Abstract
Objective: This study was performed to investigate bone deteriorations and the involvement of skeletal Eph/ephrin signaling pathway of GIOP aged mice in response to the treatment of genistein. Methods: The biomarkers in serum and urine were measured, tibias were taken for the measurement on gene and protein expression and histomorphology analysis, and femurs were taken for the measurement on bone Ca and three-dimensional architecture of trabecular bone. Results: Genistein showed a greater increase in bone Ca, BMD and significantly increased FGF-23 and OCN, reduced TRACP-5b, PTH and CTX in GIOP mice. Genistein reversed DXM-induced trabecular deleterious effects and stimulated bone remodeling. The treatment of DXM group with genistein significantly elevated the ratio of OPG/RANKL. Moreover, genistein administration down-regulated the mRNA and protein expression of Eph A2 and ephrin A2 in tibia of the GIOP mice. In contrast, the mRNA and protein expression of Eph B4 and ephrin B2 were increased in mice treated by DXM with genistein as compared to the DXM single treatment. Conclusions: DXM-induced trabecular bone micro-structure deterioration in aged mice was involved in the regulation of the Eph receptors and ephrin ligands. Genistein might represent a therapy with bone-forming as well as an anti-resorptive activity in GIOP mice. The underlying mechanism was mediated, at least partially, through regulation Eph/ephrin signaling.
Keywords: Eph/ephrin, bone, genistein, dexamethasone
Introduction
Glucocorticoids have been widely used in clinics due to their anti-inflammatory and immunomodulatory effects. However, the therapeutic use for immunosuppression after organ transplantation or for inflammatory diseases of glucocorticoids is always accompanied by substantial adverse outcomes such as diabetes, obesity, and bone deleterious deteriorations [1-3]. However, glucocorticoids-induced bone deleterious effects have been regarded as an important cause for osteoporosis and bone loss [4]. Clinical studies have shown that low-dose [5] or high-dose [6] glucocorticoid, especially dexamethasone (DXM), inhalatory therapy as a cause of bone loss in human. In vitro studies also show that glucocorticoids can induce osteoblasts and osteocyte apoptosis [1,7]. Studies also show that bone mesenchymal stem cells (BMSCs) proliferation, osteogenic differentiation, and reactive activity to an osteogenic inductor are reduced in glucocorticoid-induced osteoporosis (GIOP) rats [8,9]. Many studies have found that renin-angiotensin system (RAS) [10], hormones [11], and some transcription factors [1,12], such as nuclear factor erythroid 2-related factor 2 (Nrf2) and runt-related transcription factor 2 (Runx2), contribute to GIOP. However, the underlying signaling mechanisms accounting for GIOP are still not well characterized.
Bone cells, such as chondrocytes, osteoblasts, osteocytes and osteoclasts, expressed a variety of ephrin ligands and Eph receptors [13]. Human articular cartilage cells expressed ephrinB2 and EphB4 [14]. Cultured osteoclasts induced by RANKL, ephrinA2, B1, B2, and receptors EphA1, A2, A4 were dynamically expressed as revealed by RT-PCR [15-17]. The protein expression of ephrin ligands and Eph receptors were identified in human MSC by western blotting and immunohistochemistry [18]. Developmental deficiencies in EphB/ephrinB signaling pathway can lead to skeletal abnormal. These include defective development of the somitogenesis [19], craniofacial development [20], limb development [21], and other bone abnormalities observed in EphB2, EphB3 and ephrinB1 mutant mice, also in individuals harboring ephrinB1 mutations that cause the X-linked developmental disorder craniofrontonasal syndrome [22]. Although the effects of Eph/ephrin signaling pathway can lead to skeletal abnormal, the role of Eph/ephrin in the process of bone deteriorations induced by glucocorticoid remains largely unknown.
Genistein, an isoflavone abundant in high concentrations in soybeans, tofu, tempeh and soymilk, has been shown to have pharmacological properties beneficial for skeletal health due to its ability to bind to and activate estrogen receptors [23]. Epidemiological studies have indicated that, compared with Europeans, Asian soy-rich diets leads to lower morbidity of post-menopausal osteoporosis [24]. A soy extract containing a similar level of genistein in the form of Novasoy is improving tibial trabecular bone quality in OVX mice [25]. Moreover, genistein administration significantly improves femoral mechanical properties and alleviates femoral turnover that the anti-osteoporotic effect of genistein is partly PTH/PTHR1-dependent [26]. In contrast to previous reports, the present study shows that genistein delivered as a once daily oral supplement has no beneficial effect on the tibia in rat models for postmenopausal bone loss [27]. Therefore, this occurs with levels of dietary and supplemental intake of genistein is controversial in OVX-induced osteoporosis. However, the effects of genistein in other expreimental animal models, such as glucocorticoid-induced osteoporosis animal model, remain largely unknown.
The above research results suggested that Eph/ephrin bidirectional signaling provided an intriguing explanation of cellular and molecular mechanisms responsible for osteoblast-osteoclast coupling and maybe a potential target for osteoporosis treatment. Therefore, the aim of the present work was to investigate the protective effects of genistein in DXM-induced bone deteriorations and if Eph/ephrin was involved in mediating its protective actions.
Materials and methods
Participants
Patients ambulatory outpatients aged > 30 years were screened with written informed consent from The Second Affiliated Hospital of Anhui Medical University. Lumbar spine (L1-L4), femoral neck, or total hip BMD T-score was measured by DXA. The study was approved by the Ethics Committee of The Second Affiliated Hospital of Anhui Medical University. 63 patients had used high doses of glucocorticoid, they were collected between 02/2013 and 02/2014.
Animal treatment
Twelve-week-old female C57BL/6J mice (Guangzhou University of Traditional Chinese Medicine, Guangzhou, China) were allowed to acclimate to the environment for 1 week. All experimental procedures were carried out in accordance with the guidelines of The Second Affiliated Hospital of Anhui Medical University on Animal Care. All chemicals and reagents were purchased from Sigma (Oakville, Ontario, Canada), except where noted.
The mice were randomly divided into three groups: (1) Vehicle group (n = 12); (2) Mice were injected intramuscularly with 5 mg/kg body weight dexamethasone (DXM) three times a week for 12 weeks (DXM, n = 12); (3) Mice in the G + DXM group received genistein orally at a dose of 10 mg/kg per day combined with DXM for 12 weeks (G + DXM, n = 12).
Chemistries in serum and urine and bone Ca
The concentrations of calcium (Ca) and creatinine (Cre) from serum and urine were measured by standard colorimetric methods using a micro-plate reader (Bio-Tek, USA). The level of urine Ca was corrected by the concentration of urine Cre. Serum levels of intact parathyroid hormone (PTH 1-84), fibroblast growth factor-23 (FGF-23), tartrate resistant acid phosphatase-5b (TRACP-5b), Osteocalcin (OCN) and C-terminal telopeptide of type I collagen (CTX) were detected using rat bioactive PTH ELISA assay (Immutopics, Inc., San Clemente, CA, USA) with ELISA reader (MD SpectraMax M5, USA).
The tibias were incinerated at 800°C for 6 hours and the ash weighed. 10 mg of bone ash was then dissolved in 1 ml of 37% HCl and diluted with Millin-Q water. The calcium content was determined by the kit used for serum and urine calcium assay.
Bone histomorphology
The tibias were decalcified in 0.5 M EDTA (pH = 8.0) and then embedded in paraffin by standard histological procedures. Section of 5 μm were cut and stained with hematoxylin & eosin (H&E), and visualized under a microscope (Leica DM 2500).
The trabecular bone microarchitecture of the proximal metaphysis of the tibia was measured using a microtomography scanner (SkyScan 1076, Kontizh, Belgium) with a slice thickness of 22 μm. The volume of interest (VOI) was trabecular compartments based on 100 consecutive slices away from the distal femur growth plate. The 3D images were obtained for visualization and display. Bone morphometric parameters, including bone volume over total volume (BV/TV), trabecula number (Tb. N), trabecula thickness (Tb. Th) and bone mineral density over total volume (BMD/TV) were obtained by analyzing the VOI.
Reverse transcription-polymerase chain reaction
The tibias of each animal were crushed under liquid nitrogen conditions and RNA extraction was performed according to the TRIzol manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). RNA integrity was verified by agarose gel electrophoresis. Synthesis of cDNAs was performed by reverse transcription reactions with 2 μg of total RNA using moloney murine leukemia virus reverse transcriptase (Invitrogen) with oligo dT(15) primers (Fermentas) as described by the manufacturer. The first strand cDNAs served as the template for the regular polymerase chain reaction (PCR) performed using a DNA Engine (ABI 7300). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control was used to normalize the data to determine the relative expression of the target genes. The reaction conditions were set according to the kit instructions. The PCR primers used in this study were shown in Table 1.
Table 1.
Primers sequence used for RT-PCR analysis
| gene | Forward primer sequence (5’-3’) | Reverse primer sequence (5’-3’) |
|---|---|---|
| RANKL | tcaggagttccagctatgat | ccatcagctgaagatagtcc |
| OPG | tcactgggctgtttcttcag | tcctctttctcagggtgctt |
| EphA2 | acaacatccgcctagagg | tacttcatgccagctgcgatg |
| EphB4 | ccccagggaagaaggagagc | gcccacgagcggatgactgtg |
| EphrinB2 | gacgtccagaactagaagctgg | caccatccaatggaagcctgg |
| EphrinB4 | caacatccaatggaagcctgg | ggagttgaagaagccatcagg |
| GAPGH | gtgaggtgaccgcatcttct | cttgccgtgggtagagtcat |
Western blotting
The tibias were homogenized and extracted in NP-40 buffer, followed by 5-10 min boiling and centrifugation to obtain the supernatant. Samples containing 50 μg of protein were separated on 10% SDS-PAGE gel, transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). After saturation with 5% (w/v) non-fat dry milk in TBS and 0.1% (w/v) Tween 20 (TBST), the membranes were incubated with the following antibodies, EphA2, EphB4, ephrinA2, ephrinB2 (R&D Systems, Minneapolis, MN, USA), at dilutions ranging from 1:500 to 1:2,000 at 4°C over-night. After three washes with TBST, membranes were incubated with secondary immunoglobulins (Igs) conjugated to IRDye 800CW Infrared Dye (LI-COR), including donkey anti-goat IgG and donkey anti-mouse IgG at a dilution of 1:10,000-1:20,000. After 1 hour incubation at 37°C, membranes were washed three times with TBST. Blots were visualized by the Odyssey Infrared Imaging System (LI-COR Biotechnology). Signals were densitometrically assessed (Odyssey Application Software version 3.0) and normalized to the β-actin signals to correct for unequal loading using the mouse monoclonal anti-β-actin antibody (Bioworld Technology, USA).
Statistical analysis
The data from these experiments were reported as mean ± standard error of mean (SEM) for each group. All statistical analyses were performed using PRISM version 4.0 (GraphPad). Inter-group differences were analyzed by one-way ANOVA, and followed by Tukey’s multiple comparison test as a post test to compare the group means if overall P < 0.05. Differences with P value of < 0.05 were considered statistically significant.
Results
Patients’ baseline characteristics
A total of 63 patients, 30 male and 33 female, were screened from The Second Affiliated Hospital of Anhui Medical University. On mean age, the women were older than the men, with the median age of the women being 60, eight years older than the median age of men. With the exception of weight and height, they also tend to less aBMD, worse physical fitness index than men with long-term glucocorticoid therapy (Table 2).
Table 2.
Baseline characteristics
| Variable | Male | Female |
|---|---|---|
| N | 30 | 33 |
| Age (years), mean (SD) | 52 (14.2) | 60 (10.8) |
| Anthropometry | ||
| BMI (kg/m2), mean (SD) | 25.8 (3.8) | 27.4 (3.3) |
| Weight (kg), mean (SD) | 70.7 (14.2) | 65.6 (9.6) |
| Height (cm), mean (SD) | 167.9 (5.2) | 155.3 (5.6) |
| Body fat content (%), mean (SD) | 31.8 (9.0) | 37.2 (7.5) |
| Glucocorticoid use (years) | 5.9 (2.3) | 8.2 (4.2) |
| aBMD (by DXA) | ||
| Lumber spine (T-score) | -1.61 (0.49) | -2.62 (0.88) |
| Femoral neck (T-score) | -1.68 (0.43) | -2.03 (0.58) |
| Femur (T-score) | -1.39 (0.45) | -1.89 (0.65) |
N, number of patients with available data; aBMD, areal bone mineral density; DXA, dual-energy X-ray absorptiometry.
Physiological and biochemical properties
DXM administration through intramuscular injection for twelve weeks, glucocorticoid-treated mice showed significant increases in body weight and decreases in uterus weight compared to that of the control group (Table 3). The Ca level in serum, urine and bone was comparable in the three experimental groups. DXM could up-regulate urine Ca excretion and down-regulate the Ca content in bone and serum (Table 3). Glucocorticoid-treated could accelerate calcium outflow. Twelve weeks after the genistein treatment, the uterus weight was increased in the combination group when compared to that of the DXM single group. When comparing the results of serum, urine and bone between DXM and G + DXM groups, we could easily see that the genistein increased serum Ca, decreased urine Ca excretion and increased bone calcium content (Table 3). From these calcium metabolic data, it was well shown that genistein exerted protective effects on maintaining calcium balance of DXM-induced bone deteriorations in mice.
Table 3.
Physiological and biochemical properties
| Vehicle | DXM | G + DXM | |
|---|---|---|---|
| Body weight (g) | 24.7 ± 1.8 | 30.2 ± 2.5* | 28.3 ± 2.4 |
| Uterus weight (mg) | 56.3 ± 4.1 | 41 ± 3.2* | 54.2 ± 4.7# |
| Ca/Ash (mg/mg) | 0.42 ± 0.025 | 0.27 ± 0.017* | 0.37 ± 0.022# |
| Bone Ca (mg) | 8.7 ± 0.42 | 5.4 ± 0.35* | 7.1 ± 0.32# |
| Serum Ca (mg/dL) | 10.53 ± 0.35 | 9.08 ± 0.32* | 10.68 ± 0.49# |
| Urine Ca/Cre (mg/mg) | 0.044 ± 0.005 | 0.092 ± 0.009* | 0.053 ± 0.005# |
Values are expressed as mean ± SEM, n = 5 in each group.
P < 0.05, versus vehicle group;
P < 0.05, versus DXM group.
Micro-CT and bone histology
The loss of trabecular bone mass at the proximal metaphysis of the tibia was quantified using micro-CT scanning. Analyses of the data from the proximal metaphysis of the tibia revealed that GIOP mice exhibited significantly lower trabecular BMD/TV, BV/TV, Tb. N and Tb. Th, compared to that of the control group (Table 4). Notably, treatment with genistein for GIOP mice resulted in increasing the BV/TV ratio, Tb. N, Tb. Th and BMD/TV (Table 4). Histological analysis on trabecular bone in proximal metaphysis of mice was performed by H&E staining (Figure 1). The histology of trabecular bone below growth plate was markedly different in the three experimental groups. H&E staining showed the increased disconnections and separation among growth plate and trabecular bone network as well as the reduction of trabecular bone mass of primary and secondary spongiosa throughout the proximal metaphysis of tibia in DXM group. Importantly, genistein reversed DXM-induced trabecular deleterious effects and stimulated bone remodeling.
Table 4.
Bone parameters of proximal tibia
| Vehicle | DXM | G + DXM | |
|---|---|---|---|
| BV/TV (%) | 36 ± 3.5 | 19 ± 2.4* | 28 ± 3.2# |
| Tb. N (mm-1) | 8.6 ± 0.78 | 5.2 ± 0.36* | 7.8 ± 0.91# |
| Tb. Th (μm) | 54 ± 4.8 | 27 ± 4.2* | 50 ± 4.5# |
| BMD/TV (mg HA/cm3) | 153 ± 12.2 | 87 ± 10.6* | 129 ± 13.3# |
BV/TV, bone volume over total volume; Tb. N, trabecula number; Tb. Th, trabecula thickness; BMD/TV, bone mineral density over total volume. Values are expressed as mean ± SEM, n = 5 in each group.
P < 0.05, versus vehicle group;
P < 0.05, versus DXM group.
Figure 1.

Hematoxylin and eosin staining of the proximal metaphysis of the tibia. Trabecular bone zone below growth plate was shown.
Bone metabolic biochemical makers
Serum concentrations of bone turnover markers, like TRACP-5b as a bone resorption marker and OCN as a bone formation marker, were determined. The results showed that the serum PTH, TRACP-5b and CTX level in DXM group were significantly increased, and the serum FGF-23 and OCN level were significantly decreased when compared to that of the control group (Figure 2). The serum PTH, TRACP-5b and CTX level in the combination group (G + DXM group) were lower than DXM group (P < 0.05), and the serum FGF-23 and OCN level were significantly elevated in G + DXM group (Figure 2).
Figure 2.

Biochemical parameters analysis. PTH, parathyroid hormone; FGF-23, fibroblast growth factor-23; TRACP-5b, tartrate resistant acid phosphatase-5b; OCN, Osteocalcin; CTX, C-terminal telopeptide of type I collagen. Values are expressed as mean ± SEM, n = 7-10 in each group. *P < 0.05, versus vehicle group; #P < 0.05, versus DXM group.
Osteoprotegerin/receptor activator of nuclear factor kappa B ligand (OPG/RANKL) ratio
The maturation and formation of osteoclasts were mainly regulated by the balance of extracellular OPG and RANKL levels, thus, the ratio of OPG/RANKL expression in tibia was determined in our study. The RT-PCR result showed that the ratio of OPG/RANKL was significantly decreased, and RANKL was increased in mice treated by DXM as compared to the control group (Figure 3). The treatment of DXM group with genistein significantly elevated the ratio of OPG/RANKL. However, the mRNA expression of OPG was no obvious difference in both the DXM and G + DXM group (Figure 3).
Figure 3.
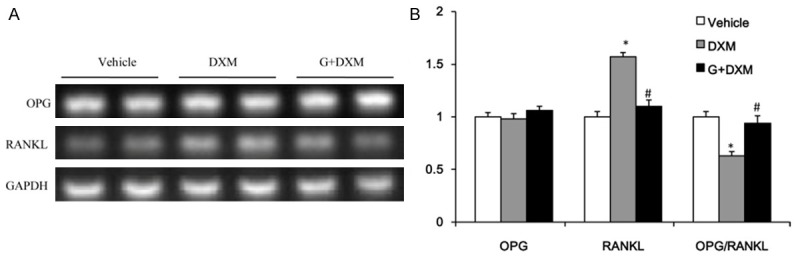
OPG/RANKL ratio in tibia. The mRNA expression of osteoprotegerin (OPG), receptor activator of nuclear factor-κB ligand (RANKL) (A) and the quantitative ratio of OPG/RANKL (B). Values are expressed as mean ± SEM, n = 6-7 in each group. *P < 0.05, versus vehicle group; #P < 0.05, versus DXM group.
mRNA and protein expression of bone Eph/ephrin
Osteoclast-osteoblast interactions, osteoclast-osteoclast or osteoblast-osteoblast interactions through Eph/ephrin can affect bone metabolism. To investigate the involvement of local bone tissue Eph/ephrin in DXM-induced bone deteriorations, the Eph/ephrin components, EphA2, EphB4, ephrinA2, ephrinB2, were determined at mRNA and protein expression level from mice tibia (Figure 4A-D). The mRNA and protein expression of EphA2 and ephrinA2 was significantly higher in tibia of DXM group than control group. On the contrary, the mRNA and protein expression of EphB4 and ephrinB2 was significantly decreased at DXM group (Figure 4A-D). The treatment by DXM induction could significantly up-regulate the mRNA and protein expression of Eph A2 and ephrin A2, Eph A2/ephrin A2 pathway negatively regulated bone formation. Interestingly, genistein administration down-regulated the mRNA and protein expression of Eph A2 and ephrin A2 in tibia of the GIOP mice. In contrast, the mRNA and protein expression of Eph B4 and ephrin B2 were increased in mice treated by DXM with genistein as compared to the DXM single treatment (Figure 4A-D).
Figure 4.

mRNA and protein expression of Eph receptors and ephrins. The mRNA expression of erythropoietin-producing hepatocyte receptor A2 (Eph A2), erythropoietin-producing hepatocyte receptor B4 (Eph B4), Eph receptor interacting protein A2 (ephrin A2), Eph receptor interacting protein B4 (ephrin B4) in the tibia (A) and densitometric quantification (B), the protein expression of Eph A2, Eph B4, ephrin A2 and ephrin B4 in the tibia (C) and densitometric quantification (D). Values are expressed as mean ± SEM, n = 6-7 in each group. *P < 0.05, versus vehicle group; #P < 0.05, versus DXM group.
Discussion
An increasing amount of clinical evidence suggests a role for the isoflavone genistein in the treatment of postmenopausal osteoporosis [25,26], osteonecrosis [28] and GOIP [29]. However, the molecular mechanisms of genistein in expreimental animal models, such as glucocorticoid-induced osteoporosis animal model, remain largely unknown. The major finding of this in vivo study was that EphA2/ephrinA2 signaling in bone was activated, and EphB4/ephrinB2 signaling was blocked in GIOP characterized by the reduction of bone volume and strength, accompanied with inhibition of bone formation and enhancement of bone resorption. Then, when the EphA2/ephrinA2 was suppressed, and EphB4/ephrinB2 was elevated by genistein, all the bone abnormalities were normalized approximately. This indicates that the local bone Eph/ephrin was involved in GIOP, which regulates both bone formation and bone resorption. This finding may contribute to a better understanding of the pathogenesis underlying GIOP.
In this study, DXM injection successfully led to bone deleterious effects. The DXM-induced increasing in bone resorption was confirmed by the increased level of CTX and the decreased level of osteocalcin in the serum, and the decreased level of serum Ca and the increased level of urinary Ca excretion. Moreover, histomorphology staining also confirmed the results. A recently identified phosphatonin, known as fibroblast growth factor 23 (FGF-23), disclosed new pathways in the pathophysiology of mineral metabolism [30]. Clinical studies had shown that the downregulation of serum FGF-23 levels in Crohn disease appeared as a secondary compensatory effect on the bone and mineral metabolism induced by chronic intestinal inflammation [31]. This study provided evidence that the down-regulation of serum FGF-23 levels in GIOP mice. The serum CTX level in the G + DXM group were lower than DXM group, and the serum FGF-23 and OCN level were significantly elevated in G + DXM group suggesting an anti-resorptive effect.
Mice overexpression the transcription factor c-Fos can induce osteoclast differentiation and develop osteoporosis [32]. Expression of Fos family proteins is essential to induce expression of the transcription factor NFATc1, the master regulator of osteoclast differentiation [33]. Several osteoclast-specific genes including tartrate-resistant acid phosphatase, cathepsin K and matrix metalloproteinase 9 are transcriptional targets of NFATc1 [34]. EphrinB2 was identified as an NFATc1 target gene in microarray screening [13]. To date, a few studies to our knowledge investigating the B-subclass Eph/ephrin, demonstrated that osteoarthritic subchondral bone metabolism involved EphB4/ephrinB2 signaling. The activation of EphB4 by ephrin-B2 inhibited the expression of factors such as MMPs, interleukins and RANKL, which resulted in reduced resorption activity leading to osteoarthritic subchondral bone [14,15]. We demonstrated that ephrinB4 reverse signaling and EphB4 forward signaling were suppressed that the mRNA and protein expression of EphB4 and ephrinB2 was significantly decreased in tibia of mice in DXM group, suggesting that suppressing the expression of EphB4 and ephrinB2 might be involved in bone deteriorations due to administration of glucocorticoid.
Osteoclast-derived factors also negatively regulate osteoblasts and antagonize coupling. Osteoclast-derived ephrinA2, which was expressed early during osteoclastogenesis, may be such a negative regulator [13]. Interestingly, differentiation of osteoblasts lacking EphA2 was enhanced along with alkaline phosphatase, Runx2, and Osterix expression, indicating that EphA2 on osteoblasts generates anti-osteoblastogenic signals presumably by up-regulating RhoA activity. EphrinA2-EphA2 interaction facilitates the initiation phase of bone remodeling by enhancing osteoclast differentiation and suppressing osteoblast differentiation [13,15]. In this study, we demonstrated that the mRNA and protein expression of EphA2 and ephrinA2 was significantly increased in tibia of mice in DXM group. Moreover, the decreased mRNA expression ratio of OPG/RANKL in tibia and increased serum TRACP-5b indicated that DXM could stimulate osteoclastogenesis in the bone of mice. This is in contrast to the transition phase of bone remodeling from bone resorption to bone formation, when ephrinB2-EphB4 interaction inhibits osteoclastogenesis with concomitant promotion of bone formation [15].
In conclusion, DXM-induced trabecular bone micro-structure deterioration in aged mice was involved in the regulation of the Eph receptors and ephrin ligands. On the basis of the present results, genistein might represent a therapy with bone-forming as well as an anti-resorptive activity in GIOP mice. The underlying mechanism was mediated, at least partially, through down-regulation of EphA2/ephrinA2 signaling and up-regulation of EphB4/ephrinB2 signaling. Moreover, these studies will identify new potential therapeutic targets to GIOP treatment.
Disclosure of conflict of interest
None.
References
- 1.Lin H, Wei B, Li G, Zheng J, Sun J, Chu J, Zeng R, Niu Y. Sulforaphane reverses glucocorticoid-induced apoptosis in osteoblastic cells through regulation of the Nrf2 pathway. Drug Des Devel Ther. 2014;8:973–982. doi: 10.2147/DDDT.S65410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323–328. doi: 10.1007/s00198-003-1548-3. [DOI] [PubMed] [Google Scholar]
- 3.Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48:3224–3229. doi: 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- 4.Elshal MF, Almalki AL, Hussein HK, Khan JA. Synergistic antiosteoporotic effect of Lepidium sativum and alendronate in glucocorticoid-induced osteoporosis in Wistar rats. Afr J Tradit Complement Altern Med. 2013;10:267–273. doi: 10.4314/ajtcam.v10i5.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capozzi A, Casa SD, Altieri B, Pontecorvi A. Chronic low-dose glucocorticoid inhalatory therapy as a cause of bone loss in a young man: case report. Clin Cases Miner Bone Metab. 2013;10:199–202. [PMC free article] [PubMed] [Google Scholar]
- 6.De Vries F, Bracke M, Leufkens HG, Lammers JW, Cooper C, Van Staa TP. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum. 2007;56:208–214. doi: 10.1002/art.22294. [DOI] [PubMed] [Google Scholar]
- 7.Jahn K, Lara-Castillo N, Brotto L, Mo CL, Johnson ML, Brotto M, Bonewald LF. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of beta-catenin. Eur Cell Mater. 2012;24:197–209. doi: 10.22203/ecm.v024a14. discussion 209-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou DA, Zheng HX, Wang CW, Shi D, Li JJ. Influence of glucocorticoids on the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. BMC Musculoskelet Disord. 2014;15:239. doi: 10.1186/1471-2474-15-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma X, Zhang X, Jia Y, Zu S, Han S, Xiao D, Sun H, Wang Y. Dexamethasone induces osteogenesis via regulation of hedgehog signalling molecules in rat mesenchymal stem cells. Int Orthop. 2013;37:1399–1404. doi: 10.1007/s00264-013-1902-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yongtao Z, Kunzheng W, Jingjing Z, Hu S, Jianqiang K, Ruiyu L, Chunsheng W. Glucocorticoids activate the local renin-angiotensin system in bone: possible mechanism for glucocorticoid-induced osteoporosis. Endocrine. 2014;47:598–608. doi: 10.1007/s12020-014-0196-z. [DOI] [PubMed] [Google Scholar]
- 11.Campbell IA, Douglas JG, Francis RM, Prescott RJ, Reid DM. Hormone replacement therapy (HRT) or etidronate for osteoporosis in postmenopausal asthmatics on glucocorticoids: a randomised factorial trial. Scott Med J. 2009;54:21–25. doi: 10.1258/rsmsmj.54.1.21. [DOI] [PubMed] [Google Scholar]
- 12.Koromila T, Baniwal SK, Song YS, Martin A, Xiong J, Frenkel B. Glucocorticoids antagonize RUNX2 during osteoblast differentiation in cultures of ST2 pluripotent mesenchymal cells. J Cell Biochem. 2014;115:27–33. doi: 10.1002/jcb.24646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adh Migr. 2012;6:148–156. doi: 10.4161/cam.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwan Tat S, Pelletier JP, Amiable N, Boileau C, Lavigne M, Martel-Pelletier J. Treatment with ephrin B2 positively impacts the abnormal metabolism of human osteoarthritic chondrocytes. Arthritis Res Ther. 2009;11:R119. doi: 10.1186/ar2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M, Iwakura Y, Suda T, Matsuo K. Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem. 2009;284:14637–14644. doi: 10.1074/jbc.M807598200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Cheng S, Zhao SL, Nelson B, Kesavan C, Qin X, Wergedal J, Mohan S, Xing W. Targeted disruption of ephrin B1 in cells of myeloid lineage increases osteoclast differentiation and bone resorption in mice. PLoS One. 2012;7:e32887. doi: 10.1371/journal.pone.0032887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arthur A, Zannettino A, Panagopoulos R, Koblar SA, Sims NA, Stylianou C, Matsuo K, Gronthos S. EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone. 2011;48:533–542. doi: 10.1016/j.bone.2010.10.180. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe T, Sato Y, Saito D, Tadokoro R, Takahashi Y. EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc Natl Acad Sci U S A. 2009;106:7467–7472. doi: 10.1073/pnas.0902859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing W, Kim J, Wergedal J, Chen ST, Mohan S. Ephrin B1 regulates bone marrow stromal cell differentiation and bone formation by influencing TAZ transactivation via complex formation with NHERF1. Mol Cell Biol. 2010;30:711–721. doi: 10.1128/MCB.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghu Nadhanan R, Skinner J, Chung R, Su YW, Howe PR, Xian CJ. Supplementation with fish oil and genistein, individually or in combination, protects bone against the adverse effects of methotrexate chemotherapy in rats. PLoS One. 2013;8:e71592. doi: 10.1371/journal.pone.0071592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirtori CR. Risks and benefits of soy phytoestrogens in cardiovascular diseases, cancer, climacteric symptoms and osteoporosis. Drug Saf. 2001;24:665–682. doi: 10.2165/00002018-200124090-00003. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Li Q, Wan HY, Helferich WG, Wong MS. Genistein and a soy extract differentially affect three-dimensional bone parameters and bone-specific gene expression in ovariectomized mice. J Nutr. 2009;139:2230–2236. doi: 10.3945/jn.109.108399. [DOI] [PubMed] [Google Scholar]
- 26.Miao Q, Li JG, Miao S, Hu N, Zhang J, Zhang S, Xie YH, Wang JB, Wang SW. The bone-protective effect of genistein in the animal model of bilateral ovariectomy: roles of phytoestrogens and PTH/PTHR1 against post-menopausal osteoporosis. Int J Mol Sci. 2012;13:56–70. doi: 10.3390/ijms13010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner RT, Iwaniec UT, Andrade JE, Branscum AJ, Neese SL, Olson DA, Wagner L, Wang VC, Schantz SL, Helferich WG. Genistein administered as a once-daily oral supplement had no beneficial effect on the tibia in rat models for postmenopausal bone loss. Menopause. 2013;20:677–686. doi: 10.1097/gme.0b013e31827d44df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bitto A, Polito F, Burnett B, Levy R, Di Stefano V, Armbruster MA, Marini H, Minutoli L, Altavilla D, Squadrito F. Protective effect of genistein aglycone on the development of osteonecrosis of the femoral head and secondary osteoporosis induced by methylprednisolone in rats. J Endocrinol. 2009;201:321–328. doi: 10.1677/JOE-08-0552. [DOI] [PubMed] [Google Scholar]
- 29.Bitto A, Burnett BP, Polito F, Levy RM, Marini H, Di Stefano V, Irrera N, Armbruster MA, Minutoli L, Altavilla D, Squadrito F. Genistein aglycone reverses glucocorticoid-induced osteoporosis and increases bone breaking strength in rats: a comparative study with alendronate. Br J Pharmacol. 2009;156:1287–1295. doi: 10.1111/j.1476-5381.2008.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oikonomou KA, Orfanidou TI, Vlychou MK, Kapsoritakis AN, Tsezou A, Malizos KN, Potamianos SP. Lower fibroblast growth factor 23 levels in young adults with Crohn disease as a possible secondary compensatory effect on the disturbance of bone and mineral metabolism. J Clin Densitom. 2014;17:177–184. doi: 10.1016/j.jocd.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Lee CH, Kwak SC, Kim JY, Oh HM, Rho MC, Yoon KH, Yoo WH, Lee MS, Oh J. Genipin inhibits RANKL-induced osteoclast differentiation through proteasome-mediated degradation of c-Fos protein and suppression of NF-kappaB activation. J Pharmacol Sci. 2014;124:344–353. doi: 10.1254/jphs.13174fp. [DOI] [PubMed] [Google Scholar]
- 33.Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H, Wagner EF. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem. 2004;279:26475–26480. doi: 10.1074/jbc.M313973200. [DOI] [PubMed] [Google Scholar]
- 34.Song I, Kim JH, Kim K, Jin HM, Youn BU, Kim N. Regulatory mechanism of NFATc1 in RANKL-induced osteoclast activation. FEBS Lett. 2009;583:2435–2440. doi: 10.1016/j.febslet.2009.06.047. [DOI] [PubMed] [Google Scholar]


