Abstract
Polycationic nanocomplexes are a robust means for achieving nucleic acid condensation and efficient intracellular gene deliveries. To enhance delivery, a multilayered nanoparticle consisting of a core of electrostatically bound elements was used. These included a histone-mimetic peptides, poly-l-arginine and poly-d-glutamic acid was coated with silicate before surface functionalization with poly-l-arginine. Transfection efficiencies and duration of expression were similar when using green fluorescent protein (GFP) plasmid DNA (pDNA) or GFP mRNA. These nanoparticles demonstrated significantly higher (>100%) and significantly longer (15 vs. 4 days) transfection efficiencies in comparison to a commercial transfection agent (Lipofectamine 2000). Reprogramming of human foreskin fibroblasts using mRNA to the Sox2 transcription factor resulted in three-fold higher neurosphere formation in comparison to the commercial reagent. These results demonstrate the potential of these nanoparticles as ideal vectors for gene delivery.
Introduction
Nanoscale technologies for drug and nucleic acid delivery have steadily improved, but they have yet to be used in reprogramming of fibroblasts to cells of neural lineage as well as in generalized stem cell applications.1–5 In recent years, nonviral methods for delivery have received particular attention due to immunogenic and toxic responses associated with viral vectors, which limit practical use and tenability.6–8 One important limitation of nonviral gene delivery is the inefficient delivery of functional nucleic acids to the nucleus.6,9 Commonly utilized polyplexes consisting of poly(ethylenimine) and DNA have a tendency to shed the majority (>90%) of themselves during cellular internalization, with the payload often bound to the remains of the polymeric cationic nanocarrier.10,11 To overcome this limitation, we developed a transiently stabilized hybrid polyplex-inorganic nanoparticulate with a hierarchical structure that is functionalized for maximal nuclear-specific unpackaging and localization. Polyplexes are polyion complexes prepared from polycations and nucleic acids, which provide an excellent means by which to deliver plasmid DNA (pDNA), antisense DNA, mRNA, siRNA, and small molecules as therapeutics.12,13 Their advantages include ease of preparation as well as versatility in biological function.
Polyplexes prepared from a combination of cationic and anionic polymer-based derivatives are reported to yield efficient gene delivery and subsequent expression through facilitated endosomal escape into the cytoplasm, based on the proton sponge effect, as well as some putative retrograde trafficking and compartment-specific unpackaging elements within the nucleus.11,14 However, several hurdles still need to be overcome for clinical applications using polyplexes. Polyplex systems often have low complex stability in a biological milieu such as serum, thereby leading to undesirable complex disassociation or aggregation and decreased transfection activity concomitant with increased adverse effects.15–17 Therefore, polyplexes that form highly stable complexes under extracellular conditions are required, especially for systemic administration. However, a drawback of polyplexes that exhibit higher stability is that intracellular release of an enclosed nucleic acid may be hindered.
Results and Discussion
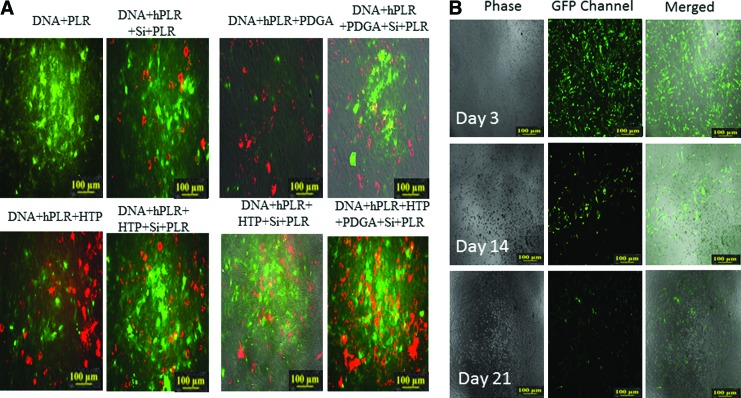
To determine particle uptake and transfection efficiency due to the presence or absence of the different layers and components, fluorescein isothiocyanate (FITC) labeled poly-l-arginine (PLR) containing particles encapsulating pDNA for mCherry (Addgene: pcDNA3.3_mCherry) were transfected into MC3T3-E1 cell lines. Cationic PLR was conjugated with pDNA in the presence of histone h3 tail peptide (HTP) and/or poly-d-glutamic acid (PDGA) followed with or without encapsulation in a silicate layer and a final layer of PLR. Histone tail peptides were used to enable loosening of pDNA from the cationic polymer (PLR) by acetylation. Silicate coating was used to stabilize and condense the quarternary core composed of pDNA/mRNA, PLR, HTP, and PDGA. An outermost layer of PLR was used to provide a positive charge to the particle so that it can effectively adhere to the negatively charged cell membrane. Confocal imaging for plasmid expression indicated that the nanoparticle with all components, that is, poly-l-arginine (PLR) coating of silicate encapsulated quarternary core, demonstrated good transfection efficiency (as observed by greater intensity of green in Fig. 1A) while showing intensive expression (as observed by greater intensity of red mCherry in Fig. 1A). Particles made using PLR complexed with pDNA demonstrated minimal expression, which was increased by layering with silicate followed by another layer of PLR. Complexation with PDGA demonstrated effective release of pDNA from the cationic polyplex, resulting in greater expression of transfected pDNA. Confocal microscopy also revealed that silicate-coated nanoparticles are extremely resilient to aggregation and dissociation in serum (no extracellular green/yellow clusters) in comparison to their binary, ternary, and quaternary core counterparts without silicate stabilization. Qualitative observation demonstrated PDGA inclusion to greatly increase the number of particles taken up by cells in both silicate and nonsilicate-coated complex transfections. The particle with the most efficient expression was used to study the duration of plasmid expression. We transfected these nanoparticles encapsulating pDNA to green fluorescent protein (GFP) into human foreskin fibroblasts (HFFs). Results indicated high expression on day 3 that was reduced by day 14 with minimal expression by day 21 (Fig. 1B).
FIG. 1.
(A) Confocal at 30 h after transfection of mCherry DNA into MC3T3-E1 osteoblasts with the noted complexes and PLR tagged with FITC at a 1:100 FITC:PLRmer stoichiometric ratio.(+Silicate): No extracellular aggregation. DNA-PDGA-PLR: Efficient gene expression. Note the increased number of clustered particles. (+Silica): No extracellular aggregation. Significantly greater intracellular clustering of nanoparticles. DNA-HTP-PLR: Efficient gene expression. (+Silicate): No extracellular aggregation. Nuclear localization of nanoparticles is observed. DNA-HTP-PDGA-PLR: Efficient gene expression. (+Silicate): No extracellular aggregation. Nuclear localization and significant cytoplasmic accumulation is observed. (B) Transfection of GFP pDNA-loaded particles into murine bone marrow-derived cells to analyze retention of these particles in the primary cells for 3 weeks. FITC, fluorescein isothiocyanate; GFP, green fluorescent protein; HTP, histone tail peptide; PDGA, poly-d-glutamic acid; pDNA, plasmid DNA. Color images available online at www.liebertpub.com/tec
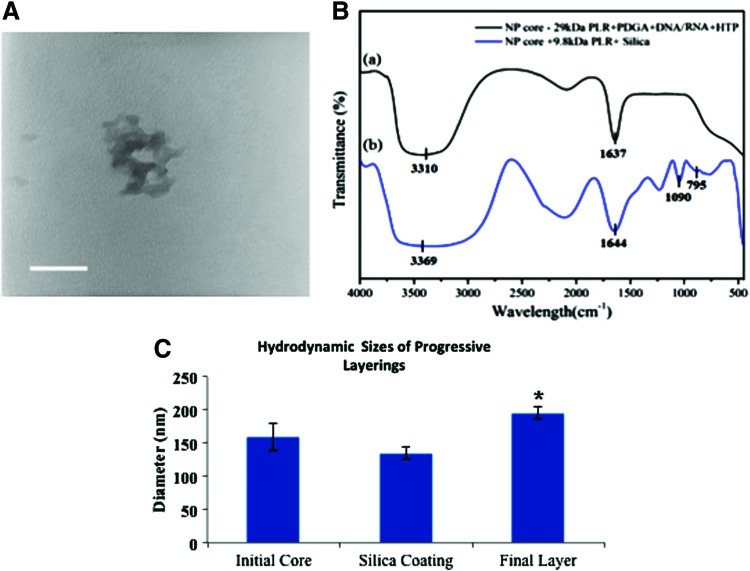
Morphologic analysis of silicate-polyplexed nanoparticles by transmission electron microscope clearly showed the core-shell structure (Fig. 2A). The average nanoparticle diameters are in the range from 120 to 170 nm with a narrow particle size distribution, which was consistent with dynamic light scattering analysis. The prepared nanoparticles were characterized by Fourier transform infrared (FTIR) spectra in (Fig. 2B). The characteristic absorbance peaks at 1637 and 3310 cm−1 corresponds to the amide group and N-H group stretching, respectively.18 In Figure 2B(b), the FTIR spectra of the core-shell particles show absorption bands at 1090 and 795 cm−1, which can be attributed to asymmetric vibration and symmetric vibration of Si-O bonds.19 The absorption peaks of the amide group and the N-H group shifted to 3369 and 1644 cm−1, which indicated their interaction with silicate shells.18 Therefore, the FTIR spectra conclusively indicated the formation of silicate coating.
FIG. 2.
(A) Transmission electron microscope of a desiccated and lyophilized nanoparticle. (B) FTIR of silica-stabilized nanoparticles and demonstration of further layering with PLR. (C) Dynamic light scattering of nanoparticles during core formation, silica stabilization, and further layering (*p<0.05). FTIR, Fourier transform infrared. Color images available online at www.liebertpub.com/tec
Silicate coating of ternary complexes was observed to condense nanoparticles (Fig. 2C). This could be explained by a number of factors, most likely due to the documented instability of nanocomplexes formed with polypeptides with heterogeneous charge distributions.20,21 Since formation of cores (152.6±8.5 nm) occurs within an acidic solution (pH ∼6), it is possible that silicate coating (140.0±5.1 nm) within an alkaline solution (pH=7.4) causes anionic components (PDGA and DNA) to increase their negative charge densities. Within the presence of anionic monomeric silicate species, the anionic nanoparticle constituents may seek to repulse themselves inward, exposing more cationic species for silicate condensation and oligomeric network formation and effectively sealing a “semi-stable” core within a shell of silicate. This phenomenon has recently been documented in the form of “dual-responsive” nanoparticles.15,22 Not unexpectedly, further layering on this silicate shell yielded a final particle with a hydrodynamic diameter increase of 15–30 nm (171.2±10.2 nm). The ostensibly loose polyplex morphology of these quaternary complexes also lends support to the idea that silation gels the polyplexes as a whole rather than merely at the surface.
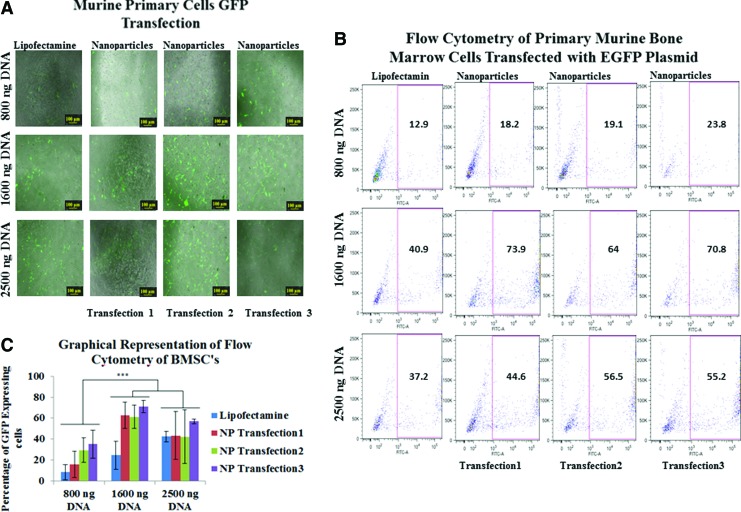
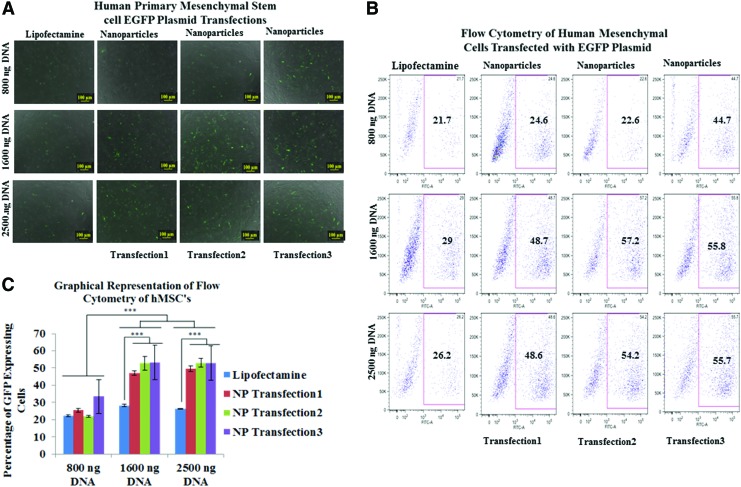
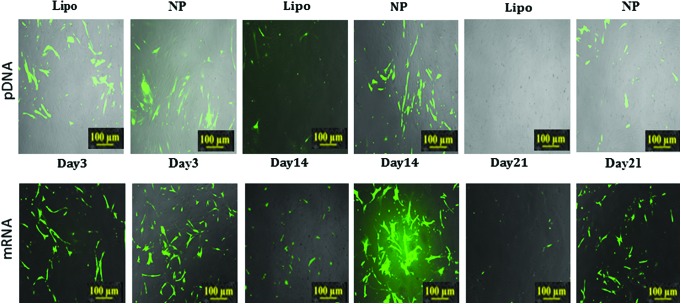
These multilayered nanoparticles demonstrated higher transfection efficiencies when compared with lipofectamine transfections in both primary bone marrow stromal cells (BMSCs) and primary human mesenchymal stem cells (HMSCs) (Figs. 3 and 4). Transfection efficiencies of pDNA in BMSCs were 1.41, 1.48, and 1.84 times higher for 800 ng; 1.8, 1.56, and 1.73 for 1600 ng; and 1.19, 1.15, and 1.48 for 2500 ng of transfected pDNA for single-, double-, and triple nanoparticle-mediated transfections (Fig. 3). Transfection efficiencies of pDNA in HMSCs were 1.14, 1.04, and 2.05 times higher for 800 ng; 1.67, 1.97, and 1.92 times for 1600 ng; and 1.85, 2.06, and 2.12 for 2500 ng (Fig. 4), compared with Lipofectamine for single, double, and triple transfections, respectively. Efficient expression of GFP in both cell types was obtained when using 1600 ng of pDNA, where expression was 130% higher in BMSCs and 70% higher in hMSCs relative to lipofectamine. There was a statistically significant difference between the percentage of GFP expressing cells transfected with pDNA using lipofectamine and nanoparticles for the single transfections whereas for repeated second and third transfection of nanoparticles, there was no statistically significant difference between the nanoparticle transfected groups for both BMSC's and HMSCs, at any of the concentrations of pDNA except for the transfection in HMSCs at 800 ng of pDNA concentration using lipofectamine compared with thrice nanoparticle transfected cell group. The pDNA and mRNA to GFP was transfected at 1600 ng concentration into the HFFs using nanoparticles and lipofectamine, and it was observed that the nanoparticle transfected cell groups showed greater expression compared with Lipofectamine over 21 days. However, due to its cytosolic burst release feature, Lipofectamine transfected cell group showed comparable transfection and expression efficacy at day 3. The duration of mRNA to GFP expression in HFFs was slightly longer as compared with duration of pDNA expression. This can be attributed to the fact that the half life of mRNA is longer than pDNA for expression of gene of interest. The GFP expression via mRNA as in case with pDNA was almost equal for lipofectamine and nanoparticle transfected cells at day 3 due to burst release profile of lipofectamine. However, again as in the case with pDNA expression, the cells transfected with nanoparticles showed a longer duration of expression (Fig. 5).
FIG. 3.
(A) Fluorescent microscopy of primary bone marrow stromal cells transfected with pDNA encoding GFP using 800, 1600, and 2500 ng of DNA introduced via a single transfection with Lipofectamine (left) and three transfections with nanoparticles on days 2, 3, and 4 (right). (B) Flow cytometry of identical transfection conditions. (C) Quantitative representation of transfection efficiency via flow cytometry (p<0.5). ***indicates that groups are statistically different from one other (p<0.5). Color images available online at www.liebertpub.com/tec
FIG. 4.
(A) Fluorescent microscopy of human mesenchymal stem cells transfected with pDNA encoding GFP using 800, 1600, and 2500 ng of DNA introduced via a single transfection with Lipofectamine 2000 (left) and three transfections with nanoparticles on days 2, 3, and 4 (right). (B) Flow cytometry of identical transfection conditions. (C) Quantitative representation of transfection efficiency via flow cytometry. Color images available online at www.liebertpub.com/tec
FIG. 5.
(Upper Panel) Fluorescent imaging of human foreskin fibroblasts (HFF) transfected with GFP pDNA—expression is assessed for more than 21 days (Lower Panel). Fluorescent imaging of HFFs transfected with GFP mRNA synthesized using in-vitro mRNA synthesis kit—expression is assessed for more than 21 days. Color images available online at www.liebertpub.com/tec
The cytotoxicity of these nanoparticles was assessed using Promokine Apoptotic/Necrotic/Healthy Cell Detection kit via flow cytometry in human dermal fibroblasts and HMSCs. There was a statistically significantly difference between the percentage of healthy cells in the cell groups transfected with nanoparticles as compared with the percentage of healthy cells in the cell groups transfected with Lipofectamine2000 with the cell group transfected with nanoparticles showing a fold higher viability as compared with Lipofectamine2000-transfected groups. This shows that these multilayered polymeric nanoparticles can be safe for delivery of functional nucleic acids in vitro as well as in vivo (Supplementary Fig. S3; Supplementary Data are available online at www.liebertpub.com/tec).
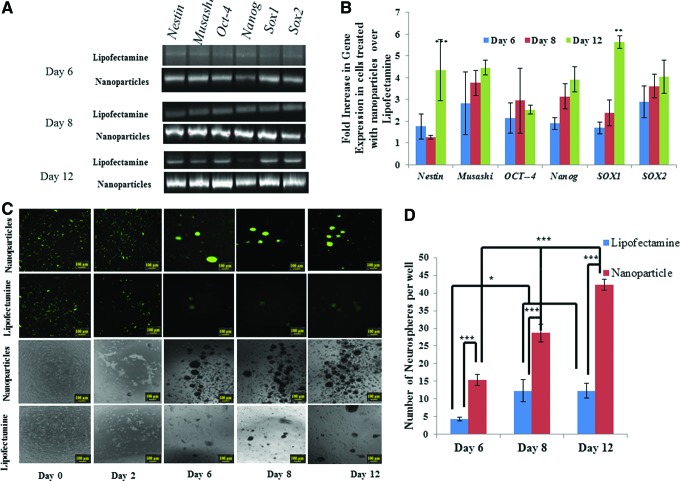
Neurospheres are free-floating spherical condensations of cells with neural stem/progenitor cell characteristics that can be derived by reprogramming of HFFs using Sox2 transcription factor.4 The efficiency of generating neurospheres on delivery ofSox2 mRNA to fibroblasts was compared using the two delivery vectors (naonoparticles and Lipofectamine). The number of neurospheres in cultures transfected with nanoparticles was found to be three-fold higher at 12 days post-transfection (Fig. 6). Nanoparticle transfection of Sox2 mRNA had altered the gene expression profile of cells in the affected cultures. Fibroblasts such as HFFs can be characterized by a gene expression profile that includes high levels of expression of the Col1a1, Col2a1, and Fap genes, but on transfection, many neural progenitor and pluripotency genes are upregulated, such as Nanog, Oct4, Sox2, Sox1, Nestin, and Musashi. Primers and Ct values for qPCR are given in Supplementary Tables S2 and S3, respectively. Expression patterns of these genes were examined through quantitative reverse transcription polymerase chain reaction analysis for days 6, 8, and 12 post-transfection. Figure 6A and B shows the expression levels of each gene after nanoparticle transfections with reference to Lipofectamine-induced transfection. Most of the neural progenitor and pluripotency genes were expressed at levels two-fold or greater in nanoparticle-transfected cultures compared with Lipofectamine-transfected cultures at all time points. All of the genes analyzed increased in expression through day 12 except Oct4, in which no significant change in expression occurred between the three time points. Sox2 expression pattern was included in the analysis to determine differential delivery patterns between Lipofectamine and nanoparticles. The steady increase in Sox2 expression levels till four-fold over Lipofectamine delivery shows that nanoparticles continue to provide Sox2 delivery through day 12, which subsequently increases expression of other marker genes, indicative of a neuroprogenitor. Sox1 is a particularly strong indicator of neural induction, as it is one of the earliest transcription factors expressed in ectodermal cells committed to a neural lineage and upregulation of Sox1 is directly correlated to neural determination and differentiation.23,24 As shown in Figure 6C, fibroblast reprogramming using nanoparticles to deliver Sox2 led to an eight-fold increase in Sox1 expression over untreated cells and a 6-fold increase over Lipofectamine by day 12. This indicated increased levels of neural differentiation in the cells treated with Sox2 pDNA via this silica-stabilized and multistage polyplex delivery system.
FIG. 6.
(A, B) Gene expression analysis of HFFs transfected with Sox2 transcription factor using nanoparticles and Lipofectamine2000. The fold increase in gene expression was determined using ΔΔCt formula normalized against gene expression of cells treated with Lipofectamine2000. (C) Transfection of HFFs with GFP mRNA and Sox2 mRNA using nanoparticles and Lipofectamine. (D) Number of neurospheres generated by transfections HFFs with Sox2 using nanoparticles and Lipofectamine2000. * and *** indicates that groups are statistically different from one other (p<0.05). Color images available online at www.liebertpub.com/tec
In this work, we demonstrate that a coating of a silicate gel core with PLR yields a cationic nanoparticle with potentially useful therapeutic nucleic acid delivery properties that include high pDNA/mRNA binding ability as well as high rates of cellular uptake and extended release into the nucleus. We have shown that pDNA/mRNA complexed with PDGA, PLR, and HTP and stabilized by silicate before a further layering by PLR can induce effective delivery of nucleic acid payloads into the cell to initiate protein translation of a pDNA sequence, as well as reprogramming of differentiated cells into a desired phenotype using time-released mRNA. In early qualitative studies, quaternary core complexes composed of (pDNA/RNA+PDGA+PLR+HTP) also demonstrated significantly enhanced transfection into the murine osteoblast cell lines (MC3T3-E1) (Fig. 1A) along with greater gene expression of mCherry pDNA.
Cationic polymers such as poly(ethylenimine), chitosan, poly(L-arginine), poly(L-ornithine), and poly(L-lysine) form relatively stable complexes that are capable of mediating effective gene transfer at various amines to phosphate ratios (n/p), where the “n” to “p” ratio determines the quantity of DNA that can be complexed with the polymer. Using improvements to mere electrostatic binding between such polymers, pDNA delivery was shown to be much more efficient compared with the commercially available transfection reagent Lipofectamine. We have successfully demonstrated the increased efficacy conferred by multilayered complexes designed for nuclear-specific unpackaging and biocompatibility.
The positive charge of PLR in the outermost coating of nanoparticles leads to a strong electrostatic interaction with the negatively charged cell surface membrane, leading to facilitated particle wrapping and uptake (Fig. 1A). This is in agreement with the recent demonstration that poly-ethyleneimine (PEI) nanoparticles, which are similarly negatively charged, are taken up into the cells with high efficiency.25 However, the latter study did not look at nucleic acid delivery but showed that the attachment of ligand such as folic acid further enhances uptake in cancer cells.26 Previous research has been done to study PEI delivery with silica as well as silica delivery of DNA to find the optimal nanoparticle composition to get enhanced transfection efficiencies.26–28 Cationic polymers bind to sulfated proteoglycans, which act as cellular receptors on cholesterol-rich lipid rafts—this characteristic makes them excellent for ubiquitous targeting in vitro.29 Carboxylate-containing polymers have been shown to further influence DNA-polymer complexation and release kinetics, where±ratios remain similar but additional negative moieties serve to promote electrostatic unpackaging during cellular uptake while increased surface area due to increased number of polyplexes facilitates transfection.30–33 This complexation is frequently observed to be reversed after exposure to the multitude of proteins, salts, and other molecules present within serum, so standard transfections frequently utilize serum-free medium; indeed, heparin, dextran sulfate, and alginate are well documented as destabilizing cationic polyplexes.
A paradox is that, with DNA constructs, the disassociation of DNA from polymer is desired within the nucleus. To achieve this compartment-specific unpackaging and efficient gene transfer to the nucleus, numerous studies have utilized cationic histone-mimetic peptides, which endogenously are nuclear localized and subjected to various modifications, including deprotonation by acetyl-CoA and histone acetyltransferase.34–36 It has been previously shown that histone H3 tail peptides (HTP) formed polyplexes that interacted with the transcriptionally activating HAT complex HBO1, and that polyplexes containing H3K4Me3 achieved fast pDNA transcription after microinjection into cellular nuclei.35,37 Building on this work, this study aimed at exploiting this very property of trimethymated histone tail peptides to gain enhanced gene transcription on delivery, holding off exposure of the moieties until endosomal internalization leads to acidifying conditions that dissolve the transiently stabilizing silicate. In addition, due to the intrinsic nature of histones and their exclusive existence within the nucleus, domains for histone winding are similar to domains for nuclear-specific targeting mediated by karyopherin and importin binding to highly conserved lysine- and arginine-rich regions.38–42 Therefore, the quaternary core complexes composed of (pDNA/mRNA+PDGA+PLR+HTP) demonstrated significantly enhanced transfection into the murine osteoblast cell line (MC3T3-E1) (Fig. 1A) along with greater gene expression of mCherry pDNA. However, it has been previously observed that trimethylated HTPs enhance the rate of gene expression but do not greatly enhance the rate of transfection as compared with nonmethylated HTPs. This might be because of the presence of histone methyltransferases in the nucleus of the cell. These methyltransferases might be interacting with and methylating the nonmethylated HTP, although at a slower rate. Therefore, this effect can potentially outweigh any initial advantages in transcriptional activation by the pretrimethylatedpolyplexes. Since the deprotonations induced by HTP's occur at key arginine and lysine residues, it is possible that poly(L-arginine) may also act as a substrate for such enzymatic activity by methyl and acetyltransferases, among others.43–45 One unfortunate effect of cationic polymer transfections is that the majority of the payload is dissociated during initial phases of uptake (perhaps through interaction with the anionic cell membrane),46 and that the remaining payload frequently remains bound to its cationic carrier during nuclear dispersion. In seeking to mediate many of the outlined inefficiencies, recent studies have demonstrated oligomeric silicate coating of bare polyplexes to effectively seal the payload during initial phases of uptake, additionally preventing destabilization by polyanions and contributing to buffering during endosomal uptake (via the proton sponge effect, a phenomenon whereby lysosomes are prematurely ruptured due to osmotic influx).15,47–49 Further layering on this anionic silicate layer presents a highly functionalized nanoparticle surface with a stable core capable of controlled nucleic acid release. Our results suggest that silicate is capable of reversibly stabilizing highly complex and otherwise readily destabilizable quaternary cores of nanoparticles, which subsequently are released into the cell and take advantage of many of the outlined benefits of histone tail peptides, cationic polymers, and anionic polymers. We posit that use of D-isomers of poly (glutamic acid) as an anionic polymer presents a time-release element to our polymeric delivery system, due to the fact that D-isomers are unlikely to be proteolytically degraded and have been documented to have limited immunogenicity.50–52 If a nanoparticle's charge becomes increasingly anionic during its residence within the cell, it will be more likely to repulse anionic nucleic acids as has been previously documented with poly(glutamic acid)'s inclusion within vectors.30,31 We observed no visible polyplex internalization 30 h after transfection with silicate-coated poly (L-arginine)-DNA complexes (despite gene expression), while similar complexes containing poly(D-glutamic acid) are capable of sustaining prolonged release over the course of roughly a week and maintaining gene expression in 55–70% transfected cells (Fig. 4). In addition, our multilayered polyplexes are stable in serum for more than 1 week, while their cores rapidly destabilize in the presence of anionic polymers alone.
Using the nanotechnology here, we demonstrate superior expression of pDNA in primary murine adherent bone marrow cells and primary human mesenchymal cells. We also demonstrate reprogramming of primary HFF cells using nonviral nanocarriers. These carriers, which consist of biomimetic polypeptides and biocompatible materials, condense genetic payloads (pDNA and mRNA) with high efficacy and help achieve an optimum extracellular stability and intracellular unpackaging and release. This technology is based on a few simple tenets: If a “semi-stable” particle on the threshold of its stability can be released into the cytosol, functionalization will greatly increase payload delivery. In addition, the trigger for this loss of stability can be anything ranging from hydrolytic and enzymatic degradation to conformational change.
Supplementary Data
Supplementary Data contains detailed methods involved in formation of the dual layered core-shell structure nanoparticle for the optimization of nanoparticle design for transfection into murine osteoblast cell line and primary bone marrow cells along with detailed calculations on the optimized concentrations of each of the components and their interactions with each other.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the funding from NIH/NIA grant R01 AG030637. They are grateful for the guidance rendered by Dr. Deanna Thompson at RPI for her help with examination of neurospheres. They are also thankful to Aniket Tolpadi for all the help with the design of figures and artwork for the nanoparticle schematic used in this article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Musick M.A., McConnell K.I., Lue J.K., Wei F., Chen C., and Suh J. Reprogramming virus nanoparticles to bind metal ions upon activation with heat. Biomacromolecules 12, 2153, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Li H., Park S.H., Reif J.H., LaBean T.H., and Yan H. DNA-templated self-assembly of protein and nanoparticle linear arrays. J Am Chem Soc 126, 418, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Seo E.J., Jang I.H., Do E.K., Cheon H.C., Heo S.C., Kwon Y.W., Jeong G.O., Kim B.R., and Kim J.H. Efficient production of retroviruses using PLGA/bPEI-DNA nanoparticles and application for reprogramming somatic cells. PLoS One 8, e76875, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ring K.L., Tong L.M., Balestra M.E., Javier R., Andrews-Zwilling Y., Li G., Walker D., Zhang W.R., Kreitzer A.C., and Huang Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 11, 100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huangfu D., Osafune K., Maehr R., Guo W., Eijkelenboom A., Chen S., Muhlestein W., and Melton D.A. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 26, 1269, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Al-Dosari M.S., and Gao X. Nonviral gene delivery: principle, limitations, and recent progress. AAPS J 11, 671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lungwitz U., Breunig M., Blunk T., and Göpferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm 60, 247, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Ditto A.J., Shah P.N., and Yun Y.H. Non-viral gene delivery using nanoparticles. Expert Opin Drug Deliv 6, 1149, 2009 [DOI] [PubMed] [Google Scholar]
- 9.De Smedt S.C., Demeester J., and Hennink W.E. Cationic polymer based gene delivery systems. Pharm Res 17, 113, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Du J.-Z., Du X.-J., Mao C.-Q., and Wang J. Tailor-made dual pH-sensitive polymer–doxorubicin nanoparticles for efficient anticancer drug delivery. J Am Chem Soc 133, 17560, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Eliyahu H., Barenholz Y., and Domb A. Polymers for DNA delivery. Molecules 10, 34, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godbey W., Wu K.K., and Mikos A.G. Tracking the intracellular path of poly (ethylenimine)/DNA complexes for gene delivery. Proc Natl Acad Sci U S A 96, 5177, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi J., Chou B., Choi J.L., Ta A.L., and Pun S.H. Investigation of polyethylenimine/DNA polyplex transfection to cultured cells using radiolabeling and subcellular fractionation methods. Mol Pharm 10, 2145, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rungsardthong U., Ehtezazi T., Bailey L., Armes S.P., Garnett M.C., and Stolnik S. Effect of polymer ionization on the interaction with DNA in nonviral gene delivery systems. Biomacromolecules 4, 683, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Miyata K., Gouda N., Takemoto H., Oba M., Lee Y., Koyama H., Yamasaki Y., Itaka K., Nishiyama N., and Kataoka K. Enhanced transfection with silica-coated polyplexes loading plasmid DNA. Biomaterials 31, 4764, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Wong S.Y., Pelet J.M., and Putnam D. Polymer systems for gene delivery—past, present, and future. Prog Polym Sci 32, 799, 2007 [Google Scholar]
- 17.Jeong J.H., Kim S.W., and Park T.G. Molecular design of functional polymers for gene therapy. Prog Polym Sci 32, 1239, 2007 [Google Scholar]
- 18.Lakard S., Herlem G., Lakard B., and Fahys B. Theoretical study of the vibrational spectra of polyethylenimine and polypropylenimine. J Mol Struct Theochem 685, 83, 2004 [Google Scholar]
- 19.Beganskienė A., Sirutkaitis V., Kurtinaitienė M., Juškėnas R., and Kareiva A. FTIR, TEM and NMR investigations of Stöber silica nanoparticles. Mater Sci (Medžiagotyra) 10, 287, 2004 [Google Scholar]
- 20.Reilly M.J., Larsen J.D., and Sullivan M.O. Polyplexes traffic through caveolae to the Golgi and endoplasmic reticulum en route to the nucleus. Mol Pharm 9, 1280, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Millili P.G., Selekman J.A., Blocker K.M., Johnson D.A., Naik U.P., and Sullivan M.O. Structural and functional consequences of poly (ethylene glycol) inclusion on DNA condensation for gene delivery. Microsc Res Tech 73, 866, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y., Litwin T., Nagaraja A.R., Kwong B., Katz J., Watson N., and Irvine D.J. Cytosolic delivery of membrane-impermeable molecules in dendritic cells using pH-responsive core-shell nanoparticles. Nano Lett 7, 3056, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Yau W.W.Y., Rujitanaroj P.-o., Lam L., and Chew S.Y. Directing stem cell fate by controlled RNA interference. Biomaterials 33, 2608, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Roper S., and Hemberger M. Defining pathways that enforce cell lineage specification in early development and stem cells. Cell Cycle 8, 1515, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Baker A., Saltik M., Lehrmann H., Killisch I., Mautner V., Lamm G., Christofori G., and Cotten M. Polyethylenimine (PEI) is a simple, inexpensive and effective reagent for condensing and linking plasmid DNA to adenovirus for gene delivery. Gene Ther 4, 773, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Xia T., Kovochich M., Liong M., Meng H., Kabehie S., George S., Zink J.I., and Nel A.E. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano 3, 3273, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corso T.D., Torres G., Goulah C., Roy I., Gambino A.S., Nayda J., Buckley T., Stachowiak E.K., Bergey E.J., and Pudavar H. Assessment of viral and non-viral gene transfer into adult rat brains using HSV-1, calcium phosphate and PEI-based methods. Folia Morphol (Warsz) 64, 130, 2005 [PubMed] [Google Scholar]
- 28.Kneuer C., Sameti M., Haltner E.G., Schiestel T., Schirra H., Schmidt H., and Lehr C.-M. Silica nanoparticles modified with aminosilanes as carriers for plasmid DNA. Int J Pharm 196, 257, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Hess G.T., Humphries IV W.H., Fay N.C., and Payne C.K. Cellular binding, motion, and internalization of synthetic gene delivery polymers. Biochim Biophys Acta 1773, 1583, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao Z.-X., Ho Y.-C., Chen H.-L., Peng S.-F., Hsiao C.-W., and Sung H.-W. Enhancement of efficiencies of the cellular uptake and gene silencing of chitosan/siRNA complexes via the inclusion of a negatively charged poly (γ-glutamic acid). Biomaterials 31, 8780, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Peng S.-F., Yang M.-J., Su C.-J., Chen H.-L., Lee P.-W., Wei M.-C., and Sung H.-W. Effects of incorporation of poly (γ-glutamic acid) in chitosan/DNA complex nanoparticles on cellular uptake and transfection efficiency. Biomaterials 30, 1797, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Douglas K.L., Piccirillo C.A., and Tabrizian M. Effects of alginate inclusion on the vector properties of chitosan-based nanoparticles. J Control Release 115, 354, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Douglas K.L., and Tabrizian M. Effect of experimental parameters on the formation of alginate–chitosan nanoparticles and evaluation of their potential application as DNA carrier. J Biomater Sci Polym Ed 16, 43, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Fritz J.D., Herweijer H., Zhang G., and Wolff J.A. Gene transfer into mammalian cells using histone-condensed plasmid DNA. Hum Gene Ther 7, 1395, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Wagstaff K.M., Glover D.J., Tremethick D.J., and Jans D.A. Histone-mediated transduction as an efficient means for gene delivery. Mol Ther 15, 721, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Li H., Luo T., Sheng R., Sun J., Wang Z., and Cao A. Endoplasmic reticulum localization of poly (ω-aminohexyl methacrylamide) s conjugated with (l-)-arginines in plasmid DNA delivery. Biomaterials 34, 7923, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Reilly M.J., Larsen J.D., and Sullivan M.O. Histone H3 tail peptides and poly (ethylenimine) have synergistic effects for gene delivery. Mol Pharm 9, 1031, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Mosammaparast N., Jackson K.R., Guo Y., Brame C.J., Shabanowitz J., Hunt D.F., and Pemberton L.F. Nuclear import of histone H2A and H2B is mediated by a network of karyopherins. J Cell Biol 153, 251, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosammaparast N., Guo Y., Shabanowitz J., Hunt D.F., and Pemberton L.F. Pathways mediating the nuclear import of histones H3 and H4 in yeast. J Biol Chem 277, 862, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Mühlhäusser P., Müller E.C., Otto A., and Kutay U. Multiple pathways contribute to nuclear import of core histones. EMBO Rep 2, 690, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmeri D., and Malim M.H. Importin β can mediate the nuclear import of an arginine-rich nuclear localization signal in the absence of importin α. Mol Cell Biol 19, 1218, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fagerlund R., Melen K., Kinnunen L., and Julkunen I. Arginine/lysine-rich nuclear localization signals mediate interactions between dimeric STATs and importin α5. J Biol Chem 277, 30072, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Smith B.C., and Denu J.M. Chemical mechanisms of histone lysine and arginine modifications. Biochim Biophys Acta 1789, 45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frankel A., Yadav N., Lee J., Branscombe T.L., Clarke S., and Bedford M.T. The novel human protein arginine N-methyltransferase PRMT6 is a nuclear enzyme displaying unique substrate specificity. J Biol Chem 277, 3537, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Onozato M.L., Tojo A., Leiper J., Fujita T., Palm F., and Wilcox C.S. Expression of NG, NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney effects of angiotensin II receptor blockers. Diabetes 57, 172, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Shi J., Chou B., Choi J.L., Ta A.L., and Pun S.H. Investigation of polyethylenimine/DNA polyplex transfection to cultured cells using radiolabeling and subcellular fractionation methods. Mol Pharm 10, 2145, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gouda N., Miyata K., Christie R.J., Suma T., Kishimura A., Fukushima S., Nomoto T., Liu X., Nishiyama N., and Kataoka K. Silica nanogelling of environment-responsive PEGylated polyplexes for enhanced stability and intracellular delivery of siRNA. Biomaterials 34, 562, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Behr J.-P. The proton sponge: a trick to enter cells the viruses did not exploit. CHIMIA Int J Chem 51, 1, 1997 [Google Scholar]
- 49.Freeman E.C., Weiland L.M., and Meng W.S. Modeling the proton sponge hypothesis: examining proton sponge effectiveness for enhancing intracellular gene delivery through multiscale modeling. J Biomater Sci Polym Ed 24, 398, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tugyi R., Uray K., Iván D., Fellinger E., Perkins A., and Hudecz F. Partial D-amino acid substitution: Improved enzymatic stability and preserved Ab recognition of a MUC2 epitope peptide. Proc Natl Acad Sci U S A 102, 413, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chu D.S., Johnson R.N., and Pun S.H. Cathepsin B-sensitive polymers for compartment-specific degradation and nucleic acid release. J Control Release 157, 445, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welch B.D., VanDemark A.P., Heroux A., Hill C.P., and Kay M.S. Potent D-peptide inhibitors of HIV-1 entry. Proc Natl Acad Sci U S A 104, 16828, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.