Abstract
Purpose
To examine the onset and outcome of ipilimumab-related hypophysitis and the response to treatment with systemic high dose corticosteroids.
Patient and Methods
Twenty-five patients who developed ipilimumab-related hypophysitis were analyzed for the incidence, time to onset, time to resolution, frequency of resolution, and the effect of systemic high-dose corticosteroids on clinical outcome. To calculate the incidence, the total number (187) of patients with metastatic melanoma treated with ipilimumab at Dana-Farber Cancer Institute (DFCI) was retrieved from the DFCI oncology database. Comparisons between corticosteroid treatment groups were performed using Fisher’s exact test. The distributions of overall survival were based on the method of Kaplan-Meier.
Results
The overall incidence of ipilimumab-related hypophysitis was 13%, with a higher rate in males (16.1%) than females (8.7%). The median time to onset of hypophysitis after initiation of ipilimumab treatment was 9 weeks (range: 5–36 weeks). Resolution of pituitary enlargement, secondary adrenal insufficiency, secondary hypothyroidism, male secondary hypogonadism, and hyponatremia occurred in 73%, 0%, 64%, 45%, and 92% of patients, respectively. Systemic high dose corticosteroid treatment did not improve the outcome of hypophysitis as measured by resolution frequency and time to resolution. One-year overall survival in the cohort of patients was 83%, and while slightly higher in patients who did not receive high dose corticosteroids, there was no statistically significant difference between treatment arms.
Conclusion
Systemic high dose corticosteroid therapy in patients with ipilimumab-related hypophysitis may not be indicated. Instead, supportive treatment of hypophysitis-related hormone deficiencies with the corresponding hormone replacement should be given.
Introduction
CTLA-4 is an immune checkpoint protein which negatively regulates T cell responses (1). In clinical trials, monoclonal antibodies against immune checkpoint proteins have demonstrated promising and durable anti-cancer effects (2–4). In 2011, the Food and Drug Administration (FDA) approved ipilimumab, a humanized monoclonal antibody against CTLA-4, for the treatment of advanced melanoma. Ipilimumab-associated hypophysitis, the most common endocrinopathy related to anti-CTLA-4 treatment, presents as either panhypopituitarism or isolated anterior pituitary hormone deficiency, with or without pituitary enlargement (7–10). Systemic high dose corticosteroids (HDS) have been recommended as a standard treatment for patients with ipilimumab-related hypophysitis (11, 12), but the benefits of this treatment are unclear. Some studies have suggested that systemic HDS do not appear to counteract the anti-cancer effects of ipilimumab (13, 14), although this has been questioned in another study (15). To the best of our knowledge, there has been no study assessing the effects of HDS on the outcome of ipilimumab-related hypophysitis. In this retrospective study, we did not identify a beneficial effect of systemic HDS treatment in patients with ipilimumab-related hypophysitis.
Patients and Methods
Patients
Patients with ipilimumab-related hypophysitis were evaluated clinically in the outpatient endocrinology clinic of Brigham and Women’s Hospital. This cohort analysis was performed retrospectively by collecting relevant data from chart reviews. The period for this study was from August 21, 2008 to February 5, 2014. Institutional review board approval was obtained for the study. There were 45 patients who developed ipilimumab-related endocrinopathies after ipilimumab therapy. To eliminate confounding influences from combined therapy, we excluded patients who also received other immune checkpoint blocking therapy, including anti-PD1 (pembrolizumab or Nivolumab) or anti-PDL1 (MPDL-3280A), or the angiogenesis inhibitor, bevacizumab. Twenty-five patients with hypophysitis who received ipilimumab monotherapy were included in this analysis. Three of these twenty-five patients were reported previously (8). All patients had stage IV melanoma except for two with stage IIIA and stage IIIC melanoma. To calculate the incidence of hypophysitis, the total number of patients who received ipilimumab monotherapy during the study period was obtained from the DFCI synergistic patient and research knowledge systems-oncology data retrieval system.
Definition of hypophysitis
Hypophysitis was diagnosed based on either imaging evidence of pituitary enlargement or biochemical evidence of anterior pituitary hormone deficiency following ipilimumab treatment. Anterior pituitary hormone deficiencies were diagnosed based on low levels of the primary target gland hormones - cortisol, thyroxine, sex steroids, and IGF1 - with low or inappropriately normal levels of the corresponding pituitary hormones, adrenocorticotropin (ACTH), thyrotropin (TSH), gonadotropins (FSH, LH), and growth hormone (GH), based on laboratory reference ranges. Endocrinopathy in this study was defined as any of the hypophysitis-related anterior pituitary hormone deficiencies.
Definition of replacement dose corticosteroids, systemic high-dose corticosteroids, time to onset, time to resolution, and survival time
Time to onset of hypophysitis was defined as the number of weeks between the administration of the first dose of ipilimumab and the diagnosis of hypophysitis, based on imaging or biochemical testing. Resolution of a particular endocrinopathy was defined as normalization of levels of the primary target gland hormone and the corresponding pituitary hormone following discontinuation of hormone replacement. The time to resolution refers to the number of weeks between the diagnosis and resolution of the endocrinopathy. Since this was a retrospective study, biochemical testing and imaging were not done at fixed, controlled intervals. Replacement dose corticosteroids was defined as a daily dose not higher than 30 mg hydrocortisone or equivalent (16), except in 2 patients who were transiently exposed to 60 mg hydrocortisone for 2–3 days following sick-day guidelines. Systemic high-dose corticosteroid treatment was defined as the administration of corticosteroids at a dose of more than 30 mg hydrocortisone (or equivalent) daily for more than one week during the course of ipilimumab treatment and/or at the time of onset of hypophysitis. Survival time was calculated from the day of initiation of ipilimumab therapy to the day of death of the patient. The follow-up of patients who were alive was censored on February 5, 2014. Toxicity grading of ipilimumab-related hypophysitis was based on the criteria defined by Corsello et al, with modification (17). Briefly, severity was categorized from 1–5: 1. Asymptomatic; clinical or diagnostic observations only; intervention not indicated. 2. Symptomatic; hormone replacement indicated; limiting instrumental activities of daily living (ADL). 3. Severe symptoms; limiting self-care for ADL; hospitalization indicated. 4. Life-threatening consequences; urgent intervention indicated. 5. Death.
Statistical analysis
Statistical analyses are primarily descriptive. Proportions are presented with 95% exact binomial confidence intervals (CI). Comparisons between corticosteroid groups were based on Fisher’s exact test. The distributions of overall survival were based on the product-limit method of Kaplan-Meier and were compared using the log-rank test. Time point estimates of survival are accompanied by 95% confidence intervals estimated using log(-log(survival)) methodology. Median follow-up was based on Kaplan-Meier estimates with an inverted censor. Statistical significance was defined as p<0.05. All analyses were conducted using SAS® version 9.3.
Results
Demographics
Of 187 patients who received ipilimumab monotherapy during the time interval of the study, 25 patients (13%, 95% CI 9% to 19%) developed ipilimumab-related hypophysitis. Of these 25 patients, 17 received 3 mg/kg ipilimumab and 8 received 10 mg/kg ipilimumab by intravenous infusion every 3 weeks. The incidence of hypophysitis was 16.1% (19/118) in males and 8.7% (6/69) in females (Table 1). Median follow-up in the cohort of patients with hypophysitis was 14.2 months (95% CI 9.7 to 18.2 months).
Table 1. Incidence of ipilimumab-related hypophysitis.
The incidence was calculated as the number of patients with hypophysitis compared to the total number of patients treated with ipilimumab monotherapy.
| Male | Female | Total | |
|---|---|---|---|
| Hypophysitis (N) | 19 | 6 | 25 |
| Total patients received ipilimumab (N) | 118 | 69 | 187 |
| Hypophysitis (%) | 16.1 | 8.7 | 13.3 |
Manifestations of hypophysitis
In light of the retrospective nature of this study, the biochemical tests and imaging performed in individual patients varied. To reflect this variability, the incidence of an endocrinopathy was calculated as the ratio of the number of patients who had abnormal test results over the total number of the patients with ipilimumab-related hypophysitis who had the tests performed. The overall incidence of pituitary enlargement was 60% (15/25) among the patients with hypophysitis. Secondary adrenal insufficiency and secondary hypothyroidism were the most common anterior pituitary insufficiencies diagnosed, with an incidence of 88% for each (Table 2). Among the 9 patients who had their prolactin measured, only one had hyperprolactinemia while four had low prolactin levels. None of the patients with a diagnosis of hypophysitis, except for two, received corticosteroids within six months prior to the onset of hypophysitis. One of these two patients who had received prior HDS had radiographic evidence of new onset pituitary enlargement. The other patient who had received prior HDS did not have pituitary enlargement on imaging, but the diagnosis of hypophysitis was made based on the presence of rapid onset central hypothyroidism. More than half of the patients with hypophysitis had co-existing hyponatremia, a finding similarly observed in another recent study (9), but not reported in earlier studies (3, 5, 19). When comparing the incidence of each anterior pituitary hormone deficiency between the groups treated with 3 mg/kg and 10 mg/kg ipilimumab, no significant difference in the frequency of each endocrinopathy was found (Table 2).
Table 2. The overall and dose-dependent incidence of ipilimumab-related pituitary enlargement, anterior pituitary hormone deficiencies, and hyponatremia.
The incidence of each anterior pituitary hormone deficiency was calculated based on gender and ipilimumab dose, taking into account the number of patients with ipilimumab-related hypophysitis in whom the measure was assessed. The incidence is shown as both the number affected and as a percentage. AI: Adrenal insufficiency; IGF1: Insulin-like growth factor 1.
| Overall | Ipilimumab (3 mg/kg) | Ipilimumab (10 mg/kg) | |||
|---|---|---|---|---|---|
|
| |||||
| Male N=19 |
Female N=6 |
Total N=25 |
Total N=17 |
Total N=8 |
|
| Pituitary enlargement | |||||
| Yes | 10 (53) | 5 (83) | 15 (60) | 10 (59) | 5 (63) |
| No | 9 (47) | 1 (17) | 10 (40) | 7 (41) | 3 (37) |
| Secondary AI | |||||
| Yes | 18 (95) | 4 (67) | 22 (88) | 15 (88) | 7 (88) |
| No | 1 (5) | 2 (33) | 3 (12) | 2 (12) | 1 (12) |
| Secondary hypothroidism | |||||
| Yes | 18 (95) | 4 (67) | 22 (88) | 15 (88) | 7 (88) |
| No | 1 (5) | 2 (33) | 3 (12) | 2 (12) | 1 (12) |
| Secondary hypogonadism | |||||
| Yes | 15 (79) | 0 (0) | 15 (60) | 10 (59) | 5 (63) |
| No | 2 (11) | 3 (50) | 5 (20) | 4 (24) | 1 (13) |
| Not measured | 2 (11) | 3 (50) | 5 (20) | 3 (18) | 2 (25) |
| Low IGF1 | |||||
| Yes | 3 (60) | 0 (0) | 3 (12) | 1 (33) | 2 (50) |
| No | 2 (40) | 2 (33) | 4 (16) | 2 (67) | 2 (50) |
| Not measured | 14 (74) | 4 (67) | 18 (72) | 14 (82) | 4 (50) |
| Low prolactin | |||||
| Yes | 4 (21) | 0 (0) | 4 (16) | 2 (12) | 2 (25) |
| No | 4 (21) | 1 (17) | 5 (20) | 2 (12) | 3 (38) |
| Not measured | 11 (58) | 5 (83) | 16 (64) | 13 (76) | 3 (38) |
| Hyponatremia | |||||
| Yes | 12 (63) | 2 (33) | 14 (56) | 10 (59) | 3 (38) |
| No | 7 (37) | 4 (67) | 11 (44) | 7 (41) | 5 (62) |
Patterns of onset and resolution of ipilimumab-related hypophysitis
Given the retrospective nature of this study, the frequency and timing of biochemical tests and imaging performed in individual patients varied, influencing these determinations. With this caveat, the median time to onset of hypophysitis after initiation of ipilimumab therapy, based on diagnostic testing, was 9 weeks, with a range of 5 to 36 weeks (Table 3). The frequency of resolution of secondary adrenal insufficiency, secondary hypothyroidism and male hypogonadotropic hypogonadism (HH) were 0%, 64% and 47%, respectively. Among the subset of patients whose endocrinopathy resolved, the median times to resolution of secondary hypothyroidism and male HH, based on biochemical testing, were 10 and 15 weeks, respectively (Table 3). Discontinuation of ipilimumab did not appear to affect the outcome of hypophysitis. In the six patients in whom ipilimumab was discontinued, the frequency of resolution of secondary hypothyroidism and secondary hypogonadism was 33% (2/6 and 1/3, respectively). The median times to resolution, based on laboratory testing, of secondary hypothyroidism and secondary hypogonadism after discontinuation of ipilimumab among these six patients were 13 and 10 weeks, respectively. Most patients in this study received 3–4 doses of ipilimumab, whereas three patients received 8, 9 and 15 doses of ipilimumab, respectively. Interestingly, among the three patients who had received prolonged ipilimumab therapy, resolution of a subset of endocrinopathies occurred during ipilimumab treatment. Normalization of pituitary size was observed in 11 of 15 patients (73%) with enlarged pituitaries in 2 to 27 weeks, based on follow-up imaging. The other 4 patients did not have repeated imaging studies before the end of this study. Six of 11 patients with resolution of enlarged pituitaries had earlier MRI studies, at 3 to 8 weeks after identification of pituitary enlargement, which did not show resolution of pituitary enlargement.
Table 3. Median time to onset, frequency of resolution, and median time to resolution.
Time is measured in weeks. The frequency of resolution is represented as the number of patients in whom resolution occurred, and as a percentage of the patients in whom the deficiency was noted. n/a: Not available. N: number of patients.
| Time to Onset (median weeks (range)) | Resolution | Time to Resolution (median weeks (range)) | ||
|---|---|---|---|---|
| N | % | |||
| Hypophysitis | 9 (5–36) | |||
| Pituitary enlargement | 8 (5–13) | 11 | 73.3 | 15 (2–27) |
| SecondaryAdrenal insufficiency | 9.5 (5–36) | 0 | 0.0 | n/a |
| Secondary hypothroidism | 9.5 (6–36) | 14 | 63.6 | 10.5 (1–44) |
| Secondary hypogonadism | 9 (5–13) | 7 | 46.7 | 15 (2–92) |
| Hyponatremia | 9 (5–36) | 12 | 92.3 | 3.5 (1–10) |
The effect of HDS therapy on the outcome of ipilimumab-related hypophysitis and on survival rate
Among 25 patients with ipilimumab-related hypophysitis, 15 patients received HDS treatment for ipilimumab-related hypophysitis (n=5), other immune-related adverse events (IrAEs) (n=8), or brain metastasis (n=2). The patients in the HDS group received dexamethasone (n=4, dose: 4–24 mg daily; duration: 3–12 weeks), prednisone (n=9, dose: 40–100 mg daily; duration: 2–14 weeks), hydrocortisone (n=1; dose: 60 mg daily; duration: 3 weeks) or dexamethasone followed by prednisone (n=1, doses: dexamethasone 8 mg daily; duration: 1 week, prednisone 40 mg daily; duration: 5 weeks). The other 10 patients did not receive HDS during the study period. Among the patients who received HDS, 6 of 15 (40%; 95% CI 16 to 68%) had grade 2 ipilimumab-related hypophysitis toxicity; 9 of 15 (60%; 95% CI 32 to 84%) had grade 3/4 toxicity (17). Among the patients who received replacement corticosteroids, 7 of 10 (70%; 95% CI 35 to 93%) had grade 2 toxicity; 3 of 10 (30%; 95% CI 7 to 65%) had grade 3/4 toxicity. Toxicity rates were comparable between the HDS and non-HDS cohorts.
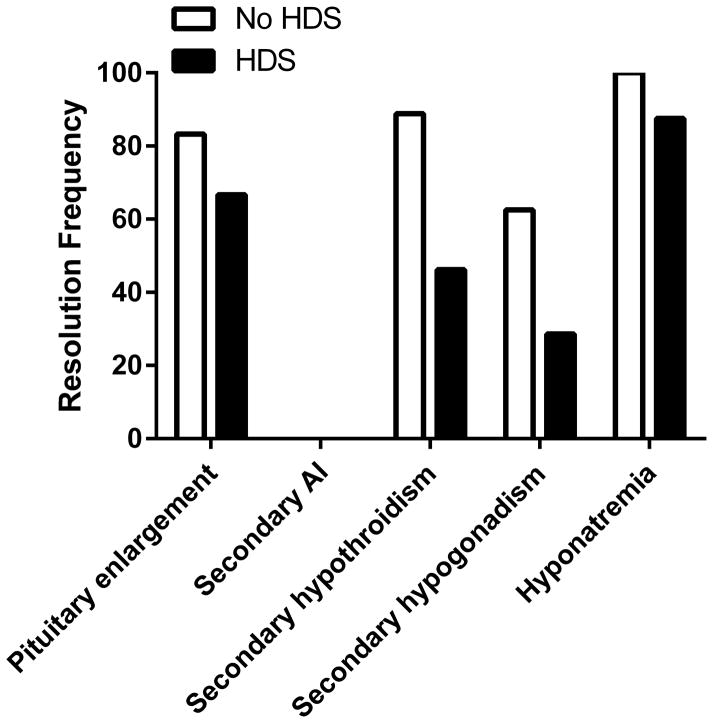
We compared the frequency of resolution and the median times to resolution, based on laboratory testing data available, between those with and without systemic HDS treatment (Table 4). There was no documented resolution of secondary adrenal insufficiency in either group. Among patients with secondary adrenal insufficiency who received HDS, 13 patients had morning paired ACTH and cortisol levels tested 2 to12 months after HDS withdrawal. All 13 patients who had adrenal function testing demonstrated persistent adrenal insufficiency. There were no statistically significant differences in the frequency of resolution of other adverse events (Table 4 and Figure 1), although the data suggest that the frequency of resolution of secondary hypothyroidism may be less in the HDS group (46% versus 89%, Fisher’s exact p=0.07) (Figure 1). In addition, median times to resolution for each adverse event were comparable between the two groups (Table 4).
Table 4. The effect of high dose corticosteroids on the frequency of resolution of ipilimumab-related adverse events.
The frequency of resolution is calculated as the number with resolution compared to the total number affected for each endocrinopathy. The time is measured in weeks. N: Number; n/a: Not applicable.
| Resolution (N with resolution/N affected)
|
Median time to resolution (Median weeks (Range))
|
|||
|---|---|---|---|---|
| High dose corticosteroids | No | Yes | No | Yes |
| Pituitary enlargement | 5/6 | 6/9 | 8 (2–27) | 16 (4–26) |
| Secondary adrenal insufficiency | 0/10 | 0/12 | n/a | n/a |
| Secondary hypothroidism | 8/9 | 6/13 | 14.5 (2–44) | 9 (1–16) |
| Secondary hypogonadism | 5/8 | 2/7 | 40 (2–92) | 12 (10–15) |
| Hyponatremia | 5/5 | 7/8 | 2 (1–7) | 4 (1–10) |
Figure 1. The effect of high dose corticosteroids on the frequency of resolution of each endocrinopathy.
The frequency of resolution is represented as the percentage of those with resolution compared to the total number affected for each endocrinopathy. HDS: high dose corticosteroids.
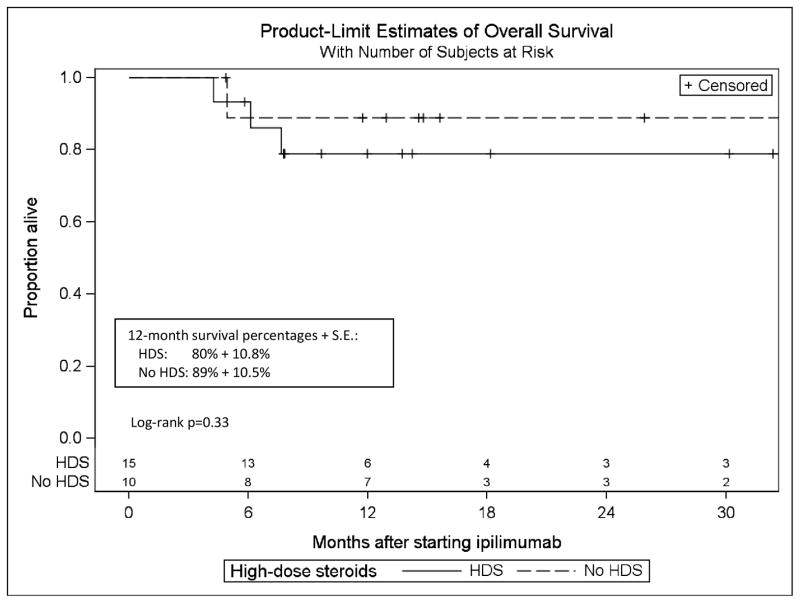
During the course of the study, four of 15 (27%) patients in the HDS treatment group died, whereas one of 10 (10%) patients in the group that did not receive HDS died. Twelve-month Kaplan-Meier estimates (S.E.) of survival were 83% (7.7%) for the full cohort, and 80% (10.8%) and 89% (10.5%) in patients who did or did not receive HDS, respectively (Figure 2). All deaths were attributed to the underlying metastatic disease.
Figure 2. The effects of high dose corticosteroids on survival rate.
Kaplan-Meier estimates of overall survival with the numbers of patients at risk. The X axis was extended to 32 months (median follow-up + 18 months), the time at which there was 20% or less of the sample remaining. HDS: high dose corticosteroids.
Discussion
Although a definitive diagnosis of autoimmune hypophysitis by ipilimumab has not been documented, an animal study has shown that injection of a CTLA-4 blocking antibody can induce lymphocytic infiltration in mouse pituitary glands (20). Non-ipilimumab related autoimmune hypophysitis is more common in females, with a male:female ratio of 1:6 for lymphocytic adenohypophysitis (18, 21). In contrast, in the current study the incidence of ipilimumab-related hypophysitis was found to be higher in males (Table 1). Similar results were found in some, but not all, prior studies (10, 17). Ipilimumab-associated diabetes insipidus has been reported (6); nonetheless, compared to anterior pituitary hormone deficiencies, posterior pituitary hormone deficiency is infrequent (7–9). In this retrospective cohort analysis, even in one patient with transient prolactin elevation suggestive of possible pituitary stalk infiltration, there was no evidence of diabetes insipidus.
The risk of hypopituitarism in patients receiving brain radiation has been documented (22). In the current study, two patients diagnosed with hypophysitis on the basis of pituitary insufficiency but without pituitary enlargement had received brain radiation therapy prior to the diagnosis of hypophysitis. One developed reversible central hypothyroidism, whereas radiation-induced hypopituitarism is usually irreversible (23). Another developed rapid onset of secondary adrenal insufficiency following ipilimumab therapy. His growth hormone and gonadal axes remained intact. Since corticortrophs are the most resistant to radiation damage (22, 23), the normal growth hormone and gonadal axes combined with severe ACTH deficiency make radiation-induced isolated ACTH deficiency unlikely. For these reasons, it was concluded that brain radiation was not the cause of the hypopituitarism in these two patients.
Pituitary enlargement is one of the major concerns in these patients, because the enlarged pituitary may compress the optic chiasm and cause visual field deficits. In very rare cases, inflammation associated with autoimmune hypophysitis (non-ipilimumab-related) was reported to extend to the cavernous sinus and result in progressive internal carotid artery stenosis as well as rapid visual deterioration (24). In the current and in another recent study (9), no patient had radiographic evidence of optic nerve compression or cavernous sinus extension. All patients had gross visual field exams and some (n=4) had formal visual field testing; none had hypophysitis-related visual field deficits. The lack of pituitary imaging prior to ipilimumab therapy and variable intervals between radiographic studies in some patients may have underestimated the incidence of pituitary enlargement, since transient and mild pituitary enlargement may occur in some patients (9). In this study, most of the enlarged pituitaries normalized in size in serial radiographic studies, in both corticosteroid replacement and HDS groups.
Although the mechanisms are not known, it appears that corticotrophs and thyrotrophs are more vulnerable to ipilimumab-related damage than other anterior pituitary cells, since ACTH and TSH deficiencies were most commonly seen in these patients (Table 2). The recovery of corticotroph function appears to be rare, since resolution of secondary adrenal insufficiency was not observed in this study and was infrequently reported in previous studies (9, 10, 13). The median time to onset of pituitary enlargement was one week earlier than that of biochemical evidence of pituitary hormone deficiency. This finding, consistent with another recent report (9), is important because the first manifestation of hypophysitis in patients on ipilimumab therapy could be the incidental finding of an enlarged pituitary gland during surveillance or restaging brain MRI scan, without initial biochemical evidence of pituitary hormone deficiency. Since secondary adrenal insufficiency, which can be life threatening, occurs in almost every patient with ipilimumab-related hypophysitis, we recommend starting these patients on replacement doses of corticosteroids proactively, even if their initial biochemical tests show normal cortisol levels. Hyponatremia occurred concomitantly with hypophysitis, suggesting a close relationship between these two conditions.
In the ipilimumab package insert, as well as in the literature, a course of HDS is the recommended treatment for patients with ipilimumab-related hypophysitis. However, there have been no compelling data to support this management approach. Similarly, although HDS are commonly used in classic autoimmune hypophysitis in an attempt to reduce inflammation, reduce mass effect symptoms related to the enlarged pituitary, and prevent or reverse pituitary hormone deficiency, the outcomes have been variable (18, 25). In the present study, among 10 patients who did not receive HDS treatment, replacement doses of corticosteroids substantially improved headaches and fatigue. The frequency of resolution and time to resolution did not significantly differ between the groups that did and did not receive HDS (Table 4 and Figure 1). Differences in the severity of hypophysitis cannot account for this finding, since the average toxicity score for the severity of hypophysitis was not different between the two groups. In the case of secondary adrenal insufficiency, no recovery was identified among our patient cohort, regardless of whether they were treated with systemic HDS or not. It is not clear if persistent adrenal insufficiency after HDS withdrawal was due to hypophysitis, adrenal suppression from HDS (26), or both. In any case, HDS did not improve the recovery of corticotroph function. Based on these findings, we conclude that systemic HDS treatment did not improve the outcome of ipilimumab-related hypophysitis.
As a result of its immunosuppressant activity, the potential effects of corticosteroids on the anti-cancer activity of immune checkpoint inhibition must be taken into consideration. In a previous study, the median duration of response was somewhat shorter in patients who received systemic HDS, although there was no statistically significant effect on the overall duration of clinical response (13). In the present study, we observed an 83% one-year survival rate in the full cohort, which surpasses survival rates reported in previously published trials of ipilimumab (3, 27). A higher rate of mortality was observed in the group treated with HDS; however, the distributions of overall survival were not statistically significantly different between groups (Figure 2). The survival outcomes of our study concur with those of previous studies (13, 15), which have questioned the null effect of HDS on survival outcome. Based on the outcome of this study (albeit retrospective), we recommend initiation of hormone replacement without routine systemic HDS in patients with ipilimumab-related hypophysitis. Our study is limited by its retrospective nature, limited size, and variability of the timing of initial and subsequent hormonal and radiographic evaluation.
Statement of translational relevance.
Ipilimumab, a monoclonal antibody against CTLA4 has demonstrated promising and durable anti-cancer effect. Consistent with its mechanism of action of de novo stimulation or enhancement of pre-existing T cell responses, a number of immune-related adverse events (irAEs) have been observed in patients treated with ipilimumab. Systemic high dose corticosteroids (HDS) have been routinely used to manage irAEs. However, the crosstalk between glucocorticoid receptor activation and CTLA-4 inhibition remains to be elucidated. We studied the onset and outcome of ipilimumab-related hypophysitis and the response to treatment with systemic HDS. We did not find any benefit effect of HDS on the outcome of hypophysitis and the malignancy. Our study implies that crosstalk between CTLA-4 inhibition and glucocorticoid receptor activation is a complex process that produces complicated effects on the clinical outcome of CTLA-4 inhibition therapy. Systemic high dose corticosteroid therapy in patients with ipilimumab-related hypophysitis may not be indicated.
Acknowledgments
This research was supported by NICHD/NIH K08 HD070957 (to L.M.).
Footnotes
Financial disclosure:
Le Min: Consulting and advisory role: Merck.
F. Stephen Hodi: Consulting and advisory role: Merck, Bristol-Myers Squibb, Genentech, Novartis. Research funding: Merck, Bristol-Myers Squibb, Genentech.
Jason J. Luke: Consulting and advisory role: Genentech, Amgen and Bayer.
Ursula Kaiser: Consulting and advisory role: Novo Nordisk, Takeda.
The remaining authors do not have financial disclosures.
References
- 1.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–94. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119:1675–82. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 6.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 7.Blansfield JA, Beck KE, Tran K, Yang JC, Hughes MS, Kammula US, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–8. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min L, Vaidya A, Becker C. Association of ipilimumab therapy for advanced melanoma with secondary adrenal insufficiency: a case series. Endocr Pract. 2012;18:351–5. doi: 10.4158/EP11273.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, et al. Ipilimumab-induced Hypophysitis: A Detailed Longitudinal Analysis in a Large Cohort of Patients with Metastatic Melanoma. J Clin Endocrinol Metab. 2014:jc20142306. doi: 10.1210/jc.2014-2306. [DOI] [PubMed] [Google Scholar]
- 10.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371–81. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JS. Practical Management of Immune-Related Adverse Events from Immune Checkpoint Protein Antibodies for the Oncologist. Am Soc Clin Oncol Educ Book. 2012;32:174–7. doi: 10.14694/EdBook_AM.2012.32.79. [DOI] [PubMed] [Google Scholar]
- 12.Corsello SM, Salvatori R, Barnabei A, De Vecchis L, Marchetti P, Torino F. Ipilimumab-induced endocrinopathies: when to start corticosteroids (or not) Cancer Chemother Pharmacol. 2013;72:489–90. doi: 10.1007/s00280-013-2213-y. [DOI] [PubMed] [Google Scholar]
- 13.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–8. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–9. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459–65. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 16.Johannsson G, Falorni A, Skrtic S, Lennernas H, Quinkler M, Monson JP, et al. Adrenal insufficiency: review of clinical outcomes with current glucocorticoid replacement therapy. Clinical endocrinology. 2014 doi: 10.1111/cen.12603. [DOI] [PubMed] [Google Scholar]
- 17.Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98:1361–75. doi: 10.1210/jc.2012-4075. [DOI] [PubMed] [Google Scholar]
- 18.Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. Autoimmune hypophysitis. Endocr Rev. 2005;26:599–614. doi: 10.1210/er.2004-0011. [DOI] [PubMed] [Google Scholar]
- 19.Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2009;13:29–38. doi: 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 20.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6:230ra45. doi: 10.1126/scitranslmed.3008002. [DOI] [PubMed] [Google Scholar]
- 21.Hamnvik OP, Laury AR, Laws ER, Jr, Kaiser UB. Lymphocytic hypophysitis with diabetes insipidus in a young man. Nat Rev Endocrinol. 2010;6:464–70. doi: 10.1038/nrendo.2010.104. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez A, Brada M, Zabuliene L, Karavitaki N, Wass JA. Radiation-induced hypopituitarism. Endocr Relat Cancer. 2009;16:733–72. doi: 10.1677/ERC-08-0231. [DOI] [PubMed] [Google Scholar]
- 23.Darzy KH. Radiation-induced hypopituitarism after cancer therapy: who, how and when to test. Nature clinical practice Endocrinology & metabolism. 2009;5:88–99. doi: 10.1038/ncpendmet1051. [DOI] [PubMed] [Google Scholar]
- 24.Buxton N, Robertson I. Lymphocytic and granulocytic hypophysitis: a single centre experience. Br J Neurosurg. 2001;15:242–5. doi: 10.1080/02688690120057664. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 25.Kristof RA, Van Roost D, Klingmuller D, Springer W, Schramm J. Lymphocytic hypophysitis: non-invasive diagnosis and treatment by high dose methylprednisolone pulse therapy? Journal of neurology, neurosurgery, and psychiatry. 1999;67:398–402. doi: 10.1136/jnnp.67.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinsen S, Baslund B, Klose M, Rasmussen AK, Friis-Hansen L, Hilsted L, et al. Why glucocorticoid withdrawal may sometimes be as dangerous as the treatment itself. European journal of internal medicine. 2013;24:714–20. doi: 10.1016/j.ejim.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. Jama. 2014;312:1744–53. doi: 10.1001/jama.2014.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]