Abstract
Objective: To determine the diagnostic features of Robertsonian (Rob) translocation (11; 13) in mice and the mechanisms underlying the effect on spermatogenesis and reproductive decline. Methods: A Rob translocation (11; 13) mouse model was established by cross-breeding, and confirmed by chromosome analysis. Chromosome aberrations and translocation patterns were identified in mice with Rob translocation (11; 13) by fluorescence in situ hybridization (FISH). Spermatogenic disorders were investigated at different stages of spermatogenesis. Immunofluorescent analysis was performed on sections of testis and epididymis specimens during spermatogenic meiosis. The weight of the testes and reproductive decline were recorded. Results: The crossed Rob translocation (11; 13) mouse has 39 chromosomes, including a fusion chromosome (included chromosomes 11 and 13) using dual color FISH. There was no difference in the distribution pattern of SYCP3 and γH2AX in spermatocytes between Rob translocation and wild-type mice; however, round haploid spermatids presented characteristic morphologic changes of apoptosis and the number of haploid spermatids was decreased. Furthermore, the immature germ cells were released into the epididymis and the number of mature sperm was reduced. Conclusions: Chromosome aberrations and spermatogenic disorders may result from apoptosis of round haploid spermatids and a reduced number of mature sperm in Rob translocation (11; 13) mice. Abnormal sperm and reduced number of sperm may be one of the main reasons for reproductive decline and male infertility in Rob translocation (11; 13) mice.
Keywords: Infertility, spermatogenesis, Robertsonian translocation, mouse
Introduction
Infertility and sterility continue to be important medical conditions worldwide in the 21st century. An estimated 10%-15% of couples of reproductive age have infertility. Male factor infertility accounts for approximately 50% of the cases associated with genetic and non-genetic conditions [1]. Chromosomal abnormalities are frequently detected in infertile men; the incidence of chromosomal abnormalities in infertile couples is approximately 7% [2], particularly in men with low sperm counts [3-6]. Robertsonian (Rob) translocations with chromosomal abnormalities are amongst the most common chromosomal rearrangements in mammals [7]. Rob translocations are created by a fusion of two acrocentric chromosomes (homologous or non-homologous chromosomes) after breakage and loss of the p-arms [8]; Rob translocations are often the cause of male infertility [9,10]. Carriers of Rob translocations are phenotypically normal, but these men are at high risk for infertility related to severe oligozoospermia, abnormal gametes, and chromosomally-unbalanced offspring [11].
Spermatogenesis is a complex differentiation process essential for all animal species that reproduce sexually. This process can be divided into three stages, as follows: mitosis in spermatogonia; meiosis in spermatocytes; and spermiogenesis [12]. During meiosis, homologous chromosomes align, pair, synapse, recombine, and separate, resulting in haploid cells with distinct sets of genetic material [13]. At the molecular level, spermatogenesis is highly organized and involves the expression and interaction of numerous enriched or specific genes. Abnormal translocation carriers may affect gene-expressed disorders, meiotic arrest, failure of spermatogenesis, and infertility [14-16]. Rob translocations can affect infertility with various degrees of sperm alterations in humans or the pregnancy outcomes of carriers; However, the diagnostic feature and mechanisms by which Rob translocations affect spermatogenesis and the reproductive decline in mammals is poorly understood. Because of the high frequency of Rob translocations, it is important to establish a mouse model to further our knowledge of this important mutational process.
In the present study we established a Rob translocation (11; 13) mouse model by cross-breeding, which was confirmed with chromosome analysis and FISH. The chromosomal aberrations of Rob translocation mice can serve as a model for analysis of the impact of the rearrangement on spermatogenesis and reproductive decline. The results showed that meiosis in the Rob translocation (11; 13) mouse model is valid; however, a number of round haploid spermatids were apoptotic and the number of haploid spermatids was decreased. Moreover, the weight of Rob translocation mouse testis was less than wild mice during the same period, including the reproductive decline. These data showed that Rob translocation may be caused by chromosome aberrations and spermatogenic disorders. Spermatogenic disorders may be one of the main reasons for reproductive decline and male infertility. These results provide additional support to understand the infertility of Rob translocation carriers in humans. Studies on spermatogenesis from translocation carriers and in mouse models may help us to understand the mechanism underlying meiotic segregation.
Materials and methods
Ethics statement
All animal experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals issued by the Peking University Third Hospital in Beijing. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Peking University Third Hospital (protocol number: 2013SZ021).
Mouse and crosses
Homozygous Rob translocation mice [2 pairs homozygous for Rb (11; 13) 4Bnr x homozygous for Rb (11; 13) 4Bnr] were purchased from Jackson Laboratory (Stock Number 000729; USA) and CD-1 mice were purchased from the Experiment Animal Center of Peking University Health Science Center. Heterozygous males were generated by reciprocal crosses between homozygous Rob mice and CD-1 mice, and F1 heterozygous carriers of a Rob translocation chromosome (11; 13). All mice were bred in a pathogen-free environment with water, fed a mouse breeder diet, and maintained in a temperature- and humidity-controlled room. Mice were sacrificed by cervical dislocation.
Karyotype and reagents
Chromosomes were prepared from lymphocyte cultures of three male mice (F1 heterozygous Rob mice). The lymphocytes were sampled aseptically and cultured for 72 h at 37.5°C in 5 ml of RPMI-1640 medium and 3 μg/ml of phytohemagglutinin (PHA), as previously reported [17]. Colchicine (0.04 μg/ml, final concentration) was added to the cultures 2 h before the cells were harvested. Cells were first hypotonized in KCl (0.075 M) for 28 min, thrice-fixed in Carnoy’s fixative (acetic acid/methanol [1:3]), and dropped onto clean microscopic slides. Then, the metaphase chromosome spreads in microscopic slides were stained by Giemsa’s stain solution. Heterozygous Rob mouse metaphase spreads samples with good quality were used for chromosome analysis. Unless specified otherwise, the general reagents were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA) and Invitrogen.
FISH
To verify the karyotype of the F1 heterozygous Rob translocations on chromosomes (11; 13), dual color FISH with specific alphoid probes for the mouse chromosome 11 whole painting probe (XMP11 green; MetaSystems GmbH, Altlussheim, Germany) and mouse chromosome 13 whole painting probe (XMP13 green; MetaSystems) were prepared in F1 Rob translocation mouse metaphase chromosome spreads and controls.
The painting probes were first denatured for 10 min at 70°C, then placed on ice. Slides with F1 Rob metaphase spreads were denatured by formamide, as described [18]. Briefly, the metaphase chromosome spreads were baked at 65°C for 1 h, then incubated for 30 min in 70% (v/v) formamide in 2 × SSC at 80°C to denature the mouse chromosome, and quenched in ice cold 70% (v/v) ethanol for 5 min at room temperature. The metaphase samples were chilled in cold 70% (v/v) ethanol for 2 min, 90% ethanol for 2 min, 100% ethanol for 5 min, and air-dried at room temperature. The hybridization solution and denatured painting probe were added to the denatured metaphase spreads and sealed and placed in a moist box at 37°C for 20 h.
After incubation, the cover slips were removed and the mouse metaphase spreads were washed twice with 50% (v/v) formamide in 2 × SSC for 10 min, 3 times in 2 × SSC for 5 min, and washed in 0.1% TritonX-100/4 × SSC for 5 min. Mouse metaphase spreads were stained using DAPI (1 μg/ml) for 5 min and mounted on microscope slides. The procedure of FISH was similar to previous reports [19,20]. For FISH signal detection, the signals were observed and analyzed in a fluorescence microscope (Axio Imager Z1; Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA) fitted with a CCD camera (AxioCam MRm; Zeiss ) and automated image software (FISH Imaging System, version 5.1; MetaSystems GmbH).
Immunohistochemistry and immunocytochemistry
Immunohistochemistry was performed using standard manipulations. The protocol was basically the same as described in our previous study [21]. Briefly, 8-µm frozen sections of mouse testis and epididymis from F1 Rob translocation mice were fixed immediately in 4% paraformaldehyde for 15 min at room temperature. After blocking, the sections were stained using anti-SYCP3 (1:200; Abcam), anti-γH2AX (1:200; Upstate), or pre-immune rabbit serum as a negative control for 1 h at room temperature. Secondary antibody was FITC-conjugated or TRITC-conjugated anti-rabbit antibody (1:500), followed by incubation with DAPI (Sigma-Aldrich).
Statistical analysis
Statistical analysis was carried out using SPSS software (SPSS Statistics 17.0.1, SPSS, Inc., Chicago, IL, USA). Each experiment was repeated at least three times and differences were considered significant at P < 0.05.
Results
Crossed and identified Rob translocation carriers
The wild type mouse karyotype is comprised of 2n = 40 telocentric chromosomes. The homozygous Rob mouse [carrying a pair of Rob (11; 13) chromosomes] were found (2n = 38) using the chromosome analysis; however, the offspring (F1) produced by the cross between a homozygous Rob mouse and a wild-type mouse have 39 chromosomes, which includes a heterozygous Rob fusion chromosome (Figure 1A) according to chromosome karyotype analysis of 30 male F1 Rob translocation and CD-1mice.
Figure 1.

Metaphase chromosome spreads from F1 Rob translocation mouse lymphocytes and FISH. A. Metaphase chromosome spreads from F1 Rob translocation mice, including a fusion chromosome; B. Hybridization signals on the Rob translocation fusion chromosome (orange and green) obtained with dual color labeled probes. Images were captured with laser confocal microscopy. The scale bars represent 10 μm for all images.
To validate the Rob translocation chromosomes, F1 Rob translocation mouse chromosomes can be characterized by FISH mapping with dual color whole painting probes. DNA probes derived from mouse chromosome13 (orange) and 11 (green) were simultaneously hybridized to the Rob translocation metaphase chromosome spreads. The hybridized signals were clearly visible with the probes (orange-and green-labeled, respectively) showing specific hybridization signals on the Rob translocation chromosome (Figure 1B), especially in the Rob fusion chromosome (orange and green). High hybridization efficiency and consistent signals were observed for all chromosome spreads. This result indicated that a single translocation carrier mouse model (11; 13) was established.
Spermatogenesis in Rob translocation mice
Spermatogenesis is a complex developmental process representing the complexity of spermatogenic gene expression. To investigate spermatogenesis in the Rob translocation, immunostaining was performed on testis sections (Figure 2). SYCP3 is essential for the formation and maintenance of the synaptonemal complex (SC) localized to the nuclei of meiosis phases of spermatocytes on the testis sections of Rob translocation mouse (Figure 2A). There was no signification difference in the SYCP3 distribution pattern in spermatocytes between Rob translocation mice and wild-type mice; however, the round haploid spermatids presented characteristic morphologic changes of apoptosis and the number of haploid spermatids was decreased.
Figure 2.
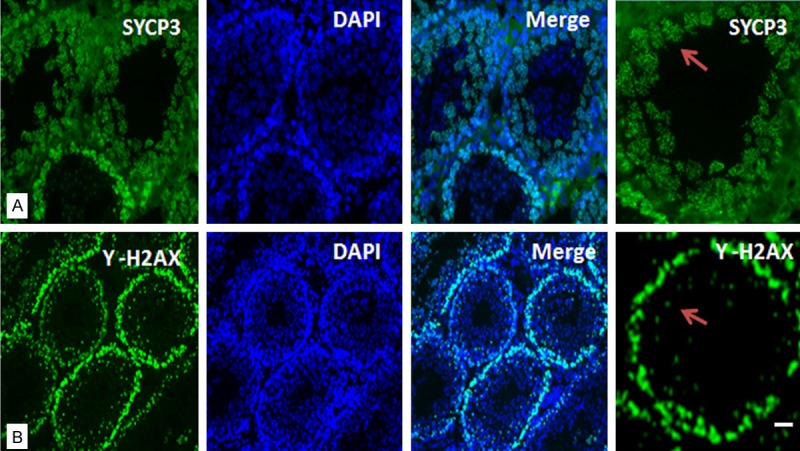
Rob translocation mouse testis sections were detected by immunostaining. A. SYCP3 (green) was used as a marker for identifying germ cells at different stages of prophase of the first division of meiosis. B. γ-H2AX was stained green. DNA was revealed by the blue DAPI staining. Low magnification and high magnification images. Images were captured with laser confocal microscopy. The scale bars represent 100 μm.
Phosphorylation of H2AX histone (γH2AX), which is a marker protein localized in the sex body during meiosis, was detected on the sections (Figure 2B) during further examination of spermatogenesis. γH2AX was detected and localized in the spermatocytes on the adult F1 Rob translocation mouse testis sections. The protein expression pattern of γH2AX matched wild-type mouse testis sections. The succession of meiosis in F1 Rob translocation mice proceeds according to the expected pattern in the spermatocyte stage.
Mature sperm was reduced in Rob translocation mice
To further examine the number of sperm in F1 Rob translocation mice, immunostaining was performed on testis and epididymis sections. Different stages in the development of sperm cell bundles were observed on the testis sections of Rob translocation mice. There were no differences in the spermatocytes between the Rob translocation mice and wild-type mice in the spermatocyte stage; however, immature germ cells were released into the epididymis under fluorescence microscopy on the epididymis sections of F1 Rob translocation mice (Figure 3A and 3B). In the same period, immature germ cells were not found in the epididymis in wild-type mice (Figure 3C). Apoptosis of the round haploid spermatids led to a reduction in immature sperm compared with normal mice.
Figure 3.

Rob translocation mouse and wild-type mouse epididymis sections were detected by immunostaining (DAPI stain). A. 4 W of F1 Rob translocation mouse epididymis sections; B. Adult F1 Rob translocation mouse epididymis sections; C. Adult wild-type mouse epididymis sections; DNA was revealed by blue DAPI staining. Low magnification and high magnification images. Images were captured with laser confocal microscopy. The scale bars represent 100 μm.
Rob translocation mice have smaller testes than wild-type mice
To further study a possible reduction in Rob mice, we analyzed the size and weight of the testes and sperm parameters. There was a significant difference between the wild mouse group and the F1 Rob translocation mouse group with respect to the size and weight of the testes. The relative size and weight of the tests in the F1 Rob translocation mouse group was smaller than the wild-type mouse group (Figure 4). There was no significant difference in sperm morphology between the F1 Rob translocation and wild-type mouse groups; however, the number of sperm with fast progressive motility and sperm count were reduced (data not shown).
Figure 4.

Weight of adult F1 Rob translocation mouse and wild-type mouse testis.
To determine whether or not the succession of maturation stages is altered in Rob translocation mice, the testis and epididymis of wild-type and Rob translocation mice were subjected to histological analysis and immunostaining. The testis and epididymis have the similar maturation stages between wild-type and Rob translocation mice during the various stages in the seminiferous epithelium cycle. Thus, the succession of maturation stages is valid.
Discussion
In mammals, Rob translocation is one of the most common chromosomal rearrangements [22]. Most Rob translocation carriers are potentially fertile, but produce high numbers of unbalanced gametes that frequently result in infertility and spontaneous abortions [23]. The diagnostic features of the Rob translocation and the mechanisms which affect spermatogenesis and reproductive decline in mammalian spermatogenesis are poorly understood. In fact, more than 100 different Rob chromosomes have been found in the house mouse involving all 19 autosomes and the X chromosome [24]. Different translocations may have a different impact on fertility and spermatogenesis. It is necessary to establish and examine the frequency and clinical relevance of the Rob translocation chromosome model in mice. Stock No.000729 mice acquired from the Jackson Laboratory carry a Robertsonian chromosome consisting of chromosomes 11 and 13, which may be useful for studies of retinal degeneration, cancer, reproduction/meiosis, birth defects, aneuploidy generation, genomic studies, or genetic mapping. What is important is that many genes map on mouse chromosome 11 and some genes are located on human chromosome 17 that are associated with male fertility. In this study, we established a Rob translocation (11; 13) mouse model, which was produced by a cross between a Rob translocation mouse [carrying a pair of Rob (11; 13) chromosomes] and a wild-type mouse. Heterozygotes for Rob chromosomes have a tendency to be infertile or to produce offspring with birth defects. To validate the established F1 heterozygous Rob translocation mouse model, mouse chromosomes which have 39 chromosomes and a fusion chromosome were characterized by dual color FISH and chromosome analysis. These results confirmed that we had established a Rob translocation (11; 13) mouse model which will extend our knowledge of infertility.
Meiosis is a complex process by which diploid germ cells from both genders of sexually-reproducing organisms produce haploid gametes such that pairs of gametes from each gender fuse during fertilization to give diploid offspring [13]. The SC and chromosomes separate and may play an important role in meiosis. SYCP3 participate in the assembly of SC, which is a key step of meiosis. In the current study we did not find significant differences between wild type and F1 Rob translocation mice during the relative frequency of early stages in the spermatocyte stage. We found the succession of maturation stages in Rob translocation mice was valid using histological analysis and immunostaining. Apoptosis of round spermatids were reduced. Thus, apoptosis of round spermatids in Rob translocation mice may have meiotic chromosomal aberrations. Robertsonian translocation carriers may have an asynapsis and meiotic disorder in spermatocytes [25]. Abnormal spermatogenesis may occur from Rob translocation and reproductive decline. In the current study we found an abundance of round haploid spermatids that presented with characteristic morphologic changes in apoptosis and the number of round haploid spermatids was decreased. Interestingly, the number of mature sperms as well as the size and weight of the testes were reduced compared with normal mice. Apoptosis of round spermatids may be one of the reasons Rob translocation (11; 13) carriers have infertility or fecundity decline.
Mouse chromosome 11 has previously been shown to contain many important genes for male and female fertility [26], such as ubiquitin-conjugating enzyme 2b (Ube2b), which results in a block in gametogenesis [27], Trp53, which determines inheritance in mouse gametes and embryo function in vitro [28], an allele of the testicular isoform of angiotensin-converting enzyme (Ace-T), which results in complete male infertility [29], growth differentiation factor-9 (GDF-9), which results in an early block in ovarian folliculogenesis [30], Wnt3, which is essential for Wnt signaling control and adult spermatogenesis [31], and Dnahc2, which is associated with a significant reduction in epididymal sperm count, abnormal epididymal sperm morphology, and a complete absence of sperm motility [32]. CATSPER1 is one of four members of the sperm-specific CATSPER voltage-gated calcium channel family, which is known to be essential for normal male fertility in mice [33]. Many genes map on mouse chromosome 11 and some genes located on human chromosome 17 are associated with male fertility. SPAG9, a single copy gene that maps to human chromosome 17q21.33 with a location on mouse chromosome 11, was previously shown to be expressed exclusively in testis and is a sperm-associated JNK-binding protein that may have a role in the spermatozoa-oocyte interaction [34]. Moreover, mouse chromosome 13 has been shown to contain many important genes for male and female fertility. In the current study we found that apoptosis of round haploid spermatids and immature sperm were reduced in the F1 Rob translocation (11; 13) mouse. Gene expression disorders may cause spermatogenic disorders. These data indicated that the Rob translocation (11; 13) may lead to some genes associated with male fertility having disturbances in transcriptional activityand gene silencing in chromosome 11 and chromosome 13. The recently reported Rob translocation carriers may have asynapsis and meiotic silencing in spermatocytes [25], oogenesis, and embryo development [35-37].
In conclusion, we have established a Rob translocation (11; 13) mouse model. The present study provides strong evidence that Rob translocations may have an impact on chromosome aberrations and also be associated with spermatogenic disorders and reproductive decline. These results provide additional support to understand the mechanism underlying chromosome aberrations and spermatogenesis, and may even help to understand the infertility of Rob translocation carriers in humans.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NO. 81070534) and National Natural Science Foundation of China for Young Scholars (NO. 81200466).
Disclosure of conflict of interest
None.
References
- 1.Almeida C, Doria S, Moreira M, Pinto J, Barros A. Normal sperm in a 2;2 homologous male translocation carrier. J Assist Reprod Genet. 2012;29:665–668. doi: 10.1007/s10815-012-9770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniely M, Aviram-Goldring A, Barkai G, Goldman B. Detection of chromosomal aberration in fetuses arising from recurrent spontaneous abortion by comparative genomic hybridization. Hum Reprod. 1998;13:805–809. doi: 10.1093/humrep/13.4.805. [DOI] [PubMed] [Google Scholar]
- 3.Davies W, Isles A, Smith R, Karunadasa D, Burrmann D, Humby T, Ojarikre O, Biggin C, Skuse D, Burgoyne P, Wilkinson L. Xlr3b is a new imprinted candidate for X-linked parent-of-origin effects on cognitive function in mice. Nat Genet. 2005;37:625–629. doi: 10.1038/ng1577. [DOI] [PubMed] [Google Scholar]
- 4.Vegetti W, Van Assche E, Frias A, Verheyen G, Bianchi MM, Bonduelle M, Liebaers I, Van Steirteghem A. Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in-situ hybridization in infertile men. Hum Reprod. 2000;15:351–365. doi: 10.1093/humrep/15.2.351. [DOI] [PubMed] [Google Scholar]
- 5.Harkonen K, Suominen J, Lahdetie J. Aneuploidy in spermatozoa of infertile men with teratozoospermia. Int J Androl. 2001;24:197–205. doi: 10.1046/j.1365-2605.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Huang J, Liu P, Qiao J. Analysis of meiotic segregation patterns and interchromosomal effects in sperm from six males with Robertsonian translocations. J Assist Reprod Genet. 2007;24:406–411. doi: 10.1007/s10815-007-9137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poongothai J, Gopenath TS, Manonayaki S. Genetics of human male infertility. Singapore Med J. 2009;50:336–347. [PubMed] [Google Scholar]
- 8.Vozdova M, Oracova E, Kasikova K, Prinosilova P, Rybar R, Horinova V, Gaillyova R, Rubes J. Balanced chromosomal translocations in men: relationships among semen parameters, chromatin integrity, sperm meiotic segregation and aneuploidy. J Assist Reprod Genet. 2013;30:391–405. doi: 10.1007/s10815-012-9921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munne S. Analysis of chromosome segregation during preimplantation genetic diagnosis in both male and female translocation heterozygotes. Cytogenet Genome Res. 2005;111:305–309. doi: 10.1159/000086904. [DOI] [PubMed] [Google Scholar]
- 10.Acloque H, Bonnet-Garnier A, Mompart F, Pinton A, Yerle-Bouissou M. Sperm nuclear architecture is locally modified in presence of a Robertsonian translocation t(13;17) PLoS One. 2013;8:e78005. doi: 10.1371/journal.pone.0078005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaffer LG. Risk estimates for uniparental disomy following prenatal detection of a nonhomologous Robertsonian translocation. Prenat Diagn. 2006;26:303–307. doi: 10.1002/pd.1384. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang XJ, Shi YQ, Xu B, Chen L, Tang WH, Huang J, Lian Y, Liu P, Qiao J. SLX2 interacting with BLOS2 is differentially expressed during mouse oocyte meiotic maturation. Cell Cycle. 2014;13:2231–7. doi: 10.4161/cc.29265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi YQ, Zhuang XJ, Xu B, Hua J, Liao SY, Shi Q, Cooke HJ, Han C. SYCP3-like X-linked 2 is expressed in meiotic germ cells and interacts with synaptonemal complex central element protein 2 and histone acetyltransferase TIP60. Gene. 2013;527:352–359. doi: 10.1016/j.gene.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Jaafar H, Gabriel-Robez O, Rumpler Y. Chromosomal anomalies and disturbance of transcriptional activity at the pachytene stage of meiosis: relationship to male sterility. Cytogenet Cell Genet. 1993;64:273–280. doi: 10.1159/000133592. [DOI] [PubMed] [Google Scholar]
- 15.Ashley T, Gaeth AP, Creemers LB, Hack AM, de Rooij DG. Correlation of meiotic events in testis sections and microspreads of mouse spermatocytes relative to the mid-pachytene checkpoint. Chromosoma. 2004;113:126–136. doi: 10.1007/s00412-004-0293-5. [DOI] [PubMed] [Google Scholar]
- 16.de Boer P, Searle AG, van der Hoeven FA, de Rooij DG, Beechey CV. Male pachytene pairing in single and double translocation heterozygotes and spermatogenic impairment in the mouse. Chromosoma. 1986;93:326–336. doi: 10.1007/BF00327591. [DOI] [PubMed] [Google Scholar]
- 17.Zhuang XJ, Lu YQ, Zhang M, Lu SS, Lu KH. Microisolation and microcloning of bovine X-chromosomes for identification of sorted buffalo (Bubalus bubalis) spermatozoa. Anim Reprod Sci. 2011;126:32–36. doi: 10.1016/j.anireprosci.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Shim SH, Kyhm JH, Chung SR, Kim SR, Park MI, Lee CH, Cho YH. Generation of FISH probes using laser microbeam microdissection and application to clinical molecular cytogenetics. J Microbiol Biotechnol. 2007;17:1079–1082. [PubMed] [Google Scholar]
- 19.Sohn SH, Cho EJ, Son WJ, Lee CY. Diagnosis of bovine freemartinism by fluorescence in situ hybridization on interphase nuclei using a bovine Y chromosome-specific DNA probe. Theriogenology. 2007;68:1003–1011. doi: 10.1016/j.theriogenology.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Hill FS, Marchetti F, Liechty M, Bishop J, Hozier J, Wyrobek AJ. A new FISH assay to simultaneously detect structural and numerical chromosomal abnormalities in mouse sperm. Mol Reprod Dev. 2003;66:172–180. doi: 10.1002/mrd.10299. [DOI] [PubMed] [Google Scholar]
- 21.Shi YQ, Liao SY, Zhuang XJ, Han CS. Mouse Fem1b interacts with and induces ubiquitin-mediated degradation of Ankrd37. Gene. 2011;485:153–159. doi: 10.1016/j.gene.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Kasikova K, Vozdova M, Prinosilova P, Gaillyova R, Hanakova M, Rubes J. Sperm meiotic segregation, aneuploidy and high risk of delivering an affected offspring in carriers of non-Robertsonian translocation t(13; 15) J Assist Reprod Genet. 2012;29:693–698. doi: 10.1007/s10815-012-9767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douet-Guilbert N, Bris MJ, Amice V, Marchetti C, Delobel B, Amice J, Braekeleer MD, Morel F. Interchromosomal effect in sperm of males with translocations: report of 6 cases and review of the literature. Int J Androl. 2005;28:372–379. doi: 10.1111/j.1365-2605.2005.00571.x. [DOI] [PubMed] [Google Scholar]
- 24.Nachman MW, Searle JB. Why is the house mouse karyotype so variable? Trends Ecol Evol. 1995;10:397–402. doi: 10.1016/s0169-5347(00)89155-7. [DOI] [PubMed] [Google Scholar]
- 25.Naumova AK, Fayer S, Leung J, Boateng KA, Camerini-Otero RD, Taketo T. Dynamics of response to asynapsis and meiotic silencing in spermatocytes from Robertsonian translocation carriers. PLoS One. 2013;8:e75970. doi: 10.1371/journal.pone.0075970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadi S, Wade-Martins R. Familial Alzheimer’s disease coding mutations reduce Presenilin-1 expression in a novel genomic locus reporter model. Neurobiol Aging. 2014;35:443.e5–443.e16. doi: 10.1016/j.neurobiolaging.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Grootegoed JA, Baarends WM, Roest HP, Hoeijmakers JH. Knockout mouse model and gametogenic failure. Mol Cell Endocrinol. 1998;145:161–166. doi: 10.1016/s0303-7207(98)00183-x. [DOI] [PubMed] [Google Scholar]
- 28.Li A, Ganeshan L, O’Neill C. The effect of Trp53 gene-dosage and parent-of-origin of inheritance on mouse gamete and embryo function in vitro. Biol Reprod. 2012;86:175. doi: 10.1095/biolreprod.111.097741. [DOI] [PubMed] [Google Scholar]
- 29.Ramaraj P, Kessler SP, Colmenares C, Sen GC. Selective restoration of male fertility in mice lacking angiotensin-converting enzymes by sperm-specific expression of the testicular isozyme. J Clin Invest. 1998;102:371–378. doi: 10.1172/JCI3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 31.Kerr GE, Young JC, Horvay K, Abud HE, Loveland KL. Regulated Wnt/beta-catenin signaling sustains adult spermatogenesis in mice. Biol Reprod. 2014;90:3. doi: 10.1095/biolreprod.112.105809. [DOI] [PubMed] [Google Scholar]
- 32.Clark AT, Firozi K, Justice MJ. Mutations in a novel locus on mouse chromosome 11 resulting in male infertility associated with defects in microtubule assembly and sperm tail function. Biol Reprod. 2004;70:1317–1324. doi: 10.1095/biolreprod.103.020628. [DOI] [PubMed] [Google Scholar]
- 33.Avenarius MR, Hildebrand MS, Zhang Y, Meyer NC, Smith LL, Kahrizi K, Najmabadi H, Smith RJ. Human male infertility caused by mutations in the CATSPER1 channel protein. Am J Hum Genet. 2009;84:505–510. doi: 10.1016/j.ajhg.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jagadish N, Rana R, Selvi R, Mishra D, Garg M, Yadav S, Herr JC, Okumura K, Hasegawa A, Koyama K, Suri A. Characterization of a novel human sperm-associated antigen 9 (SPAG9) having structural homology with c-Jun N-terminal kinase-interacting protein. Biochem J. 2005;389:73–82. doi: 10.1042/BJ20041577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Chen G, Lian Y, Gao X, Huang J, Qiao J. A normal birth following preimplantation genetic diagnosis by FISH determination in the carriers of der(15)t(Y;15)(Yq12;15p11) translocations: two case reports. J Assist Reprod Genet. 2007;24:483–488. doi: 10.1007/s10815-007-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J, Lian Y, Qiao J, Chen Y, Ren XL, Liu P. Characteristics of embryo development in Robertsonian translocations’ preimplantation genetic diagnosis cycles. Prenat Diagn. 2009;29:1167–1170. doi: 10.1002/pd.2376. [DOI] [PubMed] [Google Scholar]
- 37.Jin H, Ping L, Jie Q, Ying L, Yongjian C. Translocation chromosome karyotypes of the Robertsonian translocation carriers’ embryos. Fertil Steril. 2010;93:1061–1065. doi: 10.1016/j.fertnstert.2008.11.020. [DOI] [PubMed] [Google Scholar]


