Abstract
The key event in the pathogenesis of the transmissible spongiform encephalopathies is a template-dependent misfolding event where an infectious isoform of the prion protein (PrPSc) comes into contact with native prion protein (PrPC) and changes its conformation to PrPSc. In many extraneurally inoculated models of prion disease this PrPC misfolding event occurs in lymphoid tissues prior to neuroinvasion. The primary objective of this study was to compare levels of total PrPC in hamster lymphoid tissues involved in the early pathogenesis of prion disease. Lymphoid tissues were collected from golden Syrian hamsters and Western blot analysis was performed to quantify PrPC levels. PrPC immunohistochemistry (IHC) of paraffin embedded tissue sections was performed to identify PrPC distribution in tissues of the lymphoreticular system. Nasal associated lymphoid tissue contained the highest amount of total PrPC followed by Peyer’s patches, mesenteric and submandibular lymph nodes, and spleen. The relative levels of PrPC expression in IHC processed tissue correlated strongly with the Western blot data, with high levels of PrPC corresponding with a higher percentage of PrPC positive B cell follicles. High levels of PrPC in lymphoid tissues closely associated with the nasal cavity could contribute to the relative increased efficiency of the nasal route of entry of prions, compared to other routes of infection.
Introduction
The normal isoform of the prion protein (PrPC) is a highly conserved mammalian glycophosphatidylinositol linked membrane protein expressed in tissues throughout the body [1]. PrPC is found in highest concentrations in the central nervous system, but is also present in lower amounts in skeletal muscle, lung, intestine, autonomic ganglia, heart, and ovary [2, 3, 4]. Peripheral mucous associated lymphoid tissues, lymph nodes and spleen also express PrPC, where it has been localized to follicular dendritic cells (FDCs), intraepithelial lymphocytes and dendritic cells [3, 5]. While the highly-conserved nature and wide distribution of PrPC suggest an important function, a definitive physiological role for PrPC has not been determined and PrPC null mice fail to display an overt phenotype [6].
Infectious prions consist of PrPSc, a misfolded isoform of the host encoded PrPC, and are the causative agent of a class of progressive neurodegenerative diseases called the transmissible spongiform encephalopathies (TSEs) [7]. The TSEs include Creutzfeldt-Jakob disease in humans, scrapie in sheep and goats, bovine spongiform encephalopathy in cattle, chronic wasting disease in cervids, and transmissible mink encephalopathy in ranch raised mink. The TSEs have common characteristics that include extended incubation periods which can last years to decades, followed by development of clinical signs and a rapidly progressive disease course. PrPC is required for prion infection as PrPC knockout mice fail to replicate the agent and do not develop disease after inoculation with prions [8].
TSE diseases can be experimentally transmitted by a number of routes including intracerebral, per os, intranerve, intratongue, subcutaneous, and intraperitoneal routes of exposure [9, 10, 11, 12]. Inhalation of prion infected inoculum into the nasal cavity causes disease in hamsters, mice, sheep and deer [13, 14, 15, 16, 17]. Extraneural routes of inoculation are typically characterized by PrPSc accumulation in lymphoreticular system (LRS) tissues, particularly spleen, prior to neuroinvasion [18, 19]. Consistent with this feature, inhalation of inoculum by rodents results in early deposition of PrPSc in nasal associated lymphoid tissue (NALT), unencapsulated lymphoid tissue found directly inferior to nasal mucosa [13, 17]. This is of particular interest as inhalation of prions into the nasal cavity is 10–100 times more efficient compared to per os, considered to be the most common route of infection in natural prion disease [13, 15, 20].
The amount of PrPC available for conversion is known to affect prion disease pathogenesis. Transgenic mice that produce one half the amount of PrPC compared to wild type mice have longer incubation periods following intracerebral inoculation [21]. Aged mice express less PrPC on FDCs compared to young mice and fail to show either clinical signs of prion infection or pathology as expected within their normal life span following intraperitoneal inoculation [22]. Taken together these observations suggest that the level of PrPC available in LRS tissue has a measurable effect on the efficiency of prion infection. In this study we compared the abundance of PrPC of selected lymphoid tissues collected from uninfected hamsters. We hypothesized that relatively high amounts of PrPC in the NALT contribute to the increased efficiency of nasal cavity inoculations.
Methods
Ethics statement
This study was conducted in compliance with National Institutes of Health guidelines in the care and use of laboratory animals. All procedures involving animals were approved by the Creighton University Institutional Animal Care and Use Committee.
Animals
Adult male Syrian golden hamsters (Harlan Sprague Dawley, Indianapolis, IN) were anaesthetized with isofluorane and killed via transcardial perfusion with phosphate buffered saline containing 5 mM ethylenediaminetetracetic acid (EDTA). Animals intended for immunohistochemistry (IHC) processing were subsequently perfusion fixed with periodate-lysine-paraformaldehyde (PLP) followed by immersion of the tissue in PLP for 5–24 hours at room temperature.
Tissue collection
Lymphoid tissues including spleen (SP), Peyer’s patches (PP), submandibular lymph nodes (SLN) and mesenteric lymph nodes (MLN) were removed, placed into cassettes and stored in 70% ethanol at room temperature until processing. Heads (with jaw and tongue removed) were placed into decalcifying solution (Thermo Scientific, Kalamazoo, MI) for a total of two weeks at room temperature with a change of solution midway through the decalcifying process. Nasal cavities were blocked and embedded in paraffin as described previously [23]. Serial sections of each tissue were cut using a microtome at 7μm and collected on glass slides.
Immunohistochemistry
Following deparafinization and rehydration endogenous peroxidases were blocked by immersion in 0.3% v/v hydrogen peroxide in methanol for 20 minutes. The slides were rinsed thoroughly with tris buffered saline containing 0.05% v/v Tween 20 (TTBS) and incubated for 30 minutes with 10% v/v normal horse serum in TTBS at room temperature. Slides were incubated at 4°C overnight with anti prion antibody 3F4 (2.6 μg/mL: Millipore, Temecula, CA) with 3% v/v normal horse serum in TTBS. Slides were rinsed with TTBS and incubated with biotinylated horse anti-mouse secondary antibody (1 μg/mL: Vector laboratories, Burlingame, CA) in 3% v/v normal horse serum TTBS for 30 minutes at room temperature. Signal amplification was performed using the Vectastain Elite ABC-HRP (Vector, Burlingame, CA) and diaminobenzidine reaction was used to visualize antigen location. The following controls were used to ensure specificity of IHC: use of a mouse IgG isotype control (Abcam, Cambridge, MA) in place of primary antibody and omission of either the primary or secondary antibodies with all other steps being the same. Lymphoid tissue sections not further than 140 μm apart were processed for PrPC and examined using a Nikon Eclipse 80i light microscope. Images were captured with an Infinity 2 digital camera (Lumenera, Ottawa, ON) and ImageJ software (NIH, Bethesda, MD).
Semi-quantitative calculation of PrPC lymphoid follicles
The total number of lymphoid follicles and the number of lymphoid follicles expressing PrPC from the SP, SLN, MLN, and PP from eight uninfected hamsters were examined. Percentages were calculated as the number of immunoreactive follicles divided by the total follicles per organ for each animal and further averaged per tissue type for the entire sample group.
Tissue collection and preparation for Western blot
The SP, PP, SLN and MLN were collected, flash frozen and stored at -80°C. NALT collection technique was modified from a method previously described in mice [24]. Collection of NALT was accomplished by removing the jaw and muzzle anterior to the incisors. Tissues lateral and superior to the nasal cavity were trimmed without disturbing the nasal mucosa. The septal window was identified using a dissecting microscope and the septum was removed. The NALT, located deep to the mucosa on the floor of the nasal cavity, was removed, flash frozen and stored at -80°C. Lymphoid tissues were homogenized to 20% w/v in Dulbecco’s phosphate buffered saline, 1% v/v Triton X-100, 0.5mM EDTA and complete protease inhibitor (Roche Diagnostics, Mannheim, Germany). The homogenates were centrifuged for 30 seconds at 2000xg and the supernatant was removed and stored at -80°C. NALT samples were incubated with 2.5 U/μl benzonase (EMD Millipore, San Diego, CA) and 2mM magnesium chloride for thirty minutes on ice to decrease viscosity of the sample prior to Western blot procedures. Deglycosylation of select tissue samples was performed using PNGase F (New England Biolabs, Ipswich, MA) according to manufacturer protocol. Briefly, tissue homogenates were treated with 10% v/v 10x glycoprotein denaturing buffer at 100°C for ten minutes. The samples were incubated at 37°C with PNGase F (1 unit per 10 μg tissue) containing 10% v/v of G7 reaction buffer and 10% v/v NP-40 detergent.
SDS-PAGE and Western blot
Sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blot procedures were performed as previously described [25]. Briefly, samples were size fractionated using NuPage 4–12% Bis-tris gels (Invitrogen, Carlsbad, CA) and transferred to polyvinylidene difluoride membrane (Millipore, Billerica, MA). After blocking the membranes with 5% w/v blotting grade blocker (BioRad, Hercules, CA) in TTBS at room temperature for a minimum of 30 minutes the membranes were probed using the mouse monoclonal anti-prion protein antibody 3F4 (0.2 μg/mL: Millipore, Temecula, CA) in 5% w/v Blotto/TTBS overnight at 4°C. Following washes in TTBS the membranes were incubated with peroxidase conjugated Affinipure donkey anti mouse secondary antibody (0.32 μg/mL: Jackson ImmunoResearch, West Grove, PA) in 5% w/v Blotto/TTBS for a minimum of one hour at room temperature. Following TTBS washes the membranes were developed with Super Signal West Femto (Pierce, Rockford, IL) and were imaged using a Kodak 4000R imager (Kodak, Rochester, NY). Quantification of PrPC was performed using ImageQuant software (Kodak, Rochester, NY). Membranes were washed with TTBS then incubated with the anti β-actin mouse monoclonal antibody (0.005 μg/mL: Santa Cruz Biotechnology, Dallas, TX) for one hour at room temperature, washed with TTBS and exposed to peroxidase conjugated Affinipure donkey anti mouse secondary (0.32 μg/mL: Jackson ImmunoResearch, West Grove, PA) for one hour at room temperature. Washing, development and imaging of the membranes were performed as described above. β-actin protein abundance was used to normalize PrPC protein levels between tissue samples. All lymphoid samples were examined in triplicate. A one-twentieth μg tissue equivalent of brain was examined relative to lymphoid tissue to ensure that the level of PrPC was in the linear range of Western blot detection on all Western blots used for quantification. Normalized PrPC abundance values were calculated as a percentage of the uninfected brain PrPC intensity average in order to standardize lymphoid PrPC intensity measurements between Western blots. PrPC migration patterns were compared by plotting intensity of PrPC signal against migration distance allowing graphic visualization of the relative molecular weight populations of PrPC present in each sample.
Statistical analysis
PrPC abundance was compared using one way analysis of variance (ANOVA); significance value was set at P<0.05. Post hoc testing was performed using Tukey’s honestly significant difference (HSD) test. ANOVA and Tukey’s HSD tests were both performed using Graphpad Prism software V6.0 (San Diego, CA).
Results
The abundance of total PrPC in NALT homogenates was greater compared to the other lymphoid tissues examined
NALT contained approximately 3, 5, and 6 fold the amount of PrPC per μg equivalent of tissue compared to PP, MLN and SLN respectively (Fig. 1B). SP contained the lowest amount of PrPC per μg equivalent of the examined tissues (Fig. 1B). Analysis of the PrPC abundance with ANOVA testing indicated that lymphoid tissues from different organs contain significantly (p<.05) different amounts of PrPC. All one on one comparisons of PrPC from lymphoid tissues performed using Tukey’s honestly significant difference post hoc testing were significantly (p<0.05) different with the exception of SP versus SLN and MLN versus SLN.
Fig 1. NALT contains significantly more PrPC than other lymphoid tissues.
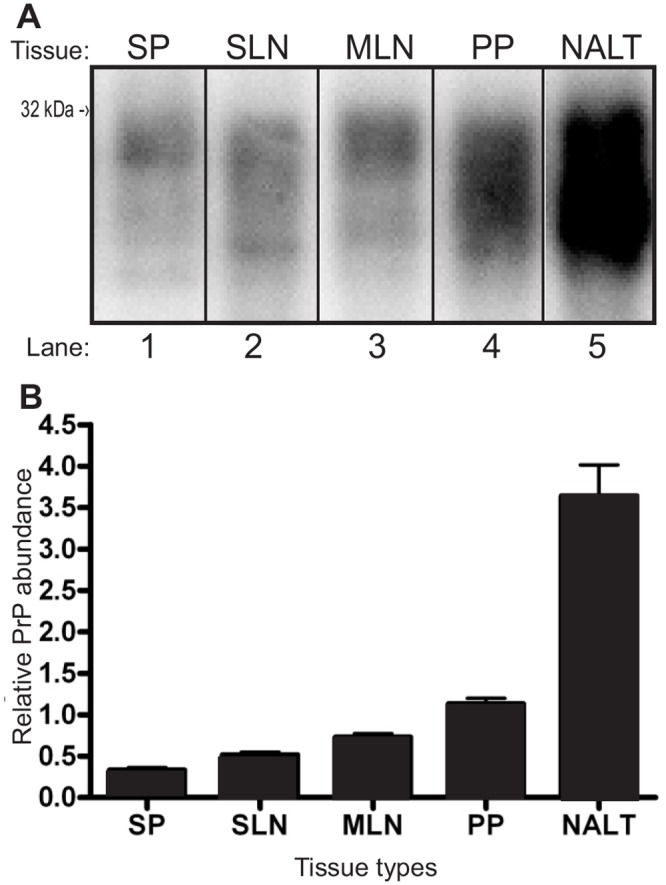
A) Western blot analysis and B) normalized quantification of lymphatic tissue PrPC abundance. SP—spleen, SLN—submandibular lymph node, MLN—mesenteric lymph node, PP—Peyer’s patch, NALT—nasal associated lymphoid tissue.
Distinctive PrPC migration patterns were observed between tissue types
PrPC from PP contained a mixed molecular weight population with no obvious prevalent group while SP and SLN PrPC contained two distinct migration patterns (Fig. 2A lanes 4–5, 2–3, and 6–7). The abundance of PrPC from SLN was equally distributed between relatively higher and lower molecular weights while PrPC from the SP was mainly comprised of a higher molecular weight population (Fig. 2B). PrPC from NALT exhibited a unique migration pattern compared to the other examined lymphoid tissues with two distinct bands but a prevalence of the lower weight population (Fig. 2C). PNGase treatment of SLN, PP and NALT indicated differences in overall ratios of full length PrPC to truncated forms (Fig. 2E). While NALT and SLN were both composed of two predominant forms of PrPC, PP total PrPC consisted of multiple truncated forms of PrPC which were between the molecular weights of full length and the truncated fragment C2.
Fig 2. PrPC migration patterns were distinct for different lymphoid tissues.

A) Migration banding patterns are consistent when comparing individual animal homogenates. SP, SLN and PP homogenates from individual animals migrate similarly. B) Line graph of lane intensity analysis demonstrating peak differences in intensity of PrPC migration. C) Western blot of lymphoid tissue (NALT, SP, and PP) and BR control. D) Line graph of lane analysis of PrPC intensity of lymphoid tissue indicates intensity difference and migration profile of NALT. E) Comparison of PNGase treated and untreated PrPC from lymphoid tissue (SLN, PP and NALT and BR) demonstrates variable levels of full length and truncated PrPC in samples. BR—brain, SP—spleen, PP—Peyer’s patch, SLN—submandibular lymph node, NALT—nasal associated lymphoid tissue.
PrPC was localized to B cell follicles in lymphoid tissue
PrPC immunoreactivity was visualized as a brown, diffuse reaction product between lymphocytes. PrPC immunoreactivity was mainly observed in B cell follicles of examined lymphoid tissues (Fig. 3B-F), however, a distinct difference in the percentage of PrPC positive follicles in the different lymphoid tissues was noted. The ratio of PrPC containing follicles was greatest in PP (89.7%±12.5%) with smaller percentages of PrPC positive follicles identified in MLN and SLN (55.2%±17.3% and 67.6%±14.2%) and the smallest ratio of PrPC positive follicles in SP (20.9%±7.7%). NALT was characterized by the widespread distribution of PrPC immunoreactivity through the entire structure (Fig. 3B). For all of the tissues PrPC immunoreactivity was consistent with the morphology and location of FDCs [26]. Omission of primary or secondary antibodies, or use of the isotype control resulted in a complete lack of staining (Fig. 3A).
Fig 3. Immunohistochemistry (IHC) of lymphoid tissues demonstrating presence and localization of PrPC within B cell follicles.

IHC was performed on A/B) NALT, C) MLN, D) SLN, E) PP and F) SP with the anti-PrP antibody 3F4 (B-F) or an isotype control (A). The tissue sections were processed identically using the same reagents at the same time to illustrate relative differences in PrPC expression between tissues. The scale bar represents 100 μm. NALT—nasal associated lymphoid tissue, MLN—mesenteric lymph node, SLN—submandibular lymph node, PP—Peyer’s patch, SP—spleen.
Discussion
The primary finding of this work is that NALT contains a relatively greater amount of total PrPC compared to other lymphoid tissues and that lymphoid tissues express distinct levels of PrPC as demonstrated by both Western blot and IHC. The importance of PrPC abundance is apparent in an in vitro model of prion infection. Protein misfolding cyclic amplification (PMCA) using brain homogenate from mice overexpressing PrPC as substrate resulted in more efficient conversion of PrPSc than substrate from wild type mice [27]. This finding indicates the level of available PrPC is a contributing factor in the conversion efficiency of endogenous PrPC to pathological PrPSc. This effect is also seen in in vivo models as mice engineered to express decreased levels of PrPC have longer incubation periods following inoculation with mouse adapted prion disease than wild type control mice [21, 28].
The relative differences in total PrPC amounts between lymphoid tissues positively correlates with the PrPSc levels in prion-infected animals. Hamsters peripherally inoculated with the hyper strain of hamster adapted transmissible mink encephalopathy (TME) demonstrate higher amounts of PrPSc in lymph nodes than spleen at clinical stage of disease [29]. This does not appear to be unique to hamsters as lymph nodes from mink subcutaneously infected with TME are infectious several months before spleen and contain higher titers of infectious agent once the clinical stage of disease has been reached [30]. Peyer’s patches and lymph nodes from terminal mice orally infected with both scrapie and bovine spongiform encephalopathy also consistently contain higher levels of PrPSc than spleen [31].
Differences in PrPC migration patterns that were observed between lymphoid tissues in hamsters may be due to a difference in glycoform ratios in the tissue homogenates. The presence of PrPC with distinctive ratios of glycoforms has been previously observed in transgenic mice that selectively expressed tissue-specific PrPC, which is consistent with our observation that different tissues possessed distinct patterns of glycosylation [35]. Glycosylation of PrPC can affect the efficiency of PrPSc formation. The effect of PrPC glycoforms on conversion to PrPSc can be influenced by the strain and species of PrPSc. For example, in hamsters, diglycosylated PrPC supports conversion of Sc237 but in mice the prion strain RML requires the presence of unglycosylated PrPC for efficient conversion [32]. Glycosylation may affect the efficiency of PrPSc formation by making the conformation of the cellular protein more similar to that of the disease associated protein. PrPC produced by cells engineered to hinder post translational modifications of the protein, including glycosylation, has biochemical properties more commonly associated with PrPSc such as resistance to protease digestion [33] and similar treatment of other glycoproteins increases their insolubility in detergents [34].
Tissue specific populations of full length and truncated forms of PrPC may contribute to the difference in PrPC migration patterns observed between lymphoid tissues. β-cleavage of PrPC occurs under normal physiological conditions resulting in the C2 truncated PrPC fragment [35]. This cleavage event can vary between tissues, leading to primary structural differences in total populations of PrPC [36]. Interestingly, deglycosylated PrPC from PP appears to consist of multiple truncated forms, suggesting distinctive β-cleavage patterns on that tissue. This is in agreement with a recent in vitro study in which enzymatically driven β-cleavage was observed at multiple sites within the octarepeat sequence of PrPC [37], however, we cannot exclude the possibility that the multiple forms of PP PrPC occur ex vivo. Our results are consistent with a study which revealed multiple species of PrPC defined by differences in post translational modifications, namely glycophosphotidylinositol moieties and glycol groups, and in primary structure of the protein itself [38]. Given the complexity of PrPC structure, additional characterization of NALT PrPC structure may increase our understanding of unique aspects of the nasal route of inoculation. Overall, the increased abundance of PrPC in the NALT may contribute to the increased efficiency of extranasal prion infection compared to the per os route.
Acknowledgments
We would like to thank Michael Tellman for excellent technical assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the National Institute for Neurological Disorders and Stroke (RO1 NS061994 and RO1 NS061994-03S1 to AEK) and the National Center for Research Resources (CO6 RR17417-01 and G20 RR024001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Stahl N, Borchelt DR, Hsiao K, Prusiner SB (1987) Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 51: 229–240. [DOI] [PubMed] [Google Scholar]
- 2. Bendheim PE, Brown HR, Rudelli RD, Scala LJ, Goller NL, et al. (1992) Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology 42: 149–156. [DOI] [PubMed] [Google Scholar]
- 3. Ford MJ, Burton LJ, Morris RJ, Hall SM (2002) Selective expression of prion protein in peripheral tissues of the adult mouse. Neuroscience 113: 177–192. [DOI] [PubMed] [Google Scholar]
- 4. Fournier JG, Escaig-Haye F, Billette de Villemeur T, Robain O, Lasmézas CI, et al. (1998) Distribution and submicroscopic immunogold localization of cellular prion protein (PrPC) in extracerebral tissues. Cell Tissue Res 292: 77–84. [DOI] [PubMed] [Google Scholar]
- 5. Horiuchi M, Yamazaki N, Ikeda T, Ishiguro N, Shinagawa M (1995) A cellular form of prion protein (PrPC) exists in many non-neuronal tissues of sheep. J Gen Virol 76: 2583–2587. [DOI] [PubMed] [Google Scholar]
- 6. Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp H, et al. (1992) Normal development and behavior of mice lacking the neuronal cell-surface PrP protein. Nature 356: 577–582. [DOI] [PubMed] [Google Scholar]
- 7. Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216: 136–144. [DOI] [PubMed] [Google Scholar]
- 8. Büeler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, et al. (1993) Mice devoid of PrP are resistant to scrapie. Cell 73: 1339–1347. [DOI] [PubMed] [Google Scholar]
- 9. Bartz JC, Kincaid AE, Bessen RA (2002) Retrograde transport of transmissible mink encephalopathy within descending motor tracts. J Virol 76: 5759–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bartz JC, Kincaid AE, Bessen RA (2003) Rapid prion neuroinvasion following tongue infection. J Virol 77: 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimberlin RH, Walker CA (1978) Pathogenesis of mouse scrapie: effect of route of inoculation on infectivity titres and dose-response curves. J Comp Pathol 88: 39–47. [DOI] [PubMed] [Google Scholar]
- 12. Kimberlin RH, Walker CA (1986) Pathogenesis of scrapie (strain 263K) in hamsters infected intracerebrally, intraperitoneally or intraocularly. J Gen Virol 67: 255–263. [DOI] [PubMed] [Google Scholar]
- 13. Kincaid AE, Bartz JC (2007) The nasal cavity is a route for prion infection in hamsters. J Virol 81: 4482–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haybaeck J, Heikenwalder M, Klevenz B, Schwarz P, Margalith I, et al. (2011) Aerosols transmit prions to immunocompetent and immunodeficient mice. PLoS Pathogens 7: 1001257 10.1371/journal.ppat.1001257 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Denker ND, Hayes-Klug J, Anderson KR, Seelig DM, Haley NJ, et al. (2013) Aerosol transmission of chronic wasting disease in white-tailed deer. J Virol 87:1890–2. 10.1128/JVI.02852-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamir AN, Kunkle RA, Richt JA, Miller JM, Greenlee JJ (2008) Experimental transmission of US scrapie agent by nasal, peritoneal, and conjunctival routes to genetically susceptible sheep. Vet Pathol 45: 7–11. 10.1354/vp.45-1-7 [DOI] [PubMed] [Google Scholar]
- 17. Sbriccoli M, Cardone F, Valanzano A, Lu M, Graziano S, et al. (2008) Neuroinvasion of the 263K scrapie strain after intranasal administration occurs through olfactory-unrelated pathways. Acta Neuropathol 117: 175–184. 10.1007/s00401-008-0474-z [DOI] [PubMed] [Google Scholar]
- 18. Eklund CM, Kennedy RC, Hadlow WJ (1967) Pathogenesis of scrapie virus infection in the mouse. J Infect Dis 117: 15–22 [DOI] [PubMed] [Google Scholar]
- 19. Bruce ME (1985) Agent replication in a long incubation period model of mouse scrapie. J Gen Virol 66: 2517–2522. [DOI] [PubMed] [Google Scholar]
- 20. Hadlow WJ, Kennedy RC, Race RE (1982) Natural infection of Suffolk sheep with scrapie virus. J Infect Dis 146: 657–664. [DOI] [PubMed] [Google Scholar]
- 21. Büeler H, Raeber A, Sailer A, Fischer M, Aguzzi A, Weissmann C (1994) High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol Med 1: 19–30. [PMC free article] [PubMed] [Google Scholar]
- 22. Brown KL, Wathne GJ, Sales J, Bruce ME, Mabbott NA (2009) The effects of host age on follicular dendritic cell status dramatically impair scrapie agent neuroinvasion in aged mice. J Immunol 183: 5199–5207. 10.4049/jimmunol.0802695 [DOI] [PubMed] [Google Scholar]
- 23. Kincaid AE, Hudson KF, Richey MW, Bartz JC (2012) Rapid transepithelial transport of prions following inhalation. J Virol 86: 12731–12740 10.1128/JVI.01930-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heritage PL, Underdown BJ, Arsenault AL, Snider DP, McDermott MR (1997) Comparison of murine nasal-associated lymphoid tissue and Peyer’s patch. Am J Respir Crit Care Med 156: 1256–1262. [DOI] [PubMed] [Google Scholar]
- 25. Shikiya RA, Ayers JI, Schutt CR, Kincaid AE, Bartz JC (2010) Co-infecting prion strains compete for a limiting cellular resource. J Virol 84: 5706–5714. 10.1128/JVI.00243-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen LL, Adams JC, Steinman RM (1978) Anatomy of germinal centers in mouse spleen, with special reference to “follicular dendritic cells”. J Cell Biol 77: 148–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mays CE, Titlow W, Seward T, Telling GC, Ryou C (2009) Enhancement of protein misfolding cyclic amplification by using concentrated cellular prion protein source. Biochem Biophysl Res Commun 388: 306–310. 10.1016/j.bbrc.2009.07.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakaguchi S, Katamine S, Shigematsu K, Nakatani A, Moriuchi R, et al. (1995) Accumulation of proteinase K-resistant prion protein (PrP) is restricted by the expression level of normal PrP in mice inoculated with a mouse-adapted strain of the Creutzfeldt-Jakob disease agent. J Virol 69: 7586–7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bartz JC, DeJoia C, Tucker T, Kincaid AE, Bessen RA (2005) Extraneural prion neuroinvasion without lymphoreticular system infection. J Virol 79: 11858–11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hadlow WJ, Race RE, Kennedy RC (1987) Temporal distribution of transmissible mink encephalopathy virus in mink inoculated subcutaneously. J Virol 61: 3235–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maignien T, Lasmézas CI, Beringue V, Dormont D, Deslys JP (1999) Pathogenesis of the oral route of infection of mice with scrapie and bovine spongiform encephalopathy agents. J Gen Virol 80: 3035–3042. [DOI] [PubMed] [Google Scholar]
- 32. Nishina KA, Deleault NR, Mahal SP, Baskakov I, Luhr T, et al. (2006) The stoichiometry of host PrPC glycoforms modulates the efficiency of PrPSc formation in vitro. Biochemistry 45: 14129–14139. [DOI] [PubMed] [Google Scholar]
- 33. Lehmann S, Harris DA (1997) Blockade of glycosylation promotes acquisition of scrapie-like properties by the prion protein in cultured cells. J Biol Chem 272: 21479–21487. [DOI] [PubMed] [Google Scholar]
- 34. Leavitt R, Schlesinger S, Kornfeld S (1977) Impaired intracellular migration and altered solubility of nonglycosylated glycoproteins of vesicular stomatitis virus and Sindbis virus. J Biol Chem 252: 9018–9023. [PubMed] [Google Scholar]
- 35. Chen SG, Teplow DB, Parchi P, Teller JK, et al. (1995) Truncated forms of the human prion protein in normal brain and in prion diseases. J Biol Chem 270:19173–19180. [DOI] [PubMed] [Google Scholar]
- 36. Dron M, Moudjou M, Chapuis J, Salamat MKF, et al. (2010) Endogenous proteolytic cleavage of disease-associated prion protein to produce C2 fragments is strongly cell- and tissue-dependent. J Biol Chem 285: 10252–10264. 10.1074/jbc.M109.083857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McDonald AJ, Dibble JP, Evans EGB, Millhauser GL (2014) A new paradigm for enzymatic control of α-cleavage and β-cleavage of the prion protein. J Biol Chem 289: 803–813. 10.1074/jbc.M113.502351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pan T, Li R, Wong B-S, Liu T, et al. (2002) Heterogeneity of normal prion protein in two-dimensional immunoblot: presence of various glycosylated and truncated forms. J Neurochem 81: 1092–1101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


