Abstract
Intrapulmonary arteriovenous anastomoses (IPAVA) have been known to exist in human lungs for over 60 years. The majority of the work in this area has largely focused on characterizing the conditions in which IPAVA blood flow ( IPAVA) is either increased, e.g. during exercise, acute normobaric hypoxia, and the intravenous infusion of catecholamines, or absent/decreased, e.g. at rest and in all conditions with alveolar hyperoxia (
IPAVA) is either increased, e.g. during exercise, acute normobaric hypoxia, and the intravenous infusion of catecholamines, or absent/decreased, e.g. at rest and in all conditions with alveolar hyperoxia ( = 1.0). Additionally,
= 1.0). Additionally,  IPAVA is present in utero and shortly after birth, but is reduced in older (>50 years) adults during exercise and with alveolar hypoxia, suggesting potential developmental origins and an effect of age. The physiological and pathophysiological roles of
IPAVA is present in utero and shortly after birth, but is reduced in older (>50 years) adults during exercise and with alveolar hypoxia, suggesting potential developmental origins and an effect of age. The physiological and pathophysiological roles of  IPAVA are only beginning to be understood and therefore these data remain controversial. Although evidence is accumulating in support of important roles in both health and disease, including associations with pulmonary arterial pressure, and adverse neurological sequelae, there is much work that remains to be done to fully understand the physiological and pathophysiological roles of IPAVA. The development of novel approaches to studying these pathways that can overcome the limitations of the currently employed techniques will greatly help to better quantify
IPAVA are only beginning to be understood and therefore these data remain controversial. Although evidence is accumulating in support of important roles in both health and disease, including associations with pulmonary arterial pressure, and adverse neurological sequelae, there is much work that remains to be done to fully understand the physiological and pathophysiological roles of IPAVA. The development of novel approaches to studying these pathways that can overcome the limitations of the currently employed techniques will greatly help to better quantify  IPAVA and identify the consequences of
IPAVA and identify the consequences of  IPAVA on physiological and pathophysiological processes. Nevertheless, based on currently published data, our proposed working model is that
IPAVA on physiological and pathophysiological processes. Nevertheless, based on currently published data, our proposed working model is that  IPAVA occurs due to passive recruitment under conditions of exercise and supine body posture, but can be further modified by active redistribution of pulmonary blood flow under hypoxic and hyperoxic conditions.
IPAVA occurs due to passive recruitment under conditions of exercise and supine body posture, but can be further modified by active redistribution of pulmonary blood flow under hypoxic and hyperoxic conditions.
Introduction
Arteriovenous anastomoses provide a vascular conduit for blood flow to bypass a capillary bed. First described over 100 years ago (Sappey, 1879), these anastomotic connections have been demonstrated to exist not only in various systemic vascular beds (Brown, 1937; Prinzmetal et al. 1947, 1948; Simkin et al. 1948; Prichard & Daniel, 1953, 1956), but also in the lungs of animals (Prinzmetal et al. 1948; Rahn et al. 1952; Sirsi & Bucher, 1953; Niden & Aviado, 1956; Niden et al. 1960; McMullan et al. 2004; Lovering et al. 2007; Stickland et al. 2007; Bates et al. 2012) including humans (Tobin & Zariquiey, 1950; Tobin, 1966; Wilkinson & Fagan, 1990; Lovering et al. 2007). Despite the anatomical evidence for the existence of intrapulmonary arteriovenous anastomoses (IPAVA), potential physiological and pathophysiological roles for these vascular conduits are only beginning to be described, and therefore, remain controversial. Current interests focus on the ideas that IPAVA blood flow ( IPAVA) may (1) be involved in regulating pulmonary pressure, (2) reduce pulmonary gas exchange efficiency, and (3) explain some forms of cryptogenic stroke. Supporting data are largely correlational, due in part to the inherent limitations associated with the available methodology used to study
IPAVA) may (1) be involved in regulating pulmonary pressure, (2) reduce pulmonary gas exchange efficiency, and (3) explain some forms of cryptogenic stroke. Supporting data are largely correlational, due in part to the inherent limitations associated with the available methodology used to study  IPAVA, and the present inability to isolate the potential effect of
IPAVA, and the present inability to isolate the potential effect of  IPAVA from other possible effects. Here we will present the case for the existence, and importance, of
IPAVA from other possible effects. Here we will present the case for the existence, and importance, of  IPAVA and attempt to reconcile the controversy surrounding the proposed physiological and pathophysiological roles of
IPAVA and attempt to reconcile the controversy surrounding the proposed physiological and pathophysiological roles of  IPAVA by presenting anatomic-based data that are consistent with physiological consequences. We also present a working model that helps to uniformly explain the accumulating data in this area of investigation, although we expect this working model to evolve as new data are published and better techniques to study
IPAVA by presenting anatomic-based data that are consistent with physiological consequences. We also present a working model that helps to uniformly explain the accumulating data in this area of investigation, although we expect this working model to evolve as new data are published and better techniques to study  IPAVA become available.
IPAVA become available.
Techniques, and their limitations, for detecting and quantifying blood flow through IPAVA
The ‘anatomic-based’ techniques that can be used to study  IPAVA, all exploit the role of the lungs as a biological filter. The premise of these techniques is that microspheres, microbubbles, or radiolabelled macroaggregates of albumin (MAA) injected into either the venous circulation or directly into the pulmonary artery are trapped by the pulmonary microcirculation unless they flow through IPAVA or a patent foramen ovale (PFO) that are larger than the objects injected. These intravenously injected objects can subsequently be either collected in the pulmonary venous effluent (microspheres), visualized in the left heart using echocardiography (microbubbles), or imaged using radioactive labels attached to the particles injected (radiolabelled MAA and microspheres). In all cases, the transpulmonary passage of microspheres, microbubbles and MAA is dependent upon the diameter of these objects being larger than pulmonary capillaries (mean diameter of 6.5 μm, maximum diameter of 13 μm; Glazier et al. 1969; Rosenzweig et al. 1970). Accordingly, if bubbles have the ability to squeeze through capillaries, or if MAA breakup into smaller pieces, or if large diameter microspheres can pass though distended capillaries or corner vessels, then these techniques may either overestimate the percentage of
IPAVA, all exploit the role of the lungs as a biological filter. The premise of these techniques is that microspheres, microbubbles, or radiolabelled macroaggregates of albumin (MAA) injected into either the venous circulation or directly into the pulmonary artery are trapped by the pulmonary microcirculation unless they flow through IPAVA or a patent foramen ovale (PFO) that are larger than the objects injected. These intravenously injected objects can subsequently be either collected in the pulmonary venous effluent (microspheres), visualized in the left heart using echocardiography (microbubbles), or imaged using radioactive labels attached to the particles injected (radiolabelled MAA and microspheres). In all cases, the transpulmonary passage of microspheres, microbubbles and MAA is dependent upon the diameter of these objects being larger than pulmonary capillaries (mean diameter of 6.5 μm, maximum diameter of 13 μm; Glazier et al. 1969; Rosenzweig et al. 1970). Accordingly, if bubbles have the ability to squeeze through capillaries, or if MAA breakup into smaller pieces, or if large diameter microspheres can pass though distended capillaries or corner vessels, then these techniques may either overestimate the percentage of  IPAVA or suggest it is present when it is not. Important to this discussion, theoretical estimates suggest that capillaries can distend 2% per 1 Torr increase in pressure (Krenz & Dawson, 2003; Reeves et al. 2005). Applying this to high intensity exercise, a 10 μm capillary may be able to increase in diameter to between 16 μm and 25 μm at mean pulmonary artery pressures between 40–60 Torr. Thus, using microspheres greater than 25 μm and/or when mean pulmonary pressure is less than 60 Torr, will allow for the use of microspheres to detect and quantify
IPAVA or suggest it is present when it is not. Important to this discussion, theoretical estimates suggest that capillaries can distend 2% per 1 Torr increase in pressure (Krenz & Dawson, 2003; Reeves et al. 2005). Applying this to high intensity exercise, a 10 μm capillary may be able to increase in diameter to between 16 μm and 25 μm at mean pulmonary artery pressures between 40–60 Torr. Thus, using microspheres greater than 25 μm and/or when mean pulmonary pressure is less than 60 Torr, will allow for the use of microspheres to detect and quantify  IPAVA assuming no pulmonary capillaries are greater than a 10 μm initial diameter. We refer the reader to previous reviews on this topic for more detailed descriptions of these techniques and their limitations (Lovering et al. 2006, 2009a, 2010; Stickland & Lovering, 2006).
IPAVA assuming no pulmonary capillaries are greater than a 10 μm initial diameter. We refer the reader to previous reviews on this topic for more detailed descriptions of these techniques and their limitations (Lovering et al. 2006, 2009a, 2010; Stickland & Lovering, 2006).
The technique used most often for investigations of  IPAVA is transthoracic saline contrast echocardiography (TTSCE). Although TTSCE does not quantify the volume of
IPAVA is transthoracic saline contrast echocardiography (TTSCE). Although TTSCE does not quantify the volume of  IPAVA, several scoring systems have been developed (Barzilai et al. 1991; Zukotynski et al. 2007; Lovering et al. 2008b; Gazzaniga et al. 2009; van Gent et al. 2009), with the intent to grade or score the degree of left heart contrast observed under different conditions. Although quantitative measures of blood flow cannot be determined from bubble scores, greater degrees of left heart contrast (i.e. increased bubble scores) appear to correspond well with an increase in the volume of blood flow through pulmonary arteriovenous malformations (PAVM) and impairments in pulmonary gas exchange efficiency (Fischer et al. 2010). Bubble grades/scores also correlate with the size of PFO determined using intracardiac echocardiography (Fenster et al. 2014). However, a direct correlation between saline contrast grades/scores and
IPAVA, several scoring systems have been developed (Barzilai et al. 1991; Zukotynski et al. 2007; Lovering et al. 2008b; Gazzaniga et al. 2009; van Gent et al. 2009), with the intent to grade or score the degree of left heart contrast observed under different conditions. Although quantitative measures of blood flow cannot be determined from bubble scores, greater degrees of left heart contrast (i.e. increased bubble scores) appear to correspond well with an increase in the volume of blood flow through pulmonary arteriovenous malformations (PAVM) and impairments in pulmonary gas exchange efficiency (Fischer et al. 2010). Bubble grades/scores also correlate with the size of PFO determined using intracardiac echocardiography (Fenster et al. 2014). However, a direct correlation between saline contrast grades/scores and  IPAVA in healthy humans remains to be established.
IPAVA in healthy humans remains to be established.
Because of the limitations with the currently used techniques, novel techniques and/or approaches that overcome this current set of limitations will be required to advance this field significantly. One place to start would be the development of biodegradable microspheres too large (>25 μm) to squeeze through pulmonary capillaries in humans, used in conjunction with novel imaging or detection techniques. Additionally, the development of novel approaches to visualize  IPAVA under various conditions in animal models will allow for mechanistically based approaches to study the direct regulation of blood flow through these pathways.
IPAVA under various conditions in animal models will allow for mechanistically based approaches to study the direct regulation of blood flow through these pathways.
Casting of the pulmonary vasculature has been done in an attempt to visualize and locate IPAVA within isolated lungs (Tobin & Zariquiey, 1950; Rahn et al. 1952; Tobin, 1966), and these studies have found that IPAVA are ‘located at the apex of and within the lobular divisions of the lung’ (Tobin & Zariquiey, 1950). Assuming a 100 μm diameter and 300 μm length of an IPAVA, there would need to be ∼200 IPAVA in order to accommodate the estimated 2% of the cardiac output  t during high-intensity exercise (cardiac output ∼25 l min-1), see exercise section below. These calculation estimates assume uniformity of size, but there may be many sizes and the sizes may be distributed differently within the lung, e.g. large diameter IPAVA at the bottom and small diameter IPAVA at the top of the lung. Recent work in human fetal lungs demonstrates the potential to create three-dimensional reconstructions of arteriovenous connections in the lung (Galambos et al. 2013, 2014, 2015), providing a novel approach for studying IPAVA. A significant amount of work remains to establish the precise size range and distribution, of IPAVA as these data are currently lacking in this area.
t during high-intensity exercise (cardiac output ∼25 l min-1), see exercise section below. These calculation estimates assume uniformity of size, but there may be many sizes and the sizes may be distributed differently within the lung, e.g. large diameter IPAVA at the bottom and small diameter IPAVA at the top of the lung. Recent work in human fetal lungs demonstrates the potential to create three-dimensional reconstructions of arteriovenous connections in the lung (Galambos et al. 2013, 2014, 2015), providing a novel approach for studying IPAVA. A significant amount of work remains to establish the precise size range and distribution, of IPAVA as these data are currently lacking in this area.
Regulation of blood flow through IPAVA in healthy humans and in animals
Rest
For detecting  IPAVA in intact healthy humans, TTSCE offers a minimally invasive, sensitive technique with the capacity to perform multiple serial injections. Using TTSCE to detect
IPAVA in intact healthy humans, TTSCE offers a minimally invasive, sensitive technique with the capacity to perform multiple serial injections. Using TTSCE to detect  IPAVA, we have recently shown that 28% of 174 healthy, young subjects demonstrated
IPAVA, we have recently shown that 28% of 174 healthy, young subjects demonstrated  IPAVA at rest, at sea level (Elliott et al. 2013). These data are supported by other work using TTSCE in otherwise healthy humans at rest with a history of migraine headache (n = 104) that shows an identical prevalence of
IPAVA at rest, at sea level (Elliott et al. 2013). These data are supported by other work using TTSCE in otherwise healthy humans at rest with a history of migraine headache (n = 104) that shows an identical prevalence of  IPAVA of 28% (Woods et al. 2010). Importantly, both of these data sets included a rigorous evaluation for PFO in their respective subject populations and both report a PFO prevalence of 38%. A very similar prevalence of PFO (35%) has also been demonstrated using TTSCE in a larger sample (n = 1162) of adults (Marriott et al. 2013).
IPAVA of 28% (Woods et al. 2010). Importantly, both of these data sets included a rigorous evaluation for PFO in their respective subject populations and both report a PFO prevalence of 38%. A very similar prevalence of PFO (35%) has also been demonstrated using TTSCE in a larger sample (n = 1162) of adults (Marriott et al. 2013).
Our data were collected in human subjects while reclined at 45 deg in the left lateral decubitus position (Elliott et al. 2013). This information on body positioning is important because there is an effect of posture on the detection of  IPAVA. First reported by Stickland et al. (2004), two out of eight subjects demonstrated
IPAVA. First reported by Stickland et al. (2004), two out of eight subjects demonstrated  IPAVA at rest in the supine position, but were clear of left-sided contrast after standing upright, suggesting that the distribution of pulmonary blood flow may be important for the detection of
IPAVA at rest in the supine position, but were clear of left-sided contrast after standing upright, suggesting that the distribution of pulmonary blood flow may be important for the detection of  IPAVA (Stickland et al. 2004). Similarly, we have shown that in 18 subjects with
IPAVA (Stickland et al. 2004). Similarly, we have shown that in 18 subjects with  IPAVA at rest when supine, 17 were clear of left-sided contrast after standing upright (Elliott et al. 2013). Note though that, although standing upright, subjects leaned forward slightly to facilitate obtaining clear apical, four-chamber echocardiograms. Why body position alters the perfusion of IPAVA in some, but not all, individuals is unknown but it may be due to changes in regional lung perfusion that accompany changes in body posture. Note also that the use of the vasodilatory drugs nitroglycerine and aminophylline in humans at rest does not induce
IPAVA at rest when supine, 17 were clear of left-sided contrast after standing upright (Elliott et al. 2013). Note though that, although standing upright, subjects leaned forward slightly to facilitate obtaining clear apical, four-chamber echocardiograms. Why body position alters the perfusion of IPAVA in some, but not all, individuals is unknown but it may be due to changes in regional lung perfusion that accompany changes in body posture. Note also that the use of the vasodilatory drugs nitroglycerine and aminophylline in humans at rest does not induce  IPAVA (Lozo et al. 2014). Additionally, the use of noradrenaline (norepinephrine) to acutely increase pulmonary artery systolic pressure (PASP) in humans at rest does not induce
IPAVA (Lozo et al. 2014). Additionally, the use of noradrenaline (norepinephrine) to acutely increase pulmonary artery systolic pressure (PASP) in humans at rest does not induce  IPAVA (Lozo et al. 2014) and the use of lower body positive pressure to acutely elevate PASP does not consistently result in
IPAVA (Lozo et al. 2014) and the use of lower body positive pressure to acutely elevate PASP does not consistently result in  IPAVA (Stickland et al. 2006). Accordingly, the consistent findings with these studies is that these vessels appear to not be perfused in the majority of subjects tested under resting conditions despite acute increases in PASP and/or in the presence of well known pulmonary vascular dilators, suggesting that something else is responsible for mediating
IPAVA (Stickland et al. 2006). Accordingly, the consistent findings with these studies is that these vessels appear to not be perfused in the majority of subjects tested under resting conditions despite acute increases in PASP and/or in the presence of well known pulmonary vascular dilators, suggesting that something else is responsible for mediating  IPAVA in humans at rest breathing air (Fig.1A). Theoretically it remains possible that saline contrast bubbles used to detect
IPAVA in humans at rest breathing air (Fig.1A). Theoretically it remains possible that saline contrast bubbles used to detect  IPAVA may be squeezing through pulmonary capillaries, and this has yet to be conclusively proven or disproven. A comprehensive review of the bubble physics literature is beyond the scope of this review, and as such we refer the reader to prior work in this area (Yang, 1971; Yang et al. 1971a,b; Butler & Hills, 1979; Meltzer et al. 1980, 1981; Roelandt, 1982; Meerbaum et al. 1993). Despite these apparent consistencies across numerous studies, the saline contrast bubbles used to detect
IPAVA may be squeezing through pulmonary capillaries, and this has yet to be conclusively proven or disproven. A comprehensive review of the bubble physics literature is beyond the scope of this review, and as such we refer the reader to prior work in this area (Yang, 1971; Yang et al. 1971a,b; Butler & Hills, 1979; Meltzer et al. 1980, 1981; Roelandt, 1982; Meerbaum et al. 1993). Despite these apparent consistencies across numerous studies, the saline contrast bubbles used to detect  IPAVA may be getting through pulmonary capillaries in some individuals but not others, for reasons that we do not yet understand.
IPAVA may be getting through pulmonary capillaries in some individuals but not others, for reasons that we do not yet understand.
Figure 1. IPAVA working model.
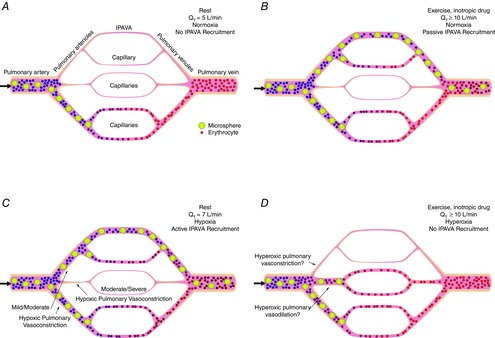
Diagram summarizing the working model for how active and passive regulation of the pulmonary circulation could determine the recruitment and perfusion of intrapulmonary arteriovenous anastomoses (IPAVA) under various conditions. IPAVA are considered to be few in number and >25 μm in diameter (top vessel branch) while capillaries are considered to be many in number and <10 μm in diameter (all others); each panel is within an isogravitational plane so we are not suggesting top or bottom blood flow preference is dependent upon gravity. Small/thin vessels with pink background void of erythrocytes and microspheres represent no pulmonary perfusion but the potential for recruitment is possible, whereas areas with erythrocytes and/or microspheres represent areas that are being perfused. Yellow spheres represent intravenously injected microspheres. A, at rest breathing air.  T is ∼5 l min-1 and there is only passive recruitment of a small portion of the available pulmonary vasculature with little, or no
T is ∼5 l min-1 and there is only passive recruitment of a small portion of the available pulmonary vasculature with little, or no  IPAVA, and consequently there is no appearance of microspheres in the pulmonary venous effluent and/or left ventricle. Large black arrow represents direction of blood flow. B, increased
IPAVA, and consequently there is no appearance of microspheres in the pulmonary venous effluent and/or left ventricle. Large black arrow represents direction of blood flow. B, increased  T during exercise or intravenous inotropic drug infusion breathing air.
T during exercise or intravenous inotropic drug infusion breathing air.  T is at least double resting values (≥10 l min−1), which causes passive recruitment of a larger proportion of the pulmonary vasculature that contains IPAVA, such that IPAVA are passively recruited/perfused. Subsequently microspheres appear in the pulmonary venous effluent and/or left ventricle and the volume of
T is at least double resting values (≥10 l min−1), which causes passive recruitment of a larger proportion of the pulmonary vasculature that contains IPAVA, such that IPAVA are passively recruited/perfused. Subsequently microspheres appear in the pulmonary venous effluent and/or left ventricle and the volume of  IPAVA increases as the intensity of exercise increases. Large black arrow represents direction of blood flow. C, at rest breathing hypoxic gas.
IPAVA increases as the intensity of exercise increases. Large black arrow represents direction of blood flow. C, at rest breathing hypoxic gas.  T is slightly increased to ∼7 l min−1, depending upon the severity of hypoxia. Hypoxia causes an active redistribution of pulmonary blood flow via varying degrees of hypoxic pulmonary vasoconstriction (or other unknown active redistribution mechanism) towards those areas of the lung with IPAVA, and consequently, IPAVA are passively recruited/perfused. Subsequently microspheres appear in the pulmonary venous effluent and/or left ventricle and the volume of
T is slightly increased to ∼7 l min−1, depending upon the severity of hypoxia. Hypoxia causes an active redistribution of pulmonary blood flow via varying degrees of hypoxic pulmonary vasoconstriction (or other unknown active redistribution mechanism) towards those areas of the lung with IPAVA, and consequently, IPAVA are passively recruited/perfused. Subsequently microspheres appear in the pulmonary venous effluent and/or left ventricle and the volume of  IPAVA increases as the severity of hypoxia increases (i.e.
IPAVA increases as the severity of hypoxia increases (i.e.  decreases). Large black arrow represents direction of blood flow. D, increased
decreases). Large black arrow represents direction of blood flow. D, increased  T during exercise or intravenous catecholamine infusion breathing 100% O2.
T during exercise or intravenous catecholamine infusion breathing 100% O2.  T is at least double resting values (≥10 l min-1), but with alveolar hyperoxia (
T is at least double resting values (≥10 l min-1), but with alveolar hyperoxia ( = 1.0). Hyperoxia causes an active redistribution of pulmonary blood flow, either hyperoxic vasoconstriction of areas containing IPAVA or hyperoxic vasodilatation of areas without IPAVA (or other unknown active redistribution mechanism). Consequently, IPAVA are not recruited/perfused and no microspheres appear in the pulmonary venous effluent and/or left ventricle. Large black arrow represents direction of blood flow.
= 1.0). Hyperoxia causes an active redistribution of pulmonary blood flow, either hyperoxic vasoconstriction of areas containing IPAVA or hyperoxic vasodilatation of areas without IPAVA (or other unknown active redistribution mechanism). Consequently, IPAVA are not recruited/perfused and no microspheres appear in the pulmonary venous effluent and/or left ventricle. Large black arrow represents direction of blood flow.
Exercise
In contrast to resting conditions, >95% of healthy humans demonstrate  IPAVA during exercise (Fig.1B). In 2004, two seminal studies using TTSCE to detect
IPAVA during exercise (Fig.1B). In 2004, two seminal studies using TTSCE to detect  IPAVA were published demonstrating in healthy humans without a PFO and/or
IPAVA were published demonstrating in healthy humans without a PFO and/or  IPAVA at rest that blood begins to flow through IPAVA during submaximal exercise and continues to flow through IPAVA up to maximal exercise at sea level (Eldridge et al. 2004; Stickland et al. 2004). Since then, several studies using subjects without a PFO have replicated these findings (Dujic et al. 2005; Lovering et al. 2008a,b; Elliott et al. 2011; Madden et al. 2013, 2014; Thom et al. 2013; Norris et al. 2014). Two of these studies using TTSCE and bubbles scores have also suggested that
IPAVA at rest that blood begins to flow through IPAVA during submaximal exercise and continues to flow through IPAVA up to maximal exercise at sea level (Eldridge et al. 2004; Stickland et al. 2004). Since then, several studies using subjects without a PFO have replicated these findings (Dujic et al. 2005; Lovering et al. 2008a,b; Elliott et al. 2011; Madden et al. 2013, 2014; Thom et al. 2013; Norris et al. 2014). Two of these studies using TTSCE and bubbles scores have also suggested that  IPAVA is increased and occurs at lower relative workloads with exercise while breathing hypoxic gas, when compared to exercise breathing air (Lovering et al. 2008a; Elliott et al. 2011). Additionally, there do not appear to be any differences between men and women in the occurrence of
IPAVA is increased and occurs at lower relative workloads with exercise while breathing hypoxic gas, when compared to exercise breathing air (Lovering et al. 2008a; Elliott et al. 2011). Additionally, there do not appear to be any differences between men and women in the occurrence of  IPAVA during exercise (Kennedy et al. 2012). A consistent finding with these studies is that during exercise, most humans (>95%) appear to have increased
IPAVA during exercise (Kennedy et al. 2012). A consistent finding with these studies is that during exercise, most humans (>95%) appear to have increased  IPAVA as detected by TTSCE (Fig.1B), but, as mentioned above with the resting studies, this may be due to bubbles getting through distended capillaries.
IPAVA as detected by TTSCE (Fig.1B), but, as mentioned above with the resting studies, this may be due to bubbles getting through distended capillaries.
The main shortcoming of these studies, and TTSCE in general, is that the volume of  IPAVA during exercise is not known nor does it provide us with definitive information about the size and location of IPAVA within the lung. However, intravenously injection of 25 and 50 μm microspheres in exercising dogs confirmed that
IPAVA during exercise is not known nor does it provide us with definitive information about the size and location of IPAVA within the lung. However, intravenously injection of 25 and 50 μm microspheres in exercising dogs confirmed that  IPAVA does occur during exercise and was calculated to be 1.4% (range 0.2–3.1%) of
IPAVA does occur during exercise and was calculated to be 1.4% (range 0.2–3.1%) of  T; (Stickland et al. 2007). Technetium-99m-labelled MAA (99mTc-MAA) is a technique that can be used in humans to quantify
T; (Stickland et al. 2007). Technetium-99m-labelled MAA (99mTc-MAA) is a technique that can be used in humans to quantify  IPAVA because the proportion of radioactivity outside of the lungs represents the proportion of MAA that were not trapped in the pulmonary capillaries, i.e. they travelled through IPAVA and became trapped in systemic capillaries. Studies in healthy male humans using 99mTc-MAA and gamma camera imaging measured
IPAVA because the proportion of radioactivity outside of the lungs represents the proportion of MAA that were not trapped in the pulmonary capillaries, i.e. they travelled through IPAVA and became trapped in systemic capillaries. Studies in healthy male humans using 99mTc-MAA and gamma camera imaging measured  IPAVA to be 1.3% (range −0.3 to 2.7%) of
IPAVA to be 1.3% (range −0.3 to 2.7%) of  t during maximal treadmill exercise (Lovering et al. 2009b). Mean
t during maximal treadmill exercise (Lovering et al. 2009b). Mean  IPAVA in seven subjects (3 female) has been measured to be 2.5% (±2.6%; range ∼ −0.5 to 7%) with
IPAVA in seven subjects (3 female) has been measured to be 2.5% (±2.6%; range ∼ −0.5 to 7%) with  IPAVA increasing in 5/7 subjects during cycle ergometer exercise at 85% of maximal capacity (Bates et al. 2014). These studies used MAA with a mean diameter of 20–40 μm and ∼90% of MAA between 10 and 90 μm in size. One study prior to these used 99mTc albumin microspheres with a size range of 7–25 μm in diameter and these authors found an increase in shunt of 2.4% of
IPAVA increasing in 5/7 subjects during cycle ergometer exercise at 85% of maximal capacity (Bates et al. 2014). These studies used MAA with a mean diameter of 20–40 μm and ∼90% of MAA between 10 and 90 μm in size. One study prior to these used 99mTc albumin microspheres with a size range of 7–25 μm in diameter and these authors found an increase in shunt of 2.4% of  T in five normal subjects during exercise, with a range of −2.4 to 5.9% (Whyte et al. 1992). A key point of summary for these studies is that the onset of, and the amount of,
T in five normal subjects during exercise, with a range of −2.4 to 5.9% (Whyte et al. 1992). A key point of summary for these studies is that the onset of, and the amount of,  IPAVA is variable between subjects but it appears to consistently increase in healthy subjects and happens in almost everyone during exercise (Fig.1B). Furthermore, and perhaps most importantly,
IPAVA is variable between subjects but it appears to consistently increase in healthy subjects and happens in almost everyone during exercise (Fig.1B). Furthermore, and perhaps most importantly,  IPAVA during exercise has been consistently detected using three different anatomically based techniques (microspheres, TTSCE, and 99mTc-MAA) that all report identical findings. Nevertheless, because of the limitations of these techniques, MAA may be breaking up and travelling through pulmonary capillaries as smaller pieces in the human studies and therefore may be overestimating
IPAVA during exercise has been consistently detected using three different anatomically based techniques (microspheres, TTSCE, and 99mTc-MAA) that all report identical findings. Nevertheless, because of the limitations of these techniques, MAA may be breaking up and travelling through pulmonary capillaries as smaller pieces in the human studies and therefore may be overestimating  IPAVA.
IPAVA.
An area of inconsistency in this field is in the exercising thoroughbred horse. In one study using microspheres, the authors found that microspheres did not travel through the lungs of thoroughbred horses during exercise (Manohar & Goetz, 2005). However, we have previously detailed the technical limitations of this study where injecting too few microspheres and/or sampling low volumes of blood may result in conditions where microsphere detection may not be possible even if IPAVA are present and being perfused (Stickland & Lovering, 2006). Nonetheless, recent work has found that bubble contrast is able to traverse the pulmonary circulation in exercising horses (La Gerche et al. 2013). Whether or not this resulted from bubble contrast travelling through IPAVA or from distended pulmonary capillaries is unknown, but future studies using the appropriate numbers of large diameter microspheres and blood sampling volume will help to answer this interesting question.
The underlying factors and mechanisms that regulate  IPAVA during exercise at sea level are currently unknown. Key suggested candidates include increased
IPAVA during exercise at sea level are currently unknown. Key suggested candidates include increased  T, pulmonary artery pressures, or left atrial pressure (Stickland et al. 2004; Bryan et al. 2012; Laurie et al. 2012; Elliott et al. 2014a). Work using intravenous catecholamine infusion to increase
T, pulmonary artery pressures, or left atrial pressure (Stickland et al. 2004; Bryan et al. 2012; Laurie et al. 2012; Elliott et al. 2014a). Work using intravenous catecholamine infusion to increase  T and PASP (Bryan et al. 2012; Laurie et al. 2012) demonstrates that, regardless of the inotropic drug used, a doubling of
T and PASP (Bryan et al. 2012; Laurie et al. 2012) demonstrates that, regardless of the inotropic drug used, a doubling of  T results in a significant increase in
T results in a significant increase in  IPAVA as detected with TTSCE (Fig.1B). Furthermore, recent work by our group suggests that increased
IPAVA as detected with TTSCE (Fig.1B). Furthermore, recent work by our group suggests that increased  T, independent of PASP, can increase
T, independent of PASP, can increase  IPAVA in human subjects breathing room air (Elliott et al. 2014a). Accordingly, increases in
IPAVA in human subjects breathing room air (Elliott et al. 2014a). Accordingly, increases in  T that accompany exercise or are induced by infusion of inotropic drugs, may allow for blood to perfuse IPAVA through simple vascular recruitment of under-perfused areas of the lung (Fig.1B). Thus, the consistent finding with these investigations using TTSCE is that increased
T that accompany exercise or are induced by infusion of inotropic drugs, may allow for blood to perfuse IPAVA through simple vascular recruitment of under-perfused areas of the lung (Fig.1B). Thus, the consistent finding with these investigations using TTSCE is that increased  T is likely important, but not increased PASP, which is consistent with the resting data obtained using TTSCE (Fig.1A and B). Despite these consistencies, until mechanistically based studies using large diameter microspheres that are not able to pass through distended pulmonary capillaries are utilized, this area of research will remain inconclusive. Clearly, performing these mechanistic studies will significantly advance this area of research.
T is likely important, but not increased PASP, which is consistent with the resting data obtained using TTSCE (Fig.1A and B). Despite these consistencies, until mechanistically based studies using large diameter microspheres that are not able to pass through distended pulmonary capillaries are utilized, this area of research will remain inconclusive. Clearly, performing these mechanistic studies will significantly advance this area of research.
Hypoxia
Using TTSCE and bubble scoring,  IPAVA is suggested to increase when healthy humans breathe hypoxic gas at sea level (normobaric hypoxia) at rest (Lovering et al. 2008a; Laurie et al. 2010; Norris et al. 2014; Tremblay et al. 2014; Fig.1C). Additionally, left-sided bubble contrast increases with greater decreases in arterial O2 saturation (Laurie et al. 2010). To date, the majority of work in humans has used TTSCE to detect
IPAVA is suggested to increase when healthy humans breathe hypoxic gas at sea level (normobaric hypoxia) at rest (Lovering et al. 2008a; Laurie et al. 2010; Norris et al. 2014; Tremblay et al. 2014; Fig.1C). Additionally, left-sided bubble contrast increases with greater decreases in arterial O2 saturation (Laurie et al. 2010). To date, the majority of work in humans has used TTSCE to detect  IPAVA; however, recent work has demonstrated that
IPAVA; however, recent work has demonstrated that  IPAVA, measured using 99mTc-MAA, is ∼5% of
IPAVA, measured using 99mTc-MAA, is ∼5% of  T in healthy humans breathing 10% O2 (Bates et al. 2014). Additionally, studies in dogs (Niden & Aviado, 1956) using microspheres 60–420 μm in diameter, and in rats (Bates et al. 2012) using microspheres 25, 50 and 70 μm in diameter, demonstrate that normobaric hypoxia also increases the proportion of large diameter microspheres that pass through IPAVA, identical to the work using TTSCE and 99mTc-MAA in humans. The consistent finding in this area is that hypoxia increases
T in healthy humans breathing 10% O2 (Bates et al. 2014). Additionally, studies in dogs (Niden & Aviado, 1956) using microspheres 60–420 μm in diameter, and in rats (Bates et al. 2012) using microspheres 25, 50 and 70 μm in diameter, demonstrate that normobaric hypoxia also increases the proportion of large diameter microspheres that pass through IPAVA, identical to the work using TTSCE and 99mTc-MAA in humans. The consistent finding in this area is that hypoxia increases  IPAVA, but because of the techniques utilized for these investigations additional work using large diameter microspheres is needed to verify if this is the case in humans and not just in dogs and rats.
IPAVA, but because of the techniques utilized for these investigations additional work using large diameter microspheres is needed to verify if this is the case in humans and not just in dogs and rats.
Interestingly, the work by Bates and colleagues demonstrated that hypoxia-induced  IPAVA was less or absent in isolated rat lungs, but was significantly greater in the intact animal, suggesting there is some inconsistency in this area. Thus, there may be either some blood-borne factor or innervation to the lung that is responsible for increasing
IPAVA was less or absent in isolated rat lungs, but was significantly greater in the intact animal, suggesting there is some inconsistency in this area. Thus, there may be either some blood-borne factor or innervation to the lung that is responsible for increasing  IPAVA in hypoxic conditions. Considering the clear difference in
IPAVA in hypoxic conditions. Considering the clear difference in  IPAVA between these two experimental conditions, it would appear most beneficial to focus time and resources on studying
IPAVA between these two experimental conditions, it would appear most beneficial to focus time and resources on studying  IPAVA in the intact animal. However, directing future work to the intact animal would not preclude studies aimed at isolating IPAVA for histological purposes in isolated lungs. Also, if neurally mediated active control plays a role in regulating
IPAVA in the intact animal. However, directing future work to the intact animal would not preclude studies aimed at isolating IPAVA for histological purposes in isolated lungs. Also, if neurally mediated active control plays a role in regulating  IPAVA then a neurologically intact preparation may be required to continue to advance this area.
IPAVA then a neurologically intact preparation may be required to continue to advance this area.
Another area of inconsistency in this area comes from data obtained in field studies. These studies at high altitude have investigated the occurrence of  IPAVA at rest in Sherpas and healthy sea level inhabitants after acclimatization to 5050 m (Foster et al. 2014). One noteworthy finding of this study was that
IPAVA at rest in Sherpas and healthy sea level inhabitants after acclimatization to 5050 m (Foster et al. 2014). One noteworthy finding of this study was that  IPAVA in hypobaric hypoxia was less than expected for the level of arterial hypoxemia compared to studies in normobaric hypoxia. Specifically, there was a reduction in bubble scores at altitude, as detected by TTSCE, compared to sea level. The authors speculated that several possibilities might explain the lower than expected
IPAVA in hypobaric hypoxia was less than expected for the level of arterial hypoxemia compared to studies in normobaric hypoxia. Specifically, there was a reduction in bubble scores at altitude, as detected by TTSCE, compared to sea level. The authors speculated that several possibilities might explain the lower than expected  IPAVA including pulmonary vascular remodelling and/or reduced
IPAVA including pulmonary vascular remodelling and/or reduced  T with acclimatization compared to acute normobaric hypoxia. Additionally, it remains possible that the in vivo microbubble dynamics of saline contrast are altered in hypobaric environments. Specifically, compared to normobaria, microbubble stability may be impaired, accelerating microbubble time to dissolution. For example, during studies at both sea level and at 5050 m all saline contrast injections consisted of 4 ml of saline combined with 1 ml of air. In accordance with the ideal gas law (PV = nRT), for the same volume of air the ∼50% reduction in barometric pressure would correspond to a ∼50% reduction in the absolute moles of gas within the 1 ml volume. Yet in all cases at altitude there was complete right heart opacification following bubble injection, suggesting that a sufficient number of bubbles were initially present in the right heart. Nevertheless, it remains unknown to what extent, or even if, this change in barometric pressure would affect microbubble stability. However, as mentioned above, due to the limitations of using TTSCE with saline contrast bubbles, future investigations should be performed using large diameter microspheres to determine if in fact
T with acclimatization compared to acute normobaric hypoxia. Additionally, it remains possible that the in vivo microbubble dynamics of saline contrast are altered in hypobaric environments. Specifically, compared to normobaria, microbubble stability may be impaired, accelerating microbubble time to dissolution. For example, during studies at both sea level and at 5050 m all saline contrast injections consisted of 4 ml of saline combined with 1 ml of air. In accordance with the ideal gas law (PV = nRT), for the same volume of air the ∼50% reduction in barometric pressure would correspond to a ∼50% reduction in the absolute moles of gas within the 1 ml volume. Yet in all cases at altitude there was complete right heart opacification following bubble injection, suggesting that a sufficient number of bubbles were initially present in the right heart. Nevertheless, it remains unknown to what extent, or even if, this change in barometric pressure would affect microbubble stability. However, as mentioned above, due to the limitations of using TTSCE with saline contrast bubbles, future investigations should be performed using large diameter microspheres to determine if in fact  IPAVA is reduced at altitude, or if it is simply an artefact of the techniques currently employed.
IPAVA is reduced at altitude, or if it is simply an artefact of the techniques currently employed.
Work by Laurie et al. was the first to suggest that  IPAVA increased with higher bubble scores as detected by TTSCE as peripheral arterial oxygen saturation (
IPAVA increased with higher bubble scores as detected by TTSCE as peripheral arterial oxygen saturation ( ) decreased (Laurie et al. 2010). Breathing hypoxic gas at rest elicits a number of physiological responses that may cause the increases in
) decreased (Laurie et al. 2010). Breathing hypoxic gas at rest elicits a number of physiological responses that may cause the increases in  IPAVA, including decreasing arterial O2 content (
IPAVA, including decreasing arterial O2 content ( ), arterial partial pressure of O2 (
), arterial partial pressure of O2 ( ), and mixed venous partial pressure of O2 (
), and mixed venous partial pressure of O2 ( ), and increasing sympathetic activity and hypoxic pulmonary vasoconstriction (HPV), but it is currently unknown how the reduction in O2 functions to regulate
), and increasing sympathetic activity and hypoxic pulmonary vasoconstriction (HPV), but it is currently unknown how the reduction in O2 functions to regulate  IPAVA. Work using intravenous propranolol in healthy humans breathing hypoxic gas (
IPAVA. Work using intravenous propranolol in healthy humans breathing hypoxic gas ( = 0.12) at rest suggests that the hypoxaemia-induced increase in
= 0.12) at rest suggests that the hypoxaemia-induced increase in  IPAVA is not a β-receptor-mediated response (Laurie et al. 2012). As with sea level exercise, increased
IPAVA is not a β-receptor-mediated response (Laurie et al. 2012). As with sea level exercise, increased  T and/or increased pulmonary artery pressure may be responsible for increasing
T and/or increased pulmonary artery pressure may be responsible for increasing  IPAVA. However, hypoxia-induced
IPAVA. However, hypoxia-induced  IPAVA is probably not due to increased left atrial pressure because it does not increase in subjects breathing hypoxic gases at rest (Groves et al. 1987). Hypoxia-induced
IPAVA is probably not due to increased left atrial pressure because it does not increase in subjects breathing hypoxic gases at rest (Groves et al. 1987). Hypoxia-induced  IPAVA could be related to HPV and a subsequent increase in pulmonary vascular pressure, specifically an uneven pulmonary vasoconstriction where some vessels dilate (Hultgren et al. 1964, 1971; Fig.1C). Hypoxia-induced
IPAVA could be related to HPV and a subsequent increase in pulmonary vascular pressure, specifically an uneven pulmonary vasoconstriction where some vessels dilate (Hultgren et al. 1964, 1971; Fig.1C). Hypoxia-induced  IPAVA has been shown to occur within 30 min of breathing hypoxic gas (Laurie et al. 2010), although acute hypoxia induces only minor increases in PASP whereas more significant increases in PASP occur at approximately 45 min and later (Talbot et al. 2005). Work using acetazolamide to block HPV found that isocapnic hypoxia-induced
IPAVA has been shown to occur within 30 min of breathing hypoxic gas (Laurie et al. 2010), although acute hypoxia induces only minor increases in PASP whereas more significant increases in PASP occur at approximately 45 min and later (Talbot et al. 2005). Work using acetazolamide to block HPV found that isocapnic hypoxia-induced  IPAVA occurred independently of significant increases in PASP (Tremblay et al. 2014). It has been shown that hypoxia alters pulmonary blood flow distribution after 10 min so it is possible that hypoxia is able to regulate
IPAVA occurred independently of significant increases in PASP (Tremblay et al. 2014). It has been shown that hypoxia alters pulmonary blood flow distribution after 10 min so it is possible that hypoxia is able to regulate  IPAVA independently of HPV or as a result of minimal increases in PASP caused by the acute phase of HPV. Bates and colleagues have suggested that a reduced
IPAVA independently of HPV or as a result of minimal increases in PASP caused by the acute phase of HPV. Bates and colleagues have suggested that a reduced  may be responsible for increasing
may be responsible for increasing  IPAVA, which may be the case with hypoxia (Bates et al. 2012). However, with inotropic drug infusion at rest,
IPAVA, which may be the case with hypoxia (Bates et al. 2012). However, with inotropic drug infusion at rest,  would increase so a reduction in
would increase so a reduction in  would not explain the increase in
would not explain the increase in  IPAVA that occurs with inotropic drugs. Future work using drugs such as acetazolamide to block HPV, and separating the effect of
IPAVA that occurs with inotropic drugs. Future work using drugs such as acetazolamide to block HPV, and separating the effect of  from
from  , may help to elucidate the mechanisms responsible for hypoxia-induced
, may help to elucidate the mechanisms responsible for hypoxia-induced  IPAVA. Developing an experimental paradigm where
IPAVA. Developing an experimental paradigm where  IPAVA can be prevented in hypoxic conditions would help to elucidate the specific mechanisms that induce blood flow through IPAVA with alveolar hypoxia. Although the mechanisms responsible for the regulation of
IPAVA can be prevented in hypoxic conditions would help to elucidate the specific mechanisms that induce blood flow through IPAVA with alveolar hypoxia. Although the mechanisms responsible for the regulation of  IPAVA remain elusive, understanding the regulation of hypoxia-induced
IPAVA remain elusive, understanding the regulation of hypoxia-induced  IPAVA could have broad implications for millions of individuals residing at high altitude and individuals who are hypoxaemic secondary to lung disease, such as COPD patients who have been reported to have intrapulmonary shunting as detected with TTSCE (Shaikh et al. 2014). Furthermore, until these investigations are performed using large diameter microspheres in humans and animals or better techniques become available, this area will remain inconclusive.
IPAVA could have broad implications for millions of individuals residing at high altitude and individuals who are hypoxaemic secondary to lung disease, such as COPD patients who have been reported to have intrapulmonary shunting as detected with TTSCE (Shaikh et al. 2014). Furthermore, until these investigations are performed using large diameter microspheres in humans and animals or better techniques become available, this area will remain inconclusive.
Hyperoxia
Of particular interest in this field are the studies demonstrating that  IPAVA is either prevented or reduced in healthy humans breathing 100% O2 with lower bubbles scores or no bubbles in the left heart as detected by TTSCE (Lovering et al. 2008b; Fig.1D). The effect of breathing 100% O2 on regulating
IPAVA is either prevented or reduced in healthy humans breathing 100% O2 with lower bubbles scores or no bubbles in the left heart as detected by TTSCE (Lovering et al. 2008b; Fig.1D). The effect of breathing 100% O2 on regulating  IPAVA has been demonstrated during exercise (Elliott et al. 2011, 2014b; Ljubkovic et al. 2012), post exercise (Madden et al. 2013) and at rest with inotropic drug infusion when breathing 100% O2 (Bryan et al. 2012; Laurie et al. 2012), but not when breathing 40% O2 (Elliott et al. 2014a). One criticism of these findings is that the reduced appearance of left-sided contrast (i.e. lower bubble scores) is due to changing the internal and/or external partial pressure environment of the intravenously injected bubbles. Elliott et al. (2011) addressed this criticism by injecting bubbles of different gaseous compositions (air, O2, CO2, N2, and He) during exercise breathing air, 14% O2, and 100% O2 and found identical bubble scores within a given
IPAVA has been demonstrated during exercise (Elliott et al. 2011, 2014b; Ljubkovic et al. 2012), post exercise (Madden et al. 2013) and at rest with inotropic drug infusion when breathing 100% O2 (Bryan et al. 2012; Laurie et al. 2012), but not when breathing 40% O2 (Elliott et al. 2014a). One criticism of these findings is that the reduced appearance of left-sided contrast (i.e. lower bubble scores) is due to changing the internal and/or external partial pressure environment of the intravenously injected bubbles. Elliott et al. (2011) addressed this criticism by injecting bubbles of different gaseous compositions (air, O2, CO2, N2, and He) during exercise breathing air, 14% O2, and 100% O2 and found identical bubble scores within a given  regardless of the injected bubble composition (Elliott et al. 2011). Similar findings with respect to the effect of hyperoxia reducing
regardless of the injected bubble composition (Elliott et al. 2011). Similar findings with respect to the effect of hyperoxia reducing  IPAVA have been demonstrated using an embolization model of increased
IPAVA have been demonstrated using an embolization model of increased  IPAVA and intravenously injected microspheres 60–420 μm in diameter to detect
IPAVA and intravenously injected microspheres 60–420 μm in diameter to detect  IPAVA (Niden & Aviado, 1956). A consistent finding of these studies using 100% O2 is that using either TTSCE or microspheres provided identical results. One area of inconsistency with these data was that using
IPAVA (Niden & Aviado, 1956). A consistent finding of these studies using 100% O2 is that using either TTSCE or microspheres provided identical results. One area of inconsistency with these data was that using  of 0.4 did not prevent
of 0.4 did not prevent  IPAVA as detected by TTSCE, suggesting that the effect of hyperoxia may be a dose-dependent phenomenon. However, the bubbles used in these studies with 40% O2 may be travelling through distended capillaries so more work in this area using large microspheres in humans and animals is needed.
IPAVA as detected by TTSCE, suggesting that the effect of hyperoxia may be a dose-dependent phenomenon. However, the bubbles used in these studies with 40% O2 may be travelling through distended capillaries so more work in this area using large microspheres in humans and animals is needed.
The mechanisms responsible for the hyperoxia-induced reduction in  IPAVA during exercise at sea level remain unknown. According to the Fick principle of mass balance, breathing 100% O2 during maximal exercise could result in a decrease in
IPAVA during exercise at sea level remain unknown. According to the Fick principle of mass balance, breathing 100% O2 during maximal exercise could result in a decrease in  T proportional to the increase in
T proportional to the increase in  , assuming a constant
, assuming a constant  . The 10% increase in
. The 10% increase in  when breathing 100% O2, could therefore correspond to a reduction in
when breathing 100% O2, could therefore correspond to a reduction in  T of no more than 10% at a given submaximal exercise intensity. If
T of no more than 10% at a given submaximal exercise intensity. If  IPAVA was in part regulated by increases in flow, then this reduction in
IPAVA was in part regulated by increases in flow, then this reduction in  T during exercise breathing 100% O2, could, theoretically, help explain the reduction in
T during exercise breathing 100% O2, could, theoretically, help explain the reduction in  IPAVA during exercise breathing 100% O2. However, with a 10% reduction in
IPAVA during exercise breathing 100% O2. However, with a 10% reduction in  T from 20 l min−1 to 18 l min−1,
T from 20 l min−1 to 18 l min−1,  IPAVA during submaximal exercise breathing air when
IPAVA during submaximal exercise breathing air when  T < 18 l min−1 is still greater than
T < 18 l min−1 is still greater than  IPAVA during exercise while breathing 100% O2 when
IPAVA during exercise while breathing 100% O2 when  T = 18 l min−1. Thus, it is unlikely that an expected ∼10% reduction in
T = 18 l min−1. Thus, it is unlikely that an expected ∼10% reduction in  T is responsible for the observed effect of hyperoxia on
T is responsible for the observed effect of hyperoxia on  IPAVA. Other possible explanations are that hyperoxia either vasoconstricts IPAVA as previously hypothesized (Lovering et al. 2009a) or pulmonary blood flow is redistributed by hyperoxia-induced vasodilatation such that blood flows through areas of the lung without IPAVA (Fig.1D). Recent work by our group using nifedipine, acetazolamide and sildenafil, independently, to target various pulmonary vascular smooth muscle pathways to prevent or reduce vasoconstriction during exercise breathing 100% O2, were ineffective in altering bubble score as detected by TTSCE. These data suggest that the mechanisms targeted by these drugs are not independently involved in the effect of 100% O2 on
IPAVA. Other possible explanations are that hyperoxia either vasoconstricts IPAVA as previously hypothesized (Lovering et al. 2009a) or pulmonary blood flow is redistributed by hyperoxia-induced vasodilatation such that blood flows through areas of the lung without IPAVA (Fig.1D). Recent work by our group using nifedipine, acetazolamide and sildenafil, independently, to target various pulmonary vascular smooth muscle pathways to prevent or reduce vasoconstriction during exercise breathing 100% O2, were ineffective in altering bubble score as detected by TTSCE. These data suggest that the mechanisms targeted by these drugs are not independently involved in the effect of 100% O2 on  IPAVA (Elliott et al. 2014b). Whether or not there are multiple redundant pathways mediating
IPAVA (Elliott et al. 2014b). Whether or not there are multiple redundant pathways mediating  IPAVA is unknown, but studies using combinations of drugs that target the pulmonary vascular pathways may be helpful in elucidating this question. There is an increase in the perfusion heterogeneity of the lung, measured using radioactive microspheres when animals are ventilated with hyperoxia (
IPAVA is unknown, but studies using combinations of drugs that target the pulmonary vascular pathways may be helpful in elucidating this question. There is an increase in the perfusion heterogeneity of the lung, measured using radioactive microspheres when animals are ventilated with hyperoxia ( = 0.4–0.5; Melsom et al. 1999; Hlastala et al. 2004), so an active redistribution of pulmonary blood flow caused by hyperoxia is not without precedent. Nevertheless, the effect of hyperoxia on the redistribution of microspheres may be due to vasodilatation of areas that are closed or vasoconstriction of areas that are open. The majority of work in this area has used TTSCE, and considering the limitations to this technique that were mentioned previously, future work in this area should seek to quantify
= 0.4–0.5; Melsom et al. 1999; Hlastala et al. 2004), so an active redistribution of pulmonary blood flow caused by hyperoxia is not without precedent. Nevertheless, the effect of hyperoxia on the redistribution of microspheres may be due to vasodilatation of areas that are closed or vasoconstriction of areas that are open. The majority of work in this area has used TTSCE, and considering the limitations to this technique that were mentioned previously, future work in this area should seek to quantify  IPAVA with large diameter microspheres (e.g. >25 μm). Furthermore, future studies should seek to determine the effect of alveolar hyperoxia on pulmonary blood flow distribution and
IPAVA with large diameter microspheres (e.g. >25 μm). Furthermore, future studies should seek to determine the effect of alveolar hyperoxia on pulmonary blood flow distribution and  IPAVA at rest and/or during exercise using the existing methodologies as well as novel approaches. Additionally, as outlined above for hypoxia, developing an experimental paradigm where
IPAVA at rest and/or during exercise using the existing methodologies as well as novel approaches. Additionally, as outlined above for hypoxia, developing an experimental paradigm where  IPAVA is possible in hyperoxic conditions would help to elucidate the specific mechanisms that induce or prevent
IPAVA is possible in hyperoxic conditions would help to elucidate the specific mechanisms that induce or prevent  IPAVA in these conditions.
IPAVA in these conditions.
Effect of age on blood flow through IPAVA
It is well known that the pulmonary vasculature changes with age (Taylor & Johnson, 2010) and this may be true of IPAVA as well. Work in a fetal lamb preparation has demonstrated that  IPAVA occurs in the fetus, and then gradually declines as the newborn lamb ages to approximately 6 months old (McMullan et al. 2004). Of particular interest, children with congenital heart diseases who underwent a ‘classical’ Glen Shunt surgical procedure (unilateral superior vena cava to right pulmonary artery shunt) developed pulmonary arteriovenous anastomoses (Lovering et al. 2013). However, if both the superior and inferior vena cavas are anastomosed to the pulmonary artery, the inclusion of the hepatic blood flow from the inferior vena cava prevents the formation of pulmonary arteriovenous anastomoses in these children, suggesting that there is an unknown hepatic factor capable of regulating pulmonary arteriovenous anastomoses. To our knowledge there have not been any studies in healthy human new-borns examining
IPAVA occurs in the fetus, and then gradually declines as the newborn lamb ages to approximately 6 months old (McMullan et al. 2004). Of particular interest, children with congenital heart diseases who underwent a ‘classical’ Glen Shunt surgical procedure (unilateral superior vena cava to right pulmonary artery shunt) developed pulmonary arteriovenous anastomoses (Lovering et al. 2013). However, if both the superior and inferior vena cavas are anastomosed to the pulmonary artery, the inclusion of the hepatic blood flow from the inferior vena cava prevents the formation of pulmonary arteriovenous anastomoses in these children, suggesting that there is an unknown hepatic factor capable of regulating pulmonary arteriovenous anastomoses. To our knowledge there have not been any studies in healthy human new-borns examining  IPAVA, but the existence of IPAVA in human fetal lungs has been characterized previously using isolated lungs and large diameter microspheres (Wilkinson & Fagan, 1990). It is intriguing that
IPAVA, but the existence of IPAVA in human fetal lungs has been characterized previously using isolated lungs and large diameter microspheres (Wilkinson & Fagan, 1990). It is intriguing that  IPAVA occurs in the fetus but that it stops with normal postnatal development. It could be hypothesized that IPAVA are part of the normal fetal circulation, which also includes the ductus arteriosus, because they are regulated similarly, with hyperoxia preventing blood flow and hypoxia allowing for blood flow (Coceani & Olley, 1988). However, the ductus closes shortly after birth whereas IPAVA appear to remain patent in humans as adults.
IPAVA occurs in the fetus but that it stops with normal postnatal development. It could be hypothesized that IPAVA are part of the normal fetal circulation, which also includes the ductus arteriosus, because they are regulated similarly, with hyperoxia preventing blood flow and hypoxia allowing for blood flow (Coceani & Olley, 1988). However, the ductus closes shortly after birth whereas IPAVA appear to remain patent in humans as adults.
Compared to humans <50 years old, lower bubble scores are detected with TTSCE in humans >50 years old during exercise breathing air and when breathing hypoxic gas at rest (Norris et al. 2014). These data suggest that  IPAVA is reduced in older subjects. In these studies, caparisons between old and young subjects were made during iso-workload exercise to avoid potential ageing confounders on exercise capacity. The consistent theme with these data is that
IPAVA is reduced in older subjects. In these studies, caparisons between old and young subjects were made during iso-workload exercise to avoid potential ageing confounders on exercise capacity. The consistent theme with these data is that  IPAVA is a normal occurrence early in life and that the body retains the ability to recruit these pathways during early life and middle age but that
IPAVA is a normal occurrence early in life and that the body retains the ability to recruit these pathways during early life and middle age but that  IPAVA declines with age, for reasons that have yet to be determined. The majority of this work in older subjects has been obtained using TTSCE, so these results may be due to bubbles getting through pulmonary capillaries and/or bubbles not getting through pulmonary capillaries in older subjects. As such, work in developmental physiology models using techniques that can quantify
IPAVA declines with age, for reasons that have yet to be determined. The majority of this work in older subjects has been obtained using TTSCE, so these results may be due to bubbles getting through pulmonary capillaries and/or bubbles not getting through pulmonary capillaries in older subjects. As such, work in developmental physiology models using techniques that can quantify  IPAVA will help to move this area forward.
IPAVA will help to move this area forward.
Proposed physiological and pathophysiological roles of IPAVA
Defining the physiological and/or pathophysiological roles of IPAVA remains an exciting and wide-open area of study because these roles are only beginning to be described. Blood flow through IPAVA is proposed to bypass the pulmonary capillaries during conditions of increased pulmonary blood flow such as exercise (Recavarren, 1966). In doing so, it has been hypothesized that there may be multiple effects of  IPAVA. First,
IPAVA. First,  IPAVA may act as a pressure relief mechanism to assist in pulmonary pressure regulation. Second, if
IPAVA may act as a pressure relief mechanism to assist in pulmonary pressure regulation. Second, if  IPAVA is bypassing the gas-exchanging units within the lung, then this blood flow may reduce pulmonary gas exchange efficiency either as a shunt or a diffusion-limited vessel. Third, if blood flow is travelling through large diameter IPAVA, then this may cause a breach in the pulmonary capillary filter allowing emboli to enter into the systemic arterial circulation whereby the embolus can reach the brain or heart subsequently causing adverse neurological and/or cardiovascular sequelae.
IPAVA is bypassing the gas-exchanging units within the lung, then this blood flow may reduce pulmonary gas exchange efficiency either as a shunt or a diffusion-limited vessel. Third, if blood flow is travelling through large diameter IPAVA, then this may cause a breach in the pulmonary capillary filter allowing emboli to enter into the systemic arterial circulation whereby the embolus can reach the brain or heart subsequently causing adverse neurological and/or cardiovascular sequelae.
Physiology
Pulmonary pressure
As mentioned above, pulmonary pressure and  IPAVA could be related (Stickland et al. 2004), such that IPAVA act as low resistance vascular conduits that help to regulate pulmonary pressure. In this manner, IPAVA would act as ‘pop-off’ valves by allowing
IPAVA could be related (Stickland et al. 2004), such that IPAVA act as low resistance vascular conduits that help to regulate pulmonary pressure. In this manner, IPAVA would act as ‘pop-off’ valves by allowing  IPAVA, thereby preventing excessive increases in pulmonary vascular resistance and pressure (Stickland et al. 2004; Norris et al. 2014). Stickland and colleagues were first to demonstrate, in one subject without a PFO, that no
IPAVA, thereby preventing excessive increases in pulmonary vascular resistance and pressure (Stickland et al. 2004; Norris et al. 2014). Stickland and colleagues were first to demonstrate, in one subject without a PFO, that no  IPAVA during exercise was associated with high pulmonary artery pressure, compared to subjects with
IPAVA during exercise was associated with high pulmonary artery pressure, compared to subjects with  IPAVA who had low pulmonary artery pressure (Stickland et al. 2004). However, it is unlikely that the volume of
IPAVA who had low pulmonary artery pressure (Stickland et al. 2004). However, it is unlikely that the volume of  IPAVA suggested to occur during exercise (<5% of the
IPAVA suggested to occur during exercise (<5% of the  T) being diverted through IPAVA would have a significant, much less measurable, effect on pulmonary artery pressure. Rather, we would offer the hypothesis that areas of the lung that are recruited during conditions of increased pulmonary blood flow may also contain IPAVA such that when these areas are recruited, they result in lower pressures and also allow for
T) being diverted through IPAVA would have a significant, much less measurable, effect on pulmonary artery pressure. Rather, we would offer the hypothesis that areas of the lung that are recruited during conditions of increased pulmonary blood flow may also contain IPAVA such that when these areas are recruited, they result in lower pressures and also allow for  IPAVA. In this way, it is the pulmonary vascular recruitment and distension that results in keeping pulmonary vascular resistance low and not the direct perfusion of IPAVA. Although
IPAVA. In this way, it is the pulmonary vascular recruitment and distension that results in keeping pulmonary vascular resistance low and not the direct perfusion of IPAVA. Although  IPAVA occurs simultaneously under these conditions, it is simply due to the association of these vessels with areas of the vasculature that are recruited under these conditions and direct
IPAVA occurs simultaneously under these conditions, it is simply due to the association of these vessels with areas of the vasculature that are recruited under these conditions and direct  IPAVA has no measureable effect on pulmonary pressure as we have previously suggested (Norris et al. 2014).
IPAVA has no measureable effect on pulmonary pressure as we have previously suggested (Norris et al. 2014).
Pulmonary capillary distension
Others have investigated the relationship of the degree of pulmonary transit of agitated succinylated gelatin contrast and pulmonary vascular resistance during exercise (La Gerche et al. 2010; Lalande et al. 2012). Both of these studies concluded that transpulmonary passage of agitated gelatin contrast was through distended pulmonary capillaries rather than IPAVA. Although these data (La Gerche et al. 2010; Lalande et al. 2012) lend support to the notion that a greater  IPAVA is related to a lower pulmonary vascular resistance during exercise, and therefore may allow for the detection of capillary distension, these authors did not report that subjects were screened for the presence of a PFO. Given the discussion above with respect to the prevalence and importance of PFO in these anatomically based approaches to collecting data, using succinylated gelatin contrast should be interpreted with the understanding that contrast could have either traversed a PFO or IPAVA, or squeezed through pulmonary capillaries as those authors suggested. The differences in in vivo saline contrast bubble dynamics and succinylated gelatin bubble contrast dynamics remains unknown, but the possibility exists that gelatin bubbles are more stable and can therefore squeeze through capillaries but saline bubbles are larger and less stable so they cannot. Studies examining these different contrast agents in conjunction with microspheres of known sizes will help to determine whether or not bubbles are squeezing through pulmonary capillaries.
IPAVA is related to a lower pulmonary vascular resistance during exercise, and therefore may allow for the detection of capillary distension, these authors did not report that subjects were screened for the presence of a PFO. Given the discussion above with respect to the prevalence and importance of PFO in these anatomically based approaches to collecting data, using succinylated gelatin contrast should be interpreted with the understanding that contrast could have either traversed a PFO or IPAVA, or squeezed through pulmonary capillaries as those authors suggested. The differences in in vivo saline contrast bubble dynamics and succinylated gelatin bubble contrast dynamics remains unknown, but the possibility exists that gelatin bubbles are more stable and can therefore squeeze through capillaries but saline bubbles are larger and less stable so they cannot. Studies examining these different contrast agents in conjunction with microspheres of known sizes will help to determine whether or not bubbles are squeezing through pulmonary capillaries.
Pulmonary gas exchange efficiency
The idea that  IPAVA may act as a shunt is not new, yet this idea remains highly contentious. Seminal work by Nomoto et al. demonstrated that intravenous infusion of adrenaline (epinephrine) increased the number of large diameter microspheres (15–30 μm) able to traverse the lung (Nomoto et al. 1974). Concomitant with an increase in microspheres travelling through IPAVA, was a reduction in
IPAVA may act as a shunt is not new, yet this idea remains highly contentious. Seminal work by Nomoto et al. demonstrated that intravenous infusion of adrenaline (epinephrine) increased the number of large diameter microspheres (15–30 μm) able to traverse the lung (Nomoto et al. 1974). Concomitant with an increase in microspheres travelling through IPAVA, was a reduction in  , suggesting the possibility that blood flowing through IPAVA contributes to pulmonary gas exchange efficiency and may act as a shunt (Nomoto et al. 1974). Stickland and colleagues were the first to show a correlation between
, suggesting the possibility that blood flowing through IPAVA contributes to pulmonary gas exchange efficiency and may act as a shunt (Nomoto et al. 1974). Stickland and colleagues were the first to show a correlation between  IPAVA detected with TTSCE, and a widening of the alveolar-to-arterial
IPAVA detected with TTSCE, and a widening of the alveolar-to-arterial  difference (
difference ( ) during exercise (Stickland et al. 2004). Work using 99mTc-MAA suggests that
) during exercise (Stickland et al. 2004). Work using 99mTc-MAA suggests that  at maximal exercise is negatively correlated with
at maximal exercise is negatively correlated with  IPAVA (Lovering et al. 2009b) and that
IPAVA (Lovering et al. 2009b) and that  IPAVA is positively correlated with the
IPAVA is positively correlated with the  (Bates et al. 2014); however, correlation does not mean causation.
(Bates et al. 2014); however, correlation does not mean causation.
Investigations in healthy humans have used intravenous infusions of inotropic drugs to increase  IPAVA while at rest breathing air at sea level. Bryan and colleagues report that with increased IPAVA blood flow there was an increase in calculated shunt fraction (
IPAVA while at rest breathing air at sea level. Bryan and colleagues report that with increased IPAVA blood flow there was an increase in calculated shunt fraction ( S/
S/ T; Bryan et al. 2012). These data are in agreement with those obtained with microsphere studies in animals (Nomoto et al. 1974), although the work by Bryan et al. did not explicitly state that their subjects were free of PFO and the increase in
T; Bryan et al. 2012). These data are in agreement with those obtained with microsphere studies in animals (Nomoto et al. 1974), although the work by Bryan et al. did not explicitly state that their subjects were free of PFO and the increase in  S/
S/ T may have been the result of blood flow through a PFO or increased ventilation to perfusion (
T may have been the result of blood flow through a PFO or increased ventilation to perfusion ( /
/ ) heterogeneity. Elliott et al. have recently used intravenous adrenaline to increase
) heterogeneity. Elliott et al. have recently used intravenous adrenaline to increase  IPAVA in subjects breathing air and 40% O2 (Elliott et al. 2014a). In this experimental paradigm,
IPAVA in subjects breathing air and 40% O2 (Elliott et al. 2014a). In this experimental paradigm,  IPAVA was the same in both conditions, yet when subjects are breathing 40% O2 contributions to the
IPAVA was the same in both conditions, yet when subjects are breathing 40% O2 contributions to the  from diffusion limitation and
from diffusion limitation and  /
/ heterogeneity are minimized or prevented (Wagner & West, 1972). These authors calculated that the venous admixture required to explain the
heterogeneity are minimized or prevented (Wagner & West, 1972). These authors calculated that the venous admixture required to explain the  was ∼2% of
was ∼2% of  T in both conditions, breathing either air or 40% O2, when
T in both conditions, breathing either air or 40% O2, when  IPAVA was present (Elliott et al. 2014a). Taken together, these data support the idea that with diffusion limitation and
IPAVA was present (Elliott et al. 2014a). Taken together, these data support the idea that with diffusion limitation and  /
/ heterogeneity minimized or prevented, only postpulmonary shunt and
heterogeneity minimized or prevented, only postpulmonary shunt and  IPAVA could have played a role in determining pulmonary gas exchange efficiency because these subjects did not have a PFO. Nevertheless, these data were obtained with TTSCE and as such the presence of bubbles in the left heart may be due to passage through pulmonary capillaries and the increase in calculated shunt fraction may have been the result of increases in postpulmonary shunt only, rather than from intrapulmonary shunt as we previously suggested (Elliott et al. 2014a). Accordingly, directly demonstrating deoxygenated blood flow travelling through these pathways and mixing with oxygenated blood will be beneficial in moving this area beyond correlational data to clearly demonstrate cause and effect. Until that time, these data will remain controversial because they are inconsistent with data obtained using the multiple inert gas elimination technique (MIGET). The contemporary explanation for determinants of pulmonary gas exchange efficiency in healthy humans suggest that intrapulmonary and/or intracardiac shunt does not significantly contribute to the
IPAVA could have played a role in determining pulmonary gas exchange efficiency because these subjects did not have a PFO. Nevertheless, these data were obtained with TTSCE and as such the presence of bubbles in the left heart may be due to passage through pulmonary capillaries and the increase in calculated shunt fraction may have been the result of increases in postpulmonary shunt only, rather than from intrapulmonary shunt as we previously suggested (Elliott et al. 2014a). Accordingly, directly demonstrating deoxygenated blood flow travelling through these pathways and mixing with oxygenated blood will be beneficial in moving this area beyond correlational data to clearly demonstrate cause and effect. Until that time, these data will remain controversial because they are inconsistent with data obtained using the multiple inert gas elimination technique (MIGET). The contemporary explanation for determinants of pulmonary gas exchange efficiency in healthy humans suggest that intrapulmonary and/or intracardiac shunt does not significantly contribute to the  (Dempsey & Wagner, 1999). This supposition is supported by data obtained using gas exchange-dependent techniques such as the MIGET and 100% O2 technique (Torre-Bueno et al. 1985; Hammond et al. 1986; Wagner et al. 1986; Vogiatzis et al. 2008). There are presently no other technical approaches with the capacity to isolate the effect of each determinant of pulmonary gas exchange efficiency. Others, have previously outlined, in detail, the limitations associated with the MIGET technique (Hlastala, 1984) and the reasons why there may be discrepancies between data obtained with anatomically based techniques and those obtained with gas exchange-dependent techniques (Genovesi, 1976; Wagner, 1995; Stickland & Lovering, 2006). Thus we refer the reader to these previous works on the discrepancies between these two technical approaches, which is beyond the scope of this review.
(Dempsey & Wagner, 1999). This supposition is supported by data obtained using gas exchange-dependent techniques such as the MIGET and 100% O2 technique (Torre-Bueno et al. 1985; Hammond et al. 1986; Wagner et al. 1986; Vogiatzis et al. 2008). There are presently no other technical approaches with the capacity to isolate the effect of each determinant of pulmonary gas exchange efficiency. Others, have previously outlined, in detail, the limitations associated with the MIGET technique (Hlastala, 1984) and the reasons why there may be discrepancies between data obtained with anatomically based techniques and those obtained with gas exchange-dependent techniques (Genovesi, 1976; Wagner, 1995; Stickland & Lovering, 2006). Thus we refer the reader to these previous works on the discrepancies between these two technical approaches, which is beyond the scope of this review.
Work by some authors using succinylated gelatin bubble contrast, rather than saline bubble contrast, has suggested that if  IPAVA participated in pulmonary gas exchange then it would ‘…immediately cause true shunt and hypoxemia’ (Lalande et al. 2012). However, this statement, and similar statements by others (La Gerche et al. 2010), do not take into account fundamental concepts of pulmonary gas exchange efficiency (Dempsey & Wagner, 1999). For example, during exercise, when subjects are hyperventilating and alveolar
IPAVA participated in pulmonary gas exchange then it would ‘…immediately cause true shunt and hypoxemia’ (Lalande et al. 2012). However, this statement, and similar statements by others (La Gerche et al. 2010), do not take into account fundamental concepts of pulmonary gas exchange efficiency (Dempsey & Wagner, 1999). For example, during exercise, when subjects are hyperventilating and alveolar 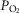 is increased,
is increased,  IPAVA may act as a true shunt resulting in a widening of the
IPAVA may act as a true shunt resulting in a widening of the  , yet
, yet  would remain unchanged from resting conditions and therefore arterial hypoxaemia would not occur (Dempsey & Wagner, 1999; Lovering et al. 2005). This would be true of any shunt, not just
would remain unchanged from resting conditions and therefore arterial hypoxaemia would not occur (Dempsey & Wagner, 1999; Lovering et al. 2005). This would be true of any shunt, not just  IPAVA. It should also be mentioned that arterial oxygen saturation (
IPAVA. It should also be mentioned that arterial oxygen saturation ( ) could be reduced under conditions of constant
) could be reduced under conditions of constant  , by reductions in pH and increases in body temperature that accompany exercise (Dempsey & Wagner, 1999; Lovering & Stickland, 2010), independent of any pulmonary gas exchange impairment.
, by reductions in pH and increases in body temperature that accompany exercise (Dempsey & Wagner, 1999; Lovering & Stickland, 2010), independent of any pulmonary gas exchange impairment.
It is possible that  IPAVA may contribute to pulmonary gas exchange efficiency in some cases, and not represent a source of true shunt in other cases. In this way, IPAVA would represent a vessel where some degree of gas exchange can occur, most likely around the perimeter of the vessel lumen (Conhaim & Staub, 1980) but would be critically dependent upon the size of the vessel and the
IPAVA may contribute to pulmonary gas exchange efficiency in some cases, and not represent a source of true shunt in other cases. In this way, IPAVA would represent a vessel where some degree of gas exchange can occur, most likely around the perimeter of the vessel lumen (Conhaim & Staub, 1980) but would be critically dependent upon the size of the vessel and the  . Pre-capillary/non-capillary gas exchange is not a new concept (Sobol et al. 1963; Conhaim & Staub, 1980) and has recently been demonstrated to occur in vivo in the murine model (Tabuchi et al. 2013). Tabuchi et al. demonstrated that during normoxic ventilation, complete O2 equilibration occurs in ∼0.1 s and begins in arterioles ≤30 μm in diameter. In light of these data, blood flow through IPAVA >50 μm in diameter would probably not participate in pulmonary gas exchange, thereby impairing pulmonary gas exchange efficiency. Whereas blood flow through IPAVA ≤30 μm in diameter may participate in pulmonary gas exchange, thereby not impairing pulmonary gas exchange efficiency. Whether or not this is the case in humans is unknown and will remain unknown until this is directly visualized as it has been in the mouse. As such, the size of IPAVA and the
. Pre-capillary/non-capillary gas exchange is not a new concept (Sobol et al. 1963; Conhaim & Staub, 1980) and has recently been demonstrated to occur in vivo in the murine model (Tabuchi et al. 2013). Tabuchi et al. demonstrated that during normoxic ventilation, complete O2 equilibration occurs in ∼0.1 s and begins in arterioles ≤30 μm in diameter. In light of these data, blood flow through IPAVA >50 μm in diameter would probably not participate in pulmonary gas exchange, thereby impairing pulmonary gas exchange efficiency. Whereas blood flow through IPAVA ≤30 μm in diameter may participate in pulmonary gas exchange, thereby not impairing pulmonary gas exchange efficiency. Whether or not this is the case in humans is unknown and will remain unknown until this is directly visualized as it has been in the mouse. As such, the size of IPAVA and the  are important and would need to be taken into consideration when determining if these vessels, or any pulmonary vessel for that matter, played a role in pulmonary gas exchange efficiency.
are important and would need to be taken into consideration when determining if these vessels, or any pulmonary vessel for that matter, played a role in pulmonary gas exchange efficiency.
Pathophysiology
Pulmonary capillaries filter out particles (i.e. emboli) >7–15 μm in diameter. IPAVA are known to be large diameter pathways and  IPAVA may represent a breach in the pulmonary capillary filter (Abushora et al. 2013). If an embolus were to flow through an IPAVA it could end up in the heart or brain and result in myocardial infarction, transient ischaemic attack (TIA), or stroke. Prizel et al. demonstrated that ∼13% of the myocardial infarcts in their autopsy study were the result of coronary artery embolic infarct (Prizel et al. 1978). In support of this, Clark et al. (2013) has recently suggested that cardiac ischaemia in Hereditary Haemorrhagic Telangiectasia (HHT) patients with known PAVMs is the result of emboli traversing the lung via PAVMs and becoming lodged in a coronary artery (Clark et al. 2013). Stoddard and colleagues demonstrated that
IPAVA may represent a breach in the pulmonary capillary filter (Abushora et al. 2013). If an embolus were to flow through an IPAVA it could end up in the heart or brain and result in myocardial infarction, transient ischaemic attack (TIA), or stroke. Prizel et al. demonstrated that ∼13% of the myocardial infarcts in their autopsy study were the result of coronary artery embolic infarct (Prizel et al. 1978). In support of this, Clark et al. (2013) has recently suggested that cardiac ischaemia in Hereditary Haemorrhagic Telangiectasia (HHT) patients with known PAVMs is the result of emboli traversing the lung via PAVMs and becoming lodged in a coronary artery (Clark et al. 2013). Stoddard and colleagues demonstrated that  IPAVA in individuals at rest breathing air occurred five times more frequently in a subgroup of individuals who had suffered a cerebrovascular accident that was cryptogenic in nature compared to healthy individuals (Abushora et al. 2013). In this study,
IPAVA in individuals at rest breathing air occurred five times more frequently in a subgroup of individuals who had suffered a cerebrovascular accident that was cryptogenic in nature compared to healthy individuals (Abushora et al. 2013). In this study,  IPAVA at rest breathing air was a stronger predictor of stroke than both hypertension and hyperlipidaemia (Abushora et al. 2013). The TTSCE data in the aforementioned studies are supported by data in rats using large diameter microspheres, where the microspheres that traversed the pulmonary microcirculation, presumably through IPAVA, became lodged in the brain (Bates et al. 2012). The presence of a breach in the pulmonary capillary filter, however, only provides a potential pathway for emboli, and by no means guarantees adverse neurological or cardiovascular sequelae.
IPAVA at rest breathing air was a stronger predictor of stroke than both hypertension and hyperlipidaemia (Abushora et al. 2013). The TTSCE data in the aforementioned studies are supported by data in rats using large diameter microspheres, where the microspheres that traversed the pulmonary microcirculation, presumably through IPAVA, became lodged in the brain (Bates et al. 2012). The presence of a breach in the pulmonary capillary filter, however, only provides a potential pathway for emboli, and by no means guarantees adverse neurological or cardiovascular sequelae.
Seemingly inconsistent with these above data, most people are reported to have  IPAVA during exercise as detected with TTSCE, yet most people do not have stroke or TIA when they exercise. Chronic aerobic activity reduces coagulation potential and therefore lowers the probability for clot formation (Womack et al. 2003) which probably explains why most healthy people do not have a stroke or TIA when they go for a run. Nevertheless, until large diameter microspheres are injected into an animal under conditions that increase
IPAVA during exercise as detected with TTSCE, yet most people do not have stroke or TIA when they exercise. Chronic aerobic activity reduces coagulation potential and therefore lowers the probability for clot formation (Womack et al. 2003) which probably explains why most healthy people do not have a stroke or TIA when they go for a run. Nevertheless, until large diameter microspheres are injected into an animal under conditions that increase  IPAVA and the animal subsequently has a stroke and/or TIA, there will be no direct demonstration of causation.
IPAVA and the animal subsequently has a stroke and/or TIA, there will be no direct demonstration of causation.
Additional support for IPAVA acting as a breach in the pulmonary capillary filter comes from work by Dujic and colleagues suggesting that  IPAVA could allow arterialization of gas emboli that formed during the diving ascent (Ljubkovic et al. 2012). In this study, all subjects with post-dive arterialization of gas emboli had larger degrees of
IPAVA could allow arterialization of gas emboli that formed during the diving ascent (Ljubkovic et al. 2012). In this study, all subjects with post-dive arterialization of gas emboli had larger degrees of  IPAVA during exercise, while subjects without post-dive arterialization of gas emboli were individuals with no
IPAVA during exercise, while subjects without post-dive arterialization of gas emboli were individuals with no  IPAVA during exercise, as assessed using TTSCE (Ljubkovic et al. 2012). Whether or not this contributes to arterial gas embolism and subsequently decompression sickness remains controversial but exercise after a dive increases arterial gas embolism (Madden et al. 2013).
IPAVA during exercise, as assessed using TTSCE (Ljubkovic et al. 2012). Whether or not this contributes to arterial gas embolism and subsequently decompression sickness remains controversial but exercise after a dive increases arterial gas embolism (Madden et al. 2013).
As mentioned above,  IPAVA has been suggested to play a role in pulmonary gas exchange efficiency in healthy humans, but this has not been directly established. Nonetheless, a recent case report of a patient with Beriberi heart implicates
IPAVA has been suggested to play a role in pulmonary gas exchange efficiency in healthy humans, but this has not been directly established. Nonetheless, a recent case report of a patient with Beriberi heart implicates  IPAVA in the pathophysiology of this disease as well (Nakano et al. 2013). At presentation the patient had a
IPAVA in the pathophysiology of this disease as well (Nakano et al. 2013). At presentation the patient had a  T of 14 l min−1 with only a mild increase in pulmonary pressure along with
T of 14 l min−1 with only a mild increase in pulmonary pressure along with  of 93% on air and
of 93% on air and  IPAVA was present as detected by TTSCE. After treatment with thiamine repletion,
IPAVA was present as detected by TTSCE. After treatment with thiamine repletion,  T decreased to 6.6 l min−1,
T decreased to 6.6 l min−1,  was 99% on air and
was 99% on air and  IPAVA was absent as detected by TTSCE. Although this is only a case report, the increase in
IPAVA was absent as detected by TTSCE. Although this is only a case report, the increase in  T concomitant with the presence of
T concomitant with the presence of  IPAVA closely resembles the conditions required to increase
IPAVA closely resembles the conditions required to increase  IPAVA in healthy humans (Elliott et al. 2014a). Using an animal model of this disease and large diameter microspheres would directly address the question of whether or not IPAVA are involved in this disease. Clearly, these and other potential pathophysiological roles of
IPAVA in healthy humans (Elliott et al. 2014a). Using an animal model of this disease and large diameter microspheres would directly address the question of whether or not IPAVA are involved in this disease. Clearly, these and other potential pathophysiological roles of  IPAVA should be investigated but much more work is required in various patient populations in this area to confirm and/or refute these roles, which at present are only hypothesized roles.
IPAVA should be investigated but much more work is required in various patient populations in this area to confirm and/or refute these roles, which at present are only hypothesized roles.
Conclusions and future directions
In summary, we have presented a case that IPAVA exist and that  IPAVA may be involved in both physiological and pathophysiological processes. However, this line of research requires more work in well-designed studies using appropriately screened subjects without PFO to continue to make advances that will establish these vessels as relevant in human health and disease. Pertinent areas of future research include determining the precise location of IPAVA in healthy human lungs using modern anatomically based techniques, which will facilitate mechanistically based approaches to determining the regulation of these pathways. For example, real-time visualization of IPAVA and
IPAVA may be involved in both physiological and pathophysiological processes. However, this line of research requires more work in well-designed studies using appropriately screened subjects without PFO to continue to make advances that will establish these vessels as relevant in human health and disease. Pertinent areas of future research include determining the precise location of IPAVA in healthy human lungs using modern anatomically based techniques, which will facilitate mechanistically based approaches to determining the regulation of these pathways. For example, real-time visualization of IPAVA and  IPAVA
in vitro and in vivo will be required for studies of these pathways during the developmental and ageing processes. Additionally, there are humans with
IPAVA
in vitro and in vivo will be required for studies of these pathways during the developmental and ageing processes. Additionally, there are humans with  IPAVA at rest who have been excluded from most studies outlined in this review. Work in these individuals may provide insight into the mechanisms regulating these blood vessels and the impact of this blood flow on physiological processes, if any. Important areas of future focus must be on quantifying
IPAVA at rest who have been excluded from most studies outlined in this review. Work in these individuals may provide insight into the mechanisms regulating these blood vessels and the impact of this blood flow on physiological processes, if any. Important areas of future focus must be on quantifying  IPAVA during various conditions in intact humans and animals and directly measuring the impact of
IPAVA during various conditions in intact humans and animals and directly measuring the impact of  IPAVA on pulmonary gas exchange efficiency. Notably, developing the ability to prevent
IPAVA on pulmonary gas exchange efficiency. Notably, developing the ability to prevent  IPAVA under conditions when it is normally present, e.g. during exercise, and measuring the accompanying effect on pulmonary gas exchange efficiency would allow for demonstration of a cause and effect, if one existed. Lastly, the development of large animal models to investigate the mechanistic regulation of IPAVA and to understand the data discrepancies between anatomically based techniques and gas exchange-dependent techniques will be instrumental in resolving existing controversies.
IPAVA under conditions when it is normally present, e.g. during exercise, and measuring the accompanying effect on pulmonary gas exchange efficiency would allow for demonstration of a cause and effect, if one existed. Lastly, the development of large animal models to investigate the mechanistic regulation of IPAVA and to understand the data discrepancies between anatomically based techniques and gas exchange-dependent techniques will be instrumental in resolving existing controversies.
Acknowledgments
We would like to thank Matthew Vu for the design work in creating Fig. 1.
Glossary
Abbreviations
- A−aDO2
alveolar-to-arterial partial pressure of O2 difference
- CaO2
arterial O2 content
- FIO2
fraction of inspired oxygen
- HHT
hereditary haemorrhagic telangiectasia
- HPV
hypoxic pulmonary vasoconstriction
- IPAVA
intrapulmonary arteriovenous anastomoses
- MAA
macroaggregates of albumin
- MIGET
multiple inert gas elimination technique
- Pv¯O2
mixed venous partial pressure of O2
- PaO2
arterial partial pressure of O2
- PASP
pulmonary artery systolic pressure
- PAVM
pulmonary arteriovenous malformation
- PFO
patent foramen ovale
- SaO2
arterial O2 saturation
- SpO2
peripheral estimate of arterial O2 saturation
- 99mTc-MAA
technetium-99m-labelled MAA
- TIA
transient ischaemic attack
- TTSCE
transthoracic saline contrast echocardiography
- Q̇T
cardiac output
- Q̇IPAVA
blood flow through IPAVA
- Q̇SQ̇T
shunt fraction
- V̇O2
O2 consumption, ventilation to perfusion ratio (V̇/Q̇)
Biographies
AndrewLovering,PhD, is an integrative physiologistwhohas investigated awide range of respiratory systemquestions from understanding how medullary respiratory neurons reconfigure their activity during hypoxia-induced periodic breathing in sleeping cats to understanding the roles of intracardiac and intrapulmonary shunt on the regulation of pulmonary gas exchange efficiency in humans exercising above 5000 m; he thinks that the patent foramen ovale is highly underrated.
 |
Joseph (JJ) Duke, PhD, was a postdoctoral fellow in Dr Lovering’s laboratory and investigated the regulation and quantification of blood flow through intrapulmonary arteriovenous anastomoses in healthy humans during exercise and at rest breathing hypoxic gas.He is currently an Assistant Professor.
Jonathan Elliott, PhD,was a graduate student in Dr Lovering’s Laboratory and investigatedmechanisms regulating blood flow through intrapulmonary arteriovenous anastomoses and quantifying the associated physiological impact of this blood flow on pulmonary gas exchange efficiency. He is currently a postdoctoral fellow.
Additional information
Competing interests
None declared.
Funding
Our research is supported by: American Heart Association Scientist Development Grant No. 228023; American Physiological Society's Giles F. Filley Memorial Award for Excellence in Respiratory Physiology & Medicine; American Lung Association in Oregon and administered through the American Thoracic Society (ATS Grant No. C-10-014); Defense Medical Research and Development Program Grant No. W81XWH-10-2-0114/No. DM1027581JTCG5 TATRC (A.T.L.); American Heart Association Pre-doctoral Fellowship (J.E.E.); Eugene and Clarissa Evonuk Graduate Fellowship in Environmental and Exercise Physiology (J.E.E.).
References
- Abushora MY, Bhatia N, Alnabki Z, Shenoy M, Alshaher M. Stoddard MF. Intrapulmonary shunt is a potentially unrecognized cause of ischemic stroke and transient ischemic attack. J Am Soc Echocardiogr. 2013;26:683–690. doi: 10.1016/j.echo.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Barzilai B, Waggoner AD, Spessert C, Picus D. Goodenberger D. Two-dimensional contrast echocardiography in the detection and follow-up of congenital pulmonary arteriovenous malformations. Am J Cardiol. 1991;68:1507–1510. doi: 10.1016/0002-9149(91)90287-u. [DOI] [PubMed] [Google Scholar]
- Bates ML, Farrell ET, Drezdon A, Jacobson JE, Perlman SB. Eldridge MW. Hypoxia and exercise increase the transpulmonary passage of 99mTc-labeled albumin particles in humans. PloS One. 2014;9:e101146. doi: 10.1371/journal.pone.0101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ML, Fulmer BR, Farrell ET, Drezdon A, Pegelow DF, Conhaim RL. Eldridge MW. Hypoxia recruits intrapulmonary arteriovenous pathways in intact rats but not isolated rat lungs. J Appl Physiol (1985) 2012;112:1915–1920. doi: 10.1152/japplphysiol.00985.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ME( The occurrence of arterio-venous anastomoses in the tongue of the dog. Anat Rec. 1937;69:287–297. [Google Scholar]
- Bryan TL, van Diepen S, Bhutani M, Shanks M, Welsh RC. Stickland MK. The effects of dobutamine and dopamine on intrapulmonary shunt and gas exchange in healthy humans. J Appl Physiol. 2012;113:541–548. doi: 10.1152/japplphysiol.00404.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler BD. Hills BA. The lung as a filter for microbubbles. J Appl Physiol. 1979;47:537–543. doi: 10.1152/jappl.1979.47.3.537. [DOI] [PubMed] [Google Scholar]
- Clark K, Pyeritz RE. Trerotola SO. Angina pectoris or myocardial infarctions, pulmonary arteriovenous malformations, hereditary hemorrhagic telangiectasia, and paradoxical emboli. Am J Cardiol. 2013;112:731–734. doi: 10.1016/j.amjcard.2013.04.052. [DOI] [PubMed] [Google Scholar]
- Coceani F. Olley PM. The control of cardiovascular shunts in the fetal and perinatal period. Can J Physiol Pharmacol. 1988;66:1129–1134. doi: 10.1139/y88-185. [DOI] [PubMed] [Google Scholar]
- Conhaim RL. Staub NC. Reflection spectrophotometric measurement of O2 uptake in pulmonary arterioles of cats. J Appl Physiol. 1980;48:848–856. doi: 10.1152/jappl.1980.48.5.848. [DOI] [PubMed] [Google Scholar]
- Dempsey JA. Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol. 1999;87:1997–2006. doi: 10.1152/jappl.1999.87.6.1997. [DOI] [PubMed] [Google Scholar]
- Dujic Z, Palada I, Obad A, Duplancic D, Brubakk AO. Valic Z. Exercise-induced intrapulmonary shunting of venous gas emboli does not occur after open-sea diving. J Appl Physiol (1985) 2005;99:944–949. doi: 10.1152/japplphysiol.01431.2004. [DOI] [PubMed] [Google Scholar]
- Eldridge MW, Dempsey JA, Haverkamp HC, Lovering AT. Hokanson JS. Exercise-induced intrapulmonary arteriovenous shunting in healthy humans. J Appl Physiol. 2004;97:797–805. doi: 10.1152/japplphysiol.00137.2004. [DOI] [PubMed] [Google Scholar]
- Elliott JE, Choi Y, Laurie SS, Yang X, Gladstone IM. Lovering AT. Effect of initial gas bubble composition on detection of inducible intrapulmonary arteriovenous shunt during exercise in normoxia, hypoxia, or hyperoxia. J Appl Physiol. 2011;110:35–45. doi: 10.1152/japplphysiol.00145.2010. [DOI] [PubMed] [Google Scholar]
- Elliott JE, Duke JW, Hawn JA, Halliwill JR. Lovering AT. Increased cardiac output, not pulmonary artery systolic pressure, increases intrapulmonary shunt in healthy humans breathing room air and 40% O2. J Physiol. 2014a;592:4537–4535. doi: 10.1113/jphysiol.2014.274829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JE, Friedman JM, Futral JE, Goodman RD. Lovering AT. Sildenafil, nifedipine, and acetazolamide do not allow for blood flow through intrapulmonary arteriovenous anastomoses during exercise breathing 100% oxygen. Exp Physiol. 2014b;99:1636–1647. doi: 10.1113/expphysiol.2014.081562. [DOI] [PubMed] [Google Scholar]
- Elliott JE, Nigam SM, Laurie SS, Beasley KM, Goodman RD, Hawn JA, Gladstone IM, Chesnutt MS. Lovering AT. Prevalence of left heart contrast in healthy, young, asymptomatic humans at rest breathing room air. Respir Physiol Neurobiol. 2013;188:71–78. doi: 10.1016/j.resp.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Fenster BE, Curran-Everett D, Freeman AM, Weinberger HD, Kern Buckner J. Carroll JD. Saline contrast echocardiography for the detection of patent foramen ovale in hypoxia: a validation study using intracardiac echocardiography. Echocardiography. 2014;31:420–427. doi: 10.1111/echo.12403. [DOI] [PubMed] [Google Scholar]
- Fischer CH, Campos O, Fernandes WB, Kondo M, Souza FL, De Andrade JL. Carvalho AC. Role of contrast-enhanced transesophageal echocardiography for detection of and scoring intrapulmonary vascular dilatation. Echocardiography. 2010;27:1233–1237. doi: 10.1111/j.1540-8175.2010.01228.x. [DOI] [PubMed] [Google Scholar]
- Foster GE, Ainslie PN, Stembridge M, Day TA, Bakker A, Lucas SJ, Lewis NC, MacLeod DB. Lovering AT. Resting pulmonary haemodynamics and shunting: a comparison of sea-level inhabitants to high altitude Sherpas. J Physiol. 2014;592:1397–1409. doi: 10.1113/jphysiol.2013.266593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos C, Sims-Lucas S. Abman SH. Histologic evidence of intrapulmonary anastomoses by three-dimensional reconstruction in severe bronchopulmonary dysplasia. Ann Am Thorac Soc. 2013;10:474–481. doi: 10.1513/AnnalsATS.201305-124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos C, Sims-Lucas S. Abman SH. Three-dimensional reconstruction identifies misaligned pulmonary veins as intrapulmonary shunt vessels in alveolar capillary dysplasia. J Pediatr. 2014;164:192–195. doi: 10.1016/j.jpeds.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos C, Sims-Lucas S, Ali N, Gien J, Dishop MK. Abman SH. Intrapulmonary vascular shunt pathways in alveolar capillary dysplasia with misalignment of pulmonary veins. Thorax. 2015;70:84–85. doi: 10.1136/thoraxjnl-2014-205851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga P, Buscarini E, Leandro G, Reduzzi L, Grosso M, Pongiglione G, Pedrinazzi C, Lanzarini L, Portugalli V, Blotta P, et al. Contrast echocardiography for pulmonary arteriovenous malformations screening: does any bubble matter? Eur J Echocardiogr: J Working Group on Echocardiogr Eur Soc Cardiol. 2009;10:513–518. doi: 10.1093/ejechocard/jen317. [DOI] [PubMed] [Google Scholar]
- Genovesi MG, Tierney DF, Taplin GV. Eisenberg H. An intravenous radionuclide method to evaluate hypoxemia caused by abnormal alveolar vessels. Am Rev Resp Dis. 1976;114:59–65. doi: 10.1164/arrd.1976.114.1.59. [DOI] [PubMed] [Google Scholar]
- Glazier JB, Hughes JM, Maloney JE. West JB. Measurements of capillary dimensions and blood volume in rapidly frozen lungs. J Appl Physiol. 1969;26:65–76. doi: 10.1152/jappl.1969.26.1.65. [DOI] [PubMed] [Google Scholar]
- Groves BM, Reeves JT, Sutton JR, Wagner PD, Cymerman A, Malconian MK, Rock PB, Young PM. Houston CS. Operation Everest II: elevated high-altitude pulmonary resistance unresponsive to oxygen. J Appl Physiol. 1987;63:521–530. doi: 10.1152/jappl.1987.63.2.521. [DOI] [PubMed] [Google Scholar]
- Hammond MD, Gale GE, Kapitan KS, Ries A. Wagner PD. Pulmonary gas exchange in humans during exercise at sea level. J Appl Physiol. 1986;60:1590–1598. doi: 10.1152/jappl.1986.60.5.1590. [DOI] [PubMed] [Google Scholar]
- Hlastala MP. Multiple inert gas elimination technique. J Appl Physiol. 1984;56:1–7. doi: 10.1152/jappl.1984.56.1.1. [DOI] [PubMed] [Google Scholar]
- Hlastala MP, Lamm WJ, Karp A, Polissar NL, Starr IR. Glenny RW. Spatial distribution of hypoxic pulmonary vasoconstriction in the supine pig. J Appl Physiol. 2004;96:1589–1599. doi: 10.1152/japplphysiol.00211.2003. [DOI] [PubMed] [Google Scholar]
- Hultgren HN, Grover RF. Hartley LH. Abnormal circulatory responses to high altitude in subjects with a previous history of high-altitude pulmonary edema. Circulation. 1971;44:759–770. doi: 10.1161/01.cir.44.5.759. [DOI] [PubMed] [Google Scholar]
- Hultgren HN, Lopez CE, Lundberg E. Miller H. Physiologic studies of pulmonary edema at high altitude. Circulation. 1964;29:393–408. doi: 10.1161/01.cir.29.3.393. [DOI] [PubMed] [Google Scholar]
- Kennedy JM, Foster GE, Koehle MS, Potts JE, Sandor GG, Potts MT, Houghton KM, Henderson WR. Sheel AW. Exercise-induced intrapulmonary arteriovenous shunt in healthy women. Respir Physiol Neurobiol. 2012;181:8–13. doi: 10.1016/j.resp.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Krenz GS. Dawson CA. Flow and pressure distributions in vascular networks consisting of distensible vessels. Am J Physiol Heart Circ Physiol. 2003;284:H2192–H2203. doi: 10.1152/ajpheart.00762.2002. [DOI] [PubMed] [Google Scholar]
- La Gerche A, Daffy JR, Mooney DJ, Forbes G. Davie AJ. Transit of micro-bubbles through the pulmonary circulation of Thoroughbred horses during exercise. Res Vet Sci. 2013;95:644–647. doi: 10.1016/j.rvsc.2013.04.002. [DOI] [PubMed] [Google Scholar]
- La Gerche A, Macisaac AI, Burns AT, Mooney DJ, Inder WJ, Voigt JU, Heidbuchel H. Prior DL. Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol. 2010;109:1307–1317. doi: 10.1152/japplphysiol.00457.2010. [DOI] [PubMed] [Google Scholar]
- Lalande S, Yerly P, Faoro V. Naeije R. Pulmonary vascular distensibility predicts aerobic capacity in healthy individuals. J Physiol. 2012;590:4279–4288. doi: 10.1113/jphysiol.2012.234310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie SS, Elliott JE, Goodman RD. Lovering AT. Catecholamine-induced opening of intrapulmonary arteriovenous anastomoses in healthy humans at rest. J Appl Physiol. 2012;113:1213–1222. doi: 10.1152/japplphysiol.00565.2012. [DOI] [PubMed] [Google Scholar]
- Laurie SS, Yang X, Elliott JE, Beasley KM. Lovering AT. Hypoxia-induced intrapulmonary arteriovenous shunting at rest in healthy humans. J Appl Physiol. 2010;109:1072–1079. doi: 10.1152/japplphysiol.00150.2010. [DOI] [PubMed] [Google Scholar]
- Ljubkovic M, Zanchi J, Breskovic T, Marinovic J, Lojpur M. Dujic Z. Determinants of arterial gas embolism after scuba diving. J Appl Physiol (1985) 2012;112:91–95. doi: 10.1152/japplphysiol.00943.2011. [DOI] [PubMed] [Google Scholar]
- Lovering AT, Eldridge MW. Stickland MK. Counterpoint: Exercise-induced intrapulmonary shunting is real. J Appl Physiol. 2009a;107:994–997. doi: 10.1152/japplphysiol.91489.2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering AT, Elliott JE, Beasley KM. Laurie SS. Pulmonary pathways and mechanisms regulating transpulmonary shunting into the general circulation: An update. Injury. 2010;41S2:S16–S23. doi: 10.1016/S0020-1383(10)70004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering AT, Haverkamp HC. Eldridge MW. Responses and limitations of the respiratory system to exercise. Clin Chest Med. 2005;26:439–457. doi: 10.1016/j.ccm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Lovering AT, Haverkamp HC, Romer LM, Hokanson JS. Eldridge MW. Transpulmonary passage of 99mTc macroaggregated albumin in healthy humans at rest and during maximal exercise. J Appl Physiol. 2009b;106:1986–1992. doi: 10.1152/japplphysiol.01357.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering AT, Riemer RK. Thebaud B. Intrapulmonary arteriovenous anastomoses. Physiological, pathophysiological, or both? Ann Am Thorac Soc. 2013;10:504–508. doi: 10.1513/AnnalsATS.201308-265ED. [DOI] [PubMed] [Google Scholar]
- Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS. Eldridge MW. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol. 2008a;104:1418–1425. doi: 10.1152/japplphysiol.00208.2007. [DOI] [PubMed] [Google Scholar]
- Lovering AT. Stickland MK. Not hearing is believing: novel insight into cardiopulmonary function using agitated contrast and ultrasound. J Appl Physiol (1985) 2010;109:1290–1291. doi: 10.1152/japplphysiol.01083.2010. [DOI] [PubMed] [Google Scholar]
- Lovering AT, Stickland MK, Amann M, Murphy JC, O'Brien MJ, Hokanson JS. Eldridge MW. Hyperoxia prevents exercise-induced intrapulmonary arteriovenous shunt in healthy humans. J Physiol. 2008b;586:4559–4565. doi: 10.1113/jphysiol.2008.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering AT, Stickland MK. Eldridge MW. Intrapulmonary shunt during normoxic and hypoxic exercise in healthy humans. Adv Exp Med Biol. 2006;588:31–45. doi: 10.1007/978-0-387-34817-9_4. [DOI] [PubMed] [Google Scholar]
- Lovering AT, Stickland MK, Kelso AJ. Eldridge MW. Direct demonstration of 25- and 50-μm arteriovenous pathways in healthy human and baboon lungs. Am J Physiol Heart Circ Physiol. 2007;292:H1777–H1781. doi: 10.1152/ajpheart.01024.2006. [DOI] [PubMed] [Google Scholar]
- Lozo M, Lojpur M, Madden D, Lozo P, Banic I. Dujic Z. The effects of nitroglycerin, norepinephrine and aminophylline on intrapulmonary arteriovenous anastomoses in healthy humans at rest. Respir Physiol Neurobiol. 2014;199:19–23. doi: 10.1016/j.resp.2014.04.007. [DOI] [PubMed] [Google Scholar]
- McMullan DM, Hanley FL, Cohen GA, Portman MA. Riemer RK. Pulmonary arteriovenous shunting in the normal fetal lung. J Am Coll Cardiol. 2004;44:1497–1500. doi: 10.1016/j.jacc.2004.06.064. [DOI] [PubMed] [Google Scholar]
- Madden D, Lozo M, Dujic Z. Ljubkovic M. Exercise after SCUBA diving increases the incidence of arterial gas embolism. J Appl Physiol (1985) 2013;115:716–722. doi: 10.1152/japplphysiol.00029.2013. [DOI] [PubMed] [Google Scholar]
- Madden D, Thom SR, Milovanova TN, Yang M, Bhopale VM, Ljubkovic M. Dujic Z. Exercise before scuba diving ameliorates decompression-induced neutrophil activation. Med Sci Sports Exerc. 2014;46:1928–1935. doi: 10.1249/MSS.0000000000000319. [DOI] [PubMed] [Google Scholar]
- Manohar M. Goetz TE. Intrapulmonary arteriovenous shunts of >15 μm in diameter probably do not contribute to arterial hypoxemia in maximally exercising Thoroughbred horses. J Appl Physiol. 2005;99:224–229. doi: 10.1152/japplphysiol.01230.2004. [DOI] [PubMed] [Google Scholar]
- Marriott K, Manins V, Forshaw A, Wright J. Pascoe R. Detection of right-to-left atrial communication using agitated saline contrast imaging: experience with 1162 patients and recommendations for echocardiography. J Am Soc Echocardiogr. 2013;26:96–102. doi: 10.1016/j.echo.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Meerbaum S, Nanda N. Schlief R. Advances in Echo Imaging Using Contrast Enhancement. The Netherla nds: Kluwer; 1993. Principles of echo contrast; pp. 9–42. [Google Scholar]
- Melsom MN, Flatebo T. Nicolaysen G. Hypoxia and hyperoxia both transiently affect distribution of pulmonary perfusion but not ventilation in awake sheep. Acta Physiol Scand. 1999;166:151–158. doi: 10.1046/j.1365-201x.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- Meltzer RS, Sartorius OE, Lancee CT, Serruys PW, Verdouw PD, Essed CE. Roelandt J. Transmission of ultrasonic contrast through the lungs. Ultrasound Med Biol. 1981;7:377–384. doi: 10.1016/0301-5629(81)90048-x. [DOI] [PubMed] [Google Scholar]
- Meltzer RS, Tickner EG. Popp RL. Why do the lungs clear ultrasonic contrast? Ultrasound Med Biol. 1980;6:263–269. doi: 10.1016/0301-5629(80)90022-8. [DOI] [PubMed] [Google Scholar]
- Nakano S, Sujino Y, Tanno J, Ariyama M, Muramatsu T, Senbonmatsu T, Nishimura S, Tamura Y. Fukuda K. Inducible intrapulmonary arteriovenous shunt in a patient with beriberi heart. Am J Respir Crit Care Med. 2013;187:332–333. doi: 10.1164/ajrccm.187.3.332. [DOI] [PubMed] [Google Scholar]
- Niden AH. Aviado DM., Jr Effects of pulmonary embolism on the pulmonary circulation with special reference to arteriovenous shunts in the lung. Circ Res. 1956;4:67–73. doi: 10.1161/01.res.4.1.67. [DOI] [PubMed] [Google Scholar]
- Niden AH, Burrows B. Barclay WR. Effects of drugs on the pulmonary circulation and ventilation as reflected by changes in the arterial oxygen saturation. Circ Res. 1960;8:509–518. doi: 10.1161/01.res.8.3.509. [DOI] [PubMed] [Google Scholar]
- Nomoto S, Berk JL, Hagen JF. Koo R. Pulmonary anatomic arteriovenous shunting caused by epinephrine. Arch Surg. 1974;108:201–204. doi: 10.1001/archsurg.1974.01350260055012. [DOI] [PubMed] [Google Scholar]
- Norris HC, Mangum TS, Duke JW, Straley TB, Hawn JA, Goodman RD. Lovering AT. Exercise- and hypoxia-induced blood flow through intrapulmonary arteriovenous anastomoses is reduced in older adults. J Appl Physiol (1985) 2014;116:1324–1333. doi: 10.1152/japplphysiol.01125.2013. [DOI] [PubMed] [Google Scholar]
- Prichard MM. Daniel PM. Arterio-venous anastomoses in the tongue of the dog. J Anat. 1953;87:66–74. [PMC free article] [PubMed] [Google Scholar]
- Prichard MM. Daniel PM. Arteriovenous anastomoses in the human external ear. J Anat. 1956;90:309–317. [PMC free article] [PubMed] [Google Scholar]
- Prinzmetal M, Ornitz ME, Simkin B. Bergman HC. Arterio-venous anastomoses in liver, spleen and lungs. Am J Physiol. 1948;152:48–52. doi: 10.1152/ajplegacy.1947.152.1.48. [DOI] [PubMed] [Google Scholar]
- Prinzmetal M, Simkin B, et al. Studies on the coronary circulation; the collateral circulation of the normal human heart by coronary perfusion with radioactive erythrocytes and glass spheres. Am Heart J. 1947;33:420–442. doi: 10.1016/0002-8703(47)90090-2. [DOI] [PubMed] [Google Scholar]
- Prizel KR, Hutchins GM. Bulkley BH. Coronary artery embolism and myocardial infarction. Ann Intern Med. 1978;88:155–161. doi: 10.7326/0003-4819-88-2-155. [DOI] [PubMed] [Google Scholar]
- Rahn H, Stroud RC. Tobin CE. Visualization of arterio-venous shunts in by cinefluorography in the lungs of normal dogs. Proc Soc Exp Biol. 1952;80:239. doi: 10.3181/00379727-80-19581. [DOI] [PubMed] [Google Scholar]
- Recavarren S. The preterminal arterioles in the pulmonary circulation of high-altitude natives. Circulation. 1966;33:177–180. doi: 10.1161/01.cir.33.2.177. [DOI] [PubMed] [Google Scholar]
- Reeves JT, Linehan JH. Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol. 2005;288:L419–L425. doi: 10.1152/ajplung.00162.2004. [DOI] [PubMed] [Google Scholar]
- Roelandt J. Contrast echocardiography. Ultrasound Med Biol. 1982;8:471–492. doi: 10.1016/0301-5629(82)90079-5. [DOI] [PubMed] [Google Scholar]
- Rosenzweig DY, Hughes JM. Glazier JB. Effects of transpulmonary and vascular pressures on pulmonary blood volume in isolated lung. J Appl Physiol. 1970;28:553–560. doi: 10.1152/jappl.1970.28.5.553. [DOI] [PubMed] [Google Scholar]
- Sappey PC. Traité d'Anatomie Descriptive. Paris: V.A. Delahaye; 1879. [Google Scholar]
- Shaikh ZF, Kelly JL, Shrikrishna D, de Villa M, Mullen MJ, Hopkinson NS, Morrell MJ. Polkey MI. Patent foramen ovale is not associated with hypoxemia in severe chronic obstructive pulmonary disease and does not impair exercise performance. Am J Respir Crit Care Med. 2014;189:540–547. doi: 10.1164/rccm.201309-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin B, Bergman HC, Silver H. Prinzmetal M. Renal arteriovenous anastomoses in rabbits, dogs and human subjects. Arch Intern Med (Chic) 1948;81:115–125. doi: 10.1001/archinte.1948.00220200003001. [DOI] [PubMed] [Google Scholar]
- Sirsi M. Bucher K. Studies on arteriovenous anastomoses in the lungs. Experientia. 1953;9:217–218. doi: 10.1007/BF02176896. [DOI] [PubMed] [Google Scholar]
- Sobol BJ, Bottex G, Emirgil C. Gissen H. Gaseous diffusion from alveoli to pulmonary vessels of considerable size. Circ Res. 1963;13:71–79. doi: 10.1161/01.res.13.1.71. [DOI] [PubMed] [Google Scholar]
- Stickland MK. Lovering AT. Exercise-induced intrapulmonary arteriovenous shunting and pulmonary gas exchange. Exerc Sport Sci Rev. 2006;34:99–106. doi: 10.1249/00003677-200607000-00003. [DOI] [PubMed] [Google Scholar]
- Stickland MK, Lovering AT. Eldridge MW. Exercise-induced arteriovenous intrapulmonary shunting in dogs. Am J Respir Crit Care Med. 2007;176:300–305. doi: 10.1164/rccm.200702-206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland MK, Welsh RC, Haykowsky MJ, Petersen SR, Anderson WD, Taylor DA, Bouffard M. Jones RL. Intra-pulmonary shunt and pulmonary gas exchange during exercise in humans. J Physiol. 2004;561:321–329. doi: 10.1113/jphysiol.2004.069302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland MK, Welsh RC, Haykowsky MJ, Petersen SR, Anderson WD, Taylor DA, Bouffard M. Jones RL. Effect of acute increases in pulmonary vascular pressures on exercise pulmonary gas exchange. J Appl Physiol. 2006;100:1910–1917. doi: 10.1152/japplphysiol.01484.2005. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Styp-Rekowska B, Slutsky AS, Wagner PD, Pries AR. Kuebler WM. Precapillary oxygenation contributes relevantly to gas exchange in the intact lung. Am J Respir Crit Care Med. 2013;188:474–481. doi: 10.1164/rccm.201212-2177OC. [DOI] [PubMed] [Google Scholar]
- Talbot NP, Balanos GM, Dorrington KL. Robbins PA. Two temporal components within the human pulmonary vascular response to approximately 2 h of isocapnic hypoxia. J Appl Physiol (1985) 2005;98:1125–1139. doi: 10.1152/japplphysiol.00903.2004. [DOI] [PubMed] [Google Scholar]
- Taylor BJ. Johnson BD. The pulmonary circulation and exercise responses in the elderly. Semin Respir Critic Care Med. 2010;31:528–538. doi: 10.1055/s-0030-1265894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom SR, Milovanova TN, Bogush M, Yang M, Bhopale VM, Pollock NW, Ljubkovic M, Denoble P, Madden D, Lozo M. Dujic Z. Bubbles, microparticles, and neutrophil activation: changes with exercise level and breathing gas during open-water SCUBA diving. J Appl Physiol (1985) 2013;114:1396–1405. doi: 10.1152/japplphysiol.00106.2013. [DOI] [PubMed] [Google Scholar]
- Tobin CE. Arteriovenous shunts in the peripheral pulmonary circulation in the human lung. Thorax. 1966;21:197–204. doi: 10.1136/thx.21.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin CE. Zariquiey MO. Arteriovenous shunts in the human lung. Proc Soc Exp Biol Med. 1950;75:827–829. doi: 10.3181/00379727-75-18360. [DOI] [PubMed] [Google Scholar]
- Torre-Bueno JR, Wagner PD, Saltzman HA, Gale GE. Moon RE. Diffusion limitation in normal humans during exercise at sea level and simulated altitude. J Appl Physiol. 1985;58:989–995. doi: 10.1152/jappl.1985.58.3.989. [DOI] [PubMed] [Google Scholar]
- Tremblay JC, Lovering AT, Ainslie PN, Stembridge M, Burgess KR, Bakker A, Donnelly J, Lucas SJ, Lewis NC, Dominelli PB, Henderson WR, Dominelli GS, Sheel AW. Foster GE. Hypoxia, not pulmonary vascular pressure induces blood flow through intrapulmonary arteriovenous anastomoses. J Physiol. 2014 doi: 10.1113/jphysiol.2014.282962. DOI: 10.1113/jphysiol.2014.282962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gent MW, Post MC, Snijder RJ, Swaans MJ, Plokker HW, Westermann CJ, Overtoom TT. Mager JJ. Grading of pulmonary right-to-left shunt with transthoracic contrast echocardiography: does it predict the indication for embolotherapy? Chest. 2009;135:1288–1292. doi: 10.1378/chest.08-1266. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Zakynthinos S, Boushel R, Athanasopoulos D, Guenette JA, Wagner H, Roussos C. Wagner PD. The contribution of intrapulmonary shunts to the alveolar-to-arterial oxygen difference during exercise is very small. J Physiol. 2008;586:2381–2391. doi: 10.1113/jphysiol.2007.150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD. Impairment of gas exchange in liver cirrhosis. Eur Respir J. 1995;8:1993–1995. doi: 10.1183/09031936.95.08121993. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW. Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol. 1986;61:260–270. doi: 10.1152/jappl.1986.61.1.260. [DOI] [PubMed] [Google Scholar]
- Wagner PD. West JB. Effects of diffusion impairment on O2 and CO2 time courses in pulmonary capillaries. J Appl Physiol. 1972;33:62–71. doi: 10.1152/jappl.1972.33.1.62. [DOI] [PubMed] [Google Scholar]
- Whyte MK, Peters AM, Hughes JM, Henderson BL, Bellingan GJ, Jackson JE. Chilvers ER. Quantification of right to left shunt at rest and during exercise in patients with pulmonary arteriovenous malformations. Thorax. 1992;47:790–796. doi: 10.1136/thx.47.10.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MJ. Fagan DG. Postmortem demonstration of intrapulmonary arteriovenous shunting. Arch Dis Child. 1990;65:435–437. doi: 10.1136/adc.65.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack CJ, Nagelkirk PR. Coughlin AM. Exercise-induced changes in coagulation and fibrinolysis in healthy populations and patients with cardiovascular disease. Sports Med. 2003;33:795–807. doi: 10.2165/00007256-200333110-00002. [DOI] [PubMed] [Google Scholar]
- Woods TD, Harmann L, Purath T, Ramamurthy S, Subramanian S, Jackson S. Tarima S. Small- and moderate-size right-to-left shunts identified by saline contrast echocardiography are normal and unrelated to migraine headache. Chest. 2010;138:264–269. doi: 10.1378/chest.09-2797. [DOI] [PubMed] [Google Scholar]
- Yang WJ. Dynamics of gas bubbles in whole blood and plasma. J Biomech. 1971;4:119–125. doi: 10.1016/0021-9290(71)90022-4. [DOI] [PubMed] [Google Scholar]
- Yang WJ, Echigo R, Wotton DR. Hwang JB. Experimental studies of the dissolution of gas bubbles in whole blood and plasma. I. Stationary bubbles. J Biomech. 1971a;4:275–281. doi: 10.1016/0021-9290(71)90033-9. [DOI] [PubMed] [Google Scholar]
- Yang WJ, Echigo R, Wotton DR. Hwang JB. Experimental studies of the dissolution of gas bubbles in whole blood and plasma. II. Moving bubbles or liquids. J Biomech. 1971b;4:283–288. doi: 10.1016/0021-9290(71)90034-0. [DOI] [PubMed] [Google Scholar]
- Zukotynski K, Chan RP, Chow CM, Cohen JH. Faughnan ME. Contrast echocardiography grading predicts pulmonary arteriovenous malformations on CT. Chest. 2007;132:18–23. doi: 10.1378/chest.06-2356. [DOI] [PubMed] [Google Scholar]


