Abstract
Objective: The goal of this study was to evaluate the effect of protein energy malnutrition on protein glycosylation by investigating transferrin isoform pattern and its relationship to the degree of malnutrition and the biochemical markers of nutritional status in children.
Methods: Forty one children with mild (n=23) and severely/moderately (n=18) acute malnutrition and 29 controls were enrolled in the study. Serum transferrin isoforms were determined by isoelectric focusing electrophoresis. Transferrin, prealbumin, zinc, iron and insulin-like growth factor-1 (IGF-1) were measured using automated analyzers.
Findings : Asialotransferrin and disialotransferrin were significantly higher in severely/moderately malnourished patients compared to controls (P=0.04 and P=0.04, respectively). Other transferrin isoform patterns were not different among three groups. Serum IGF-1, transferrin and iron levels of severely/ moderately malnourished group were significantly lower than tose of controls (P=0.001, 0.02 and 0.03, respectively). Serum prealbumin and zinc levels were similar in all three groups. Serum IGF-1, transferrin and iron levels, and all transferrin isoform patterns were not significantly different in mildly malnutrition group from other two groups.
Conclusion: The changes in transferrin isoform pattern observed in malnourished patients may indicate that malnutrition is a catabolic state which has effects on glycosylation.
Key Words: Glycosylation; Isoelectric Focusing; Protein Energy Malnutrition; Transferrin; Isoform
Introduction
Protein glycosylation, a result of many complex reactions in Golgi and endoplasmic reticulum[1], is closely related to physiological processes such as cell adhesion, migration, cell growth and cell differentiation[2]. Therefore, defects in glycosylation are associated with several pathological conditions including inflammation, rheumatoid arthritis, cancer, liver diseases[3], galactosemia and fructosemia[4], sepsis[5], bacterial meningitis[6], drugs[7] and chronic alcohol abuse[8,9] in addition to congenital disorders of glycosylation[10-12].
Protein energy malnutrition is the insufficient and imbalanced consumption of nutrients, characterized by catabolism and consequently, severe morbidity and a high mortality rate[13-16]. Since malnutrition is considered as a catabolic state, it may influence the glycosylation process and this effect may be related to the severity of malnutrition.
To date, many studies have been done to define the complex mechanisms of glycosylation and to detect disorders of glycosylation by analyzing different glycoproteins. Isoelectrofocusing of serum transferrin (Tf) is the most widely used screening test for altered glycosylation[11,12]. Based on its sialic acid (SA) content, human Tf contains at least six different isoforms (glycoforms): pentasialo-, tetrasialo-, trisialo-, disialo-, monosialo-, and asialo-transferrin. The asialo, monosialo and disialo isoforms of transferrin constitute the carbohydrate-deficient transferrin (CDT)[7]. CDT is widely known as a laboratory marker of chronic alcohol abuse for over twenty years. There are only few studies that investigated the effect of catabolic state on glycosylation. In these studies, CDT has been detected by different methods[17-19]. The analysis of all Tf isoforms separately was performed in only one of these studies[19]. To our knowledge, there is no study investigating the relationship between protein energy malnutrition and transferrin isoforms in children in the literature.
The aim of this study was to evaluate the effect of acute malnutrition on glycosylation by investigating the possible changes in transferrin isoform pattern in malnutrition and its relationship to the severity of malnutrition in children.
Subjects and Methods
Study population
This study was conducted in Dokuz Eylül University Faculty of Medicine, Department of Pediatrics, Izmir Turkey. Patients admitted with the complaints of feeding difficulties and/or failure to thrive aged between 6 months and 5 years were enrolled in the study. Height for age and weight for height were calculated for the patients and the severity of acute malnutrition was determined according to Waterlow classification[20,21]. Sex- and age-matched healthy children admitted to well child outpatient clinic for routine visits constituted the controls. Children were grouped as healthy controls (n=29); mildly (n=23) and severely/ moderately (n=18) [moderately (n=12) and severely (n= 6)] acute malnourished patients.
The study protocol was approved by the Ethical Committee of the Dokuz Eylul University Faculty of Medicine and informed consent was obtained from all parents/legal representatives. Chronic hepatic, intestinal, renal, neurologic, metabolic and rheumatologic diseases, acute inflammation and drug usage for any disease are considered as criteria for exclusion.
Biochemical analysis
Venous blood (5 mL) was drawn from a peripheral vein, the samples centrifuged at 1500g for 10 minutes and the sera were frozen at -70˚C until analyzed. Tf and prealbumin concentrations were determined immunoturbidimetrically on an auto analyzer (Architect c8000, Abbott Diag., USA). Zinc and iron levels were measured in serum spectrophotometrically on Architect c8000. Serum insulin-like growth factor-1 (IGF-1) was measured by an automated chemiluminescence immunoassay analyzer (Immulite 2500 immunoassay system from Siemens Healthcare Diag, USA).
Isoelectric focusing (IEF) analysis
Tf isoform analysis was performed by isoelectric focusing (IEF) method[22-26]. Serum aliquots were incubated at room temperature for 30 min with FeIII solution buffer to saturate the Tf with iron. Isoelectric focusing was performed on Multiphor II electrophoresis unit (Amersham Pharmacia Biotech, Sweden), followed by immunofixation and staining. In short, immobiline dry plate gels, pH 4–7 (GE Healthcare), were rehydrated and then placed on the ceramic cooling plate of the electrofocusing unit (15˚C) and prefocused for 3500 Vh. After prefocusing, samples (3 μL of each sample) were applied 1.5 cm away from the cathodic end of the gels. Tf isoforms were separated at high voltages. Following IEF, immunofixation was performed using polyclonal rabbit anti-human Tf antibody (Dako) and subsequently Coomassie Brilliant Blue G-250 (CBB G-250, Amresco) staining was applied as described by Blakesley et al[27]. Densitometric analysis of transferrin isoforms was performed using “Image Master ID Elite Software” (Amersham). Ratio of each Tf isoform to total Tf (%) was determined separately and CDT (sum of asialo-, monosialo-, and disialo-Tf): total Tf ratio (%) was also calculated. The imprecision of our method was calculated for disialotransferrin, the major constituent of CDT isoforms, and for tetrasialotransferrin, the major glycoform of all transferrin isoforms, using two levels of control material (Recipe). The coefficients of variation (CV) were determined to be <3.5% for within run (n=7) and <7% for between run (n=20) imprecision.
Statistical methods
Data was evaluated using the Statistical Package for Social Sciences (SPSS) 16.0 program for Windows. Data were expressed as mean±standard deviation. Kruskal Wallis test was used for analyzing group averages among three groups. Mann-Whitney U-test was used for comparing two group averages as a post hoc test. Chi-square test was used for comparing group ratios. Intercorrelations between parameters were computed through the Spearman’s correlation analysis. Correlation coefficient indicated low correlation at 0.10–0.29, medium correlation at 0.30–0.49, and high correlation at ≥0.50. All P-values were two-tailed and group differences or correlations with P<0.05 were considered to be statistically significant.
Findings
The mean age and gender were not significantly different among three groups (Table 1). Weight for height of each group was significantly different from that of the other groups. Besides, height for age of both patient groups was lower than that of the control group (Table 1).
Table 1.
Demographic and anthropometric features of patients and controls
| Parameter |
Severe/moderate
Malnutrition (SMM) (n=18) |
Mild
Malnutrition (MM) (n=23) |
Control group
(CG) (n=29) |
Group comparisons
( P. value) |
|---|---|---|---|---|
| Age (month) | 15.2 (5.48) | 20.78 (11.55) | 20.66 (12.64) | SMM-MM (0.2) SMM-CG (0.06) MM-CG (0.4) |
|
Gender
(Female/Male) |
14/4 | 17/6 | 15/14 | 0.06 |
| Height for age | 88.51 (6.82) | 91.56 (5.98) | 98.8 (3.55) | SMM-MM (0.08) SMM-CG (<0.001) MM-CG (<0.001) |
| Weight for height | 73.47 (4.90) | 84.83 (2.85) | 97.38 (5.00) | SMM-MM (<0.001) SMM-CG (<0.001) MM-CG (<0.001) |
All data presented as mean ± standard deviation and range
Serum IGF-1, transferrin and iron levels of severely/moderately malnourished group were significantly lower than those of controls (P=0.001, 0.02 and 0.03, respectively) (Table 2). On the other hand, these parameters were not significantly different in mildly malnourished group than in the other groups (Table 2). Serum prealbumin and zinc levels were similar in three groups.
Table 2.
Levels of serum nutritional markers and micronutrients in patients and controls (All data presented as mean ± standard deviation and range)
| Parameter |
Severe/moderate
Malnutrition (SMM) (n=18) |
Mild
Malnutrition (MM) (n=23) |
Control group
(CG) (n=29) |
Group comparisons
( P- value) |
|---|---|---|---|---|
|
Transferrin
(mg/dL) |
243.00 (54.90) (133-375) |
275.24 (57.30) (174-378) |
275.75 (41.85) (183-382) |
SMM-MM (0.09) SMM-CG (0.02) MM-CG (0.6) |
|
Prealbumin
(mg/dL) |
17.66 ( 4.71) (10-28) |
17.77 (4.37) (10-31) |
19.03 (3.54) (15-26) |
SMM-MM (0.7) SMM-CG (0.1) MM-CG (0.3) |
|
IGF-1
(ng/mL) |
33.17 ( 11.14) (25-62) |
44.33 (26.56) (25-126) |
57.15 (30.23) (25-130) |
SMM-MM (0.4) SMM-CG (0.001) MM-CG (0.07) |
|
Zinc
(μg/dL) |
57.37 (17.61) (35-98) |
58.96 (16.44) (26-98) |
62.16 (16.12) (34-90) |
SMM-MM (0.5) SMM-CG (0.2) MM-CG (0.5) |
|
Iron
(μg/dL) |
41.00 (20.82) (10-79) |
48.00 (21.42) (17-97) |
63.77 (31.20) (24-121) |
SMM-MM (0.5) SMM-CG (0.03) MM-CG (0.1) |
IGF-1: Insulin-like growth factor-1
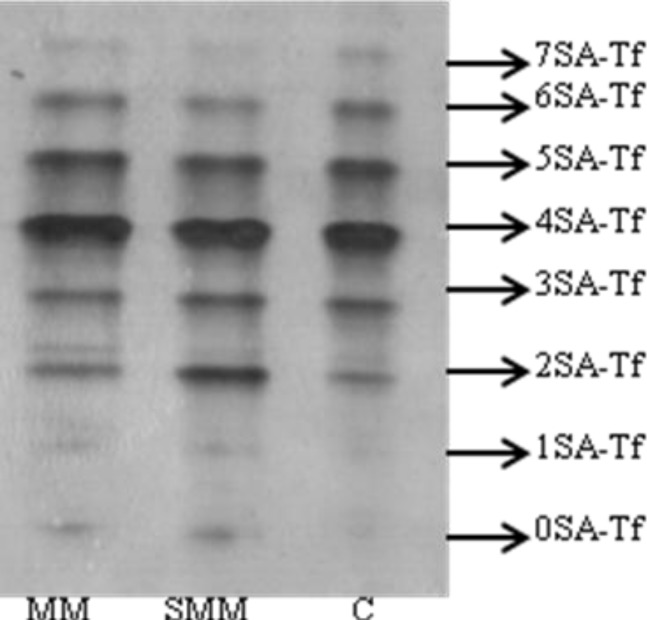
Table 3 and Fig. 1 demonstrate the isoform patterns of Tf in the patients and controls. Significantly higher values were observed for asialotransferrin and disialotransferrin in severely/moderately malnourished group compared to controls (P=0.04 and P=0.04, respectively). Although monosialotransferrin in severely/moderately malnourished group was higher than in control group, there was no statistically significant difference. Other Tf isoform patterns were not significantly different among three groups. Also, asialotransferrin and disialotransferrin values were not significantly different in mildly malnourished group than in other groups.
Table 3.
Transferrin isoforms and carbohydrate-deficient transferrin values of the patients and controls (All data presented as mean ± standard deviation and range)
| Parameter |
Severe/moderate
Malnutrition (SMM) (n=18) |
Mild
Malnutrition
(MM) (n=23) |
Control group
(CG) (n=29) |
Group comparisons
( P- value) |
|---|---|---|---|---|
| 0SA-Tf (%) | 1.76 (1.91) (0.56-8.99) |
1.22 (0.70) (0.08-2.59) |
1.01 (0.51) (0.04-1.86) |
SMM-MM (0.6) SMM-CG (0.04) MM-CG (0.4) |
| 1SA-Tf (%) | 0.89 (0.78) (0.00-3.30) |
0.51 (0.43) (0.02-1.55) |
0.62 (0.30) (0.06-1.16) |
SMM-MM (0.1) SMM-CG (0.5) MM-CG (0.2) |
| 2SA-Tf (%) | 5.57 (2.87) (3.53-16) |
4.85 (1.58) (2.21-7.89) |
4.27 (1.15) (2.28-6.47) |
SMM-MM (0.8) SMM-CG (0.04) MM-CG (0.2) |
| 3SA-Tf (%) | 8.21 (2.22) (3.82-11.40) |
8.58 (2.42) (4.10-12.35) |
8.86 (2.70) (4.35-13.91) |
SMM-MM (0.6) SMM-CG (0.4) MM-CG (0.9) |
| 4SA-Tf (%) | 57.72 (7.54) (32.81-65.27) |
56.38 (5.78) (38.84-64.84) |
57.51 (4.84) (45.62-65.95) |
SMM-MM (0.8) SMM-CG (0.3) MM-CG (0.5) |
| 5SA-Tf (%) | 20.97 (2.55) (16.85-24.56) |
21.71 (3.49) (16.25-33.39) |
20.34 (3.14) (14.20-26.82) |
SMM-MM (0.7) SMM-CG (0.4) MM-CG (0.2) |
| 6SA-Tf (%) | 6.72 (2.42) (3.38-11.74 |
5.75 (1.98) (3.27-11.70) |
6.29 (2.04) (1.92-12.36) |
SMM-MM (0.2) SMM-CG (0.7) MM-CG (0.3) |
| 7SA-Tf (%) | 1.11 (0.75) (0.04-3.31) |
0.82 (0.65) (0.00-2.90) |
1.04 (0.57) (0.16-2.47) |
SMM-MM (0.2) SMM-CG (0.7) MM-CG (0.2) |
SA: sialic acid; TF: transferrin
Fig. 1.
Isoform patterns of Tf in the patients and controls. Numbers (0SA-Tf to 7SA-Tf) on the right indicate different sialotransferrins.
MM: A mildly malnourished patient, SMM: A severely/ moderately malnourished patient, C: Control
Weight for height was positively correlated with IGF-1, prealbumin and iron levels in the whole study group. Similarly, iron was positively correlated with prealbumin, IGF-1, zinc, and transferrin levels. Prealbumin was positively correlated with IGF-1 and transferin. Also, IGF-1 was positively correlated with transferin (Table 4).
Table 4.
Correlation between anthropometric features and nutritional laboratory parameters in patients and controls (n=70)
| weight for height | Iron | Prealbumin | IGF-1 | Zinc | |
|---|---|---|---|---|---|
| Iron | 0.29* | ||||
| Prealbumin | 0.25* | 0.35‡ | |||
| IGF-1 | 0.39‡ | 0.59‡ | 0.45‡ | ||
| Zinc | 0.24 | 0.29* | 0.20 | -0.08 | |
| Transferrin | 0.24 | 0.27* | 0.25* | 0.46‡ | 0.10 |
IGF-1: Insulin-like growth factor-1
Correlation is significant at the 0.05 level;
Correlation is significant at the 0.01 level
There was a positive correlation between transferrin levels and monosialotransferrin and disialotransferrin in the whole study group. In contrast, transferrin was negatively correlated with tetrasialotransferrin (Table 5). No correlation was detected between iron, IGF-1, prealbumin, zinc and transferrin isoforms (Data not shown).
Table 5.
Correlation between serum transferrin levels and transferrin isoforms
| 0SA-Tf | 1SA-Tf | 2SA-Tf | 3SA-Tf | 4SA-Tf | 5SA-Tf | 6SA-Tf | 7SA-Tf | |
|---|---|---|---|---|---|---|---|---|
| Transferrin (n=70) | 0.16 | 0.28* | 0.33‡ | 0.18 | -0.31‡ | -0.11 | 0.32* | 0.03 |
Correlation is significant at the 0.05 level/
Correlation is significant at the 0.01 level
Discussion
In this study, the analysis of Tf isoforms by IEF revealed that, serum asialotransferrin and disialotransferrin levels (carbohydrate-deficient transferrins) were significantly increased in severely/moderately malnourished patients in comparison to controls.
Our results confirmed that CDT isoforms increase in malnutrition and these findings support the opinion that a relationship exists between catabolic diseases and protein glycosylation. To our knowledge, this is the first study in the literature investigating the relationship between protein energy malnutrition and transferrin isoforms in children.
CDT was reported to be a promising marker for assessment of nutritional status in catabolic patients in different studies. All of these studies showed that low body mass index correlated with higher CDT levels[17,28-31] or high body mass index was associated with lower CDT levels[32]. There appears to be an inverse relationship between catabolic states due to psychiatric disorders (in 63% of the patients) distinct from alcoholism[17].
Glycosyltransferases, enzymes which are involved in the glycosylation of Tf, are inhibited by the ethanol metabolite acetaldehyde. It is suggested that the biochemical mechanism underlying the CDT elevation in catabolic patients may be the structural similarities between acetoacetate and acetaldehyde. Therefore, it is possible that acetoacetate could also inhibit glycosyl-transferases resulting in an increase of CDT.
Another study by Reif et al showed that anorexia nervosa (AN) patients had elevated CDT values in 57% of cases; on the other hand, bulimia nervosa patients had normal CDT levels[18]. The body mass index and CDT levels[7]. Reif et al have shown that CDT levels increased significantly in difference between CDT levels in the two patient groups was explained by the fact that catabolic metabolism is more marked in AN.
Furthermore, patients with initially elevated CDT tended to be more seriously ill than those without. Another issue is that during therapy, as the body mass index of anorexia nervosa patients increased to normal levels, CDT inversely declined[18]. In a recent study by Arndt et al, CDT was analyzed in AN patients by three different methods including high performance liquid chromatography (HPLC), capillary electrophoresis (CE) and immunoassay. However, in contrast to the immunoassay tests, no elevation of CDT in anorexia nervosa patients was detected by HPLC and CE[19]. An interesting finding of this study was increased trisialotransferrin isoform that was detected by HPLC in 67% of AN patients.
Thus, it is suggested that elevated CDT values from the past studies using immunoassay method are most likely due to an incomplete separation of trisialotransferrin from CDT on the previous fractionation step and thus overdetermination of CDT. On the other hand, these results indicate that trisialotransferrin fraction may be used as a laboratory marker of (hypocaloric) malnutrition in this study[19].
In our study, transferrin was the only laboratory parameter showing correlation with transferrin isoforms. It was positively correlated with monosialotransferrin, disialotransferrin (CDT group), and hexasialotransferrin while negatively correlated with tetrasialotransferrin. It seems that the latter condition can be relevant to analytical process rather than the clinical status. Tetrasialotransferrin overload occurs when adequate sera for detecting all isoforms were applied to gel medium. Consequently, the intensity of tetrasialotransferrin bands would not be correlated with tetrasialotransferrin concentration. Relatively low tetrasialotransferrin isoform in higher transferrin concentrations has been explained by this analytical condition. The effect of serum iron and transferrin levels on CDT is not clear. Low iron and increased transferrin levels can increase total levels of CDT and possibly %CDT levels[7]. In our study, although both iron and transferrin levels decreased in the severely/moderately malnourished groups, asialotransferrin and disialotransferrin increased in these patients. Moreover, in our study, there was no correlation between iron and transferrin isoforms. So, it can be concluded that transferin levels can affect the di- and hexasialotransferrin levels in these groups. Brathen et al found in their study that both low and high serum transferrin levels could be associated with elevated CDT levels[28]. In the literature, three adult studies showed inverse relationship between iron and CDT levels[31,33,34], whereas Stauber et al found no relationship between iron levels and CDT values[35]. There is insufficient data to draw any conclusions about the effect of iron and transferrin on CDT values; for this reason, clinicians should analyze the elevated CDT results carefully in the patients with low iron levels, particularly in malnourished patients.
One major limitation of our study is the low number of severely (n=6) malnourished patients. In a larger patient group with severe malnutrition and in other disease states with markedly increased catabolism, transferrin isoform analysis with isoelectric focusing may reveal the alterations in protein glycosylation. Another limitation of this study is the case-control design of the study. Prospective studies, which analyze the alterations of transferrin isoforms during therapy and investigate the relationship between the weight gain and tranferrin isoforms are needed.
Conclusion
The results of this study point out that malnutrition may affect glycosylation in children. The mechanisms underlying the alterations in glycosylation during catabolic process and its outcomes need to be elucidated with further clinical and laboratory studies.
Authors Contribution
O. Bilen and N. Arslan have primary responsibility for protocol development, patient screening, enrollment, laboratory experiments, data analysis and writing the manuscript.
Z. Altun and P. Akan participated in the development of the protocol and analytic framework for the study.
B. Onvural and C. Çoker supervised the design of the study, performed the final data analyses.
All Authors approved the final version of the paper.
Acknowledgment
We thank all the patients and parents who contributed to this study.
Conflict of Interest: None
References
- 1.Jaeken J. Komrower Lecture. Congenital disorders of glycosylation (CDG): it's all in it! J Inherit Metab Dis. 2003;26(2-3):99–118. doi: 10.1023/a:1024431131208. [DOI] [PubMed] [Google Scholar]
- 2.Gu J, Taniguchi N. Potential of N-glycan in cell adhesion and migration as either a positive or negative regulator. Cell Adh Migr. 2008;2(4):243–5. doi: 10.4161/cam.2.4.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrostek L, Cylwik B, Gruszewska E, et al. N-Latex CDT results in liver diseases. Alcohol Alcohol. 2012;47(4):428–32. doi: 10.1093/alcalc/ags053. [DOI] [PubMed] [Google Scholar]
- 4.Pronicka E, Adamowicz M, Kowalik A, et al. Elevated carbohydrate-deficient transferrin (CDT) and its normalization on dietary treatment as a useful biochemical test for hereditary fructose intolerance and galactosemia. Pediatr Res. 2007;62(1):101–5. doi: 10.1203/PDR.0b013e318068641a. [DOI] [PubMed] [Google Scholar]
- 5.Piagnerelli M, Boudjeltia KZ, Nuyens V, et al. Rapid alterations in transferrin sialylation during sepsis. Shock. 2005;24(1):48–52. doi: 10.1097/01.shk.0000168524.20588.67. [DOI] [PubMed] [Google Scholar]
- 6.Quintana E, Gala S, Cazorla AG, et al. Secondary alteration of the transferrin isoelectric focusing pattern in a case of bacterial meningitis. J Inherit Metab Dis. 2007;30(2):267. doi: 10.1007/s10545-007-0530-1. [DOI] [PubMed] [Google Scholar]
- 7.Fleming MF, Anton RF, Spies CD. A review of genetic, biological, pharmacological, and clinical factors that affect carbohydrate-deficient transferrin levels. Alcohol Clin Exp Res. 2004;28:1347–55. doi: 10.1097/01.alc.0000139815.89794.be. [DOI] [PubMed] [Google Scholar]
- 8.Bortolotti F, De Paoli G, Tagliaro F. Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: A critical review of the literature 2001–2005. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841(1-2):96–109. doi: 10.1016/j.jchromb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Binkhorst M, Wortmann SB, Funke S, et al. Glycosylation defects underlying fetal alcohol spectrum disorder: a novel pathogenetic model. "When the wine goes in, strange things come out". J Inherit Metab Dis. 2012;35(3):399–405. doi: 10.1007/s10545-011-9425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Brien JF. Methods for detection of carbohydrate-deficient glycoprotein syndromes. Semin Pediatr Neurol. 2005;12(3):159–62. doi: 10.1016/j.spen.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Jaeken J. Congenital disorders of glycosylation. Ann NY Acad Sci. 2010;1214:190–8. doi: 10.1111/j.1749-6632.2010.05840.x. [DOI] [PubMed] [Google Scholar]
- 12.Marklova E, Albahri Z. Screening and diagnosis of congenital disorders of glycosylation. Clin Chim Acta. 2007;385(1-2):6–20. doi: 10.1016/j.cca.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Olson DL, Schwenk WF. Part I. Malnutrition in the pediatric population. Dis Mon. 2002;48(11):703–12. doi: 10.1067/mda.2002.130133. [DOI] [PubMed] [Google Scholar]
- 14.Chisti MJ, Tebruegge M, La Vincente S, et al. Pneumonia in severely malnourished children in developing countries - mortality risk, aetiology and validity of WHO clinical signs: a systematic review. Trop Med Int Health. 2009;14(10):1173–89. doi: 10.1111/j.1365-3156.2009.02364.x. [DOI] [PubMed] [Google Scholar]
- 15.Irena AH, Mwambazi M, Mulenga V. Diarrhea is a major killer of children with severe acute malnutrition admitted to inpatient set-up in Lusaka, Zambia. Nutr J. 2011;10:110. doi: 10.1186/1475-2891-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garenne M, Willie D, Maire B, et al. Incidence and duration of severe wasting in two African populations. Public Health Nutr. 2009;12(11):1974–82. doi: 10.1017/S1368980009004972. [DOI] [PubMed] [Google Scholar]
- 17.Reif A, Keller H, Schneider M, et al. Carbohydrate-deficient transferrin is elevated in catabolic female patients. Alcohol Alcohol. 2001;36(6):603–7. doi: 10.1093/alcalc/36.6.603. [DOI] [PubMed] [Google Scholar]
- 18.Reif A, Fallgatter AJ, Schmidtke A. Carbohydrate-deficient transferrin parallels disease severity in anorexia nervosa. Psychiatry Res. 2005;137(1-2):143–6. doi: 10.1016/j.psychres.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Arndt T, Erkens M, Holtkamp K, et al. High prevalence of increased trisialotransferrin concentrations in patients with anorexia nervosa: implications for determination of carbohydrate-deficient transferrin. Clin Chim Acta. 2007;379(1-2):150–3. doi: 10.1016/j.cca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Waterlow JC. Evolution of kwashiorkor and marasmus. Lancet. 1974;2(7882):712. doi: 10.1016/s0140-6736(74)93283-8. [DOI] [PubMed] [Google Scholar]
- 21.Waterlow JC. Classification and definition of protein calorie malnutrition. Br Med J. 1972;3:566–9. doi: 10.1136/bmj.3.5826.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Alessandro AM, D'Andrea G, Oratore A. Different patterns of human serum transferrin on isoelectric focusing using synthetic carrier ampholytes or immobilized pH gradients. Electrophoresis. 1988;9(2):80–3. doi: 10.1002/elps.1150090204. [DOI] [PubMed] [Google Scholar]
- 23.Pascali VL, Dobosz M, Destro-Bisol G, D'Aloja E. Characterization of genetic variants of human serum transferrin by isoelectric focusing: comparison between conventional and immobilized pH gradients, and application to a protocol for paternity testing. Electrophoresis. 1988;9(8):411–7. doi: 10.1002/elps.1150090811. [DOI] [PubMed] [Google Scholar]
- 24.de Jong G, van Eijk HG. Microheterogeneity of human serum transferrin: a biological phenomenon studied by isoelectric focusing in immobilized pH gradients. Electrophoresis. 1988;9(9):589–98. doi: 10.1002/elps.1150090921. [DOI] [PubMed] [Google Scholar]
- 25.Strahler JR, Hanash SM, Somerlot L, et al. Effect of salt on the performance of immobilized pH gradient isoelectric focusing gels. Electrophoresis. 1988;9(2):74–80. doi: 10.1002/elps.1150090203. [DOI] [PubMed] [Google Scholar]
- 26.Petren S, Vesterberg O. Separation of different forms of transferrin by isoelectric focusing to detect effects on the liver caused by xenobiotics. Electrophoresis. 1989;10(8-9):600–4. doi: 10.1002/elps.1150100812. [DOI] [PubMed] [Google Scholar]
- 27.Blakesley RW, Boezi JA. A new staining technique for proteins in polyacrylamide gels using coomassie brilliant blue G250. Anal Biochem. 1977;82:580–2. doi: 10.1016/0003-2697(77)90197-x. [DOI] [PubMed] [Google Scholar]
- 28.Brathen G, Bjerve K, Brodtkorb E, et al. Detection of alcohol abuse in neurological patients: variables of clinical relevance to the accuracy of the %CDT-TIA and CDTect methods. Alcohol Clin Exp Res. 2001;25(1):46–53. [PubMed] [Google Scholar]
- 29.Conigrave KM, Degenhardt LJ, Whitfield JB, et al. CDT, GGT, and AST as markers of alcohol use: the WHO/ISBRA collaborative project. Alcohol Clin Exp Res. 2002;26(3):332–9. [PubMed] [Google Scholar]
- 30.Sillanaukee P, Aalto M, Seppa K. Carbohydrate-deficient transferin and conventional alcohol markers as indicators for brief intervention among heavy drinkers in primary care health. Alcohol Clin Exp Res. 1998;22(4):892–6. [PubMed] [Google Scholar]
- 31.Whitfield JB, Zhu G, Heath AC, et al. Effects of alcohol consumption on indices of iron stores and of iron stores on alcohol intake markers. Alcohol Clin Exp Res. 2001;25(7):1037–45. [PubMed] [Google Scholar]
- 32.Sillanaukee P, Strid N, Jousilahti P, et al. Association of self-reported diseases and health care use with commonly used laboratory markers for alcohol consumption. Alcohol Alcohol. 2001;36(4):339–45. doi: 10.1093/alcalc/36.4.339. [DOI] [PubMed] [Google Scholar]
- 33.Jensen PD, Peterslund NA, Poulsen JH, et al. The effect of iron overload and iron reductive treatment on the serum concentration of carbohydrate-deficient transferrin. Br J Haematol. 1994;88(1):56–63. doi: 10.1111/j.1365-2141.1994.tb04977.x. [DOI] [PubMed] [Google Scholar]
- 34.De Feo TM, Fargion S, Duca L, et al. Carbohydrate-deficient transferrin, a sensitive marker of chronic alcohol abuse, is highly influenced by body iron. Hepatology. 1999;29(3):658–63. doi: 10.1002/hep.510290326. [DOI] [PubMed] [Google Scholar]
- 35.Stauber RE, Vollmann H, Pesserl I, et al. Carbohydrate-deficient transferrin in healthy women: relation to estrogens and iron status. Alcohol Clin Exp Res. 1996;20(6):1114–7. doi: 10.1111/j.1530-0277.1996.tb01955.x. [DOI] [PubMed] [Google Scholar]