Abstract
Autophagy is a fundamental cellular recycling process vulnerable to compromise in neurodegeneration. We now report that a cell-penetrating neurotrophic and neuroprotective derivative of the central nervous system (CNS) metabolite, lanthionine ketimine (LK), stimulates autophagy in RG2 glioma and SH-SY5Y neuroblastoma cells at concentrations within or below pharmacological levels reported in previous mouse studies. Autophagy stimulation was evidenced by increased lipidation of microtubule-associated protein 1 light chain 3 (LC3) both in the absence and presence of bafilomycin-A1 which discriminates between effects on autophagic flux versus blockage of autophagy clearance. LKE treatment caused changes in protein level or phosphorylation state of multiple autophagy pathway proteins including mTOR; p70S6 kinase; unc-51-like-kinase-1 (ULK1); beclin-1 and LC3 in a manner essentially identical to effects observed after rapamycin treatment. The LKE site of action was near mTOR because neither LKE nor the mTOR inhibitor rapamycin affected tuberous sclerosis complex (TSC) phosphorylation status upstream from mTOR. Confocal immunofluorescence imaging revealed that LKE specifically decreased mTOR (but not TSC2) colocalization with LAMP2+ lysosomes in RG2 cells, a necessary event for mTORC1-mediated autophagy suppression, whereas rapamycin had no effect. Suppression of the LK-binding adaptor protein CRMP2 (collapsin response mediator protein-2) by means of shRNA resulted in diminished autophagy flux, suggesting that the LKE action on mTOR localization may occur through a novel mechanism involving CRMP2-mediated intracellular trafficking. These findings clarify the mechanism-of-action for LKE in preclinical models of CNS disease, while suggesting possible roles for natural lanthionine metabolites in regulating CNS autophagy.
Keywords: autophagy, mTOR complex (mTORC), CRMP2, DPYSL2, lanthionine ketimine
Introduction
Autophagy is a normal catabolic process whereby cells recycle damaged proteins, lipid deposits and entire organelles. Macro-autophagy (hereby referred to simply as autophagy) is a common sub-type of autophagy involving the regulated creation, trafficking and fusion of autophagic vesicles (AVs) with lysosomes to form autophagolysosomes (APLs) where the recycling occurs (Dall’Armi et al., 2013; Gallagher and Chan, 2013; Klionsky et al., 2012; Ravikumar et al., 2010; Wirth et al., 2008;). Autophagy is a complex process involving many different protein components, but predominant amongst its regulatory elements are the mammalian target of rapamycin complex-1 (mTORC1) and unc-51-like kinase-1 (ULK1) (Ravikumar et al., 2010; Wirth et al., 2008; Gallagher and Chan, 2013). mTORC1 is itself a kinase that inhibits ULK1 (Gallagher and Chan, 2013; Wirth et al., 2008). When nutrients and cell energy are limiting, mTORC1 is suppressed by the coordinated actions of tuberous sclerosis complex (TSC) proteins 1 and 2 (TSC1/2) and Rheb (Ras homolog enriched in brain) (Ravikumar et al., 2010). This allows disinhibition of ULK1 which then associates with beclin-1 protein to form pre-autophagosomal structures (Gallagher and Chan, 2013; Wirth et al., 2008). Autophagosomes mature in a process marked by phosphatidylethanolamine conjugation to microtubule-associated protein 1 light chain 3 (LC3-I→LC3-II conversion) (Dall’Armi et al., 2013; Klionsky et al., 2012). After lysosomal fusion the autophagy components are turned over and the cycle completes.
Impaired autophagy is becoming widely associated with a variety of neoplastic and central nervous system (CNS) pathologies. In some cases the molecular pathology is clear. For instance, mutation of TSC1 or TSC2 causes tuberous sclerosis, a multi-organ system pathology characterized by brain and kidney tumors, developmental delays and neuropsychological deficits (Inoki et al., 2005). In other cases autophagy is clearly perturbed but the root cause is not known. For example, neurons in Alzheimer’s disease (AD) brain and mouse models accumulate AVs concomitant with the build-up of potentially neurotoxic proteins and peptides such as amyloid beta (Aβ) peptides (Nixon and Yang, 2011) and beclin-1 decreases by up to 70% in frank AD (Pickford et al., 2008), indicative of a profound autophagic dysfunction. Autophagy is proving so fundamental for tissue maintenance, even in normal tissue, that it has been invoked to explain why caloric restriction reliably slows natural aging (Lamming et al., 2013).
Today autophagy is pharmacologically manipulated using derivatives of the microbial macrolide rapamycin (serolimus) which forms a ternary complex with the protein FKBP12 that binds and inhibits mTORC1. Rapamycin derivatives (rapalogs) are now used as immunosuppresants during kidney transplantation; in the clinical management of TSC, renal cell carcinoma (RCC), pancreatic cancer, and hormone receptor positive/HER2 negative breast cancer; and are in clinical trials for other indications (Hasskarl, 2014). In preclinical studies rapalogs have shown efficacy against a wide variety of pathologies, and notably, decrease Aβ burden and improve cognition in AD mouse models (Spilman, et al., 2010; Tan et al., 2014). No endogenous mammalian substance has been reported that engages the mTOR pathway to produce rapamycin-like effects, although given the fundamental importance of regulating the mTOR pathway, such substance(s) might be suspected to exist.
Lanthionine ketimine (LK) is a mammalian sulfur amino acid metabolite with no known natural function, which is thought to form through promiscuous reactions of transsulfuration pathway enzymes coupled to subsequent transaminations; and also possibly through glutathione-dependent chemistries mediated by lanthionine cyclase-like protein-1 (LanCL1) (Cavallini et al., 1991; Chung et al., 2007; Cooper, 2004; Fontana et al., 1997; Hensley, 2010a,b; Hensley and Denton, 2015; Zhang et al., 2009). A membrane-penetrating LK ester derivative (LKE) has shown potent neuroprotective and neurotrophic activities (Hensley et al., 2010a,b, 2011; Hubbard et al., 2013; Nada et al., 2012) and growth-suppressive effects towards glioma cells cortically-implanted in Fisher 344 rats (Floyd et al., 2013). Chronic oral LKE treatment diminishes amyloid burden and tau hyperphosphorylation while enhancing cognition in the 3xTg-AD mouse model of AD (Hensley et al., 2013). The precise mechanism-of-action for LKE has been unclear but is likely mediated in part through LK or LKE binding to the microtubule-associated protein, CRMP2 (collapsin response mediator protein-2) (Hensley et al., 2010a,b, 2011; Nada et al., 2012; Floyd et al., 2013; Hubbard et al., 2013). We now show that LKE stimulates autophagy in mammalian CNS cells, acting in a fashion that is functionally analogous to rapamycin but through a distinct mechanism that affects mTOR localization at the lysosome. These results indicate a possible role for LK and CRMP2 in the natural regulation of mTOR-dependent CNS autophagy, and suggest a use for LK derivatives in managing diseases of autophagy impairment.
Materials and Methods
Chemistry
LKE [R-5-ethyl-(2H-1,4-thiazine-5,6-dihydro-3,5-dicarboxylic acid)] was synthesized from 3-bromopyruvate (TCI-America, B-1153) and L-cysteine-ethyl ester HCl (Alfa-Aesar, L06328) as previously described (Hensley et al. 2010, 2011).
Cell Cultures
SH-SY5Y cells (human neuroblastoma; ATCC® CRL-2266™; ATCC) were maintained in complete culture medium [1:1 F-12K (ATCC 30-2004) and EMEM (ATCC 30-2003) with 15% fetal bovine serum (FBS; Life Technologies, 10437-077) and penicillin/streptomycin (100 IU; ATCC 30-2300) Cells were routinely passed by trypsinization. Cells were plated onto 24-well culture plates at 150,000 cells/well or T25 flasks at 1.8 × 106 cells/flask and maintained for four days before treatment. LKE was added to cell culture medium from a concentrated stock solution (in saline) to achieve final concentrations.
Rat glioma cells RG2[D74] (ATCC® CRL-2433™; RG2 cells) were cultured at 37°C in 5% C02 in air atmosphere in Dulbecco’s Modified Eagle’s Medium (Corning Celgro, 10-013-CV) supplemented with 10% FBS and penicillin/streptomycin (MP Biomedics,1670049). For experiments cells were plated in 100 mm2 tissue culture-treated dishes and the medium was changed every 2–3 days until the cells became 85–90% confluent. At the time of treatment, LKE (dissolved in saline and neutralized) was diluted into culture medium to the desired final concentrations. The medium in each of the dishes was replaced with medium containing LKE or vehicle (saline), cells were left to equilibrate for 15 min, then bafilomycin-A1 (10 nM; LC Laboratories, B1793-2UG) was added and cells were incubated for additional 4h. At termination the cell culture medium was removed, cells were washed with PBS (Sigma, P4417-100TAB) and lysed on ice in Pierce RIPA buffer (Thermo Scientific, 89901) containing protease and phosphatase inhibitors. A similar approach was used in experiments wherein cells were treated with rapamycin (LC Laboratories, Y-22989).
For each experiment, four to five flasks of cells per treatment group were lysed by addition of 0.5 mL cell lysis buffer [RIPA buffer plus, 1 mM sodium orthovanadate (Sigma, 13721-39-6), 5 mM β-glycerophosphate (Sigma, 154804-51-0) and 1:200 mammalian protease inhibitor cocktail (Sigma, P8340) to the frozen cells followed by scraping and sonication of the pooled lysate. Samples were centrifuged 5 min at 16,000×g in a benchtop centrifuge and supernatant removed for Lowry protein assay. Samples were adjusted to final concentration of 10 mg/mL with lysis buffer, mixed 1:1 with SDS-PAGE loading dye (BioRad, 161-0737) containing 2% β-mercaptoethanol (Acros organics, 125472500), boiled and frozen at −80°C until use.
Lentiviral transduction
SH-SY5Y cells were stably transduced with CRMP2 shRNA (h) lentiviral particles according to manufacturer’s protocol with minor deviations. SH-SY5Y cells were plated onto 12-well plates at a density of 30,000 cells/wells. Medium was replaced with a mixture of complete culture medium containing 5µg/ml Polybrene®, Santa Cruz Biotech, Inc.; sc-134220). CRMP2 shRNA lentiviral particles (Santa Cruz Biotech, Inc.; sc44485-v) or negative scrambled shRNA sequence control lentiviral particles (Santa Cruz Biotech,Inc.; sc108080) were added to the cells at a multiplicity of infection (MOI) of 2.5. Twenty four hours later, culture medium was removed and replaced with complete culture medium without Polybrene®. Cells were split 1:2 and stable clones expressing the shRNA were obtained via puromycin dihydrochloride (2 µg/ml) (Santa Cruz Biotech Inc.; sc-205821) selection. For experiments using these transduced cell lines (CRMP2-KD and vector control), cells were plated on T25 flasks in complete culture medium and grown to approximately 50% confluency. LKE was diluted into flasks from a concentrated stock solution in saline. Bafilomycin A1 was used at 10 nM from a 10 µM stock solution in DMSO (Mediatech, INC., 25-950-COC). Equivalent DMSO volumes were added to controls.
Immunochemistry
Samples containing 25 µg protein (5 µL/sample) were electrophoresed across 4–20% precast polyacrylamide gels (BioRad, Cat# 456-8093) using pre-stained molecular weight markers (BioRad, 161-0305; 161-0324) to follow the process in real time. Generally electrophoresis employed a Tris-glycine running buffer (BioRad, 161-0732) pH 8 operating at 90V throughout the run, but for LC3 assays (25 µg protein) the voltage was reduced to 60V for the first three-quarters of the electrophoresis then increased to 90V until the lowest molecular weight marker (aprotinin, 5 kDa) approached within 2 mm of the lower edge of the gel. Gels were wet-blotted to polyvinylidine difluoride (PVDF) membranes (Millipore, ISEQ00010) for 2h at 60V, blocked overnight in 4% bovine serum albumin (BSA, Santa Cruz Biotechnology, 9048-46-8) and developed using antibodies listed in Table I (1:1000 dilution each). For LC3 analyses the same PVDF-blotted membranes were cut horizontally at the 38 kDa marker so that the lower half could be blotted for LC3 while the upper half could be blotted simultaneously for actin to ensure equality of protein loading and transfer across samples. Blots were developed with enhanced chemiluminescence reagents (Amersham, RPN2132). Antibodies used for immunoblotting are listed in Table I, and were all used at 1:1000 dilution.
Table I.
Antibodies used in the study.
| Protein (epitope) | Clone | Species | Supplier (catalog number) or antigen |
|---|---|---|---|
| actin | AC-15 | Ms-MAB | Sigma-Aldrich (A1978) |
| AKT | n/a | Rb | Cell Signaling Technology (9272) |
| AKT(pS473) | n/a | Rb | Cell Signaling Technology (9271) |
| Atg-5 | D5F5U | Rb-MAB | Cell Signaling Technology (12994) |
| Atg-7 | D12B11 | Rb-MAB | Cell Signaling Technology (8558) |
| Atg-12 | D88H11 | Rb-MAB | Cell Signaling Technology (4180) |
| beclin-1 | n/a | Rb | Sigma-Aldrich (B6186) |
| CRMP2 (DPYSL2) | 1F11 | Ms-MAB | Sigma-Aldrich (AB102756) |
| LAMP2 | ABL-93 | Rt-MAB | Abcam (25339) |
| LC3A | D50G8-XP® | Rb-MAB | Cell Signaling Technology (4599) |
| mTOR | 7C10 | Rb-MAB | Cell Signaling Technology (2983) |
| mTOR | n/a | Rb | Santa Cruz Biotechnology (8319; H-266) |
| mTOR(pS2448) | D9C2-XP® | Rb-MAB | Cell Signaling Technology (5536) |
| mTOR(pS2481) | n/a | Rb | Cell Signaling Technology (2974) |
| p70S6K | 49D7 | Rb-MAB | Cell Signaling Technology (2708) |
| p70S5K(T421,S424) | n/a | Rb | Cell Signaling Technology (9204) |
| raptor | 24C12 | Rb-MAB | Cell Signaling Technology (2280) |
| TSC2 | D93F12-XP® | Rb-MAB | Cell Signaling Technology (4308) |
| TSC2 | n/a | Rb | Santa Cruz Biotechnology (893; C-20) |
| TSC2(pS939) | n/a | Rb | Cell Signaling Technology (3615) |
| TSC2(pT1462) | n/a | Rb | Cell Signaling Technology (3611) |
| ULK1 | D8H5 | Rb-MAB | Cell Signaling Technology (8054) |
| ULK1(pS555) | D1H4 | Rb-MAB | Cell Signaling Technology (5869) |
| ULK1(pS757) | n/a | Rb | Cell Signaling Technology (6880) |
mTOR or TSC2 localization relative to lysosomes was assessed using a microscopic technique involving fluorescent double-labeling of mTOR or TSC2 and the lysosomal marker LAMP2 (Sancak eta al., 2010). Cultured rat glioma cells (RG2) treated with 50 nM rapamycin and 10 µM LKE for 24 h were fixed in 4% formaldehyde (Sigma, 47608) for 30 min, permeabilized for 30 min in 0.1% Triton X-100 (Sigma, T-9284) in PBS at room temperature; blocking of nonspecific binding sites was carried out with 2% B-PBS (BSA in PBS). Immunolabeling with rabbit anti-mTOR (Santa Cruz Biotechnology; sc-8319) (1:100), or rabbit anti-tuberin (TSC2) (Santa Cruz Biotechnology; sc-893) (1:100), and rat anti-LAMP2 mAb (Abcam, ab25339) (1:100) was carried out for 2h at room temperature. The secondary antibodies used were Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probes, A11008, 1:100) and Alexa Fluor 594 goat anti-rat IgG (Molecular Probes, A11007, 1:100) applied for 30 min at room temperature. An Olympus IX 71 fluorescence microscope was used for observation and image collection. Images were captured with a charge-coupled device (CCD) camera (QImaging, Canada), recorded with Slidebook 4.2 digital microscopy software, and later analyzed using MetaMorph for Olympus Basic Offline software. A minimum of four independent experiments, each including all treatments and controls, were used for assessments of TSC2 or mTOR colocalization with LAMP2+ structures. The images were acquired at 20x magnification. Colocalization of the fluorescent tags (“green” for mTOR or TSC2 and “red” for LAMP2) as overlapped (“yellow”) area was assessed. Data from analysis of 120 images from 12 slides of 4 experiments per treatment were analyzed.
Statistics
All data were graphically presented as mean ± SEM unless otherwise specified. In the case of single mean comparisons, data were analyzed by two-tailed unpaired t-tests or Mann-Whitney tests appropriate to data distributions. In case of multiple comparisons, data were analyzed by one-or two-way ANOVA with post-hoc Bonferroni multiple comparisons using GraphPad Prism Software (GraphPad).
Results
LKE stimulates autophagy flux in cell culture
Though many measurable cellular protein changes correlate with autophagy, one must be careful in the interpretation of autophagy biomarkers. An increase in autophagy components can be caused by a true increase in autophagic flux, or by late-stage blockade of autophagy clearance (Klionsky et al., 2012). A broad scientific consensus has emerged that one of the most reliable ways to determine whether a treatment increases true autophagy flux is to measure LC3-I to LC3-II conversion in the absence and presence of the vacuolar ATPase inhibitor bafilomycin-A1 (Klionsky et al., 2012). Bafilomycin neutralizes lysosomal pH, preventing autophagosome-lysosomal fusion, and traps LC3-II at a point prior to its turnover in mature autophagolysosomes (Klionsky et al., 2012). Therefore a manipulation that increases autophagy flux will increase LC3-II in the presence of a bafilomycin block, whereas a treatment that blocks autophagy clearance will have no effect on LC3-II under these conditions (Klionsky et al., 2012).
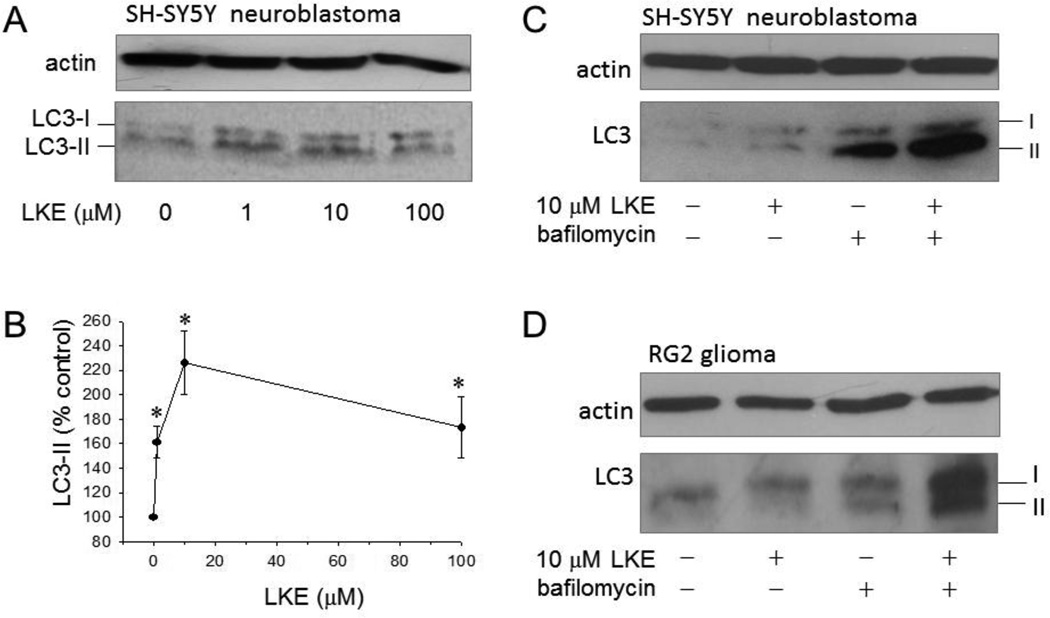
Chronic oral administration of LKE to mice results in blood and brain LKE concentrations reaching 10–15 µM with no histological, toxicological or behavioral evidence of adverse effects (Harris-White et al. 2013 and unpublished observations). Treatment with LKE at pharmacologically-relevant (Hensley et al., 2013), low µM concentrations increased autophagic lipidation of LC3-I to LC3-II by 2 to 3-fold in SH-SY5Y cells with similar effects observed in RG2 glioma cells (Figs. 1–2). The LC3-II conversion was evident within 4 hours and was maintained in SH-SHSY5Y cells for at least 4 days (Fig. 1). In the presence of bafilomycin, LKE likewise increased LC3-II by 3.5 to 4-fold (Fig. 1) consistent with acceleration of autophagic flux and inconsistent with late-stage autophagy inhibition.
Figure 1. LKE stimulates autophagy in SH-SY5Y neuroblastoma and RG2 glioma cells.
A: SH-SY5Y cells were treated for 4d then lysed and blotted for LC3. B: The ratio of LC3-II/actin was expressed as % of control for six independent experiments; *P<0.05. C,D: Confirmation that the LC3-II increase was cause by increased autophagy flux rather than blockage of late-stage autophagy clearance steps was accomplished by co-treating cells 4h with 10 µM LKE in the presence or absence of bafilomycin A-1. Persistence of the LKE-induced LC3-II increase in the presence of bafilomycin was taken to indicate that the LKE effect was due to autophagy stimulation rather than clearance inhibition.
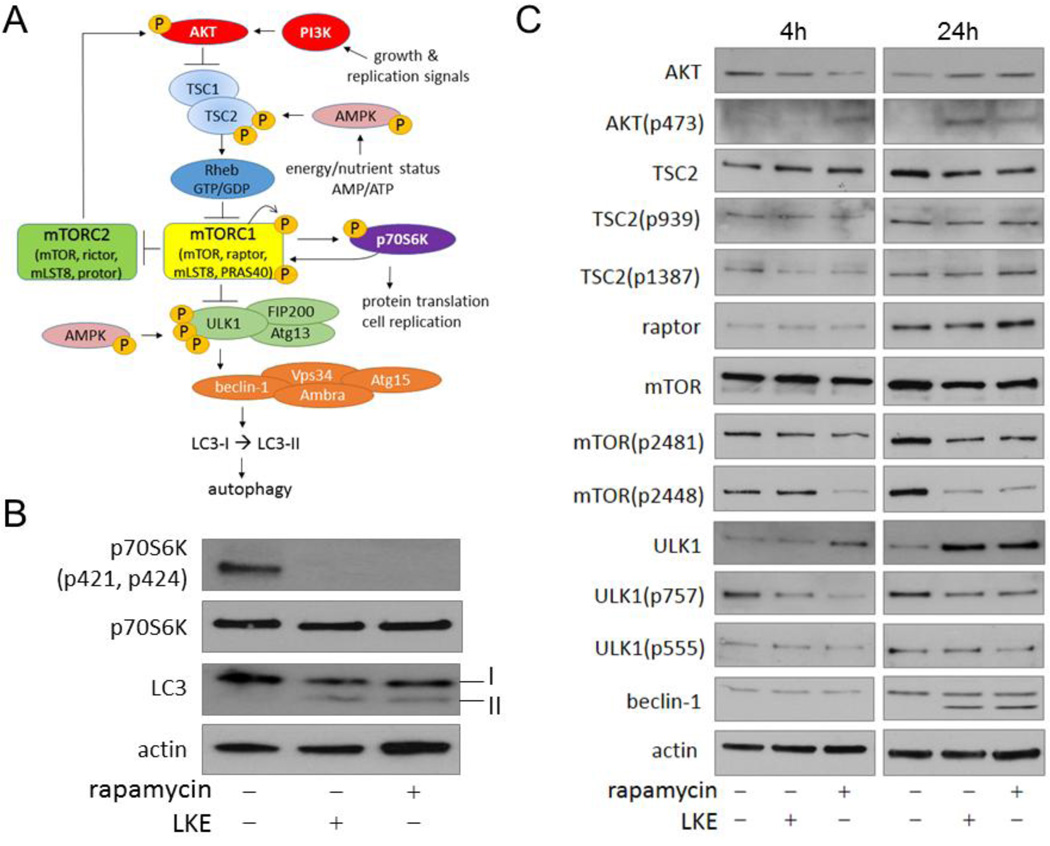
Figure 2. LKE stimulation of autophagy is due to inhibition of mTOR pathway signaling and maps to a site of action near the mTORC1.
RG2 glioma cells were treated 4h or 24h with 50 nM rapamycin or 10 µM LKE, lysed and blotted with antibodies against the indicated proteins and phospho-epitopes. A: The current autophagy regulation model, inspired by but substantially modified from Ravikumar et al. (2010). B: Action of LKE and rapamycin on the archetypical mTORC1 target, p70S6 kinase; C: Pathway analysis to map site of action for LKE relative to rapamycin. Note that the first common site of action for LKE and rapamycin is at mTOR phosphorylation on residues 2448 (autophosphorylation site) and 2481 (S6K feedback phosphorylation site) but no effects on TSC1/2 occur that would be consistent with a pro-autophagic activity. The stimulation of AKT(p403) by rapamycin is known to occur through disinibition of mTORC2 after mTORC1 inhibition (Guertin et al., 2006).
LKE stimulation of autophagy mirrors biochemical effects of rapamycin and maps to a similar site of action near mTORC
We reasoned that one mechanism by which LKE could promote autophagy would be through antagonism of mTORC1. In initial experiments to test this hypothesis, RG2 cells were treated 24h with LKE or the archetypical mTORC1 inhibitor, rapamycin, and the canonical mTORC1 phosphorylation target, p70S6K, was assessed. After 24h treatment both LKE and rapamycin decreased p70S6K phosphorylation on the mTORC1 target residues pT421/pS424 concomitant with induction of LC3 lipidation (Fig. 2). This prompted a subsequent study to more fully map the site of LKE action on the mTOR pathway relative to that of rapamycin (Fig. 2). RG2 glioma cells were treated 4 or 24h with 50 nM rapamycin or 10 µM LKE and autophagy pathway markers were assessed by western blots using protein and phosphorylation state-specific antibodies (Fig. 2). LKE had the same biochemical effects as rapamycin at every level of analysis, including mTOR autophosphorylation at S2481 and feedback S6K-dependent phosphorylation at S2448 (Chiang and Abraham, 2005; Soliman et al., 2010). Both LKE and rapamycin decreased ULK1 phosphorylation on the mTORC1 target site S757 while increasing total ULK1 protein (Fig. 2). Furthermore, both LKE and rapamycin caused the appearance of a truncated beclin-1 band in RG2 lysates at 24h (Fig. 2). However, no effects of either drug were seen at the level of TSC1/2 protein or phosphorylation at AKT target sites TSC2-S939 or the AMPK target site TSC2-S1387 (Inoki et al., 2006), indicating that mTOR inhibition is not a consequence of TSC1/2 activation by AMPK. Indeed both total and phosphorylated TSC2 decreased slightly at 24h treatment with either LKE or rapamycin, which would tend to disfavor autophagy (Fig. 2); but the relative ratios of phosphorylated/total TSC1/2 remained unchanged (Fig. 2). Likewise neither LKE nor rapamycin altered ULK1 phosphorylation on its AMPK target site, T555 (Fig. 2) (Bach et al., 2011). Both LKE and rapamycin increased AKT phosphorylation at S473, which is a well-known phenomenon in rapamycin-treated cells, caused by loss of mTORC1 inhibition of mTORC2 which then acts reciprocally on AKT (Guertin et al., 2006) (Fig. 2).
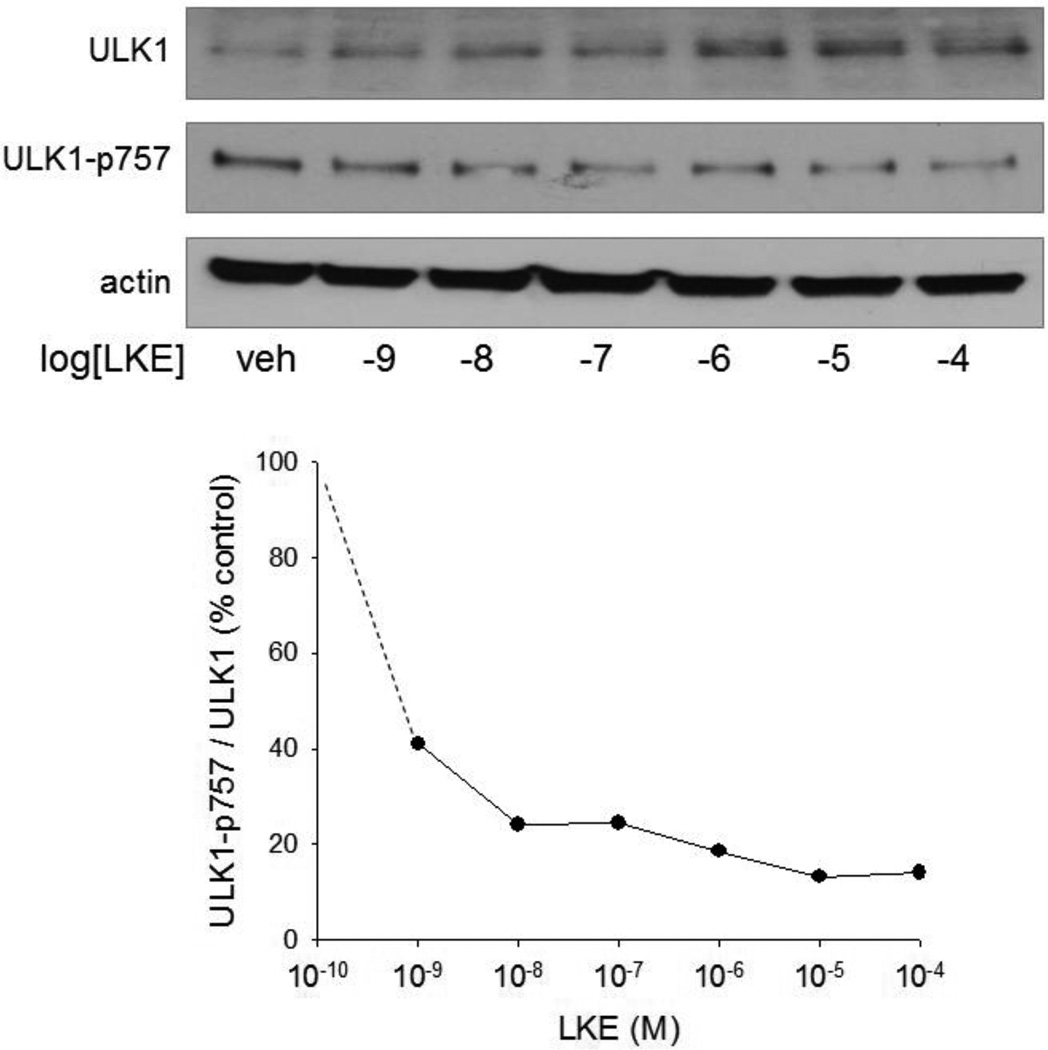
The phenomenon that ULK1 increases upon mTOR inhibition has been recently reported and likely represents a feed-forward stabilization mechanism involving altered ULK1 binding to Ambra and ultimately K63-linked ubiquitination (Nazio et al., 2013). This circumstance allowed a sensitive estimation of LKE potency upon the mTOR pathway to be achieved by monitoring the simultaneous change in ULK1 increase and ULK1(p757) decrease. At 24h in RG2 cells, LKE affected the ULK1 system with EC50 of approximately 50 nM (Fig. 3) which is 10–100 fold lower than pharmacologically achievable brain concentrations (Hensley et al., 2013) and near the reported brain physiological concentration of LK (Fontana et al., 1997). This is also within the concentration range at which LKE potentiates neurite outgrowth in NSC-34 motor neuron-like cells and primary dorsal root ganglial neurons (Hensley et al. 2011; Hensley and Denton 2015).
Figure 3. LKE effect on ULK1 is a sensitive indicator of autophagy activation.
A: RG2 glioma cells were treated 24 h with the indicated concentration of LKE, lysed and probed for total ULK1 or ULK1 phosphorylated on the mTOR target residue 757. B: The ratio of ULK1/ULK1(p757) band densities was plotted as a function of LKE concentration.
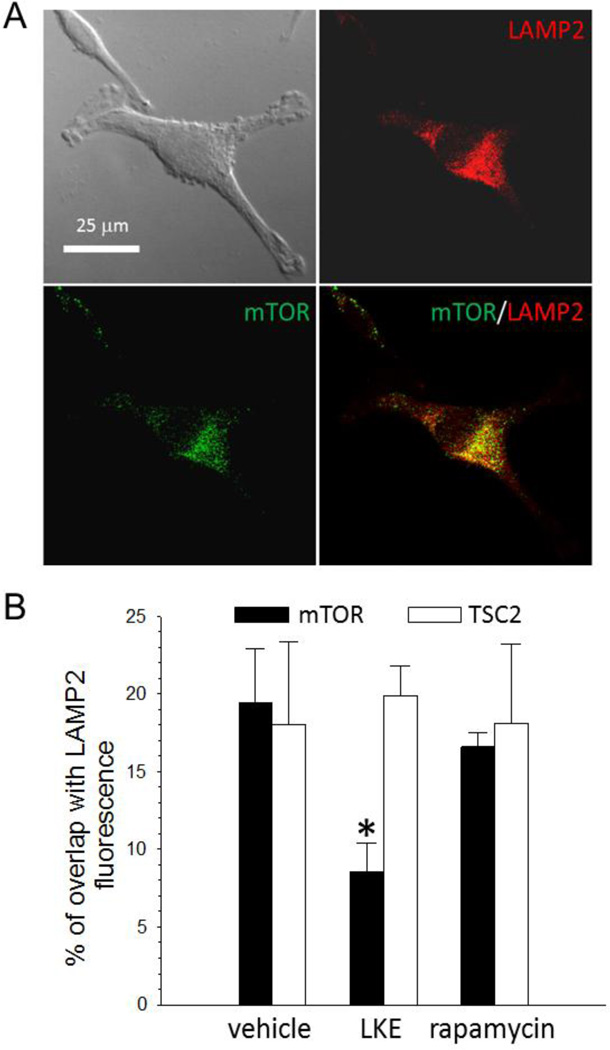
LKE decreases mTOR localization, but not TSC2 localization, at the lysosomes whereas rapamycin has no effect
In recent years it has become apparent that early events in autophagy are regulated largely by compartmentalization of components. mTORC1 assembles on the surface of lysosomes in complex with Rag GTPases and other components to form an active complex that negatively regulates autophagy (Bar-Peled et al., 2012), whereas TSC1/2 also is recruited to the same vicinity in order to negatively regulate mTORC1 (Jewell et al., 2013; Menon et al., 2014; Sancak et al., 2010). Because we previously found that LKE binds and functionally augments CRMP2,20,24 an adaptor protein involved with (amongst other processes) protein trafficking along microtubules (Khanna et al., 2012), we reasoned that LKE might be affecting autophagy by altering mTOR or TSC1/2 localization at lysosomes. Immunofluorescence studies were performed to test this working hypothesis by co-labeling RG2 with antibodies against mTOR or TSC2 and the lysosomal protein marker LAMP2. Positioning of mTOR and TSC2 at lysosomes was assessed by the overlap of mTOR or TSC1/2 markers with LAMP2 fluorescent puncta. The mTOR/LAMP2 overlap was significantly diminished by LKE treatment whereas the TSC2/LAMP2 overlap was not affected (Fig. 4). Contrastingly, rapamycin treatment did not affect either mTOR or TSC2 overlap with LAMP2 (Fig. 4). This data demonstrates that despite LKE and rapamycin producing functionally similar effects on mTOR pathway-dependent autophagy, the two compounds do so through markedly different mechanisms of action.
Figure 4. LKE, but not rapamycin, specifically decreases mTOR localization at lysosomes.
Confocal images of RG2 glioma cells double-immunofluorescently labeled for mTOR (green) and the lysosomal marker LAMP2 (red). Co-localization of the two markers (yellow) indicates positioning of mTOR at lysosomal sites. LKE (10 µM, overnight) significantly reduced the mTOR/LAMP2 co-localization but did not affect TSC2 localization relative to lysosomes. Rapamycin (50 nM, overnight) had no significant effect on either mTOR or TSC2 localization. Data represent analyses of 120 images from 12 slides of 4 experiments per treatment are presented in graph.
CRMP2 protein level sets the pace for autophagic flux in SH-SY5Y cells
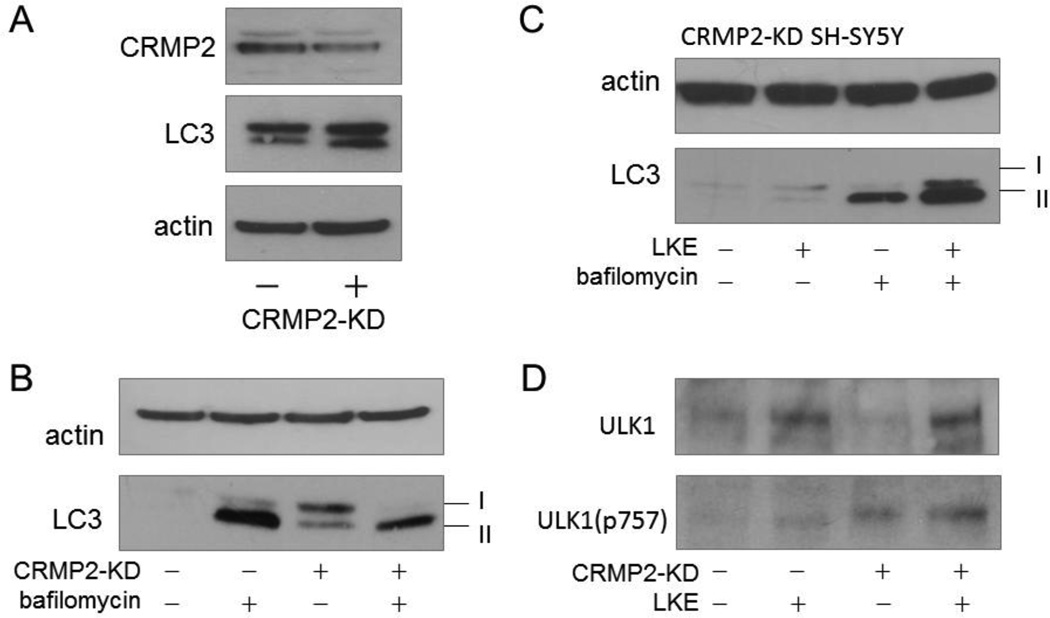
In previous work we have shown that LK binds CRMP2 or CRMP2 complexes (Hensley and Denton, 2015) and that LKE acts like a functional CRMP2-enhancer in mammalian cells and nematodes hypomorphic for the CRMP2-homolog Unc-33 (Floyd et al., 2013; Hensley et al., 2010; Hensley et al., 2011; Hubbard et al., 2013; Nada et al., 2012). Studies were therefore undertaken to ascertain whether CRMP2 functionally influences autophagic processes. SH-SY5Y cells were stably transduced with shRNA directed against CRMP2 to create a cell line stably expressing CRMP2 at 50 ± 10% (P<0.01) the level of vector control SH-SY5Y cells (CRMP2-KD; Fig. 5). Basal LC3-II was substantially increased in CRMP2-KD cells, relative to vector control SH-SY5Y cells, in the absence of bafilomycin treatment; however the bafilomycin-induced increase of LC3-II in CRMP2-KD cells was many-fold smaller than the corresponding effect observed in vector control cells (Fig. 5). This is consistent with the interpretation that CRMP2-KD slows autophagy flux upstream from the bafilomycin-sensitive lysosomal-autophagosomal fusion step (Klionsky et al., 2012) though the data do not rule out additional effects of CRMP2 on late-stage autophagolysosome clearance events. LKE increased LC3-II lipidation in CRMP2-KD cells as well as vector control cells, suggesting that if LKE effects are mediated through CRMP2, the 50% CRMP2 that was refractory to shRNA knock-down was sufficient to allow the LKE effect on autophagy to fully manifest (Fig. 5).
Figure 5. Partial knock-down of the LK binding, cargo protein CRMP2 slows autophagy flux in SH-SY5Y neuroblastoma cells.
A: Relative to vector control infected cells, SH-SY5Y cells stably transfected with shRNA against CRMP2 expressed 50 ± 10% (P<0.01) as much CRMP2 protein, and LC3-II was relatively more elevated in the basal condition. B: CRMP2-knockdown (CRMP2-KD) cells had more LC3-II in the basal state than vector control cells, but displayed proportionally less response to bafilomycin-A, suggesting the increased LC3-II was likely due to impaired autophagy clearance or generally slower autophagy flux. C: LKE increased LC3-II in CRMP2-KD cells both with and without bafilomycin co-treatment, indicating increased autophagy flux induced by the drug even in the presence of diminished CRMP2. D: ULK1 protein was decreased in CRMP2-KD cells but increased by LKE treatment in both vector control and CRMP2-KD cells.
Western blots of ULK1 indicated that SH-SY5Y cells expressed relatively less of this protein than did RG2 glioma cells, but CRMP2 knockdown tended to decrease ULK1, whereas LKE increased ULK1 protein in SH-SY5Y cells much as it did in the RG2 cells (Fig. 5).
Discussion
In the present study we report that a membrane-penetrating derivative of the brain amino acid metabolite lanthionine ketimine acts potently on the mTOR pathway to stimulate autophagic flux in both neurotypic and glial cell cultures. The findings suggest that brain lanthionine metabolites may not be mere metabolic waste but rather, important endogenous small molecule regulators of the mTORC1 pathway, the existence of which has been wholly unforeseen. Formal proof of such a natural function of endogenous lanthionines would require genetically or pharmacologically manipulating lanthionine production in vivo, which unfortunately is not currently possible due to limitations in our present knowledge of the biochemical origins of these molecules. However, considering that mTOR is a highly integrated sensor of both energy status and amino acid supply, it is intuitively reasonable that any endogenous mTOR pathway regulator would likely derive from limiting brain amino acid products.
Natural lanthionine ketimine is thought to form through promiscuous functions of the transsulfuration pathway and linked kynurenine pathway (Cavallini et al., 1991; Chung et al., 2007; Cooper, 2004; Fontana et al., 1997; Hensley, 2010b; Hensley and Denton, 2015; Zhang et al., 2009), and in fact, represents only one member of a class of thioether ketimines with uncertain origin and function pathway (Cavallini et al., 1991; Cooper, 2004; Fontana et al., 1997; Hensley, 2010; et al., 2009; Hensley and Denton, 2015). Mammalian brain also contains an unusual glutathione and LK-binding protein called LanCL1 (Chung et al., 2007; Hensley and Denton, 2015; Hensley et al., 2010a; Zhang et al., 2009) which is highly homologous to prokaryotic lanthionine synthase enzymes and which we have speculated might represent an alternative source of these compounds (Cooper, 2004; Chung et al., 2007; Hensley, 2010b; Hensley and Denton, 2015; Zhang et al., 2009). Interestingly, LanCL1 knock-out mice develop severe neuroinflammation and neurodegeneration beginning at 8 weeks of age but autophagy has yet to be assessed in these animals (Huang et al., 2014). LanCL1 is upregulated in some models of neurodegeneration including the SOD1G93A mouse model of amyotrophic lateral sclerosis (ALS) (Chung et al., 2007); and natural variations in LanCL1 expression predict mouse strain sensitivity to the Parkinsonian neurotoxin, MPTP, with higher LanCL1 expression associated with resistance to the neurotoxin (Jones., 2013).
Besides LanCL1, there exists a peripheral LanCL2 isoform which was recently reported to modulate Akt phosphorylation via mTORC2 binding (Zeng et al., 2014). Taken together these findings argue the need for further, detailed investigations of brain lanthionine origins and the roles that might be played by lanthionine metabolites and LanCL proteins in neurological disease. These topics are the subject of ongoing research in our labs.
The present work also offers first evidence that the LK-interacting protein CRMP2 influences basal autophagy pace in cell cultures. Evidence that LK effects may be mediated via CRMP2 is as follows. First, immobilized LK previously was found to bind CRMP2 as well as LanCL1 and STXBP1 (syntaxin binding protein-1) in affinity proteomics experiments (Hensley et al., 2010a). Second, LK was previously shown to promote neurite outgrowth as one would expect of a functional CRMP2 enhancer (Hensley et al., 2010a; Hensley et al., 2011; Khanna et al., 2012; Nada et al., 2012; Hubbard et al., 2013). Third, when nematodes engineered to express a partial loss-of-function Unc-33 (CRMP2) homolog mutation were allowed to develop in the presence of LKE, the compound allowed a partial rescue of these animals’ neuroanatomic phenotype (Hubbard et al., 2013). Fourth, LKE treatment normalized changes in hippocampal CRMP2 protein that occur during aging in the 3xTg-AD mouse (Hensley et al., 2013). CRMP2 is a microtubule-associated protein (MAP) that was first described as a mediator for axon retraction signals in developing neurons (Kawano et al., 2005). CRMP2 has diverse functions including direct stabilization of microtubules and connection of kinesin and dynein motors to vesicle docking proteins during microtubule transport (Kawano et al., 2005; Rahajeng et al., 2010). CRMP2 function as a cargo adaptor protein in microtubule-directed protein trafficking may be particularly salient to its role in autophagy because mTSC1/2, mTORC1 and downstream complex functions require carefully orchestrated intracellular movements of both complex components and the substrates upon which the complex enzymes act (Jewell et al., 2013; Menon et al., 2014; Sancak et al., 2010). Given the role of CRMP2 in protein trafficking, it is plausible that CRMP2 may be involved with some such relocation events. Currently CRMP2 is reported to bind more than twenty different proteins, with the implications of most of these interactions being poorly understood (Khanna et al., 2012). Interestingly, CRMP2 is a substrate for phosphorylation by glycogen synthase kinase-3β (GSK3β) and cyclin-dependent kinase-5 (Cdk5), the same kinases implicated in MAP-tau hyperphosphorylation leading to neurofibrillary tangles (NFTs) in AD brain (Uchida et al., 2005). Like phospho-Tau (pTau), pCRMP2 collects in NFTs (Cole et al., 2007; Rahajeng et al., 2010; Uchida et al., 2005). It has been hypothesized, but not established, that CRMP2 association with nascent NFTs might deplete neurons of functional CRMP2 pools thus destabilizing microtubules and/or compromising other crucial CRMP2-dependent phenomena (Yoshida et al., 1998). Our data predict that any such CRMP2 depletion would affect autophagy with likely adverse consequences, such as are observed in AD (Pickford et al., 2008; Nixon and Yang, 2011). Conversely, agents that functionally enhance CRMP2 might mitigate some autophagy defects and may explain why LKE produced benefits on amyloid and tau burden and cognition in 3xTg-AD mice (Hensley et al., 2013) similar to effects of rapamycin in other AD models (Spilman et al., 2010; Tan et al., 2014).
At this time we are unable to ascertain exactly how, at the protein structural level, LK or LKE influences autophagy though there are several plausible mechanisms. Available evidence (Hensley et al., 2010a) (and Figs. 5–6) suggests that LK(E) may affect CRMP2 : protein binding partner interactions to alter the functionally available pool of CRMP2 in the cell; or to alter the known dynamics of CRMP2 interaction with microtubule motor proteins. This could increase the rate of some subset of vesicle transport processes, encouraging autophagy initiation or autophagosome maturation. Indeed, it is recognized that mTORC1 activity is largely regulated by its localization with respect to TSC1/2, Rheb, and lysosomes (Bar-Peled et al., 2012; Jewell et al., 2013; Menon et al., 2014; Sancak et al., 2010). Alternatively, LK(E) may act downstream from mTORC1 on ULK1, beclin-1 or other proteins that then feedback to negatively regulate mTORC1.
A better understanding of autophagy regulation by LKE and similar compounds could lead to improved treatment for a host of CNS and peripheral conditions where autophagy dysfunction recently has been implicated, including such diverse conditions as Batten disease (Thelen et al., 2012); diabetic neuropathy (Qu et al., 2014); hearing loss (Menardo et al., 2012); lysosomal storage diseases (Pivtoraiko et al., 2009); muscular dystrophies (De Palma et al., 2012; Bibee et al., 2014); spinal cord injury (Wang et al., 2014); and traumatic brain injury (Sarkar et al., 2014). Work is underway in our laboratory, and others, to test LK derivatives in appropriate preclinical models of these pathologies.
Highlights.
Ester of the neural metabolite lanthionine ketimine (LKE) is neuroprotective.
LKE promotes autophagy in glial and neuronal cell lines.
LKE acts below pharmacological concentrations previously achieved in 3xTg-AD mice.
LKE affects autophagy through localization of mTOR and downstream events.
The LK-binding protein CRMP2 is necessary for proper autophagy.
Acknowledgments
Funding and disclosure
This work was supported in part by the University of Toledo Foundation Biomedical Innovation Award (KH); Veterans Administration Merit funding (MHW); a grant from the Muscular Dystrophy Association (MDA217526; KH); and the National Institutes of Health (NS082283; KH). KH is the inventor on U.S. patent 7,683,055 covering composition and use of lanthionine ketimine derivatives including lanthionine ketimine-ester (LKE) and holds equity in a company engaged in commercial development of the technology.
Abbreviations
- AD
Alzheimer’s disease
- AKT
AKT phosphotidylinositol-3-kinase (protein kinase B)
- AMPK
AMP-activated protein kinase
- APLs
autophagolysosomes
- Atg
autophagy protein
- AVs
autophagic vesicles
- CNS
central nervous system
- CRMP2
collapsin-response mediator protein 2
- DPYSL2
dihydropyrimidinase-like protein-2
- LanCL1
lanthionine cyclase-like protein-1
- LC3
microtubule-associated protein 1A/1B-light chain 3 (LC3)
- LK
lanthionine ketimine
- LKE
lanthionine ketimine-ethyl ester
- mTOR
mammalian target of rapamycin
- mTORC1(2)
mTOR complex-1(2)
- p70S6K
p70S6 ribosomal protein-directed protein kinase
- TSC
tuberous sclerosis complex
- Tsc1
tuberous sclerosis complex gene-1(hamartin)
- Tsc2
tuberous sclerosis complex gene-2 (tuberin)
- ULK1
uncordinated 51-like autophagy-regulating kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bach M, Larance M, James DE, Ramm G. The serine/threonine kinase ULK1 is a target of multiple phosphorylation events. Biochem. J. 2011;440:283–291. doi: 10.1042/BJ20101894. [DOI] [PubMed] [Google Scholar]
- Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. An expanded ragulator is a GEF for the RAG GTPases that signal amino acid levels to mTORC1. Cell. 2012;150:1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibee KP, Cheng YJ, Ching JK, Marsh JN, Li AJ, Keeling RM, Connolly AM, Golumbek PT, Myerson JW, Hu G, Chen J, Shannon WD, Lanza GM, Weihl CC, Wickline SA. Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function. FASEB J. 2014;28:2047–2061. doi: 10.1096/fj.13-237388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallini D, Ricci G, Dupre S, Pecci L, Costa M, Matarese RM, Pensa B, Antonuci A, Solinas SP, Fontana M. Sulfur-containing cyclic ketimines and imino acids. A novel family of endogenous products in search for a role. Eur. J. Biochem. 1991;202:217–223. doi: 10.1111/j.1432-1033.1991.tb16365.x. [DOI] [PubMed] [Google Scholar]
- Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J. Biol. Chem. 2005;280:25485–25490. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- Chung C, Kurien BT, Mehta P, Mhatre MC, Mou S, Pye QN, Stewart CA, West MS, Williamson KS, Post J, Liu L, Wang R, Hensley K. Identification of lanthionine synthase C-like protein 1 (LanCL1) as a prominent glutathione binding protein expressed in the mammalian central nervous System. Biochemistry. 2007;46:3262–3269. doi: 10.1021/bi061888s. [DOI] [PubMed] [Google Scholar]
- Cole AR, Noble W, van Aalten L, Plattner F, Meimaridou R, Hogan D, Taylor M, LaFrancois J, Gunn-Moore F, Verkhratski A, Oddo S, LaFerla F, Giese KP, Dineley KT, Duff K, Richardson JC, Yan SD, Hanger DP, Allan SM, Sutherland C. Collapsin response mediator protein-2 hyperphosphorylation is an early event in Alzheimer’s disease progression. J. Neurochem. 2007;103:1132–1144. doi: 10.1111/j.1471-4159.2007.04829.x. [DOI] [PubMed] [Google Scholar]
- Cooper AJ. The role of glutamine transaminase K (GTK) in sulfur and alpha-keto acid metabolism in the brain, and in the possible bioactivation of neurotoxicants. Neurochem. Int. 2004;44:557–577. doi: 10.1016/j.neuint.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Dall’Armi C, Devereaux KA, Di Paolo G. The role of lipids in the control of autophagy. Curr. Biol. 2013;23:R33–R45. doi: 10.1016/j.cub.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma C, Morisi F, Cheli S, Pambianco S, Cappello V, Vezzoli M, Rovere-Querini P, Moggio M, Ripolone M, Francolini M, Sandri M, Clemeni E. Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis. 2012;3:e418. doi: 10.1038/cddis.2012.159. doi:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R, Castro HC, Neto F, Zimmerman GA, Hensley K, Towner R. Nitrone-based therapeutics for neurodegenerative diseases. Their use alone or in combination with lanthionines. Free Rad. Biol. Med. 2013;51:931–941. doi: 10.1016/j.freeradbiomed.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana M, Brunori A, Costa M, Antonucci A. Detection of cystathionine ketimine and lanthionine ketimine in human brain. Neurochem. Res. 1997;22:821–844. doi: 10.1023/a:1022083809994. [DOI] [PubMed] [Google Scholar]
- Gallagher LE, Chan EY. Early signaling events of autophagy. Essays Biochem. 2013;55:1–15. doi: 10.1042/bse0550001. [DOI] [PubMed] [Google Scholar]
- Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKC-alpha, but not S6K1. Dev. Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Hasskarl J. Everolimus. Recent Results Cancer Res. 2014;201:373–392. doi: 10.1007/978-3-642-54490-3_23. [DOI] [PubMed] [Google Scholar]
- Hensley K, Christov A, Kamat S, Zhang XC, Jackson KW, Snow S, Post J. Proteomic identification of binding partners for the brain metabolite lanthionine ketimine (LK) and documentation of LK effects on microglia and motoneuron cell cultures. J. Neurosci. 2010a;30:2979–2988. doi: 10.1523/JNEUROSCI.5247-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K. Emerging biological importance of central nervous system lanthionines. Molecules. 2010b;15:5581–5594. doi: 10.3390/molecules15085581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, Venkova K, Christov A, Gunning W, Park J. Collapsin response mediator protein-2: An emerging pathologic feature and therapeutic target for neurodisease indications. Mol. Neurobiol. 2011;43:180–191. doi: 10.1007/s12035-011-8166-4. [DOI] [PubMed] [Google Scholar]
- Hensley K, Venkova K, Christov A, Johnson M, Eslami P, Gabbitta SP, Harris-White M. A derivative of the brain metabolite lanthionine ketimine improves cognition and diminishes pathology in the 3xTg-AD mouse model of Alzheimer’s disease. J. Neuropath. Exp. Neurol. 2013;72:955–969. doi: 10.1097/NEN.0b013e3182a74372. [DOI] [PubMed] [Google Scholar]
- Hensley K, Denton TT. Alternative functions of the brain transsulfuration pathway represent an underappreciated aspect of brain redox biochemistry with significant potential for therapeutic manipulation. Free Rad. Biol. Med. 2015;78C:123–134. doi: 10.1016/j.freeradbiomed.2014.10.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Chen M, Pang D, Bi D, Zou Y, Zia X, Yang W, Luo L, Deng R, Tan H, Zhou L, Yu S, Guo L, Du X, Cui Y, Hu J, Mao Q, Worley PF, Xiao B. Developmental and activity-dependent expression of LanCL1 confers antioxidant activity required for neuronal survival. Developmental Cell. 2014;30:479–487. doi: 10.1016/j.devcel.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard C, Benda E, Hardin T, Baxter T, St. John E, O’Brien S, Hensley K, Holgado A. Lanthionine ketimine ethyl ester partially rescues neuro-developmental defects in unc-33 (DPYSL2/CRMP2) mutants. J. Neurosci. Res. 2013;91:1183–1190. doi: 10.1002/jnr.23239. [DOI] [PubMed] [Google Scholar]
- Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat. Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Jewell JL, Russell RC, Guan KL. Amino acid signaling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BC, Miller DB, O’Callaghan JP, Lu L, Unger EL, Alam G, Williams RW. Systems analysis of genetic variation in MPTP neurotoxicity in mice. Neurotoxicology. 2013;27:26–34. doi: 10.1016/j.neuro.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Yoshimura T, Tsuboi D, Kawabata S, Kaneko-Kawano T, Shirataki H, Takenawa T, Kaibuchi K. CRMP-2 is involved in kinesin-1-dependent transport of the Swa-1/WAVE1 complex and axon formation. Moll. Cell. Biol. 2005;25:9920–9935. doi: 10.1128/MCB.25.22.9920-9935.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Wilson SM, Brittain JM, Weimer J, Sultana R, Butterfield DA, Hensley K. Opening Pandora’s jar: A primer on the putative roles of collapsin response mediator protein 2 (CRMP2) in a panoply of neurodegenerative, sensory and motor neuron, and central disorders. Future Neurol. 2012;7:749–771. doi: 10.2217/FNL.12.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J. Clin. Invest. 2013;123:980–989. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menardo J, Tang Y, Ladrech S, Lenoir M, Casas F, Michel C, Bourien J, Ruel J, Rebillard G, Maurice T, Puel JL, Wang J. Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse cochlea. Antioxid Redox Signal. 2012;16:263–274. doi: 10.1089/ars.2011.4037. [DOI] [PubMed] [Google Scholar]
- Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, Cantley LC, Manning BD. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada SE, Raghava A, Tulsulkar J, Hensley K, Shah ZA. A derivative of the CRMP2 binding compound lanthionine ketimine provides neuroprotection in a mouse model of cerebral ischemia. Neurochem. Int. 2012;61:1357–1363. doi: 10.1016/j.neuint.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazio F, Strappazon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M, Gretzmeier C, Dengjel J, Piacentini M, Fimia GM, Cecconi F. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat. Cell Biol. 2013;15:406–416. doi: 10.1038/ncb2708. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Yang DS. Autophagy failure in Alzheimer’s disease-locating the primary defect. Neurobiol. Dis. 2011;43:38–45. doi: 10.1016/j.nbd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickford F, Masliah E, Britschgi M, Lucin K, Marasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J. Clin. Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivtoraiko VN, Stone, S.L, Roth KA, Shacka JJ. Oxidative stress and autophagy in the regulation of lysosome-dependnet neuron death. Antioxid. Redox Signal. 2009;11:481–496. doi: 10.1089/ars.2008.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L, Liang X, Gu B, Liu W. Quercetin alleviates high glucose-induced Schwann cell damage by autophagy. Neural Regen. Res. 2014;9:1195–1203. doi: 10.4103/1673-5374.135328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahajeng J, Giridharan SS, Naslavsky N, Caplan S. Collapsin response mediator protein-2 (CRMP2) regulates trafficking by linking endocytic regulatory proteins to dynein motors. J. Biol. Chem. 2010;285:31918–31922. doi: 10.1074/jbc.C110.166066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM. Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy. 2014;10:2208–2222. doi: 10.4161/15548627.2014.981787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, Fingar DC. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J. Biol. Chem. 2010;285:7866–7879. doi: 10.1074/jbc.M109.096222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Yu JT, Zhu XC, Tan MS, Wang HF, Cao L, Zhang QQ, Shi JQ, Gao L, Qin H, Zhang YD, Tan L. Temsirolimus promotes autophagic clearance of amyloid-β and provides protective effects in cellular and animal models of Alzheimer’s disease. Pharmacol. Res. 2014;81C:54–63. doi: 10.1016/j.phrs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Thelen M, Damme M, Schweizer M, Hagel C, Wong AM, Cooper JD, Braulke T, Galliciotti G. Disruption of the autophagy-lysosome pathway is involved in neuropathology of the nclf mouse model of neuronal ceroid lipofuscinosis. PLoS One. 2012;7(4):e35493. doi: 10.1371/journal.pone.0035493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Ohshima T, Sasaki Y, Suzuki H, Yanai S, Yamashita N, Nakamura F, Takei K, Ihara Y, Mikoshiba K, Kolattukudy P, Honnorat J, Goshima Y. Semaphorin3A signaling is mediated via sequential cdk5 and GSK3β phosphorylation of CRMP2: Implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes Cells. 2005;10:165–179. doi: 10.1111/j.1365-2443.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Liu WG, Muharram A, Wu ZY, Lin JH. Neuroprotective effects of autophagy induced by rapamycin in rat acute spinal cord injury model. Neuroimmunomodulation. 2014;21:257–267. doi: 10.1159/000357382. [DOI] [PubMed] [Google Scholar]
- Wirth M, Juachim J, Tooze SA. Autophagosome formation – The role of ULK1 and beclin1-PI3KC3 complexes in setting the stage. Sem. Cancer Biol. 2008;23:301–309. doi: 10.1016/j.semcancer.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Watanabe A, Ihara Y. Collapsin response mediator protein-2 is associated with neurofibrillary tangles in Alzheimer’s disease. J. Biol. Chem. 1998;273:9761–9768. doi: 10.1074/jbc.273.16.9761. [DOI] [PubMed] [Google Scholar]
- Zeng M, van der Donk WA, Chen J. Lantibiotic cyclase-like protein 2 (LanCL2) is a novel regulator of Akt. Mol. Biol. Cell. 2014;25:3954–3961. doi: 10.1091/mbc.E14-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang L, Liu Y, Xu J, Zhu G-X, Cang H, Li X, Bartlam M, Hensley K, Li G, Rao Z, Zhang X-C. Structure of human lanthionine synthetase C-like protein 1 and its interaction with Eps8 and glutathione. Genes and Development. 2009;23:1387–1392. doi: 10.1101/gad.1789209. [DOI] [PMC free article] [PubMed] [Google Scholar]