Abstract
Aims
The World Heart Federation (WHF) guidelines for rheumatic heart disease (RHD) are designed for a standard portable echocardiography (STAND) machine. A recent study in a tertiary care centre demonstrated that they also had good sensitivity and specificity when modified for use with handheld echocardiography (HAND). Our study aimed to evaluate the performance of HAND for early RHD diagnosis in the setting of a large-scale field screening.
Methods and results
STAND was performed in 4773 children in Gulu, Uganda, with 10% randomly assigned to also undergo HAND. Additionally, any child with mitral or aortic regurgitation also underwent HAND. Studies were performed by experienced echocardiographers and blindly reviewed by cardiologists using 2012 WHF criteria, which were modified slightly for HAND—due to the lack of spectral Doppler capability. Paired echocardiograms were performed in 1420 children (mean age 10.8 and 53% female), resulting in 1234 children who were normal, 133 who met criteria for borderline RHD, 47 who met criteria for definite RHD, and 6 who had other diagnoses. HAND had good sensitivity and specificity for RHD detection (78.9 and 87.2%, respectively), but was most sensitive for definite RHD (97.9%). Inter- and intra-reviewer agreement ranged between 66–83 and 71.4–94.1%, respectively.
Conclusions
HAND has good sensitivity and specificity for diagnosis of early RHD, performing best for definite RHD. Protocols for RHD detection utilizing HAND will need to include confirmation by STAND to avoid over-diagnosis. Strategies that evaluate simplified screening protocols and training of non-physicians hold promise for more wide spread deployment of HAND-based protocols.
Keywords: Rheumatic heart disease, Handheld echocardiography, Screening, Epidemiology
Introduction
Rheumatic heart disease (RHD) results in significant morbidity and mortality in low-resource settings.1 It is currently estimated that at least 15.6 million people have clinically recognized RHD,1 which has an annual mortality rate between 3 and 12.5%.2–4 Even more concerning, is the potential volume of unrecognized cases detectable by echocardiographic screening.5 Prevalence studies from across four continents have shown 1.5–5.7% of asymptomatic primary schoolchildren in high-risk settings demonstrate echocardiographic evidence of RHD.6–11 Armed with this evidence, the World Health Organization recommends echocardiographic screening for RHD when feasible in RHD ‘endemic’ areas12 and the World Heart Federation (WHF) has provided evidence-based guidelines.13
However, the feasibility of such screening programmes remains in question14 and there are few examples of national programmes.15 One barrier to implementation of echocardiographic screening is the expense of standard portable echocardiography (STAND) machines. Handheld echocardiography (HAND) machines offer the promise of sensitive case detection at a fraction of the expense. Nevertheless, limited data exist on their use in RHD, and the current guidelines for echocardiographic diagnosis of RHD 13 depend on a fully functional system.
In the single study comparing HAND with STAND for diagnosis of RHD, HAND was found to have both good sensitivity (90.2%) and specificity (92.9%).16 However, this study was conducted on a small subset of children, in a tertiary setting, with an artificially high prevalence of advanced disease. The present study aimed to evaluate the performance of HAND, compared with STAND, for early RHD diagnosis under real-world conditions, involving large numbers of asymptomatic children in a field screening in Ugandan primary schools.
Methods
Study setting, population, and organization
We prospectively evaluated the sensitivity and specificity of HAND compared with STAND (gold standard) for diagnosis of RHD using the 2012 WHF criteria.13 The study was conducted over 5 days. Government schools in Gulu, Uganda, were evaluated and five were selected to ensure adequate population numbers. Gulu was the primary location of the fighting between the Ugandan army and the Lord's Resistance Army, but there has been peace in the region since 2005. The recent conflict, however, has resulted in poorer healthcare indicators than those in other regions of the country.17 While the prevalence of RHD in Gulu was not known prior to this study, it was assumed to be at least as high, if not higher than the prevalence in Kampala of 1.5%.6
Organization and intake were coordinated by 11 local volunteers, 2 nurses, and 1 school site champion. Echocardiographic screening was performed by five attending paediatric cardiologists, four paediatric cardiology fellows, and three senior echocardiography technicians. STAND echocardiograms occurred at eight stations, ∼8 h a day, for an average of 64 total screening hours per day. HAND echocardiograms were performed at one station per school. Electricity was not universally available, and portable generators, surge protectors, and power inverters were used to provide consistent power at all schools.
All participating children underwent STAND evaluation, which was used as the gold standard for diagnosis. Ten percent were randomly preselected (through study ID number) to undergo paired HAND imaging. Additionally, any child noted to have any mitral regurgitation (MR) or aortic regurgitation (AR) during STAND was also sent for paired HAND imaging. Those acquiring HAND images did so in a separate area and were blinded to the results of the STAND study.
For purposes of clinical follow-up, children with an MR or AR colour Doppler length of >1.5 or >0.5 cm, respectively, or any other concerning finding (morphological features of RHD, congenital/acquired heart disease, etc.) on STAND were referred for complete echocardiogram and clinical evaluation at the RHD clinic at Gulu Regional Referral Hospital.
Image interpretation and echo protocols
The WHF formed an international group of RHD experts in 2009 to provide a comprehensive review of the existing literature and expert opinion on the echocardiographic features of RHD. The product of this working group was published in 2012.13 These criteria were designed for use in RHD endemic populations to identify asymptomatic individuals who had no clinical history of acute rheumatic fever. According to the criteria, echocardiographic diagnosis of RHD can be definite or borderline (Table 1). Letter designations provide further details, with four subcategories for definite RHD (A–D) and three subcategories for borderline RHD (A–C, Table 1).
Table 1.
2012 WHF criteria for echocardiographic diagnosis of RHD (<20 years of age)13
| Definite RHD | |
| A. Pathological MR and at least two morphological features of RHD of the mitral valve | |
| B. Mitral stenosis with mean gradient ≥4 mmHg | |
| C. Pathological AR and at least two morphological features of RHD of the aortic valve | |
| D. Borderline disease of both the aortic and mitral valves | |
| Borderline RHD | |
| A. At least two morphological features of RHD of the mitral valve | |
| B. Pathological MR | |
| C. Pathological AR | |
| Pathological MR (all criteria must be met) | Pathological AR (all criteria must be met) |
| Seen in two views | Seen in two views |
| Jet length ≥2 cm (in at least one view) | Jet length ≥1 cm (in at least one view) |
| Velocity ≥3 m/s for one complete envelopea | Velocity ≥3 m/s for one complete envelopea |
| Pan-systolic jet in at least one envelopeb | Pan-diastolic jet in at least one envelopeb |
| Morphological features of the mitral valve | Morphological features of the aortic valve |
| Anterior leaflet thickening ≥3 mm | Irregular or focal thickening |
| Chordal thickening | Coaptation defect |
| Restricted leaflet motion | Restricted leaflet motion |
| Excessive leaflet tip motion during systole | Prolapse |
aNot required in our modified criteria for HAND.
bSeen in greater than or equal to two consecutive frames.
STAND
Eight STAND machines (four per site) were used to acquire images (seven General Electric Vivid Q/I, Milwaukee, WI, USA; one CX-50, Philips, Best, Netherlands). Multifrequency transducers were used with a frequency range of 2–5 MHz. Pre-sets were programmed to record 1.5 s loops and to optimize 2D and colour Doppler gain, depth, and resolution (highest frequency without harmonic imaging), although sonographers could adjust settings to optimize image quality. A 7-image acquisition protocol that focused on left-sided valve pathology and function was used for studies not pre-assigned for a paired HAND study. This protocol included parasternal long and apical four-chamber views in 2D and colour Doppler. An extension protocol of five additional images was added to this standardized acquisition protocol, including the addition of parasternal short images and continuous-wave Doppler across the mitral inflow and aortic outflow, for studies pre-assigned to the paired HAND study, and in any study with evidence of MR or AR.
Images were downloaded to local PACS systems in the USA and interpreted by six experienced paediatric cardiologists using the 2012 WHF guidelines (Table 1). Overall categorization (normal, borderline RHD, definite RHD, or other), sub-categorization, and the individual morphological and functional components comprising these diagnoses were recorded. Studies categorized as borderline RHD or definite RHD were placed back in the reading pool for blinded second interpretation, with disagreements prompting a third blinded review to establish consensus.
Handheld echocardiography
There was a single HAND station at each school, with three HAND machines (General Electric, VScan, Milwaukee, WI, USA) and extra batteries available. The HAND machine provides 2D and colour imaging on a 3.5-inch display and a 1.7- to 3.4-MHz transducer. Spectral Doppler is not available. Images were acquired based on the pre-set ‘auto-cycle’ function that allows for automatic detection of a single cardiac cycle beginning with end-diastole. Pre-set depth and gain were according to the device, but sonographers could adjust to maximize image quality. An 11-image standardized acquisition protocol was used, which was identical to the longer STAND protocol with the exception of spectral Doppler.
Images were downloaded onto computers and interpreted using the Vscan Gateway software by the same six paediatric cardiologists. Reviewers were blinded to the paired STAND study and the reason for HAND. As previously described, the 2012 WHF guidelines were modified secondary to the lack of continuous-wave Doppler capabilities.16 Absent spectral Doppler, a regurgitant jet was considered pan-systolic or pan-diastolic if it was seen in more than one consecutive frame (Table 1).
Ethical considerations
Ethical approval was obtained from the institutional review boards of Makerere University (Uganda), Uganda National Council for Science and Technology, Children's National Medical Centre (Washington DC, USA), and the University of Michigan (Ann Arbor, MI, USA). Permission was obtained from the Gulu Municipal Council Education Office and the headmaster of each school. Individual or parent written consent and assent was obtained according to local standards.
Statistical analyses
Study data were collected and managed using the REDCap electronic data-capture system hosted at Children's National Medical Center.18 Participant characteristics are described as median [IQR], mean [SD], or proportions where appropriate. Sensitivity and specificity, with 95% confidence intervals, were calculated for HAND compared with the gold standard of STAND. As this study sought to examine HAND as a screening tool, borderline or definite RHD on HAND was collectively considered a positive screen and indicated as ‘All Disease’. Sample size calculations were based on formula derived of studies of new diagnostic tests. A reasonable minimum value for HAND sensitivity was set at 80% for borderline RHD and 90% for definite RHD with a reasonable specificity set at 80%. Based on an estimated RHD prevalence of 2% (0.5% definite and 1.5% borderline), the projected sample size needed to produce a two-sided 90% confidence interval with width ±10% required 100 positive cases (at least 25 definite RHD and 75 borderline RHD). To reach these numbers, we estimated that it would require the screening of 5034 children.
Performance of HAND for detecting disease was compared with the following parameters on STAND: ‘All disease’ (borderline RHD and definite RHD), only borderline RHD, or only definite RHD. Additionally, the sensitivity and specificity of HAND for the quantitative individual criteria of the 2012 WHF guidelines (MR ≥2 cm, AR ≥1 cm, and thickness of anterior mitral leaflet ≥3 mm) were compared with STAND. Intra- and inter-reviewer agreement was evaluated by kappa.
Statement of responsibility
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
STAND studies were performed in 4773 primary students (Table 2). There was echocardiographic evidence of definite RHD in 52 (1.1%) and borderline RHD in 140 (2.9%), with other diagnoses (congenital and acquired heart disease) in 37 (0.8%). Paired echocardiograms (STAND and HAND) were obtained in 1420 children, with 477 (10% of STAND) preselected based on the study ID number and 943 referred for mitral and/or aortic regurgitation (mean age 10.8, SD 2.6, and 53% female). This resulted in 1234 children who were normal (86.9%), 133 who met criteria for borderline RHD (9.4%), 47 (3.3%) who met criteria for definite RHD, and 6 (4.2%) who had other diagnoses. Example comparisons of paired STAND and HAND studies are shown in Figure 1A–D and in Supplementary data online, Videos.
Table 2.
Demographics and echocardiographic parameters by STAND diagnosis
| Normal (n = 1234) | Borderline RHD (n = 133) | Definite RHD (n = 47) | |
|---|---|---|---|
| Age, mean (SD) | 10.7 (2.6) | 11.5 (2.3) | 11.3 (2.3) |
| Gender (% female) | 668 (54.1%) | 64 (48.1%) | 25 (53.1%) |
| Mitral regurgitation (cm) | |||
| 1.5–1.9 | 197 (16%) | 31 (23.3%) | 1 (2.1%) |
| ≥2.0 | 23 (1.9%) | 71 (53.4%) | 41 (87.2%) |
| Mitral stenosis (mean gradient >4 mmHg) | 0 | 0 | 4 (8.5%) |
| Aortic regurgitation (cm) | |||
| 0.5–0.9 | 28 (2.3%) | 2 (1.5%) | 1 (2.1%) |
| ≥1.0 | 0 | 13 (9.8%) | 11 (23.4%) |
| Borderline category† | |||
| Borderline A | 46 (34.6%) | ||
| Borderline B | 74 (55.6%) | ||
| Borderline C | 13 (9.8%) | ||
| Definite category‡ | |||
| Definite A | 34 (72.3%) | ||
| Definite B | 4 (8.5%) | ||
| Definite C | 1 (2.2%) | ||
| Definite D | 8 (17%) | ||
†,‡ According to the 2012 WHF criteria.
Figure 1.
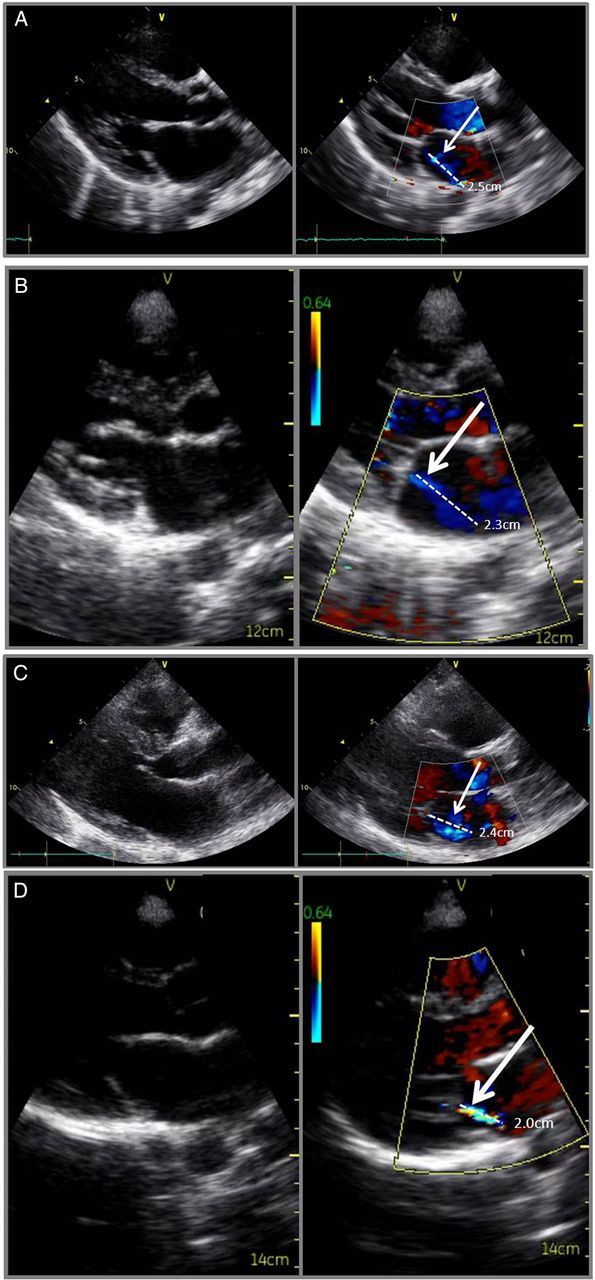
Paired STAND/HAND echocardiographic images in patients with RHD. (A and B) Definite RHD. (C and D) Borderline RHD. White arrows point to the dashed white line showing the length of valvular regurgitation.
The majority of children with borderline or definite RHD (n = 180) had isolated mitral valve disease (158, 87.8%; Table 2). HAND proved 78.9% sensitive and 87.2% specific for detection of all disease (Table 3). The sensitivity of HAND improved further with more advanced disease, being 97.9% sensitive for definite RHD. Subcategory criteria were less sensitive (Table 3).
Table 3.
Sensitivity and specificity of HAND compared with STAND
| Prevalence in those with paired echocardiograms (%) | Sensitivity | Specificity | |
|---|---|---|---|
| Screen positive for RHD | |||
| aAll Disease STAND vs. aAll Disease HAND | 12.7 | 78.9% (72.0–84.5) | 87.2% (85.2–88.9) |
| Borderline RHD STAND vs. aAll Disease HAND | 9.4 | 72.2% (63.6–79.4) | 87.2% (85.2–89) |
| Definite RHD STAND vs. aAll Disease HAND | 3.3 | 97.9% (87.3–99.9) | 87.2% (85.2–88.9) |
| Anterior mitral valve leaflet thickness ≥3 mm | 18.7 | 48.3% (42.1–54.5) | 72.1% (69.4–74.7) |
| MR ≥2 cm | 9.5 | 54.1% (45.3–62.6) | 95.8% (94.4–96.8) |
| AR ≥1 cm | 1.8 | 80.8% (60.0–92.7) | 98.8% (98.0–99.3) |
aAll Disease = borderline RHD + definite RHD.
Table 4 compares the disease categorization by STAND and HAND. In all but two cases, where borderline B was missed, MR was visualized by HAND but measured at less than the 2-cm pathological cut-off (range 0.9–1.8 cm and median 1.3 cm). HAND missed only one case of definite RHD. HAND also diagnosed 158 cases of RHD (142 borderline and 16 definite) that were determined by STAND to be normal. In 125 cases, the mitral valve morphology was assessed as abnormal by HAND (at least two morphological criteria), and normal by STAND (<2 morphological criteria). In all but 15 of these cases, the measurement of the thickness of the anterior mitral leaflet contributed to the over-diagnosis (Table 5). No cases were over-diagnosed due to change in interpretation of pan-systolic regurgitation without spectral Doppler.
Table 4.
A comparison of HAND with STAND on disease categorization (six children with other diagnosis excluded)
| HAND | STAND |
||
|---|---|---|---|
| Normal | Borderline RHD | Definite RHD | |
| Normal | 1076 | 38 | 1 |
| Borderline RHD | 142 | 83 | 15 |
| Definite RHD | 16 | 12 | 31 |
Table 5.
Reasons for non-agreement between STAND and HAND
| Reason for non-agreement on HAND | |
|---|---|
| RHD positive on STAND and normal by HAND (n = 39) | |
| Borderline A (n = 10) | Less than two morphological abnormalities (n = 10) |
| Borderline B (n = 24) | Length of MR <2 cm (n = 22) |
| MR not seen (n = 2) | |
| Borderline C (n = 4) | Length of AR <1 cm (n = 2) |
| AR not seen (n = 2) | |
| Definite (n = 1) | MR <2 and <2 cm morphological abnormalities identified by HAND (n = 1) |
| Reason for non-agreement on STAND | |
| RHD cases positive by HAND and normal by STAND (n = 158) | |
| Borderline A (n = 110) | Less than two morphological abnormalities identified by STAND |
| Borderline B (n = 27) | Length of MR <2 cm (n = 11) |
| MR not seen (n = 16) | |
| Borderline C (n = 5) | Length of AR <1 cm (n = 1) |
| AR not seen (n = 4) | |
| Definite (n = 16) | Less than two morphological abnormalities of the mitral valve PLUS |
| Length of MR <2 cm (n = 11) | |
| MR not seen (n = 2) | |
| Length of AR <1 cm (n = 3) | |
MR, mitral regurgitation; AR, aortic regurgitation.
Ten percent of HAND studies were evaluated for intra- and inter-reviewer reliability. Self-agreement ranged between 71.4 and 94.1% (κ 0.47–0.84); between-reviewer agreement ranged from 66.7 to 82.8% (κ 0.34–0.46).
There were no technical issues that resulted in reduction in the number of children screened. HAND battery life ranged from 60 to 90 min and took approximately the same length of time to recharge. Overheating was also a problem with HAND machines, particularly when placed on a flat surface, such as a table. Holding the machine in one's cupped hand or placing it on an elevated, ventilated surface, as well as the addition of a fan for cooling, mediated this issue.
Discussion
This study represents a critical follow-up to our original paper, the only published data on the use of HAND in RHD, which evaluated the use of HAND in a tertiary setting.16 Here, we look at the performance of HAND in a large population of asymptomatic children. In this screening environment, a large-scale, fast-paced evaluation of children in Ugandan schools, HAND had good sensitivity (78.9%) and specificity (87.2%). Importantly, performance of HAND for detection of definite RHD was nearly perfect, with a sensitivity of 97.9%.
We chose to use the complete 2012 WHF guidelines for both our gold standard STAND diagnosis and interpretation of HAND studies. Concerns have been justifiably raised that these criteria may be too complex for field application.19–21 Since their development in 2012, there have been only two published prospective screening studies utilizing these criteria.22,23 Only one of these, by Roberts et al.22, reported the inter-reviewer reliability among experts, which was found to be 83.9% (κ 0.3) for the question ‘is the pathology RHD’. Our reliability data are comparable, suggesting that consistency for applying these criteria, even among experts, may be far from perfect.
Expectedly, sensitivity and specificity both suffered slightly when HAND was moved to a screening environment. Our data show that two-thirds of missed cases were missed secondary to a regurgitant jet (aortic or mitral) that was not seen or was underestimated. All but one of these cases was borderline RHD, suggesting that less severe regurgitation was missed most frequently. The aetiology of these ‘misses’ is likely multifactorial. First, environmental variables (imperfect lighting, ergonomics) and the nature of high-volume screening (fast-pace, limited views) likely play a role in sub-optimal image acquisition. Secondly, it is the nature of criteria that there must be a cut-off which separates continuous data (regurgitant jet length) into a categorical value—normal and abnormal. But, we have seen through longitudinal follow-up of latent RHD patients that children with very mild regurgitant jets can ‘bounce’ between jet lengths classified as normal (<2 cm) and those classified as abnormal (≥2 cm), while continuing to meet the other three criteria that are necessary for pathological regurgitation.24 This likely does not reflect any clinical change in those patients, only a subtle change in cardiac loading conditions—like those that could have occurred between paired studies.
The most common reason for false-positive screening studies on HAND was over-diagnosis of morphological abnormalities. In the majority of cases (92%), the anterior mitral leaflet measurement contributed one of the two morphological abnormalities needed to be deemed ‘abnormal’. As seen in our previous study,16 approximately two-thirds of those overestimations could be at least in part attributed to rounding errors as the current HAND software is limited by 1 mm measurements. However, also similar was the general tendency for structures to appear thicker on HAND, leading to false diagnosis of chordal thickening and restricted leaflet motion. These over-diagnoses reduce the specificity of HAND, leading to our continued recommendation for a two-step screening approach, including a confirmatory STAND study, when feasible.
Despite these challenges, it is important to highlight that identification of definite RHD by HAND was almost perfect. This is significant given those with definite RHD have more advanced disease. Limited longitudinal data on the natural history of latent RHD suggest that patients with definite RHD are unlikely to show improvement.7,10,24,25 We believe that these data support the importance of requiring near perfect sensitivity for definite RHD. In contrast, ∼90% of patients with borderline RHD remain stable or improve, at least up to 2 years following diagnosis.10,24 This data suggest that a lower acceptable sensitivity for borderline RHD may be reasonable. A further study into the long-term outcome of borderline RHD is needed, as is research focused on refining screening protocols to determine the best age(s) for screening to maximize case detection.
We intentionally used experts in both image acquisition and interpretation to focus on the performance of HAND technology and eliminate the potential confounder of varied experience. While ‘use of experts’ is an important initial step, it is unlikely that experts would be first line for either in a real-world situation. Several studies have looked at the use of HAND in other contexts by non-experts, with mixed results.26,27 In the only study looking at RHD, final year medical students imaged 14 patients with advanced RHD and 31 controls using a first-generation HAND device (Philips Optigo). After receiving 8 h of focused training, the sensitivity for detection of MR or stenosis was very poor for all students, ranging between 21 and 33%.28 While not tested in HAND devices, there are some limited but promising examples of successful use of STAND by non-experts for RHD screening, in particular a pilot programme in Fiji.15 However, replication studies, standardized training protocols, and competency testing are needed prior to advocating wide spread non-physician led screening.
Similarly, interpretation of images occurred off-line, using a PACS system and the dedicated HAND software. Again, in real-world situations, interpretation would be best accomplished ‘in the field’ both to conserve resources and to provide immediate feedback to those being screened. This is particularly important in contexts where opportunities for confirmatory evaluation may be limited or unavailable. Further studies will be needed to see how these variables affect the sensitivity and specificity of HAND.
Our study has several limitations. First, absent a history of acute rheumatic fever, there is no confirmatory test for RHD diagnosed through echocardiography. This uncertainty necessarily leads to a flaw in our ‘gold standard’—STAND. However, with echocardiographic screening gaining momentum, STAND equipment is now being used almost ubiquitously in places conducting RHD screening, and thus serves as the best comparison. Secondly, our data show that underestimation or missed mild regurgitation jets (false-negative screens) may be similarly problematic between STAND and HAND, as 50 cases graded as normal by STAND were diagnosed with RHD by HAND (49 of them borderline) based on the presence of pathological regurgitation. This raises an important consideration that the true ‘false-negative’ rate of STAND is not known; there are no examples of field screenings with paired STAND studies. What can be assumed is that the sensitivity of a single STAND study is not 100%. It is likely that some disease detected by HAND and not by STAND (in particular that based on objective regurgitant jet length) represents the interval increase in sensitivity produced by multiple testing. As multiple testing is not realistic, expanding acquisition protocols to include more than one colour Doppler loop in each view may increase the sensitivity of both machines for detection of pathological valvular regurgitation.
The results in this study should also be put in the perspective that HAND technology is quickly evolving and gaining functionality. It is likely that technical enhancements will lead to performance improvement. Examples of potential improvements include enhanced colour Doppler, spectral Doppler, longer battery life, WiFi or Mobile (4G) connectivity, and applications—‘apps’—built into the system (e.g. RHD guidelines). Obstetrical ultrasound transducers that connect to tablets and smart phones are already in use. It is expected that this technology will be available for cardiac ultrasound in the near future; this development could dramatically decrease the price and increase the ability to share data. These developments provide great promise for widespread utilization of HAND.
In conclusion, comparison of HAND with STAND in a large-scale field screening setting shows that HAND has good sensitivity and specificity for diagnosis of early RHD, performing best for definite RHD. As data begin to accumulate on the cost-effectiveness of echo-based screening, HAND provides a less expensive alternative to STAND. Additionally, its increased portability may contribute to extending the reach of screening efforts. Strategies that evaluate simplified screening protocols and training of non-physicians are needed before wide spread deployment of HAND-based protocols.
Funding
This project was supported by award numbers UL1TR000075 and KL2TR000076 from the National Institutes of Health National Center for Advancing Translational Sciences. Its contents are the responsibility of the authors and do not necessarily represent the views of the National Center for Advancing Translational Sciences or the National Institutes of Health. This study was also funded in part by grants from the WHF and General Electric.
Acknowledgements
The authors thank the Rotary Club of Gulu for organizational and logistical support throughout this project, as well as the children and families who consented for participation.
Conflict of interest: none declared.
References
- 1.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005;5:685–94. [DOI] [PubMed] [Google Scholar]
- 2.Jaiyesimi F, Antia AU. Childhood rheumatic heart disease in Nigeria. Trop Geogr Med 1981;33:8–13. [PubMed] [Google Scholar]
- 3.Gunther G, Asmera J, Parry E. Death from rheumatic heart disease in rural Ethiopia. Lancet 2006;367:391. [DOI] [PubMed] [Google Scholar]
- 4.Kumar R, Raizada A, Aggarwal AK, Ganguly NK. A community-based rheumatic fever/rheumatic heart disease cohort: twelve-year experience. Indian Heart J 2002;54:54–8. [PubMed] [Google Scholar]
- 5.Sliwa K, Zilla P. Rheumatic heart disease: the tip of the iceberg. Circulation 2012;125:3060–2. [DOI] [PubMed] [Google Scholar]
- 6.Beaton A, Okello E, Lwabi P, Mondo C, McCarter R, Sable C. Echocardiography screening for rheumatic heart disease in Ugandan schoolchildren. Circulation 2012;125:3127–32. [DOI] [PubMed] [Google Scholar]
- 7.Saxena A, Ramakrishnan S, Roy A, Seth S, Krishnan A, Misra P, et al. Prevalence and outcome of subclinical rheumatic heart disease in India: the RHEUMATIC (Rheumatic Heart Echo Utilisation and Monitoring Actuarial Trends in Indian Children) study. Heart 2011;97:2018–22. [DOI] [PubMed] [Google Scholar]
- 8.Webb RH, Wilson NJ, Lennon DR, Wilson EM, Nicholson RW, Gentles TL, et al. Optimising echocardiographic screening for rheumatic heart disease in New Zealand: not all valve disease is rheumatic. Cardiol Young 2011;21:436–43. [DOI] [PubMed] [Google Scholar]
- 9.Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med 2007;357:470–6. [DOI] [PubMed] [Google Scholar]
- 10.Paar JA, Berrios NM, Rose JD, Caceres M, Pena R, Perez W, et al. Prevalence of rheumatic heart disease in children and young adults in Nicaragua. Am J Cardiol 2010;105:1809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carapetis JR, Hardy M, Fakakovikaetau T, Taib R, Wilkinson L, Penny DJ, et al. Evaluation of a screening protocol using auscultation and portable echocardiography to detect asymptomatic rheumatic heart disease in Tongan schoolchildren. Nat Clin Pract Cardiovasc Med 2008;5:411–7. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Rheumatic fever and rheumatic heart disease. World Health Organization Technical Report Series 923. Geneva: World Health Organization; 2004. pp. 1–122. [PubMed]
- 13.Remenyi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol 2012;9:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts K, Colquhoun S, Steer A, Remenyi B, Carapetis J. Screening for rheumatic heart disease: current approaches and controversies. Nat Rev Cardiol 2013;10:49–58. [DOI] [PubMed] [Google Scholar]
- 15.Colquhoun SM, Carapetis JR, Kado JH, Reeves BM, Remenyi B, May W, et al. Pilot study of nurse-led rheumatic heart disease echocardiography screening in Fiji—a novel approach in a resource-poor setting. Cardiol Young 2013;23:546–52. [DOI] [PubMed] [Google Scholar]
- 16.Beaton A, Aliku T, Okello E, Lubega S, McCarter R, Lwabi P, et al. The utility of handheld echocardiography for early diagnosis of rheumatic heart disease. J Am Soc Echocardiogr 2014;27:42–9. [DOI] [PubMed] [Google Scholar]
- 17.Uganda 2006: results from the demographic and health survey. Stud Fam Plann 2009;40:161–6. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirabel M, Celermajer DS, Ferreira B, Tafflet M, Perier MC, Karam N, et al. Screening for rheumatic heart disease: evaluation of a simplified echocardiography-based approach. Eur Heart J Cardiovasc Imaging 2012;13:1024–9. [DOI] [PubMed] [Google Scholar]
- 20.Shah B, Sharma M, Kumar R, Brahmadathan KN, Abraham VJ, Tandon R. Rheumatic heart disease: progress and challenges in India. Indian J Pediatr 2013;80(Suppl 1):S77–86. [DOI] [PubMed] [Google Scholar]
- 21.Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet 2012;379:953–64. [DOI] [PubMed] [Google Scholar]
- 22.Roberts K, Maguire G, Brown A, Atkinson D, Remenyi B, Wheaton G, et al. Echocardiographic screening for rheumatic heart disease in high and low risk Australian children. Circulation 2014;129:1953–61. [DOI] [PubMed] [Google Scholar]
- 23.Shrestha NR, Kalesan B, Karki P, Sherpa K, Basnet A, Urban P, et al. Rheumatic heart disease: pilot study for a population-based evaluation of prevalence and cardiovascular outcomes among schoolchildren in Nepal. BMJ Open 2012;2:e001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaton A, Okello E, Aliku T, Lubega S, Lwabi P, Mondo C, et al. Latent rheumatic heart disease: outcomes 2 years after echocardiographic detection. Pediatr Cardiol 2014;35:1259–67. [DOI] [PubMed] [Google Scholar]
- 25.Bhaya M, Beniwal R, Panwar S, Panwar RB. Two years of follow-up validates the echocardiographic criteria for the diagnosis and screening of rheumatic heart disease in asymptomatic populations. Echocardiography 2011;28:929–33. [DOI] [PubMed] [Google Scholar]
- 26.Panoulas VF, Daigeler AL, Malaweera AS, Lota AS, Baskaran D, Rahman S, et al. Pocket-size hand-held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors. Eur Heart J Cardiovasc Imaging 2013;14:323–30. [DOI] [PubMed] [Google Scholar]
- 27.Mjolstad OC, Andersen GN, Dalen H, Graven T, Skjetne K, Kleinau JO, et al. Feasibility and reliability of point-of-care pocket-size echocardiography performed by medical residents. Eur Heart J Cardiovasc Imaging 2013;14:1195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shmueli H, Burstein Y, Sagy I, Perry ZH, Ilia R, Henkin Y, et al. Briefly trained medical students can effectively identify rheumatic mitral valve injury using a hand-carried ultrasound. Echocardiography 2013;30:621–6. [DOI] [PubMed] [Google Scholar]


