Abstract
Older adults often suffer from functional impairments that affect their ability to perform everyday tasks. To detect the onset and changes in abilities, healthcare professionals administer standardized assessments. Recently, technology has been utilized to complement these clinical assessments to gain a more objective and detailed view of functionality. In the clinic and at home, technology is able to provide more information about patient performance and reduce subjectivity in outcome measures. The timed up and go (TUG) test is one such assessment recently instrumented with technology in several studies, yielding promising results towards the future of automating clinical assessments. Potential benefits of technological TUG implementations include additional performance parameters, generated reports, and the ability to be self-administered in the home. In this paper, we provide an overview of the TUG test and technologies utilized for TUG instrumentation. We then critically review the technological advancements and follow up with an evaluation of the benefits and limitations of each approach. Finally, we analyze the gaps in the implementations and discuss challenges for future research towards automated, self-administered assessment in the home.
Keywords: Timed up and go (TUG), rehabilitation, gait analysis, clinical assessment, accelerometry
I. Introduction
In recent years, there has been an increase in life expectancy which has resulted in a global aging of the population. In 2050 there will be an estimated 88.5 million individuals aged 65 and older in the United States alone, a 120% increase over the elderly population in 2010 [1]. This growing older population is placing a heavy burden on our healthcare systems. According to the Association of American Medical Colleges, the increasing demand for healthcare will cause a shortage of 124,400 physicians by 2025 [2]. The future of healthcare availability and quality of services is uncertain. In order to meet these demands, healthcare needs to scale and utilize technology more than ever before. To address this problem, proposed technological solutions have flooded research and the healthcare market. For example, to aid individuals in living safely and independently in their homes, ambient intelligence and smart environments are being heavily researched and prototyped. Therapy and rehabilitation in the home is becoming more and more prevalent with inexpensive teleconferencing systems and networked gaming platforms like the Nintendo WiiU. Mobile applications for smartphones and tablets are being developed to assist individuals with cognitive impairment as they navigate their activities of daily living (ADLs). Wearable technology is attempting to ubiquitously collect daily living data and assess functional ability. All of these technologies are moving towards scaling healthcare assessments and rehabilitation to meet demands.
The area of instrumenting clinical assessments with technology is being explored by interdisciplinary researchers to gain a more detailed view of functionality. In the clinic and at home, technology is able to provide more details and insight about patient performance. In addition, carrying out these assessments in the home instead of the clinic is believed to be more representative of an individual’s capabilities [3]. One such exam is the extensively researched timed up and go (TUG) test, which has been widely used in the clinic and in the home to assess functionality for over 20 years [4]. The TUG test has recently been instrumented with technology in several studies, yielding promising results towards the future of automating clinical assessments. A few benefits of the TUG technology implementations include additional performance parameters, generated reports, and the ability to be self-administered in the home. These are steps in the right direction that technology needs to take to address the healthcare crisis and become widely adopted by clinicians and patients alike.
A. Goal of the Current Paper
While developments in technology have made a direct impact on the future of automated assessments, these advances have not been summarized or compared in the medical or engineering literature. There is no defined consensus on what information has been gained by technology, how valuable that information is, or which technologies are appropriate and which ones are not. If we want technology to be utilized in the clinic and deployed in the home, we need to demonstrate how it can reliably and accurately advance rehabilitation measurements and alleviate the burden on clinicians and caregivers.
The TUG test is widely used to provide valuable information on falls risk assessment, functional decline, and changes exhibited amongst populations with different conditions. We believe the technologies applied to the TUG test are representative of the current state of technology-infused clinical assessments. In this paper, we overview the timed up and go exam and analyze its importance in functional assessment. We review current technologies that are used for instrumenting TUG tests and analyze their contributions to the advancement of technical clinical assessments. Finally, we discuss the gaps in the research, challenges for engineers and clinicians, and provide suggestions for future directions towards self-administered, automated assessments.
II. Timed Up and Go Overview
The timed up and go test is a widely used method of evaluating basic mobility maneuvers [5]. It is based on the get up and go (GUG) test that was originally proposed by Mathias et al. [6] in 1986. The GUG test begins with the subject seated in an armchair. The subject rises from the chair, walks 3 meters in a linear path, performs a 180° turn, walks back to the chair, and sits down (see Fig. 1). Typical instructions given to the subject are: “When I say ‘go’, I want you to stand up and walk to the line, turn, and then walk back to the chair and sit down again. Walk at your normal pace” [4]. GUG performance is subjectively evaluated by the observer on a five-point ordinal scale: “normal”, “very slightly abnormal”, “mildly abnormal”, “moderately abnormal”, and “severely abnormal” [6]. The TUG is a timed version of the GUG that attempts to address the subjectivity of the ordinal scale with the introduction of an objective measure, the total time to complete the task [4]. For the TUG, an examiner records the number of seconds it takes for the subject to perform the task using a stopwatch. Several clinical trials and research has discovered that this duration measure is representative of an individual’s ambulatory abilities, balance, and possibly risk of falling [5].
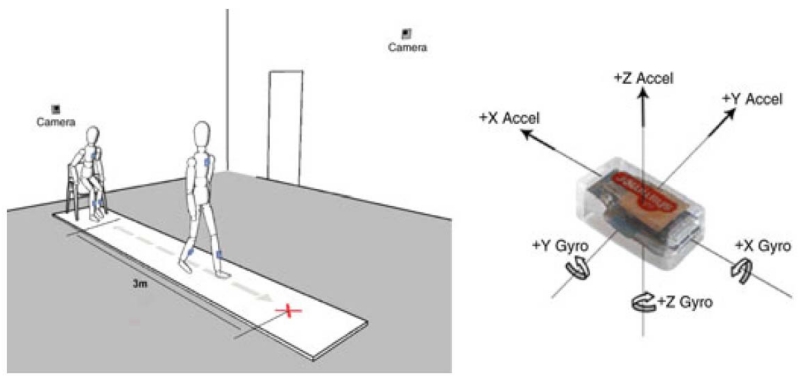
Fig. 1.
Experimental setup for instrumenting the timed up and go with inertial sensors and cameras (left). The red cross on the floor denotes the turnaround point. Shimmer inertial sensor (5.4 cm × 1.9 cm × 3.2 cm) with coordinate axes (right). Greene and Kenny (2012) [74].
The TUG test has become one of the most popular functional assessments for several reasons. First off, the TUG tests several different mobility skills. These include sit-to-stand and stand-to-sit chair transitions, turning, straight-ahead gait, balance control, and the ability to sequence tasks [7]-[9]. The TUG requires minimal materials and setup. All that is required is a chair, 3 meters of walking space, and tape for marking the turnaround point. Furthermore, the TUG is simple to score, requiring minimal training and no expertise in mobility analysis. In the seminal TUG paper, Podsiadlo et al. [4] found the TUG to have good test-retest reliability, inter-rater reliability, and concurrent validity. More recently, Hafsteinsdottir et al. [10] and Rydwik et al. [11] reviewed TUG studies for analysis of test reliability, validity, and responsiveness.
A. TUG Case Study: Parkinson’s Disease
Parkinson’s disease (PD) is a degenerative disorder of the nervous system that can cause slowed movement, tremor, impaired posture and balance, and rigid muscles [12], [13]. Because of the turning, gait, and sequencing involved, the TUG test has been deemed a highly suitable examination for assessing motor symptoms in PD [14]. Gait in particular is sensitive to changes in PD: patients have slower walking speeds, take short shuffle steps, and higher inter-stride variability [15]. In general, patients with PD have higher TUG times than healthy individuals [16]-[18]. Several studies have found the duration to complete the TUG test correlates well with moderate-to-severe PD [16], [19]–[21], but does not correlate well with early-to-moderate stage PD [18], [22]. Later we discuss how technology can address this insensitivity of the original TUG and compute gait parameters to quantify PD-affected movement.
B. TUG Case Study: Falls Risk Assessment
As the elderly population continues to grow, fall prevention is becoming more and more paramount. Several clinical assessments have been developed to quantify an individual’s risk of falling [23] or have been found to be correlated with falls risk [24]. The TUG test is one such examination that is utilized extensively in the clinic for falls risk assessment [25]-[27] ; however, the validity of the TUG as a viable fall predictor has been argued, with evidence provided that supports both sides of the debate. In support of TUG-based falls assessment, Shimada et al. [24] demonstrated that fallers take significantly longer (p = 0.011) than non-fallers to complete the TUG test. The correlation between TUG time and fall likelihood has been indicated by other studies as well [28]-[31]. In addition, certain components of the TUG have been demonstrated to correlate with fall risk, including the 180° turn [32] and the sit-to-stand movement [33], [34]. On the contrary, studies have reported that the time to complete the TUG was not significantly different amongst fallers and non-fallers [35], [36] and did not predict falls among relatively well-functioning older adults [37], [38].
C. Limitations
It is evident that the TUG test is an important standardized test with several benefits, but the TUG is not without limitations:
The duration measure is not always sensitive to falls risk in healthy older populations [35]-[39].
With three highly different subtasks (chair transition, straight-ahead gait, and 180° turn) there is opportunity for various movement strategies. For example, the 180° turn introduces variability as people with different gait and balance impairments compensate during turns differently. The subject may turn on the spot or in a curve, as is often the case with the use of an assistive device such as a walker [40].
Movement deficiencies exhibited on the complex subtasks are ignored. The effects of a new medication or therapy could go unnoticed when only analyzing the course-grained measurement of duration [41].
Three meters is not long enough to produce high reliability and discriminate amongst healthy and PD populations [42].
The TUG is fairly sensitive to subject and environmental conditions. For example, test-retest reliability is low when subjects wear different footwear [43]. A similar conclusion has been formed regarding the usage of assistive devices during the TUG test [44].
The choice of chair can introduce variability. For example, if the chair has arms they can be used for assistance rising from or lowering to the chair [41]. For this reason, several studies opt to explicitly use armless chairs [4], [6], [45], [46].
There are several limitations that are not specific to the TUG and are common amongst other clinical assessments, including variability amongst instructions given, subjectivity amongst examiners, and documentation differences [47]. It is known that performance in a lab setting does not fully represent the abilities of an individual [40], as it does not replicate ecological conditions [14]. People are more aware of the “test” situation in a laboratory or clinical setting and thus are more conscientious of their performance, often resulting in better performance [3].
D. TUG Variations
Several variations of the TUG have been proposed to address the limitations of the standard TUG and to perform additional assessments. A second task has been added to the TUG, producing timed up and go-dual tasks (TUG-DT) [31]. For several studies, the second task involves a cognitive component. Beauchet et al. [48] reported that gait parameters are affected by which cognitive task is chosen as the secondary task to walking. The countdown task requires the use of working memory [49], which consequently has the highest perturbation on gait parameters [48]. See Table I for a summary of proposed TUG variants and their descriptions.
TABLE I.
Summary of TUG variations in chronological order.
| Article | TUG Variant | Description |
|---|---|---|
| O’Brien et al. (1997) [100] |
Extra chair | Added an additional chair at the 3 meter mark. |
| Lundin-Olsson et al.
(1998) [101] |
TUG-manual | Subjects carry a glass of water while walking and place it back on the table when returning to the chair. |
| Wall et al. (2000) [46] |
Expanded TUG | Increased length to 10 meters and times each of the subtasks of the TUG separately. |
| Shumway-Cook et al. (2000) [31] |
TUG-dual task w/cognitive |
Subjects count backwards by 3’s from a randomly chosen number in the range 20 to 100. |
| Medley and Thompson (2005) [102] |
TUG-environment | Subjects walk over varying carpet thickness. Simulated by 2 foam mats, 1 meter wide and ½ and ¾ inch thick respectively. |
| Silsupadol et al. (2006) [103] |
TUG-dual task w/cognitive |
Subjects were asked simple arithmetic questions such as “What is 3 plus 2?” |
| Vaillant et al. (2006) [104] |
Extra turning | Subjects walk around the chair before sitting down. |
| Demura and Uchiyama (2007) [105] |
TUG-obstacle | Subjects step over a box, turn 180°, step back over the box and return to the chair. Increased length to 5 meters. |
| Nordin et al. (2008) [106] |
GUG-modified | Additional descriptions to each of the items on the original fall-risk 5-point ordinal scale. |
| Maranhão-Filho et al.
(2011) [107] |
TUG-dual task w/cognitive |
Subjects to recite alternating letters of the alphabet while they performed the TUG. |
| Cuesta-Vargas et al.
(2013) [108] |
Water-TUG | TUG inside a 1 meter-deep swimming pool. |
| Sprint et al. (2014) [109] |
Ecological TUG | TUG in an ecological environment including a vehicle transfer at the 180° turnaround point. |
III. Search Strategy
Three formal searches were carried out in the Institute of Electrical and Electronics Engineers (IEEE) Xplore digital library, the Association for Computing Machinery (ACM) digital library, and the National Center for Biotechnology Information PubMed digital library. Keywords searched included various combinations of: 1) one term describing the TUG test (“timed up and go”, “get up and go”, “TUG”, “GUG”, or “GUGT”) and 2) one term related to technology (“instrument”, “automate”, “sensor”, “inertial”, “accelerometer”, “gyroscope”, “ambient”, “video”, “camera”, or “Kinect”). Of the papers gathered via search, the references to related work were followed recursively if they involved instrumenting the TUG test or one of its variations.
A systematic literature search was performed and studies were included based on the following criteria: 1) an English version of the publication was available; 2) the study involved the timed up and go or an assessment similar to the TUG sequence of actions (including at least a chair transfer and ambulation); 3) the study utilized a technology to extract additional TUG information beyond the total duration; and 4) dissertations, theses, and reviews were not included. Studies that included human participants and provided reference data were sought but not required. Often there were several studies published by a set of authors using the same technological implementation for instrumenting the TUG test. For these cases, we discuss the paper that best represents the implementation and provide references for further reading. In total, 30 uniquely instrumented TUG papers satisfied these criteria and were included in the review.
IV. TUG Technologies
In the recent decade, technology has become more advanced and inexpensive than ever before. Recent research has focused on custom designing technology and adapting off-the-shelf solutions to medical applications. For this paper, technology utilized for the timed up and go test has been divided into three main categories: video-based (7 studies), wearable (18 studies), smartphone-based (4 studies), and ambient technologies (1 study). The following sections provide a brief description and discussion of the feasibility of each technology for automated assessment in the home and clinic. For the benefits and limitations of each technology, see Table II.
TABLE II.
Benefits and limitations of technologies utilized for instrumenting the TUG.
| Technology | Benefits | Limitations |
|---|---|---|
| Video-based | Hardware is not required to be attached to or worn on the body. |
Cameras need to be well-positioned. |
| Synchronized with other technologies. | Viewing area can become blocked. | |
| Often wall-powered, so no need to change batteries. |
Difficulties can arise with multiple people in the area [52], [56], [57]. |
|
| Facilitates tele-assessment. | Need sufficient lighting. | |
| Re-playable for clinician scoring at a later time. |
Surface of the floor can be problematic for depth estimation using Kinect [59]. |
|
| Depth information is available. | Privacy is a concern in the home [55]. | |
|
| ||
| Wearable inertial sensors |
Small form factor. | Need to be routinely charged. |
| Comfortable attachment minimizes user awareness. |
Difficult to self-mount sensors on one’s own body. |
|
| Units contain several sensors (i.e. accelerometers, gyroscopes, etc.). |
Need to be well-positioned and oriented. | |
| Do not require skin surface contact. | Sensors are noisy and suffer from drift. | |
| Inexpensive. | May require calibration. | |
| Attachable anywhere on the body. | May be easily noticeable. | |
| Portable; the testing space is not constrained. |
May be uncomfortable or interfere with natural movement. |
|
| Wireless. | Low processing power. | |
|
| ||
| Smartphone- based |
Contains a superset of the sensors in an IMU. |
Need to be routinely charged. |
| Mounted on the body or carried in pockets or bags. |
Need to be well-positioned and oriented. | |
| Automatically sync via the internet connection. |
To upload data, WiFi or a cell phone service is required. |
|
| An interactive display. | Training is required. | |
| High processing power. | May require calibration. | |
| Easy to use. The elderly population is learning how to use them [110]. |
Large in size. | |
|
| ||
| Ambient sensors | Hardware is not required to be attached to or worn on the body. |
Data collection is limited to the environment they are mounted in. |
| Facilitates continuous monitoring. | Requires technical installation. | |
| Integrates into the environment and can be unnoticeable. |
Multiple people or pets in the area can cause complications. |
|
| Ability to track home-related metrics such as electricity consumption. |
Low resolution for computing movement parameters such as stride length. |
|
A. Video-based
In the growing area of tele-assessment, Durfee et al. [50] explored the feasibility of a video conferencing system for a two-way connection between different locations. At one location, a therapist administered and evaluated joint range-of-motion, manual muscle test, Berg balance scale (BBS) sit-to-stand, BBS forward reach, and the TUG test. At the remote location, another therapist scored the assessments via the video conferencing system. For all assessments except the range of motion test, no significant differences between the co-located score and the remote score were found. Tele-assessments and tele-rehabilitation are viable options to allow people to receive medical services in their home.
Berrada et al. [51] implemented a video camera system to research the feasibility of automating the TUG in the home with a no-configuration-required technology. The TUG test utilized a couch in the living room of a test-home. A camera was mounted to provide a side view of the TUG area in front of the couch. The system appeared to struggle with accurate test timing; however, a benefit of this approach includes the use of a couch as it is a more ecological representation of chair transfers performed in the home.
Two years later, Skrba et al. [52] also performed research on automating the TUG test using video cameras. For this study, two webcams captured subject side and back views. From the side view, total walk duration and number of steps taken were automatically calculated. Stability into and then out of the turn was computed from the rear facing camera. One of the results of the study was significant classification of fallers and non-fallers by use of the walk duration and the time between turning and sitting back down in the chair. A stability factor to describe the balance of the turn was computed by extracting the subject’s silhouette out of the video data and tracking the center of the head. The lateral motion and maximum displacement during the turn are a few of the metrics used to compute the stability score.
Similarly, Wang et al. [53] utilized video-based technology to specifically analyze the turn portion of the TUG test. Two webcams were used to compute the number of turn steps, time to complete the turn, and a measure called “appears steady?” described as “moving fluently without hesitation”, to quantify the 180° turn. The turn times as computed by the camera system were compared to physical therapist scored times for seven participants, yielding a mean difference of 0.11 seconds and standard deviation of 0.27 seconds.
1) TUG and Kinects
In 2010 Microsoft released the Kinect, a webcam-like motion sensor for the Xbox 360. The Kinect was a major advancement for natural user interfaces, as it no longer required a game controller or any hardware attached to the body. In addition, the Kinect offers skeleton tracking and depth sensing features that can potentially alleviate a portion of the privacy concerns of video cameras [54]. A study by Demiris et al. [55] on older adults’ perceptions of video cameras found shape extraction and silhouette images to be an acceptable form of in-home, video-based technology.
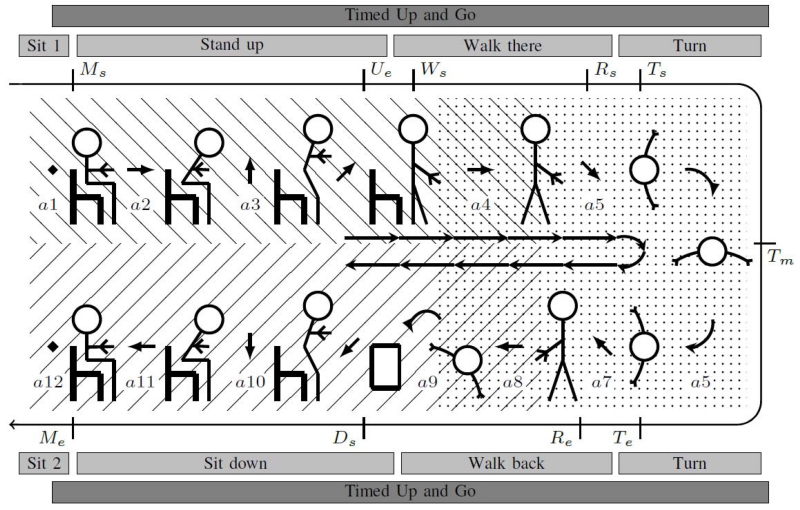
The first TUG study using Kinects was Lohmann et al. [56] and their proposed Skeleton TUG (sTUG) test. Two Kinect sensors and their skeleton tracking modes were used to record the standard 3 meter TUG test (see Fig. 2). The researchers detected ten events of the TUG: start moving (Ms), end uprising (Ue), start walking (Ws), start rotating (Rs), start turning (Ts), max turn (Tm), end turning (Te), end rotating (Re), start lowering (Ds), and end moving (Me). The events start moving, start walking, start turning, end turning, and end moving were manually labeled in the video sequence and compared to the sTUG computed times. The results yielded a mean difference of 0.1 seconds and a much lower standard deviation at 0.15 seconds compared to 0.68 seconds for stopwatch measures for five sTUG participants.
Fig. 2.
The Skeleton TUG. The dashed and dotted areas are the view and detection area of Kinect one and two respectively. Labels Ms – Me correspond to component detected events. Labels al – a12 are TUG actions. Lohmann et al. (2012) [56].
Kitsunezaki et al. [57] augmented the TUG and 10 meter walk test with a Kinect. Like Lohmann and colleagues, it was reported their Kinect processing algorithms have high precision, 0.33 seconds average difference when compared to stopwatch times. Additionally, this study compared three different possible locations for the Kinect: in front of, to the side of, and above the chair. It was concluded that placing the Kinect 4 meters directly in front of the chair minimized timing errors. Since this distance is only 1 meter greater than the required walking length of the TUG, the system setup is small enough to be installed in homes.
Another clinical assessment similar to the TUG, the Tinetti test [58], has also been instrumented with Kinect sensors. Recently, Cippitelli et al. [59] used a single Kinect sensor to track the specific Tinetti tasks of sitting in the chair, rising from the chair, and beginning to walk. With the Kinect system, joint angles for the head, shoulder, knee, ankle, hip, and elbow are reported. Joint angles are especially important to track over time as decreased range of motion is associated with falls in the elderly [60].
2) Summary
Table III summarizes the research contributions with this technology. Of the seven video-based TUG studies reviewed, three utilized Kinect sensors (42.86%). Automatically and accurately computing mobility-related parameters by video cameras are laying the ground work for longitudinal functional assessment. For example, if testing could be self-administered in the home then duration, stability factor, and number of turn steps could be collected regularly. The rate of data acquisition could be daily, a frequency much higher than trips to the clinic. Changes in these values could be tracked over time and present a detailed model of an individual’s health. Improvement or decline detected in these parameters could be indicative of cognitive or functional debility and noticed at onset.
TABLE III.
Summary of instrumenting the TUG test with video-based technologies in chronological order. M = male, D = dimensional.
| Article | Population and Sample Size |
Description of TUG Technology |
Study Findings |
|---|---|---|---|
| Berrada et al. (2007) [51] |
N/A | 1 video camera. | Timing in the video sequences is noisy. |
| Durfee et al. (2007) [50] |
10 healthy (2M, 18-35 years). |
Video and audio conferencing system. |
No significant differences between TUG scores assessed locally and remotely. |
| Skrba et al. (2009) [52] |
29 fallers and 23 non- fallers (18M, mean age 70.9 years). |
BioMOBIUS with 2 webcams. |
Discrimination of fallers and non- fallers by using automated component timings and head positioning during turning. |
| Wang et al. (2011) [53] |
7 healthy (age range 25- 88 years). |
2 calibrated digital cameras. |
180° turn time extracted from video exhibited a mean difference of 0.11 seconds compared with therapist times. |
| Lohmann et al. (2012) [56] |
5 age-related medical conditions (2M, 70-84 years). 4 healthy (4M, 29- 31 years). |
2 Kinects. | High timing precision when compared with human stopwatch time. Timing of subtask components. |
| Kitsunezaki et al. (2013) [57] |
6 healthy. | 1 Kinect. | The differences between Kinect and human scored stopwatch times and the times were averaged to 0.33 seconds. |
| Cippitelli et al. (2014) [59] |
N/A | 1 Kinect. | Analysis of head, shoulder, knee, ankle, hip, and elbow joint angles. |
One of the major benefits of video-based sensing solutions is there is no requirement to place any sensors on the body. This is especially important for the future of automated clinical assessments in the home. As we have seen, several video-based TUG researchers are already working on installing TUG technology in the home.
B. Wearable Sensors
With the advent of wireless technologies such as Bluetooth, WiFi, and Zigbee, wearable sensors have become quite popular for activity logging and healthcare applications. For example, inertial measurement units (IMUs) are movement tracking devices that contain accelerometers and gyroscopes. An accelerometer measures acceleration in meters/second2 and a gyroscope measures angular velocity in degrees/second. IMUs have been utilized quite extensively for instrumenting the TUG test. The studies have been grouped together based on the subject populations the technology was used to investigate.
1) Parkinson’s Disease
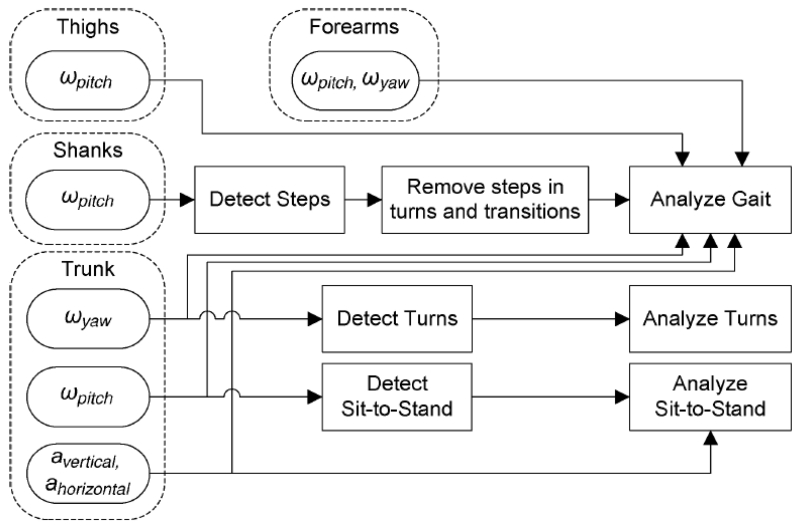
The largest body of research published on instrumenting the TUG test comes from Salarian and colleagues working on iTUG, the instrumented timed up and go [22], [41], [61]-[65]. The iTUG is comprehensively represented by Salarian et al. [41]. The iTUG was extended from the standard 3 meter TUG length to 7 meters to allow for more gait cycles during the walking phase. To observe total body movement, several inertial sensors are mounted on the body: one bi-axial gyroscope on each forearm, one uni-axial gyroscope on each shank, one uni-axial gyroscope on each thigh, and one bi-axial gyroscope and tri-axial accelerometer on the sternum (see Fig. 3).
Fig. 3.
Diagram of how inertial sensors were used for the iTUG analysis algorithms. Acceleration is represented by α and angular velocity by ω. Salarian et al. (2010) [41].
The iTUG is broken down into four sections: sit-to-stand, steady-state gait, turning, and turn-to-sit. Each component is automatically detected in the sensor signals and has a set of parameters computed for each body part involved. For example, the 180° turning phase consists of duration, trunk peak angular velocity, average step time, maximum step time, last step time before turn, and number of steps. For the full set of computed metrics, see [41, Tab. 1]. The system has been utilized to study 12 subjects with idiopathic, early-to-moderate stage PD and 12 healthy age-matched controls. Gait, turns, and turn-to sit sections of the iTUG demonstrated significant differences between the two populations. Cadence was found to be the most reliable metric (ρ = 0.94) and other measures of gait exhibited high reliability as well. With the additional sensors on the arms that are not included in most technological TUG studies, the authors discovered range and amplitude of arm-swing to be sensitive to the early stages of PD, whereas the standard TUG total duration was not sensitive enough to pick up on the early PD changes. The research and success surrounding iTUG led to a commercial sensing company, APDM [66]. APDM has an extensive customer list, greatly contributing to the use of iTUG in the clinic and in research.
2) Falls Risk
The TUG test has widely been used to determine falls risk and to classify fallers and non-fallers. Narayanan and colleagues [67]-[69] were one of the first to instrument the TUG test with inertial sensors for falls risk detection. They proposed a battery of common clinical assessment that could be self-administered and performed daily in the home. They called this set the “directed routine” and it consisted of the following five clinical assessments: 3 meter TUG test, near-tandem standing balance, alternative step test, five times sit-to-stand chair transfer, and simple reaction time. While performing these assessments subjects wear a PreventaFall Ambulatory Monitor (PFAM) which contains a single tri-axial accelerometer on their waist. To start the routine, the subject presses a button on the PFAM and audio cues begin guiding them. The collected acceleration signals are uploaded each evening and processed on a remote server. Physicians can then access the data and monitor the status of subjects via a web interface.
The total TUG duration is computed by analyzing the mediolateral acceleration signal. The acceleration signal is divided into each TUG component: time to stand, time to reach the 3 meter turnaround point, time to turn around, time to reach the chair, and time to sit down in the chair. The system was evaluated with 36 elderly participants. An estimation of falls risk with a linear least squares model achieved a root mean squared error of 0.69 (ρ = 0.58, p < 0.0002) [68].
In another seminal paper, Greene et al. [70] proposed the qTUG, the quantitative timed up and go test, to compute falls risk. Two tri-axial IMUs were placed on the front of the midpoint of each shank (see Fig. 1). Temporal gait parameters including cadence, number of gait cycles, stride time, swing time, stance time, step time, double support percent, and single support percent were computed from the inertial signals. Additional temporal parameters from the TUG components were calculated: TUG time, walk time, turn time, return time, walk-turn time ratio. Forty-four parameters from the sensors were computed in total, 29 of which were able to differentiate between fallers and non-fallers with statistical significance (p < 0.05). For a complete listing of these parameters and measured values, see [70, Tab. 3]. Additionally, using the computed IMU metrics the authors were able to achieve a mean test accuracy of 76.8% for retrospectively estimating falls risk. This method is reported as more accurate than the total TUG duration and BBS prediction. An additional paper discussing the reliability of qTUG for falls risk assessment was published by McGrath et al. [71].
3) Hemiplegia
For investigating Hemiplegic subjects, Higashi et al. [45] attached one IMU on the lower back and another IMU on the upper thigh of the leg that takes the first step when initiating gait. In total, 30 participants, 10 healthy and 20 hemiplegic, performed the TUG while wearing the sensors. Of the 20 hemiplegic participants, 10 had gait levels classified as independent and the other 10 were classified as supervised. The TUG tests were video recorded for scoring by a therapist at a later time. In addition to total TUG duration, therapists were instructed to record the following component times: standing-up, walking, turning, and sitting-down. These times were measured by analysis of the sensor signals and compared to the therapist times. A good correlation (r = 0.998) was found between the two scoring mechanisms. In addition, metrics such as cadence, acceleration root mean square (RMS), and acceleration coefficient of variation (CV) were calculated for the gait component of the test. Using the RMS and CV values, the hemiplegic participants with independent gait were able to be distinguished from the supervised hemiplegic participants with statistical significance (p < 0.01).
4) Disability Levels
SankarPandi et al. [72] recently undertook a study to investigate the predictive utility of a single wrist worn accelerometer for disability levels. The disability level was computed by summing 17 responses to questions regarding ADLs. If the participant and/or caregiver stated they could perform the task in question, such as walking at least 400 yards, a one was scored, zero otherwise. Forty features extracted from the wrist acceleration signals were used to classify disability levels with a mean accuracy of 62.16%, which was higher than the 39.10% accuracy achieved by the total TUG duration alone. Sixteen features were representative enough of the population to discriminate all disability levels and different features were found to be more significant depending on the subject’s gender.
5) Cognitive Impairment
Gillain et al. [73] investigated an IMU-based TUG on populations of healthy controls, participants with mild cognitive impairment (MCI), and participants with Alzheimer’s disease (AD). A single Locometrix tri-axial accelerometer was attached on the lower back at the level of the L3 vertebra. The gait parameters of speed, stride frequency, stride length, stride regularity, and stride symmetry were reported for the TUG test and for TUG-DT (counting down sequentially from 50). Several gait parameters were useful for differentiating amongst the three subject groups. For example, TUG-DT gait speed was found to differentiate the three participant groups. AD participants had lower stride length and gait regularity than MCI and healthy groups. MCI participants exhibited lower stride frequency than the healthy participants.
Furthermore in the area of cognitive impairment research, work by Greene et al. [70] served as a foundation for a longitudinal study published in 2012 by Greene and Kenny [74]. In this work, the qTUG was utilized to predict cognitive decline as measured by the mini mental state examination.
6) Summary
Table IV summarizes the 18 wearable sensor studies reviewed. Of these studies, eight used only accelerometers (44.44%), seven used IMUs (38.89%), one used inertial sensors plus magnetometers (5.56%), one used an accelerometer and magnetometer (5.55%), and one used surface electromyography (EMG) (5.55%). Eleven of the studies utilized only a single sensing unit (61.11%), two studies utilized two sensors (11.11%), and five studies utilized three or more sensors (27.78%). The most common location to mount a sensor was the lower back (55.56%), close to the center of mass. The next most common choice was the lower limbs, a choice which yields a high number of gait parameters. Five studies [41], [72], [75]-[77] investigated accelerometers or gyroscopes on the upper limbs and only one study [78] researched movements of the head.
TABLE IV.
Summary of instrumenting the TUG test with wearable sensors in chronological order. M = male, D = dimensional.
| Article | Population and Sample Size |
Description of TUG Technology |
Study Findings |
|---|---|---|---|
| Narayanan et al. (2007) [67] |
N/A | 1 3D accelerometer mounted at the waist. |
At home, self-administered TUG. Data automatically uploaded and accessible via a web interface. |
| Higashi et al.
(2008) [45] |
10 healthy. 20 hemiplegic. |
1 IMU at L2 vertebra and 1 IMU on 1 upper thigh. A video camera records the tests. |
Using acceleration signal metrics, hemiplegic subjects with different gait levels could be differentiated. |
| Gillain et al.
(2009) [73] |
14 healthy (mean age 73.53 years years). 14 MCI (mean age 72.85 years). 6 Alzheimer’s (mean age 73.66 years). |
1 3D accelerometer at L3 vertebra. |
Several gait parameters in the single and dual task TUG tests were different amongst the 3 population groups. |
| Marschollek et al. (2009) [111], [112] |
110 geriatric (29M). | 1 3D accelerometer located at the trunk. |
Able to classify fallers and non-fallers by utilizing gait parameters extracted from the acceleration signals. |
| Greene et al.
(2010) [70] |
142 non-fallers and 207 fallers. (103M, 72.4 ± 7.4 years) |
1 IMU mounted on the front of each shank at the midpoint level. A video camera records the tests. |
Large set of TUG component and gait parameters. Retrospectively estimated falls risk with 76.8% accuracy. |
| King et al.
(2010) [78] |
12 healthy (24-35 years). 16 fallers (9M, 79.2 ± 9.24 years) |
1 3D accelerometer (e-AR) worn on the ear. |
Metrics computed could differentiate between fallers and non-fallers. |
| Salarian et al.
(2010) [41] |
12 early-to-moderate stage PD (7M, 60.4 ± 8.5 years). 12 age- matched healthy (3M, 60.2 ± 8.2 years). |
7 inertial sensors attached on the forearms (2D gyroscope), shanks (1D gyroscope), thighs (1D gyroscope) and sternum (3D accelerometer and 2D gyroscope). Small data-logger in a waist-worn pack. |
Each TUG component is automatically detected. Gait, turns, and turn-to sit sections of the iTUG demonstrated significant differences between the 2 populations. |
| Weiss et al.
(2010) [18] |
17 PD (15M, 66.8 ± 5.9 years). 15 age- matched healthy (5M, 67.6 ± 9.6 years). |
1 3D accelerometer worn on the lower back between the L3 and L5 vertebrae. |
Analysis of sit-to-stand and stand-to-sit movements with parameters such as range, jerk, duration, and median standard deviation. |
| Chiari (2011) [113] |
20 early-to-mid PD. 20 healthy. |
1 accelerometer mounted at the L5 vertebra. |
92.5% classification accuracy for discriminating the PD and the non-PD populations. |
| Jallon et al.
(2011) [114] |
19 subjects. | 1 3D accelerometer and magnetometer on the chest. |
Graph-based Bayesian classifier distinguished TUG phases with near 85% accuracy. |
| Al-jawad and colleagues (2012) [115], [116] |
10 healthy (4M, 63.2 ± 10.1 years). 10 early stage PD (8M, 58.8 ± 9.5 years). 10 advanced stage PD (7M, 66.2 ± 4.8 years). |
1 IMU placed on the lower back. |
Able to detect different TUG subtasks with small mean absolute errors. |
| Cuesta-Vargas et al. (2013) [108] |
10 healthy (5M, 22 ± 3.1 years). |
7 EMG sensors on the right side of the body. |
Different maximum voluntary isometric contractions on land versus water TUG. |
| Mariani et al.
(2013) [14] |
10 mild-to-moderate PD (64 ± 7 years). 10 age-matched healthy (66 ± 7 years). |
1 3D IMU mounted to the upper shoe. |
Parameters computed are able to distinguish between the control subjects and the PD subjects. |
| Najafi et al.
(2013) [76] |
8 peripheral neuropathy (2M, 77 ± 7 years). |
1 accelerometer integrated into a shirt at the chest level. |
Falls risk group took significantly longer to perform stand-to-sit task. A 0.40 second (0.85%) systematic error for TUG duration was achieved. |
| Strohrmann et al. (2013) [77] |
3 children with cerebral palsy or stroke. |
10 IMUs attached to the waist, torso, and limbs. |
Computed gait parameters are predictors of a motor assessment score. |
| Tmaura et al.
(2013)[117] |
40 elderly (age ≥ 65 years). |
3D accelerometer and 3 1D gyroscopes were attached near L2 vertebra and to both thighs. |
The high falls risk subjects took significantly longer (15.77 ± 1.41 seconds) compared to the lower falls risk subjects (10.09 ± 1.86 seconds). |
| Caldara et al. (2014) [75] |
13 PD (64.6 ± 9 years). 4 healthy (64.3 ± 4 years). |
3D accelerometers, gyroscopes, and magnetometers were placed on the spine, each forearm, and each lower leg. |
Several features were computed. |
| SankarPandi et al. (2014) [72] |
321 elderly (122M, mean age 88 years). |
1 accelerometer mounted on the right wrist. |
Forty features were used to classify disability levels with a mean accuracy of 62.16%. |
Several of the studies report high numbers of computed metrics, with the most common parameters being TUG subtask durations, number of steps, cadence, stride length, and peak angular velocity. For most of the studies these parameters are post-processed and do not generate a performance report (hard copy or digitally). Ideally, we would prefer results to be generated automatically by the system, as a fully automated assessment requires. The commercial solution APDM is a system containing wireless IMUs, an access point, docking station, and software to be run on a laptop. The software automatically creates a report on the laptop that includes comparisons to normative TUG data. The entire instrumented TUG testing process from beginning to results takes less than 5 minutes [79]. Although the system is clinician-administered, the APDM solution is a vital step towards self-administered, automated assessment in the home.
C. Smartphones
The advent of smartphones has incurred a large shift in research efforts to focus on mobile computing and utilizing the sensors embedded in the device. There are several advantages of smartphones for clinical assessment (see Table II). For example, similar to IMUs, smartphones contain tri-axial accelerometers and gyroscopes. Five studies have already been published in the last 3 years utilizing the inertial sensors in smartphones to augment the TUG test.
Mellone and colleagues [80]-[83] utilized the accelerometer in an Android-based smartphone and designed an elderly-friendly user interface to self-administer the TUG test. The interface contains a button to start the exam and data collection and a button to stop. The authors intend to automatically upload the data to a remote server for access and analysis by interested parties such as clinicians. The smartphone was attached to the lower back of 49 healthy adults as they completed a 7 meter TUG test [82]. Twenty-eight parameters were computed from the acceleration signals. The implementation did not make use of the phone screen to display results or allow for automated assessment.
Fontecha et al. [84] attached a smartphone to the waist of 20 elderly subjects. The authors developed an application that runs on the phone and collects data for the TUG test and the gait portion of the Tinetti test. The acceleration data is processed to produce “dispersion measures” which include acceleration mean, standard deviation, amplitude, absolute mean difference, variance, and CV. These metrics are combined with patient medical information to produce a frailty assessment. Future work involves displaying the results on the screen for medical professionals to analyze. Another study by Cuesta-Vargas [85] explored the ability of the iPhone inertial sensors to discriminate between two groups of elderly subjects as they performed the 10 meter extended TUG.
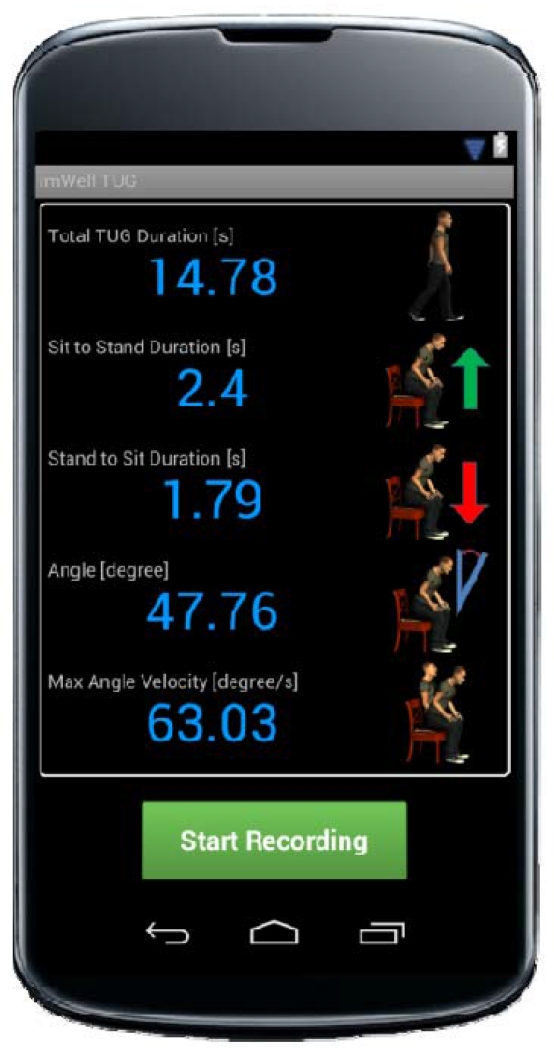
The closest implementation to a self-administered, automated TUG application for smartphones is called smartphone-TUG (sTUG) by Milosevic et al. [86]. The sTUG is composed of a user interface for controlling the test and displaying the results to the user (see Fig. 4). Parameters reported include total duration, component durations, maximum trunk angle change, and maximum angular velocity during sit-to-stand and stand-to sit. User experience reports were not included in the study so it is unclear how feasible the solution is for the elderly to use on their own.
Fig. 4.
Smartphone TUG application displaying the TUG parameters computed. Milosevic et al. (2013) [86].
1) Summary
Table V summarizes the smartphone implementations of the TUG test. Android is the most commonly chosen platform for instrumenting the TUG (80%). Of the several sensors available in smartphones, only the accelerometer and gyroscope were utilized. The smartphone solutions presented are working towards a self-administered, automated TUG test that can be performed at home. Milosevic et al. [86] was able to produce this application and report on ten parameters related to durations and angular velocities; however, the system did not quantify how the elderly handled administering the exams themselves. Connectivity to healthcare professionals who are able to interpret the changes exhibited in TUG performance would also strengthen the automated solution.
TABLE V.
Summary of instrumenting the TUG test with smartphone devices in chronological order. M = male, D = dimensional.
| Article | Population and Sample Size |
Description of TUG Technology |
Study Findings |
|---|---|---|---|
| Palmerini et al. (2011) [82] |
49 healthy (58.9 ± 16.5 years). |
Android smartphone worn on the lower back by means of an elastic belt. Utilized the accelerometer. |
Parameters were computed and analyzed with principle component analysis. |
| Fontecha et al. (2012) [84] |
20 healthy elderly (10M mean age 81.8 years, 10F mean age 85.6 years). |
Android smartphone attached to the waist. Utilized the accelerometer. |
Acceleration metrics are computed and combined with patient records to provide a frailty assessment. |
| Cuesta-Vargas (2013) [85] |
N/A | iPhone. Utilized the accelerometer and gyroscope. |
Higher degree of precision in differentiating between frail elderly subjects and elderly, physically active subjects. |
| Milosevic et al. (2013) |
3 PD. 4 healthy. | Android smartphone attached to the chest. Utilized the accelerometer and gyroscope. |
Self-administered, automated TUG application. Records inertial data during the TUG and displays the results to the user. |
Smartphone solutions, like all technology solutions seen thus far, offer computation of several additional parameters beyond total TUG duration. With the screen on the smartphone, these results have the potential to immediately be available for the examinee to interpret; however, research has not yet determined of what interest these parameters are to the elderly who perform the tests. Do individuals understand what “maximum angular velocity during the lift up phase” [86] is and how to interpret changes in these values? Is there interest in understanding these values? Now that we can collect this data, process it, and produce measurements, perhaps the next step is to critically analyze what this means for our elderly and subject populations.
D. Ambient Sensors
The last sensing modality is ambient sensors, which are neither video-based nor are required to be worn on the body. Ambient sensors include temperature, infrared motion, light, door, object, and pressure sensors. There is a large body of research surrounding the application of ambient sensors in smart homes [87]. Ambient technology has been utilized to estimate gait velocity, specifically using infrared motion sensors [88], [89], but the work has not been extrapolated to automating the TUG test (though mapping in-home gait speed to TUG duration using a Kinect has been researched [90]). There are several reasons for why this could be the case, such as the starting and stopping of the test would be difficult to determine without someone explicitly notifying the system. Also, multiple people in the sensor field of view could cause anomalies in the data stream.
The only ambient-based technological TUG (that is, not based on video recording or wearables) we found is ambient-TUG (aTUG) proposed by Frenken and colleagues [40], [91]. Frenken and colleagues sought to develop a TUG technology to more accurately assess functional ability by testing in the home. To do this, they augmented a chair with several sensors. Four force sensors are placed in the legs of the chair to monitor weight distribution. An infrared light barrier under the armrests detects when the examinee’s back has made or left contact with the chair backrest. Under the chair a laser range scanner estimates the distance the subject is from the chair. All of the data is collected and processed with a microcontroller system mounted to the chair. The system was validated by comparing the aTUG trials of five elderly subjects to stopwatch and video camera measurements. The aTUG had a mean error of 0.05 seconds and mean standard deviation of 0.59 seconds. It was also concluded that only a single light barrier on the backrest of the chair is required to automate total TUG duration.
An assessment-specific device such as the aTUG could be of use to a clinic where several TUG tests occur daily. Since the aTUG chair does not require any wearable sensors or caregiver supervision, it could be a viable technology for elderly to self-administer; however, the setup requires a special piece of furniture and optionally a light barrier to be installed at the 3 meter mark. To increase the utility of a technology-infused chair, aTUG features could be incorporated into a “smart chair”. Such a chair could have several functions including collecting biometrics (heart rate, blood pressure, etc.) and tracking physical activity to justify its size and cost in the home.
V. Discussion
Of the highlighted TUG research described previously, several trends exist. Most studies are examining the proposed technology in cross-sectional studies with PD and falls risk participants (see Table VI for a breakdown of participant populations); however, there is potential for technological assessment beyond the information gained in a snap shot. The parameters computed from the instrumented TUG and other clinical assessments can be useful to track changes in cognition and functionality over time. Greene et al. [74] explored the applications of the instrumented TUG for assessing cognitive decline over a period of 2 years. The authors alluded the results of the study “may also form part of a tool for longitudinal monitoring of cognitive function” [74]. The future of research can take this next step and move towards continuous monitoring systems for longitudinal assessment.
TABLE VI.
TUG test studies based on a population with a medical condition.
| Population | Article |
|---|---|
| PD | Salarian et al. (2008) [62], Horak et al. (2009) [61], Zampieri et al. (2009) [63], Salarian et al. (2010) [41], Weiss et al. (2010) [18], Zampieri et al. (2010) [22], Chiari et al. (2011) [113], Zampieri et al. (2011) [3], Al-jawad et al. (2012) [115], Mancini et al. (2012) [65], Mariani et al. (2013) [14], Milosevic et al. (2013), Palmerini et al. (2013) [42], Caldara et al. (2014) [75] |
| Fallers/Falls Risk |
Narayanan et al. (2008) [68], Skrba et al. (2009) [52], Greene et al. (2010) [70], King et al. (2010) [78], Narayanan et al. (2010) [69], McGrath et al. (2011) [71], Weiss et al. (2011) [39] |
| Cognitive Impairment |
Gillain et al. (2009) [73], Greene et al. (2012) [74] |
| Hemiplegia | Higashi et al. (2008) [45] |
| Multiple Sclerosis |
Spain et al. (2012) [64] |
| Neuropathy | Najafi et al. (2013) [76] |
A. Component Analysis of Timed Up and Go
The original TUG provided only one performance metric, the total duration. Even before technology infiltrated the TUG assessment several variations had been proposed to time individual components of the test [46], [92]. This provided finer-grained assessment and more insight into the mobility issues different populations struggle with. For example, strength could be quantified during the sit-to-stand and stand-to-sit movements, gait could be analyzed by computing velocity over the 3 meter distance, and balance could be assessed in the time and approach taken to complete the 180° turn. By adding video cameras or inertial sensors to the TUG test, parameters related to timing, gait, balance, and even limb tremor are extracted out of a test that previously only yielded the total duration. These metrics are able to be mined out of the sensor signals because the signals are effectively partitioned into the individual components of the TUG test: the chair transitions, linear walking, and 180° turn. Several of the TUG studies included individual analysis of each component because each of these subtasks can be viewed as standalone activities. For example, in regards to chair transfers, Millor et al. [93] recently published a review of sit-to-stand and stand-to-sit movements instrumented with motions sensors. Furthermore, linear walking has been studied comprehensively in the literature [94], [95].
B. Towards Automated Assessment
The rise of the internet and web of things has greatly advanced the feasibility of self-administered assessments. Tele-rehabilitation using webcams and an internet connection are allowing people to participate in therapy sessions and meet with their physicians from home. We saw this technology used for a new area of healthcare, tele-assessment, with the TUG research performed by Durfee et al. [50]. Data collected by means of any technology, such as object sensors, can be automatically uploaded to a remote server. The data can then be processed and viewed by experienced medical professionals to gain additional insight into patient progress.
The innovation of the smartphone further advanced this process by putting a microprocessor powerful enough to do data crunching into the hands of the patient. The solution is mobile, allowing exercises and assessments to be instrumented, logged, and analyzed anywhere. The smartphone system can even be self-contained, requiring no extra sensors or internet to compute TUG performance metrics and display them to the user. Milosevic et al. [86] demonstrated this with the sTUG. Technology needs to push towards these simple, self-contained systems if we want people to use our hardware and software. Automated assessment of rehabilitation exercises, therapy programs, and clinical measures are a large part of the future of scalable healthcare. The systems need to strive for portability, ease of use, and clinically significant information. To meet these requirements we need to look to medical professionals and seek out what tools would be beneficial. How do clinicians envision themselves using technology to assist their profession? How do clinicians envision their patients using the technology? Due to cost limitations such as money and time (training and in practical use), the automated systems we develop in the future need to have a high utility to cost ratio. This is critical if we hope to see technology in the clinic and in the homes of those who wish to live independently longer than if the technology was not available.
C. Challenges for Future Work
Technologies that aim to address the needs of healthcare have to meet several criteria in order to be valuable to medical professionals and accepted by users. Often there is a division between how the engineer and the clinician view these requirements. To bridge this gap, there are several difficulties along the path towards automated assessment:
Developing technology with a high acceptance rate in the home, specifically for elderly and disabled populations [55], [96], [97].
Designing wearable sensors to be smaller, lighter, and easier to use for elderly and disabled populations. Ease of use includes low maintenance and an exceptionally long battery life, to the point of no replacement/recharge required.
Designing creative methods of attaching wearable sensors to the body. Elderly individuals are more enthusiastic about sensors embedded into their clothing or accessories than about wearing the technology separately [96]. The fairly new areas of smart garments and sensor embedded patches/bandages are directions to look for in the future [98].
Integrating and blending technology into the environment so users are not directly aware of it. As Mark Weiser stated, “The most profound technologies are those that disappear. They weave themselves into the fabric of everyday life until they are indistinguishable from it” [99].
Extending monitoring and assessment beyond the home and clinic into community environments.
Minimizing the required number of sensors to decrease the cost, complexity, and maintenance.
Driving the cost of these systems down for the individual. Seniors are most concerned with the expense of ambient technology and they emphasize they do not want to pay for it [96].
Securing data and protecting privacy. Although a majority of seniors are not concerned with their health-related information being transmitted wirelessly [96], data about daily routines, functional and cognitive status, and medical information are potentially personally identifiable and need to be protected.
Designing creative user interfaces. A screen is only one way to convey information and we need to explore newer designs such as artificially intelligent avatars or haptic interfaces.
Presenting healthcare information to patients. People of all ages are often interested in seeing data collected about them. We need to provide intuitive, simple interfaces for people of all technical-abilities to be able to access and understand their data.
Educating/training clinicians and patients to use the software and hardware.
Ensuring patient adherence to data collection protocols for data integrity purposes.
As exhibited by this literature review, the combination of technology and a popular clinical assessment has proven to provide beneficial additional information to healthcare providers. This information is being used to provide finer-grained assessment, medical population classification, and fall risk prediction. Furthermore, the technology is enabling healthcare data to be collected in the home via tele-assessment and self-administered exams. The interdisciplinary nature of this area incurs additional challenges, but the results are beneficial for patient care.
References
- [1].Vincent G, Velkoff V. The next four decades-The older population in the United States: 2010 to 2050. U.S. Census Bureau; 2010. [Google Scholar]
- [2].Dill M, Salsberg E. The Complexities of Physician Supply and Demand: Projects Through 2025. Center for Workforce Studies, Association of American Medical Colleges; 2008. [Google Scholar]
- [3].Zampieri C, Salarian A, Carlson-Kuhta P, Nutt JG, Horak FB. Assessing mobility at home in people with early Parkinson’s disease using an instrumented Timed Up and Go test. Parkinsonism Relat. Disord. 2011 May;17(4):277–280. doi: 10.1016/j.parkreldis.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991 Feb.39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- [5].Cole B, Basmajian J, Association CP. Physical rehabilitation outcome measures. Canadian Physiotherapy Assn; 1994. [Google Scholar]
- [6].Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the ‘get-up and go’ test. Arch. Phys. Med. Rehabil. 1986 Jun.67(6):387–389. [PubMed] [Google Scholar]
- [7].Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson’s disease. Brain J. Neurol. 1987 Apr.110(Pt 2):361–379. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- [8].Rogers MA, Phillips JG, Bradshaw JL, Iansek R, Jones D. Provision of external cues and movement sequencing in Parkinson’s disease. Motor Control. 1998;2(2):125–132. doi: 10.1123/mcj.2.2.125. [DOI] [PubMed] [Google Scholar]
- [9].Bloem BR, Valkenburg VV, Slabbekoorn M, van Dijk JG. The multiple tasks test. Strategies in Parkinson’s disease. Exp. Brain Res. 2001 Apr.137(3-4):478–486. doi: 10.1007/s002210000672. [DOI] [PubMed] [Google Scholar]
- [10].Hafsteinsdottir T, Rensink M, Schuurmans M. Clinimetric Properties of the Timed Up and Go Test for Patients With Stroke: A Systematic Review. Top. Stroke Rehabil. 2014 Jan.21(3):197–210. doi: 10.1310/tsr2103-197. [DOI] [PubMed] [Google Scholar]
- [11].Rydwik E, Bergland A, Forsen L, Frandin K. Psychometric Properties of Timed Up and Go in Elderly People: A Systematic Review. Phys. Occup. Ther. Geriatr. 2011 May;29(2):102125. [Google Scholar]
- [12].Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N. Engl. J. Med. 1998 Oct.339(15):1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- [13].Lang AE, Lozano AM. Parkinson’s disease. Second of two parts. N. Engl. J. Med. 1998 Oct.339(16):1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- [14].Mariani B, Jimenez MC, Vingerhoets FJG, Aminian K. On-Shoe Wearable Sensors for Gait and Turning Assessment of Patients With Parkinson’s Disease. IEEE Trans. Biomed. Eng. 2013 Jan.60(1):155–158. doi: 10.1109/TBME.2012.2227317. [DOI] [PubMed] [Google Scholar]
- [15].Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: Stride-to-stride variations of gait cycle timing in parkinson’s disease and Huntington’s disease. Mov. Disord. 1998;13(3):428–437. doi: 10.1002/mds.870130310. [DOI] [PubMed] [Google Scholar]
- [16].Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the Timed ‘Up & Go’ test in people with Parkinson disease. Phys. Ther. 2001;81(2):810–818. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- [17].Rossi M, Soto A, Santos S, Sesar A, Labella T. A prospective study of alterations in balance among patients with Parkinson’s Disease. Protocol of the postural evaluation. Eur. Neurol. 2009;61(3):171–176. doi: 10.1159/000189270. [DOI] [PubMed] [Google Scholar]
- [18].Weiss A, Herman T, Plotnik M, Brozgol M, Maidan I, Giladi N, Gurevich T, Hausdorff JM. Can an accelerometer enhance the utility of the Timed Up & Go Test when evaluating patients with Parkinson’s disease? Med. Eng. Phys. 2010 Mar.32(2):119–125. doi: 10.1016/j.medengphy.2009.10.015. [DOI] [PubMed] [Google Scholar]
- [19].Brusse KJ, Zimdars S, Zalewski KR, Steffen TM. Testing functional performance in people with Parkinson disease. Phys. Ther. 2005 Feb.85(2):134–141. [PubMed] [Google Scholar]
- [20].Martinez-Martin P, Garcia Urra D, del Ser Quijano T, Balseiro Gomez J, Gomez Utrero E, Pineiro R, Andres MT. A new clinical tool for gait evaluation in Parkinson’s disease. Clin. Neuropharmacol. 1997 Jun.20(3):183–194. doi: 10.1097/00002826-199706000-00001. [DOI] [PubMed] [Google Scholar]
- [21].Dibble LE, Lange M. Predicting falls in individuals with Parkinson disease: a reconsideration of clinical balance measures. J. Neurol. Phys. Ther. JNPT. 2006 Jun.30(2):6067. doi: 10.1097/01.npt.0000282569.70920.dc. [DOI] [PubMed] [Google Scholar]
- [22].Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB. The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2010 Feb.81(2):171–176. doi: 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Perell KL, Nelson A, Goldman RL, Luther SL, Prieto-Lewis N, Rubenstein LZ. Fall risk assessment measures an analytic review. J. Gerontol. A. Biol. Sci. Med. Sci. 2001;56(12):M761–M766. doi: 10.1093/gerona/56.12.m761. [DOI] [PubMed] [Google Scholar]
- [24].Shimada H, Suzukawa M, Tiedemann A, Kobayashi K, Yoshida H, Suzuki T. Which neuromuscular or cognitive test is the optimal screening tool to predict falls in frail community-dwelling older people? Gerontology. 2009;55(5):532–538. doi: 10.1159/000236364. [DOI] [PubMed] [Google Scholar]
- [25].Barry E, Galvin R, Keogh C, Horgan F, Fahey T. Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and metaanalysis. BMC Geriatr. 2014 Feb.14(1):14. doi: 10.1186/1471-2318-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schoene D, Wu SM-S, Mikolaizak AS, Menant JC, Smith ST, Delbaere K, Lord SR. Discriminative Ability and Predictive Validity of the Timed Up and Go Test in Identifying Older People Who Fall: Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2013 Feb.61(2):202–208. doi: 10.1111/jgs.12106. [DOI] [PubMed] [Google Scholar]
- [27].Beauchet O, Fantino B, Allali G, Muir SW, Montero-Odasso M, Annweiler C. Timed Up and Go test and risk of falls in older adults: a systematic review. J. Nutr. Health Aging. 2011;15(10):933–938. doi: 10.1007/s12603-011-0062-0. [DOI] [PubMed] [Google Scholar]
- [28].Oliver D. Risk factors and risk assessment tools for falls in hospital in-patients: a systematic review. Age Ageing. 2004 Mar.33(2):122–130. doi: 10.1093/ageing/afh017. [DOI] [PubMed] [Google Scholar]
- [29].Large J, Gan N, Basic D, Jennings N. Using the Timed Up and Go Test to stratify elderly inpatients at risk of falls. Clin. Rehabil. 2006 May;20(5):421–428. doi: 10.1191/0269215506cr959oa. [DOI] [PubMed] [Google Scholar]
- [30].Whitney SL, Marchetti GF, Schade A, Wrisley DM. The sensitivity and specificity of the Timed ‘Up & Go’ and the Dynamic Gait Index for self-reported falls in persons with vestibular disorders. J. Vestib. Res. Equilib. Orientat. 2004;14(5):397–409. [PubMed] [Google Scholar]
- [31].Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- [32].Dite W, Temple VA. Development of a clinical measure of turning for older adults. Am. J. Phys. Med. Rehabil. Assoc. Acad. Physiatr. 2002 Nov.81(11):857–866. doi: 10.1097/00002060-200211000-00010. quiz 867-868. [DOI] [PubMed] [Google Scholar]
- [33].Najafi B, Aminian K, Loew F, Blanc Y, Robert PA. Measurement of stand-sit and sit-stand transitions using a miniature gyroscope and its application in fall risk evaluation in the elderly. IEEE Trans. Biomed. Eng. 2002 Aug.49(8):843–851. doi: 10.1109/TBME.2002.800763. [DOI] [PubMed] [Google Scholar]
- [34].Cheng PT, Liaw MY, Wong MK, Tang FT, Lee MY, Lin PS. The sit-to-stand movement in stroke patients and its correlation with falling. Arch. Phys. Med. Rehabil. 1998 Sep.79(9):1043–1046. doi: 10.1016/s0003-9993(98)90168-x. [DOI] [PubMed] [Google Scholar]
- [35].Shahar D, Levi M, Kurtz I, Shany S, Zvili I, Mualleme E, Shahar A, Sarid O, Melzer I. Nutritional status in relation to balance and falls in the elderly: a preliminary look at serum folate. Ann. Nutr. Metab. 2009;54(1):59–66. doi: 10.1159/000207356. [DOI] [PubMed] [Google Scholar]
- [36].Thrane G, Joakimsen RM, Thornquist E. The association between timed up and go test and history of falls: the Tromso study. BMC Geriatr. 2007;7(1):1. doi: 10.1186/1471-2318-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Buatois S, Gueguen R, Gauchard GC, A Benetos, Perrin PP. Posturography and risk of recurrent falls in healthy non-institutionalized persons aged over 65. Gerontology. 2006;52(6):345–352. doi: 10.1159/000094983. [DOI] [PubMed] [Google Scholar]
- [38].Boulgarides LK, McGinty SM, Willett JA, Barnes CW. Use of clinical and impairment-based tests to predict falls by community-dwelling older adults. Phys. Ther. 2003;83(4):328–339. [PubMed] [Google Scholar]
- [39].Weiss A, Herman T, Plotnik M, Brozgol M, Giladi N, Hausdorff JM. An instrumented timed up and go: the added value of an accelerometer for identifying fall risk in idiopathic fallers. Physiol. Meas. 2011 Dec.32(12):2003–2018. doi: 10.1088/0967-3334/32/12/009. [DOI] [PubMed] [Google Scholar]
- [40].Frenken T, Vester B, Brell M, Hein A. aTUG: Fully-automated timed up and go assessment using ambient sensor technologies. 2011 5th International Conference on Pervasive Computing Technologies for Healthcare (PervasiveHealth) 2011:55–62. [Google Scholar]
- [41].Salarian A, Horak FB, Zampieri C, Carlson-Kuhta P, Nutt JG, Aminian K. iTUG, a Sensitive and Reliable Measure of Mobility. IEEE Trans. Neural Syst. Rehabil. Eng. 2010 Jun.18(3):303–310. doi: 10.1109/TNSRE.2010.2047606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Palmerini L, Mellone S, Avanzolini G, Valzania F, Chiari L. Quantification of Motor Impairment in Parkinson’s Disease Using an Instrumented Timed Up and Go Test. IEEE Trans. Neural Syst. Rehabil. Eng. 2013 Jul.21(4):664–673. doi: 10.1109/TNSRE.2012.2236577. [DOI] [PubMed] [Google Scholar]
- [43].Arnadottir SA, Mercer VS. Effects of footwear on measurements of balance and gait in women between the ages of 65 and 93 years. Phys. Ther. 2000;80(1):17–27. [PubMed] [Google Scholar]
- [44].Medley A, Thompson M. The effect of assistive devices on the performance of community dwelling elderly on the timed up and go test. Issues Aging. 1997;20:3–8. [Google Scholar]
- [45].Higashi Y, Yamakoshi K, Fujimoto T, Sekine M, Tamura T. Quantitative evaluation of movement using the timed up-and-go test. IEEE Eng. Med. Biol. Mag. 2008 Jul.27(4):38–46. [Google Scholar]
- [46].Wall JC, Bell C, Campbell S, Davis J. The Timed Get-up-and-Go test revisited: measurement of the component tasks. J. Rehabil. Res. Dev. 2000 Feb.37(1):109–113. [PubMed] [Google Scholar]
- [47].Frenken T, Wilken O, Hein A. Technical approaches to unobtrusive geriatric assessments in domestic environments. Proceedings of the 5th Workshop on Behaviour Monitoring and Interpretation, BMI. 2010;10:63–74. [Google Scholar]
- [48].Beauchet O, Dubost V, Aminian K, Gonthier R, Kressig RW. Dual-task-related gait changes in the elderly: does the type of cognitive task matter? J. Mot. Behav. 2005 Jul.37(4):259–264. [PubMed] [Google Scholar]
- [49].Hittmair-Delazer M, Semenza C, Denes G. Concepts and facts in calculation. Brain J. Neurol. 1994 Aug.117(Pt 4):715–728. doi: 10.1093/brain/117.4.715. [DOI] [PubMed] [Google Scholar]
- [50].Durfee WK, Savard L, Weinstein S. Technical Feasibility of Teleassessments for Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2007 Mar.15(1):23–29. doi: 10.1109/TNSRE.2007.891400. [DOI] [PubMed] [Google Scholar]
- [51].Berrada D, Romero M, Abowd G, Blount M, Davis J. Automatic administration of the get up and go test. Proceedings of the 1st ACM SIGMOBILE international workshop on Systems and networking support for healthcare and assisted living environments. 2007:73–75. [Google Scholar]
- [52].Skrba Z, O’Mullane B, Greene BR, Scanaill CN, Fan CW, Quigley A, Nixon P. Objective real-time assessment of walking and turning in elderly adults. Engineering in Medicine and Biology Society, 2009. EMBC 2009. Annual International Conference of the IEEE. 2009:807–810. doi: 10.1109/IEMBS.2009.5333934. [DOI] [PubMed] [Google Scholar]
- [53].Wang F, Skubic M, Abbott C, Keller JM. Quantitative analysis of 180 degree turns for fall risk assessment using video sensors. Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE. 2011:7606–7609. doi: 10.1109/IEMBS.2011.6092005. [DOI] [PubMed] [Google Scholar]
- [54].Ghose A, Sinha P, Bhaumik C, Sinha A, Agrawal A, Dutta Choudhury A. Proceedings of the 2013 ACM Conference on Pervasive and Ubiquitous Computing Adjunct Publication. New York, NY, USA: 2013. UbiHeld: Ubiquitous Healthcare Monitoring System for Elderly and Chronic Patients; pp. 1255–1264. [Google Scholar]
- [55].Demiris G, Oliver DP, Giger J, Skubic M, Rantz M. Older adults’ privacy considerations for vision based recognition methods of eldercare applications. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2009;17(1):41–48. doi: 10.3233/THC-2009-0530. [DOI] [PubMed] [Google Scholar]
- [56].Lohmann O, Luhmann T, Hein A. Skeleton Timed Up and Go. Bioinformatics and Biomedicine (BIBM), 2012 IEEE International Conference on. 2012:1–5. [Google Scholar]
- [57].Kitsunezaki N, Adachi E, Masuda T, Mizusawa J. Kinect applications for the physical rehabilitation. Medical Measurements and Applications Proceedings (MeMeA), 2013 IEEE International Symposium on. 2013:294–299. [Google Scholar]
- [58].Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J. Am. Geriatr. Soc. 1986 Feb.34(2):119–126. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- [59].Cippitelli E, Gasparrini S, Gambi E, Spinsante S. A depth-based joints estimation algorithm for get up and go test using Kinect. 2014 IEEE International Conference on Consumer Electronics (ICCE) 2014:226–227. [Google Scholar]
- [60].Michael Chiacchiero BD. The Relationship Between Range of Movement, Flexibility, and Balance in the Elderly. Top. Geriatr. Rehabil. 2010;26(2):148–155. [Google Scholar]
- [61].Horak F, Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt J. Longitudinal monitoring of gait and mobility in Parkinson’s disease (PD) using an instrumented timed up and go test (iTUG) Mov. Disord. 2009;24(Suppl 1):S403. [Google Scholar]
- [62].Salarian A, Zampieri C, Horak F, Carlson-Kuhta P, Aminian K. 2nd International Congress on Gait & Mental Function. Amsterdam, Netherlands: 2008. Objective evaluation of Get-up and Go test in patients with Parkinson’s disease using kinematic sensors. [Google Scholar]
- [63].Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB. An Instrumented Timed Up and Go Test Characterizes Gait and Postural Transitions in Untreated Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry. 2009 Sep. doi: 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Spain RI, St. George RJ, Salarian A, Mancini M, Wagner JM, Horak FB, Bourdette D. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture. 2012 Apr.35(4):573–578. doi: 10.1016/j.gaitpost.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mancini M, Priest KC, Nutt JG, Horak FB. Quantifying freezing of gait in Parkinson’s disease during the instrumented timed up and go test. Engineering in Medicine and Biology Society (EMBC), 2012 Annual International Conference of the IEEE. 2012:1198–1201. doi: 10.1109/EMBC.2012.6346151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mancini M, King L, Salarian A, Holmstrom L, McNames J, Horak F. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. J. Bioeng. Biomed. Sci. 2013 doi: 10.4172/2155-9538.S1-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Narayanan MR, Lord SR, Budge MM, Celler BG, Lovell NH. Falls management: detection and prevention, using a waist-mounted triaxial accelerometer. Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf. 2007;2007:40374040. doi: 10.1109/IEMBS.2007.4353219. [DOI] [PubMed] [Google Scholar]
- [68].Narayanan MR, Scalzi ME, Redmond SJ, Lord SR, Celler BG, Lovell NH. A wearable triaxial accelerometry system for longitudinal assessment of falls risk. Engineering in Medicine and Biology Society, 2008. EMBS 2008. 30th Annual International Conference of the IEEE. 2008:2840–2843. doi: 10.1109/IEMBS.2008.4649794. [DOI] [PubMed] [Google Scholar]
- [69].Narayanan MR, Redmond SJ, Scalzi ME, Lord SR, Celler BG, Lovell NH. Longitudinal Falls-Risk Estimation Using Triaxial Accelerometry. IEEE Trans. Biomed. Eng. 2010 Mar.57(3):534–541. doi: 10.1109/TBME.2009.2033038. [DOI] [PubMed] [Google Scholar]
- [70].Greene BR, Donovan AO, Romero-Ortuno R, Cogan L, Ni Scanaill C, Kenny RA. Quantitative Falls Risk Assessment Using the Timed Up and Go Test. IEEE Trans. Biomed. Eng. 2010 Dec.57(12):2918–2926. doi: 10.1109/TBME.2010.2083659. [DOI] [PubMed] [Google Scholar]
- [71].McGrath D, Greene BR, Doheny EP, McKeown DJ, De Vito G, Caulfield B. Reliability of quantitative TUG measures of mobility for use in falls risk assessment. Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE. 2011:466–469. doi: 10.1109/IEMBS.2011.6090066. [DOI] [PubMed] [Google Scholar]
- [72].SankarPandi SK, Dlay S, Woo WL, Catt M. Predicting disability levels of community dwelling older individuals using single wrist mounted accelerometer. Biomedical and Health Informatics (BHI), 2014IEEE-EMBSInternational Conference on. 2014:720–723. [Google Scholar]
- [73].Gillain S, Warzee E, Lekeu F, Wojtasik V, Maquet D, Croisier J-L, Salmon E, Petermans J. The value of instrumental gait analysis in elderly healthy, MCI or Alzheimer’s disease subjects and a comparison with other clinical tests used in single and dual-task conditions. Ann. Phys. Rehabil. Med. 2009 Jul.52(6):453–474. doi: 10.1016/j.rehab.2008.10.004. [DOI] [PubMed] [Google Scholar]
- [74].Greene BR, Kenny RA. Assessment of Cognitive Decline Through Quantitative Analysis of the Timed Up and Go Test. IEEE Trans. Biomed. Eng. 2012 Apr.59(4):988–995. doi: 10.1109/TBME.2011.2181844. [DOI] [PubMed] [Google Scholar]
- [75].Caldara M, Comotti D, Galizzi M, Locatelli P, Re V, Alimonti D, Poloni M, Rizzetti MC. A Novel Body Sensor Network for Parkinson’s Disease Patients Rehabilitation Assessment. 2014 11th International Conference on Wearable and Implantable Body Sensor Networks (BSN) 2014:81–86. [Google Scholar]
- [76].Najafi B, Armstrong DG, Mohler J. Novel wearable technology for assessing spontaneous daily physical activity and risk of falling in older adults with diabetes. J. Diabetes Sci. Technol. 2013 Sep.7(5):1147–1160. doi: 10.1177/193229681300700507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Strohrmann C, Labruyere R, Gerber CN, van Hedel HJ, Arnrich B, Troster G. Monitoring motor capacity changes of children during rehabilitation using body-worn sensors. J. NeuroEngineeringRehabil. 2013 Jul.10(1):83. doi: 10.1186/1743-0003-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].King RC, Atallah L, Wong C, Miskelly F, Yang G-Z. Elderly Risk Assessment of Falls with BSN. 2010 International Conference on Body Sensor Networks (BSN) 2010:30–35. [Google Scholar]
- [79].APDM Movement Monitoring Solutions. [Online]. Available: www.apdm.com.
- [80].Tacconi C, Mellone S, Chiari L. Smartphone-based applications for investigating falls and mobility. 2011 5 th International Conference on Pervasive Computing Technologies for Healthcare (PervasiveHealth) 2011:258–261. [Google Scholar]
- [81].Mellone S, Tacconi C, Chiari L. Suitability of a Smartphone accelerometer to instrument the Timed Up and Go test: A preliminary study. Gait Posture. 2011;33:S50–S51. [Google Scholar]
- [82].Palmerini L, Mellone S, Rocchi L, Chiari L. Dimensionality reduction for the quantitative evaluation of a smartphone-based Timed Up and Go test. Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE. 2011:7179–7182. doi: 10.1109/IEMBS.2011.6091814. [DOI] [PubMed] [Google Scholar]
- [83].Mellone S, Tacconi C, Schwickert L, Klenk J, Becker C, Chiari L. Smartphone-based solutions for fall detection and prevention: the FARSEEING approach. Z. Fur Gerontol. Geriatr. 2012 Dec.45(8):722–727. doi: 10.1007/s00391-012-0404-5. [DOI] [PubMed] [Google Scholar]
- [84].Fontecha J, Navarro FJ, Hervas R, Bravo J. Elderly frailty detection by using accelerometer-enabled smartphones and clinical information records. Pers. Ubiquitous Comput. 2012 May;17(6):1073–1083. [Google Scholar]
- [85].Cuesta-Vargas A. [27-Aug-2014];MTUG: an Instrumented Timed Up and Go Extended Test. 25-Oct-2013. [Online]. Available: http://riuma.uma.es/xmlui/handle/10630/6164.
- [86].Milosevic M, Jovanov E, Milenkovic A. Quantifying Timed-Up-and-Go test: A smartphone implementation. Body Sensor Networks (BSN), 2013 IEEE International Conference on. 2013:1–6. [Google Scholar]
- [87].Acampora G, Cook DJ, Rashidi P, Vasilakos AV. A Survey on Ambient Intelligence in Healthcare. Proc. IEEE. 2013 Dec.101(12):2470–2494. doi: 10.1109/JPROC.2013.2262913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Austin D, Hayes TL, Kaye J, Mattek N, Pavel M. Unobtrusive monitoring of the longitudinal evolution of in-home gait velocity data with applications to elder care. Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE. 2011:6495–6498. doi: 10.1109/IEMBS.2011.6091603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hagler S, Austin D, Hayes TL, Kaye J, Pavel M. Unobtrusive and Ubiquitous In-Home Monitoring: A Methodology for Continuous Assessment of Gait Velocity in Elders. IEEE Trans. Biomed. Eng. 2010 Apr.57(4):813–820. doi: 10.1109/TBME.2009.2036732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Stone EE, Skubic M. Mapping Kinect-based in-home gait speed to TUG time: A methodology to facilitate clinical interpretation. 2013 7th International Conference on Pervasive Computing Technologies for Healthcare (PervasiveHealth) 2013:57–64. [Google Scholar]
- [91].Frenken T, Lipprandt M, Brell M, Govercin M, Wegel S, Steinhagen-Thiessen E, Hein A. Novel approach to unsupervised mobility assessment tests: Field trial for aTUG. 2012 6th International Conference on Pervasive Computing Technologies for Healthcare (PervasiveHealth) 2012:131–138. [Google Scholar]
- [92].Botolfsen P, Helbostad JL, Moe-nilssen R, Wall JC. Reliability and concurrent validity of the Expanded Timed Up-and-Go test in older people with impaired mobility. Physiother. Res. Int. 2008 Jun.13(2):94–106. doi: 10.1002/pri.394. [DOI] [PubMed] [Google Scholar]
- [93].Millor N, Lecumberri P, Gomez M, Martinez-Ramirez A, Izquierdo M. Kinematic Parameters to Evaluate Functional Performance of Sit-to-Stand and Stand-to-Sit Transitions Using Motion Sensor Devices: A Systematic Review. IEEE Trans. Neural Syst. Rehabil. Eng. 2014 Sep.22(5):926–936. doi: 10.1109/TNSRE.2014.2331895. [DOI] [PubMed] [Google Scholar]
- [94].Tao W, Liu T, Zheng R, Feng H. Gait Analysis Using Wearable Sensors. Sensors. 2012 Feb.12(12):2255–2283. doi: 10.3390/s120202255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Whittle MW. Clinical gait analysis: A review. Hum. Mov. Sci. 1996 Jun.15(3):369–387. [Google Scholar]
- [96].Steele R, Lo A, Secombe C, Wong YK. Elderly persons’ perception and acceptance of using wireless sensor networks to assist healthcare. Int. J. Med. Inf. 2009 Dec.78(12):788–801. doi: 10.1016/j.ijmedinf.2009.08.001. [DOI] [PubMed] [Google Scholar]
- [97].Demiris G, Rantz MJ, Aud MA, Marek KD, Tyrer HW, Skubic M, Hussam AA. Older adults’ attitudes towards and perceptions of ‘smart home’ technologies: a pilot study. Inform. Health Soc. Care. 2004 Jan.29(2):87–94. doi: 10.1080/14639230410001684387. [DOI] [PubMed] [Google Scholar]
- [98].Li C-Y, Yen C-H, Wang K-C, You C-W, Lau S-Y, Chen CC-H, Huang P, Chu H-H. Proceedings of the 2014 ACM International Joint Conference on Pervasive and Ubiquitous Computing. New York, NY, USA: 2014. BioScope: An Extensible Bandage System for Facilitating Data Collection in Nursing Assessments; pp. 477–480. [Google Scholar]
- [99].Weiser M. The computer for the 21st century. Sci. Am. 1991;265(3):94–104. [Google Scholar]
- [100].K O’Brien, Culham E, Pickles B. Balance and skeletal alignment in a group of elderly female fallers and nonfallers. J. Gerontol. A. Biol. Sci. Med. Sci. 1997 Jul.52(4):B221–226. doi: 10.1093/gerona/52a.4.b221. [DOI] [PubMed] [Google Scholar]
- [101].Lundin-Olsson L, Nyberg L, Gustafson Y. Attention, frailty, and falls: the effect of a manual task on basic mobility. J. Am. Geriatr. Soc. 1998 Jun.46(6):758–761. doi: 10.1111/j.1532-5415.1998.tb03813.x. [DOI] [PubMed] [Google Scholar]
- [102].Medley A, Thompson M. Usefulness of Variations of the Timed Up and Go in Apparently Healthy Individuals. Phys. Occup. Ther. Geriatr. 2005 Dec.23(4):1–23. [Google Scholar]
- [103].Silsupadol P, Siu K-C, Shumway-Cook A, Woollacott MH. Training of balance under single-and dual-task conditions in older adults with balance impairment. Phys. Ther. 2006;86(2):269–281. [PubMed] [Google Scholar]
- [104].Vaillant J, Martigne P, Vuillerme N, Caillat-Miousse J-L, Parisot J, Juvin R, Nougier V. Prediction of falls with performance on Timed ‘Up-and-Go’ and one-leg-balance tests and additional cognitive tasks. Ann. Readapt. Medecine Phys. Rev. Sci. Societe Fr. Reeduc. Fonct. Readapt. Medecine Phys. 2006 Feb.49(1):1–7. doi: 10.1016/j.annrmp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- [105].Demura S, Uchiyama M. Proper assessment of the falling risk in the elderly by a physical mobility test with an obstacle. Tohoku J. Exp. Med. 2007 May;212(1):13–20. doi: 10.1620/tjem.212.13. [DOI] [PubMed] [Google Scholar]
- [106].Nordin E, Lindelof N, Rosendahl E, Jensen J, Lundin-olsson L. Prognostic validity of the Timed Up-and-Go test, a modified Get-Up-and-Go test, staff’s global judgement and fall history in evaluating fall risk in residential care facilities. Age Ageing. 2008 Feb.37(4):442–448. doi: 10.1093/ageing/afn101. [DOI] [PubMed] [Google Scholar]
- [107].Maranhao-Filho PA, Maranhao ET, Lima MA, da Silva MM. Rethinking the neurological examination II: dynamic balance assessment. Arq. Neuropsiquiatr. 2011;69(6):959–963. doi: 10.1590/s0004-282x2011000700022. [DOI] [PubMed] [Google Scholar]
- [108].Cuesta-Vargas AI, Cano-Herrera C, Formosa D, Burkett B. Electromyographic responses during time get up and go test in water (wTUG) SpringerPlus. 2013 May;2 doi: 10.1186/2193-1801-2-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sprint G, Weeks D, Borisov V, Cook D. Proceedings of the 2014 ACM International Joint Conference on Pervasive and Ubiquitous Computing: Adjunct Publication. New York, NY, USA: 2014. Wearable Sensors in Ecological Rehabilitation Environments; pp. 163–166. [Google Scholar]
- [110].Chen M-J. Case Report: Retirees’ Acceptance and Perceived Contribution of Smartphone in Chronic Disease Management. J. Biosci. Med. 2014;02(06):1–4. [Google Scholar]
- [111].Marschollek M, Nemitz G, Gietzelt M, Wolf KH, Meyer Zu Schwabedissen H, Haux R. Predicting in-patient falls in a geriatric clinic: a clinical study combining assessment data and simple sensory gait measurements. Z. Fur Gerontol. Geriatr. 2009 Aug.42(4):317–321. doi: 10.1007/s00391-009-0035-7. [DOI] [PubMed] [Google Scholar]
- [112].Marschollek M, Wolf K-H, Gietzelt M, Nemitz G, Meyer zu Schwabedissen H, Haux R. Assessing elderly persons’ fall risk using spectral analysis on accelerometric data - a clinical evaluation study. 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2008. EMBS 2008. 2008:3682–3685. doi: 10.1109/IEMBS.2008.4650008. [DOI] [PubMed] [Google Scholar]
- [113].Chiari L. Wearable systems with minimal set-up for monitoring and training of balance and mobility. Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE. 2011:5828–5832. doi: 10.1109/IEMBS.2011.6091442. [DOI] [PubMed] [Google Scholar]
- [114].Jallon P, Dupre B, Antonakios M. A graph based method for timed up & go test qualification using inertial sensors. Acoustics, Speech and Signal Processing (ICASSP), 2011 IEEE International Conference on. 2011:689–692. [Google Scholar]
- [115].Al-Jawad A, Adame MR, Romanovas M, Hobert M, Maetzler W, Traechtler M, Moeller K, Manoli Y. Using multi-dimensional dynamic time warping for TUG test instrumentation with inertial sensors. Multisensor Fusion and Integration for Intelligent Systems (MFI), 2012 IEEE Conference on. 2012:212–218. [Google Scholar]
- [116].Adame MR, Al-Jawad A, Romanovas M, Hobert M, Maetzler W, Moeller K, Manoli Y. TUG test instrumentation for Parkinson’s disease patients using inertial sensors and dynamic time warping. Biomed. Tech. 2012 Sep.57:1071–1074. [Google Scholar]
- [117].Tmaura T, Zakaria NA, Kuwae Y, Sekine M, Minato K, Yoshida M. Quantitative analysis of the fall-risk assessment test with wearable inertia sensors. Engineering in Medicine and Biology Society (EMBC), 2013 35th Annual International Conference of the IEEE. 2013:7217–7220. doi: 10.1109/EMBC.2013.6611223. [DOI] [PubMed] [Google Scholar]