Background: The effects of ALS on bone homeostasis are poorly understood.
Results: ALS mice with muscle atrophy have reduced bone mass associated with multiple impairments of osteoblast properties and striking acceleration of osteoclast formation in bone.
Conclusion: Abnormal bone remodeling results in dramatic bone loss during the progression of muscle atrophy in ALS mice.
Significance: This study may help to define therapeutic targets for ALS patients.
Keywords: Amyotrophic Lateral Sclerosis (ALS) (Lou Gehrig Disease), Bone, Muscle Atrophy, Osteoblast, Osteoclast
Abstract
There is an intimate relationship between muscle and bone throughout life. However, how alterations in muscle functions in disease impact bone homeostasis is poorly understood. Amyotrophic lateral sclerosis (ALS) is a neuromuscular disease characterized by progressive muscle atrophy. In this study we analyzed the effects of ALS on bone using the well established G93A transgenic mouse model, which harbors an ALS-causing mutation in the gene encoding superoxide dismutase 1. We found that 4-month-old G93A mice with severe muscle atrophy had dramatically reduced trabecular and cortical bone mass compared with their sex-matched wild type (WT) control littermates. Mechanically, we found that multiple osteoblast properties, such as the formation of osteoprogenitors, activation of Akt and Erk1/2 pathways, and osteoblast differentiation capacity, were severely impaired in primary cultures and bones from G93A relative to WT mice; this could contribute to reduced bone formation in the mutant mice. Conversely, osteoclast formation and bone resorption were strikingly enhanced in primary bone marrow cultures and bones of G93A mice compared with WT mice. Furthermore, sclerostin and RANKL expression in osteocytes embedded in the bone matrix were greatly up-regulated, and β-catenin was down-regulated in osteoblasts from G93A mice when compared with those of WT mice. Interestingly, calvarial bone that does not load and long bones from 2-month-old G93A mice without muscle atrophy displayed no detectable changes in parameters for osteoblast and osteoclast functions. Thus, for the first time to our knowledge, we have demonstrated that ALS causes abnormal bone remodeling and defined the underlying molecular and cellular mechanisms.
Introduction
Amyotrophic lateral sclerosis (ALS)3 is a fatal neurological disease characterized by death of motor neurons and progressive muscle atrophy. ALS is epidemiologically classified into two forms, sporadic and familial ALS. Ninety percent of cases of ALS are sporadic ALS with the remaining 10% being familial ALS (1). Both sporadic and familial ALS manifest similar pathological and clinical phenotypes, suggesting that different initiating causes lead to a mechanistically similar neurodegenerative pathway. Mutations in the gene that encodes the superoxide dismutase 1 (SOD1) are linked to ALS (1–3), although it remains unclear how mutations in the SOD1 gene cause motor neuron degeneration. Mouse models expressing ALS-linked SOD1 mutations effectively recapitulate many features of the human disease and have been extensively used to investigate pathogenic mechanisms of ALS (4). The initial clinical symptoms of ALS may be subtle. However, as ALS progresses, muscle weakness and atrophy become severe and lead to death from respiratory failure usually within 3–5 years from the onset of symptoms. There is currently no cure for ALS. A better understanding of molecular mechanisms underlying the pathogenesis of ALS would help to define potential new therapeutic targets for treating this catastrophic disease.
A spatiotemporal association between muscle and bone is observed throughout growth, development, and aging (5–9). Skeletal muscle loading provides a primary source of anabolic mechanical stimuli for bone (8–12). Conversely, skeletal unloading results in significant bone loss (12–14). Mechanical signals exert important influences on bone cells (12, 15–25). Osteocytes embedded in the bone matrix are thought to coordinate osteoblast and osteoclast activity in response to mechanical stimuli, translating mechanical strain into biochemical signals that ultimately regulate bone remodeling (i.e. bone resorption and formation) (9, 17–21, 25, 26). Accumulative evidence suggests that Wnt/β-catenin signaling plays a critical role in mediation of the mechanical regulation of bone homeostasis (17–21, 25). Osteocyte-derived sclerostin, which is encoded by the Sost gene (27), can be induced by mechanical unloading and inhibits Wnt/β-catenin signaling in osteoblasts (12, 28, 29). These studies suggest that Wnt/β-catenin signaling is an integral part of the mechanotransduction cascade in bone. In addition, muscle is an important, local source of growth factors for bone. Muscle produces osteogenic growth factors, such as fibroblast growth factor (FGF-2) and insulin-like growth factor (IGF-1) (30, 31). Furthermore, muscle can provide muscle-derived osteogenic stem cells (30, 32, 33).
Clinically, osteopenia and fractures are common in patients with muscular atrophy (34–37). For example, patients with Duchenne muscular dystrophy, which is due to a mutation in the gene encoding the dystrophin protein, have reduced bone mass and are at risk for fractures (34, 35). Similarly, patients affected by spinal muscular atrophy show decreased bone mineral density (38). Collectively, these observations suggest that muscle atrophy in disease may impair bone integrity. The aim of this study was to determine whether, and how, bone homeostasis is impaired in a G93A ALS mouse model during the progression of muscle atrophy.
EXPERIMENTAL PROCEDURES
Reagents
Tissue culture media and fetal bovine serum (FBS) were obtained from Thermo Scientific HyClone (Logan, UT). Alizarin red, cetylpyridinium chloride, l-ascorbic acid, and β-glycerophosphate were purchased from Sigma, recombinant human macrophage colony stimulating factor and RANKL were from R&D Systems (Minneapolis, MN), and the BrdU immunostaining kit was from Invitrogen. All other chemicals were of analytical grade.
G93A Transgenic Mice
G93A transgenic mice expressing a G93A mutant form of human SOD1 in a C57B6XSJL background have been previously described (39, 40). Two-month-old (without muscle atrophy) and 4-month-old (with severe muscle atrophy) G93A female mice and their sex-matched littermate controls (hereinafter referred to as WT) were used in this study. All animals were bred and housed in the same facility at Rush University Medical Center. Mice were fed standard rodent chow and water ad libitum in sterile cages with a 12-h light/dark cycle. We used only female mice in this study; male G93A mice were saved for breeding to maintain the G93A colony because female G93A mice are infertile. All research protocols were approved by the Institutional Animal Care and Use Committee of Rush University Medical Center, where this study was conducted.
Bone Morphometric Analyses by Micro-computerized Tomography (μCT)
After sacrifice, mouse femurs were isolated and fixed in 70% ethanol for 2 days. Non-demineralized femurs from each group were scanned and measured by μCT (μCT35, SCANCO Medical AG, Wayne, PA) with an isotropic resolution of 7.0 μm following the standards of techniques and terminology recommended by the American Society for Bone and Mineral Research (41). For trabecular bone parameters, transverse slices were obtained in the region of interest in the axial direction from the trabecular bone 0.1 mm below the growth plate (bottom of the primary spongiosa). Contours were defined and drawn close to the cortical bone. The trabecular bone was then removed and analyzed separately. A three-dimensional analysis was performed on 250 trabecular bone slices. A 1.75-mm section was used to obtain mid-femoral cortical bone thickness. The analysis of the specimens involved the following bone measurements: bone volume fraction/total tissue volume (BV/TV, %), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular spacing (Tb.Sp), and cortical thickness (Cort.Th).
Bone Sample Processing, Bone Histology, and Immunohistochemical (IHC) Staining
After euthanasia, WT and G93A mice tibiae were fixed in 10% formalin at 4 °C for 2 days, decalcified in 10% EDTA (pH 7.4) for 3 weeks, and embedded in paraffin. 5-μm bone sections were stained with toluidine blue. Osteoblast surface/bone surface and osteoblast numbers/bone perimeter of trabecular bones were measured using Image Pro Plus 7.0 software (Rockville, MD). For IHC, 5-μm sections of tibiae were stained with antibodies against Runx2 (ab102711; Abcam, Cambridge, MA), osterix (Osx) (ab22552; Abcam), osteocalcin (Ocn) (sc-30044; Santa Cruz, Santa Cruz, CA), β-catenin (9562S; Cell Signaling, Beverly, MA), RANKL (sc-9073; Santa Cruz), or control IgG using the EnVision+ System-HRP (Dako North America, Inc., Carpinteria, CA) as previously described (42, 43). Approximately 6–8 sections were obtained from each sample, and the same region was analyzed from all samples.
Mineralization Apposition Rate (MAR), Mineralizing Surface per Bone Surface (MS/BS), and Bone Formation Rate (BFR)
MAR, MS/BS, and BFR were measured after the guidelines recommended by the American Society for Bone and Mineral Research (44). Briefly, mice were injected intraperitoneally with calcein (20 mg/kg) at days 6 and 2 before sacrifice. After μCT analyses, non-demineralized femurs were embedded using an Osteo-Bed Bone Embedding kit (EM0200; Sigma) and sectioned at 5 μm. Calcein labeling was visualized using EVOS fl microscopy (Life Technologies, Carlsbad, CA). Metaphyseal trabecular bones protruding into the bone marrow area and diaphyseal cortical bones were evaluated, and the distance between the double-labeled bands was measured using Image Pro Plus 7.0 software (Rockville, MD). The MAR was calculated as the mean distance between the double labels divided by the number of the days between the calcein injections. The MS/BS was calculated as the percentage of bone surface exhibiting mineralizing activity. MS/BS = (dLS + sLS/2)/BS, where dLS is the number of double-labeled surfaces, and sLS is the number of single-labeled surfaces. The bone formation rate per bone surface (BFR/BS or BFR) is the volume of mineralized bone formed per unit time and per unit bone surface. BFR/BS is calculated as BFR/BS = MAR × (MS/BS).
Isolation of Bone Marrow Cells, Colony-forming Unit-Fibroblast (CFU-F) Assay, and Colony-forming Unit-Osteoblast (CFU-OB) Assay
The long bones of the mice were isolated, and bone marrow cells were flushed with α-minimum Eagle's medium containing 20% FBS and 1% penicillin/streptomycin into a 100-mm dish. The cell suspension was aspirated to break up clusters of bone marrow cells and then cultured in the same medium for 8–10 days. The CFU-F assay was performed as previously described (45). Briefly, 1 × 106 bone marrow cells/well were seeded in a 6-well dish with 2 ml of complete MesenCult medium (Stemcell Technologies, Vancouver, BC, Canada) for 10 days. The cell colonies were fixed with ice-cold 70% ethanol for 1 h, and stained with 0.02% Giemsa solution. The CFU-OB assay was performed as previously described (45). Briefly, 2 × 106 bone marrow cells were plated in a 60-mm dish with 4 ml of osteoblast differentiation medium (α-minimum Eagle's medium containing 10% FBS, 1% penicillin/streptomycin, 50 μg/ml ascorbic acid, and 5 mm β-glycerophosphate). Media were changed every 2 days. After 21 days, media were aspirated, and the cells were fixed with ice-cold 70% ethanol for 1 h and stained with 40 mm Alizarin red (pH 4.2) at room temperature for 10 min. Cells were rinsed 5 times with nanopure water to remove unbound Alizarin red and washed with 1× phosphate-buffered saline to further remove nonspecific staining.
MTS Assay and BrdU Staining
The MTS assay was used to measure the growth of bone marrow stromal cells (BMSCs) as previously described (46). Briefly, cells were seeded into a 96-well plate (1 × 104 cells/well) in 100 μl of proliferation medium (α-minimum Eagle's medium containing 10% FBS and 1% penicillin/streptomycin). Cells were incubated at 37 °C for 24 h to allow attachment. The media were changed every 48 h. At specified time points, 20 μl of CellTitre96AQ solution reagent (Promega, Madison, WI) was added into each well and incubated for 2 h. The absorbance was recorded at 490 nm using a 96-well plate reader. The BrdU labeling reagent was purchased from Invitrogen. BrdU staining was performed using cells cultured in 8-well culture chambers (Nalgene Nunc, Rochester NY). Cells were cultured in 8-well chambers at a density of 105 cells/well in 400 μl of proliferation medium. After 2 days, cells from each group were labeled with BrdU (1:100 dilution) overnight in the same medium. To identify actively proliferating cells, nuclei that had incorporated BrdU were detected using a BrdU immunostaining kit according to the manufacturer's instructions. BrdU-positive cells were normalized to the total cell numbers.
Quantitative Real-time RT-PCR (RT-qPCR) Analysis and Western Blot Analysis
RNA isolation and reverse transcription (RT) have been previously described (47). RT-qPCR analysis was performed to measure the relative mRNA levels using the SYBR Green kit (Bio-Rad). Melting curve analyses were used to confirm the specificity of the PCR products. The levels of mRNA were calculated by the ddCt method. Samples were normalized to Gapdh expression as previously described (48). The DNA sequences of primers used for RT-qPCR are summarized in Table 1. Western blot analyses were performed as previously described (49). Primary antibodies against Runx2 (sc-10758), Osx (sc-22538), and secondary anti-rabbit or anti-mouse IgG antibodies conjugated with horseradish peroxidase were from Santa Cruz. The antibody against β-catenin (ab6302) was from Abcam, antibodies recognizing ERK1/2 (9102), phospho-ERK1/2 (Thr-202/Tyr-204) (9101S), AKT (9272S), phospho-AKT (Ser-473) (4060S), p38 (9212), and phospho-p38 (Thr-180/Tyr-182) (9216S) were from Cell Signaling, and the mouse monoclonal antibody against β-actin (A2228) was from Sigma.
TABLE 1.
Mouse RT-qPCR primers
| Name | 5′-Primer | 3′-Primer |
|---|---|---|
| Alp | TCCCACGTTTTCACATTCGG | CCCGTTACCATATAGGATGGCC |
| Ap2 | GATGAAATCACCGCAGACGACAGGA | CACCACCAGCTTGTCACCATCTCG |
| Atf4 | GAGCTTCCTGAACAGCGAAGTG | TGGCCACCTCCAGATAGTCATC |
| Bsp | AAGAGGAAGAAAATGAGAACGA | GCTTCTTCTCCGTTGTCTCC |
| Cat K | AATACGTGCAGCAGAACGGAGGC | CTCGTTCCCCACAGGAATCTCTCTGTAC |
| Cebpβ | CTT CAG CCC CTA CCT GGA GC | CCG AGG CTC ACG TAA CCG TA |
| Csf1r | CCTCCTCTGGTCCTGCTGCTGG | GCTCACACATCGCAGGGTCACC |
| Col1a1 | AGATTGAGAACATCCGCAGCC | TCCAGTACTCTCCGCTCTTCCA |
| Dmp1 | AGTGAGTCATCAGAAGAAAGTC | TACTGGCCTCTGTCGTAGCC |
| Lrp5 | GGGTCCACAAGGTCAAGGC | GCACCCTCCATTTCCATCC |
| Lrp6 | GCCCACTACTCCCTGAATGCTG | TGTGGATAGGAAGGATGATGTCAGG |
| Pparγ | GGGGATGTCTCACAATGCCATCA | TGCCAGGGCTCGCAGATCAG |
| Gapdh | CAGTGCCAGCCTCGTCCCGTAGA | CTGCAAATGGCAGCCCTGGTGAC |
| Mmp-9 | TGCCCTGGAACTCACACGACATCTTC | TCCACCTGGTTCACCTCATGGTCC |
| Ocn | AGGGAGGATCAAGTCCCG | GAACAGACTCCGGCGCTA |
| Osx | AGAGGTTCACTCGCTCTGACGA | TTGCTCAAGTGGTCGCTTCTG |
| Rank | AGAGGGGAGCCTCAGGGTCC | AAGTTCATCACCTGCCCGCTAGA |
| Rankl | GCCGAGGAAGGGAGAGAACG | CCCATCTCCTCCGAGCTGC |
| Runx2 | TAAAGTGACAGTGGACGGTCCC | TGCGCCCTAAATCACTGAGG |
| Sost | TCCTCCTGAGAACAACCAGAC | TGTCAGGAAGCGGGTGTAGTG |
| Trap | CACTCCCACCCTGAGATTTGTG | ACGGTTCTGGCGATCTCTTTG |
In Vitro and in Vivo Osteoclast Differentiation
In vitro osteoclast differentiation was performed as previously described (50). Briefly, nonadherent bone marrow-derived macrophages (BMMs) were harvested and seeded into a 96-well plate in osteoclast proliferation medium (α-minimum Eagle's medium containing 10% FBS and 1% penicillin/streptomycin and 10 ng/ml recombinant human macrophage colony stimulating factor (216-MC-025, R&D Systems) for 3 days, then changed into differentiation medium (proliferation medium plus 50 ng/ml recombinant human RANKL (390-TN-010, R&D Systems) for 5–7 days. Cells were fixed in 10% formalin for 30 min at room temperature and then subjected to tartrate-resistant acid phosphatase (TRAP) staining as previous described (50). TRAP-positive multinucleated cells (MNCs) per well were counted using light microscopy. The TRAP staining technique for bone sections has been previously described (50). The osteoclast surface/bone surface (Oc.S/BS) and osteoclast numbers/bone perimeter (Oc.N/BPm) in both primary and secondary spongiosa of tibiae were measured using Image Pro Plus 7.0 software as previously described (45, 50). Serum levels of CTX (C-telopeptide), degradation products from type I collagen during osteoclastic bone resorption, were measured using the RatLaps EIA kit according to the manufacturer's instructions (AC-06F1; Immunodiagnostic Systems Ltd., Scottsdale, AZ) as previously described (47).
Statistical Analysis
The data were analyzed using GraphPad Prism software (4.0) (San Diego, CA). A one-way analysis of variance analysis was used followed by the Tukey test. Student's t test was used to test for differences as needed. Results are expressed as the mean ± S.D. Differences with a p < 0.05 are considered statistically significant. All in vitro experiments were repeated at least three times.
RESULTS
Four-month-old G93A Mice with Severe Muscle Atrophy Have Reduced Trabecular and Cortical Bone Mass with Impaired Osteoblast Function
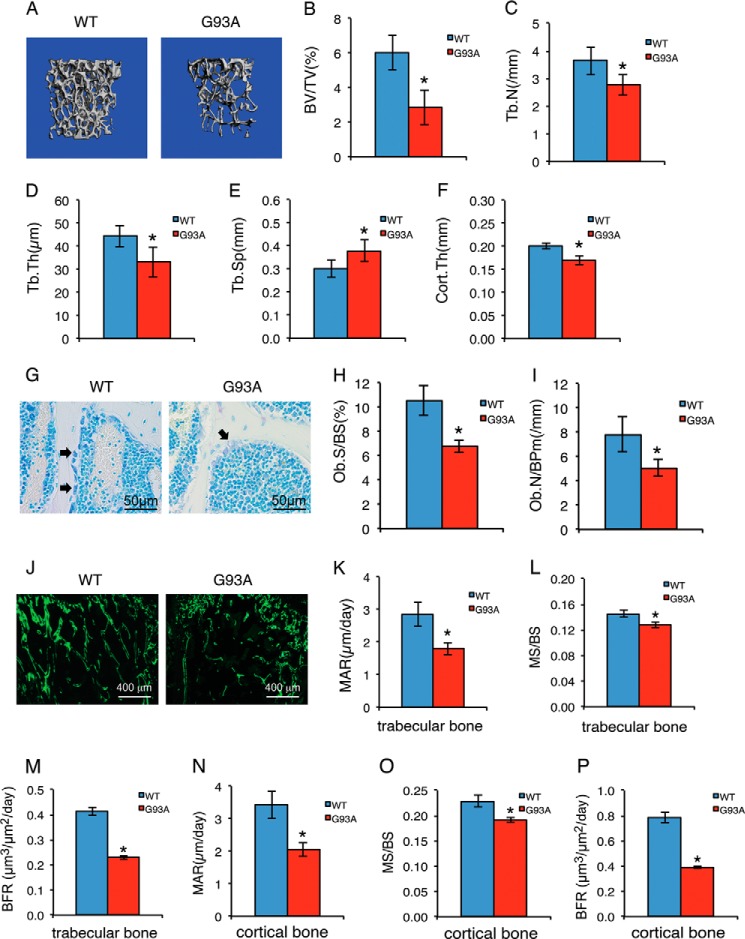
For this study, we used a G93A ALS mouse model that globally expresses the SOD1G93A mutant protein (51). G93A mice displayed muscle weakness and atrophy starting at around 3 months of age that worsened over time. The majority of these mice died at around 5 months of age due to respiratory failure. To examine whether bone integrity is impaired in ALS mice, we performed quantitative μCT analyses of femur histomorphometric parameters in 4-month-old G93A female mice compared with their sex-matched WT littermates. The results showed that G93A mice had reductions in both trabecular and cortical bones. Specifically, bone parameters that decreased significantly compared with WT were BV/TV by 52.3% (p = 0.000022), Tb.N by 24.1% (p = 0.00026), Tb.Th by 27.3% (p = 0.00016), and Cort.Th by 17.4% (p = 0.0066); in contrast, Tb.Sp was significantly increased by 28.4% (p = 0.00019) (Fig. 1, A–F). Toluidine blue-stained tibial sections of the two genotypes revealed significant reductions in osteoblast surface/bone surface (p = 0.00059) and osteoblast numbers/bone perimeter (p = 0.00019) in G93A mice (Fig. 1, G–I). Results from calcein double-labeling show that the MAR, MS/BS, and BFR, all indicators of in vivo osteoblast function, were significantly reduced in G93A mice in femur metaphyseal trabecular bones (MAR, p = 1.40E-06; MS/BS, p = 0.0049; BFR, p = 1.64E-05) and femur diaphyseal cortical bones (MAR, p = 1.42E-08; MS/BS, p = 0.028; BFR, p = 3.5E-06 (Fig. 1, J–P). Collectively, these results demonstrate that osteoblast function is severely impaired, resulting in an osteopenic phenotype in G93A mice with muscle atrophy.
FIGURE 1.
Four-month-old G93A mice with muscle atrophy show reduced trabecular and cortical bone mass associated with impaired osteoblast function. A, three-dimensional reconstructions from μCT scans of femurs from 4-month-old G93A (n = 10) and WT (n = 10) female mice. B–F, quantitative analyses of BV/TV, Tb.N, Tb.Th, Tb.Sp), and Cort.Th. *, p < 0.01 (versus WT). G–I, 5-μm tibial sections were stained with toluidine blue (G). Quantitative analyses of osteoblast surface/bone surface (Ob.S/BS) (H) and osteoblast numbers/bone perimeter (Ob.N/BPm) (I) are shown. *, p < 0.01 (versus WT), n = 7 for both WT and G93A. Arrowheads indicate osteoblasts located on trabecular surfaces. J–P, MAR, MS/BS, and BFR assays. Sections of non-demineralized femurs were used for MAR, MS/BS, and BRF assays. Magnification: 40×. Quantitative MAR, MS/BS, and BFR data for metaphyseal trabecular bones (K–M) and diaphyseal cortical bones (N–P). *, p < 0.01 (versus WT), n = 10 for both WT and G93A.
Osteoblast Differentiation Is Suppressed in Primary Cultures and Bones of 4-Month-old G93A Mice
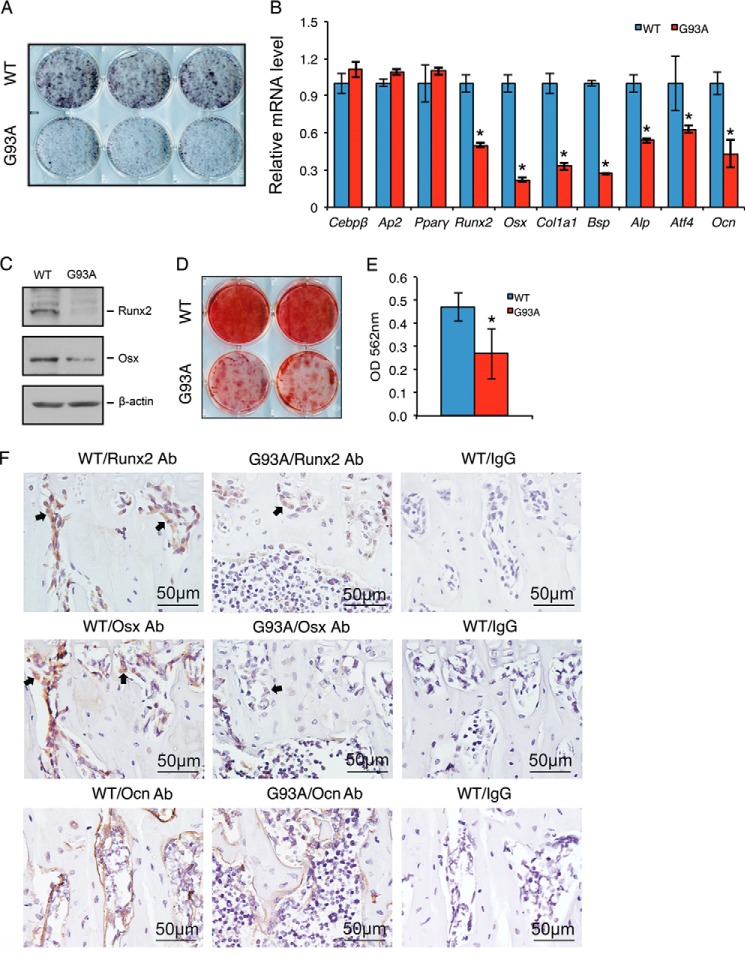
To determine whether osteoblast differentiation is impaired in ALS mice, primary BMSCs were isolated from 4-month-old G93A and WT mice and differentiated in the presence of ascorbic acid. Alkaline phosphatase activity, an early marker of osteoblast differentiation, was markedly reduced from WT levels in G93A cultures (Fig. 2A). RT-qPCR analysis showed decreased expression of osteoblast differentiation marker genes, including those encoding Runx2, Osx, type I collagen (Col1a1), bone sialoprotein (Bsp), alkaline phosphatase, activating transcription factor 4 (Atf4), and Ocn, in G93A mice compared with WT mice (Fig. 2B). In contrast, the mRNA levels of CCAAT enhancer binding proteins β (Cebpβ), adipocyte protein 2 (Ap2), and peroxisome proliferator-activated receptor γ (Pparγ), all adipogenic differentiation markers, were not significantly altered in G93A cultures compared with WT cultures (Fig. 2B). Western blot analyses revealed reduced Runx2 and Osx proteins in G93A osteoblasts (Fig. 2C). Alizarin red staining of the differentiated osteoblast cultures of the two genotypes showed reduced osteoblast mineralization in G93A cultures compared with WT cultures (Fig. 2, D and E). IHC staining of tibial sections of the two genotypes showed that the levels of Runx2, Osx, and Ocn proteins were markedly decreased in osteoblasts of G93A bones compared with WT bones (Fig. 2F). It should be noted that Runx2 and Osx expression per osteoblast were dramatically reduced in G93A bones compared with WT bones.
FIGURE 2.
Osteoblast differentiation is suppressed in primary cultures and bones of 4-month-old G93A mice with muscle atrophy. A–E, in vitro osteoblast differentiation. Primary BMSCs from 4-month-old WT and G93A female mice were plated at a density of 4 × 105/dish in 35-mm dishes in differentiation media for 7 days followed by alkaline phosphatase staining (A), RT-qPCR analyses (B), or Western blot analyses (C) or for 14 days followed by Alizarin red staining (D). Gapdh mRNA was used as internal control for RT-qPCR analyses, and β-actin was used as loading control for Western blotting. Quantitative data of D are shown in E. Experiments were repeated three times in triplicate (A), quadruplicate (B), or in duplicate (D). *, p < 0.05 (versus WT). F, IHC, 5-μm tibial sections were stained with antibodies (Ab) against Runx2 (top), Osx (middle), and Ocn (bottom) or a control IgG (left panels). Runx2- and Osx-positive cells were stained brown (see arrowheads), and negative cells were stained blue. Cebpβ, CCAAT enhancer binding proteins β; Ap2, adipocyte protein 2; Pparγ, peroxisome proliferator-activated receptor γ; Col1a1, type I collagen; Bsp, bone sialoprotein; Atf4, activating transcription factor 4.
The Formation and Expansion of Bone Marrow Mesenchymal Stem Cells and Osteoprogenitors Are Reduced in Bone Marrow Cultures from 4-Month-old G93A Mice
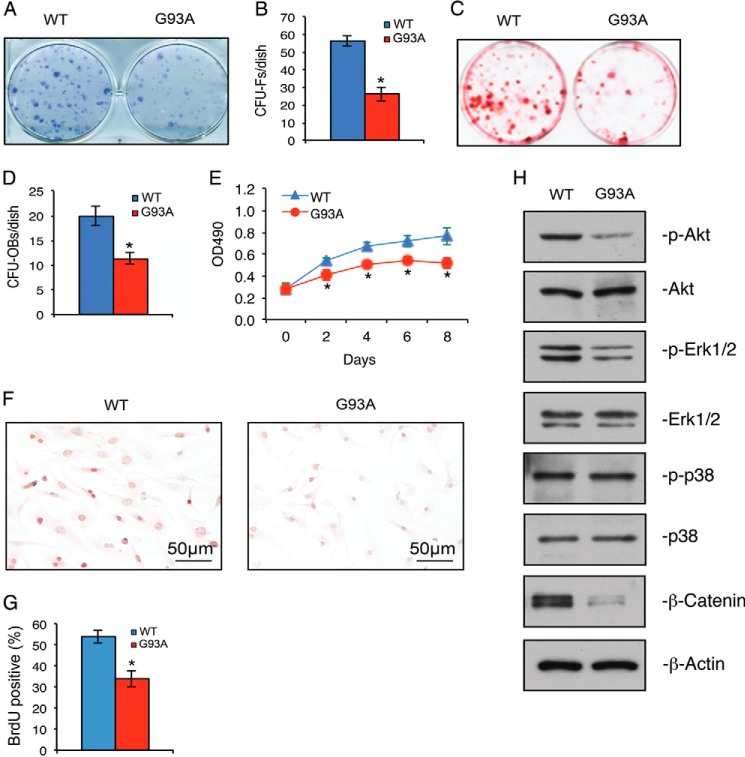
We performed CFU-F assays to measure the formation and expansion of mesenchymal stem cells in bone marrow cultures of WT and G93A mice. The results showed that the number of CFU-Fs in G93A bone marrow cultures was significantly lower than in the WT cultures (Fig. 3, A and B). In addition, the size of the CFU-F colonies formed in the G93A group was markedly smaller than that of WT group (Fig. 3A). We next examined whether ALS impairs the formation and expansion of bone marrow osteoprogenitors using CFU-OB assays. The results showed that the number of CFU-OBs was dramatically reduced in G93A cultures compared with WT cultures (Fig. 3, C and D).
FIGURE 3.
The numbers of CFU-Fs and CFU-OBs and of Akt and Erk1/2 phosphorylation, cell proliferation, and β-catenin expression are down-regulated in primary BMSCs from 4-month-old G93A mice with muscle atrophy. A and B, CFU-F assay. 1 × 106 bone marrow nucleated cells from 4-month-old WT and G93A female mice were cultured in complete MesenCult medium (Stemcell Technologies) for 10 days followed by Giemsa solution. CFU-Fs from each group were quantified. *, p < 0.05 (versus WT). Experiments were repeated three times in triplicate. C and D, CFU-OB assay. 2 × 106 bone marrow nucleated cells from 4-month-old WT and G93A female mice were cultured in osteoblast differentiation media for 21 days followed by Alizarin red staining. The number of CFU-OB colonies was quantified. *, p < 0.05 (versus WT). Experiments were repeated three times in triplicate. E, MTS assay. Primary BMSCs from 4-month-old WT and G93A female mice were seeded at a density of 1 × 104/well in 96-well plates in proliferation media. MTS assays were performed on days 0, 2, 4, 6, and 8 as indicated. *, p < 0.05 (versus WT). Experiments were repeated three times in quadruplicate. F and G, BrdU labeling. Primary BMSCs from 4-month-old WT and G93A female mice were seeded at 105 cells/well in 8-well chambers and cultured in proliferation media for 2 days followed by BrdU staining. Experiments were repeated three times in quadruplicate. H, Western blot analysis. Primary BMSCs from 4-month-old WT and G93A female mice were plated at a density of 4 × 105/dish in 35-mm dishes and cultured in proliferation media for 2 days. Experiments were repeated three times.
Impaired Cell Growth and Proliferation, Defective Activation of Akt and Erk1/2 Pathways, and Reduced β-Catenin in Primary BMSCs of 4-Month-old G93A Mice
The results from the MTS assays showed that the growth rate of primary BMSCs from G93A mice was significantly reduced in a time-dependent manner (Fig. 3E). BrdU labeling experiments revealed that the proliferation of primary BMSCs from G93A mice was reduced compared with that of WT cells (Fig. 3, F and G). Cell apoptosis, as measured by TUNEL staining, was low and not different between primary BMSC cultures of the two genotypes (data not shown). Western blot analyses showed that the levels of phospho-Akt and phospho-Erk1/2 proteins were reduced in G93A BMSCs (Fig. 3H). No significant changes in total Akt, Erk1/2, p38, and phospho-p38 proteins were observed in G93A relative to WT BMSCs (Fig. 3H). Interestingly, the level of β-catenin, which is required for mesenchymal stem cell differentiation toward the osteoblastic lineage and bone acquisition (52–54), was drastically reduced in G93A BMSCs (Fig. 3H).
Osteoclast Formation and Bone Resorption Are Strikingly Enhanced in Primary Bone Marrow Monocyte Cultures and Bones of 4-Month-old G93A Mice
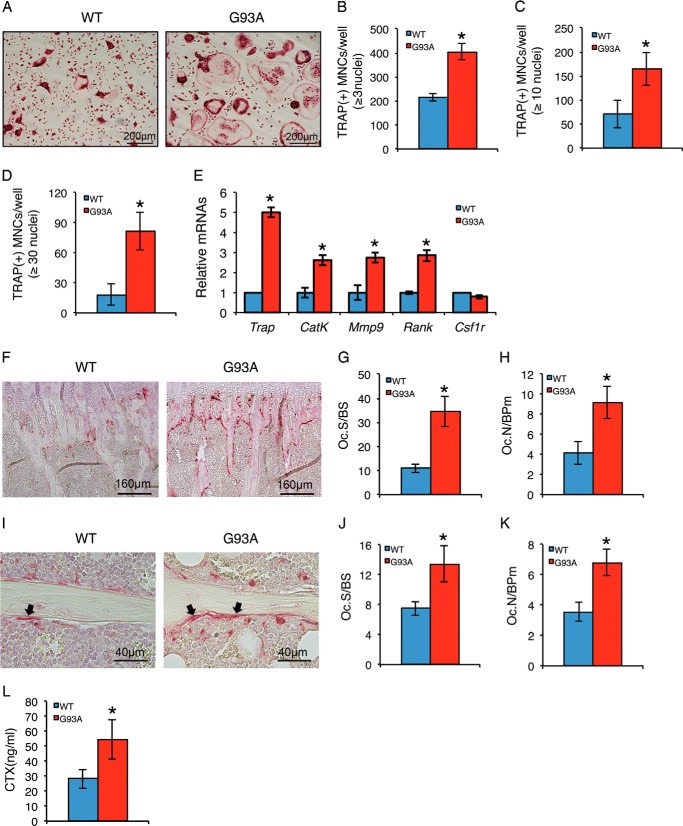
We next evaluated osteoclast formation after the addition of exogenous RANKL to WT and G93A BMMs in vitro by measuring the number of TRAP-positive MNCs generated by each genotype. The results showed a significant increase in TRAP-positive MNCs in G93A BMMs relative to BMM cultures (Fig. 4, A and B). Osteoclasts formed in G93A BMM cultures were considerably larger than those in WT BMM cultures, as demonstrated by dramatic increases in MNCs with >10 (Fig. 4C) and 30 nuclei per osteoclast (Fig. 4D) in G93A cultures. RT-qPCR analysis revealed significant increases in the expression of osteoclast differentiation marker genes, including those encoding TRAP (Trap), cathepsin K (CatK), matrix metallopeptidase 9 (Mmp-9), and receptor activator of nuclear factor κB (Rank) in G93A cultures (Fig. 4E). In contrast, the mRNA level of colony stimulating factor 1 receptor (Csf1r), an early marker for osteoclast precursors and macrophages, was not increased in G93A BMM cultures (Fig. 4E).
FIGURE 4.
Osteoclast formation is enhanced in primary BMM cultures and in bones of 4-month-old G93A mice with muscle atrophy. A–D, in vitro osteoclast formation. Primary BMMs isolated from 4-month-old wild type (WT) and G93A female mice were differentiated with macrophage colony stimulating factor (M-CSF) (10 ng/ml) and RANKL (50 ng/ml) for 5 days followed by TRAP staining. TRAP-positive MNCs (B, ≥3 nuclei; C, ≥10 nuclei; D, ≥30 nuclei) per well were scored (B–D). *, p < 0.05 (versus WT). Experiments were repeated three times with six samples per group. E, RT-qPCR analysis. Primary BMMs were differentiated for 5 days followed by RT-qPCR analysis using primers for Trap, Cat K, Mmp9, Rank, and Csf1r. Gapdh mRNA was used as internal control. *, p < 0.05 (versus WT). Experiments were repeated three times in quadruplicate. F–K, in vivo osteoclast formation. Tibial sections of 4-month-old WT and G93A female mice were stained for TRAP activity. Arrowheads indicate osteoclasts on trabecular surfaces. Quantitative data of Oc.S/BS (G and J) and Oc.N/BPm (H and K) in primary (F–H) and secondary (I–K) spongiosa. *, p < 0.05 (versus WT), n = 7. L, serum C-terminal telopeptide of type 1 collagen (CTX) assay. Serum CTX levels were assayed. *, p < 0.05 (versus WT), n = 7. CatK, cathepsin K; Mmp9, matrix metallopeptidase 9; Rank, receptor activator of nuclear factor κB; Csf1r, colony stimulating factor 1 receptor.
We next analyzed whether osteoclast formation was increased in the bones of G93A mice. Sections of tibiae from 4-month-old WT and G93A mice were subjected to TRAP staining. The results showed that in vivo osteoclast formation was strikingly increased in G93A bones with dramatic increases in Oc.S/BS and Oc.N/BPm in both primary (Fig. 4, F–H) and secondary (Fig. 4, I–K) spongiosa areas. The level of serum C-terminal telopeptide of type 1 collagen (CTX) was significantly elevated in G93A mice relative to WT mice (Fig. 4L), suggesting increased bone resorption in G93A mice.
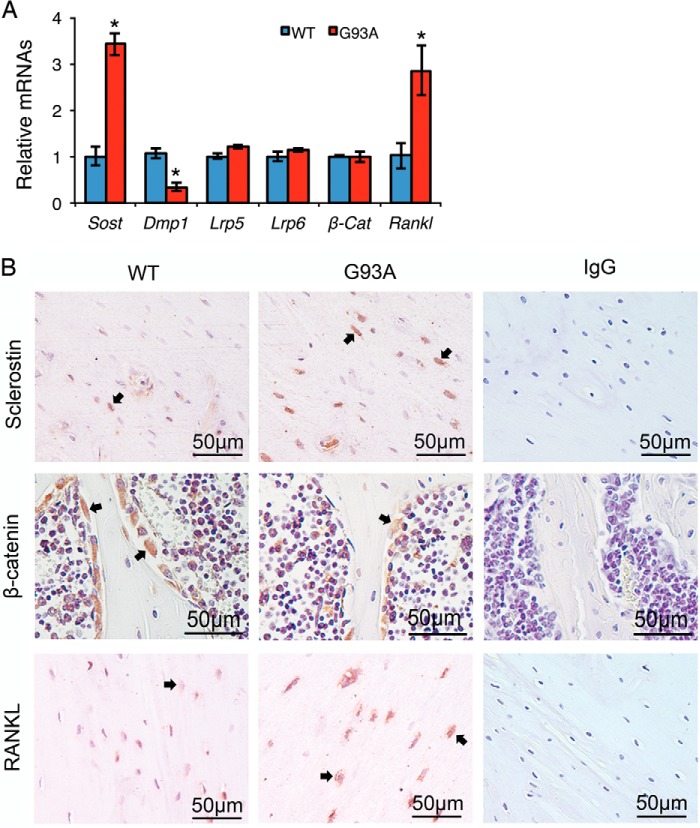
Four-month-old G93A Mice Show Dramatic Increases in Sclerostin and RANKL Expression in Osteocytes Embedded in the Bone Matrix and a Decrease in β-Catenin Expression in Osteoblasts on Bone Surfaces
Osteocyte-derived sclerostin, which is encoded by the Sost gene (27), has been reported to mediate the mechanical unloading induction of bone loss by inhibiting Wnt/β-catenin signaling, a key pathway for bone acquisition (12, 28, 29). We examined the expression of sclerostin and Wnt/β-catenin signaling molecules in G93A and WT bones. RT-qPCR analysis showed a significant increase of sclerostin mRNA in G93A compared with WT bones (Fig. 5A). In contrast, the mRNA levels of β-catenin and the upstream Lrp5 and Lrp6 Wnt coreceptors were not significantly altered in G93A compared with WT bones (Fig. 5A). In addition, the expression of the Dmp-1 (dentin matrix protein-1) gene, a marker gene for osteocytes, was reduced in G93A bones relative to WT bones, suggesting an overall impairment of osteocyte function in G93A mice (Fig. 5A). IHC staining of tibial sections from the two genotypes revealed an increase in sclerostin protein in osteocytes embedded in the bone matrix (Fig. 5B, top) and a decrease in β-catenin in osteoblasts located on trabecular bone surfaces (Fig. 5B, middle) in G93A mice. It should be noted that the β-catenin signal per osteoblast was markedly reduced in G93A compared with WT bones. Furthermore, the levels of RANKL mRNA and protein, a major osteoclastogenic factor, were increased in G93A bones (Fig. 5A) and osteocytes (Fig. 5B, bottom) relative to WT bones and osteocytes.
FIGURE 5.
Sclerostin and RANKL expression in osteocytes in the bone matrix are up-regulated and β-catenin expression in osteoblasts on bone surfaces is down-regulated in 4-month-old G93A mice with muscle atrophy. A, RT-qPCR analysis. Total RNA isolated from 4-month-old WT and G93A female tibiae were subjected to RT-qPCR analysis using primers for Sost, Lrp5, Lrp6, Dmp-1, Rankl, and β-Cat. Gapdh mRNA was used as internal control. *, p < 0.05 (versus WT). Experiments were repeated three times in quadruplicate. B, immunohistochemistry. 5-μm tibial sections from 4-month-old WT and G93A female mice were stained with antibodies against sclerostin (top), β-catenin (middle), and RANKL (bottom) or a control IgG (left panels). Sclerostin-, β-catenin-, and RANKL-positive cells were stained brown (see arrowheads), and negative cells were stained blue. Note: transverse tibial sections (top and bottom) were used to show sclerostin and RANKL expression in osteocytes embedded in the bone matrix, and longitudinal tibial sections (middle) were used to display β-catenin expression in osteoblasts on trabecular bone surfaces. Sost, sclerostin; Lrp5, low density lipoprotein receptor-related protein 5; Dmp-1, dentin matrix acidic phosphoprotein 1; RANKL, receptor activator of nuclear factor κB ligand; β-Cat, β-catenin.
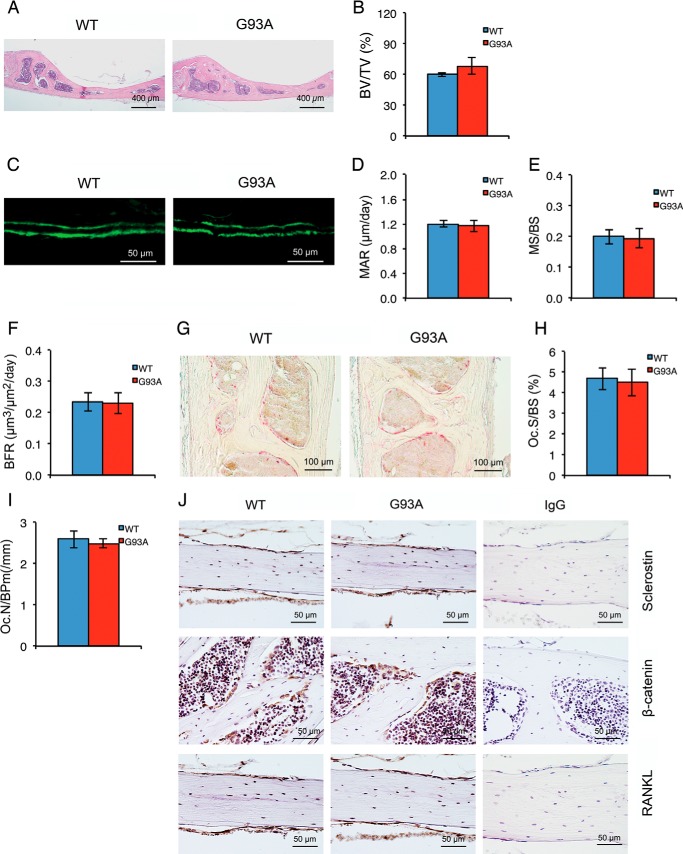
Calvarial Bone Is Not Affected in Four-month-old G93A Mice with Muscle Atrophy
We next sought to determine whether the calvarial bone that is not close to muscle is affected in 4-month-old G93A mice. In contrast to results from long bones, the calvarial BV/TV was not reduced in 4-month-old G93A mice compared with WT mice (Fig. 6, A and B). Similarly, MAR, MS/BS, and BFR of calvariae were not significantly reduced in 4-month-old G93A mice compared with WT mice (Fig. 6, C–F). Furthermore, osteoclast formation in calvarial bone was not increased in 4-month-old G93A mice (Fig. 6, G–I). Finally, the expression of Sost, RANKL, and β-catenin was not markedly altered in 4-month-old G93A versus WT calvariae (Fig. 6J).
FIGURE 6.
Calvarial bone is not affected in four-month-old G93A mice with severe muscle atrophy. A and B, H&E staining. Sections of calvarial bones from 4-month-old G93A and WT female mice were subjected to H&E staining. BV/TV was measured (B). *, p < 0.01 (versus WT), n = 9 for both WT and G93A. C–F, MAR, MS/BS, and BFR assays. Sections of non-demineralized calvariae of the two genotypes were used for MAR, MS/BS, and BFR assays. Magnification: 40×. Quantitative MAR, MS/BS, and BFR data (D–F). p > 0.05 (versus WT), n = 9 for both WT and G93A. G–I, TRAP staining. Calvarial sections of 4-month-old WT and G93A female mice were stained for TRAP activity. Quantitative data of Oc.S/BS and Oc.N/BPm (I) are shown. p > 0.05 (versus WT), n = 7. J, immunohistochemistry. 5-μm calvarial sections from 4-month-old WT and G93A female mice were stained with antibodies against sclerostin (top), β-catenin (middle), and RANKL (bottom) or a control IgG (left panels). Sclerostin-, β-catenin-, and RANKL-positive cells were stained brown, and negative cells were stained blue.
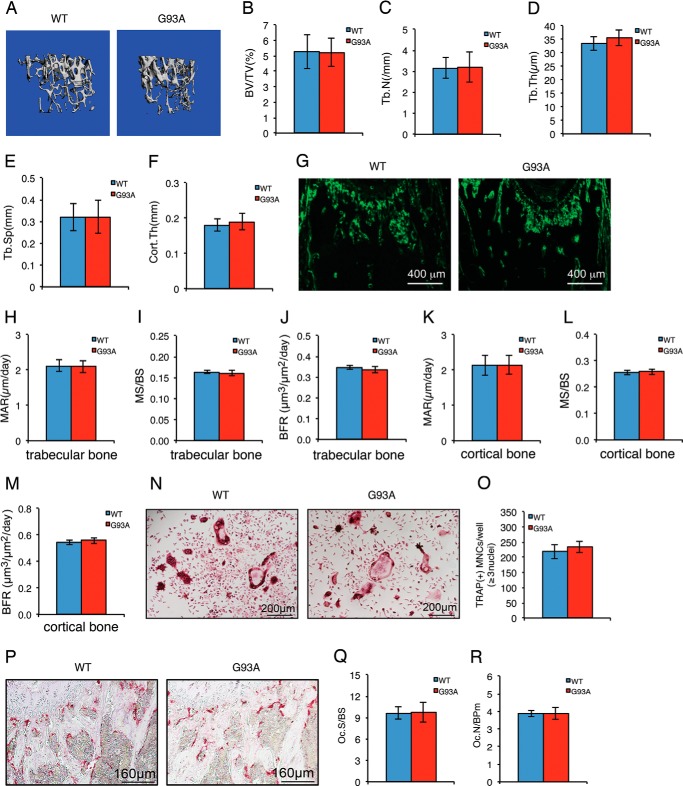
Two-month-old G93A Mice without Muscle Atrophy Have Normal Bone Mass with No Detectable Impairment in Osteoblast and Osteoclast Functions
We sought to determine whether the skeletal impairment occurred before muscle atrophy by analyzing the skeletal phenotypes of 2-month-old G93A female mice that did not show any muscle atrophy. μCT analyses of femurs of these mice revealed no significant alterations in BV/TV, Tb.N, Tb.Th, Tb.Sp, or Cort.Th compared with their sex-matched WT littermates (Fig. 7, A–F). There were no significant changes in MAR, MS/BS, and BFR in both metaphyseal trabecular bones and diaphyseal cortical bones in 2-month-old G93A mice (Fig. 7, G–M). In vitro osteoclast formation induced by exogenously supplied recombinant human RANKL in primary BMM cultures from 2-month-old G93A mice was not different from that of WT BMM cultures (Fig. 7, N and Q). Finally, in vivo osteoclast formation was not enhanced in 2-month-old G93A bones (Fig. 7, P–Q). Collectively, these results indicate that both osteoblast and osteoclast functions are intact in 2-month-old G93A mice without muscle atrophy.
FIGURE 7.
Two-month-old G93A mice without muscle atrophy have normal bone mass with no detectable changes in parameters for osteoblast and osteoclast function. A, three-dimensional reconstruction from μCT scans of femurs from 2-month-old G93A and WT female mice. B–F, quantitative analyses of BV/TV, Tb.Th, Tb.Sp, and Cort.Th. G–M, MAR, MS/BS, and BFR assays. Sections of undecalcified femurs of 2-month-old G93A (n = 8) and WT (n = 8) female mice were used for MAR, MS/BS, and BFR assays. Quantitative data of MAR, MS/BS, and BFR for metaphyseal trabecular bones (H–J) and diaphyseal cortical bones (K–M). Magnification: 40×. N and O, in vitro osteoclast formation. Primary BMMs from 2-month-old WT and G93A female mice were differentiated for 5 days followed by TRAP staining. TRAP-positive MNCs (≥3 nuclei) per well were scored (K). Experiments were repeated three times with six samples per group. P–R, in vivo osteoclast formation. Representative TRAP staining images of tibial sections of 2-month-old G93A and WT female mice (P) are shown. Quantitative analyses of Oc.S/BS and Oc.N/BPm (R) of secondary spongiosa of tibiae are shown. n = 8.
DISCUSSION
This study is the first systematic investigation of skeletal phenotypes during the progression of muscle atrophy using a well-established ALS mouse model with an ALS-causing mutation in the gene encoding superoxide dismutase 1 (SOD1G93A). We have discovered severe bone defects in the G93A ALS mice. We found that 4-month-old G93A ALS mice with severe muscle atrophy have reduced bone mass associated with accelerated osteoclast formation and bone resorption and suppressed osteoblast function and bone formation. We further found that 2-month-old G93A ALS mice without muscle atrophy have normal bone mass compared with WT control mice. These findings suggest that abnormal bone remodeling causes dramatic bone loss during the progression of muscle atrophy in ALS.
One important finding of the present study is our demonstration of multiple severe defects in osteoblasts and their precursors in the G93A ALS mice with muscle atrophy. The formation and expansion of mesenchymal stem cells and osteoprogenitors are impaired in G93A mice; this could eventually reduce the numbers of osteoblasts and suppress bone formation. Osteoblast differentiation is also suppressed in G93A mice. Several potential molecular mechanisms may be involved in the inhibition of osteoblast function and bone formation in G93A mice. First, the defective activation of the AKT pathway could impair osteoblast survival, proliferation, and differentiation (55). Second, the reduced β-catenin in BMSCs and osteoblasts in G93A mice could inhibit mesenchymal stem cell differentiation toward the osteoblast lineage as well as bone acquisition (52–54). Finally, the defect in the activation of the ERK/MAPK pathway in G93A BMSCs could negatively impact Runx2 activity and osteoblast differentiation (56–58). Together, these defects would contribute greatly to the reduced bone formation observed in ALS mice.
Another important finding from this study is the demonstration that osteoclast formation and bone resorption are strikingly enhanced in G93A mice with muscle atrophy. In vitro osteoclast formation induced by exogenously supplied recombinant human RANKL protein is stimulated in primary G93A BMM cultures, which suggests intrinsic activation of the osteoclast differentiation program in G93A mice. In addition, increased RANKL expression in G93A osteocytes (Fig. 5B), which was recently shown to be a major source of RANKL and to play a critical role in promotion of bone resorption (59, 60), may partially contribute to the accelerated osteoclast formation and bone resorption in G93A mice. Interestingly, Rufo et al. (34) reported a similar acceleration of osteoclast formation and bone resorption in dystrophic MDX mice with muscle atrophy. Furthermore, Warner et al. (61) showed that muscle paralysis in mice induced by administration of botulinum toxin A (Botox) rapidly degrades bone primarily by bone resorption. Collectively, these studies suggest that muscle-generated mechanical force may play a critical role in inhibition of osteoclast formation and bone resorption.
Previous studies have demonstrated that osteocyte-derived sclerostin binds to LRP5/6 receptors and inhibits Wnt signaling and, thereby, bone formation (28, 62). Furthermore, mice with a loss-of-function mutation in Lrp5 failed to respond anabolically to mechanical stimulation (63). In contrast, mechanical loading using a rodent ulna loading model was shown to reduce sclerostin expression, particularly in high strain regions of the bone (12). Results from our study suggest that sclerostin induced by muscle atrophy in osteocytes inhibits osteoblast function and bone formation, probably by down-regulating the Wnt/β-catenin pathway in ALS mice.
Although results from the present study strongly support our hypothesis that skeletal unloading caused by muscle atrophy plays a critical role in mediation of ALS-induced bone loss, there are several other possibilities that could contribute to the skeletal phenotypes of the mutant mice. First, there could be a direct effect of the mutant SOD1G93A protein expression on bone cells. A recent study showed that cytoplasmic reactive oxygen species and SOD1 regulate bone mass during mechanical unloading (64). It will be interesting to determine the direct effect of bone-specific transgenic overexpression of SOD1G93A mutant protein on bone in the future. Second, the reduced production of muscle-derived osteogenic factors, such as FGF-2 and IGF-1 (30, 31), and third, the lack of physical activity and/or altered feeding habits of the mutant mice, could negatively impact bone homeostasis. The fact that calvarial bone, which is not in close proximity to muscle, is not affected in 4-month-old ALS mice suggests that bone loss in ALS mice is not caused by a paracrine or systemic defect. This study provides guidance for future studies to identify potential therapeutic targets for alleviating muscle atrophy and bone loss in ALS.
This work was supported, in whole or in part, by National Institutes of Health Grants AR059647 (to G. X.), GM65188 (to C. W.), and AR057404 (to J. Z.). This work was in part supported by National Natural Science Foundation of China Grant 81472049 (to G. X.) and Muscular Dystrophy Association Research Grant MDA-4351 (to J. Z.).
- ALS
- amyotrophic lateral sclerosis
- SOD1
- superoxide dismutase 1
- μCT
- micro-computerized tomography
- BV/TV
- bone volume fraction/total tissue volume
- Tb.N
- trabecular number
- Tb.Th
- trabecular thickness
- Tb.Sp
- trabecular spacing
- Cort.Th
- cortical thickness
- IHC
- immunohistochemical
- Osx
- osterix
- Ocn
- osteocalcin
- MAR
- mineralization apposition rate
- MS/BS
- mineralizing surface per bone surface
- BFR
- bone formation rate
- CFU-F
- colony-forming unit-fibroblast
- CFU-OB
- colony-forming unit-osteoblast
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- BMSC
- bone marrow stromal cell
- RT-qPCR
- quantitative real-time RT-PCR
- TRAP
- tartrate-resistant acid phosphatase
- MNC
- multinucleated cells
- Oc.S/BS
- osteoclast surface/bone surface
- Oc.N/BPm
- osteoclast numbers/bone perimeter
- CTX
- C-terminal telopeptide of type 1 collagen
- BMM
- bone marrow-derived macrophage.
REFERENCES
- 1. Pasinelli P., Brown R. H. (2006) Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat. Rev. Neurosci. 7, 710–723 [DOI] [PubMed] [Google Scholar]
- 2. Borchelt D. R., Lee M. K., Slunt H. S., Guarnieri M., Xu Z. S., Wong P. C., Brown R. H., Jr., Price D. L., Sisodia S. S., Cleveland D. W. (1994) Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc. Natl. Acad. Sci. U.S.A. 91, 8292–8296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. (1993) Mutations in Cu2+/Zn2+ superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 [DOI] [PubMed] [Google Scholar]
- 4. McGoldrick P., Joyce P. I., Fisher E. M., Greensmith L. (2013) Rodent models of amyotrophic lateral sclerosis. Biochim. Biophys. Acta 1832, 1421–1436 [DOI] [PubMed] [Google Scholar]
- 5. Kaji H. (2014) Interaction between muscle and bone. J. Bone Metab. 21, 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lebrasseur N. K., Achenbach S. J., Melton L. J., 3rd, Amin S., Khosla S. (2012) Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. J. Bone Miner. Res. 27, 2159–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edwards M. H., Gregson C. L., Patel H. P., Jameson K. A., Harvey N. C., Sayer A. A., Dennison E. M., Cooper C. (2013) Muscle size, strength, and physical performance and their associations with bone structure in the Hertfordshire Cohort Study. J. Bone Miner. Res. 28, 2295–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamrick M. (2010) JMNI special issue: basic science and mechanisms of muscle-bone interactions. J. Musculoskelet. Neuronal Interact. 10, 1–2 [PubMed] [Google Scholar]
- 9. Bonewald L. F., Kiel D. P., Clemens T. L., Esser K., Orwoll E. S., O'Keefe R. J., Fielding R. A. (2013) Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop. J. Bone Miner. Res. 28, 1857–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lloyd S. A., Lang C. H., Zhang Y., Paul E. M., Laufenberg L. J., Lewis G. S., Donahue H. J. (2014) Interdependence of muscle atrophy and bone loss induced by mechanical unloading. J. Bone Miner. Res. 29, 1118–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ausk B. J., Huber P., Srinivasan S., Bain S. D., Kwon R. Y., McNamara E. A., Poliachik S. L., Sybrowsky C. L., Gross T. S. (2013) Metaphyseal and diaphyseal bone loss in the tibia following transient muscle paralysis are spatiotemporally distinct resorption events. Bone 57, 413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robling A. G., Niziolek P. J., Baldridge L. A., Condon K. W., Allen M. R., Alam I., Mantila S. M., Gluhak-Heinrich J., Bellido T. M., Harris S. E., Turner C. H. (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 283, 5866–5875 [DOI] [PubMed] [Google Scholar]
- 13. Bikle D. D., Sakata T., Halloran B. P. (2003) The impact of skeletal unloading on bone formation. Gravit. Space Biol. Bull. 16, 45–54 [PubMed] [Google Scholar]
- 14. Shackelford L. C., LeBlanc A. D., Driscoll T. B., Evans H. J., Rianon N. J., Smith S. M., Spector E., Feeback D. L., Lai D. (2004) Resistance exercise as a countermeasure to disuse-induced bone loss. J. Appl. Physiol. 97, 119–129 [DOI] [PubMed] [Google Scholar]
- 15. Sato K., Adachi T., Ueda D., Hojo M., Tomita Y. (2007) Measurement of local strain on cell membrane at initiation point of calcium signaling response to applied mechanical stimulus in osteoblastic cells. J. Biomech. 40, 1246–1255 [DOI] [PubMed] [Google Scholar]
- 16. Jansen J. H., Weyts F. A., Westbroek I., Jahr H., Chiba H., Pols H. A., Verhaar J. A., van Leeuwen J. P., Weinans H. (2004) Stretch-induced phosphorylation of ERK1/2 depends on differentiation stage of osteoblasts. J. Cell. Biochem. 93, 542–551 [DOI] [PubMed] [Google Scholar]
- 17. Javaheri B., Stern A. R., Lara N., Dallas M., Zhao H., Liu Y., Bonewald L. F., Johnson M. L. (2014) Deletion of a single β-catenin allele in osteocytes abolishes the bone anabolic response to loading. J. Bone Miner. Res. 29, 705–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bivi N., Pacheco-Costa R., Brun L. R., Murphy T. R., Farlow N. R., Robling A. G., Bellido T., Plotkin L. I. (2013) Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J. Orthop. Res. 31, 1075–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao L., Shim J. W., Dodge T. R., Robling A. G., Yokota H. (2013) Inactivation of Lrp5 in osteocytes reduces young's modulus and responsiveness to the mechanical loading. Bone 54, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tu X., Rhee Y., Condon K. W., Bivi N., Allen M. R., Dwyer D., Stolina M., Turner C. H., Robling A. G., Plotkin L. I., Bellido T. (2012) Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 50, 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galli C., Passeri G., Macaluso G. M. (2010) Osteocytes and WNT: the mechanical control of bone formation. J. Dent. Res. 89, 331–343 [DOI] [PubMed] [Google Scholar]
- 22. Kostenuik P. J., Halloran B. P., Morey-Holton E. R., Bikle D. D. (1997) Skeletal unloading inhibits the in vitro proliferation and differentiation of rat osteoprogenitor cells. Am. J. Physiol. 273, E1133–E1139 [DOI] [PubMed] [Google Scholar]
- 23. Ishijima M., Rittling S. R., Yamashita T., Tsuji K., Kurosawa H., Nifuji A., Denhardt D. T., Noda M. (2001) Enhancement of osteoclastic bone resorption and suppression of osteoblastic bone formation in response to reduced mechanical stress do not occur in the absence of osteopontin. J. Exp. Med. 193, 399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kondo H., Nifuji A., Takeda S., Ezura Y., Rittling S. R., Denhardt D. T., Nakashima K., Karsenty G., Noda M. (2005) Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss via sympathetic nervous system. J. Biol. Chem. 280, 30192–30200 [DOI] [PubMed] [Google Scholar]
- 25. Lin C., Jiang X., Dai Z., Guo X., Weng T., Wang J., Li Y., Feng G., Gao X., He L. (2009) Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/β-catenin signaling. J. Bone Miner. Res. 24, 1651–1661 [DOI] [PubMed] [Google Scholar]
- 26. Di Monaco M., Vallero F., Di Monaco R., Tappero R. (2011) Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch. Gerontol. Geriatr. 52, 71–74 [DOI] [PubMed] [Google Scholar]
- 27. Brunkow M. E., Gardner J. C., Van Ness J., Paeper B. W., Kovacevich B. R., Proll S., Skonier J. E., Zhao L., Sabo P. J., Fu Y., Alisch R. S., Gillett L., Colbert T., Tacconi P., Galas D., Hamersma H., Beighton P., Mulligan J. (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 68, 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li X., Zhang Y., Kang H., Liu W., Liu P., Zhang J., Harris S. E., Wu D. (2005) Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 280, 19883–19887 [DOI] [PubMed] [Google Scholar]
- 29. ten Dijke P., Krause C., de Gorter D. J., Lowik C. W., van Bezooijen R. L. (2008) Osteocyte-derived sclerostin inhibits bone formation: its role in bone morphogenetic protein and Wnt signaling. J. Bone Joint Surg. Am. 90, 31–35 [DOI] [PubMed] [Google Scholar]
- 30. Lang T. F. (2011) The bone-muscle relationship in men and women. J. Osteoporos 2011, 702735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hamrick M. W., McNeil P. L., Patterson S. L. (2010) Role of muscle-derived growth factors in bone formation. J. Musculoskelet. Neuronal Interact. 10, 64–70 [PMC free article] [PubMed] [Google Scholar]
- 32. Lee J. Y., Qu-Petersen Z., Cao B., Kimura S., Jankowski R., Cummins J., Usas A., Gates C., Robbins P., Wernig A., Huard J. (2000) Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J. Cell Biol. 150, 1085–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Südkamp N. P., Haas N. P., Sinnig M., Sottmann G., Tscherne H. (1993) Incidence of pseudarthroses in open fractures: analysis of 948 open fractures. Aktuelle Traumatol. 23, 59–67 [PubMed] [Google Scholar]
- 34. Rufo A., Del Fattore A., Capulli M., Carvello F., De Pasquale L., Ferrari S., Pierroz D., Morandi L., De Simone M., Rucci N., Bertini E., Bianchi M. L., De Benedetti F., Teti A. (2011) Mechanisms inducing low bone density in Duchenne muscular dystrophy in mice and humans. J. Bone Miner. Res. 26, 1891–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sbrocchi A. M., Rauch F., Jacob P., McCormick A., McMillan H. J., Matzinger M. A., Ward L. M. (2012) The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporos. Int. 23, 2703–2711 [DOI] [PubMed] [Google Scholar]
- 36. Vestergaard P., Glerup H., Steffensen B. F., Rejnmark L., Rahbek J., Moseklide L. (2001) Fracture risk in patients with muscular dystrophy and spinal muscular atrophy. J. Rehabil. Med. 33, 150–155 [PubMed] [Google Scholar]
- 37. Larson C. M., Henderson R. C. (2000) Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J. pediatr. Orthop. 20, 71–74 [PubMed] [Google Scholar]
- 38. Khatri I. A., Chaudhry U. S., Seikaly M. G., Browne R. H., Iannaccone S. T. (2008) Low bone mineral density in spinal muscular atrophy. J. Clin. Neuromuscul. Dis. 10, 11–17 [DOI] [PubMed] [Google Scholar]
- 39. Zhou J., Yi J., Fu R., Liu E., Siddique T., Ríos E., Deng H. X. (2010) Hyperactive intracellular calcium signaling associated with localized mitochondrial defects in skeletal muscle of an animal model of amyotrophic lateral sclerosis. J. Biol. Chem. 285, 705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X. (1994) Motor neuron degeneration in mice that express a human Cu2+,Zn2+ superoxide dismutase mutation. Science 264, 1772–1775 [DOI] [PubMed] [Google Scholar]
- 41. Bouxsein M. L., Boyd S. K., Christiansen B. A., Guldberg R. E., Jepsen K. J., Müller R. (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 25, 1468–1486 [DOI] [PubMed] [Google Scholar]
- 42. Yu S., Franceschi R. T., Luo M., Fan J., Jiang D., Cao H., Kwon T. G., Lai Y., Zhang J., Patrene K., Hankenson K., Roodman G. D., Xiao G. (2009) Critical role of activating transcription factor 4 in the anabolic actions of parathyroid hormone in bone. PloS ONE 4, e7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu S., Zhu K., Lai Y., Zhao Z., Fan J., Im H. J., Chen D., Xiao G.(2013) ATF4 Promotes β-catenin expression and osteoblastic differentiation of bone marrow mesenchymal stem cells. Int. J. Biol. Sci. 9, 256–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dempster D. W., Compston J. E., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R., Parfitt A. M. (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 28, 2–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xiao G., Cheng H., Cao H., Chen K., Tu Y., Yu S., Jiao H., Yang S., Im H. J., Chen D., Chen J., Wu C. (2012) Critical role of filamin-binding LIM protein 1 (FBLP-1)/migfilin in regulation of bone remodeling. J. Biol. Chem. 287, 21450–21460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang X., Yu S., Galson D. L., Luo M., Fan J., Zhang J., Guan Y., Xiao G. (2008) Activating transcription factor 4 is critical for proliferation and survival in primary bone marrow stromal cells and calvarial osteoblasts. J. Cell. Biochem. 105, 885–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhu K., Jiao H., Li S., Cao H., Galson D. L., Zhao Z., Zhao X., Lai Y., Fan J., Im H. J., Chen D., Xiao G. (2013) ATF4 promotes bone angiogenesis by increasing vegf expression and release in the bone environment. J. Bone Miner. Res. 28, 1870–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cao H., Zhu K., Qiu L., Li S., Niu H., Hao M., Yang S., Zhao Z., Lai Y., Anderson J. L., Fan J., Im H. J., Chen D., Roodman G. D., Xiao G. (2013) Critical role of AKT protein in myeloma-induced osteoclast formation and osteolysis. J. Biol. Chem. 288, 30399–30410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang S., Xu H., Yu S., Cao H., Fan J., Ge C., Fransceschi R. T., Dong H. H., Xiao G. (2011) Foxo1 mediates insulin-like growth factor 1 (IGF1)/insulin regulation of osteocalcin expression by antagonizing Runx2 in osteoblasts. J. Biol. Chem. 286, 19149–19158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cao H., Yu S., Yao Z., Galson D. L., Jiang Y., Zhang X., Fan J., Lu B., Guan Y., Luo M., Lai Y., Zhu Y., Kurihara N., Patrene K., Roodman G. D., Xiao G. (2010) Activating transcription factor 4 regulates osteoclast differentiation in mice. J. Clin. Invest. 120, 2755–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gonzalez de Aguilar J. L., Niederhauser-Wiederkehr C., Halter B., De Tapia M., Di Scala F., Demougin P., Dupuis L., Primig M., Meininger V., Loeffler J. P. (2008) Gene profiling of skeletal muscle in an amyotrophic lateral sclerosis mouse model. Physiol. Genomics 32, 207–218 [DOI] [PubMed] [Google Scholar]
- 52. Day T. F., Guo X., Garrett-Beal L., Yang Y. (2005) Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 8, 739–750 [DOI] [PubMed] [Google Scholar]
- 53. Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 54. Clevers H., Nusse R. (2012) Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 [DOI] [PubMed] [Google Scholar]
- 55. Kawamura N., Kugimiya F., Oshima Y., Ohba S., Ikeda T., Saito T., Shinoda Y., Kawasaki Y., Ogata N., Hoshi K., Akiyama T., Chen W. S., Hay N., Tobe K., Kadowaki T., Azuma Y., Tanaka S., Nakamura K., Chung U. I., Kawaguchi H. (2007) Akt1 in osteoblasts and osteoclasts controls bone remodeling. PloS ONE 2, e1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xiao G., Jiang D., Thomas P., Benson M. D., Guan K., Karsenty G., Franceschi R. T. (2000) MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J. Biol. Chem. 275, 4453–4459 [DOI] [PubMed] [Google Scholar]
- 57. Ge C., Xiao G., Jiang D., Franceschi R. T. (2007) Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 176, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim H. J., Kim J. H., Bae S. C., Choi J. Y., Kim H. J., Ryoo H. M. (2003) The protein kinase C pathway plays a central role in the fibroblast growth factor-stimulated expression and transactivation activity of Runx2. J. Biol. Chem. 278, 319–326 [DOI] [PubMed] [Google Scholar]
- 59. Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J. Q., Bonewald L. F., Kodama T., Wutz A., Wagner E. F., Penninger J. M., Takayanagi H. (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 [DOI] [PubMed] [Google Scholar]
- 60. Xiong J., Onal M., Jilka R. L., Weinstein R. S., Manolagas S. C., O'Brien C. A. (2011) Matrix-embedded cells control osteoclast formation. Nat. Med. 17, 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Warner S. E., Sanford D. A., Becker B. A., Bain S. D., Srinivasan S., Gross T. S. (2006) Botox induced muscle paralysis rapidly degrades bone. Bone 38, 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ellies D. L., Viviano B., McCarthy J., Rey J. P., Itasaki N., Saunders S., Krumlauf R. (2006) Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J. Bone Miner. Res. 21, 1738–1749 [DOI] [PubMed] [Google Scholar]
- 63. Sawakami K., Robling A. G., Ai M., Pitner N. D., Liu D., Warden S. J., Li J., Maye P., Rowe D. W., Duncan R. L., Warman M. L., Turner C. H. (2006) The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J. Biol. Chem. 281, 23698–23711 [DOI] [PubMed] [Google Scholar]
- 64. Morikawa D., Nojiri H., Saita Y., Kobayashi K., Watanabe K., Ozawa Y., Koike M., Asou Y., Takaku T., Kaneko K., Shimizu T. (2013) Cytoplasmic reactive oxygen species and SOD1 regulate bone mass during mechanical unloading. J. Bone Miner. Res. 28, 2368–2380 [DOI] [PubMed] [Google Scholar]