Abstract
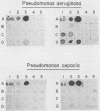
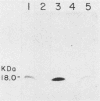
Much of the morbidity and mortality in patients with cystic fibrosis (CF) is secondary to pulmonary infections with Pseudomonas aeruginosa and, more recently, with Pseudomonas cepacia. Prevention of colonization and subsequent infection would be a useful therapeutic strategy. The pili (fimbriae) of P. aeruginosa are a potential vaccine antigen, as they have been implicated in binding to respiratory epithelium and appear to have limited antigenic diversity. Monoclonal antibodies (MAbs) raised to P. aeruginosa pilin demonstrated significant cross-reactivity, as four of five P. aeruginosa strains with known pilin sequences and 10 of 15 P. aeruginosa clinical isolates hybridized by immunoblot with at least one of the three MAbs tested. The P. cepacia strains demonstrated minimal cross-reactivity with these MAbs, as only 2 of 16 strains hybridized immunologically. The three MAbs decreased the adherence of 35S-labeled P. aeruginosa PA1244 to bovine tracheal cells by 56, 45, and 31%. One of these MAbs decreased the adherence of strains P. aeruginosa PAO1 and P. cepacia 249 to CF epithelial cells by 46 and 25%, respectively. While antibodies to Pseudomonas pili must be shown to be protective in patients with CF, these studies give support for a multivalent vaccine strategy using P. aeruginosa pilin as the immunogen.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman W., Lessie T. G. Response of Pseudomonas cepacia to beta-Lactam antibiotics: utilization of penicillin G as the carbon source. J Bacteriol. 1979 Dec;140(3):1126–1128. doi: 10.1128/jb.140.3.1126-1128.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castric P. A., Sidberry H. F., Sadoff J. C. Cloning and sequencing of the Pseudomonas aeruginosa 1244 pilin structural gene. Mol Gen Genet. 1989 Mar;216(1):75–80. doi: 10.1007/BF00332233. [DOI] [PubMed] [Google Scholar]
- Doig P., Todd T., Sastry P. A., Lee K. K., Hodges R. S., Paranchych W., Irvin R. T. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect Immun. 1988 Jun;56(6):1641–1646. doi: 10.1128/iai.56.6.1641-1646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T. C. Pilins of Bacteroides nodosus: molecular basis of serotypic variation and relationships to other bacterial pilins. Microbiol Rev. 1988 Jun;52(2):233–247. doi: 10.1128/mr.52.2.233-247.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann D. A., Klinger J. D. Pseudomonas cepacia: biology, mechanisms of virulence, epidemiology. J Pediatr. 1986 May;108(5 Pt 2):806–812. doi: 10.1016/s0022-3476(86)80749-1. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K., Parker M. L., Lory S. Nucleotide sequence and transcriptional initiation site of two Pseudomonas aeruginosa pilin genes. J Biol Chem. 1986 Nov 25;261(33):15703–15708. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lee K. K., Sastry P. A., Paranchych W., Hodges R. S. Immunological studies of the disulfide bridge region of Pseudomonas aeruginosa PAK and PAO pilins, using anti-PAK pilus and antipeptide antibodies. Infect Immun. 1989 Feb;57(2):520–526. doi: 10.1128/iai.57.2.520-526.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. W., Alexander B., McGowan B. Purification, characterization, and serologic characteristics of Bacteroides nodosus pili and use of a purified pili vaccine in sheep. Am J Vet Res. 1983 Sep;44(9):1676–1681. [PubMed] [Google Scholar]
- Paranchych W., Frost L. S. The physiology and biochemistry of pili. Adv Microb Physiol. 1988;29:53–114. doi: 10.1016/s0065-2911(08)60346-x. [DOI] [PubMed] [Google Scholar]
- Pasloske B. L., Finlay B. B., Paranchych W. Cloning and sequencing of the Pseudomonas aeruginosa PAK pilin gene. FEBS Lett. 1985 Apr 22;183(2):408–412. doi: 10.1016/0014-5793(85)80821-8. [DOI] [PubMed] [Google Scholar]
- Pasloske B. L., Joffe A. M., Sun Q., Volpel K., Paranchych W., Eftekhar F., Speert D. P. Serial isolates of Pseudomonas aeruginosa from a cystic fibrosis patient have identical pilin sequences. Infect Immun. 1988 Mar;56(3):665–672. doi: 10.1128/iai.56.3.665-672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasloske B. L., Sastry P. A., Finlay B. B., Paranchych W. Two unusual pilin sequences from different isolates of Pseudomonas aeruginosa. J Bacteriol. 1988 Aug;170(8):3738–3741. doi: 10.1128/jb.170.8.3738-3741.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J Infect Dis. 1985 Apr;151(4):575–580. doi: 10.1093/infdis/151.4.575. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Sadoff J. C., Pyle M., Silipigni J. D. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect Immun. 1984 Apr;44(1):38–40. doi: 10.1128/iai.44.1.38-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon J., Morrison S. L., Kabat E. A. Detection of specific hybridoma clones by replica immunoadsorption of their secreted antibodies. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1420–1424. doi: 10.1073/pnas.76.3.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M., Billyard E., Deal C., Getzoff E., Hagblom P., Meyer T. F., Segal E., Tainer J. Gonococcal pilus: genetics and structure. Curr Top Microbiol Immunol. 1985;118:13–28. doi: 10.1007/978-3-642-70586-1_2. [DOI] [PubMed] [Google Scholar]
- Spence J. E., Buffone G. J., Rosenbloom C. L., Fernbach S. D., Curry M. R., Carpenter R. J., Ledbetter D. H., O'Brien W. E., Beaudet A. L. Prenatal diagnosis of cystic fibrosis using linked DNA markers and microvillar intestinal enzyme analysis. Hum Genet. 1987 May;76(1):5–10. doi: 10.1007/BF00283042. [DOI] [PubMed] [Google Scholar]
- Tramont E. C., Boslego J. W. Pilus vaccines. Vaccine. 1985 Mar;3(1):3–10. doi: 10.1016/0264-410x(85)90003-9. [DOI] [PubMed] [Google Scholar]
- Tsao M. C., Walthall B. J., Ham R. G. Clonal growth of normal human epidermal keratinocytes in a defined medium. J Cell Physiol. 1982 Feb;110(2):219–229. doi: 10.1002/jcp.1041100217. [DOI] [PubMed] [Google Scholar]
- Valdivia H. H., Dubinsky W. P., Coronado R. Reconstitution and phosphorylation of chloride channels from airway epithelium membranes. Science. 1988 Dec 9;242(4884):1441–1444. doi: 10.1126/science.2462280. [DOI] [PubMed] [Google Scholar]
- Wheeler W. B., Williams M., Matthews W. J., Jr, Colten H. R. Progression of cystic fibrosis lung disease as a function of serum immunoglobulin G levels: a 5-year longitudinal study. J Pediatr. 1984 May;104(5):695–699. doi: 10.1016/s0022-3476(84)80946-4. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H., Coleman D. L., Finkbeiner W. E., Tuet I. K. Electrical properties of monolayers cultured from cells of human tracheal mucosa. J Appl Physiol (1985) 1985 May;58(5):1729–1735. doi: 10.1152/jappl.1985.58.5.1729. [DOI] [PubMed] [Google Scholar]
- Wilcken B., Brown A. R., Urwin R., Brown D. A. Cystic fibrosis screening by dried blood spot trypsin assay: results in 75,000 newborn infants. J Pediatr. 1983 Mar;102(3):383–387. doi: 10.1016/s0022-3476(83)80653-2. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Yankaskas J., Cheng E., Knowles M. R., Boucher R. Growth and differentiation of human nasal epithelial cells in culture. Serum-free, hormone-supplemented medium and proteoglycan synthesis. Am Rev Respir Dis. 1985 Aug;132(2):311–320. doi: 10.1164/arrd.1985.132.2.311. [DOI] [PubMed] [Google Scholar]