Abstract
The adoptive transfer of T cells engineered to express artificial chimeric antigen receptors (CARs) that target a tumor cell surface molecule has emerged as an exciting new approach for cancer immunotherapy. Clinical trials in patients with advanced B cell malignancies treated with CD19-specific CAR-modified T cells (CAR-T) have shown impressive antitumor efficacy, leading to optimism that this approach will be useful for treating common solid tumors. Because CAR-T cells recognize tumor cells independent of their expression of human leukocyte antigen (HLA) molecules, tumors that escape conventional T cells by downregulating HLA and/or mutating components of the antigen processing machinery can be eliminated. The ability to introduce or delete additional genes in T cells has the potential to provide therapeutic cell products with novel attributes that overcome impediments to immune mediated tumor elimination in immunosuppressive tumor microenvironments. This review will discuss recent concepts in the development of effective and safe synthetic CARs for adoptive T cell therapy (ACT).
Introduction
The adoptive transfer of T cells engineered to express artificial chimeric antigen receptors (CARs) that target a tumor cell surface molecule is an exciting new approach for cancer immunotherapy. Clinical trials in patients with advanced B cell malignancies treated with CD19-specific CAR-modified T cells (CAR-T) have shown impressive antitumor efficacy [1-5], leading to optimism that this approach can be applied to treat common solid tumors [6]. This review will discuss recent advances in the development of effective and safe synthetic CARs for adoptive T cell therapy (ACT).
Structural elements of chimeric antigen receptors
Ligand binding
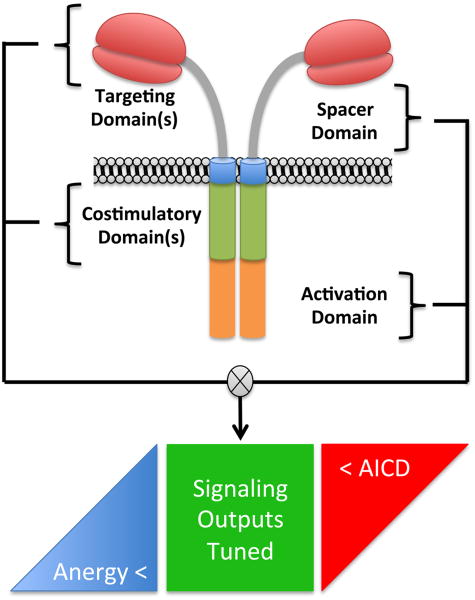
CARs consist of fusion molecules and are typically comprised of an extracellular single chain variable fragment (scFv) of a monoclonal antibody (mAb) specific for a surface molecule on the tumor cell, a spacer domain that provides flexibility and optimizes T cell and target cell engagement, a transmembrane domain, and signaling modules that trigger T cell effector functions (Figure 1). The use of scFvs for ligand binding takes advantage of the high specificity and prevalence of mAbs for tumor associated molecules, although other novel ligand binding domains have been utilized or are under development for clinical applications [7].
Figure 1. Structural Elements of Chimeric Antigen Receptors.
Biophysical components of chimeric antigen receptors work in concert to affect qualitative and quantitative signaling outputs to the T cell. Signal output tuning based on scFv binding affinity, extracellular spacer size adapted to the target epitope location, and cytoplasmic costimulatory and ITAM elements are interdependent. The net effect of any combination of components is to tune the CAR to be compatible with effector T cell physiology between thresholds that enforce hypoactivity (anergy) and hyperactivity (activation induced cell death).
In contrast to T cell receptors (TCRs) that have been perfected through evolution to safely and efficiently distinguish self from non-self, CARs are constructed synthetically and assembly of an optimal receptor construct is largely empiric (Box 1). Ligand binding of a CAR differs from that of a TCR binding to peptide/MHC (pMHC) in receptor affinity, antigen density, and spatial properties; and experimental approaches to designing an optimal CAR for a specific target molecule have relied on functional assays of transduced T cells in vitro or in human tumor xenograft models. Few studies have evaluated the effect of affinity by designing CARs from scFvs of the same specificity but with different affinities. In one study, a CAR constructed from a higher affinity scFv specific for an epitope in the Ig-like/Frizzled region of ROR1 exhibited superior antitumor activity against human tumor xenografts than a lower affinity CAR specific for the same region of ROR1 [8]. However, based on studies of class I restricted TCRs that revealed a threshold of affinity beyond which antigen and CD8 co-receptor engagement result in activation induced T cell death and loss of therapeutic activity [9], it is likely that for each target molecule there will be an affinity threshold for CARs, beyond which T cell effector function and/or survival may be compromised.
Box 1. CAR Assembly.
Select scFv binding domains to membrane proximal epitopes in target molecules
Examine scFvs of different affinities
Screen spacer length variants for optimal function in vitro and in NSG mice
Screen costimulatory domains for desired function in vitro and in NSG mice
Alter CAR fusion sites to minimize potential immunogenicity
Safety testing in animal models if feasible
The number of molecules that are expressed on the tumor cell surface and available to bind the CAR can vary substantially for individual targets, and is typically much higher than the number of pMHC molecules available for binding of TCRs. Because it is unlikely that CARs will serially engage target molecules and cluster in organized synapses as is observed with TCR/pMHC recognition, it is assumed that a higher ligand density is required for CAR recognition than for TCRs [10]. TCR signaling is further enhanced by the small size of the TCR/pMHC complex, which results in their physical segregation from tyrosine phosphatases that have large ectodomains [11,12]. By contrast, the spatial interaction between a CAR-T cell and its target cell differs depending on the structure and location of the epitope on the target molecule and the design of the extracellular domain of the CAR. Indeed, studies have shown that it is critical to tailor the length and composition of the extracellular spacer domain for individual target molecules to optimize tumor cell recognition, T cell proliferation, and cytokine production [13,14].
Tumor escape from CAR-T cells can occur by the selective outgrowth of antigen loss variants and might be overcome by targeting two different tumor associated molecules. It is feasible to express two ligand binding domains in tandem separated by a flexible linker, or as single CAR constructs in the same or different T cells [15]. The expression of scFvs in tandem is appealing and has been shown to work in principle. However, different spatial requirements for CAR binding for each target may limit the applicability of this approach for some targets or compromise the efficacy of tandem CARs that do not optimally signal through one of the binding domains.
Signaling Modules
Distinct constellations of intracellular signaling domains have been incorporated into CARs to activate effector functions in the T cell. CARs were initially designed with CD3ζ or FcR domains as the only intracellular signaling module, but clinical trials of ACT with such “first” generation CAR-T cells targeting L1CAM and CD20 did not confer significant antitumor activity or result in proliferation and persistence of CAR-T cells in vivo [16,17]. Subsequently, CARs were designed to enhance cytokine production by incorporating one or more costimulatory domains fused to CD3ζ, such as CD28, CD137, or OX-40, and these “second” and “third” generation receptors were superior for inducing cytokine production and proliferation of CAR-T cells in vitro, and for mediating tumor regression in xenograft models in Nod/Scid/γc-/- (NSG) mice compared to CARs with CD3ζ alone [18-23]. Direct comparison of a CD19-specific CAR containing CD28/CD3ζ and one containing only CD3ζ has been performed in one clinical trial and as predicted from preclinical data, T cells expressing the CD28/CD3ζ construct exhibited superior persistence [24]. Surprisingly, antitumor activity was not as dramatic in this study as reported for other trials with CD19-specific CAR-T cells, potentially reflecting other variations in CAR design and/or T cell product composition. Strategies for further augmenting potency and supporting survival of CAR-T cells by co-expressing additional costimulatory receptors, their ligands, and/or cytokines may be necessary for effective CAR-T cell therapy in solid tumors where suppressive tumor microenvironments interfere with T cell function [25,26].
Types of T cells to engineer with CARs for adoptive immunotherapy
CD8+ and CD4+ T cells consist of phenotypically distinct naïve and memory subsets that vary in frequency in the blood and serve different roles in adaptive immunity. The availability of methods for selecting specific T cell subsets using antibodies for constellations of surface markers that distinguish them, and the efficiency of gene transfer using retroviral, lentiviral or transposon based gene delivery makes it feasible to express CARs in specific subsets of T cells and use defined formulations for therapy (Figure 2). Current data on CD8+ T cell differentiation are consistent with a progressive differentiation model such that naïve (TN) and central memory (TCM) cells have superior proliferative capacity, longer telomeres, and improved survival after adoptive transfer compared to effector memory (TEM) and effector (TE) T cells [27-29]. This would suggest that selecting TN and/or TCM for genetic modification might provide products capable of the greatest proliferation and in vivo persistence. However, other factors such as homing receptor expression that dictate trafficking to sites of tumor and the need for immediate effector functions to control rapidly progressive tumors might influence how engineered T cell products might be formulated for optimal efficacy. Current approaches to generate CAR-T cell products by genetically modifying all of the T cells obtained by leukapheresis result in large variability between patients and may not fully capitalize on the proliferation, migration, survival, and functional properties of individual subsets.
Figure 2. Formulating Engineered T Cell Products of Defined Composition.
The composition of T cells present in peripheral blood is heterogeneous and prone to fluctuations based on patient age, disease status and prior therapies. Without formulation of T cell products of defined composition, each patient will receive a different product with potentially significant bariance in potency and safety. CAR trials are now underway that are examining the impact of formulating products of defined ratios of CD4 and CD8 T cells derived from different precursors (TN= naïve, TCM= central memory), as well as, purifying CAR T cells based on purity and expression level of the CAR.
Support for the differential capacity of effector T cells derived from different CD8+ T cell subsets to eradicate tumors after ACT comes both from studies with murine T cells and with human T cells in NSG mice [30-32]. Purifying even relatively rare human T cell subsets such as CD8+ TCM from the blood can be accomplished using a combination of positive and negative immunomagnetic selection or positive serial positive serial enrichment with reversible Fab streptamers [33,34]. Thus, the use of defined T cell subsets in ACT in clinical protocols is now beginning to be evaluated. Although the preclinical data and rationale are appealing, it remains to be determined if selecting defined subsets for modification with CARs will enhance efficacy and/or reduce toxicity. Resolving these issues may require comparative clinical trials of defined composition T cell products.
Enhancing efficacy and function of CAR-T cells
The robust clinical activity of ACT with CD19-specific CAR-T cells in ALL might be attributed to uniform, high-level expression of CD19, the vascularized distribution of tumor cells, and unique qualities of lymphoid cells that may make them more susceptible to T cell effector mechanisms [1,3,5,35]. In treating solid tumors, significant challenges are anticipated beyond the identification of safe targets and design of functional CARs, and additional engineering of T cells for efficient homing, retention of viability, and maintenance of function within the immunosuppressive tumor microenvironment may be necessary for optimal efficacy [36]. Transferring or editing additional genes in T cells to affect cell function and potentially enhance efficacy might provide essential attributes to optimize antitumor activity [37,38]. Cell-intrinsic resistance to immunologic checkpoints imposed in the tumor microenvironment is an area of particular interest and strategies for attenuating TGF-beta signaling and deflecting signaling from inhibitory checkpoint co-receptors such as PD-1 by expression of chimeric receptors that are composed of extracellular PD-1 fused to cytoplasmic CD28 have been described [39-41]. Permanent gene editing of CAR-T cells using zinc finger nucleases, TALENs, or CRISPR-Cas technologies are being developed and can be achieved by mRNA transfection to transiently express editing construct(s). Recently, library screens of shRNA have been employed to identify novel endogenous genes that when knocked down improve the efficacy of anti-tumor T cells, and may provide additional targets for modification of CAR-T cells [42]. CAR-T cells can also deliver secreted recombinant effector proteins to the tumor site, best illustrated by the use of IL-12 secreting CAR-T cells to modify cells in the tumor microenvironment to support the anti-tumor activity of CAR T cells [26,43,44],
Safety
Treatment of refractory leukemia patients with CD19 CAR-T cells has revealed significant and sometimes life-threatening toxicities arising from synchronous CAR-T cell activation and production of effector cytokines, including tumor necrosis factor, interferon gamma, and interleukin 2 [1,3,45]. The secretion of cytokines causes fever, hypotension that requires intravenous fluid and pressors, transient neurologic dysfunction, and a macrophage activation syndrome characterized by high interleukin 6 levels and hemophagocytosis. In addition to supportive care, treatment with anti interleukin 6 antibodies and with corticosteroids may be necessary in severe cases. Targeting molecules on solid tumors that may not be strictly tumor specific with CAR-T cells carries the additional risk of damage to normal tissues due to on-target, off-tumor recognition [46]. Transfecting T cells with mRNA encoding the CAR to provide self-limited expression of the CAR in transferred T cells may be useful to screen for immediate toxicity, but the need for repeated infusions to achieve optimal antitumor effects has resulted in anaphylaxis related to the development of an IgE antibody response to the CAR, which was derived from a murine monoclonal antibody [47,48]. Dual targeting with split receptor systems has been developed to achieve selective recognition of tumor cells where the target molecule on the tumor is also expressed on a subset of normal cells. One strategy is to co-express two CARs that operate as logic gates such that recognition of one target antigen is not sufficient to elicit a full T cell activation signal. This can be achieved by expressing a CAR specific for a molecule shared by the tumor and some normal cells but designed to have attenuated signaling by using an scFv of low affinity, an extracellular spacer that is suboptimal, or a CD3ζ construct that is mutated in one or more of the immune receptor tyrosine activation motifs (ITAMs). A second CAR specific for a target on the tumor but not the normal cells with the target for the activating CAR provides the full T cell activation signal and selective tumor recognition [49,50]. An alternative strategy for achieving tumor selectivity is to employ both activating and inhibitory CARs. In this scenario, the activating CAR targets a molecule on both tumor cells and some normal cells, while the inhibitory CAR is specific for a molecule that is expressed only on normal cells. When the CAR-T cell encounters a normal cell, signaling through the activating receptor is inhibited, thereby preventing damage to normal tissues [51]. These combinatorial systems, while not yet evaluated in the clinic, could allow for effective targeting of a greater number of targets that are expressed on solid tumors and a subset of normal tissues.
Equipping CAR T cells through their genetic modification to exert orthogonal effector outputs that enhance potency must be matched with stringent safety attributes that allow for the regulation of these cells in the patient. Genetic platforms that act as transgene transcriptional and/or translational regulatory systems in T cells that respond to clinically acceptable small molecule drugs are under development and might serve to modulate the activity of CAR-T cells in near real time in a clinician controlled context. Ultimately, the provision of a suicide construct for rapid and complete elimination of CAR-T cells is the most robust safety strategy for controlling adverse events associated with prolonged engraftment and potentially mitigating acute toxicities from CAR-T cells. The ability of cell ablation to attenuate acute toxicities such as cytokine storm once initiated remains to be assessed clinically. Several suicide constructs have now been advanced to the clinic, in particular the dimerizable iCasp9, which when activated can result in the elimination of the vast majority of transferred T cells [52,53]. EGFRt is a cell surface tag that retains the binding epitope for cetuximab and is used for immunomagnetic cell selection and cell tracking in clinical trials [54]. In vitro and murine models demonstrate that co-expressing a cell surface tag comprised of a truncated epidermal growth factor receptor (EGFRt) can allow T cells to be eliminated by cetuximab administration, however the utility of this construct for cell ablation in humans is the subject of evaluation.
Challenges and Future Perspectives
Adoptive T cell therapy for cancer has entered a new and accelerated phase of clinical translation based on applying advances in synthetic biology and genetic engineering to derive therapeutic cell products. The design of CAR-T cell therapies remains largely empiric in target selection, defining the requirements for optimal signaling of T cell effector functions, and in formulating therapeutic products. Many questions are unresolved (Box 2), and it remains to be determined whether the striking success of this approach in the treatment of B cell malignancies can be recapitulated in solid tumors. However, careful interpretation of the results of well-designed clinical trials combined with advances in the development of functional, regulated synthetic receptors and T cell biology suggests that significant progress will continue to be made in applying principles of synthetic biology to cancer therapy.
Box 2. Unresolved Questions in CAR Therapeutics.
What are the optimal formulations of engineered T cells and is it the same in different cancers?
Are optimal costimulatory domains the same in CD4+ and CD8+ T cell subsets?
Can split receptor systems provide for adequate safety without facilitating tumor escape?
Can transgene immunogenicity be effectively mitigated for repeated cell dosing in less heavily treated patient populations?
Will suicide constructs enable attenuation of off target toxicities once they become clinically evident?
Highlights.
Description of structural elements of chimeric antigen receptors used to engineer T cells
Differences between T cell receptor and chimeric antigen receptor recognition
Types of T cells that can be engineered to target tumor cells
Strategies for augmenting function and selectivity of chimeric antigen receptors
Strategies for improving safety of chimeric antigen receptor modified T cells
Acknowledgments
The authors thank past and present members of our laboratories and the support of NIH grants CA136551, CA114536, AI053193, and P50CA132393.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- **1.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science translational medicine. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. This paper describes the results of treating advanced acute lymphoblastic leukemia with autologous T cells modified with a CD19-specific CAR containing the CD28 and CD3ζ signaling domains. An algorithm for identifying and managing patients with cytokine release syndrome is provided. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, et al. Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies Can Be Effectively Treated With Autologous T Cells Expressing an Anti-CD19 Chimeric Antigen Receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **3.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. The study reports results in pediatric acute lymphoblastic leukemia demonstrating that treatment with CD19 specific chimeric antigen receptor-modified T cells against CD19 was was associated with a high remission rate, even among patients for whom stem-cell transplantation had failed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Textor A, Listopad JJ, Wuhrmann LL, Perez C, Kruschinski A, Chmielewski M, Abken H, Blankenstein T, Charo J. Efficacy of CAR T-cell Therapy in Large Tumors Relies upon Stromal Targeting by IFNgamma. Cancer research. 2014 doi: 10.1158/0008-5472.CAN-14-0079. [DOI] [PubMed] [Google Scholar]
- 7.Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer research. 2004;64:9160–9166. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 8.Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, Rader C, Riddell SR. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engels B, Chervin AS, Sant AJ, Kranz DM, Schreiber H. Long-term persistence of CD4(+) but rapid disappearance of CD8(+) T cells expressing an MHC class I-restricted TCR of nanomolar affinity. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:652–660. doi: 10.1038/mt.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choudhuri K, Llodra J, Roth EW, Tsai J, Gordo S, Wucherpfennig KW, Kam LC, Stokes DL, Dustin ML. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507:118–123. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choudhuri K, Wiseman D, Brown MH, Gould K, van der Merwe PA. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature. 2005;436:578–582. doi: 10.1038/nature03843. [DOI] [PubMed] [Google Scholar]
- 12.Choudhuri K, Parker M, Milicic A, Cole DK, Shaw MK, Sewell AK, Stewart-Jones G, Dong T, Gould KG, van der Merwe PA. Peptide-major histocompatibility complex dimensions control proximal kinase-phosphatase balance during T cell activation. The Journal of biological chemistry. 2009;284:26096–26105. doi: 10.1074/jbc.M109.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O'Neill A, Irlam J, Chester KA, Kemshead JT, Shaw DM, et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. Journal of immunotherapy. 2005;28:203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- *14.Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, Jensen MC, Riddell SR. The non-signaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer immunology research. 2014 doi: 10.1158/2326-6066.CIR-14-0127. This study examining the length and composition of the extracellular spacer domain for CARs that target different cell surface molecules demonstrates that the length of this region can be tailored for optimal CAR function and identifies mutations in immunoglobulin Fc spacers that are necessary to abrogate Fc receptor binding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, Corder A, Schonfeld K, Koch J, Dotti G, et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Molecular therapy Nucleic acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg JR, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 18.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 19.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, Quintas-Cardama A, Larson SM, Sadelain M. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 21.Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, Smith DD, Forman SJ, Jensen MC, Cooper LJ. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer research. 2006;66:10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 22.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, Samanta M, Lakhal M, Gloss B, Danet-Desnoyers G, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. The Journal of clinical investigation. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer research. 2011;71:5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 26.Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerlach C, Rohr JC, Perie L, van Rooij N, van Heijst JW, Velds A, Urbanus J, Naik SH, Jacobs H, Beltman JB, et al. Heterogeneous differentiation patterns of individual CD8+ T cells. Science. 2013;340:635–639. doi: 10.1126/science.1235487. [DOI] [PubMed] [Google Scholar]
- 28.Crompton JG, Sukumar M, Restifo NP. Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy. Immunological reviews. 2014;257:264–276. doi: 10.1111/imr.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Buchholz VR, Flossdorf M, Hensel I, Kretschmer L, Weissbrich B, Graf P, Verschoor A, Schiemann M, Hofer T, Busch DH. Disparate individual fates compose robust CD8+ T cell immunity. Science. 2013;340:630–635. doi: 10.1126/science.1235454. This study shows that acute and recall immunity mediated by CD8+ T cells requires the initial recruitment of multiple precursors, and provides data demonstrating a linear developmental path for CD8+ T cell differentiation that progresses from slowly proliferating long-lived to rapidly expanding short-lived subsets. [DOI] [PubMed] [Google Scholar]
- 30.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, et al. A human memory T cell subset with stem cell-like properties. Nature medicine. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, Borman ZA, Kerkar SP, Scott CD, Finkelstein SE, et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:5343–5352. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stemberger C, Dreher S, Tschulik C, Piossek C, Bet J, Yamamoto TN, Schiemann M, Neuenhahn M, Martin K, Schlapschy M, et al. Novel serial positive enrichment technology enables clinical multiparameter cell sorting. PloS one. 2012;7:e35798. doi: 10.1371/journal.pone.0035798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terakura S, Yamamoto TN, Gardner RA, Turtle CJ, Jensen MC, Riddell SR. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119:72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramos CA, Savoldo B, Dotti G. CD19-CAR trials. Cancer journal. 2014;20:112–118. doi: 10.1097/PPO.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, Kapoor V, Scholler J, Pure E, Milone MC, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:4262–4273. doi: 10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen MC, Riddell SR. Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells. Immunological reviews. 2014;257:127–144. doi: 10.1111/imr.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vonderheide RH, June CH. Engineering T cells for cancer: our synthetic future. Immunological reviews. 2014;257:7–13. doi: 10.1111/imr.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prosser ME, Brown CE, Shami AF, Forman SJ, Jensen MC. Tumor PD-L1 co-stimulates primary human CD8(+) cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Molecular immunology. 2012;51:263–272. doi: 10.1016/j.molimm.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Ankri C, Cohen CJ. Out of the bitter came forth sweet: Activating CD28-dependent co-stimulation via PD-1 ligands. Oncoimmunology. 2014;3:e27399. doi: 10.4161/onci.27399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou P, Shaffer DR, Alvarez Arias DA, Nakazaki Y, Pos W, Torres AJ, Cremasco V, Dougan SK, Cowley GS, Elpek K, et al. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature. 2014;506:52–57. doi: 10.1038/nature12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chmielewski M, Hombach AA, Abken H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunological reviews. 2014;257:83–90. doi: 10.1111/imr.12125. [DOI] [PubMed] [Google Scholar]
- 44.Chinnasamy D, Yu Z, Kerkar SP, Zhang L, Morgan RA, Restifo NP, Rosenberg SA. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:1672–1683. doi: 10.1158/1078-0432.CCR-11-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, Chew A, Carroll RG, Scholler J, Levine BL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer research. 2010;70:9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, Zhao Y, Kalos M, June CH. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer immunology research. 2013;1:26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nature biotechnology. 2013;31:71–75. doi: 10.1038/nbt.2459. This paper demonstrates how combinatorial signaling through two CARs can be used to impose a requirement for expression of two molecules on tumor cells for efficient recognition, potentially improving the selectivity of CAR T cell therapy for tumors where target molecules may be shared with normal tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, Powell DJ., Jr Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer immunology research. 2013;1:43–53. doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *51.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Science translational medicine. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. This study describes a strategy for reducing on target, off tumor recognition by CAR T cells by co-expressing an CAR that delivers an inhibitory signal with one that delivers an activating signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Budde LE, Berger C, Lin Y, Wang J, Lin X, Frayo SE, Brouns SA, Spencer DM, Till BG, Jensen MC, et al. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PloS one. 2013;8:e82742. doi: 10.1371/journal.pone.0082742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. The New England journal of medicine. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Chang WC, Wong CW, Colcher D, Sherman M, Ostberg JR, Forman SJ, Riddell SR, Jensen MC. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]