Abstract
The Hippo signaling pathway is important for controlling organ size and tissue homeostasis. Originally identified in Drosophila melanogaster, the core components of the Hippo pathway are highly conserved in mammals. The Hippo pathway can be modulated by a wide range of stimuli, including G protein coupled receptor (GPCR) signaling, changes in the actin cytoskeleton, cell-cell contact, and cell polarity. When activated, the Hippo pathway functions as a tumor suppressor to limit cell growth. However, dysregulation by genetic inactivation of core pathway components, or amplification or gene fusion of its downstream effectors, results in increased cell proliferation and decreased apoptosis and differentiation. Not surprisingly, this can lead to tissue overgrowth, tumorigenesis, and many other diseases.
Keywords: Hippo, YAP, TAZ, cancer, disease
The Hippo pathway: a kinase cascade to regulate YAP/TAZ
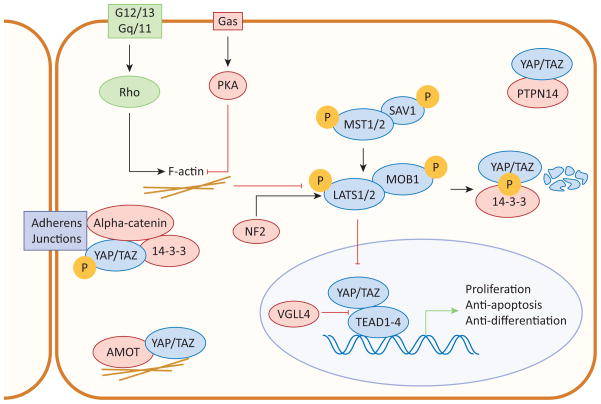
In mammals the Hippo pathway consists of a kinase cascade of Mammalian STE20-like 1/2 (MST1/2; see Glossary) and Large tumor suppressor 1/2 (LATS1/2; Figure 1). MST forms a heterodimer with the adaptor protein Salvador 1 (SAV1), which enhances MST kinase activity and facilitates MST-LATS interaction [1, 2]. MST then directly phosphorylates Mob1 homolog (MOB1) and LATS. Phosphorylated MOB1 binds to the autoinhibitory region of LATS, enabling LATS phosphorylation and activation [3, 4]. Once activated, LATS phosphorylates the main effectors of the Hippo pathway, transcriptional co-activators Yes-associated protein (YAP) and WW domain containing transcription factor (WWTR1 or TAZ) [5–7]. LATS kinase activity inhibits YAP/TAZ transcriptional activity by altering YAP/TAZ localization and protein stability.
Figure 1. The Hippo signaling pathway.
When the Hippo pathway is activated, MST1/2 in complex with SAV1 phosphorylate and activate LATS1/2 and MOB1. When activated, LATS1/2 phosphorylates YAP/TAZ, the primary effectors of the Hippo pathway. When phosphorylated, YAP/TAZ are sequestered in the cytoplasm or degraded. When YAP/TAZ are dephosphorylated, they translocate to the nucleus where they interact with TEAD1-4 to induce transcription and promote cell proliferation and inhibit apoptosis. Arrows and blunt ends indicate activation and inhibition, respectively.
Phosphorylated YAP/TAZ are sequestered in the cytoplasm by binding to 14-3-3 and are ubiquitinated and degraded [8–10]. It was also found in Drosophila that phosphorylated Yorkie (the Drosophila ortholog of YAP/TAZ) is actively excluded from the nucleus in an Exportin 1 (XPO1)-dependent manner [11]. When LATS are inactive, dephosphorylated YAP/TAZ translocate to the nucleus to initiate transcription [7, 11–13]. YAP/TAZ do not contain their own DNA-binding motifs and initiate transcription by interacting with the DNA-binding transcription factors TEA domain family members 1–4 (TEAD1-4) [14–17]. Through these interactions, YAP/TAZ induce the expression of genes regulating proliferation, differentiation, and apoptosis. YAP/TAZ also interact with other transcription factors including SMAD family members (Smad), p63/p73, Paired box 3 (Pax3), and T-box transcription factor 5 (TBX5) [18, 19]. However, the roles of these transcription factors in mediating the growth promoting activities of YAP/TAZ have not yet been established.
The Hippo pathway can be regulated at many levels. For instance, YAP/TAZ nuclear localization can be modulated by cell-cell contact [8, 20]. Neurofibromin 2 (NF2) is a tumor suppressor localized near adherens and tight junctions. NF2 mediates contact inhibition by recruiting LATS to the cell membrane, where it is phosphorylated and activated by MST and SAV1 [21]. In addition, nuclear NF2 inhibits E3 ubiquitin ligase CRL4DCAF1-mediated LATS degradation, resulting in LATS accumulation and YAP phosphorylation and inactivation [22]. Several components of adherens and tight junctions, including Angiomotin (AMOT), α-catenin, and protein tyrosine phosphatase non-receptor 14 (PTPN14) also directly interact with the Hippo pathway. AMOT induces LATS2-mediated YAP phosphorylation and sequesters YAP/TAZ to tight junctions, inhibiting YAP’s co-transcriptional activity [23–26]. However, a recent report found that AMOT can stimulate YAP activity by two mechanisms: (i) binding YAP in the cytoplasm and preventing its phosphorylation by LATS; and (ii) by forming a transcriptional complex with YAP and TEAD in the nucleus to induce transcription of YAP downstream target genes [27]. These seemingly contradictory results could be due to tissue or context-specific roles of AMOT in regulating the Hippo pathway. α-catenin forms a trimeric complex with phosphorylated YAP and 14-3-3, sequestering YAP to adherens junctions and preventing its dephosphorylation [28]. PTPN14 directly binds and sequesters YAP in the cytoplasm [29, 30]. Together, these findings illustrate how the cell’s surroundings tightly regulate the Hippo pathway.
Wnt signaling and extracellular hormones can also regulate YAP/TAZ activity [31]. A large number of hormones act through G protein coupled receptors (GPCRs) to either activate or inhibit YAP/TAZ. Serum, Lysophosphatidic acid (LPA), Sphingosine-1-phosphate (S1P), and thrombin signal via G12/13 and Gq/11 to activate downstream Rho GTPases, modulate the actin cytoskeleton, and activate YAP/TAZ [32]. Conversely, epinephrine and glucagon signal via Gαs to activate protein kinase A (PKA), modulate the actin cytoskeleton, and induce YAP/TAZ phosphorylation [32]. Although it is clear that changes in actin cytoskeleton dynamics are important for mediating upstream signals to regulate YAP/TAZ, the full mechanism is unknown. GPCRs play an important role in modulating a wide range of cellular processes, including cell proliferation and survival, and it is likely that some of these functions are mediated through the Hippo pathway.
The Hippo pathway in regeneration and development
The Hippo pathway in liver regeneration
The liver has a remarkable ability to regenerate following injury. Although the primary source of new tissue during regeneration is proliferating hepatocytes, hepatocytes are quiescent under normal conditions. MST1/2 inactivation is required to wake hepatocytes out of quiescence, suggesting the Hippo pathway and active YAP/TAZ play a necessary role in initiating regeneration [33]. Hepatocyte-specific MST1/2 knockout is sufficient to dramatically increase hepatocyte proliferation, resulting in massive liver overgrowth due to aberrant YAP activity [34]. In addition, liver overgrowth caused by inducible, liver-specific YAP overexpression is due to an increase in hepatocyte cell number, not cell size, suggesting that regulation of YAP/TAZ is sufficient to initiate cell proliferation in quiescent hepatocytes [13]. Indeed, recent work has shown that inducing expression of a constitutively active YAP in hepatocytes in vivo can cause hepatocytes to dedifferentiate back into progenitor cells, which may have important implications in our understanding of the mechanisms behind liver regeneration [35].
YAP protein levels are markedly increased during liver regeneration in humans [36, 37], as well as during regeneration following hepatectomy [38], bile acid-induced injury [39], and bile duct ligation-induced injury [37] in mice. Following bile duct ligation, liver-specific YAP knockout mice are more susceptible to injury and show reduced hepatocyte proliferation and increased necrosis, indicating that YAP is required for regeneration [37]. Deleting MST1/2 to activate YAP also protects the liver from acetaminophen-induced liver injury [40]. A recent study found that following a partial hepatectomy in rats YAP activation is accompanied by MST1/2, LATS1/2, and MOB1 inactivation [41]. However, once the liver reaches its pre-hepatectomy size, MST1/2 activity is restored followed by YAP inactivation [41]. This study highlights the importance of canonical Hippo pathway components in dynamically regulating YAP during regeneration and maintenance of final liver size. In other genetic models, AMOT can increase YAP activity by preventing YAP phosphorylation and increasing YAP-TEAD transcriptional activity, so it is not surprising that liver-specific AMOT knockout mice also have reduced cell proliferation and regeneration following toxin-induced injury [27]. Liver-specific knockdown of α-catenin also results in increased YAP activity and liver overgrowth following partial hepatectomy, indicating that α-catenin may play a role in inactivating YAP following regeneration [42]. Taken together, these findings suggest that YAP-TEAD transcriptional activity is activated in response to multiple types of injury and is required for initiating cell proliferation and for complete hepatic recovery. Following complete regeneration, YAP activity is regulated at multiple levels, including by canonical Hippo pathway kinases MST1/2, LATS1/2, and MOB1, as well as α-catenin, to inactivate YAP and prevent liver overgrowth.
The Hippo pathway in pancreatic development
Intact Hippo signaling is also required for normal pancreatic development. Knocking down YAP is sufficient to block pancreatic progenitor cell proliferation [43]. miR-375 can also regulate pancreatic progenitor cell proliferation by inhibiting translation of YAP mRNA via binding to the 3′ UTR, further supporting a role for YAP/TAZ in pancreatic development [43]. YAP’s role in pancreatic development appears to be compartment specific. Pancreas-specific MST1/2 knockout and ectopic YAP overexpression both result in decreased pancreas size [44, 45]. Dysregulation of the Hippo pathway does not appear to play a significant role for the endocrine compartment, and MST1/2 knockout mice have normal fed blood glucose levels [45]. However, the exocrine compartment of MST1/2 knockout mice shows a dramatic increase in cell proliferation, accompanied by a similar increase in cell death [44]. The decrease in pancreas size is primarily due to loss of tissue architecture in the exocrine compartment due to dedifferentiation of acinar cells back into ductal cells [44, 45]. This appears to be YAP-dependent because deleting a single allele of YAP in the MST1/2 knockout mice results in improved pancreatic growth and structure [44]. The difference between endocrine and exocrine compartments is probably due to expression levels, since YAP is not expressed in the endocrine compartment following differentiation. Nevertheless, it will be interesting to see how YAP becomes differentially regulated in the endocrine compartment, as well as whether TAZ is similarly regulated.
The Hippo pathway in ocular development
YAP/TAZ are stimulated by both mechanical and biochemical signals to regulate ocular development, regeneration, and disease. For instance, Sveinsson’s Chorioretinal Atrophy (SCRA), a rare genetic disease resulting in degeneration of the choroid and retina, is caused by a mutation in TEAD1 [46]. This mutant TEAD1 is defective in YAP/TAZ binding and has no transcriptional activity [46]. Interestingly, this work raised the possibility that YAP-TEAD transcriptional activity is important for cell-cell and cell-matrix adhesions, as SCRA is caused by tearing of the retinal pigment epithelium (RPE) [46]. In other cellular contexts, increased YAP expression is correlated with epithelial to mesenchymal transition (EMT) and the loss of cell-cell junctions. In fact, it has been shown in RPE cells that TAZ-TEAD1 transcriptional activity results in ZEB1 expression, loss of cell-cell contact, and EMT [47]. So the observation that defective YAP-TEAD transcriptional activity is also correlated with defective cell-cell and cell-matrix adhesions may lead to a more dynamic understanding of the role of YAP in regulating cell-cell interactions. In contrast, NF2 knockout mice develop cataracts caused by disorganization and accumulation of cells in the lens epithelium due to abnormal tissue growth [48]. This is rescued by deleting YAP, indicating that this phenotype is dependent on the Hippo pathway. This phenotype aligns with the current understanding of elevated YAP activity resulting in overgrowth, as seen in other tissues and organs.
The Hippo pathway in intestinal regeneration
The Hippo pathway is important for maintaining intestinal homeostasis. The intestinal lining is constantly exposed to a harsh environment and must continually regenerate to replace dying cells. Intestine-specific YAP knockout mice show no major effects during development or on normal homeostasis [49]. However, YAP plays an important role in regeneration following injury. In a Dextran Sodium Sulfate (DSS)-induced colonic regeneration model, YAP protein levels are increased during regeneration. Deleting YAP blocks regeneration in this model and results in substantial intestinal damage and increased mortality [49]. However, another study looked at intestinal regeneration following whole body irradiation and found that intestine-specific YAP overexpression resulted in impaired regeneration, and intestine-specific YAP knockout mice developed hyperplasia [50]. They also found that expressing constitutively active YAP can suppress growth of colorectal cancer (CRC) xenografts, suggesting YAP acts as a tumor suppressor. These conflicting reports may be due to multiple factors. The role of YAP in regeneration may be injury specific, since the two studies utilized different injury models to induce regeneration. Some differences might also be explained by the involvement of other signaling pathways. For instance, YAP overexpression-induced dysplasia can be blocked by γ-Secretase, a Notch signaling inhibitor [51]. Barry et al. found that YAP is silenced in a subset of human CRC, and YAP blocks regeneration by inhibiting Wnt signaling and preventing Dishevelled (DVL) nuclear translocation [50, 52]. Loss of YAP results in Wnt hypersensitivity during regeneration and causes hyperplasia [50, 53]. Further work is needed to fully understand how these different pathways interface in specific cellular niches and how they may regulate each other during regeneration.
The Hippo pathway in cardiomyocyte regeneration
During development the heart grows dramatically due to cardiomyocyte proliferation. However, a week after birth cardiomyocytes stop proliferating and any subsequent growth is due to cardiomyocyte hypertrophy [54]. As such, the adult heart has a limited ability to regenerate following injury. Instead, the myocardium replaces lost cardiomyocytes with fibrotic scar tissue, which reduces heart contractility and function. Recent studies have identified a potential role for the Hippo pathway in enhancing cardiomyocyte proliferation following injury. For instance, conditional MST1 overexpression in the heart results in increased cardiomyocyte apoptosis in vitro and dilated cardiomyopathy in vivo [55]. Conversely, overexpressing dominant-negative MST1 or LATS2 showed improved cardiac function following either myocardial infarction or ischemia and reperfusion [56, 57]. In addition, SAV1 deficient cardiomyocytes can re-enter the cell cycle and undergo cell division, and SAV1 heart-specific knockout mice show improved recovery following ischemia with ejection fraction and fractional shortening values comparable to control, non-ischemic mice [58]. These reports suggest that Hippo-deficient hearts exhibit increased regenerative potential. YAP transgenic mice also show increased regeneration and decreased fibrosis following heart injury [59]. More specifically, Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit beta (PIK3CB), a catalytic subunit of PI3K, is a direct target of YAP in promoting cardiomyocyte proliferation [60]. Thus, manipulating the Hippo pathway following injury could be key to improving heart regeneration, decreasing fibrosis, and increasing survival.
Dysregulation of the Hippo pathway in human cancer
While YAP/TAZ activity is important for cell proliferation and regeneration, the Hippo pathway must be tightly regulated. Not surprisingly, dysregulation of the Hippo pathway can lead to uncontrolled proliferation, resulting in a wide range of diseases (Boxes 1–3) and cancers. The Hippo pathway can become dysregulated by a variety of mechanisms, including YAP gene amplification, methylation or deletion of upstream Hippo pathway components, mutations in upstream GPCRs, or by crosstalk with other signaling pathways including Wnt signaling.
Box 1. The Hippo pathway in polycystic kidney disease.
Polycystic kidney disease (PKD) is a life-threatening disease caused by cyst formation throughout the kidneys, and is frequently caused by inactivating mutations in either the PKD1 or PKD2 [53]. Interestingly, YAP and TAZ appear to serve different functions in PKD progression. The planar cell polarity component Four-jointed (Fjx1) is required for regeneration following tubular epithelial injury, but is decreased in a PKD1-inducible knockout model for PKD [113]. Fjx1 is a transcriptional target of YAP, and Fjx1 and YAP expression are both increased during regeneration following injury in both control and PKD1 knockout mice. However, in PKD1 knockout mice YAP nuclear localization and transcriptional activity continued to persist after recovery, resulting in cyst formation [113]. Increased YAP expression was also observed in human PKD patients [113]. Thus, while YAP seems to play a role in kidney recovery following injury, sustained signaling may cause PKD.
TAZ appears to have a more direct contribution to PKD. TAZ forms a complex with Polycystin-2 (PC2, the protein product of PKD1), thereby targeting it for ubiquitination and degradation. TAZ knockout results in PC2 accumulation, leading to PKD [53], and also results in the down-regulation of other genes necessary for proper cilia development and function [114]. The lack of functional cilia also contributes to cyst formation. In fact, TAZ knockout mice begin developing cysts as early as embryonic day 15.5, possibly due to a combination of these factors [115]. This phenotype was also seen in a mouse model with TAZ conditionally knocked out in nephrons [116].
Box 3. The Hippo pathway in neurological disease.
Hippo pathway components are involved in non-cancer neurological diseases,. For instance, studies reported that YAP/TAZ mediate gene transcription induced by AβPP, the precursor of Amyloid β which is thought to drive Alzheimer’s disease [119]. In addition, MST1 is a key mediator of Amyotrophic Lateral Sclerosis (ALS). MST1 activity is increased in motor neurons from SOD1(G93A) mice, an ALS mouse model. MST1 activates p38 and Caspase-3 and -9, resulting in autophagosome accumulation and motor neuron death. When MST1 is knocked out in these mice, they show increased motor neuron viability, delayed symptom onset, and extended survival, although it is not clear whether YAP or TAZ are involved in this phenotype [120]. These findings demonstrate the importance of the Hippo pathway in some neurodegenerative diseases and can hopefully be expanded in the near future.
Box 2. The Hippo pathway in arrhythmogenic cardiomyopathy and Holt-Oram syndrome.
The Hippo pathway has also been implicated in several heart diseases. Arrhythmogenic right ventricular cardiomyopathy (ARVC) is characterized by thinning of the right ventricular walls, replacement of the myocardium with fibroadipocytes, and arrhythmias. ARVC is caused by the loss of intact desmosomes. Recent work has shown that MST1/2, LATS1/2, and YAP are phosphorylated in human ARVC hearts, as well as in knockout mouse models for obligatory desmosome components DSP and JUP [117]. In addition, knocking down LATS1/2 or overexpressing a constitutively active YAP mutant in cardiomyocytes results in adipogenesis, further supporting a causal role for the Hippo pathway in ARVC [117].
The Hippo pathway is also involved in Holt-Oram syndrome, which consists of heart defects and abnormalities of the upper limbs. TBX5, which is essential for cardiac and limb development, is often mutated in Holt-Oram patients [118]. TBX5 normally interacts with YAP/TAZ, but mutations in TBX5 prevent its binding to TAZ and results in a congenital heart defect called Tetralogy of Fallot (TOF) [19]. Taken together, the Hippo pathway plays an important role in heart development. Future work is needed to determine whether the Hippo pathway can be manipulated or therapeutically targeted to improve regeneration following injury.
The Hippo pathway in liver cancer
YAP is frequently amplified in hepatocellular carcinoma (HCC) and is required to sustain increased cell proliferation and tumor growth [61]. Risk factors for HCC include hepatitis infection and exposure to xenobiotics, and these have also been implicated in activating YAP. The Hepatitis B virus X protein (HBx) directly increases YAP expression by enhancing YAP gene transcription [62]. 1,4-bis[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP) is a xenobiotic mimic that activates constitutive androstane receptor (CAR) to increase YAP protein levels and induce HCC [63]. In addition, GA-binding protein (GABP), which is involved in antioxidant defense, can directly promote YAP transcription [40]. Increased GABP nuclear localization and YAP expression are both correlated in liver cancer, so it is possible that high GABP levels promote increased YAP expression in HCC [40].
Inducing YAP overexpression in a liver-specific transgenic model causes abnormal hepatocyte proliferation and suppressed apoptosis, resulting in increased liver size and HCC [13, 51]. These findings are consistent with knockouts of other Hippo pathway components. One study deleted MOB1A, with a heterozygous mutation for MOB1B, and found that these mice have an increased lifetime chance of developing HCC [64]. Increased liver growth and HCC have also been reported in liver-specific SAV1 knockout, NF2 knockout, and MST1/2 knockout mice [48, 65, 66]. These findings implicate the Hippo pathway in both controlling liver size and preventing tumorigenesis. In addition, NF2 knockout mice show reduced tumorigenesis when crossed with liver-specific AMOT knockout mice, suggesting AMOT-YAP interaction is also important for YAP-driven tumorigenesis [27]. However, much of the current understanding of the Hippo pathway in HCC has been derived from genetic models, and few mutations or deletions in Hippo pathway components have been observed in human HCC [67]. While the mouse work has established a clear role for the Hippo pathway in HCC, future work should focus on how the Hippo pathway becomes dysregulated in human HCC.
YAP/TAZ gene fusion in epithelioid hemangioendothelioma
Epithelioid hemangioendothelioma (EHE) is a rare vascular tumor most commonly found in the lung, bone, and skin. Recently, it has been shown that YAP/TAZ chromosome translocations occur in virtually all EHE cases [68]. These chromosome translocations result in a fusion protein between either TAZ and Calmodulin binding transcription activator 1 (CAMTA1), TAZ and FBJ murine osteosarcoma viral oncogene homolog B (FOSB), or YAP and Transcription factor binding to IGHM enhancer 3 (TFE3) [69–71]. While the fusion proteins retain their YAP/TAZ TEAD-binding domains, they are missing key phosphorylation sites required by LATS to inactivate YAP/TAZ, so these fusion proteins may act as constitutively active transcription factors. Although research on the role of YAP/TAZ in EHE is at its infancy, the observation that YAP/TAZ chromosome translocations occur in virtually all cases of EHE strongly suggest that dysregulated YAP/TAZ fusion proteins may act as cancer drivers.
The Hippo pathway in breast cancer
YAP/TAZ activity has been correlated with increased risk of metastasis and reduced survival across all human breast cancer subtypes [72]. However, the role of the Hippo pathway in breast cancer progression remains controversial. On one hand, TAZ is highly expressed in invasive breast cancer cell lines and primary breast cancers, and TAZ overexpression is sufficient to induce cell proliferation, transformation, and EMT in breast cancer cell lines [6, 73]. Similarly, overexpressing YAP in breast cancer cell lines induces tumor formation and growth in xenograft experiments [74], and deleting YAP prevents tumor growth in an oncogene-induced breast cancer model [75]. In addition, leukemia inhibitory factor receptor (LIFR) has been identified as a tumor suppressor that acts through the Hippo pathway to inactivate YAP both in vitro and in vivo [76]. These reports support an oncogenic role for YAP/TAZ. On the other hand, there are reports that suggest YAP acts as a tumor suppressor. YAP protein expression is decreased in luminal breast cancer tissues, and YAP knockdown in breast cancer cell lines actually enhances tumor migration, invasion, and tumor growth in nude mice [77]. A recent study reported that hyperactivation of YAP alone is not sufficient to drive mammary tumorigenesis in vivo, and YAP-induced oncogenic growth may be dependent on the presence of additional mutations or amplifications [75]. Additional work is needed to determine whether these conflicting reports may be due to cell type-specific differences.
The Hippo pathway in lung cancer
YAP/TAZ are both highly expressed in non-small cell lung cancer (NSCLC) in humans, and knockdown of either YAP or TAZ in NSCLC cells is sufficient to suppress proliferation, invasion, and tumor growth in mice [78, 79]. High YAP expression is correlated with advanced stage, lymph node metastasis, and decreased survival [78]. In fact, it has been shown that knockdown of either YAP or TAZ is sufficient to decrease cell migration in vitro and metastasis in vivo, and expression of constitutively active YAP is sufficient to drive lung cancer progression in vivo [80]. However, although these studies strongly point towards an oncogenic function for YAP/TAZ, the mechanisms by which they become dysregulated in NSCLC progression was not known until recently.
Overexpression of MST1 is sufficient to inhibit cell proliferation and apoptosis in NSCLC cells [81]. This is mostly likely due to MST activation of LATS, preventing YAP/TAZ nuclear localization. LATS1 protein levels are frequently decreased in NSCLC tissues, and loss of LATS1 expression is correlated with advanced stage, lymph node metastasis, and decreased survival [82]. In addition, other non-canonical Hippo pathway components have also been identified to interact with YAP/TAZ in lung cancer. Vestigial-like family member 4 (VGLL4) is frequently down-regulated in lung cancer, and expressing VGLL4 in lung cancer cells suppresses cell proliferation and tumor growth in mice by competitively inhibiting YAP-TEAD binding and transcriptional activity [83]. Another study found that high YAP expression was correlated with increased AXL receptor tyrosine kinase (Axl) expression in lung adenocarcinomas, and that knocking down YAP also resulted in loss of Axl, proliferating cell nuclear antigen (PCNA), and matrix metalloproteinase-9 (MMP-9) [84]. This study further confirmed that knocking down YAP inhibits proliferation and invasion of lung cancer cells, an effect which is potentially mediated through Axl. Finally, miR-135b expression increases lung cancer metastasis by targeting LATS2, and inhibiting miR-135b suppresses tumor growth and metastasis [85]. Expression of miR-135b is regulated by DNA demethylation and nuclear factor-kappa B (NFKb) signaling, raising the possibility that inflammatory and epigenetic modifications may regulate expression of miR-135b, thereby resulting in LATS2 inhibition, YAP/TAZ nuclear translocation, and cancer [85].
The Hippo pathway in malignant mesothelioma
Malignant mesothelioma is a rare cancer of the mesothelium, the lining which covers many of the body’s internal organs, and often comes with a poor prognosis. Recent work has found that homozygous deletion or inactivating mutations in NF2, SAV1, or LATS2 are frequently observed in human malignant mesothelioma tissues and cell lines [86, 87]. Moreover, Ajuba LIM protein (AJUBA) can inactivate YAP through signaling via LATS, and down-regulation of AJUBA has also been associated with malignant mesothelioma [88]. These deletions or mutations contribute to increased YAP protein levels and aberrant YAP-TEAD transcriptional activity, which drive increased cell proliferation and anchorage-independent growth by up-regulating the cell cycle-promoting Cyclin D1 and Forkhead box M1 [87]. Knocking down YAP in malignant mesothelioma cells is sufficient to inhibit cell proliferation and anchorage-independent growth [87]. Together, these findings strongly implicate dysregulation of the Hippo pathway in malignant mesothelioma and YAP as a potential therapeutic target. As cases of malignant mesothelioma are primarily associated with asbestos exposure, it may be informative to determine whether there is something about asbestos that is pre-inclined to inducing mutations in Hippo pathway components.
The Hippo pathway in pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) has one of the worst prognoses of all cancers because often the patient does not experience symptoms until the cancer has reached an advanced stage. PDAC tissues often have increased YAP expression and nuclear localization, and elevated YAP expression is correlated with poor prognosis [89]. Moreover, in pancreatic cancer cells, YAP knockdown results in reduced proliferation and reduced anchorage-independent growth, suggesting YAP may play an important role in PDAC progression [89]. These findings are supported by work done in KRAS transgenic mice. KRAS is frequently mutated in PDAC, but in a mouse model expressing mutated KRAS, deleting YAP is sufficient to prevent PDAC. In addition, deleting YAP in pancreatic cancer cells harboring the mutant KRAS is sufficient to prevent proliferation and growth in mice [90]. A similar study found that in an inducible KRAS-driven PDAC mouse model where removal of KRAS resulted in complete tumor regression, some mice later developed spontaneous tumors due to YAP amplification and increased YAP-TEAD2 transcriptional activity [91]. This finding suggests that KRAS-driven tumors may acquire additional mechanisms to further increase proliferation and growth, and YAP may play an important role in enabling PDAC to escape KRAS addiction.
The Hippo pathway in Kaposi sarcoma
YAP/TAZ play a driving role in Kaposi sarcoma (KS), a tumor caused by the Kaposi sarcoma-associated herpesvirus (KSHV). KS results in cutaneous lesions which can spread throughout the skin, mouth, gastrointestinal, and respiratory tracts. Tissue samples from human KS patients show elevated levels of YAP/TAZ [92]. Recently, it was shown that KSHV encodes a viral GPCR (vGPCR), which signals through Gq/11 and G12/13 to RhoA, inactivating LATS1/2 and activating YAP/TAZ [92]. In addition, cells overexpressing vGPCR failed to grow in a xenograft mouse model when YAP/TAZ were depleted, indicating that YAP/TAZ are necessary for KSHV-induced tumorigenesis.
The Hippo pathway in uveal melanoma
Uveal melanoma (UM) is the most common type of eye cancer in adults, with approximately 80% of UM cases characterized by activating mutations in either GNAQ or GNA11. Although overexpression of mutant Gq/11 is sufficient to transform melanocytes [93], the signaling events downstream of Gq/11 were unknown. Two studies showed that Gq/11 can activate YAP by inhibiting LATS1/2 and disrupting AMOT-YAP interaction [94, 95]. Importantly, both papers demonstrate that treating UM with Verteporfin, a drug which blocks YAP-TEAD interaction (therefore inhibiting YAP transcriptional activity), is sufficient to inhibit UM tumor growth in mice [93, 96]. This is an important finding not only for treating UM, but also has broad implications for how YAP may be involved in other GPCR-associated cancers.
The Hippo pathway in renal cell carcinoma
YAP has also been implicated in renal cell carcinoma (RCC). A recent report found that the LATS1 promoter is frequently methylated in RCC, resulting in down-regulation of LATS1 and increased YAP activity [97]. Indeed, RCC tissues show elevated levels of YAP, and knocking down YAP in RCC cell lines blocks cell proliferation and increases apoptosis [98]. Although more work is needed to assess the role of YAP in RCC initiation and progression in vivo and whether YAP is essential for RCC survival, this report raises the possibility that YAP may be a useful therapeutic target for RCC.
The Hippo pathway in colorectal cancer
Although the most common mutations in CRC involve adenomatous polyposis coli (APC) and dysregulated β-catenin signaling, YAP/TAZ may be required downstream mediators of these mutations. YAP/TAZ are reported to be degraded by the β-catenin destruction complex, along with APC, Axin, and glycogen synthase kinase 3 (GSK3) [99]. Beta-catenin is required to recruit TAZ to the destruction complex, and the absence of Wnt signaling results in both β-catenin and YAP/TAZ cytoplasmic sequestration, phosphorylation, and degradation [31]. Cytoplasmic YAP/TAZ can also directly interact with DVL and β-catenin, inhibiting DVL phosphorylation and preventing β-catenin nuclear translocation [50]. Conversely, activation of Wnt signaling results in both β-catenin and TAZ accumulation, and YAP/TAZ co-transcriptional activity is required for many of the Wnt transcriptional responses [31]. In fact, one study found that β-catenin-driven tumors require YAP and TBX5 to induce expression of genes required to inhibit apoptosis and promote tumor survival [100]. Finally, β-catenin can interact with TCF/LEF to directly induce YAP gene transcription [101]. It is clear that crosstalk between the Hippo and Wnt signaling pathways plays an important role in CRC, and this must be taken into consideration when therapeutically targeting either pathway.
YAP is often overexpressed in CRC, and YAP/TAZ activity is correlated with decreased survival [102]. LATS1 promoter methylation has also been reported in CRC, which may lead to increased YAP activity [103]. In mice, inducing YAP overexpression in the intestine results in dysplasia after two days, although the intestine regenerates once induction is stopped [51]. Similar phenotypes were also seen in MST1/2 and SAV1 knockout mice, which developed adenomas after 13 weeks and polyps after 13 months, respectively [49, 104]. Both of these phenotypes were blocked by deleting YAP, indicating that these pathologies are YAP-dependent. In addition, one report suggests that YAP may play an important role in causing CRC cells to become dormant during chemotherapy treatment and active during relapse [105]. Cells resistant to 5-fluorouracil (5FU) express high levels of YAP, which becomes phosphorylated and cytoplasmic when the cells are exposed to 5FU, causing the cells to enter quiescence. Increased YAP protein levels were also seen in human CRC liver metastases and were correlated with CRC relapse [105]. Although the authors did not show whether removing 5FU causes increased YAP nuclear localization and cell proliferation, these findings are highly significant and may hugely impact the paradigm for treating CRC and preventing relapse.
The Hippo pathway in multiple myeloma
The Hippo pathway plays an important role in regulating lymphocyte apoptosis. YAP acts as a tumor suppressor in several hematological cancers, including multiple myeloma (MM), lymphoma, and leukemia [106]. These cancers are typically characterized by genetic instability and inactivating mutations in tumor protein p53 (TP53). In human MM patient samples, YAP is also frequently deleted or down-regulated [106]. YAP interacts with ABL1 to induce p53-independent apoptosis, and inhibiting MST1 in MM cells is sufficient to up-regulate YAP protein levels and induce apoptosis, both in vitro and in vivo [106]. This report raises the possibility that YAP may act as a tumor suppressor and proposes a novel therapeutic strategy for targeting the Hippo pathway in hematological cancer. Little is known about YAP/TAZ in hematological cells, and any role of YAP/TAZ as tumor suppressors would challenge the current paradigm that YAP/TAZ act as oncogenes.
The Hippo pathway in the nervous system
The Hippo pathway is involved in several nervous system tumors. Loss of function mutations in NF2 causes Neurofibromatosis Type 2, a genetic disorder characterized by the development of schwannomas and meningiomas with increased YAP expression and nuclear localization [107, 108]. NF2 inhibits YAP activity by promoting LATS activation and inhibiting LATS ubiquitination and degradation [21, 22]. Loss of function mutations in NF2 results in increased LATS degradation and YAP accumulation, so loss of NF2 and subsequent tumor growth could be due to aberrant YAP activity. In the central nervous system, NF2 expression is also significantly reduced in human malignant gliomas, and expression of NF2 has been shown to inhibit human glioma growth both in vitro and in vivo [109]. Likewise, YAP is highly expressed in many human brain tumors including infiltrating gliomas, and YAP overexpression promotes glioblastoma growth in vitro [110].
Concluding remarks
The Hippo signaling pathway plays an important role in regulating key cellular functions, including cell proliferation, apoptosis, and differentiation. Recognized for its driving contribution in a wide variety of diseases and cancers (Figure 2, Table 1), research into identifying new ways to therapeutically target the Hippo pathway has expanded tremendously in recent years. A screen of FDA approved drugs found that Verteporfin can bind YAP and prevent YAP-TEAD interaction [96]. As discussed earlier, Verteporfin can block tumor growth in UM, as well as suppress tumor growth in a NF2 knockout or YAP overexpression liver cancer model [94–96]. A cell-based screen identified dobutamine, a beta-adrenergic receptor agonist, as another YAP inhibitor, inducing LATS-independent YAP phosphorylation [111]. A recent report designed cyclic YAP-like peptides to prevent YAP-TEAD interaction, although it remains to be seen whether these peptides can block YAP transcriptional activity [112]. Finally, the finding that some GPCR ligands induce YAP phosphorylation opens the possibility that YAP activity may be therapeutically altered by modulating GPCR signaling [32]. This is an exciting time when our knowledge of the Hippo field is expanding tremendously and will hopefully lead to the development of specific drugs to manipulate YAP/TAZ activity.
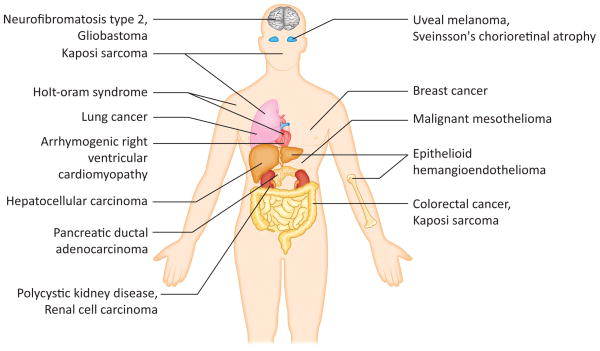
Figure 2. Disease implications of the Hippo pathway.
Dysregulation of the Hippo pathway has been implicated in a number of cancers and diseases throughout the body. Dysregulation may be due to genetic inactivation of core pathway components or amplification or gene fusion of its downstream effectors YAP and TAZ. Here we briefly summarize some of the diseases covered in this review.
Table 1.
Diseases characterized by dysregulated Hippo signaling
| Disease | YAP/TAZ status in humans | Known genetic alterations in human | Mouse genetic models | References |
|---|---|---|---|---|
| Diseases characterized by increased YAP expression | ||||
| Hepatocellular Carcinoma (HCC) | Increased YAP expression | YAP amplification | YAP overexpression, liver-specific SAV1, NF2, MST1/2 knockout mice develop HCC | [13, 48, 51, 61, 65, 66] |
| Lung cancer | Increased YAP/TAZ expression is correlated with lymph node metastasis, decreased survival | Loss of LATS1 is correlated with lymph node metastasis, decreased survival | YAP/TAZ knockdown is sufficient to suppress tumor growth; expressing constitutively active YAP is sufficient to drive cancer progression | [78, 80, 82] |
| Pancreatic Ductal Adenocarcinoma (PDAC) | Increased YAP expression | YAP knockout prevents PDAC growth | [89] | |
| Colorectal Cancer (CRC) | YAP/TAZ are often overexpressed; increased YAP/TAZ is correlated with decreased survival | Frequently caused by mutations in APC that may activate YAP/TAZ; LATS1 promoter is frequently methylated | YAP overexpression, SAV1, and MST1/2 knockout mice develop CRC | [49, 51, 102–104] |
| Renal Cell Carcinoma (RCC) | Increased YAP expression | LATS1 is frequently methylated, resulting in reduced LATS1 expression | [97, 98] | |
| Neurofibromatosis Type 2 | Increased YAP expression | Loss of function mutations in NF2 | [107, 108] | |
| Polycystic Kidney Disease (PKD) | Increased YAP expression | Caused by mutations in PKD1 or PKD2 | Sustained YAP activity following injury in PKD1 knockout mice results in cysts; TAZ knockout results in improper cilia development, cyst formation, and PKD | [53, 113] |
| Diseases characterized by changes in YAP expression | ||||
| Breast cancer | Increased YAP/TAZ activity is correlated with increased risk of metastasis, decreased survival; YAP protein levels are decreased in luminal breast cancer tissues | Overexpressing YAP induces tumor formation | [72, 74, 77] | |
| Diseases characterized by decreased YAP expression | ||||
| Multiple Myeloma (MM) | YAP is frequently deleted or down-regulated | Inhibiting MST1 to up- regulate YAP is sufficient to induce apoptosis in vivo | [106] | |
| Diseases characterized by chromosome translocations | ||||
| Epithelioid Hemangioendothelioma (EHE) | Chromosome translocations between either TAZ- CAMTA1, TAZ-FOSB, or YAP-TFE3 | [69–71] | ||
| Diseases characterized by mutations or genetic alterations in other Hippo pathway components | ||||
| Malignant Mesothelioma | Deletion or inactivating mutations in NF2, SAV1, or LATS2 are common | [86, 87] | ||
| Kaposi Sarcoma (KS) | KSHV encodes a vGPCR which activates YAP/TAZ | YAP/TAZ knockdown prevents KSHV- induced tumorigenesis | [92] | |
| Uveal Melanoma (UM) | Caused by mutations in GNAQ and GNA11 | Treating UM cells with Vertoporfin prevents tumor growth | [94, 95] | |
| Sveinsson’s Chorioretinal Atrophy (SCRA) | Caused by mutations in TEAD1 which prevent YAP-TEAD interaction | [46] | ||
| Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) | YAP is phosphorylated | Caused by mutations in desmosome components DSP, JUP, DSC2, DSG2, and PKP2 | LATS1/2 knockdown or expressing constitutively active YAP causes ARVC; DSP or JUP KO mice show increased MST1/2, LATS1/2, and YAP phosphorylation | [117] |
| Holt-Oram Syndrome | Caused by mutations in TBX5, which prevents binding to TAZ | [19, 118] | ||
However, many important and fundamental questions still remain (Box 4). Further elucidating the crosstalk between the Hippo pathway and Wnt, TGF-β, Notch, Ras, mTOR, and Sonic hedgehog signaling remains a priority. Additional areas of future research include further elucidating whether YAP/TAZ always function as oncoproteins, or whether they also have context-specific tumor suppressing functions. Finally, it will be important to dissect whether YAP/TAZ are differentially regulated and whether they activate different transcription profiles. Addressing these questions may help open the door to the next wave of discoveries in the Hippo field.
Box 4. Outstanding Questions.
Do all cell growth/tumor suppressor functions of LATS go through YAP/TAZ? If not, what are the other physiological substrates of LATS?
How do changes in the actin cytoskeleton regulate YAP/TAZ phosphorylation, localization, and transcriptional activity?
Are YAP/TAZ differentially regulated, and do they initiate different transcription profiles?
Do YAP/TAZ always function as oncoproteins, or do they also have a tumor suppressing function?
How does the Hippo pathway crosstalk with other signaling pathways, including the Wnt, TGF-β, Notch, Ras, mTOR, and Sonic Hedgehog signaling pathways?
Highlights.
Dysregulation of the Hippo pathway is implicated in a variety of cancers and diseases
Hippo pathway may be a therapeutic target in a number of cancers
Manipulating the Hippo pathway may improve regeneration following injury
Acknowledgments
We apologize to our colleagues who have made many important contributions to the Hippo field but whose work could not be cited due to space constraints. This work was supported by grants from the National Institutes of Health (NIH) to K.L.G. (CA132809, EYO226116, and P30CA023100). S.W.P. and A.W.H. were supported in part by the UCSD Graduate Training Program in Cellular and Molecular Pharmacology training grant (T32 GM007752).
Glossary
- 14-3-3
a binding protein that binds and sequesters phosphorylated YAP/TAZ in the cytoplasm
- Angiomotin (AMOT)
AMOT can induce LATS2-mediated YAP phosphorylation and sequester YAP/TAZ to the actin cytoskeleton, preventing their translocation into the nucleus
- Adenomatous polyposis coli (APC)
a tumor suppressor that helps regulate levels of β-catenin. APC is frequently mutated in colorectal cancer
- Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC)
a genetic disease caused by mutations in desmosome components. ARVC is characterized by thinning of the right ventricular wall, replacement of the myocardium with fibroadipocytes, and arrhythmias
- Constitutive Androstane Receptor (CAR)
CAR is a nuclear receptor which senses xenobiotics and upregulates expression of proteins to metabolize them
- Calmudulin Binding Transcription Activator 1 (CAMTA1)
a transcription factor that is frequently fused with TAZ due to a chromosomal translocation in epithelioid hemangioendothelioma
- Desmosome
complex intercellular junctions consisting of multiple protein subunits and responsible for providing the surrounding tissue with the resistance necessary to withstand mechanical stress. Mutations in desmosome components are frequently observed in ARVC
- Desmoplakin (DSP)
a required component for intact desmosome assembly. DSP anchors the rest of the desmosome complex to intermediate filaments within the cell
- Dishevelled (DVL)
DVL is a component of the Wnt signaling pathway, acting downstream of the Frizzled receptor. YAP may block intestinal regeneration by preventing DVL nuclear translocation
- FBJ Murine Osteosarcoma Viral Oncogene Homolog B (FOSB)
a transcription factor that is frequently fused with TAZ due to a chromosomal translocation in epithelioid hemangioendothelioma
- Gq/11
GNAQ and GNA11 encode the GPCR subunits Gq and G11. Mutations in GNAQ and GNA11 are frequently observed in uveal melanoma
- G-Protein Coupled Receptors (GPCR)
GPCRs make up the largest class of receptors in the cell, and are responsible for regulating a variety of important cellular responses including cell proliferation and survival
- Plakoglobin (JUP)
Plakoglobin is a required component for intact desmosome assembly. JUP helps link the intermediate filaments and DSP to the desmosome cadherins. Mutations in JUP are associated with ARVC
- Large Tumor Suppressor 1/2 (LATS1/2)
LATS1 and 2 are serine/threonine kinases that are phosphorylated and activated by MST1/2 and MOB1. Once activated, LATS1/2 phosphorylate and inactivate YAP
- Lysophosphatidic Acid (LPA)
a potent inducer of YAP activity via signaling through GPCRs
- Mob1 Homolog (MOB1)
MOB1 is phosphorylated by MST1/2. Once phosphorylated, MOB1 binds the autoinhibitory region of LATS1/2, enabling LATS1/2 phosphorylation and activation
- Mammalian STE20-like 1/2 (MST1/2)
MST1/2 serine/threonine kinases form a heterodimer with SAV1 to phosphorylate and activate LATS1/2 and MOB1
- Neurofibromin 2 (NF2)
NF2 can induce LATS2 phosphorylation and activation, resulting in YAP inactivation. NF2 is frequently mutated in Neurofibromatosis Type 2
- Polycystic Kidney Disease 1/2 (PKD1/2)
PKD1/2 encode proteins Polycystin 1 (PC1) and Polycystin 2 (PC2), respectively. PC1 and PC2 are membrane proteins that may be involved in signal transduction and enable the cell to sense the surrounding matrix. PC2 interacts with TAZ, which targets PC2 for ubiquitination and degradation
- Sphingosine-1-Phosphate (S1P)
a potent inducer of YAP activity via signaling through GPCRs
- Salvador 1 (SAV1)
an adaptor protein which enhances MST1/2 kinase activity and enables MST1/2-LATS1/2 interaction
- WW Domain Containing Transcription Factor (TAZ)
TAZ is a transcriptional co-activator and downstream effector of the Hippo pathway. Similar to YAP, phosphorylated TAZ is sequestered in the cytoplasm and degraded. When dephosphorylated, TAZ translocates to the nucleus and interacts with other transcription factors to drive transcription
- T-Box Transcription Factor 5 (TBX5)
TBX5 is a transcription factor which interacts with YAP/TAZ. Mutations in TBX5 are associated with Holt-Oram Syndrome
- TEA Domain Family Members 1-4 (TEAD1-4)
the main transcription factors that interact with YAP/TAZ to drive transcription. Although YAP/TAZ are known to interact with other transcription factors besides TEAD1-4, their role in mediating the growth promoting activities of YAP/TAZ have not been established
- Transcription Factor Binding to IGHM Enhancer 3 (TFE3)
a transcription factor that is frequently fused with YAP due to a chromosomal translocation in epithelioid hemangioendothelioma
- Verteporfin
Verteporfin is an FDA-approved drug which blocks YAP-TEAD interaction, thereby inhibiting YAP transcriptional activity
- Yes-Associated Protein (YAP)
YAP is a transcriptional co-activator and downstream effector of the Hippo pathway. When phosphorylated, YAP is sequestered in the cytoplasm by binding to 14-3-3, ubiquitinated, and targeted for degradation. When dephosphorylated, YAP translocates to the nucleus and interacts with a variety of transcription factors, including TEAD1-4, to induce gene expression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Callus BA, et al. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273:4264–4276. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- 2.Tapon N, et al. salvador Promotes Both Cell Cycle Exit and Apoptosis in Drosophila and Is Mutated in Human Cancer Cell Lines. Cell. 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 3.Chan EH, et al. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 4.Praskova M, et al. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18:311–321. doi: 10.1016/j.cub.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, et al. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Lei QY, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Molecular and cellular biology. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008;135:1081–1088. doi: 10.1242/dev.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & development. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu CY, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. The Journal of biological chemistry. 2010;285:37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao B, et al. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes & development. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren F, et al. Hippo signaling regulates Yorkie nuclear localization and activity through 14-3-3 dependent and independent mechanisms. Dev Biol. 2010;337:303–312. doi: 10.1016/j.ydbio.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanai F, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vassilev A, et al. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes & development. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu S, et al. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, et al. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes & development. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrigno O, et al. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling. Oncogene. 2002;21:4879–4884. doi: 10.1038/sj.onc.1205623. [DOI] [PubMed] [Google Scholar]
- 19.Murakami M, et al. A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt-Oram syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18034–18039. doi: 10.1073/pnas.0509109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135:4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- 21.Yin F, et al. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, et al. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer cell. 2014;26:48–60. doi: 10.1016/j.ccr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao B, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes and Development. 2010;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan SW, et al. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. The Journal of biological chemistry. 2011;286:7018–7026. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paramasivam M, et al. Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Molecular biology of the cell. 2011;22:3725–3733. doi: 10.1091/mbc.E11-04-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, et al. Angiomotin-like proteins associate with and negatively regulate YAP1. The Journal of biological chemistry. 2011;286:4364–4370. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi C, et al. The p130 isoform of angiomotin is required for Yap-mediated hepatic epithelial cell proliferation and tumorigenesis. Sci Signal. 2013;6:ra77. doi: 10.1126/scisignal.2004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlegelmilch K, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, et al. PTPN14 interacts with and negatively regulates the oncogenic function of YAP. Oncogene. 2013;32:1266–1273. doi: 10.1038/onc.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, et al. PTPN14 is required for the density-dependent control of YAP1. Genes & development. 2012;26:1959–1971. doi: 10.1101/gad.192955.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azzolin L, et al. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 32.Yu FX, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avruch J, et al. Mst1/2 signalling to Yap: gatekeeper for liver size and tumour development. Br J Cancer. 2011;104:24–32. doi: 10.1038/sj.bjc.6606011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou D, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yimlamai D, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, et al. Differences in Yes-associated protein and mRNA levels in regenerating liver and hepatocellular carcinoma. Mol Med Rep. 2012;5:410–414. doi: 10.3892/mmr.2011.640. [DOI] [PubMed] [Google Scholar]
- 37.Bai H, et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012;56:1097–1107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apte U, et al. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology. 2009;50:844–851. doi: 10.1002/hep.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anakk S, et al. Bile acids activate YAP to promote liver carcinogenesis. Cell Rep. 2013;5:1060–1069. doi: 10.1016/j.celrep.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H, et al. The Ets transcription factor GABP is a component of the hippo pathway essential for growth and antioxidant defense. Cell Rep. 2013;3:1663–1677. doi: 10.1016/j.celrep.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grijalva JL, et al. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014;307:G196–204. doi: 10.1152/ajpgi.00077.2014. [DOI] [PubMed] [Google Scholar]
- 42.Herr KJ, et al. Loss of alpha-catenin elicits a cholestatic response and impairs liver regeneration. Sci Rep. 2014;4:6835. doi: 10.1038/srep06835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang ZW, et al. miR-375 inhibits proliferation of mouse pancreatic progenitor cells by targeting YAP1. Cell Physiol Biochem. 2013;32:1808–1817. doi: 10.1159/000356614. [DOI] [PubMed] [Google Scholar]
- 44.Gao T, et al. Hippo signaling regulates differentiation and maintenance in the exocrine pancreas. Gastroenterology. 2013;144:1543–1553. 1553 e1541. doi: 10.1053/j.gastro.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.George NM, et al. Hippo signaling regulates pancreas development through inactivation of Yap. Mol Cell Biol. 2012;32:5116–5128. doi: 10.1128/MCB.01034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitagawa M. A Sveinsson’s chorioretinal atrophy-associated missense mutation in mouse Tead1 affects its interaction with the co-factors YAP and TAZ. Biochem Biophys Res Commun. 2007;361:1022–1026. doi: 10.1016/j.bbrc.2007.07.129. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, et al. Taz-tead1 links cell-cell contact to zeb1 expression, proliferation, and dedifferentiation in retinal pigment epithelial cells. Investigative ophthalmology & visual science. 2010;51:3372–3378. doi: 10.1167/iovs.09-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang N, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai J, et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes & development. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barry ER, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camargo FD, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 52.Varelas X, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837–848. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- 53.Tian Y, et al. TAZ promotes PC2 degradation through a SCFbeta-Trcp E3 ligase complex. Mol Cell Biol. 2007;27:6383–6395. doi: 10.1128/MCB.00254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahuja P, et al. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiological reviews. 2007;87:521–544. doi: 10.1152/physrev.00032.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto S, et al. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. The Journal of clinical investigation. 2003;111:1463–1474. doi: 10.1172/JCI17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Odashima M, et al. Inhibition of endogenous Mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circulation research. 2007;100:1344–1352. doi: 10.1161/01.RES.0000265846.23485.7a. [DOI] [PubMed] [Google Scholar]
- 57.Shao D, et al. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nat Commun. 2014;5:3315. doi: 10.1038/ncomms4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heallen T, et al. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–4690. doi: 10.1242/dev.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xin M, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin Z, et al. Pi3kcb Links Hippo-YAP and PI3K-AKT Signaling Pathways to Promote Cardiomyocyte Proliferation and Survival. Circulation research. 2014 doi: 10.1161/CIRCRESAHA.115.304457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zender L, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang T, et al. Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology. 2012;56:2051–2059. doi: 10.1002/hep.25899. [DOI] [PubMed] [Google Scholar]
- 63.Kowalik MA, et al. Yes-associated protein regulation of adaptive liver enlargement and hepatocellular carcinoma development in mice. Hepatology. 2011;53:2086–2096. doi: 10.1002/hep.24289. [DOI] [PubMed] [Google Scholar]
- 64.Nishio M, et al. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. J Clin Invest. 2012;122:4505–4518. doi: 10.1172/JCI63735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee KP, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu L, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science signaling. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flucke U, et al. Epithelioid Hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagnostic pathology. 2014;9:131. doi: 10.1186/1746-1596-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Antonescu CR, et al. ZFP36-FOSB fusion defines a subset of epithelioid hemangioma with atypical features. Genes Chromosomes Cancer. 2014;53:951–959. doi: 10.1002/gcc.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Antonescu CR, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes, chromosomes & cancer. 2013;52:775–784. doi: 10.1002/gcc.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanas MR, et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Science translational medicine. 2011;3:98ra82. doi: 10.1126/scitranslmed.3002409. [DOI] [PubMed] [Google Scholar]
- 72.Cordenonsi M, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 73.Chan SW, et al. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer research. 2008;68:2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- 74.Wang X, et al. Yes-associated protein promotes tumour development in luminal epithelial derived breast cancer. European journal of cancer. 2012;48:1227–1234. doi: 10.1016/j.ejca.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Chen Q, et al. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes & development. 2014;28:432–437. doi: 10.1101/gad.233676.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen D, et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nature medicine. 2012;18:1511–1517. doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan M, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell death and differentiation. 2008;15:1752–1759. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, et al. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer science. 2010;101:1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou Z, et al. TAZ is a novel oncogene in non-small cell lung cancer. Oncogene. 2011;30:2181–2186. doi: 10.1038/onc.2010.606. [DOI] [PubMed] [Google Scholar]
- 80.Lau AN, et al. Tumor-propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis. EMBO J. 2014;33:468–481. doi: 10.1002/embj.201386082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu CM, et al. Mst1 overexpression inhibited the growth of human non-small cell lung cancer in vitro and in vivo. Cancer Gene Ther. 2013;20:453–460. doi: 10.1038/cgt.2013.40. [DOI] [PubMed] [Google Scholar]
- 82.Lin XY, et al. Expression of LATS1 contributes to good prognosis and can negatively regulate YAP oncoprotein in non-small-cell lung cancer. Tumour Biol. 2014;35:6435–6443. doi: 10.1007/s13277-014-1826-z. [DOI] [PubMed] [Google Scholar]
- 83.Zhang W, et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014;24:331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cui ZL, et al. YES-associated protein 1 promotes adenocarcinoma growth and metastasis through activation of the receptor tyrosine kinase Axl. Int J Immunopathol Pharmacol. 2012;25:989–1001. doi: 10.1177/039463201202500416. [DOI] [PubMed] [Google Scholar]
- 85.Lin CW, et al. MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat Commun. 2013;4:1877. doi: 10.1038/ncomms2876. [DOI] [PubMed] [Google Scholar]
- 86.Murakami H, et al. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 2011;71:873–883. doi: 10.1158/0008-5472.CAN-10-2164. [DOI] [PubMed] [Google Scholar]
- 87.Mizuno T, et al. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene. 2012;31:5117–5122. doi: 10.1038/onc.2012.5. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka I, et al. LIM-domain protein AJUBA suppresses malignant mesothelioma cell proliferation via Hippo signaling cascade. Oncogene. 2013 doi: 10.1038/onc.2013.528. [DOI] [PubMed] [Google Scholar]
- 89.Diep CH, et al. Down-regulation of Yes Associated Protein 1 expression reduces cell proliferation and clonogenicity of pancreatic cancer cells. PLoS One. 2012;7:e32783. doi: 10.1371/journal.pone.0032783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang W, et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Science signaling. 2014;7:ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kapoor A, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu G, et al. Kaposi sarcoma-associated herpesvirus promotes tumorigenesis by modulating the Hippo pathway. Oncogene. 2014 doi: 10.1038/onc.2014.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Raamsdonk CD, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu FX, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feng X, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu-Chittenden Y, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes & development. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen KH, et al. Methylationassociated inactivation of LATS1 and its effect on demethylation or overexpression on YAP and cell biological function in human renal cell carcinoma. Int J Oncol. 2014 doi: 10.3892/ijo.2014.2687. [DOI] [PubMed] [Google Scholar]
- 98.Cao JJ, et al. YAP is overexpressed in clear cell renal cell carcinoma and its knockdown reduces cell proliferation and induces cell cycle arrest and apoptosis. Oncol Rep. 2014;32:1594–1600. doi: 10.3892/or.2014.3349. [DOI] [PubMed] [Google Scholar]
- 99.Azzolin L, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 100.Rosenbluh J, et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Konsavage WM, Jr, et al. Wnt/beta-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. The Journal of biological chemistry. 2012;287:11730–11739. doi: 10.1074/jbc.M111.327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu FX, et al. Hippo Pathway Regulation of Gastrointestinal Tissues. Annu Rev Physiol. 2015;77:8.1–8.27. doi: 10.1146/annurev-physiol-021014-071733. [DOI] [PubMed] [Google Scholar]
- 103.Wierzbicki PM, et al. Underexpression of LATS1 TSG in colorectal cancer is associated with promoter hypermethylation. World J Gastroenterol. 2013;19:4363–4373. doi: 10.3748/wjg.v19.i27.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou D, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A. 2011;108:E1312–1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Touil Y, et al. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin Cancer Res. 2014;20:837–846. doi: 10.1158/1078-0432.CCR-13-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cottini F, et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nature medicine. 2014;20:599–606. doi: 10.1038/nm.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schulz A, et al. A neuronal function of the tumor suppressor protein merlin. Acta neuropathologica communications. 2014;2:82. doi: 10.1186/s40478-014-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Striedinger K, et al. The neurofibromatosis 2 tumor suppressor gene product, merlin, regulates human meningioma cell growth by signaling through YAP. Neoplasia. 2008;10:1204–1212. doi: 10.1593/neo.08642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lau YK, et al. Merlin is a potent inhibitor of glioma growth. Cancer research. 2008;68:5733–5742. doi: 10.1158/0008-5472.CAN-08-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Orr BA, et al. Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. Journal of neuropathology and experimental neurology. 2011;70:568–577. doi: 10.1097/NEN.0b013e31821ff8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bao Y, et al. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. J Biochem. 2011;150:199–208. doi: 10.1093/jb/mvr063. [DOI] [PubMed] [Google Scholar]
- 112.Zhou Z, et al. Targeting Hippo pathway by specific interruption of YAP-TEAD interaction using cyclic YAP-like peptides. FASEB J. 2014 doi: 10.1096/fj.14-262980. [DOI] [PubMed] [Google Scholar]
- 113.Happe H, et al. Altered Hippo signalling in polycystic kidney disease. J Pathol. 2011;224:133–142. doi: 10.1002/path.2856. [DOI] [PubMed] [Google Scholar]
- 114.Hossain Z, et al. Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc Natl Acad Sci U S A. 2007;104:1631–1636. doi: 10.1073/pnas.0605266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Makita R, et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol. 2008;294:F542–553. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- 116.Reginensi A, et al. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 2013;9:e1003380. doi: 10.1371/journal.pgen.1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen SN, et al. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circulation research. 2014;114:454–468. doi: 10.1161/CIRCRESAHA.114.302810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Basson CT, et al. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nature genetics. 1997;15:30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- 119.Orcholski ME, et al. Signaling via amyloid precursor-like proteins APLP1 and APLP2. Journal of Alzheimer’s disease: JAD. 2011;23:689–699. doi: 10.3233/JAD-2010-101470. [DOI] [PubMed] [Google Scholar]
- 120.Lee JK, et al. MST1 functions as a key modulator of neurodegeneration in a mouse model of ALS. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12066–12071. doi: 10.1073/pnas.1300894110. [DOI] [PMC free article] [PubMed] [Google Scholar]