Summary
Familial cerebral cavernous malformations are autosomal dominant conditions that can result in significant morbidity. A two-hit mechanism is accepted as likely responsible for formation of these malformations. We present 2 patients with this disease who received therapeutic radiation and developed very high numbers of malformations within the radiation ports, supporting radiation as an accelerator of lesion formation and suggesting implications for risks of radiation in this disease.
Introduction
Familial cerebral cavernous malformations (fCCMs) constitute a rare autosomal dominant disease, due to mutations in any of 3 separate genes, which causes significant morbidity and mortality in affected patients, including seizures, hemorrhage and strokes. We present 2 fCCM patients who developed exceptionally large numbers of cerebral cavernous malformations (CCMs) within a radiation port, suggesting radiation may accelerate lesion formation in this disease.
Case reports
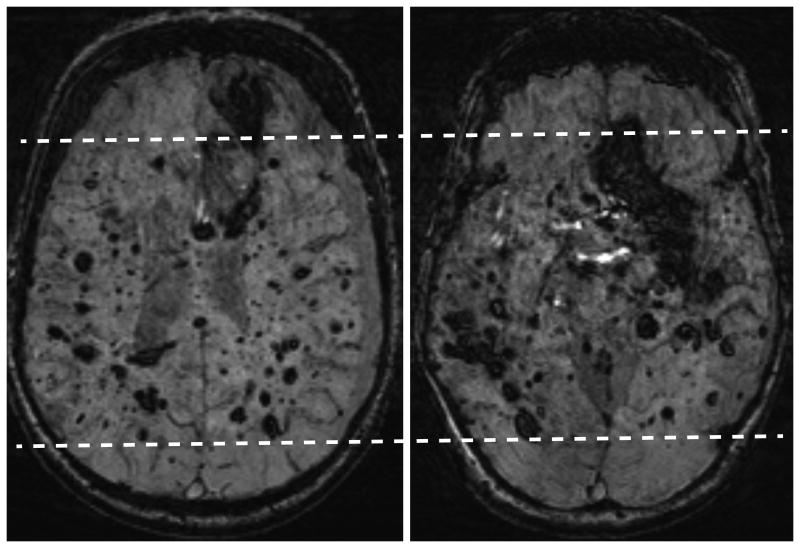
Patient 1 is a 46 year old man with genetically proven CCM1 mutation. He was treated in 1979 with a full course of therapeutic radiation in opposed lateral portals for a brainstem “mass,” likely an unrecognized CCM. His current brain MRI (Figure 1 A and B) reveals more than 500 CCMs.
Figure 1.
A (left) and B (right): Susceptibility-weighted MRI axial images show extensive CCM burden (both large and small) in the brain with relative sparing of the anterior and posterior cerebrum, indicating a central brain radiation port was used (dashed lines). As a result of his extensive disease burden, this patient has poor neurological function, residing in a group home and using a walker for ambulation. There is a chronic left subdural hematoma caused by lack of coordination and balance with numerous related falls.
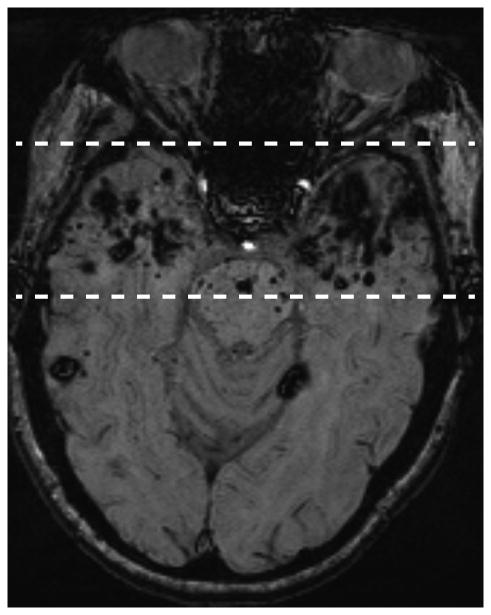
Patient 2 is a 49 year old woman who was treated with radiation therapy in 1989 for a nasopharyngeal carcinoma (radiation estimated at 70 Gy for the temporal lobes and 50 Gy for the cerebellum, both fractionated). Although CCMs are present throughout the brain, there is a high burden in the radiated temporal lobes and the inferior cerebellum, included in treatment of upper neck lymph nodes (Figure 2).
Figure 2.
A (left) and B (right): Axial MRI susceptibility-weighted images. Nasopharyngeal carcinoma treatment resulted in bilateral temporal lobe exposure to therapeutic doses of radiation (estimated 70 Gy). Note the much higher density of CCM lesions (both large and small) in the temporal lobes (dashed lines) compared to the remaining brain (A). The cerebellum was also exposed to therapeutic radiation (estimated 50 Gy) and likewise has a heavy CCM burden (B).
Discussion
CCMs can occur sporadically (many of which are associated with developmental venous anomalies), from genetic mutations, and in small numbers after radiation. There is increasing evidence to support a two-hit mechanism in fCCMs.1,2 A two-hit mechanism is familiar in tumor formation,3 but CCMs are composed of morphologically abnormal endothelia and not considered neoplasms. We are not aware of previous reports of radiation as a cause of second hits in fCCMs, but radiation is well known to be a risk factor for tumor development in some tumor syndromes. Patients with familial CCMs already have one germline mutation. Although radiation is known to cause CCMs after radiation for brain tumors, especially in children, these are usually few in number.4,5 These 2 patients are unique among the several hundred fCCM patients we have seen. The development of hundreds of CCMs in these 2 fCCM patients, and especially the evident geographic pattern corresponding to a radiation port, suggest that radiation therapy could induce a somatic gene mutation, the second “hit”, resulting in subsequent CCM development.
Available information in 2 cases does not permit conclusions about rate of development of CCMs nor dose dependence. Further cases would be helpful, and such information would be welcome. However, the findings suggest some practical implications. Proposed radiation therapy to patients with familial CCM gene mutations should be carefully considered. Genetic testing may be prudent in patients considered for radiation therapy who have a family history of autosomal dominant fCCM.
Extra consideration should be given to the clinical need for CT in familial CCM patients or mutation carriers. Although radiation doses from CT are far lower than therapy doses, multiple diagnostic CT scans can summate to more significant radiation doses. MRI, which is better than CT for evaluation of CCMs, should always be considered as a first option for imaging fCCM patients.
Footnotes
Conflicts of Interest: none
Contributor Information
Michael Golden, Dept of Radiology at the Univ of New Mexico. Albuquerque, NM, USA.
Saba Saeidi, Dept of Radiology at the Univ of New Mexico. Albuquerque, NM, USA.
Benny Liem, Dept of Internal Medicine at the Univ of New Mexico. Albuquerque, NM, USA.
Eric Marchand, Dept of Neurosurgery at the Univ of New Mexico. Albuquerque, NM, USA.
Leslie Morrison, Dept of Neurology at the Univ of New Mexico. Albuquerque, NM, USA.
Blaine Hart, Dept of Radiology at the Univ of New Mexico. Albuquerque, NM, USA.
References
- 1.Gault J, Shenkar R, Recksiek P, Awad IA. Biallelic somatic and germ line CCM1 truncating mutations in a cerebral cavernous malformation lesion. Stroke J Cereb Circ. 2005;36:872–4. doi: 10.1161/01.STR.0000157586.20479.fd. [DOI] [PubMed] [Google Scholar]
- 2.Pagenstecher A, Stahl S, Sure U, Felbor U. A two-hit mechanism causes cerebral cavernous malformations: complete inactivation of CCM1, CCM2 or CCM3 in affected endothelial cells. Hum Mol Genet. 2009;18:911–8. doi: 10.1093/hmg/ddn420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knudson AG. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68:820–3. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heckl S, Aschoff A, Kunze S. Radiation-induced cavernous hemangiomas of the brain: a late effect predominantly in children. Cancer. 2002;94:3285–91. doi: 10.1002/cncr.10596. [DOI] [PubMed] [Google Scholar]
- 5.Jain R, Robertson PL, Gandhi D, Gujar SK, Muraszko KM, Gebarski S. Radiation-induced cavernomas of the brain. AJNR Am J Neuroradiol. 2005;26:1158–62. [PMC free article] [PubMed] [Google Scholar]