chiu et al., 2012), adolescents (Bosch et al., 2012), and adults (Entringer et al., 2011; Entringer et al., 2008a; Entringer et al., 2008b; Ziol-Guest et al., 2009). Some of this work suggests that gestation or early childhood is a “sensitive period” for the effects on social adversity on later health. For example, in a nationally representative prospective study, family income during the prenatal and first year of life was associated with greater body mass index in adulthood, whereas no association was observed for household income between 1 to 5 years and 6 to 10 years of age (Ziol-Guest et al., 2009).
In conclusion, using a prospective population-based birth cohort that has reached middle adulthood, high prenatal adversity predicted elevated inflammation, as indicated by CRP. Our results suggest that social adversity during the prenatal period may influence the development of stress response systems independent of later experiences in early childhood. Future studies of the physiologic consequences of social adversity should therefore search as early in the life course as possible for the developmental origins of adult disease. Mechanisms for the observed associations also need to be discovered; based on existing knowledge, they will exist across multiple domains including social experiences later in childhood, neurological and psychological development, and as well as behavioral pathways that include physical growth trajectories (Appleton et al., 2011; Non, 2014).
Supplementary Material
Highlights.
Prenatal adversity is associated with adult CRP, independent of childhood adversity.
High prenatal adversity was associated with a 3-fold elevated odds of high-risk CRP.
It remains necessary to identify underlying mechanisms for the observed associations.
Acknowledgments
This research was supported by grants from the National Institutes of Health (MH087544, PI: Gilman; AG023397, PI: Buka), the Robert Wood Johnson Foundation, and the W. K. Kellogg Foundation. We appreciate the contributions of Ms. Kathleen McGaffigan, our expert analyst and data manager.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
There has been no financial support for this work that could have influenced its outcome.
References
- Appleton AA, Buka SL, McCormick MC, Koenen KC, Loucks EB, Gilman SE, Kubzansky LD. Emotional Functioning at Age 7 Years is Associated With C-Reactive Protein in Middle Adulthood. Psychosomatic Medicine. 2011;73:295–303. doi: 10.1097/PSY.0b013e31821534f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton AA, Buka SL, McCormick MC, Koenen KC, Loucks EB, Kubzansky LD. The Association Between Childhood Emotional Functioning and Adulthood Inflammation Is Modified by Early-Life Socioeconomic Status. Health Psychology. 2012;31:413–422. doi: 10.1037/a0027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int. J. Epidemiol. 2002;31:285–293. [PubMed] [Google Scholar]
- Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, Rich-Edwards JW. Inflammation and Early-Life Abuse in Women. Am. J. Prev. Med. 2012;43:611–620. doi: 10.1016/j.amepre.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH. Sensitive periods in development: Structural characteristics and causal interpretations. Psychological Bulletin. 1989;105:179–197. doi: 10.1037/0033-2909.105.2.179. [DOI] [PubMed] [Google Scholar]
- Bosch NM, Riese H, Reijneveld SA, Bakker MP, Verhulst FC, Ormel J, Oldehinkel AJ. Timing matters: Long term effects of adversities from prenatal period up to adolescence on adolescents’ cortisol stress response. The TRAILS study. Psychoneuroendocrinology. 2012;37:1439–1447. doi: 10.1016/j.psyneuen.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Broman SH, Nichols PI, Kennedy WA. Preschool IQ: Prenatal and Early Developmental Correlates. New York: Hallstead Press; 1975. [Google Scholar]
- Brown J, Cohen P, Johnson JG, Salzinger S. A longitudinal analysis of risk factors for child maltreatment: findings of a 17-year prospective study of officially recorded and self-reported child abuse and neglect. Child Abuse Negl. 1998;22:1065–1078. doi: 10.1016/s0145-2134(98)00087-8. [DOI] [PubMed] [Google Scholar]
- Cook NR, Buring JE, Ridker PM. The Effect of Including C-Reactive Protein in Cardiovascular Risk Prediction Models for Women. Annals of Internal Medicine. 2006;145:21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain, Behavior, and Immunity. 2007;21:343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GDO, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. New England Journal of Medicine. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Zajacova A, Aiello A. Early origins of health disparities: Burden of infection, health, and socioeconomic status in US children. Social Science & Medicine. 2009;68:699–707. doi: 10.1016/j.socscimed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S. Impact of stress and stress physiology during pregnancy on child metabolic function and obesity risk. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:320–327. doi: 10.1097/MCO.0b013e32835e8d80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr. Opin. Endocrinol. Diabetes Obes. 2010;17:507–516. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, Wüst S, Wadhwa PD. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proceedings of the National Academy of Sciences. 2011;108:E513–E518. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wüst S. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Hormones and Behavior. 2009;55:292–298. doi: 10.1016/j.yhbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Entringer S, Kumsta R, Nelson EL, Hellhammer DH, Wadhwa PD, Wüst S. Influence of prenatal psychosocial stress on cytokine production in adult women. Developmental Psychobiology. 2008a;50:579–587. doi: 10.1002/dev.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Wüst S, Kumsta R, Layes IM, Nelson EL, Hellhammer DH, Wadhwa PD. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. Am. J. Obstet. Gynecol. 2008b;199:498.e491–498.e497. doi: 10.1016/j.ajog.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Li D, Sepanski Whipple S. Cumulative Risk and Child Development. Psychological Bulletin. 2013 doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- Fox SE, Levitt P, Nelson CA. How the Timing and Quality of Early Experiences Influence the Development of Brain Architecture. Child Development. 2010;81:28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman HE, Corey CR. Insurance status and access to health-services among poor persons. Health Serv. Res. 1993;28:531–541. [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Martin LT, Abrams DB, Kawachi I, Kubzansky L, Loucks EB, Rende R, Rudd R, Buka SL. Educational attainment and cigarette smoking: a causal association? Int. J. Epidemiol. 2008;37:615–624. doi: 10.1093/ije/dym250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. The Lancet. 1998;352:707–708. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: A life history and evolutionary perspective. American Journal of Human Biology. 2007;19:1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- Glymour MM, Tzourio C, Dufouil C. Is Cognitive Aging Predicted by One’s Own or One’s Parents’ Educational Level? Results From the Three-City Study. American Journal of Epidemiology. 2012;175:750–759. doi: 10.1093/aje/kwr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic Medicine. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Johnson SB, Riley AW, Granger DA, Riis J. The Science of Early Life Toxic Stress for Pediatric Practice and Advocacy. Pediatrics. 2013;131:319–327. doi: 10.1542/peds.2012-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juonala M, Viikari JSA, Ronnemaa T, Taittonen L, Marniemi J, Raitakari OT. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood - The Cardiovascular Risk in Young Finns Study. Arteriosclerosis Thrombosis and Vascular Biology. 2006;26:1883–1888. doi: 10.1161/01.ATV.0000228818.11968.7a. [DOI] [PubMed] [Google Scholar]
- Koenker R, Bassett G., Jr. Regression Quantiles. Econometrica. 1978;46:33–50. [Google Scholar]
- Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. Journal of Epidemiology and Community Health. 2003;57:778–783. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Olsen J, Vestergaard M, Obel C, Baker JL, Sørensen TIA. Prenatal Stress Exposure Related to Maternal Bereavement and Risk of Childhood Overweight. Plos One. 2010;5:e11896. doi: 10.1371/journal.pone.0011896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Olsen J, Vestergaard M, Obel C, Kristensen JK, Virk J. Prenatal Exposure to Bereavement and Type-2 Diabetes: A Danish Longitudinal Population Based Study. Plos One. 2012;7:e43508. doi: 10.1371/journal.pone.0043508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and Atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Liu SY, Kawachi I, Glymour MM. Education and Inequalities in Risk Scores for Coronary Heart Disease and Body Mass Index Evidence for a Population Strategy. Epidemiology. 2012;23:657–664. doi: 10.1097/EDE.0b013e318261c7cc. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Buka SL, Rogers ML, Liu T, Kawachi I, Kubzansky LD, Martin LT, Gilman SE. Education and Coronary Heart Disease Risk Associations May be Affected by Early-Life Common Prior Causes: A Propensity Matching Analysis. Annals of Epidemiology. 2012;22:221–232. doi: 10.1016/j.annepidem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathilda Chiu Y-H, Coull BA, Cohen S, Wooley A, Wright RJ. Prenatal and Postnatal Maternal Stress and Wheeze in Urban Children. American Journal of Respiratory and Critical Care Medicine. 2012;186:147–154. doi: 10.1164/rccm.201201-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Annu Rev Psychol. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswander KR, Gordon M. The Women and Their Pregnancies. Washington, DC: U.S. Government Printing Office; 1972. [Google Scholar]
- Non AL, Rewak M, Kawachi I, Gilman SE, Loucks EB, Appleton AA, Roman JC, Buka SL, Kubzansky LD. Childhood social disadvantage, cardiometabolic risk, and chronic disease in adulthood. American Journal of Epidemiology. 2014;180:263–271. doi: 10.1093/aje/kwu127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behavior and Immunity. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K, O’Connor TG, Glover V. Prenatal Stress and Neurodevelopment of the Child: Focus on the HPA Axis and Role of the Placenta. Developmental Neuroscience. 2009;31:285–292. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease application to clinical and public health practice - A statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Rehkopf DH. Quantile Regression for Hypothesis Testing and Hypothesis Screening at the Dawn of Big Data. Epidemiology. 2012;23:665–667. doi: 10.1097/EDE.0b013e318261f7be. [DOI] [PubMed] [Google Scholar]
- Ridker PM. C-Reactive Protein: A Simple Test to Help Predict Risk of Heart Attack and Stroke. Circulation. 2003;108:e81–e85. doi: 10.1161/01.CIR.0000093381.57779.67. [DOI] [PubMed] [Google Scholar]
- Ridker PM. C-Reactive Protein and the Prediction of Cardiovascular Events Among Those at Intermediate Risk: Moving an Inflammatory Hypothesis Toward Consensus. J. Am. Coll. Cardiol. 2007;49:2129–2138. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. New England Journal of Medicine. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- Rogers LK, Velten M. Maternal inflammation, growth retardation, and preterm birth: Insights into adult cardiovascular disease. Life Sciences. 2011;89:417–421. doi: 10.1016/j.lfs.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 2006;82:485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ. Environmental Risk Factors in Infancy. Pediatrics. 1998;102:1287–1292. [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid Programming. Annals of the New York Academy of Sciences. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Sidebotham P, Heron J, Golding J. Child maltreatment in the “Children of the Nineties:” deprivation, class, and social networks in a UK sample. Child Abuse Negl. 2002;26:1243–1259. doi: 10.1016/s0145-2134(02)00415-5. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, Koenen KC. Childhood adversity and inflammatory and immune biomarkers associated with cardiovascular risk in youth: a systematic review. Brain, Behavior, and Immunity. 2011;26:239–250. doi: 10.1016/j.bbi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, Koenen KC. Internalizing and externalizing behaviors predict elevated inflammatory markers in childhood. Psychoneuroendocrinology. 2013;38:2854–2862. doi: 10.1016/j.psyneuen.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Ziol-Guest KM, Duncan GJ, Kalil A. Early Childhood Poverty and Adult Body Mass Index. American Journal of Public Health. 2009;99:527–532. doi: 10.2105/AJPH.2007.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.