Abstract
Cauliflower mosaic virus gene VI product (P6) is an essential protein that forms cytoplasmic, inclusion bodies (IBs). P6 contains four regions involved in self-association, termed D1–D4. D3 binds to D1, along with D4 and contains a spacer region (termed D3b) between two RNA-binding domains. Here we show D3b binds full-length P6 along with D1 and D4. Full-length P6s harboring single amino acid substitutions within D3b showed reduced binding to both D1 and D4. Full-length P6s containing D3b mutations and fused with green fluorescent protein formed inclusion-like bodies (IL-Bs) when expressed in Nicotiana benthamiana leaves. However, mutant P6s with reduced binding to D1 and D4, showed smaller IL-Bs, than wild type. Likewise, viruses containing these mutations, showed a decrease in inoculated leaf viral DNA levels and reduced efficiency of systemic infection. These data suggest that mutations influencing P6 self-association alter IB formation and reduce virus infection.
Keywords: CaMV, TAV, Gene VI product, Inclusion Bodies
Introduction
Proteins encoded by viral genomes are often multi-functional (Hull, 2002). For example, the plant pararetrovirus Cauliflower mosaic virus (CaMV) encodes a protein, P6 (the product of gene VI), that has been implicated in a variety of functions including: translational transactivation (TAV), host range determination, symptom formation movement, replication, and silencing suppression (Acosta-Leal et al., 2011; Bonneville et al., 1989; Haas et al., 2008; Harries et al., 2009; Kobayashi and Hohn, 2003; Laird et al., 2013; Love et al., 2007; Schoelz et al., 1986; Schoelz and Wintermantel, 1993). Many of these activities are likely mediated via interactions of P6 with viral and host proteins.
Indeed, P6 binds to other CaMV proteins such as P1 (movement protein) and P4 (coat protein) (Hapiak et al., 2008; Himmelbach et al., 1996). Similarly, P6 interacts with a variety of host proteins including large ribosomal subunit proteins RL13, RL18, and RL24 as well as translation factor eIF3g (Bureau et al., 2004; Leh et al., 2000; Park et al., 2001). These interactions may be important for the TAV function of P6. P6 also interacts with CHUP1, a plant protein localized to the outer membrane of chloroplasts that is essential for chloroplast movement on microfilaments in response to changes in light intensity (Angel et al., 2013). The interaction of P6 with CHUP1 likely contributes to intracellular movement of CaMV for delivery of virions to the plasmodesmata (Rodriguez et al., 2014).
P6 also self-associates (Haas et al., 2005; Li and Leisner, 2002) and this interaction involves four regions, termed D1–D4 (Fig. 1), all of which bind to the full-length protein. D1 (amino acids 1-110) is essential for P6 self-association (Haas et al., 2005) and this region can self-associate independent of the rest of P6. D2 (amino acids 156-253) may bind inefficiently to D3 (Li and Leisner, 2002) and contains the minimal TAV domain required for production of CaMV proteins from the polycistronic 35S RNA (De Tapia et al., 1993). D3 (amino acids 249-379) binds efficiently to D1 (amino acids 1-110) and D4 (amino acids 414-520), but not itself (Li and Leisner, 2002). Deletion of gene VI sequences encoding D3 from the CaMV genome resulted in a non-infectious virus.
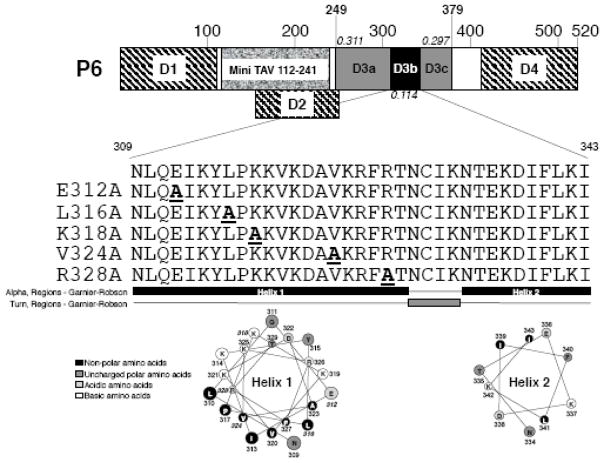
Fig. 1.
Schematic diagram of P6 and location of mutations. The 520 amino acid P6 protein is indicated; large numbers above box indicate amino acids. Hatched areas, P6 regions involved in self-association) (Haas et al., 2005; Li and Leisner, 2002); granular region Mini-TAV region (De Tapia et al., 1993); D1 (amino acids 1-110); D2 (amino acids 156-253); shaded area, D3 (amino acids 249-379); D4 (amino acids 414-520). D3a and D3c are indicated (note both contain RNA-binding domains); black area, D3b examined in this study. Numbers in italics: number of variable amino acid positions within that portion of P6 per amino acid. The amino acid sequence of the D3b region is shown below the P6 cartoon as well as the amino acid changes for the various mutants, single letter amino acid designations are given. The D3b region is likely α-helical (black) with an intervening turn (gray) below the sequences as predicted by Garnier-Robson model in the Protean software contained within the Lasergene Software package. Helical wheels for both helices (predicted by the Protean software package) are indicated below the secondary structure prediction; black, non-polar amino acids; dark gray, uncharged polar amino acids; light gray, acidic amino acids; white, basic amino acids; amino acid numbers are given; bold italic numbers, amino acids mutated.
D3 possesses a tripartite organization: The N-terminal portion (amino acids 249-308; D3a) contains a non-sequence specific RNA-binding domain (De Tapia et al., 1993). This region also contains the binding sites for RL24 and eIF3g (Park et al., 2001). The central portion (amino acids 309-343; termed D3b) contains part of the P4 (capsid protein) binding site (Ryabova et al., 2002) and was proposed to play a role in P6 interactions required for TAV (De Tapia et al., 1993). The C-terminal portion (amino acids 344-379; D3c) contains the remainder of the P4 binding site and another non-sequence specific RNA-binding domain (De Tapia et al., 1993; Ryabova et al., 2002).
In addition to its many functions P6 is also a major constituent of viral-induced inclusion bodies (IBs) (Covey and Hull, 1981; Shockey et al., 1980). Viruses infecting both animal and plant hosts induce the formation of IBs (Knipe, 1990; Martelli and Russo, 1977). Interestingly, viruses may induce formation of more than one type of IB in infected cells, each with different functions. For example, the CaMV genome encodes two proteins that can form different types of IBs: P6 and P2 (Bak et al., 2013; Espinoza et al., 1991). P2, encoded by CaMV gene II, forms electron-lucent IBs that are dynamic, either aggregating or dissociating in response to various stimuli (Armour et al., 1983; Blanc et al., 1993; Espinoza et al., 1991; Khelifa et al., 2007; Martiniere et al., 2013; Woolston et al., 1983). These electron-lucent IBs are essential for insect vectoring of CaMV and have been termed Transmission Bodies (TBs). TBs are not required for viral infection within a plant and strains of CaMV exist that either lack most of the P2 coding sequence or contain gene II mutations resulting in P2 proteins incapable of forming native TBs (Howarth et al., 1981; Khelifa et al., 2007; Nakayashiki et al., 1993). For example, CaMV isolate CM1841 harbors a G94R mutation in gene II that has been shown to cause production of aberrant TBs in other CaMV strains and so may account for the failure of this virus to be aphid-transmissible.
In contrast, the IBs formed by P6 are electron-dense (edIBs) and are required for viral propagation within infected plants (Covey and Hull, 1981; Shockey et al., 1980). P6 edIBs are sites where CaMV protein synthesis, genome replication, and virion assembly are thought to occur (Haas et al., 2002; Hull, 2002; Mazzolini et al., 1989). P6 IBs are also dynamic, releasing virus particles in response to certain stimuli (Bak et al., 2013). P6 may contain several self-association domains (Li and Leisner, 2002) to permit simultaneous interaction with other P6s, permitting aggregation into edIBs. Thus, we predict that mutations affecting the regions of P6 involved in self-association may impair edIB formation and reduce viral infection.
In this paper, single amino acid substitutions were generated within D3b to examine their effects on P6 binding to the self-association domains. These mutations were also tested for their effects on the formation of inclusion–like bodies (I-LBs) and viruses harboring these substitution mutations within the gene VI sequence were tested for their ability to propagate in plants.
Materials and Methods
Secondary Structure Prediction and Calculation of Sequence Variability
The secondary structure of the D3b region of CaMV isolate CM1841 (Gardner et al., 1981) was predicted using the Garnier-Robson model in the Protean software contained within the Lasergene Software package (DNA STAR INC, Madison, WI). Helical wheels were constructed using the same software. Multiple sequence alignment (Supplementary Fig. S1) of the D3 region amino acid sequences for 20 CaMV isolates was performed using the Megalign Program within the Lasergene Software package. The variability of each residue within a segment of D3 was estimated dividing the total number of amino acids that varied by the total number of residues.
Enzymes, Plasmids, Bacteria and Yeast
All restriction enzymes, Taq DNA polymerase, and T4 DNA ligase were purchased from Promega Corporation (Madison, WI) and were used following the manufacturer’s protocol. Pfu DNA Polymerase was purchased from Stratagene (La Jolla, CA). The cloned genome of CaMV isolate CM1841 (pCaMV10) was provided by Dr. Stephen Howell (Iowa State University, Ames, IA). Yeast plasmids pEG202, pJG4-5, and pSH18-34, along with yeast strain EGY48, were gifts from Dr. Roger Brent (Molecular Sciences Institute, Berkeley, CA). Expression vectors pSITE4NB-eIF3g, W260 P6-GFP, P19, along with A. tumefaciens strain AGL-1 are described in Angel et al., (2011) and Angel et al., (2013). The pENTR plasmid was purchased from Invitrogen (Invitrogen, Carlsbad CA). The pSITE plasmids, pSITE-2NB, pSITE-4CA, and pSITE-4NB (Chakrabarty et al., 2007) were purchased from TAIR (The Arabidopsis Information Resource). Yeast media was purchased from Clonetech (Palo Alto, CA). All plasmid constructions made in this manuscript were confirmed by sequencing (DNA Sequencing Core Facility, Ann Arbor, MI).
Gateway cloning
Full-length gene VI lacking a stop codon was PCR amplified from CaMV isolate CM1841 (pCaMV10) (Gardner et al., 1981) using primers GW CaMVP6-1F and GW CaMVP6-2CR (Supplementary Table 1). The PCR product was then inserted into pENTR TOPO-D vector (Invitrogen, Carlsbad CA) following the manufacturer’s protocol. Site-directed mutagenesis was performed on this construct (see below) and the mutant constructs were mobilized into pSITE2NB using Gateway technology LR Clonase II reaction (Invitrogen, Carlsbad CA), following the manufacturer’s protocol. This produced agroinfectious plant vectors capable of expressing full-length wild type or mutant P6s fused at their C-terminus to the N-terminus of green fluorescent protein (GFP).
Site-directed mutagenesis and yeast two-hybrid analyses
Site-directed mutagenesis of D3b was performed using the QuikChange® XL site-directed mutagenesis kit from Stratagene (La Jolla, CA). Single-stranded DNA oligonucleotide primers (synthesized by Integrated DNA Technologies, Inc. Coralville, IA) were designed to individually change each of five pre-determined amino acids to alanines (Supplementary Table 1). Site-directed mutagenesis reactions using the D3 coding region inserted into pJG4-5 (Li and Leisner, 2002) as a template, were performed according to the manufacturer’s specifications in an Eppendorf Mastercycler thermocycler. The D3 fragments harboring the mutations were excised from pJG4-5 by NcoI digestion and ligated into FL-ΔN (Li and Leisner, 2002) reconstituting a full-length gene VI harboring D3b mutations in pJG4-5.
Site-directed mutagenesis of full-length P6 cloned in pENTR was performed using the QuikChange® XL site-directed mutagenesis kit from Stratagene (La Jolla, CA). The primers in Supplementary Table 1 were used to generate the same point mutations within the D3b coding region of full-length gene VI.
Yeast two-hybrid analyses
Yeast transformations were performed according to the LiOAc method (Ausubel et al., 1993; Gyuris et al., 1993). Yeast lines containing pEG202 plasmids harboring gene VI, D1 and D4, as well as pJG4-5 containing full-length gene VI were described previously (Li and Leisner, 2002). Yeast lines harboring pEG202 plasmids were established first and then the pJG4-5 plasmids were introduced. Transformants were evaluated for their ability to grow in the absence of leucine and the β-galactosidase activity of four individual colonies per transformation, was determined (Ausubel et al., 1993; Gyuris et al., 1993).
Cauliflower mosaic virus mutant construction and infection
Each of the pJG4-5 plasmids harboring a single amino acid substitution mutation within gene VI was digested with NcoI and the D3 coding fragments (395 bp) were ligated into that site within pCaMV10-NH-BB-N plasmid (Li and Leisner, 2002). The resulting plasmids were then digested with SacI and SpeI (sites are present within genes VI and VII, respectively) and inserted into those sites within pCaMV10-NcoI-DE, to generate CaMV genomes containing single amino acid substitution mutations within the D3b coding region of gene VI.
To construct a viral genome lacking D3b, a fragment of gene VI (nucleotides 6448-6709) was amplified from pCaMV10 with the primers KL-1F and DI-2R, the PCR product was digested with EcoRI and XhoI and ligated into those sites within pJG4-5, to generate the plasmid pD3-5. A second fragment (pCaMV10 nucleotides 6788-7350) was amplified using the DI-1F and FL-2R PCR primers, digested with XhoI and inserted into that site of pD3-5 to generate the plasmid pD3-53. pD3-53 contains the complete 3′ end (nucleotides 6448-7350) of gene VI but lacking virtually all of the D3b coding region. The pD3-53 plasmid was then digested with NcoI and the 302 bp fragment (encoding D3 lacking D3b) was ligated into that site of the pCaMV10-NH-BB-N. This plasmid was then digested with SacI and SpeI, the 2.3 kbp fragment was inserted into those sites within pCaMV10-NcoI-DE. The resulting plasmid was termed pCaMV10-ΔD3b.
The wild type and mutant CaMV genomes were excised from the vector by SalI digestion and individually inoculated onto plants. Six week old turnips (Brassica rapa L. cv. Just Right) were mechanically inoculated with 10 μg of SalI-digested virus clone per plant in a solution of 2X SSC and washed celite. Plants were propagated as described previously (Agama et al., 2002).
PCR Detection of CaMV
To detect virus, inoculated and systemic leaf tissues were harvested at 35 days post-inoculation (DPI). Leaf tissue was ground and total DNA was extracted (Li and Leisner, 2002). CaMV was detected by PCR using the GIIF and GIIR primers as described previously.
To quantify virus levels, real-time PCR was used as described by Love et al. (2005). Briefly, the inoculated leaves of virus-infected plants were harvested at 35 DPI. Five infected plants were analyzed per virus (except for the L316A in which three infected plants were used due to lower infectivity). Inoculated leaf tissue was ground, treated with Proteinase K (250 μg/ml), and total DNA was isolated using the Qiagen DNeasy plant mini kit (Valencia, CA) as described in (Cecchini et al., 2002).
Total DNA isolated from inoculated leaves was assayed by quantitative PCR using a Bio-Rad real-time PCR machine. Reactions were carried out in triplicate in a total volume of 50 μl using a Promega qPCR kit, according to the manufacturer’s specifications. Each reaction contained 5 ng of total DNA extracted from inoculated leaves and the primer concentrations were 0.2 μM. Primers used to amplify the CaMV genome were Q7-F and Q1-R, derived from regions found in ORFs VII and I, respectively as described by Love et al. (2005). To normalize these data, primers 18S-F and 18S-R were used to amplify a portion of the 18S ribosomal RNA gene. The qPCR results were analyzed using the comparative threshold cycle method and normalized against 18S rDNA. As mentioned by Love et al. (2005), the 18S rRNA genes are useful normalization standards since they are present at a fixed number of copies per genome and accurately reflect the amounts of total DNA present. The R-program (Team, 2005) was used to remove all outliers and GraphPad Prism 5 Software was used to perform a one-way analysis of variance (ANOVA) and Tukey HSD test to determine statistical significance of differences between the mutants and wild type.
Fluorescence microscopy
All gene VI constructs, except those made in the pSITE vectors, were inserted into Agrobacterium tumefaciens binary vector pKYLX7 (Schardl et al., 1987) and individually introduced into A. tumefaciens strain AGL-1 by electroporation (Mattanovich et al., 1989). The pSITE constructs were also introduced into A. tumefaciens strain AGL-1 by electroporation. After confirming the identity of the A. tumefaciens strains, the bacteria were infiltrated into the leaves of four to six week-old Nicotiana benthamiana as described in Angel et al. (2013). All experiments consisted of infiltrating three leaves per plant and each experiment was repeated in triplicate.
Three days post-infiltration N. benthamiana leaves were observed using a Leica SP8 Confocal Laser Scanning Microscope at the University of Toledo. GFP and RFP were excited at wavelengths of 488 and 543 nm, respectively (Chakrabarty et al., 2007). Images were acquired sequentially line-by-line when using multiple fluorophores in concert. The Leica oil immersion 63x lens was used to acquire images at a resolution of 1024-by-1024 pixels. Images were processed using Leica software. Twelve Z-stacks were analyzed per construct.
The Leica processing software was used to measure the area of inclusion-like bodies (I-LBs) for 1841 and W260 P6. For all experiments, a minimum of 78 I-LBs was analyzed to determine average size. The R-program was used to perform the unpaired t-test. ASSESS software (American Phytopathological Society Press) was used to determine the average area of I-LBs formed by wild type and mutant CM1841 P6s. The R-program was used to perform a one-way analysis of variance (ANOVA) and TukeyHSD test. Mutant I-LB size was then normalized to the size of wild type IBs, which was set to 1.0 in our studies.
Protein expression analyses
To determine expression levels for wild type and mutant P6s, agroinfiltrated N. benthamiana leaves were harvested three days post-infiltration, ground in extraction buffer and protein extracts were prepared as described in (Angel et al., 2013). Protein extracts were separated by electrophoresis through a 10% polyacrylamide gel at 180 volts in a Mini-PROTEAN® 3 Cell (Bio-Rad Laboratories, Inc., Hercules, CA). Proteins were then electrophoretically transferred to a nitrocellulose membrane in a mini trans-blot® transfer cell (Bio-Rad Laboratories, Inc.). The membrane was washed with 1 X TBS (20 mM Tris-HCl, pH 7.6, 0.14 M NaCl), and blocked with 5% non-fat dry milk for 2 hours at room temperature. The membrane was cut into an upper and lower portion to allow for detection of both P6:GFP (upper) and neomycin phosphotransferase II (NPTII; lower) on the same blot. The Neomycin phosphotransferase II gene is encoded on the same piece of T-DNA as the P6-GFP constructions and serves as a loading control.
The membranes were incubated with either goat-anti-GFP (Santa Cruz, Biotechnology, Santa Cruz, CA) (1:1000 dilution) or rabbit-anti-NPTII (Sigma-Aldrich, ST Louis, MO; 2 μg/ml) primary antibodies. The membranes were washed three times with 1 X TBS containing 0.05% Tween-20, and incubated with either donkey-anti-goat (Sigma-Aldrich, ST Louis, MO) at 1:5000 dilution or goat-anti-rabbit (Santa Cruz, Biotechnology, Santa Cruz, CA) secondary antibody at 1:5000 dilution, respectively. The membranes were washed with 1X TBS containing 0.05% Tween-20, covered with luminol/peroxide chemiluminescent reagent (Millipore, Inc., Billerica, MA) and exposed to X-ray film. Films (HyBlot CL autoradiography film, Danville Scientific, Inc.) were developed using a Konica SRX-101® developer.
Results
Identification of a short conserved amino acid segment within P6 and its requirement for infectivity
D3 is essential for P6 self-association (Li and Leisner, 2002) and is comprised of a tripartite structure (Fig. 1) with a central portion (termed D3b) located between N-terminal (termed D3a) and C-terminal (termed D3c) RNA-binding domains (De Tapia et al., 1993). Multiple sequence alignment of D3 amino acid sequences showed that the D3a and D3c portions of D3 contained 0.311 and 0.297 variable amino acid positions per amino acid, while the value for D3b was 0.114 (Supplementary Fig. 1). Hence, D3b (P6 amino acids 309-343) was more well-conserved than the two RNA binding domains. The D3b region is polar (21/35 amino acids) and charged (4/35 amino acids are acidic, while 10/35 are basic). Computer prediction of secondary structure suggests that D3b contains two α-helices separated by a four amino acid turn and the N-terminal helix appears to be amphipathic.
Deletion of the D3 coding region within gene VI in CaMV resulted in a non-infectious virus (Li and Leisner, 2002). Turnips inoculated with a CM1841 virus lacking the D3b coding region (pCaMV10ΔD3b) showed no symptoms at 65 days post-inoculation. Deletion mutant viral DNA was undetectable by PCR in either inoculated or upper non-inoculated leaves for any of the ten plants inoculated (Fig. 2A). By contrast, viral DNA was easily detectable in both the inoculated and upper non-inoculated leaves of plants infected with wild type CM1841.
Fig. 2.
Role of the D3b region in CaMV infectivity and protein binding. A, Gel electrophoresis of PCR products from virus-infected plants. L indicates 100 bp ladder. I represents inoculated leaves; U, upper non-inoculated leaves for representative plants inoculated with either the D3b deletion mutant (Mutant; pCaMV10ΔD3b) or with wild type (Wild Type; CM1841) virus DNA. DNA indicates the PCR performed with pCaMV10 DNA (positive control); M, procedure performed on mock-inoculated plant; arrow indicates position of CaMV PCR amplification product. B, Schematic diagram of the constructs tested for leucine-independent growth and β-galactosidase activity (for C, D, and E). Black box, LexA DBD in pEG202; hatched box, B42 TAD in pJG4-5; white box, full-length or portions of CaMV P6; numbers to the left of each pair of constructions correspond to the plates in C and to the abscissa of the β-galactosidase assays shown in E. C, Growth of yeast transformants on media with (+L) and without (−L) leucine. D, key for the plates in C. E, β-galactosidase activity (units) of yeast transformants expressing constructs for three different experiments along with the standard deviation.
Interactions of D3b with full length P6 and its self-association regions
D3 was previously shown to interact with D1, D4, and possibly inefficiently with D2, but not with itself (Li and Leisner, 2002). Therefore the ability of D3b to associate with full-length P6 as well as individual self-association regions was investigated. Yeast two-hybrid data (Fig. 2B–E) indicated that D3b interacted efficiently with full-length P6 and significantly but more inefficiently with the D1 and D4 P6 self-association regions. The D3b interactions with D2 were inconsistent and this interaction was not pursued further. However, all (except negative control) including D2 (not shown), showed leucine-independent growth indicative of an interaction.
Binding of D3b mutants to P6 interaction regions
To examine the role of D3b in P6 interactions in more detail, single amino acid substitutions (Fig. 1) were generated in this portion of gene VI in the context of the full-length protein. Each of these point mutations individually changed a specific amino acid to an alanine. None of these mutants, when co-transformed with empty pEG202, permitted either leucine-independent growth, or induced β-galactosidase activity (for colonies grown on plates containing leucine) above control levels (Supplementary Fig. S3).
Based on leucine-independent growth (Fig. 3A,C,D), the D1 P6 self-association domain interacted with all five of the single amino acid substitution mutants. However, β-galactosidase activity (Fig. 3B) generated from interaction of D1 with each mutant was lower than induced by interactions with the wild type protein. The two mutations near the N-terminus of D3b (E312A and L316A) showed a much more drastic decrease in β-galactosidase activity than the remaining mutants (K318A, V324A and R328A).
Fig. 3.
Interactions of D3b mutant P6s with P6 self-association region, D1. A, Schematic diagram of the constructs tested for leucine independent growth and β-galactosidase activity (for B, C, and D). Black box, LexA DBD in pEG202; hatched box, B42 TAD in pJG4-5; white box, full-length or portions of CaMV P6; numbers to the left of each pair of constructions correspond to abscissa of the β-galactosidase assays shown in B and the plates in C. B, β-galactosidase activity of yeast transformants expressing constructs as represented in A. C, Growth of yeast transformants on media with (+L) and without (−L) leucine. D, key for the plates in C.
Interaction of all five mutant P6s with interaction region D4 (Fig. 4) resembled that with D1. Again, mutations near the N-terminal end of D3b (E312A and L316A) exhibited a strong reduction on D4 binding with the mutant P6s based on β-galactosidase activity (Fig. 4B). Interestingly, the β-galactosidase activity generated from the R328A mutation (at the other end of the tested region) in combination with D4 was similar to that of the E312A mutant. At the other end of the spectrum, the K318A mutant exhibited wild type β-galactosidase activity and the V324A mutant, showed nine-fold less than the wild type.
Fig. 4.
Interactions of D3b mutant P6s with P6 self-association domain, D4. A, Schematic diagram of the constructs tested for β-galactosidase activity and leucine independent growth (for B, C, and D). Black box, LexA DBD in pEG202; hatched box, B42 TAD in pJG4-5; white box, full-length or portions of CaMV P6; numbers to the left of each pair of constructions correspond to the abscissa of the β-galactosidase assays shown in B and the plates in C. B, β-galactosidase activity (units) of yeast transformants for three different experiments along with the standard deviation. C, Growth of yeast transformants on media with (+L) and without (−L) leucine. D, key for the plates in C.
Analysis of fluorescent-labeled IBs in plant cells
Harries and coworkers (Harries et al., 2009) fused the GFP gene to the 3′ end of CaMV strain W260 gene VI in a plant expression vector, agroinfiltrated this construct into N. benthamiana leaves and observed the formation of fluorescently-labeled inclusion-like bodies (I-LBs) within three days post-infiltration (dpinf). The I-LBs are formed in plant cells that express only P6. Therefore, I-LBs are equivalent to edIBs and not TBs, since P2 is not expressed in the infiltrated cells. To determine if P6 from CaMV isolate CM1841 forms similar I-LBs, CM1841 P6 was tagged at its C-terminal end with GFP. Similar to its W260 counterpart, leaves agroinfiltrated with constructs expressing CM1841 P6-GFP (Fig. 5A) showed a punctate distribution of GFP fluorescence whereas, agroinfiltration of constructs expressing GFP alone exhibited a diffuse cytoplasmic pattern. This difference in GFP distribution suggests that the punctate distribution shown with CM1841 P6 was due to I-LB formation rather than GFP aggregation due to over-expression.
Fig. 5.
Fluorescent inclusion-like bodies (I-LBs) formed by constructs expressing CM1841 P6-GFP in Nicotiana benthamiana. Constructs were agroinfiltrated into N. benthamiana leaves and examined by fluorescence microscopy three days post-infiltration. Magnification bar for A and C is 10 μm. A. Distribution of green fluorescent protein (GFP) alone (top three panels); W260 P6 fused to GFP (center three panels) and CM1841 P6 fused to GFP (bottom three panels). Left panels show GFP fluorescence alone (GFP); center panels, show bright field (BF) images of the same panels; and right, bright field overlay of GFP fluorescence (MERGE). B. Average size of W260 P6-GFP and CM1841 P6-GFP I-LBs. Z-stacks obtained by fluorescence microscopy were delineated and analyzed for size differences. Error bars indicate standard error of the mean and the star indicates a statistically-significant difference (p<0.0001). C. Co-localization of P6 and eIF3g. Left panels; P6-GFP fluorescence, middle panels; eIF3g-RFP, and right panels; overlay of GFP and RFP fluorescence. Top 3 panels, with W260 P6-GFP; bottom three panels, with CM1841 P6-GFP.
CaMV edIBs in infected plants are associated with the host translational machinery (Park et al., 2001) and W260 P6-GFP fluorescence was reported to co-localize with the translation factor eIF3g tagged with RFP (eIF3g-RFP) expressed in agroinfiltrated cells (Angel et al., 2013). As with W260 P6-GFP, CM1841 P6-GFP fluorescence co-localized with eIF3g-RFP (Fig. 5C) when expressed in agroinfiltrated cells. This suggests that CM1841 P6-GFP puncta are authentic edIBs. During the course of these studies, CM1841 I-LBs were found to be significantly smaller than those formed by their W260 counterpart (Fig. 5B). Interestingly, Shalla and coworkers (Shalla et al., 1980) had noted that the size of P6 inclusion bodies in CaMV-infected turnip leaves was determined in part by the strain of the virus. The analysis of the P6-GFP I-LBs of W260 and CM1841 support the concept that P6 edIB size is a trait determined by the P6 coding sequence.
To determine if the mutations introduced into CM1841 P6 influenced I-LB size, the D3b mutants were each tagged at the C-terminal end with GFP and then examined after expression in agroinfiltrated leaves. Leaves agroinfiltrated with constructs individually expressing each of the D3b mutants (Fig. 6A) showed a punctate distribution of GFP fluorescence, indicative of I-LB formation. However, distinct differences in I-LB size (Fig. 6B) were observed among the D3b mutants. The D3b mutants could be roughly divided into two classes based on I-LB size when compared to wild-type. The mutants K318A and V324A formed the first class, showing I-LBs similar in size to those induced by wild-type P6. In contrast, the E312A, L316A, and R328A mutants formed the second class, showing significantly smaller I-LBs when compared to wild-type. The I-LBs formed by the L316A mutant P6-GFPs were particularly small and were magnified three-fold to increase visibility (Fig. 6 C).
Fig. 6.
Fluorescence microscopy of mutant P6 I-LBs. Constructs were agroinfiltrated into N. benthamiana leaves and examined by fluorescence microscopy. three days post-infiltration. Magnification bar for A and C is 10 μm. A. Distribution of fluorescence for wild type CM1841 P6 (WT) and the D3b mutants (P6-GFPs analyzed are given to left of each set of panels). Left panels, GFP fluorescence (GFP); middle panels, bright field (BF) images of the same panels; and right, bright field overlay of left and middle panels (MERGE). B. Average size of GFP-labeled I-LBs formed by P6 mutants relative to those formed by wild type is given. This was determined from the z-stacks obtained by fluorescence microscopy. Average values were normalized to the size of wild type I-LBs, that was set to 1 and standard error of the mean are given. Different letters indicate statistically significant differences (p< 0.015). C. Protein gel blot analysis of wild type and mutant P6s expressed by agroinfiltration in N. benthamiana leaves. Upper panel, blot of wild type and mutant P6-GFP fusion proteins using anti-GFP antibodies; lower panel, the neomycin phosphotransferase type II (NPTII) was detected using anti-NPTII antibodies, served as a loading control.
Western blot analyses (Fig. 6D) were performed to ensure that the fluorescence was due to an intact P6-GFP fusion protein. Earlier studies (Supplementary Fig. S3) had shown that expression of CM1841 P6 with an N-terminal GFP tag in infiltrated leaves resulted in a diffuse cytoplasmic pattern of fluorescence, resembling that observed in leaves expressing GFP alone (Supplementary Fig. S3A). Western blot analysis (Supplementary Fig. S3B) of protein extracts from these leaves showed that GFP was cleaved from the N-terminus of P6 when those fusion proteins were expressed in infiltrated leaves. Western blot analyses of wild type and all mutant P6s fused at the C-terminus to GFP (Fig. 6D upper panel) showed that the fusion proteins were expressed and intact. The levels of the various P6-GFP fusion proteins expressed in infiltrated leaves were different. However, given the variation in transgene-derived protein expression in agroinfiltrated leaves, the differences in P6-FGP fusion protein levels amongst the mutants were not significant. The neomycin phosphotransferase II protein (Fig. 6D, lower panel) was used as a loading control (this protein is expressed from the same T-DNA as the P6-GFP genes).
Effects of D3b mutations on CaMV infectivity
To investigate their effects on CaMV infections, each of the mutations within the D3b coding region was incorporated into the CM1841 genome. The virus genomes were then inoculated to turnips at ten plants per virus and the infections were then permitted to progress. Inoculated leaves were harvested at 35 DPI and total viral DNA levels were determined by Real-time PCR (Fig. 7A). Based on inoculated leaf levels, the D3b mutants could be classified into two categories: those that were not significantly different from wild type (K318A and V324A) and those that showed drastically reduced levels of viral DNA (E312A, L316A and R328A).
Fig. 7.
Propagation of CaMVs harboring D3b mutations in turnips. A, propagation of virus in inoculated leaves as determined by real-time PCR. Inoculated leaves were harvested at 35 days post-inoculation, DNA was isolated and real time PCR was performed, normalizing virus DNA levels against levels of 18S rDNA genes. Average values and standard error of the mean are given. Different letters indicate statistically-significant differences (p<0.001 for A). B, Average number of leaves exhibiting systemic symptoms per plant. A total of ten plants were inoculated with each virus and the average number of leaves showing systemic symptoms per plant was determined at 35 days post-inoculation. Both average and standard error of the mean are indicated; different letters indicate statistically-significant differences (p<0.03).
Plants inoculated with the wild type and all mutant viruses generally showed systemic symptoms by 30–35 days post-inoculation (dpi) (Supplementary Fig. S4). However, the infection rate varied. By 35 dpi, 100% of the plants inoculated with wild type as well as the K318A and V324A mutants exhibited systemic symptoms. By this time, 70% and 80% of the plants inoculated with the E312A and R328A mutants, respectively, showed systemic symptoms. The infection rate for the L316A mutant was reduced, with only 30% of the plants showing systemic symptoms.
At 35 dpi, all of the plants inoculated with wild type virus exhibited vein clearing/vein banding symptoms characteristic of CaMV infection and a large number of upper non-inoculated leaves per plant were symptomatic (Fig. 7B). Plants inoculated with the K318A and V324A mutants also showed results similar to wild-type.
In contrast, viruses containing the E312A, L316A and R328A mutations induced dramatically different symptoms. For the most part, the systemic symptoms displayed by plants infected with these mutants were mainly chlorotic lesions on systemic leaves (Supplementary Fig. S5). The L316A mutant appeared to be the most restricted, exhibiting only a few chlorotic lesions on some systemically-infected leaves. Plants inoculated with the E312A and R328A mutants typically showed a limited number of chlorotic lesions on systemically-infected leaves. Limited vein clearing symptoms were occasionally observed later in infection. Interestingly, plants inoculated with the E312A, L316A, and R328A mutants that showed systemic chlorotic lesions, exhibited these symptoms on older systemically-infected leaves, but not young leaves. CaMV DNA could be detected in both symptomatic older leaves as well as younger leaves. However, the levels of virus DNA detected in the younger asymptomatic leaves were much lower. As the asymptomatic young leaves aged, they eventually exhibited chlorotic lesions.
At higher concentrations of DNA inoculum, turnips infected with the E312A and R328A mutants showed two types of symptoms. About half of the E312A and about three-fourths of the R328A symptomatic plants showed exclusively chlorotic lesions on systemically-infected leaves. The remaining symptomatic plants showed vein clearing and vein banding symptoms typical of wild-type. In both cases, the number of leaves showing systemic symptoms was the same but was significantly reduced compared to plants infected with wild-type virus.
Sequencing virus DNA isolated from leaves infected with the K318A and V324A mutants showed that both viruses maintained the original mutations. The same was true of the other three mutants in plants exhibiting the systemic chlorotic lesion phenotype. However, sequencing the virus genome of plants inoculated with the E312A and R328A mutants that induced wild type symptoms showed that the mutations had reverted.
Discussion
Many viral processes occur in IBs (Hull, 2002; Knipe, 1990; Martelli and Russo, 1977), and it is likely that the integrity of these structures is important for infectivity. The major protein comprising CaMV edIBs is P6 (Covey and Hull, 1981; Shockey et al., 1980), a multifunctional protein required for viral infection (Bonneville et al., 1989; Haas et al., 2008; Kobayashi and Hohn, 2003; Love et al., 2007; Schoelz et al., 1986; Schoelz and Wintermantel, 1993). Indeed the stability of edIBs does appear to influence the CaMV life cycle (Anderson et al., 1992). Hence, P6 self-association may be a key determinant for some of the functions this protein performs. Previously, four portions of P6 (D1–D4) were found to play a role in self-association (Haas et al., 2005; Li and Leisner, 2002). Here we investigated D3, a region termed (amino acids 249-379) involved in P6 self-association.
D3 has been implicated in a variety of activities and is essential for viral infectivity (Li and Leisner, 2002). D3 contains the two P6 non-sequence specific RNA binding domains, separated by a spacer region (De Tapia et al., 1993). The upstream RNA-binding domain (amino acids 249-309) also plays a role in interactions with eIF3g and RL24 (Park et al., 2001). The downstream RNA-binding domain (343-379) has also been implicated in interactions with the P4 CaMV capsid protein (Ryabova et al., 2002). Interestingly, the spacer region (D3b) is the most highly conserved portion of D3 and was investigated for its potential role in P6 self-association and viral infection.
D3b interacted with full-length P6. These data may indicate that D3b plays role in P6 self-association functions identified for D3 (Li and Leisner, 2002). The inefficient interaction of D3b with the individual domains may suggest that these domains require a particular structural context for appropriate interactions. Underscoring the importance of D3b was the observation that CaMVs lacking this region were non-infectious. This also agrees with the data from other workers (Kobayashi and Hohn, 2003) who showed that a larger deletion of P6, spanning D3b and beyond (amino acids 306-366), was non-infectious.
Because deletion mutations can have drastic effects on protein structure, single amino acid substitutions were generated within D3b that were not predicted to change protein secondary structure. These mutants were then evaluated for their effects on binding to P6 self-association domains, I-LB formation, and viral infection. The two most N-terminal mutations had a detrimental effect on binding to both D1 and D4. The C-terminal-most mutation showed lower than wild-type activity in binding to D1 and D4 as well. The two central mutations appeared less severe in their effects on binding to D4 and to some extent D1. Taken together, these data suggest that certain mutations within D3b influence binding of P6 to domains involved in self-association and likely influence self-interaction.
Mutations that affect P6 self-association may also influence formation of edIBs. Therefore, the ability of CM1841 P6 to form I-LBs was examined by fluorescence microscopy. The ability of CM1841 P6 to form fluorescent I-LBs was compared with that of W260 P6-GFP, as a reference (Angel et al., 2013; Harries et al., 2009). As with W260 P6-GFP, the CM1841 counterpart resulted in fluorescent puncta within agroinfiltrated cells. Both the W260 and CM1841 P6-GFP proteins co-localized with eIF3g-RFP (Angel et al., 2013 and this study), an association expected of authentic edIBs (Park et al., 2001). To determine if the D3b mutants affected I-LB formation, fluorescence microscopy was performed. All of the mutants generated punctate distributions of GFP indicative of I-LBs. However, these studies indicated that the mutants fell into two classes. Some mutants (K318A and V324A) generated fluorescent P6 I-LBs that were not significantly different in size compared to those produced by wild type CM1841 P6-GFP. The other mutants (E312A, L316A, and R328A) induced formation of P6 I-LBs that were significantly smaller in size than wild type.
Some of these mutations, when incorporated into the CaMV genome, also influenced virus propagation and systemic infection. The virus levels for the K318A and V324A mutants in inoculated leaves were not significantly different from wild type, while those for the E312A, L316A, and R328A mutants were significantly reduced. This is interesting since a region of P6 spanning all of D3b along with additional downstream sequences was proposed to be essential in basic viral replication (Kobayashi and Hohn, 2003). The inoculated leaf propagation data were also reflected in the formation of systemic symptoms. The K318A and V324A mutant viruses appeared essentially wild type in their ability to elicit viral systemic symptoms. However, the E312A, L316A, and R328A mutant viruses were debilitated in their ability to induce systemic symptoms.
Taking these data together, we conclude that the mutations that most strongly affect D3b binding to the P6 self-association regions (especially those affecting D4) have the strongest reduction in I-LB size and inoculated leaf virus DNA levels. This resulted in a reduced ability to induce a systemic infection. One possible explanation for all of these data revolves around the ability of P6 to form edIBs.
The formation of P6 edIBs begins with the generation of small clusters of ribosomes associated with electron-dense material, but containing few or no virus particles (Martelli and Russo, 1977). Those clusters are thought to then accrete to form small and then larger IBs that do contain virus particles. Interestingly, larger edIBs appear to have different properties from smaller ones. Smaller P6 edIBs, termed, “small bodies” (Champagne et al., 2004) contain unprocessed (N-terminal-containing) P4 coat protein, while the larger edIBs do not. The N-terminal 76 amino acids of P4 are removed by the protease located at the N-terminal end of P5. This protease must be excised from P5 in order for the rest of the protein comprising the reverse transcriptase/RNase H enzyme, to be activated (Hohn and Futterer, 1997; Takatsuji et al., 1992; Torruella et al., 1989). If the N-terminus of P4 is present in “small bodies”, then it is likely that the protease is not very active in those structures and probably has not been processed from P5 efficiently. Hence, “small bodies” would be expected to have less active reverse transcriptase/RNase H activity compared with large edIBs. All of the D3b mutants analyzed in this study likely have the ability to form “small bodies” because a punctate distribution of fluorescent-P6 is observed. However, the mutants (E312A, L316A, and R328A) most strongly affected in binding to the P6 self-association domains also form the smallest IBs in agroinfiltrated leaves. This suggests that the E312A, L316A, and R328A may be “trapped” in the “small body” stage and are unable to efficiently fuse to form larger edIBs. Hence, these three mutants should have the lowest replication in inoculated leaves, which is exactly what is observed. Because of the lower levels of virus in inoculated leaves, the efficiency of systemic infection would also be compromised in the small body-trapped mutants. Alternatively, the inability to cleave the N-terminus of the capsid protein in small bodies might also be expected to inhibit the maturation of virions. A recent study indicated that P6 edIBs may have a role in delivery of virion to plasmodesmata (Rodriguez et al., 2014). Consequently, CaMV mutants trapped in the small body stage might be less efficient in movement of virions to adjacent cells. Nonetheless, these data suggest that fusion of “small bodies” into larger edIBs may require P6 interactions that involve D3b and for CM1841 at least, the ability to form proper IBs is important for the infection process.
In summary, we find that mutations within a self-association domain of CaMV P6 protein (D3) that affect binding to the other self-association domains result in smaller I-LBs, reduced propagation in inoculated leaves and a more restricted systemic infection.
Supplementary Material
Highlights.
The D3b region of CaMV P6 binds to the full-length protein.
Point mutations in D3b reduce binding to P6 self-association domains.
P6 proteins harboring D3b mutations form smaller inclusion-like bodies.
Viruses harboring these mutations show reduced infectivity.
Acknowledgments
The authors thank Dr. Roger Brent (Molecular Sciences Institute, Berkeley, CA) for plasmids pEG202 and pJG4-5, along with yeast strain EGY48 harboring pSH18-34. The authors also thank Drs. Richard Komuniecki and Song-Tao Liu (University of Toledo, Toledo, OH, USA) for many of the vectors used in this study. In addition, the authors thank Dr. Wendy Zellner of the USDA-ARS for her help with statistical analyses. This work was supported in part by NIH Grant number 1R15AI50641-01 and USDA-ARS Specific Cooperative Agreement: 58-3607-1-193.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Leal R, Duffy S, Xiong Z, Hammond RW, Elena SF. Advances in plant virus evolution: translating evolutionary insights into better disease management. Phytopathology. 2011;101:1136–1148. doi: 10.1094/PHYTO-01-11-0017. [DOI] [PubMed] [Google Scholar]
- Agama K, Beach S, Schoelz J, Leisner SM. the 5′ third of Cauliflower mosaic virus gene VI conditions resistance breakage in Arabidopsis ecotype Tsu-0. Phytopathology. 2002;92:190–196. doi: 10.1094/PHYTO.2002.92.2.190. [DOI] [PubMed] [Google Scholar]
- Anderson EJ, Trese AT, Sehgal OP, Schoelz JE. Characterization of a chimeric Cauliflower mosaic virus isolate that is more severe and accumulates to higher concentrations the either of the strains from which it was derived. Mol Plant-Microbe Interact. 1992;5:48–54. [Google Scholar]
- Angel CA, Lutz L, Yang X, Rodriguez A, Adair A, Zhang Y, Leisner SM, Nelson RS, Schoelz JE. The P6 protein of Cauliflower mosaic virus interacts with CHUP1, a plant protein which moves chloroplasts on actin microfilaments. Virology. 2013;443:363–374. doi: 10.1016/j.virol.2013.05.028. [DOI] [PubMed] [Google Scholar]
- Armour SL, Melcher U, Pirone TP, Lyttle DJ, Essenberg RC. Helper component for aphid transmission encoded by region II of Cauliflower mosaic virus DNA. Virology. 1983;129:25–30. doi: 10.1016/0042-6822(83)90392-6. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. Greene Publishing Associates and John Wiley & Sons, INC; Cambridge MA USA: 1993. [Google Scholar]
- Bak A, Gargani D, Macia J-L, Malouvet E, Vernerey M-S, Blanc S, Drucker M. Virus factories of Cauliflower mosaic virus are virion reservoirs that engage actively in vector transmission. J Virol. 2013;87:12207–12215. doi: 10.1128/JVI.01883-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc S, Cerutti M, Usmany M, Vlak JM, Hull R. Biological activity of the Cauliflower mosaic virus aphid transmission expressed in a heterologous system. Virology. 1993;192:643–650. doi: 10.1006/viro.1993.1080. [DOI] [PubMed] [Google Scholar]
- Bonneville JM, Sanfacon H, Futterer J, Hohn T. Posttranscriptional trans-activation in cauliflower mosaic virus. Cell. 1989;59:1135–1143. doi: 10.1016/0092-8674(89)90769-1. [DOI] [PubMed] [Google Scholar]
- Bureau M, Leh V, Haas M, Geldreich A, Ryabova L, Yot P, Keller M. P6 protein of Cauliflower mosaic virus, a translation reinitiator, interacts with ribosomal protein L13 from Arabidopsis thaliana. J Gen Virol. 2004;85:3765–3775. doi: 10.1099/vir.0.80242-0. [DOI] [PubMed] [Google Scholar]
- Cecchini E, Geri C, Love AJ, Coupland G, Covey SN, Milner JL. Mutations that delay flowering in Arabidopsis de-couple symptom response from Cauliflower mosaic virus accumulation during infection. Mol Plant Pathol. 2002;3:81–90. doi: 10.1046/j.1464-6722.2001.00097.x. [DOI] [PubMed] [Google Scholar]
- Chakrabarty R, Banerjee R, Chung SM, Farman M, Citovsky V, Hogenhout SA, Tzfira T, Goodin M. pSITE vectors for stable integration or transient expression of autofluorescent protein fusions in plants: probing Nicotiana benthamiana-virus interactions. Mol Plant-Microbe Interact. 2007;20:740–750. doi: 10.1094/MPMI-20-7-0740. [DOI] [PubMed] [Google Scholar]
- Champagne J, Benhamou N, Leclerc D. Localization of the N-terminal domain of cauliflower mosaic virus coat protein precursor. Virology. 2004;324:257–262. doi: 10.1016/j.virol.2004.04.014. [DOI] [PubMed] [Google Scholar]
- De Tapia M, Himmelbach A, Hohn T. Molecular dissection of the cauliflower mosaic virus translation transactivator. EMBO J. 1993;12:3305–3314. doi: 10.1002/j.1460-2075.1993.tb06000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza AM, Medina V, Hull R, Markham PG. Cauliflower mosaic virus gene II product forms distinct inclusion bodies in infected plant cells. Virology. 1991;185:337–344. doi: 10.1016/0042-6822(91)90781-6. [DOI] [PubMed] [Google Scholar]
- Gardner RC, Howarth AJ, Hahn P, Brown-Luedi M, Shepherd RJ, Messing J. The complete nucleotide sequence of an infectious clone of cauliflower mosaic virus by M13mp7 shotgun sequencing. Nucleic Acids Res. 1981;9:2871–2888. doi: 10.1093/nar/9.12.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- Haas G, Azevedo J, Moissiard G, Geldreich A, Himber C, Bureau M, Fukuhara T, Keller M, Voinnet O. Nuclear import of CaMV P6 is required for infection, and suppression of the RNA silencing factor DRB4. EMBO, J. 2008;27:2102–2112. doi: 10.1038/emboj.2008.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M, Bureau M, Geldreich A, Yot P, Keller M. Cauliflower mosaic virus: still in the news. Mol Plant Pathol. 2002;3:419–429. doi: 10.1046/j.1364-3703.2002.00136.x. [DOI] [PubMed] [Google Scholar]
- Haas M, Geldreich A, Bureau M, Dupuis L, Leh V, Vetter G, Kobayashi K, Hohn T, Ryabova L, Yot P, Keller M. The open reading frame VI product of Cauliflower mosaic virus is a nucleocytoplasmic protein: its N terminus mediates its nuclear export and formation of electron-dense viroplasms. Plant Cell. 2005;17:927–943. doi: 10.1105/tpc.104.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapiak M, Li Y, Agama K, Swade S, Okenka G, Falk J, Khandekar S, Raikhy G, Anderson AJP, Zellner W, Schoelz JE, Leisner SM. Cauliflower mosaic virus gene VI product N-terminus contains regions involved in resistance-breakage, self-association, and interactions with movement protein. Virus Res. 2008;138:119–129. doi: 10.1016/j.virusres.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries PA, Palanichelvam K, Yu W, Schoelz JE, Nelson RS. The Cauliflower mosaic virtus protein 6 forms motile inclusions that traffic along actin microfilaments and stabilize microtubules. Plant Physiol. 2009;149:1005–1016. doi: 10.1104/pp.108.131755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelbach A, Chapdelaine Y, Hohn T. Interaction between cauliflower mosaic virus inclusion body protein and capsid protein: Implications for viral assembly. Virology. 1996;217:147–157. doi: 10.1006/viro.1996.0102. [DOI] [PubMed] [Google Scholar]
- Hohn T, Futterer J. The proteins and functions of plant pararetroviruses: knowns and unknowns. Cr Rev Plant Sci. 1997;16:133–161. [Google Scholar]
- Howarth AJ, Gardner RC, Messing J, Shepherd RJ. Nucleotide sequence of naturally occurring deletion mutants of cauliflower mosaic virus. Virology. 1981;112:678–685. doi: 10.1016/0042-6822(81)90313-5. [DOI] [PubMed] [Google Scholar]
- Hull R. Matthews’ Plant Virology. 4. Academic Press; San Diego: 2002. [Google Scholar]
- Khelifa M, Journou S, Krishnan K, Gargani D, Esperandieu P, Blanc S, Drucker M. Electron-lucent inclusion bodies are structures specialized for aphid transmission of Cauliflower mosaic virus. J Gen Virol. 2007;88:2872–2880. doi: 10.1099/vir.0.83009-0. [DOI] [PubMed] [Google Scholar]
- Knipe D. Virus-host interactions. In: Fields BN, Knipe DM, editors. Fields Virology. Raven Press; New York: 1990. pp. 293–316. [Google Scholar]
- Kobayashi K, Hohn T. Dissection of Cauliflower mosaic virus transactivator/viroplasmin reveals distinct essential functions in basic virus replication. J Virol. 2003;77:8577–8583. doi: 10.1128/JVI.77.15.8577-8583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird J, McInally C, Carr C, Doddiah S, Yates G, Chrysanthou E, Khattab A, Love AJ, Geri C, Sadanandom A, Smith BO, Kobayashi K, Milner JJ. Identification of the domains of Cauliflower mosaic virus protein P6 responsible for suppression of RNA silencing and salicylic acid signaling. J Gen Virol. 2013;94:2777–2772. 2789. doi: 10.1099/vir.0.057729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leh V, Yot P, Keller M. The cauliflower mosaic virus translational transactivator interacts with the 60S ribosomal subunit protein L18 of Arabidopsis thaliana. Virology. 2000;266:1–7. doi: 10.1006/viro.1999.0073. [DOI] [PubMed] [Google Scholar]
- Li Y, Leisner SM. Multiple domains within the Cauliflower mosaic virus gene VI product interact with the full-length protein. Mol Plant-Microbe Interact. 2002;15:1050–1057. doi: 10.1094/MPMI.2002.15.10.1050. [DOI] [PubMed] [Google Scholar]
- Love AJ, Laird J, Holt J, Hamilton AJ, Sadanandom A, Milner JJ. Cauliflower mosaic virus protein P6 is a suppressor of RNA silencing. J Gen Virol. 2007;88:3439–3444. doi: 10.1099/vir.0.83090-0. [DOI] [PubMed] [Google Scholar]
- Martelli GP, Russo M. Plant Virus Inclusion Bodies. Adv Virus Res. 1977;21:175–266. doi: 10.1016/s0065-3527(08)60763-0. [DOI] [PubMed] [Google Scholar]
- Martiniere A, Bak A, Macia JL, Lautredou N, Gargani D, Doumayrou J, Garzo E, Moreno A, Fereres A, Blanc S, Drucker M. A virus responds instantly to the presence of the vector on the host and forms transmission morphs. eLife. 2013;2:e00183. doi: 10.7554/eLife.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattanovich D, Rucker F, da Camara Machado A, Laimer M, Regner F, Steinkellner H, Himmler G, Katinger H. Efficient transformation of Agrobacterium spp. by electroporation. Nucleic Acids Res. 1989;17:6747. doi: 10.1093/nar/17.16.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzolini L, Dabos P, Constantin S, Yot P. Further evidence that viroplasms are the site of cauliflower mosaic virus genome replication by reverse transcription during viral infection. J Gen Virol. 1989;70:3439–3449. [Google Scholar]
- Nakayashiki H, Tsuge S, Kobayashi K, Okuno T, Furusawa I. Reasons for the low accumulation level of aphid transmission factor protein in infected leaves with an aphid-non-transmissible cauliflower mosaic virus isolate, CM1841. J Gen Virol. 1993;74:2469–2472. doi: 10.1099/0022-1317-74-11-2469. [DOI] [PubMed] [Google Scholar]
- Park HS, Himmelbach A, Browning KS, Hohn T, Ryabova LA. A plant viral reinitiation factor interacts with the host translational machinery. Cell. 2001;106:723–733. doi: 10.1016/s0092-8674(01)00487-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Angel CA, Lutz L, Leisner SM, Nelson RS, Schoelz JE. Association of the P6 protein of Cauliflower mosaic virus with plasmodesmata and plasmodesmal proteins. Plant Physiol. 2014;166:1345–1358. doi: 10.1104/pp.114.249250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabova LA, Pooggin MM, Hohn T. Viral strategies of translation initiation, ribosomal shunt and reinitiation. Prog Nucleic Acid Res. 2002;72:1–39. doi: 10.1016/S0079-6603(02)72066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoelz J, Shepherd RJ, Daubert S. Region VI of cauliflower mosaic virus encodes a host range determinant. Mol Cell Biol. 1986;6:2632–2637. doi: 10.1128/mcb.6.7.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoelz JE, Wintermantel WM. Expansion of viral host range through complementation and recombination in transgenic plants. Plant Cell. 1993;5:1669–1679. doi: 10.1105/tpc.5.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalla TA, Shepherd RJ, Petersen LJ. Comparative cytology of nine isolates of cauliflower mosaic virus. Virology. 1980;102:381–388. doi: 10.1016/0042-6822(80)90105-1. [DOI] [PubMed] [Google Scholar]
- Takatsuji H, Yamauchi H, Watanabe S-I, Kato H, Ikeda J-E. Cauliflower mosaic virus reverse transcriptase. J Biol Chem. 1992;267:11579–11585. [PubMed] [Google Scholar]
- Team, R.D.C. R: A language and environment for statistical computing, reference 2† index version 2.2.1. R Foundation for Statistical Computing; Vienna, Austria: 2005. [Google Scholar]
- Torruella M, Gordon K, Hohn T. Cauliflower mosaic virus produces an aspartic proteinase to cleave its polyproteins. EMBO J. 1989;8:2819–2825. doi: 10.1002/j.1460-2075.1989.tb08428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolston CJ, Covey SN, Penswick JR, Davies JW. Aphid transmission and a polypeptide are specified by a defined region of the Cauliflower mosaic virus genome. Gene. 1983;23:15–23. doi: 10.1016/0378-1119(83)90212-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.