Abstract
Long before its chemical identity was known, the phytohormone auxin was postulated to regulate plant growth. In the late 1800s, Sachs hypothesized that plant growth regulators, present in small amounts, move differentially throughout the plant to regulate growth. Concurrently, Charles Darwin and Francis Darwin were discovering that light and gravity were perceived by the tips of shoots and roots and that the stimulus was transmitted to other tissues, which underwent a growth response. These ideas were improved upon by Boysen-Jensen and Paál and were later developed into the Cholodny–Went hypothesis that tropisms were caused by the asymmetric distribution of a growth-promoting substance. These observations led to many efforts to identify this elusive growth-promoting substance, which we now know as auxin. In this review of auxin field advances over the past century, we start with a seminal paper by Kenneth Thimann and Charles Schneider titled “The relative activities of different auxins” from the American Journal of Botany, in which they compare the growth altering properties of several auxinic compounds. From this point, we explore the modern molecular understanding of auxin—including its biosynthesis, transport, and perception. Finally, we end this review with a discussion of outstanding questions and future directions in the auxin field. Over the past 100 yr, much of our progress in understanding auxin biology has relied on the steady and collective advance of the field of auxin researchers; we expect that the next 100 yr of auxin research will likewise make many exciting advances.
Keywords: auxin, auxin history, metabolism, signaling, transport
This American Journal of Botany centennial review considers the history of auxin, with a focus on an AJB classic paper about auxin activity by Kenneth Thimann and Charles Schneider titled “The relative activities of different auxins” (Thimann and Schneider, 1939). Since the publication of this seminal paper in 1939, many of its reported observations can be explained using the knowledge gained about auxin biology in the last 75 yr. In this review, we describe the history of the auxin field, discuss a significant study published in the American Journal of Botany, summarize what we have learned in more recent years about auxin biology, and conclude with a discussion of unanswered questions in the field. We are hopeful that the next 100 yr of auxin research will reveal answers to these questions.
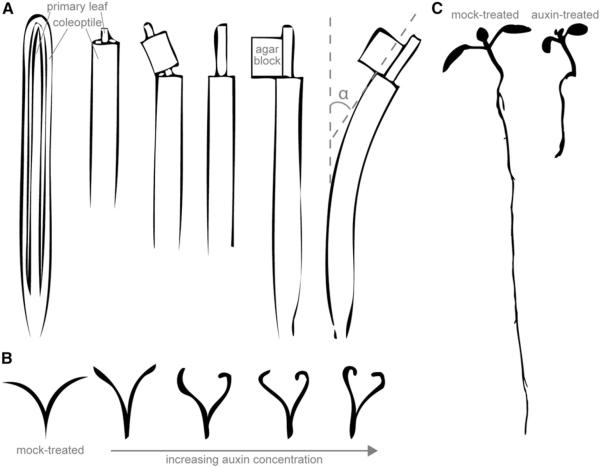
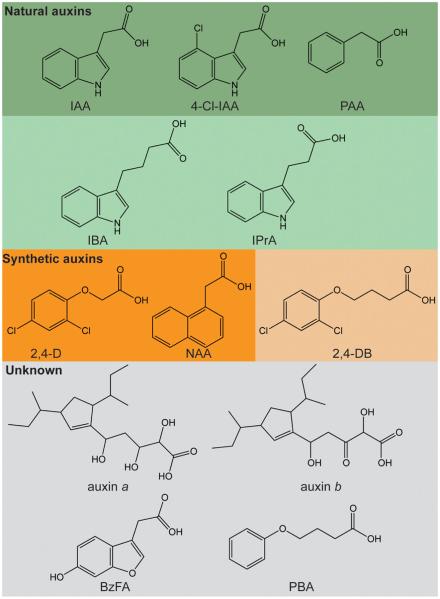
The term “auxin” is derived from the Greek word “auxein” meaning “to grow”. Discovery of auxinic compounds (both naturally occurring and synthetic auxins) was facilitated by the Avena test (Fig. 1A) developed by Went (1928). The Avena test consists of placing an agar block containing test compounds on one side of excised Avena sativa coleoptile tips and measuring the ensuing curvature (reviewed in Went and Thimann, 1937). Thus, the classic defining feature of auxinic compounds is elicitation of a physiological response in the Avena test. The first compounds to be considered auxins were auxin a (auxenotriolic acid) and auxin b (auxenolonic acid) (Kögl et al., 1933, 1934a, b). However, as time went on, many laboratories were unable to isolate these compounds and they fell out of favor as potential auxins (reviewed in Went and Thimann, 1937). For a modern critique of the history of auxin a and auxin b, we recommend Wildman (1997). Indole-3-acetic acid (IAA or heteroauxin) was later favored as the strongest auxin because of its potency as a plant growth regulator; many additional compounds (Fig. 2) were also being used as auxins, including indole-3-butyric acid (IBA), indole-3-propionic acid (IPrA), and naphthalene acetic acid (NAA), among others.
Fig. 1.
Auxin response assays. (A) The Avena test. Compounds are tested for auxin activity in Avena sativa seedlings mounted on a test apparatus, and the tip of the coleoptile removed. After a few hours of growth, a larger portion of the coleoptile is removed, and the primary leaf pulled upward to detach it from the base. A block of agar containing the compound to be tested is placed on one side of the cut surface. After incubation, the curvature induced by the diffusion and transport of auxin from the agar block into the coleoptile of the Avena seedling is measured. Image modified from Went and Thimann (1937). (B) The Pisum test. Compounds are tested for auxin activity by removing the top of 7-d-old Pisum sativum seedlings below the terminal bud and the stem split lengthwise for 3 cm. The split stem section is excised a few millimeters below the split and split stems incubated in solutions containing compounds to be tested. After incubation, the curvature angles induced from the auxins causing cell expansion on one side of the coleoptile are measured. Image modified from Went and Thimann (1937). (C) The Arabidopsis root elongation test. Compounds are tested for auxin activity by plating sterilized seed of Arabidopsis thaliana on agar-solidified media containing compounds of interest. After 7–10 d of growth, seedling root lengths are measured.
Fig. 2.
Structures of naturally occurring and synthetic auxins. Auxins found in plants include the active auxins indole-3-acetic acid (IAA), 4-Cl-IAA, and phenylacetic acid (PAA), as well as the inactive auxin precursors indole-3-butyric acid (IBA), and indole-3-propionic acid (IPrA). Synthetic auxins include the active 2,4-dichlorophenoxyacetic acid (2,4-D) and naphthalene acetic acid (NAA), and the inactive precursor 2,4-dichlorophenoxybutyric acid (2,4-DB). The occurrence of auxin a (auxenotriolic acid), auxin b (auxenolonic acid), benzofurane-3-acetic acid (BzFA), and phenyl-butyric acid (PBA) in plants or other organisms has been debated or is unknown.
Although IAA was recognized for its effective ability to induce cell expansion in plants, it was first isolated, not from plants, but from human urine (Kögl et al., 1934b), yeast (Kögl and Kostermans, 1934), and Rhizopus suinus (Thimann, 1935). Although IAA was the standard to which other auxin compounds were compared in Thimann and Schneider (1939), at the time of their study, IAA was not believed to be a native plant hormone. The elusive auxin a was thought to be the auxin present in Vicia faba, Zea mays, and Avena sativa (reviewed in Went and Thimann, 1937), whereas IAA was believed to be the natural auxin in fungal species such as Aspergillus, Rhizopus, and yeast (reviewed in Went and Thimann, 1937). Indeed, IAA was not isolated from higher plants until the early 1940s (Haagen-Smit et al., 1941). During this time, additional compounds with auxin activity were also discovered, either through chemical synthesis or extraction from biological sources. Structure–activity studies were beginning to emerge, and it was determined that a ring system containing a double bond as the nucleus of a molecule, a side chain, a carboxyl group, and a particular space relationship were necessary for cell elongation in the Avena test of auxin activity (Koepfli et al., 1938).
The burgeoning field of auxin research was littered with opposing opinions on the mechanism of auxin action and what compounds were considered auxins. However, the field eventually settled at least some of these disputes and auxin researchers Went and Thimann (1937, in Phytohormones, p. 5) provided insightful commentary on how such progress was made: “…in regard to the question of priority of the discovery of the auxins. We feel that the gradual unfolding of the current conceptions and the cooperation of different workers has made it impossible to credit any one person with such a discovery, and it is to be hoped that the reader … will gain the impression of a steady and collective advance rather than of individual contributions.”
THIMANN AND SCHNEIDER, 1939
Thimann and Schneider (1939) compared the activities of several compounds that had been recently identified for auxin activity in the Avena assay. This comparison eventually led to structure–activity relationship studies by Thimann's laboratory and others that would reveal the defining characteristics of auxin molecules. The activity of indole-3-acetic acid (IAA), the most investigated auxin at the time of this study, was compared with the activity of six other auxin molecules: indole-3-butyric acid (IBA), naphthalene acetic acid (NAA), indole-3-propionic acid (IPrA), phenyl-acetic acid (PAA or ΦAA), phenyl-butyric acid (PBA or ΦBA), and benzofurane-3-acetic acid (BzFA) (Fig. 2).
Standard auxin assays at this time consisted of either incubating etiolated stem sections of Avena or Pisum coleoptiles in auxin solutions and measuring coleoptile elongation or splitting etiolated Pisum stems and measuring their degree of curvature after incubation in an auxin solution (Fig. 1B). In these assays, Pisum was more sensitive to the auxins than Avena; however, the tested auxins displayed the same relative activity in both species, with slightly different response curves. The major conclusion from Thimann and Schneider's straight growth experiments was that both the absolute activities and the activities relative to IAA depended on the species of plant and that Avena and Pisum did not respond to auxin in a similar way. These findings were contrary to the belief at the time that species may respond to auxins differently, but those differences would always be in the same proportions. The idea that each species may have drastically different sensitivities to different auxins was first demonstrated in this seminal paper.
Many barriers limited early auxin research. For example, Thimann and Schneider recognized that differences in the purity in their auxin stocks may have played a role in the differing auxin activities they observed, a problem that plagued the field at that time. Metabolism of the applied molecules into other compounds within the plant (for example, production of IAA conjugates and oxIAA, conversion of IBA to IAA) was difficult to track. Additionally, molecular genetic differences between species had not yet been uncovered, making understanding of the response differences between Avena and Pisum difficult.
AUXIN RESEARCH AFTER WENT, THIMANN, AND SCHNEIDER
Phytohormones (Went and Thimann, 1937) lays out an excellent snapshot of the knowledge of the auxin field in the late 1930s. However, many important discoveries were made between 1939 and the molecular revolution. Until the 1980s, auxin researchers were limited by the techniques of the time and were restricted to mostly investigating the physiological effects of auxin application. However, as more sophisticated techniques were developed, more mechanistic observations were made, such as the characterization of a two-phase growth response to auxin, the characterization of auxin polar transport, and the contributions of transcription and protein synthesis to auxin response (reviewed by Goldsmith, 1977; Key, 1989; Thimann, 1977). These larger-picture characterizations laid the groundwork for the molecular revolution and provided clues as to what categories of genes could be involved in auxin response.
MODERN-DAY MOLECULAR UNDERSTANDING OF AUXIN ACTION
Our molecular understanding of auxin activity has exploded over the past 20 yr. After the establishment of Arabidopsis as a model organism for plant molecular biology, it quickly became a target of auxin research. The modern Arabidopsis equivalent to the classic Avena (Fig. 1A) and Pisum split stem (Fig. 1B) tests is the measurement of auxin inhibition of root elongation in Arabidopsis (Fig. 1C), based on the inhibitory properties of auxin on root elongation (reviewed by Scott, 1972). Use of Arabidopsis mutant screens based on resistance to the inhibitory effects of auxin on root elongation uncovered many of the factors required for the three main modes of regulation: metabolism, transport, and signaling. In the following three sections, we summarize our current understanding of these three modes of regulation of auxin activity (metabolism, transport, and signaling) and outline possible areas of future studies to gain a deeper understanding of auxin biology.
AUXIN METABOLISM
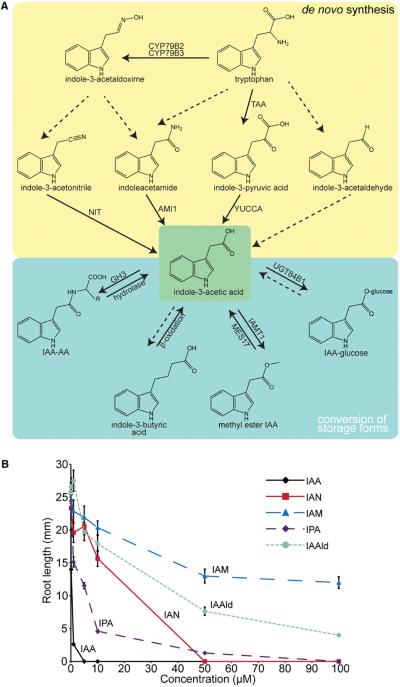
The natural auxin indole-3-acetic acid (IAA) is synthesized through both tryptophan (Trp)-dependent and Trp-independent pathways (reviewed by Korasick et al., 2013; Tivendale et al., 2014). Five major naturally occurring compounds are recognized as IAA precursors, including indole-3-pyruvic acid (IPyA), indole-3-acetaldoxime (IAOx), indole-3-acetonitrile (IAN), indole-3-acetamide (IAM), and indole-3-acetaldehyde (IAAld) (Fig. 3A). The IPyA pathway is considered the “main” auxin biosynthetic pathway (reviewed by Zhao, 2012). Arabidopsis responds differently to these auxin precursors, displaying the greatest sensitivity to IPyA and the least sensitivity to IAM in root elongation assays (Fig. 3B).
Fig. 3.
Auxin biosynthesis and storage forms in higher plants. (A) Possible pathways for plant auxin biosynthesis. Solid arrows indicate those steps for which enzymes are known. Dashed arrows indicate those steps for which no enzyme has been identified or the enzyme identity is in question. (B) Mean primary root lengths (±SE) of 8-d-old Arabidopsis seedlings grown in the presence of the indicated auxin or auxin precursor.
Auxin was hypothesized to be derived from tryptophan as early as the 1930s (reviewed by Went and Thimann, 1937). However, the major mechanism of conversion from tryptophan to IAA remained elusive until relatively recently. The IPyA pathway appears to be the main contributor to IAA (reviewed by Korasick et al., 2013; Tivendale et al., 2014) and is the only completely described pathway to date. The TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS (TAA) family of enzymes converts tryptophan to IPyA (Stepanova et al., 2008; Tao et al., 2008; Yamada et al., 2009), and the YUCCA (YUC) family of enzymes converts IPyA to IAA (Mashiguchi et al., 2011; Stepanova et al., 2011; Won et al., 2011; Dai et al., 2013), creating a simple, two-step conversion of Trp to IAA. The discovery of this two-step IAA biosynthesis pathway caused great excitement within the auxin community because it simplified the possible models for auxin biosynthesis (Fig. 3A).
The IAOx pathway may be restricted to the Brassicaceae (Quittenden et al., 2009; Sugawara et al., 2009). In Arabidopsis, the cytochrome P450 enzymes CYP79B2 and CYP79B3 convert tryptophan to IAOx (Hull et al., 2000; Mikkelsen et al., 2000; Zhao et al., 2002). IAOx is largely used for production of indole glucosinolate defense compounds (Bak et al., 2001; Zhao et al., 2002; Mikkelsen et al., 2004), with only a small portion converted to IAA (Zhao et al., 2002; Sugawara et al., 2009). A minor role for the IAOx pathway in IAA production has been suggested because the cyp79b2 cyp79b3 double mutant has little effect on IAA levels (Ljung et al., 2005; Sugawara et al., 2009). However, hyperaccumulation of IAOx in the superroot1 and superroot2 mutants results in greatly increased levels of IAA (Mikkelsen et al., 2004), suggesting that, at least under some conditions, IAOx has the potential of substantially contributing to the pool of IAA. The cyp79b2 cyp79b3 double mutant displays decreased IAN (Zhao et al., 2002; Sugawara et al., 2009) and IAM (Sugawara et al., 2009) levels, suggesting IAOx can be converted to both IAN and IAM. The enzymatic steps between IAOx and IAN remain unknown at this time; however, it appears that IAN can be converted to IAA through the activity of nitrolases (Schmidt et al., 1996; Normanly et al., 1997).
Indole-3-acetamide (IAM) has been identified as an endogenous auxin precursor in many species throughout the plant kingdom (reviewed by Korasick et al., 2013). In Arabidopsis, IAM may be produced from IAOx (Sugawara et al., 2009). Intriguingly, IAM has been detected in many species in which IAOx has not been detected (reviewed by Korasick et al., 2013), suggesting that IAM can be produced from precursors other than IAOx (Sugawara et al., 2009). Conversion of IAM to IAA may be facilitated by AMIDASE1 (AMI1) (Pollmann et al., 2003).
Our understanding of tryptophan-dependent de novo auxin biosynthesis has been greatly improved by the discovery of the two-step IPyA pathway. However, much less attention has been paid to the tryptophan-independent pathways of IAA biosynthesis. For reviews of Trp-independent auxin biosynthesis, please see Korasick et al. (2013) and Tivendale et al. (2014).
In addition to de novo IAA biosynthesis, many inactive IAA storage forms, including IAA conjugates, methyl ester IAA (MeIAA), and IBA can be rapidly converted to free IAA to regulate auxin homeostasis (reviewed by Korasick et al., 2013). These auxin storage forms are postulated to influence auxin sensitivity, transport, and compartmentalization (Cohen and Bandurski, 1982). Although these storage forms can accumulate to high levels in the plant (Table 1), specific roles for auxin derived from these inactive molecules are only now beginning to be understood. For example, altering IBA-to-IAA conversion results in an array of seedling developmental defects, including defects in cotyledon and root hair cell expansion, apical hook curvature, high-temperature-induced hypocotyl elongation, lateral root production, and root meristem maintenance, without obvious effects on adult plant morphology (Strader et al., 2010, 2011) whereas altering IBA conjugation to glucose alters shoot branching and drought tolerance in adult plant tissues (Tognetti et al., 2010). In addition, blocking conversion of IAA-amino acid conjugates to free IAA results in decreased lateral root production (Rampey et al., 2004), decreased hypocotyl elongation (Rampey et al., 2004), and decreased root hair elongation (Strader et al., 2010), suggesting that conjugate-derived auxin plays roles in these developmental processes. Altering IAA conversion to MeIAA by either altering expression of IAA CAROXYMETHYLTRANSFERASE1 (IAMT1) or mutating METHYL ESTERASE17 (MES17), results in phenotypes that suggest that MeIAA-derived auxin plays roles in gravitropism, leaf epinasty, plant height, and fertility (Qin et al., 2005; Yang et al., 2008). Intriguingly, blocking conversion of multiple auxin storage forms to free IAA results in compensating increased activity of the IPyA pathway (Spiess et al., 2014). Specific roles for auxin derived from these storage forms is only now being appreciated, and more work will be necessary to understand the conditions under which plants use de novo auxin biosynthesis vs. conversion of storage forms to increase free auxin levels.
Table 1.
IAA and IAA precursor levels in Arabidopsis thaliana.
| Molecule | Level (ng/gfw) | Source |
|---|---|---|
| IAOx | 1.7 | Sugawara et al., 2009 |
| IAN | 9720.0 | Sugawara et al., 2009 |
| IAM | 9.9 | Sugawara et al., 2009 |
| IPyA | 56.0 | Mashiguchi et al., 2011 |
| IAAld | 11.3 | Mashiguchi et al., 2011 |
| IBA | 1.3 | Strader et al., 2010 |
| IAA-Asp | 17.4 | Tam et al., 2000 |
| IAA-Glu | 3.5 | Tam et al., 2000 |
| IAA-Glc | 12.0 | Tam et al., 2000 |
| IAA | 11.2 | Sugawara et al., 2009 |
In addition to IAA, 4-Cl-IAA and phenylacetic acid (PAA) are active, naturally occurring auxins (reviewed by Korasick et al., 2013). In legumes, 4-Cl-IAA appears to be the major active auxin, whereas PAA occurrence is more widespread. Intriguingly, both 4-Cl-IAA and PAA appear to be synthesized by the TAA1 and YUCCA families of enzymes from 4-Cl-Trp and phenylalanine, respectively (Tivendale et al., 2012; Dai et al., 2013). The importance of these auxin molecules, which are present at physiologically relevant levels, is starting to be appreciated (see Strader and Nemhauser, 2013), although much research is still necessary to understand specific roles for these active auxin molecules in the plant.
Auxin metabolism has had a complicated history (Tivendale et al., 2014). As we learn more about how auxin is synthesized, stored, and degraded, we will come closer to a more complete understanding of how these pathways integrate to facilitate auxin-regulated growth responses. For example, do certain auxin biosynthetic pathways contribute more strongly to auxin homeostasis than the IPyA pathway under specific environmental or developmental contexts? Comparing differences in auxin metabolism in different plant species will surely help us reach this goal. Uncovering the biological significance of these pathways will undoubtedly be a challenging goal for the coming century.
AUXIN TRANSPORT
Long before the chemical identity of auxin was known, the rapid and polar transport of auxin was known to be important to growth responses. In Phytohormones (1937), Went and Thimann speculated that auxin is not transported by simple diffusion, but by some other mechanism. We now know that specialized carriers mediate auxin transport. Members of the PIN-FORMED (PIN) and ATP-BINDING CASSETTE SUBFAMILY B (ABCB) protein families facilitate the efflux of IAA, whereas members of the AUXIN RESISTANT1/LIKE AUX1 (AUX1/LAX) transporters facilitate IAA uptake (Fig. 4; reviewed by Zazimalova et al., 2010; Peer et al., 2011).
Fig. 4.
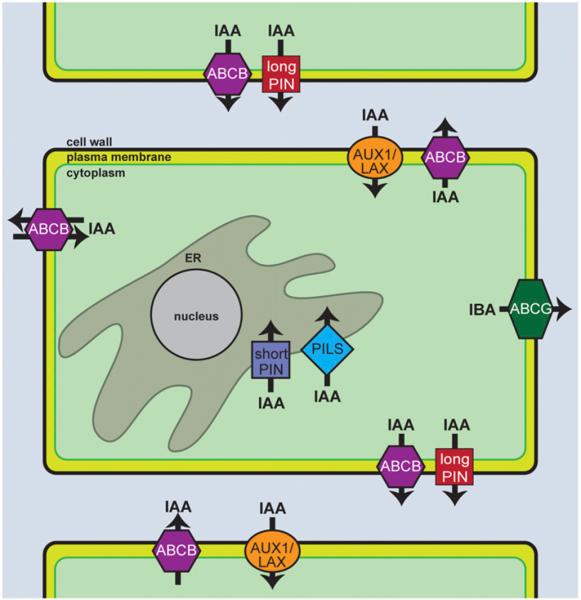
Auxin transport mechanisms. AUX/LAX proteins localize to different faces of the cell depending on the particular cell type, where they act to influx auxin from the apoplast into the cytoplasm (depicted in green). The long PIN proteins localize to either the apical or basal face of the cell in the root to efflux auxin and establish auxin gradients. The short PIN proteins and PILS proteins localize to the ER, where they efflux auxin from the cytoplasm into the ER lumen, presumably to regulate auxin activity via compartmentalization, and possibly metabolism. The ABCB family of auxin transporters localize to the plasma membrane to efflux auxin outside the cell. Some members of the ABCB family have been shown to display both efflux and influx activity based on the cytoplasmic concentration of auxin. Two members of the ABCG family localize to the outer lateral domain in the epidermis, and transport IBA into the surrounding environment.
AUX1/LAX family members, which resemble amino acid permeases, include the plasma-membrane localized H+ symporters AUX1, LAX1, LAX2, and LAX3. AUX1 facilitates uptake of both the natural auxin IAA and the synthetic auxin 2,4-dichlorophenoxyacetic acid (2,4-D) (Maher and Martindale, 1980; Yamamoto and Yamamoto, 1998; Marchant et al., 1999; Swarup et al., 2004; Yang et al., 2006), but not the synthetic auxin NAA (Yamamoto and Yamamoto, 1998; Marchant et al., 1999; Yang et al., 2006) or the natural auxin precursor IBA (reviewed by Strader and Bartel, 2011). LAX1 (Péret et al., 2012) and LAX3 (Swarup et al., 2008) are influx transporters of the natural auxin IAA and the synthetic auxin 2,4-D, but not the synthetic auxin NAA. The transport specificities and locations of AUX1 family members may contribute to auxin response differences.
The diversity of phenotypes displayed by mutants defective in members of the AUX/LAX family reveal distinct roles for these auxin carriers. The activity of AUX1 is necessary for plant gravitropic responses (Bennett et al., 1996; Marchant et al., 1999), root hair development (Grebe et al., 2002; Jones et al., 2009), apical hook development (Vandenbussche et al., 2010), and phyllotaxis (Reinhardt et al., 2003; Bainbridge et al., 2008). The lax3 mutant seedlings display decreased lateral root emergence, increased numbers of lateral root primordia, and decreased expression of cell-wall-modifying enzymes (Swarup et al., 2008), suggesting that LAX3 plays roles in auxin-dependent cell wall modification for cortical cell separation and lateral root emergence. Additionally, LAX3 is involved in apical hook development (Vandenbussche et al., 2010). LAX1 and LAX2 regulate phyllotaxis (Bainbridge et al., 2008), and LAX2 also regulates vascular differentiation in cotyledons (Péret et al., 2012). These auxin influx carriers are critical for polar auxin transport and play roles in diverse aspects of plant development.
PIN and ABCB family members mediate auxin efflux from the cell. PIN proteins are gradient-driven auxin efflux carriers that are unique to plants. There are eight members of the PIN family in Arabidopsis, which are divided into “long” and “short” PIN categories. Long PIN proteins include PIN1, PIN2, PIN3, PIN4, and PIN7, which polarly localize to the plasma membrane (reviewed in Zazimalova et al., 2010). Direct transport of IAA has been shown for the plasma-membrane-localized PIN1 (Petrášek et al., 2006; Yang and Murphy, 2009), PIN2 (Chen et al., 1998; Petrášek et al., 2006; Yang and Murphy, 2009), PIN3 (Friml et al., 2002a), PIN4 (Friml et al., 2002b), and PIN7 (Petrášek et al., 2006; Yang and Murphy, 2009). Intriguingly, each of these PIN proteins displays specific and distinct localization. In root tissues, PIN1 is localized to the downward face of stele, endodermal, and pericycle cells (Friml et al., 2002b; Blakeslee et al., 2007). PIN2 is localized in cortical and epidermal cells (Müller et al., 1998; Blakeslee et al., 2007) and the lateral root cap (Friml, 2003), whereas PIN3 is present throughout the root pericycle and columella (Friml et al., 2002a). PIN4 localizes to the root meristem (Friml et al., 2002b), and PIN7 is present in the columella and stele (Blilou et al., 2005). The specific expression patterns and polar localization of these PIN proteins are necessary to establish differential distribution of auxin to regulate aspects of plant development (reviewed by Zazimalova et al., 2010).
Although all examined PIN proteins transport IAA, PIN family members differ in their ability to transport other auxinic compounds. For example, PIN2 and PIN7 efflux 2,4-D (Yang and Murphy, 2009), whereas PIN1 does not display this ability (Yang and Murphy, 2009). Additionally, PIN4 and PIN7 transport NAA (Petrášek et al., 2006), whereas PIN1 and PIN2 do not (Blakeslee et al., 2007). These differing substrate specificities may contribute to differences in whole-plant responses to varying auxinic compounds.
Unlike the “long” PIN proteins involved in polar transport of IAA, the “short” PIN proteins, PIN5 (Mravec et al., 2009; Ganguly et al., 2010; Ding et al., 2012), PIN6 (Mravec et al., 2009; Sawchuk et al., 2013), and PIN8 (Mravec et al., 2009; Ganguly et al., 2010; Dal Bosco et al., 2012; Ding et al., 2012; Sawchuk et al., 2013) localize to the endoplasmic reticulum (ER) to transport IAA from the cytoplasm into the ER (Mravec et al., 2009; Dal Bosco et al., 2012; Ding et al., 2012; Sawchuk et al., 2013). PIN5, PIN6, and PIN8 additionally transport NAA (Petrášek et al., 2006; Mravec et al., 2009; Ganguly et al., 2010). At this time, specific roles for transport of auxin into the ER to alter plant growth are unknown; however, it seems likely that this may serve to alter cytoplasmic levels of free IAA. In addition to PIN5, PIN6, and PIN8, the PIN-LIKES (PILS) also facilitate auxin influx into the ER and may have roles in regulating the cytoplasmic pool of free auxin and auxin–amino acid conjugates (Barbez et al., 2012). Future research may reveal exciting roles for these ER-localized transporters in regulating auxin levels under certain conditions.
Study of the polar localization of PIN proteins has been instrumental in understanding plant cell polarity. The polar localization of these PIN transporters requires endocytosis and selective recycling back to the plasma membrane (Geldner et al., 2001; Kleine-Vehn and Friml, 2008). Recycling of PIN proteins presumably allows for rapid relocalization in response to environmental or developmental cues, such as gravitropism or embryonic development (reviewed by Lofke et al., 2013).
In addition to PIN-mediated IAA efflux, several ABCB transporters are required for IAA efflux, including ABCB1, ABCB4, ABCB19, and ABCB21 (reviewed by Remy and Duque, 2014). Both ABCB1 and ABCB19 facilitate the efflux of IAA (Noh et al., 2001; Geisler et al., 2005; Lewis et al., 2007) and 2,4-D (Yang and Murphy, 2009). ABCB4 functions in the efflux of IAA (Santelia et al., 2005) and NAA (Cho et al., 2007) and localizes to the plasma membrane and endomembrane compartments of the root (Terasaka et al., 2005; Cho et al., 2007). Intriguingly, ABCB4 and ABCB21 may switch from influx to efflux functions, depending on internal auxin concentrations, importing auxin at low cytoplasmic concentrations, and exporting auxin at higher concentrations (Yang and Murphy, 2009; Kamimoto et al., 2012; Kubes et al., 2012). Structural differences in ABCB4 and ABCB21 from other ABCB proteins may allow for this unique activity (Yang and Murphy, 2009; Kamimoto et al., 2012; Kubes et al., 2012). In addition, ABCB14 and ABCB15 are required for polar auxin transport (Kaneda et al., 2011), although it is unknown whether auxin is a substrate for these transporters. Phenotypes of mutants defective in ABCB family members suggest that these auxin transporters play key roles in plant development (reviewed in Petrášek and Friml, 2009; Peer et al., 2011).
In addition to transport of active auxins (i.e., IAA, 2,4-D, and NAA), plants also transport the endogenous auxin precursor IBA (reviewed by Strader and Bartel, 2011; Michniewicz et al., 2014). Early IBA transport assays relied on the ability of IBA to affect plant morphology distant from the site of application (Went and White, 1939; Leopold and Lam, 1961; Yang and Davies, 1999), raising the question of whether IBA itself or IBA-derived IAA moved through these tissues to create these morphological changes. Later studies using radiolabeled or heavy IBA demonstrated that IBA and/or IBA conjugates may travel long distances through plant tissues (Ludwig-Müller et al., 1995a; Rashotte et al., 2003; Poupart et al., 2005; Liu et al., 2012). Because IAA and IBA are chemically similar, one might hypothesize that these compounds are transported by the same mechanism. However, examined IAA carriers, including AUX1, PIN2, PIN7, ABCB1, and ABCB19, do not transport IBA (reviewed in Strader and Bartel, 2011), suggesting that unique carriers are required for IBA transport.
Several members of the Arabidopsis ABCG family of transporters are required for IBA, but not IAA, transport (reviewed in Michniewicz et al., 2014). ABCG36 is likely an efflux transporter of IBA but not IAA (Strader and Bartel, 2009; Růžička et al., 2010), whereas ABCG37 is an efflux carrier of IBA and 2,4-D but not IAA (Ito and Gray, 2006; Strader et al., 2008b; Růžička et al., 2010). Intriguingly, ABCG36 and ABCG37 are enriched at the outer lateral domain of epidermal cells (reviewed by Strader and Bartel, 2011; Michniewicz et al., 2014), suggesting that these transporters export IBA and/or other substrates out of the plant for an unknown purpose. Little is known about the transport of auxin precursors and auxin storage forms; it will be fascinating in the coming years to see whether potential transport of these inactive auxin-related molecules are important for regulating auxin homeostasis.
Despite our progress since Thimann and Schneider's 1939 speculations on auxin polar transport, there is still much left to be elucidated. For example, are other auxins and auxin storage forms, such as the naturally occurring PAA, 4-Cl-IAA, or IAA-conjugates, transported similarly to IAA or IBA? Understanding more about auxin transport will aid us in reaching Thimann's goal of determining the importance of auxin in the context of the “wholeness of the plant”.
AUXIN SIGNAL TRANSDUCTION
Auxin responses are governed by two separate signaling pathways—the TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX (TIR1/AFB) auxin response pathway (Fig. 5) and the AUXIN BINDING PROTEIN1 (ABP1) auxin response pathway (Fig. 6). Although the TIR1 pathway is well studied (reviewed by Chapman and Estelle, 2009), much remains to be determined about the ABP1 pathway.
Fig. 5.
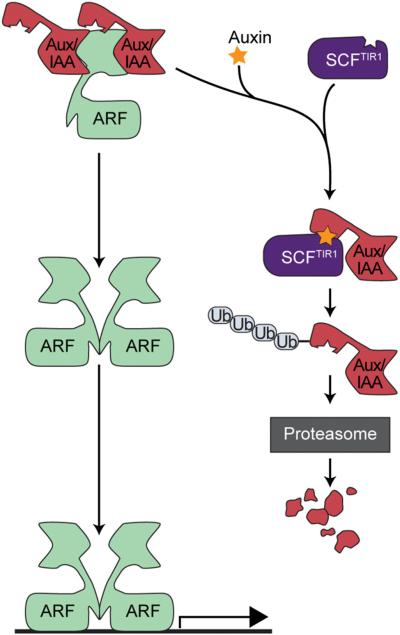
The TIR1/AFB auxin signaling pathway. Under low auxin conditions, ARF activity is repressed by multimerization with Aux/IAA repressor proteins. In the presence of auxin, an Aux/IAA protein and a TIR1/AFB protein form an auxin coreceptor, the Aux/IAA protein is then polyubiqutiylated by the SCFTIR1/AFB complex and targeted to the proteasome for degradation. This degradation of the Aux/IAA repressor relieves the repression of the ARF transcription factor to allow auxin-responsive gene transcription.
Fig. 6.
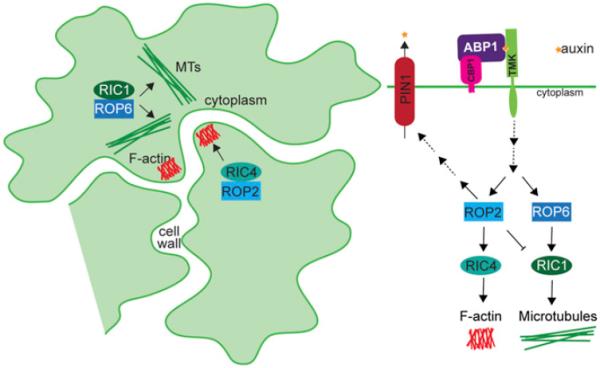
The ABP1 auxin signaling pathway. Extracellular-localized ABP1 is tethered to the plasma membrane by CBP1. ABP1 interacts with TMK receptors in an auxin-dependent manner. ROP activity is regulated downstream of auxin perception by the ABP1-TMK complex. In the epidermal pavement cells of the Arabidopsis cotyledon, ROP2 and RIC4 positively regulate actin polymerization to form lobes and also affect PIN1 protein localization to alter auxin efflux. Alternatively, ROP6 and RIC1 positively regulate microtubule polymerization, leading to constrictions that ultimately form indentations. The outcome of this pathway leads to the puzzle-piece morphology of these cells.
The TIR1/AFB auxin signaling pathway controls transcriptional responses to auxin. Under low auxin concentrations, Auxin/INDOLE-3-ACETIC ACID (Aux/IAA) proteins repress the activity of AUXIN RESPONSE FACTOR (ARF) transcription factors (reviewed by Chapman and Estelle, 2009). When auxin levels increase, a TIR1/AFB F-box protein and an Aux/IAA protein come together to form the auxin coreceptor and directly bind auxin (Dharmasiri et al., 2005; Kepinski and Leyser, 2005; Chapman and Estelle, 2009). The TIR1/AFB F-box protein, which participates in a Skp1-Cullin-F-box (SCF) E3 ubiquitin ligase complex, then polyubiquitylates the Aux/IAA repressor protein, targeting it for degradation by the 26S proteasome. This degradation of the Aux/IAA protein relieves ARF repression, allowing for auxin responsive gene transcription to occur (Ramos et al., 2001; Zenser et al., 2001; Dreher et al., 2006). This receptor–ligand interaction allows a very short signal transduction chain to facilitate rapid transcriptional responses to auxin.
In Arabidopsis, there are 29 Aux/IAAs and six TIR1/AFBs that may participate in auxin coreceptor pairs. Interestingly, different Aux/IAA–TIR1/AFB combinations display different affinities for one another and for different auxins, including both synthetic and natural forms (Calderón-Villalobos et al., 2012; Lee et al., 2014; Shimizu-Mitao and Kakimoto, 2014). These varying affinities may allow the plant to fine-tune auxin responses based on the particular set of Aux/IAA and TIR1/AFB proteins present and the type of auxin present within a given cell. This combinatorial approach with varying auxin affinities is a likely mechanism for auxin to regulate a variety of plant responses and distinct aspects of plant development. Additionally, there are 22 full-length ARF proteins in Arabidopsis, five of which activate transcription, whereas the remaining act to repress transcription (reviewed by Guilfoyle and Hagen, 2007). Complicating this system is the recent discovery that ARF and Aux/IAA proteins may multimerize, rather than interacting in simple dimerization pairs, to regulate auxin responses (Korasick et al., 2014; Nanao et al., 2014), suggesting that the ARF–Aux/IAA repression complex may incorporate many different ARF and Aux/IAA proteins to further fine-tune auxin responses. These complexities may account for the ability of the relatively simple TIR1/AFB signal transduction pathways to regulate diverse developmental processes. The combinatorial nature of the TIR1/AFB auxin signaling pathway will likely provide an endless supply of experiments for the auxin community in the coming century.
ABP1 has been studied since the 1970s (Hertel et al., 1972), but has only recently gained wide acceptance as an auxin receptor. In this pathway, auxin is likely perceived outside the plasma membrane by ABP1 (reviewed by Shi and Yang, 2011; Sauer et al., 2013). Although most of the ABP1 protein is localized to the ER, it is thought that the small percentage localized outside the cell is the active pool, because ABP1 displays low affinity for auxin at the pH of the ER (Jones and Herman, 1993; Tian et al., 1995; Henderson et al., 1997; Klode et al., 2011). ABP1 is anchored to the plasma membrane by interaction with C-TERMINAL PEPTIDE-BINDING PROTEIN1 (CBP1) (Shimomura, 2006), and it interacts with TRANSMEMBRANE KINASE (TMK) proteins in an auxin-dependent manner (Xu et al., 2014). These TMK receptor-like kinases likely serve to transduce the ABP1 signal. ABP1 and auxin are required for the activation of two RHO-LIKE GTPASES OF PLANTS (ROP), ROP2 and ROP6 (Xu et al., 2010). However, the steps between ABP1 binding auxin, TMK activity, and ROP activation have not yet been elucidated.
ABP1-dependent ROP activation is required for proper cell lobing and PIN1-GFP localization in epidermal pavement cells (Xu et al., 2010). ROP2 activation leads to actin cytoskeleton polymerization to promote pavement cell lobing through the activity of ROP-INTERACTIVE CRIB MOTIF-CONTAINING PROTEIN4 (RIC4) (Fu et al., 2005; Xu et al., 2010). In addition, ROP6 activation leads to the formation of necks by the alignment of microtubules perpendicular to the lobe outgrowth, through the action of RIC1 (Fu et al., 2005, 2009; Xu et al., 2010). These ROPs and RICs also alter the localization of PIN1 proteins along the lobes of pavement cells to drive lobe expansion (Robert et al., 2010; Xu et al., 2010). The ABP1-controlled opposing actions of ROP2 and ROP6 alter the cytoskeleton and PIN transporter localization to result in the distinct puzzle-piece morphology of the Arabidopsis epidermis. This pathway could account for many auxin responses that occur too quickly to be the result of the TIR1-regulated transcriptional pathway, such as PIN protein localization changes, modification of ion fluxes, and cell expansion (reviewed by Sauer and Kleine-Vehn, 2011). Many of the components of this pathway have yet to be identified or characterized. Discovery of additional factors required for ABP1-mediated auxin response will allow for greater dissection of ABP1 pathway roles in modulating plant growth and development.
Although the TIR1/AFB and ABP1 auxin response pathways are currently the best-established pathways, additional signaling pathways may regulate auxin responses. Several auxin response factors have been identified that, as of yet, have no assigned roles in the TIR1/AFB or ABP1 pathways. For example, S-PHASE KINASE-ASSOCIATED PROTEIN 2A (SKP2A) is required for auxin-regulated cell division and binds to the natural auxin IAA, the synthetic auxin NAA, and the synthetic auxin 2,4-D (Jurado et al., 2010), suggesting that it may serve as an auxin receptor. Currently, very little is known about this pathway; its future characterization will enhance our understanding of the connection between auxin and the cell cycle. Additionally, the dual-specificity protein phosphatase IBA RESPONSE5 (IBR5) affects auxin-responsive gene transcription by a TIR1-independent mechanism (Monroe-Augustus et al., 2003; Strader et al., 2008a) and may interact with MITOGEN ACTIVATED PROTEIN KINASE12 (MPK12) (Lee et al., 2009) to modulate auxin responses. ROP3/RAC1 GTPases are activated by auxin (Tao et al., 2002, 2005), but have not yet been implicated in any described auxin signaling pathways. Auxin-responsive phospholipase activation also appears to act independently of the TIR1/AFB pathway (Holk and Scherer, 1998; Scherer et al., 2007); however, the mechanism of this activation is still unknown. Determining roles for these orphan auxin response factors will undoubtedly enrich our understanding of control of auxin responses.
With the identification of auxin receptors, our definition of auxin may now be modernized to include molecules that directly bind these receptors (reviewed by Kepinski, 2007) and elicit an auxin response, rather than the purely physiological definition of Thimann's time, which would have included any auxin precursors or storage forms that elicit an auxin response. Investigating crosstalk between auxin signaling pathways, as well as other hormone signaling pathways, will deepen our understanding of how plants coordinate growth as a whole multicellular organism.
DIFFERENCES IN PLANT RESPONSES TO IAA AND 2,4-D
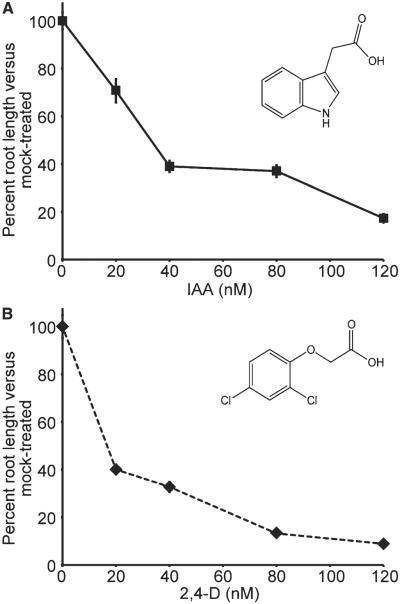
In Arabidopsis, the inhibitory effects of the natural auxin IAA and its synthetic homolog 2,4-D occur at similar concentrations. However, the dose response curves to these naturally occurring and synthetic auxins are very different—root elongation responses to 2,4-D occur in a steeper dose-response curve than to IAA (Fig. 7), suggesting the plant responses to IAA are slightly dampened in comparison to 2,4-D responses. Three possible explanations may account for these differences, including differences in perception, differences in transport, and differences in metabolism.
Fig. 7.
Arabidopsis root elongation responses to IAA and 2,4-D. Normalized mean primary root lengths (±SE) of 8-d-old Arabidopsis seedlings grown in the presence of the indicated concentration of (A) IAA or (B) 2,4-D.
Differences in receptor affinities for IAA and 2,4-D may account for the growth response differences in root elongation assays for these two compounds (Fig. 7). There are currently two described auxin receptor systems—ABP1 and TIR1. ABP1 purified from Zea mays binds IAA an order of magnitude better than 2,4-D (Löbler and Klämbt, 1985), although the affinity of either auxin for the recently described ABP1-TMK complex (Xu et al., 2014) is currently unknown. In addition, 2,4-D binds about half as well as IAA to TIR1/IAA7, and only about one third as well as IAA to AFB5/IAA7 (Tan et al., 2007; Lee et al., 2014). Investigations for synthetic auxins similar to the recent comprehensive study of TIR1/AFB and Aux/IAA binding for natural auxins will be valuable (Shimizu-Mitao and Kakimoto, 2014). Although affinities for both IAA and 2,4-D for all known auxin receptors are unknown, it is unlikely that auxin perception accounts for the greater potency of 2,4-D over IAA; for all examined receptors, IAA is a better substrate than 2,4-D.
Transport differences may account for the differences in IAA and 2,4-D growth response curves. In tobacco cells, 2,4-D shows low affinity for auxin efflux and a lower affinity for auxin influx carriers than IAA (Delbarre et al., 1996). PIN2 and PIN7 appear to transport both IAA and 2,4-D; however, PIN1 appears to only transport IAA (Yang and Murphy, 2009). Both AUX1 and LAX3 appear to transport both IAA and 2,4-D (Yang et al., 2006; Swarup et al., 2008). Long-distance transport studies directly comparing the rootward and shootward transport of IAA and 2,4-D would facilitate our understanding of the summary of the differences in individual transporters and how these differences affect overall transport of different auxins. However, based on the described data, differential transport between these two auxins is also unlikely to be responsible for the observed effects.
Transport and perception differences do not seem to be great enough to account for the higher sensitivity of Arabidopsis to 2,4-D over IAA; 2,4-D tends to be a poorer substrate for these processes, raising the possibility that differences in metabolism may account for differences in 2,4-D and IAA sensitivity. Because IAA is such a potent hormone, plants have evolved many mechanisms to regulate its homeostasis (see earlier Auxin Metabolism section). 2,4-D, on the other hand, is a synthetic molecule and therefore may not be metabolically regulated by plants. Indeed, 2,4-D does not appear to be conjugated to amino acids by the examined GH3 family of enzymes (J. Jez and A. Sherp, Washington University, personal communication; Staswick et al., 2005), suggesting that inability to conjugate 2,4-D to amino acids may prevent buffering of 2,4-D effects on growth inhibition. However, 2,4-D appears to be metabolized into unidentified products in tobacco cells (Hosek et al., 2012), suggesting that plants may at least partially dampen 2,4-D effects by decreasing the pool of active 2,4-D. Future work will be necessary to elucidate the answer to this question, but at the moment, it appears that differences in plant buffering of exogenous IAA and 2,4-D after uptake may account for differences in the effects of these molecules.
OUTSTANDING QUESTIONS IN THE FIELD OF AUXIN BIOLOGY
The past century of auxin research has yielded great insight into the roles this hormone plays in plant growth and development as well as the mechanisms of auxin action. However, many unanswered auxin questions remain; we are hopeful that the next century of auxin research will reveal the answers to the following questions.
Do additional “undiscovered” auxins or auxin precursors exist?
At the time of the classic paper about auxin activity by Kenneth Thimann and Charles Schneider (1939) titled “The relative activities of different auxins” in the American Journal of Botany, many studies were focused on structure-activity relationships of molecules with auxin activity. Since this time, only three naturally occurring endogenous active auxins, IAA, 4-Cl-IAA, and PAA, have been discovered in plants. In addition, multiple naturally occurring auxin precursors have been identified (Table 1; reviewed by Korasick et al., 2013). Other auxins or auxin precursors may have remained elusive to current detection technologies due to low abundance or chemical composition. Further, some auxins or auxin precursors may be present only in certain species (for example, 4-Cl-IAA) or during certain developmental events. Identifying all possible forms of auxin and auxin precursors will be instrumental in dissecting potential roles for different auxins and auxin biosynthesis pathways in regulating plant growth and development.
Are the naturally occurring compounds with auxin activity biologically relevant?
Although the IPyA pathway has emerged as the “main” auxin biosynthesis pathway (reviewed by Zhao, 2012), it cannot be denied that other naturally occurring precursors, including auxin conjugates and IBA, contribute to auxin homeostasis. In addition, conversion of these precursors may play roles in specific developmental events, such as IBA-derived auxin driving lateral root development (Strader et al., 2011; De Rybel et al., 2012). To determine potential roles for these other pathways and precursors in plant growth and development, we must first identify the mechanism of conversion to active auxin, then block this conversion, either by mutation or by pharmacological means, and observe the effects on plant morphology. Genetic, physiological, and biochemical studies will help unravel potential roles for specific auxin biosynthetic pathways and for uncharacterized active auxins such as PAA.
Can IBA or other auxin precursors act as signaling molecules?
In many species, IBA is more effective than IAA or synthetic auxins in promoting lateral root and adventitious root formation, causing speculation that IBA itself can act as a signaling molecule in these processes (reviewed by Ludwig-Müller, 2000). However, IBA is ineffective at inducing lateral roots in Arabidopsis mutants defective in IBA to IAA conversion (Zolman et al., 2000, 2007, 2008; Strader et al., 2011), and strong IBA conversion mutants display reduced lateral root formation in the absence of treatment (Strader et al., 2011), suggesting that the effects of IBA on lateral root induction are through its conversion to IAA in Arabidopsis. Intriguingly, a recent report suggests that both the IBA-derived auxin and the nitric oxide produced in the peroxisomal IBA to IAA conversion process contribute to lateral root induction in Arabidopsis and maize (Schlicht et al., 2013). This nitric oxide produced during IBA to IAA conversion may account for the increased effectiveness of IBA compared with other auxins in inducing lateral roots. Additional evidence suggesting that IBA can act as a signaling molecule includes stress-induced IBA (but not IAA) synthesis (Ludwig-Müller et al., 1995b) and increased IBA (but not IAA) concentrations after inoculation of maize roots with arbuscular mycorrhizal fungi (Ludwig-Müller et al., 1997). In the future, testing the possibility that IBA or other auxin precursors may act as signaling molecules will require use of mutants blocked in the ability to convert the molecule in question to active auxin, followed by examination of the effects of treatment with the auxin precursor.
Are auxin precursors and storage forms transported? If so, for what purpose?
In addition to IAA transport, transport of the naturally occurring auxin precursor IBA occurs in plants (Strader and Bartel, 2011). Because IBA transport is by a transport system independent of IAA transport, it may be possible that additional independent transport systems are used to move other forms of auxin, such as PAA, or other auxin precursors, such as auxin conjugated to amino acids or sugars, throughout the plant. Further research will be necessary to determine whether additional auxins or auxin storage forms are transported, the molecular details of these transport mechanisms, and roles transport of the auxin-related molecules may play in plant development.
How do the TIR1/AFB and ABP1 pathways regulate the broad range of growth and developmental regimes in plants?
Because auxin seemingly is involved in the regulation of nearly every aspect of plant growth and development, it is likely that additional inputs are necessary to modulate or fine-tune these responses to drive plant growth and development. This fine-tuning may be achieved through cross-talk with other hormone response pathways and will undoubtedly be affected by the combinatorial nature of the ARF and Aux/IAA interaction system.
Do all plant species use the same mechanisms for auxin response?
Intriguingly, different species display varying responses to the many auxin-related molecules. Differences in auxin response proteins or transport mechanisms may account for these response differences, such as those observed in the Pisum and Avena auxin response curves of Thimann and Schneider (1939). In the future, understanding the molecular basis of species-specific differential auxin responses could inform our understanding of the relationship between evolution and development.
How does auxin cross-talk with other hormones?
A major question that we are in the beginning stages of answering is how plants integrate the responses to different hormones to ultimately grow and respond to their environment. For example, we now know how auxin and ethylene biosynthesis pathways overlap through the VAS1 aminotransferase (Zheng et al., 2013). As we begin to unravel this complicated topic of research, we will inch toward Thimann's dream of “visualizing the wholeness of the plant.”
The next 100 yr of auxin research will undoubtedly answer many of these questions. Similar to the early discovery of auxin described by Went and Thimann (1937) in Phytohormones and today's molecular understanding of auxin action, future advances will rely on the “steady and collective advance” of the field of auxin researchers. Thimann (1963, p. 15) was wise in his observations in his 1963 review on plant growth substances in saying, “Some time soon we shall be visualizing any one of the organs of a plant as a veritable Times Square of intersecting streams of traffic, with specific hormones crossing and recrossing on predictable paths, some entering a cell together, there to activate specific biochemical processes, others accumulating or decaying, and every external influence playing its part in changing their fate. And while we may thus see the machinery so much more completely, the problem of visualizing the wholeness of the plant—the balanced and integrated organism—will be as elusive as ever.” We hope the next 100 years will be as prosperous for auxin biology as the previous 100 years has been (Fig. 8) !
Fig. 8.
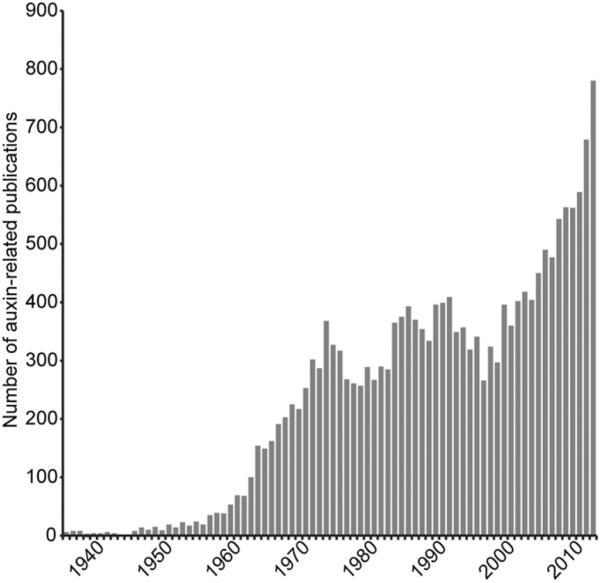
Auxin-related publications. Frequency of auxin-related publications from 1936 to 2013 (based on a PubMed search).
Box 1. Interview with Barbara Pickard.
Kenneth V. Thimann is one of the 25 most published AJB authors, with 29 papers between 1936 and 1992. His legacy of auxin research laid the groundwork for everything we know today. To get an idea of what Thimann's professional life was like, we interviewed a past Thimann graduate student (1959–1964) and current emerita professor at Washington University in St. Louis, Barbara Pickard.
THOUGHTS ON THIMANN AS A SCIENTIST…
Kenneth Vivian Thimann seemed to me, as to many across the world who shared an era with him, to be the dominant force in physiological plant biology. Although he and his students made important studies on a wide variety of topics, he was scientifically most influential for the first fully acceptable chemical identification of the “moving stimulus” described by Charles Darwin in control of differential, phototropic, growth. With all our knowledge and technical capabilities today, it is hard to realize that was not an easy or readily accepted accomplishment. Both the chemistry and physiology of indoleacetic acid, or auxin, were challenged at every step of the way.
On the one hand, Thimann persisted for decades in elaborating evidence favoring the reality of auxin and “nailing the lid on the coffin” (as he put it) on the controversy about its validity. He and student Bill Porter were not satisfied to identify indoleacetic acid or IAA as auxin, but pressed on studying homologs to learn what electron configurations were essential for its activity. On the other hand, Thimann took on the controversy about the function of auxin in phototropism and gravitropism that Darwin's interest set off—the ideas and controversy brought to a head by the famous experiments on polar and lateral movement of auxin during phototropism performed by Fritz Went and during gravitropism by Hermann Dolk. He was still working to round out the proof that transported auxin controlled both symmetric and asymmetric growth when I was lucky enough to join his circle of graduate and postgraduate students.
In Thimann's laboratory, Bruce Stowe labeled the carboxyl group of IAA with 14C, and Mary Helen Goldsmith worked out methods of following its basipetal transport. The relative ease of following 14C-labeled auxin compared to the bioassay established by Fritz Went tempted others to carry out experiments on tropism that challenged the theory built up around auxin. Most notably perhaps, coleoptiles were supplied at the apical end with radioactive auxin, set on their sides for a while, and bisected to collect counts in tissue on the two sides. No difference was found. The tissue was overloaded with hormone. Goldsmith and Thimann had been careful to bioassay the amount of native auxin actually moving through the tissue and provided plants an amount of radioactive auxin to duplicate it. They were also careful to account at the end of the experiment for the fate of 100% of the radioactivity provided—taken up, transported, and retained in the tissue. Thimann eagerly pushed the application of this method to tropism, and ultimately confirmed not only Dolk's work, which had been very precise, but also Went's, which had been less so. Ultimately, a variety of other workers found the same outcome using at least a dozen different geometries of IAA provision and measurement. Again, transportable IAA won the day.
As proof and general acceptance was building for the fundamental role of auxin, Thimann and associates of course branched out to study other effects of IAA. From the start, Thimann understood that other hormones would be found, and he was particularly interested in the interplay between them and auxin.
THOUGHTS ON THIMANN AS A MENTOR…
Working in the laboratory of Kenneth Vivian Thimann was a much-sought and most-rewarding experience for many of us aspiring plant scientists in the days of what I regard as the first, long, chapter of auxinology.
Thimann was trained as a chemist, came from a refined English social class and had a superb education with an unusually broad background. Thimann had an aura of command and control. He just looked and spoke like a world famous professor. Moreover, according to my view, he lived in a very different scientific world than we do today. Life was different in his laboratory than in laboratories today… research methods were simpler, funding was not a usual problem, publication seemed relatively easy, life was more relaxed and gracious. Thimann and others took a lot of interest in national problems and thus spent considerable time away from home base, but although professorial absence was the subject then as now of gentle jokes, the whole system was supportive and there was very little apparent stress in the laboratory.
Many people were critical of a central role for IAA and the Went and Cholodny theory. This created an atmosphere in which Thimann thrived—he traveled all over the world, promoting his forward-looking ideas; and didn't mind publishing a lot in smaller, relatively local, journals. At a time when travel was less common, this gave him access to people and universities hither, thither, and yon. As he went throughout the world scientists flocked to him. He usually had about 15 people in his very international laboratory. The Harvard Biology building was divided up so that there were lots of beautiful little laboratories, each of which held two students. And the pair kind of advised each other—often a big brother/big sister thing. In fact, there was a lot of helpful camaraderie in the whole laboratory.
Thus, if Professor Thimann was convinced that you were adequately serious and perseverant—and convincing was quite necessary—you became part of an extremely friendly research family gathered from all over the world to participate in the elucidation of how IAA, a single small organic molecule, could control or participate in the control of so many plant processes.
Thimann's command, scientific and social presence dominated our lives in a comfortable way. I think most of us saw him as a father figure. He conveyed a very warm regard for everyone in his research group, and made sure that tea—properly prepared in the English style—was available at 5:00 of every weekday, where Thimann was present for conversation if he was in town, where all the laboratory members gathered, and where plant biology visitors from around the world were frequently introduced and joined in discussion of new ideas. And when you met people they would go look at your data in your own little laboratory. So you had a tremendous opportunity to participate in the plant biology community. You felt like you had resources.
Once a month, Kenneth and Ann Thimann hosted a Sunday Evening Party for the group at their Cambridge home at 14 Gray Gardens West. No one wanted to miss these events, where there was always lively chatter and where parlor games such as charades were often played enthusiastically.
I am sure that the women members of the Thimann laboratory were all grateful for the completely fair acceptance of equal aspirations of both sexes. He maintained the equal treatment despite apparently having little or no expectation that the women would go on, as did almost all the men, to gain professorships. Such a liberal attitude, one must note, was not always evident in the hallowed halls of Harvard, where women sometimes complained of sexist treatment. His attitudes may have been shaped by bringing up three very capable daughters. I really think that his fairness and generosity toward women was a very important feature of his teaching style.
After Thimann and Mary Helen Goldsmith trained me in the IAA methods they had worked out, and I published a little scientific note, Thimann let me play with divergent new ideas and methods; but some obstacles slowed me down. So Thimann said “Well my dear you need to get a degree. Why don't you settle the [still] controversial problems in auxin transport during gravitropism and phototropism.” So I did. And after each long, late experiment, I would run to Thimann's office door and tape my data on it. Then go and sleep. Nothing really happened—I had finished what he had asked me to do. I achieved what I considered a reasonably solid proof, and still consider a reasonably solid proof. I didn't know what to do but there I was having a good time. I had friends at Harvard and I could go to lectures and read in the libraries and develop new projects. Nobody was paying any attention to my progress. I had graduate student grants. I taught the laboratory for Thimann's plant physiology course. I didn't like his outline so I ripped it up and wrote my own—it was a lot of work, but fun. Thimann was happy with the outcome, and it was a great experience for me. In Thimann's laboratory, I felt very free.
One day he said, “My dear, how long have you been here?” He said we really must get you a degree. And he said why don't you just solve this problem. Well a year or two ago that is what he had said then. I said, “Dr. Thimann, stay here four minutes.” And I rushed down the stairs—he had two floors of laboratories—I brought back the data book and kept turning page after page. He said “My dear, why don't we write this up? And you'll have a degree!” And we did. (In the process he patiently taught me a huge amount about writing.)
IN SUMMARY…
Specific memories will be forgotten as new generations take over and, with powerful new technology, advance the second long chapter in the history of auxinology, but the influence of Kenneth Thimann still undergirds the developing story. And in summary, Thimann was a very gracious and wonderful teacher loved by immense numbers of people and I guess auxin is good stuff.
Acknowledgments
We thank S. Wyatt for the invitation to write this review and E. Frick, A. Muehler-Sherp, M. Michniewicz, D. Korasick, and two anonymous reviewers for critical comments on the manuscript. This work was supported by the National Science Foundation (DGE-1143954 to T.A.E.) and the National Institutes of Health (R00 GM089987 to L.C.S.). We apologize to anyone whose work we have overlooked (see Fig. 8).
Footnotes
Manuscript received 23 June 2014; revision accepted 9 January 2015.
LITERATURE CITED
- Bainbridge K, Guyomarc'h S, Bayer E, Swarup R, Bennett M, Mandel T, Kuhlemeier C. Auxin influx carriers stabilize phyllotactic patterning. Genes & Development. 2008;22:810–823. doi: 10.1101/gad.462608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak S, Tax FE, Feldmann KA, Galbraith DW, Feyereisen R. CYP83B1, a cytochrome P450 at the metabolic branch point in auxin and indole glucosinolate biosynthesis in Arabidopsis. Plant Cell. 2001;13:101–111. doi: 10.1105/tpc.13.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbez E, Kubes M, Rolcik J, Beziat C, Pencik A, Wang B, Rosquete MR, et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature. 2012;485:119–122. doi: 10.1038/nature11001. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, et al. Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science. 1996;273:948–950. doi: 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, Makam SN, et al. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell. 2007;19:131–147. doi: 10.1105/tpc.106.040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Calderón-Villalobos LI, Lee S, De Oliveira C, Ivetac A, Brandt W, Armitage L, Sheard LB, et al. A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxin. Nature Chemical Biology. 2012;8:477–485. doi: 10.1038/nchembio.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman EJ, Estelle M. Mechanism of auxin-regulated gene expression in plants. Annual Review of Genetics. 2009;43:265–285. doi: 10.1146/annurev-genet-102108-134148. [DOI] [PubMed] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proceedings of the National Academy of Sciences, USA. 1998;95:15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M, Lee SH, Cho H-T. P-glycoprotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells. Plant Cell. 2007;19:3930–3943. doi: 10.1105/tpc.107.054288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Bandurski RS. Chemistry and physiology of the bound auxins. Annual Review of Plant Physiology. 1982;33:403–430. [Google Scholar]
- Dai X, Mashiguchi K, Chen Q, Kasahara H, Kamiya Y, Ojha S, Dubois J, et al. The biochemical mechanism of auxin biosynthesis by an Arabidopsis YUCCA flavin-containing monooxygenase. Journal of Biological Chemistry. 2013;288:1448–1457. doi: 10.1074/jbc.M112.424077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Bosco C, Dovzhenko A, Liu X, Woerner N, Rensch T, Eismann M, Eimer S, et al. The endoplasmic reticulum localized PIN8 is a pollen-specific auxin carrier involved in intracellular auxin homeostasis. Plant Journal. 2012;71:860–870. doi: 10.1111/j.1365-313X.2012.05037.x. [DOI] [PubMed] [Google Scholar]
- De Rybel B, Audenaert D, Xuan W, Overvoorde P, Strader LC, Kepinski S, Hoye R, et al. A role for the root cap in root branching revealed by the non-auxin probe naxillin. Nature Chemical Biology. 2012;8:798–805. doi: 10.1038/nchembio.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre A, Muller P, Imhoff V, Guern J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta. 1996;198:532–541. doi: 10.1007/BF00262639. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Ding Z, Wang B, Moreno I, Duplakova N, Simon S, Carraro N, Reemmer J, et al. ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nature Communications. 2012;3:941. doi: 10.1038/ncomms1941. [DOI] [PubMed] [Google Scholar]
- Dreher KA, Brown J, Saw RE, Callis J. The Arabidopsis Aux/IAA protein family has diversified in degradation and auxin responsiveness. Plant Cell. 2006;18:699–714. doi: 10.1105/tpc.105.039172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J. Auxin transport—Shaping the plant. Current Opinion in Plant Biology. 2003;6:7–12. doi: 10.1016/s1369526602000031. [DOI] [PubMed] [Google Scholar]
- Friml J, Benková E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002b;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002a;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005;120:687–700. doi: 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Fu Y, Xu T, Zhu L, Wen M, Yang Z. A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Current Biology. 2009;19:1827–1832. doi: 10.1016/j.cub.2009.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Lee SH, Cho M, Lee OR, Yoo H, Cho HT. Differential auxin-transporting activities of PIN-FORMED proteins in Arabidopsis root hair cells. Plant Physiology. 2010;153:1046–1061. doi: 10.1104/pp.110.156505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant Journal. 2005;44:179–194. doi: 10.1111/j.1365-313X.2005.02519.x. [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof Y-D, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- Goldsmith MHM. The polar transport of auxin. Annual Review of Plant Physiology. 1977;28:439–478. [Google Scholar]
- Grebe M, Friml J, Swarup K, Ljung K, Sandberg G, Terlou M, Palme K, et al. Cell polarity signaling in Arabidopsis involves a BFA-sensitive auxin influx Pathway. Current Biology. 2002;12:329–334. doi: 10.1016/s0960-9822(02)00654-1. [DOI] [PubMed] [Google Scholar]
- Guilfoyle TJ, Hagen G. Auxin response factors. Current Opinion in Plant Biology. 2007;10:453–460. doi: 10.1016/j.pbi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Haagen-Smit AJ, Leech WD, Bergen WR. Estimation, isolation and identification of auxins in plant material. Science. 1941;93:624–625. doi: 10.1126/science.93.2426.624. [DOI] [PubMed] [Google Scholar]
- Henderson J, Bauly JM, Ashford DA, Oliver SC, Hawes CR, Lazarus CM, Venis MA, Napier RM. Retention of maize auxin-binding protein in the endoplasmic reticulum: Quantifying escape and the role of auxin. Planta. 1997;202:313–323. doi: 10.1007/s004250050133. [DOI] [PubMed] [Google Scholar]
- Hertel R, Thomson KS, Russo VE. In-vitro auxin binding to particulate cell fractions from corn coleoptiles. Planta. 1972;107:325–340. doi: 10.1007/BF00386394. [DOI] [PubMed] [Google Scholar]
- Holk RU, Scherer GF. Fatty acids and lysophospholipids as potential second messengers in auxin action. Rapid activation of phospholipase A2 activity by auxin in suspension-cultured parsley and soybean cells. Plant Journal. 1998;16:601–611. [Google Scholar]
- Hosek P, Kubes M, Lankova M, Dobrev PI, Klima P, Kohoutova M, Petrasek J, et al. Auxin transport at cellular level: New insights supported by mathematical modelling. Journal of Experimental Botany. 2012;63:3815–3828. doi: 10.1093/jxb/ers074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull AK, Vij R, Celenza JL. Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proceedings of the National Academy of Sciences, USA. 2000;97:2379–2384. doi: 10.1073/pnas.040569997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Gray WM. A gain-of-function mutation in the Arabidopsis pleiotropic drug resistance transporter PDR9 confers resistance to auxinic herbicides. Plant Physiology. 2006;142:63–74. doi: 10.1104/pp.106.084533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Herman E. KDEL-containing auxin-binding protein is secreted by plasma membrane and cell wall. Plant Physiology. 1993;101:595–606. doi: 10.1104/pp.101.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AR, Kramer EM, Knox K, Swarup R, Bennett MJ, Lazarus CM, Leyser HM, Grierson CS. Auxin transport through non-hair cells sustains root-hair development. Nature Cell Biology. 2009;11:78–84. doi: 10.1038/ncb1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado S, Abraham Z, Manzano C, Lopez-Torrejon G, Pacios LF, Del Pozo JC. The Arabidopsis cell cycle F-box protein SKP2A binds to auxin. Plant Cell. 2010;22:3891–3904. doi: 10.1105/tpc.110.078972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimoto Y, Terasaka K, Hamamoto M, Takanashi K, Fukuda S, Shitan N, Sugiyama A, et al. Arabidopsis ABCB21 is a facultative auxin importer/exporter regulated by cytoplasmic auxin concentration. Plant & Cell Physiology. 2012;53:2090–2100. doi: 10.1093/pcp/pcs149. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Schuetz M, Lin BS, Chanis C, Hamberger B, Western TL, Ehlting J, Samuels AL. ABC transporters coordinately expressed during lignification of Arabidopsis stems include a set of ABCBs associated with auxin transport. Journal of Experimental Botany. 2011;62:2063–2077. doi: 10.1093/jxb/erq416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S. The anatomy of auxin perception. BioEssays. 2007;29:953–956. doi: 10.1002/bies.20657. [DOI] [PubMed] [Google Scholar]
- Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- Key JL. Modulation of gene expression by auxin. BioEssays. 1989;11:52–55. doi: 10.1002/bies.950110204. [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Friml J. Polar targeting and endocytic recycling in auxin-dependent plant development. Annual Review of Cell and Developmental Biology. 2008;24:447–473. doi: 10.1146/annurev.cellbio.24.110707.175254. [DOI] [PubMed] [Google Scholar]
- Klode M, Dahlke RI, Sauter M, Steffens B. Expression and subcellular localization of Arabidopsis thaliana auxin-binding protein 1 (ABP1) Journal of Plant Growth Regulation. 2011;30:416–424. [Google Scholar]
- Koepfli JB, Thimann KV, Went FW. Phytohormones: Structure and physiological activity. I. Journal of Biological Chemistry. 1938;122:763–780. [Google Scholar]
- Kögl F, Haagen-Smit AJ, Erxleben H. Über ein phytohormon der zellstreckung. Reindarstellung des auxins aus menschlichem harn. 4. Mitteilung über pflanzliche Wachstumsstoffe. Hoppe-Seyler's Zeitschrift fur Physiologische Chemie. 1933;214:241–261. [Google Scholar]
- Kögl F, Erxleben H, Haagen-Smit AJ. Über die Isolierung der Auxine a und b aus pflanzlichen Materialien. 9. Mitteilung über pflanzliche Wachstumsstoffe. Hoppe-Seyler's Zeitschrift fur Physiologische Chemie. 1934a;225:215–229. [Google Scholar]
- Kögl F, Haagen-Smit AJ, Erxleben H. Über ein neues Auxin (Heteroauxin) aus Harn. XI. Mitteilung über pflanzliche Wachstumsstoffe. Hoppe-Seyler's Zeitschrift fur Physiologische Chemie. 1934b;228:90–103. [Google Scholar]
- Kögl F, Kostermans DGFR. Hetero-auxin als Stoffwechselprodukt niederer pflanzlicher Organismen. Isolierung aus Hefe. 13. Mitteilung über pflanzliche Wachstumsstoffe. Hoppe-Seyler's Zeitschrift fur Physiologische Chemie. 1934;228:113–121. [Google Scholar]
- Korasick DA, Enders TA, Strader LC. Auxin biosynthesis and storage forms. Journal of Experimental Botany. 2013;64:2541–2555. doi: 10.1093/jxb/ert080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korasick DA, Westfall CS, Lee SG, Nanao MH, Dumas R, Hagen G, Guilfoyle TJ, et al. Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repression. Proceedings of the National Academy of Sciences, USA. 2014;111:5427–5432. doi: 10.1073/pnas.1400074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes M, Yang H, Richter GL, Cheng Y, Mlodzinska E, Wang X, Blakeslee JJ, et al. The Arabidopsis concentration-dependent influx/efflux transporter ABCB4 regulates cellular auxin levels in the root epidermis. Plant Journal. 2012;69:640–654. doi: 10.1111/j.1365-313X.2011.04818.x. [DOI] [PubMed] [Google Scholar]
- Lee JS, Wang S, Sritubtim S, Chen JG, Ellis BE. Arabidopsis mitogen-activated protein kinase MPK12 interacts with the MAPK phosphatase IBR5 and regulates auxin signaling. Plant Journal. 2009;57:975–985. doi: 10.1111/j.1365-313X.2008.03741.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Sundaram S, Armitage L, Evans JP, Hawkes T, Kepinski S, Ferro N, Napier RM. Defining binding efficiency and specificity of auxins for SCF(TIR1/AFB)-Aux/IAA co-receptor complex formation. ACS Chemical Biology. 2014;9:673–682. doi: 10.1021/cb400618m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold AC, Lam SL. Polar transport of three auxins. In: Klein RM, editor. 4th International Conference on Plant Growth Regulators, New York, New York; Ames, Iowa, USA: Iowa State University Press; 1961. pp. 411–418. [Google Scholar]
- Lewis DR, Miller ND, Splitt BL, Wu G, Spalding EP. Separating the roles of acropetal and basipetal auxin transport on gravitropism with mutations in two Arabidopsis multidrug resistance-like ABC transporter genes. Plant Cell. 2007;19:1838–1850. doi: 10.1105/tpc.107.051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Barkawi L, Gardner G, Cohen JD. Transport of indole-3-butyric acid and indole-3-acetic acid in Arabidopsis hypocotyls using stable isotope labeling. Plant Physiology. 2012;158:1988–2000. doi: 10.1104/pp.111.191288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg G. Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell. 2005;17:1090–1104. doi: 10.1105/tpc.104.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löbler M, Klämbt D. Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). I. Purification by immunological methods and characterization. Journal of Biological Chemistry. 1985;260:9848–9853. [PubMed] [Google Scholar]
- Lofke C, Luschnig C, Kleine-Vehn J. Posttranslational modification and trafficking of PIN auxin efflux carriers. Mechanisms of Development. 2013;130:82–94. doi: 10.1016/j.mod.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J. Indole-3-butyric acid in plant growth and development. Plant Growth Regulation. 2000;32:219–230. [Google Scholar]
- Ludwig-Müller J, Kaldorf M, Sutter EG, Epstein E. Indole-3-butyric acid (IBA) is enhanced in young maize (Zea mays L.) roots colonized with the arbuscular mycorrhizal fungus Glomus intraradices. Plant Science. 1997;125:153–162. [Google Scholar]
- Ludwig-Müller J, Raisig A, Hilgenberg W. Uptake and transport of indole-3-butyric acid in Arabidopsis thaliana: Comparison with other natural and synthetic auxins. Journal of Plant Physiology. 1995a;147:351–354. [Google Scholar]
- Ludwig-Müller J, Schubert B, Pieper K. Regulation of IBA synthetase from maize (Zea mays L.) by drought stress and ABA. Journal of Experimental Botany. 1995b;46:423–432. [Google Scholar]
- Maher EP, Martindale SJB. Mutants of Arabidopsis thaliana with altered responses to auxins and gravity. Biochemical Genetics. 1980;18:1041–1053. doi: 10.1007/BF00484337. [DOI] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO Journal. 1999;18:2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, et al. The main auxin biosynthesis pathway in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2011;108:18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michniewicz M, Powers SK, Strader LC. IBA transport by PDR proteins. In: Geisler M, editor. Plant ABC transporters, vol. 22, Signaling and communication in plants. Springer International Publishing; Cham, Switzerland: 2014. [Google Scholar]
- Mikkelsen MD, Naur P, Halkier BA. Arabidopsis mutants in the C-S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. Plant Journal. 2004;37:770–777. doi: 10.1111/j.1365-313x.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- Mikkelsen MD, Hansen CH, Wittstock U, Halkier BA. Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. Journal of Biological Chemistry. 2000;275:33712–33717. doi: 10.1074/jbc.M001667200. [DOI] [PubMed] [Google Scholar]
- Monroe-Augustus M, Zolman BK, Bartel B. IBR5, a dual-specificity phosphatase-like protein modulating auxin and abscisic acid responsiveness in Arabidopsis. Plant Cell. 2003;15:2979–2991. doi: 10.1105/tpc.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec J, Skůpa P, Bailly A, Hoyerová K, Křeček P, Bielach A, Petrášek J, et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature. 2009;459:1136–1140. doi: 10.1038/nature08066. [DOI] [PubMed] [Google Scholar]
- Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Parry G, et al. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO Journal. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanao MH, Vinos-Poyo T, Brunoud G, Thevenon E, Mazzoleni M, Mast D, Laine S, et al. Structural basis for oligomerization of auxin transcriptional regulators. Nature Communications. 2014;5:3617. doi: 10.1038/ncomms4617. [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP. Multidrug Resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell. 2001;13:2441–2454. doi: 10.1105/tpc.010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J, Grisafi P, Fink GR, Bartel B. Arabidopsis mutants resistant to the auxin effects of indole-3-acetonitrile are defective in the nitrilase encoded by the NIT1 gene. Plant Cell. 1997;9:1781–1790. doi: 10.1105/tpc.9.10.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Blakeslee JJ, Yang H, Murphy AS. Seven things we think we know about auxin transport. Molecular Plant. 2011;4:487–504. doi: 10.1093/mp/ssr034. [DOI] [PubMed] [Google Scholar]
- Péret B, Swarup K, Ferguson A, Seth M, Yang Y, Dhondt S, James N, et al. AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell. 2012;24:2874–2885. doi: 10.1105/tpc.112.097766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrášek J, Friml J. Auxin transport routes in plant development. Development. 2009;136:2675–2688. doi: 10.1242/dev.030353. [DOI] [PubMed] [Google Scholar]
- Petrášek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, Wisniewska J, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- Pollmann S, Neu D, Weiler EW. Molecular cloning and characterization of an amidase from Arabidopsis thaliana capable of converting indole-3-acetamide into the plant growth hormone, indole-3-acetic acid. Phytochemistry. 2003;62:293–300. doi: 10.1016/s0031-9422(02)00563-0. [DOI] [PubMed] [Google Scholar]
- Poupart J, Rashotte AM, Muday GK, Waddell CS. The rib1 mutant of Arabidopsis has alterations in indole-3-butyric acid transport, hypocotyl elongation, and root architecture. Plant Physiology. 2005;139:1460–1471. doi: 10.1104/pp.105.067967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G, Gu H, Zhao Y, Ma Z, Shi G, Yang Y, Pichersky E, et al. An indole-3-acetic acid carboxyl methyltransferase regulates Arabidopsis leaf development. Plant Cell. 2005;17:2693–2704. doi: 10.1105/tpc.105.034959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quittenden LJ, Davies NW, Smith JA, Molesworth PP, Tivendale ND, Ross JJ. Auxin biosynthesis in pea: Characterization of the tryptamine pathway. Plant Physiology. 2009;151:1130–1138. doi: 10.1104/pp.109.141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JA, Zenser N, Leyser O, Callis J. Rapid degradation of auxin/indoleacetic acid proteins requires conserved amino acids of domain II and is proteasome dependent. Plant Cell. 2001;13:2349–2360. doi: 10.1105/tpc.010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampey RA, Leclere S, Kowalczyk M, Ljung K, Sandberg G, Bartel B. A family of auxin-conjugate hydrolases that contributes to free indole-3-acetic acid levels during Arabidopsis germination. Plant Physiology. 2004;135:978–988. doi: 10.1104/pp.104.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Poupart J, Waddell CS, Muday GK. Transport of the two natural auxins, indole-3-butyric acid and indole-3-acetic acid, in Arabidopsis. Plant Physiology. 2003;133:761–772. doi: 10.1104/pp.103.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- Remy E, Duque P. Beyond cellular detoxification: A plethora of physiological roles for MDR transporter homologs in plants. Frontiers in Physiology. 2014;5:201. doi: 10.3389/fphys.2014.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Kleine-Vehn J, Barbez E, Sauer M, Paciorek T, Baster P, Vanneste S, et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell. 2010;143:111–121. doi: 10.1016/j.cell.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růžička K, Strader LC, Bailly A, Yang H, Blakeslee J, Łangowski Ł, Nejedlá E, et al. Arabidopsis PIS1 encodes the ABCG37 transporter of auxinic compounds including the auxin precursor indole-3-butyric acid. Proceedings of the National Academy of Sciences, USA. 2010;107:10749–10753. doi: 10.1073/pnas.1005878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia D, Vincenzetti V, Azzarello E, Bovet L, Fukao Y, Duchtig P, Mancuso S, et al. MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Letters. 2005;579:5399–5406. doi: 10.1016/j.febslet.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Sauer M, Kleine-Vehn J. AUXIN BINDING PROTEIN1: The outsider. Plant Cell. 2011;23:2033–2043. doi: 10.1105/tpc.111.087064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M, Robert S, Kleine-Vehn J. Auxin: Simply complicated. Journal of Experimental Botany. 2013;64:2565–2577. doi: 10.1093/jxb/ert139. [DOI] [PubMed] [Google Scholar]
- Sawchuk MG, Edgar A, Scarpella E. Patterning of leaf vein networks by convergent auxin transport pathways. PLOS Genetics. 2013;9:e1003294. doi: 10.1371/journal.pgen.1003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer GF, Zahn M, Callis J, Jones AM. A role for phospholipase A in auxin-regulated gene expression. FEBS Letters. 2007;581:4205–4211. doi: 10.1016/j.febslet.2007.07.059. [DOI] [PubMed] [Google Scholar]
- Schmidt R-C, Müller A, Hain R, Bartling D, Weiler EW. Transgenic tobacco plants expressing the Arabidopsis thaliana nitrilase II enzyme. Plant Journal. 1996;9:683–691. doi: 10.1046/j.1365-313x.1996.9050683.x. [DOI] [PubMed] [Google Scholar]
- Schlicht M, Ludwig-Müller J, Burbach C, Volkmann D, Baluska F. Indole-3-butyric acid induces lateral root formation via peroxisome-derived indole-3-acetic acid and nitric oxide. New Phytologist. 2013;200:473–482. doi: 10.1111/nph.12377. [DOI] [PubMed] [Google Scholar]
- Scott TK. Auxins and roots. Annual Review of Plant Physiology. 1972;23:235–258. [Google Scholar]
- Shi JH, Yang ZB. Is ABP1 an auxin receptor yet? Molecular Plant. 2011;4:635–640. doi: 10.1093/mp/ssr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Mitao Y, Kakimoto T. Auxin sensitivities of all Arabidopsis Aux/IAAs for degradation in the presence of every TIR1/AFB. Plant & Cell Physiology. 2014;55:1405–1459. doi: 10.1093/pcp/pcu077. [DOI] [PubMed] [Google Scholar]
- Shimomura S. Identification of a glycosylphosphatidylinositol-anchored plasma membrane protein interacting with the C-terminus of auxin-binding protein 1: A photoaffinity crosslinking study. Plant Molecular Biology. 2006;60:663–677. doi: 10.1007/s11103-005-5471-1. [DOI] [PubMed] [Google Scholar]
- Spiess GM, Hausman A, Yu P, Cohen JD, Rampey RA, Zolman BK. Auxin-input pathway disruptions are mitigated by changes in auxin biosynthetic gene expression in Arabidopsis thaliana. Plant Physiology. 2014 doi: 10.1104/pp.114.236026. doi:10.1104/pp.114.236026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 2005;17:616–627. doi: 10.1105/tpc.104.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, et al. TAA1 -mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133:177–191. doi: 10.1016/j.cell.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Robles LM, Novak O, He W, Guo H, Ljung K, Alonso JM. The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell. 2011;23:3961–3973. doi: 10.1105/tpc.111.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Bartel B. The Arabidopsis PLEIOTROPIC DRUG RESISTANCE8/ABCG36 ATP binding cassette transporter modulates sensitivity to the auxin precursor indole-3-butyric acid. Plant Cell. 2009;21:1992–2007. doi: 10.1105/tpc.109.065821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Bartel B. Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid. Molecular Plant. 2011;4:477–486. doi: 10.1093/mp/ssr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Hendrickson Culler A, Cohen JD, Bartel B. Conversion of endogenous indole-3-butyric acid to indole-3-acetic acid drives cell expansion in Arabidopsis seedlings. Plant Physiology. 2010;153:1577–1586. doi: 10.1104/pp.110.157461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Monroe-Augustus M, Bartel B. The IBR5 phosphatase promotes Arabidopsis auxin responses through a novel mechanism distinct from TIR1-mediated repressor degradation. BMC Plant Biology. 2008a;8:41. doi: 10.1186/1471-2229-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Monroe-Augustus M, Rogers KC, Lin GL, Bartel B. Arabidopsis iba response5 (ibr5) suppressors separate responses to various hormones. Genetics. 2008b;180:2019–2031. doi: 10.1534/genetics.108.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Nemhauser JL. Auxin 2012: A rich mea ho'oulu. Development. 2013;140:1153–1157. doi: 10.1242/dev.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Wheeler DL, Christensen SE, Berens JC, Cohen JD, Rampey RA, Bartel B. Multiple facets of Arabidopsis seedling development require indole-3-butyric acid-derived auxin. Plant Cell. 2011;23:984–999. doi: 10.1105/tpc.111.083071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara S, Hishiyama S, Jikumaru Y, Hanada A, Nishimura T, Koshiba T, Zhao Y, et al. Biochemical analyses of indole-3-acetaldoxime-dependent auxin biosynthesis in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2009;106:5430–5435. doi: 10.1073/pnas.0811226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nature Cell Biology. 2008;10:946–954. doi: 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- Swarup R, Kargul J, Marchant A, Zadik D, Rahman A, Mills R, Yemm A, et al. Structure–function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell. 2004;16:3069–3083. doi: 10.1105/tpc.104.024737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam YY, Epstein E, Normanly J. Characterization of auxin conjugates in Arabidopsis. Low steady-state levels of indole-3-acetylaspartate, indole-3-acetyl-glutamate, and indole-3-acetyl-glucose. Plant Physiology. 2000;123:589–595. doi: 10.1104/pp.123.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- Tao L, Cheung AY, Wu H. Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. Plant Cell. 2002;14:2745–2760. doi: 10.1105/tpc.006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao LZ, Cheung AY, Nibau C, Wu HM. RAC GTPases in tobacco and Arabidopsis mediate auxin-induced formation of proteolytically active nuclear protein bodies that contain AUX/IAA proteins. Plant Cell. 2005;17:2369–2383. doi: 10.1105/tpc.105.032987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaka K, Blakeslee JJ, Titapiwatanakun B, Peer WA, Bandyopadhyay A, Makam SN, Lee OR, et al. PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell. 2005;17:2922–2939. doi: 10.1105/tpc.105.035816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann KV. On the plant growth hormone produced by Rhizopus suinus. Journal of Biological Chemistry. 1935;109:279–291. [Google Scholar]
- Thimann KV. Plant growth substances; past, present and future. Annual Review of Plant Physiology. 1963;14:1–19. [Google Scholar]
- Thimann KV. Hormone action in the whole life of plants. University of Massachusetts Press; Amherst, Massachusetts, USA: 1977. [Google Scholar]
- Thimann KV, Schneider CA. The relative activities of different auxins. American Journal of Botany. 1939;26:328–333. [Google Scholar]
- Tian H, Klämbt D, Jones AM. Auxin-binding protein 1 does not bind auxin within the endoplasmic reticulum despite this being the predominant subcellular location for this hormone receptor. Journal of Biological Chemistry. 1995;270:26962–26969. doi: 10.1074/jbc.270.45.26962. [DOI] [PubMed] [Google Scholar]
- Tivendale ND, Ross JJ, Cohen JD. The shifting paradigms of auxin biosynthesis. Trends in Plant Science. 2014;19:44–51. doi: 10.1016/j.tplants.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Tivendale ND, Davidson SE, Davies NW, Smith JA, Dalmais M, Bendahmane AI, Quittenden LJ, et al. Biosynthesis of the halogenated auxin, 4-chloroindole-3-acetic acid. Plant Physiology. 2012;159:1055–1063. doi: 10.1104/pp.112.198457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetti VB, Van Aken O, Morreel K, Vandenbroucke K, van de Cotte B, De Clercq I, Chiwocha S, et al. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell. 2010;22:2660–2679. doi: 10.1105/tpc.109.071316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Petrášek J, Žádníková P, Hoyerová K, Pešek B, Raz V, Swarup R, et al. The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development. 2010;137:597–606. doi: 10.1242/dev.040790. [DOI] [PubMed] [Google Scholar]
- Went FW. Wuchsstoff und Wachstum. Extrait du Recueil des Travaux Botaniques Neerlandais. 1928;25:1–116. [Google Scholar]
- Went FW, Thimann KV. Phytohormones. Macmillan; New York, New York, USA: 1937. [Google Scholar]
- Went F, White R. Experiments of the transport of auxin. Botanical Gazette. 1939;100:465–484. [Google Scholar]
- Wildman SG. The auxin-A, B enigma: Scientific fraud or ineptitude? Plant Growth Regulation. 1997;22:37–68. [Google Scholar]
- Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, et al. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2011;108:18518–18523. doi: 10.1073/pnas.1108436108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Dai N, Chen J, Nagawa S, Cao M, Li H, Zhou Z, et al. Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science. 2014;343:1025–1028. doi: 10.1126/science.1245125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, Perrot-Rechenmann C, et al. Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell. 2010;143:99–110. doi: 10.1016/j.cell.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Greenham K, Prigge MJ, Jensen PJ, Estelle M. The TRANSPORT INHIBITOR RESPONSE2 gene is required for auxin synthesis and diverse aspects of plant development. Plant Physiology. 2009;151:168–179. doi: 10.1104/pp.109.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Yamamoto KT. Differential effects of 1-naphthaleneacetic acid, indole-3-acetic acid and 2,4-dichlorophenoxyacetic acid on the gravitropic response of roots in an auxin-resistant mutant of Arabidopsis, aux1. Plant & Cell Physiology. 1998;39:660–664. doi: 10.1093/oxfordjournals.pcp.a029419. [DOI] [PubMed] [Google Scholar]
- Yang H, Murphy AS. Functional expression and characterization of Arabidopsis ABCB, AUX 1 and PIN auxin transporters in Schizosaccharomyces pombe. Plant Journal. 2009;59:179–191. doi: 10.1111/j.1365-313X.2009.03856.x. [DOI] [PubMed] [Google Scholar]
- Yang T, Davies PJ. Promotion of stem elongation by indole-3-butyric acid in intact plants of Pisum sativum L. Plant Growth Regulation. 1999;27:157–160. [Google Scholar]
- Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E. High-affinity auxin transport by the AUX1 influx carrier protein. Current Biology. 2006;16:1123–1127. doi: 10.1016/j.cub.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Yang Y, Xu R, Ma CJ, Vlot AC, Klessig DF, Pichersky E. Inactive methyl indole-3-acetic acid ester can be hydrolyzed and activated by several esterases belonging to the AtMES esterase family of Arabidopsis. Plant Physiology. 2008;147:1034–1045. doi: 10.1104/pp.108.118224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazimalova E, Murphy AS, Yang H, Hoyerova K, Hosek P. Auxin transporters—Why so many? Cold Spring Harbor Perspectives in Biology. 2010;2:a001552. doi: 10.1101/cshperspect.a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenser N, Ellsmore A, Leasure C, Callis J. Auxin modulates the degradation rate of Aux/IAA proteins. Proceedings of the National Academy of Sciences, USA. 2001;98:11795–11800. doi: 10.1073/pnas.211312798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Molecular Plant. 2012;5:334–338. doi: 10.1093/mp/ssr104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, Normanly J, et al. Trp-dependent auxin biosynthesis in Arabidopsis: Involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes & Development. 2002;16:3100–3112. doi: 10.1101/gad.1035402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Guo Y, Novak O, Dai X, Zhao Y, Ljung K, Noel JP, Chory J. Coordination of auxin and ethylene biosynthesis by the aminotransferase VAS1. Nature Chemical Biology. 2013;9:244–246. doi: 10.1038/nchembio.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Martinez N, Millius A, Adham AR, Bartel B. Identification and characterization of Arabidopsis indole-3-butyric acid response mutants defective in novel peroxisomal enzymes. Genetics. 2008;180:237–251. doi: 10.1534/genetics.108.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Nyberg M, Bartel B. IBR3, a novel peroxisomal acyl-CoA dehydrogenase-like protein required for indole-3-butyric acid response. Plant Molecular Biology. 2007;64:59–72. doi: 10.1007/s11103-007-9134-2. [DOI] [PubMed] [Google Scholar]
- Zolman BK, Yoder A, Bartel B. Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics. 2000;156:1323–1337. doi: 10.1093/genetics/156.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]