Abstract
Carotid artery atherosclerosis is an important source of mortality and morbidity in the Western world with significant socioeconomic implications. The quest for the early identification of the vulnerable carotid plaque is already in its third decade and traditional measures, such as the sonographic degree of stenosis, are not selective enough to distinguish those who would really benefit from a carotid endarterectomy. MRI of the carotid plaque enables the visualization of plaque composition and specific plaque components that have been linked to a higher risk of subsequent embolic events. Blood suppressed T1 and T2 weighted and proton density-weighted fast spin echo, gradient echo and time-of-flight sequences are typically used to quantify plaque components such as lipid-rich necrotic core, intraplaque haemorrhage, calcification and surface defects including erosion, disruption and ulceration. The purpose of this article is to review the most important recent advances in MRI technology to enable better diagnostic carotid imaging.
Internal carotid artery atherosclerosis is a significant source of mortality and morbidity in the Western world.1,2 MRI enables the visualization of plaque composition, and specific plaque constituents have been linked to a higher risk of subsequent embolic events. Currently, either the sonographic or angiographic degree of carotid stenosis is used as a marker of severity for assessing carotid disease and risk of stroke.3,4 However, there is mounting evidence that suggests that the degree of stenosis is not enough to accurately characterize carotid plaque burden and vulnerability.
High-resolution, multicontrast carotid MRI protocols have been used to depict atherosclerotic components within carotid plaques, the validity of these protocols have been evaluated using histopathology, and the protocols have been applied in multicentre trials.5–7 Blood suppressed T1 and T2 weighted and proton density (PD)-weighted fast spin echo (FSE) and gradient echo time-of-flight sequences are typically used8 to quantify plaque components such as lipid-rich necrotic core (LRNC),5,9–11 intraplaque haemorrhage (IPH),5,12–15 calcification5,9 and surface defects including erosions, disruption and ulceration.11,16–18 In addition, the intrareader, interreader14,19–21 and the interscan reproducibility20,22,23 of quantitative measures associated with both morphology and composition have been reported.
Novel MR-defined plaque features of vulnerability are emerging and appear promising for the identification of the vulnerable plaque. A recent systematic review of 9 studies with 779 subjects demonstrated that the presence of IPH, LRNC and thinning/rupture of the fibrous cap (FC) is linked to an increased risk of future stroke or transient ischaemic attack (TIA).24 The hazard ratios for each of them as predictors of subsequent ischaemic events were 4.59 [95% confidence interval (CI), 2.91–7.24], 3.00 (95% CI, 1.51–5.95) and 5.93 (95% CI, 2.65–13.20), respectively. In a separate meta-analysis focusing on IPH that pooled 8 clinical studies of 689 patients, the hazard ratio of MRI-depicted IPH in symptomatic patients was 11.7.25 This evidence indicates that dedicated MRI-depicted plaque composition offers stroke risk information beyond measurement of luminal stenosis.
Although MRI holds promise, the clinical application for plaque characterization will require further consensus regarding MRI protocols and new MRI techniques. A recent overview of 17 studies that assessed the agreement between MRI and histology indicated that MRI could characterize calcification, FC, IPH and LRNC with moderate-to-good sensitivity and specificity.26 It suggests that further effort is needed to use MRI as a routine clinical imaging modality to assess carotid atherosclerosis characteristics. One pragmatic issue is the requirement of a dedicated receiver coil.
The purpose of this article is to review the most important recent advances in MRI technology to enable better diagnostic carotid imaging. This article also reviews recent advances in material stress analysis and molecular imaging relevant to carotid atherosclerosis.
MR sequences for carotid plaque composition analysis
The de facto standard for blood suppressed carotid imaging is double inversion recovery (DIR) preparation. Intraluminal blood signal is suppressed by applying a 180° non-selective pulse to invert the magnetization within the transmit volume, which is immediately followed by a 180° slice selective pulse to revert the magnetization within the imaging plane. The magnetization in the blood outside the imaging plane reaches a null point after a period of time defined as the inversion time. Also after a period of time, for any given flow rate, inflowing blood from outside the imaging plane will replace in the intraluminal space within the defined imaging plane.
DIR is typically implemented as a gated single slice technique as black blood (BB) imaging is originally developed for cardiac application where gating is a fundamental prerequisite. However, gated acquisitions lead to variations in T1 relaxation and the signal slice implementation results in scan times, which preclude clinical adoption. In response, Yarnykh and Yuan27 implemented an ungated multislice DIR protocol; this in effect accelerates the acquisition as a function of the number of slices that are simultaneously acquired. However, DIR is known to be sensitive at producing plaque-mimicking artefacts. This is understood to frequently occur at the carotid bifurcation, as complex flow patterns exist where blood signal within the imaging plane is not fully displaced from the imaging plane.
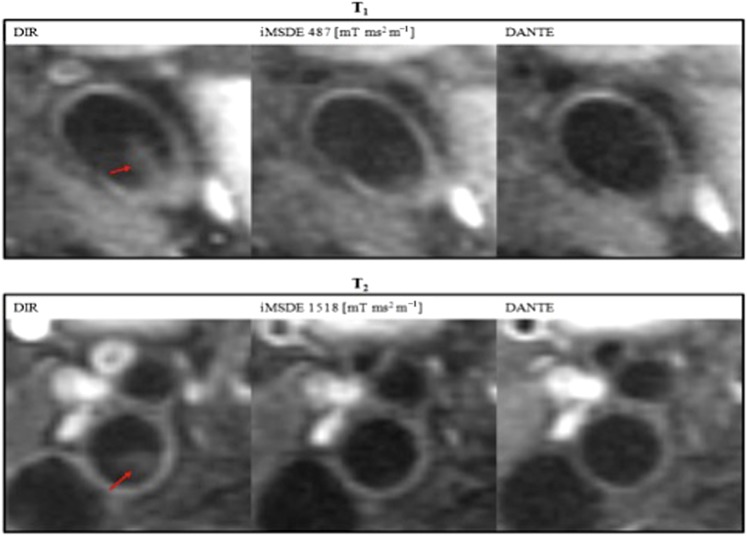
More advanced methods for blood suppression have subsequently been proposed, which are even more time efficient and can null residual blood signal. Wang et al28 presented a technique known as improved motion-sensitized driven-equilibrium (iMSDE), in which blood suppression can be directly controlled by the size of the motion-sensitizing gradients. This method effectively suppresses blood signal, however, the technique induces additional T2 decay. In 2012, Li et al29 presented Delays Alternating with Nutations for Tailored Excitation (DANTE). This study demonstrated how blood signal could be suppressed by both a function of the DANTE preparation echo-train length and flip angle. To date, there are no studies that have systematically compared all three methods; however, results of our initial validation studies are presented in Figure 1.
Figure 1.
Normal volunteer: comparison of advanced blood suppression techniques. The double inversion recovery preparation commonly exhibits plaque-mimicking artefacts (arrow). Both improved motion-sensitized driven-equilibrium (iMSDE) and Delays Alternating with Nutations for Tailored Excitation (DANTE) are more time efficient and can mitigate these artefacts. DIR, double inversion recovery.
Currently, the majority of MRI is performed on 1.5-T systems. The continuous advances in MR technology have allowed carotid imaging at higher magnetic strengths, and multiphase coils increase the potential for parallel imaging. Preliminary experience with eight-channel coils at 3.0 T indicates an increase in signal-to-noise ratio (SNR) to contrast-to-noise ratio (CNR) compared with 1.5 T. In a study by Young et al,30 18 subjects with carotid atherosclerosis were imaged on both 1.5- and 3.0-T systems using the same receiver coil. T1, T2 and PD images were obtained, and multiple slices were prescribed to encompass both the carotid bifurcation and the plaque. In this study, the mean improvements in SNR were 1.9, 2.1 and 2.1, respectively, which were statistical significant.
Yuan et al,31 recently reported on 19 patients at 3.0 T and noted significant associations between the presence of thin/ruptured FC (100% vs 36%; p = 0.006) and LRNC (100% vs 39%; p = 0.022), with a borderline association with haemorrhage (86% vs 33%; p = 0.055).
Assessment of carotid plaque morphology using three-dimensional MR sequences
In a three-dimensional (3D) sequence, a radiofrequency (RF) pulse is applied to excite an imaging volume. Phase encoding is performed in both the convention phase encoding axis and in the slice select direction. 3D sequences typically result in higher SNR efficiency. In a study by Balu et al,32 a total of 18 subjects with significant carotid stenosis were imaged with two-dimensional (2D) (2-mm slice thickness) and 3D [1-mm/0.5-mm (acquired/interpolated) slice thickness] T1 weighted FSE BB imaging sequences with DIR blood suppression. Morphological measurements, SNR in the wall and lumen, and wall-lumen CNR were compared between the 2D and 3D images. The lumen SNR, wall SNR and CNR were comparable between the two methods. There was also no difference in average volumetric measurements between 2D/3D. By contrast, the distributions of small plaque components such as calcification were better characterized by the 3D acquisition while there was a higher sensitivity to motion artefacts with 3D imaging, resulting in a small number of examinations with low image quality. The conclusion from this study was that 2D and 3D protocols can provide comparable morphometric and volumetric measurements of the carotid artery with the 3D imaging offering an advantage in terms of small plaque component visualization but with the price of lower reliability for image quality.
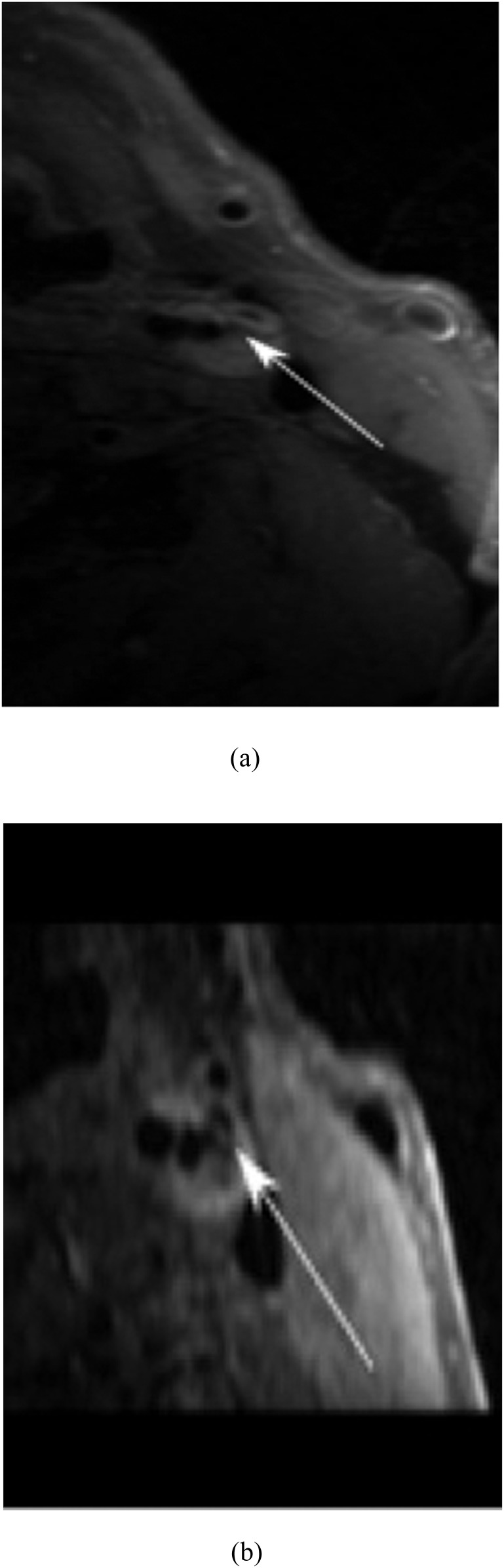
In another study by Takano et al,33 22 patients scheduled for carotid endarterectomy underwent carotid plaque MRI with both a conventional FSE 2D sequence and 3D FSE sequence with variable refocusing flip angles. This 3D sequence enables the acquisition of longer echo-train lengths whilst reducing the T2 blurring effect that would typically accrue by attempting to maintain magnetization across the train of refocusing pulse. Image quality and SNR were evaluated; observations were compared for each plaque component according to the histological category of the plaque between 2D and 3D sequences (Figure 2). No significant differences were observed among the overall imaging quality scores of the two modalities, although 3D sequences allowed visualization in random orientations, as well as better depiction of small plaque components such as ulcerations and calcifications. The SNR of the plaque to the submandibular gland on T1 weighted 3D sequence was significantly higher than that on the 2D sequence. In addition, it was shown that the SNR of the plaque to the submandibular gland of histology-defined soft-plaque components were significantly higher on T1 weighted 3D sequence than on 2D sequence. From this study, it was concluded that 3D variable-flip-angle turbo spin echo (TSE) is a promising tool for diagnostic imaging.
Figure 2.
T1 weighted MRI of (a) two-dimensional (2D) fast spin echo (FSE) with 3-mm thickness, and (b) three-dimensional (3D) variable flip angle FSE with 0.6-mm thickness. The lipid core is clearly seen in 3D FSE (arrow, b), while the partial volume effect limits the resolution of 2D FSE (arrow, a).
In 2011, Balu et al34 proposed a BB 3D gradient echo sequence (3D-MERGE), the sequence was evaluated on nine patients with carotid atheroma. The proposed sequence incorporated BB preparation using iMSDE preparation prior to 3D spoiled gradient echo readout. The proposed gradient sequence enabled isotropic resolution (0.7 mm3) and was more time efficient than the 3D FSE alternatives (imaging time, approximately 2 min).
In addition, the advances in MR technology allow the use of non-gated sequences instead of gated sequences for atherosclerotic vessel wall imaging without compromising image quality. This may shorten examination time and improve patient comfort.35 Other examples of technological advances include the 3D diffusion-prepared segmented steady–steady free precession cardiovascular MR sequence for BB,36 which allows for 3D acquisition of thin and contiguous slices with BB image contrast. Finally, TSE-based motion-sensitized driven-equilibrium sequence has been developed as an alternative to BB carotid MRI providing a more accurate depiction of the lumen boundaries by eliminating plaque-mimicking artefacts in carotid plaque imaging.37
Identification of intraplaque haemorrhage
The characterization of IPH using a 3D gradient echo sequence with a selective water excitation pulse was first presented by Moody et al13 in 2003. The sequence was coined MR direct thrombus imaging. Their study demonstrates using histopathology validation, the sequence utility in detecting thrombus. A subsequent variant of this technique was proposed by Zhu et al38 in 2010. This sequence referred to as 3D SHINE (3D spoiled gradient-recalled echo pulse sequence for haemorrhage assessment using inversion recovery and multiple echos) enables the simultaneous detection and ageing of thrombus based on its respective T1 and T2* relaxation properties. The differentiation between IPH and thrombus can be challenging, and a general guide can be seen at Tables 1 and 2.
Table 1.
MRI characteristics of various carotid plaque components
| Plaque characteristics | T1 weighted | T2 weighted | Proton density | Contrast-enhanced T1* weighted | |
|---|---|---|---|---|---|
| Fibrous tissue | o | o | o | o | |
| Calcification | – | – | – | – | |
| Lipid-rich necrotic core | o | o | – | –/o | |
| Haemorrhage | + | + | −/+ | −/+ |
+, bright signal; o, no signal.
Table 2.
MRI and histology criteria used in the classification of plaque haemorrhage
| Haemorrhage age | T1 weighted | T2 weighted | Proton density | Time of flight |
|---|---|---|---|---|
| Acute | + | –/o | –/o | + |
| Recent | + | + | + | + |
| Chronic | – | – | – | – |
+, bright signal; o, no signal.
THE EVALUATION OF FIBROUS CAP THICKNESS
Wang et al,11 previously demonstrated that different types of stroke can be identified using standard brain MR protocols prior to invasive therapies. In this study, 102 consecutive subjects with and without a history of cerebrovascular events underwent contrast-enhanced carotid and brain MR protocols as well as MR angiography. This study demonstrated that of 63 patients with mild-to-moderate stenosis (≤70%), 44 (69.8%) had Types IV and V vulnerable plaques, which was significantly higher than those of patients with severe stenosis (>70%; p < 0.001). In stroke, the number of patients with a thin or ruptured FC was twice that of those with a thick and intact FC. Contrast-enhanced MRI may therefore have important applications in clinical risk evaluations in atherosclerosis.
A study by Douek et al,12 reported on 69 patients scheduled for a carotid endarterectomy using 3.0-T contrast-enhanced MRI. The carotid plaque enhancement was assessed on T1 weighted images performed pre- and post-contrast administration. Histological analysis was performed on the entire plaque and on an area with matched contrast enhancement. Gadolinium enhancement was observed in >50% of the patients. There were three types of carotid plaques that were identified depending on the enhancement pattern; in other words, they were grouped according to whether it was the shoulder region, the shoulder and FC or the central part of the plaque enhanced most. FC rupture, IPH and plaque gadolinium enhancement were significantly more frequent in symptomatic than in asymptomatic patients (p = 0.0430, p < 0.0001 and p = 0.0340, respectively). After histological analysis, gadolinium enhancement was significantly associated with vulnerable plaque (American Heart Association VI, p = 0.006), neovascularization (p < 0.0001), macrophages (p = 0.030) and loose fibrosis (p < 0.0001). The presence of neovessels, macrophages and fibrosis in the enhancing area was 97%, 87% and 80%, respectively, and was different depending on the enhancement location in the plaque. From this study, it appears that the enhancement of carotid plaque is associated with vulnerable plaque phenotypes and related to inflammatory process.
Imaging intraplaque inflammation
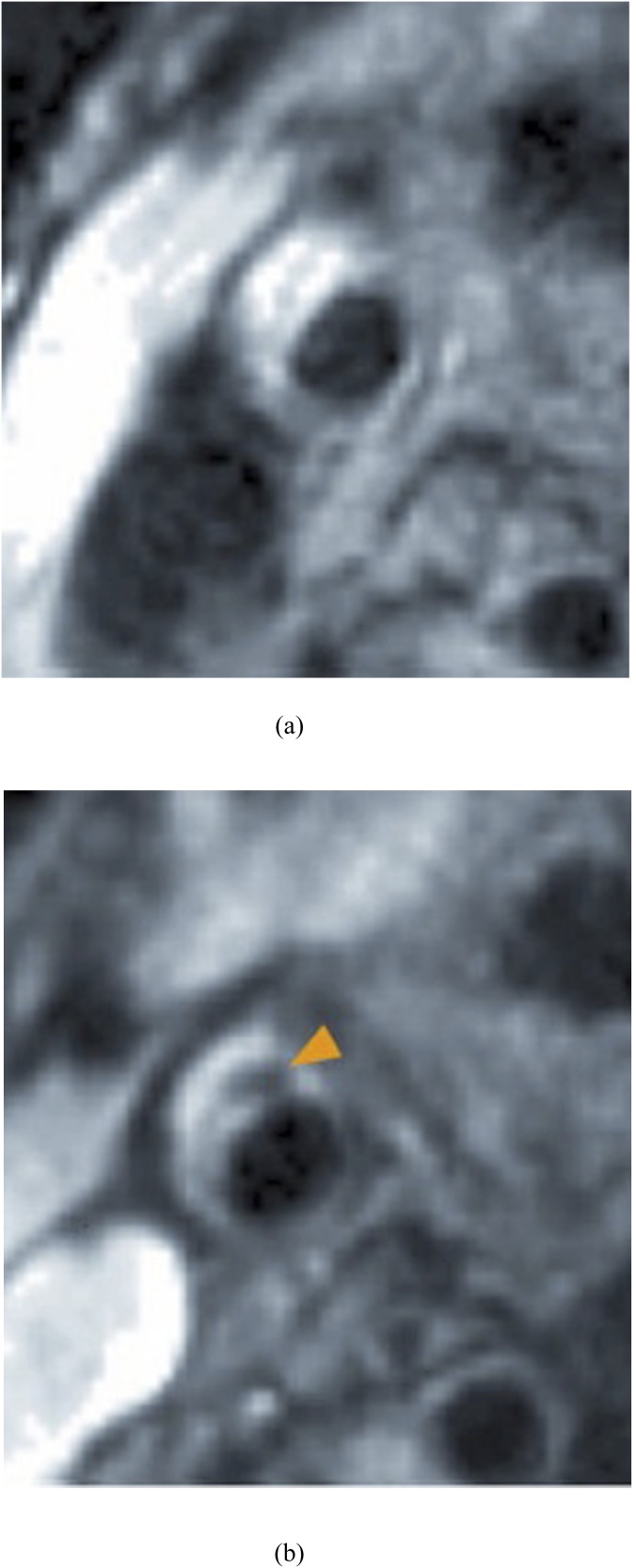
Ferumoxtran-10 (Sinerem®; Laboratoire Guerbet, Paris, France) is an ultrasmall superparamagnetic iron oxide (USPIO), which undergoes phagocytosis by macrophages and thus acts as a marker of inflammation. The application of USPIO contrast-enhanced MRI has enabled the detection of macrophage activity in carotid atherosclerosis (Figure 3). In a study by Tang et al,13 20 symptomatic patients underwent multisequence MRI before and 36 h after USPIO infusion.13 The symptomatic patients demonstrated increased USPIO uptake relative to the contralateral side. A subsequent study by Tang et al15 examined the relationship between the degree of MR-defined inflammation using USPIOs and the severity of luminal stenosis in asymptomatic carotid plaques. It was shown that there was no significant relationship between the degree of inflammation and the degree of luminal stenosis, supporting the theory that the currently used gold standard, degree of stenosis, is inadequate for stroke risk evaluation.15
Figure 3.
Carotid imaging pre (a) and post (b) T1 weighted ultrasmall superparamagnetic iron oxide administration showing significant signal loss in the post-imaging phase (arrowhead).
In a study by Kwee et al,39 50 patients with symptomatic carotid stenosis were assessed with CT, MR and fluorine-18 fludeoxyglucose positron emission tomography (18F-FDG-PET) imaging, and the agreement between them was compared. It was shown that correlations between 18F-FDG-PET and CT/MRI findings are weak; however, correlations between CT and MRI measurements appear to be moderate to strong but with considerable variation in absolute differences. In a more recent study by Izquierdo-Garcia et al,40 it was shown that MR-guided 18F-FDG-PET is a highly reproducible technique in the carotid artery providing excellent anatomic detail and that the standardized uptake value is the most reproducible parameter irrespective of other confounders.
Finally, in a study by Davies et al41 high 18F-FDG uptake was evident in only 7 out of the 12 patients waiting for endarterectomy. In the remaining five patients, 18F-FDG uptake in the targeted lesion was low. In these five patients, three had additional non-stenotic lesions identified on high-resolution MRI that exhibited a high level of 18F-FDG uptake. All three of the highly inflamed non-stenotic lesions were located in a vascular territory compatible with the patients' presenting symptoms. This was one of the first studies to suggest that angiography may not always identify the culprit lesion. Combined 18F-FDG-PET and high-resolution MRI shown to be able to assess the degree of inflammation in stenotic and non-stenotic plaques and could potentially be used to identify lesions responsible for embolic events even when the degree of stenosis is <70%.
Again, it seems that the combination of MRI and PET may add significant information useful for plaque characterization. However, the especially high cost of these two techniques combined makes the evaluation of their cost effectiveness mandatory.
Assessment of carotid intraplaque neovascularization
The theory of intraplaque neovascularization being associated with active intraplaque inflammation was demonstrated by Kerwin et al42 in 2006. Their study of 30 patients examined the relationship between quantitative pharmacokinetic measures of dynamic contrast enhancement and measures of macrophage, neovasculature and loose matrix content obtained following histopathological assessment. This study demonstrated a highly significant relationship between Ktrans and macrophage count (r = 0.750; p < 0.001)
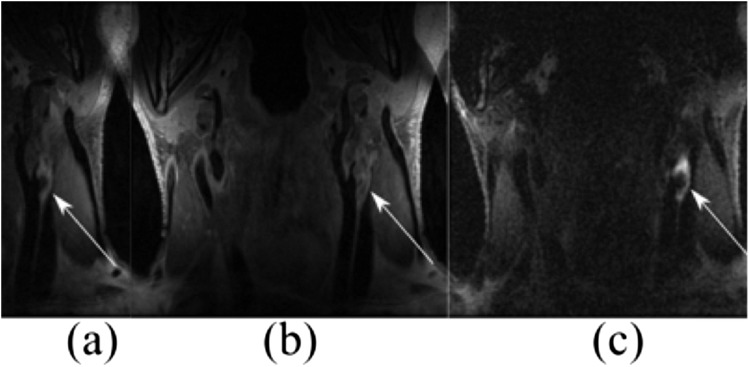
In the past, dynamic contrast-enhanced (DCE) MRI was extensively utilized to study the vascularity and neovascularization within tumours.42 Recently though, further applications came to light with delayed and DCE MRI being introduced as non-invasive tools to assess the extent of plaque neovascularization in animals and patients with atherosclerosis.43 Gadolinium is a contrast agent typically administered for routine MR angiography and can be used to improve plaque characterization (Figure 4). Gadolinium has favourable properties that allow enhancement of the FC, more reliable vessel wall measurements and better visualization of the intraplaque neovessels. The above properties combined with the pre-contrast series can help in the identification of IPH.
Figure 4.
Arrows highlight the lesion of a 74-year-old patient in coronal slices of multicontrast carotid plaque MRI: (a) pre-contrast T1 weighted three-dimensional (3D) fast spin echo (FSE); (b) post-contrast T1 weighted 3D FSE and (c) direct thrombus imaging.
However, there are currently a limited number of studies on the utility of assessing neovasculature as an indirect marker of inflammation.
CAROTID PLAQUE BIOMECHANICAL ANALYSIS USING MR IMAGING
Both IPH and FC rupture have been identified as high-risk features associated with clinical symptoms and recurrent cerebrovascular ischaemic events.25,44,45 Results from 29 histology-based studies indicated that 70.3% symptomatic carotid plaque presented with IPH.46 FC rupture is also a common feature in symptomatic patients with a prevalence of approximately 60%.45 In vivo imaging-based studies also demonstrated an association between FC rupture and subsequent events in symptomatic patients.47,48 Event rates are increased when combinations of these two features are present.46 By contrast, only about 15% symptomatic patients will experience a recurrent event at 1 year.48–51 It is clear that imaging-detected IPH or FC alone or in combination cannot serve as a robust marker for prospective cerebrovascular risk, and additional analyses or biomarkers are required. Under physiological conditions, carotid plaques are subjected to mechanical loading from pulsatile blood pressure. FC rupture may occur when this loading exceeds its material strength (Figure 5). Indeed, plaques with high mechanical stress concentrations are associated with fissuring in both coronary48 and carotid52,53 plaques.
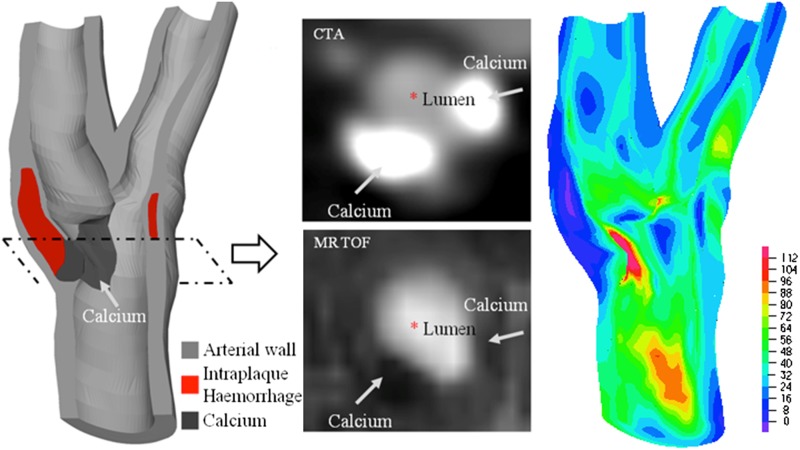
Figure 5.
Superficial calcium was found in 4–5% of ruptured plaques in the coronary, possibly the same proportion in the carotid, which could be because of the high stress concentration over the fibrous cap owing to the presence of superficial calcium. (Left: the reconstructed plaque geometry of a plaque located around carotid bifurcation from a symptomatic patient; middle: the co-registered CT and MR images showing juxtaluminal calcium; right: high stress concentration appears around the calcium; unit, kPa.) CTA, CT angiography; MRTOF, MR time of flight.
The first in vivo MRI-based study aiming to access the difference in mechanical stress in symptomatic and asymptomatic individuals possibly was performed by Li et al54 in 2007. The result obtained from 30 patients using 2D structure-only modelling indicated that the stress level in symptomatic individuals was about twice as high as that of the asymptomatic cohort. This observation was further confirmed by a study with a slightly larger patient cohort55 (n = 40). Sadat et al56,57 and Zhu et al58 found that stress level right after the onset of symptom was much higher than that of recently symptomatic and asymptomatic patients. Effort is still being made to examine the difference of stress level in symptomatic and asymptomatic individuals with more sophisticated 3D fluid structure interaction simulation models.59
A large-scale study including patients who suffered from TIA (n = 1247) indicates that 1.2% of patients had recurrent strokes within 6 h, and 2.1% and 5.1% had recurrent strokes within 12 and 24 h, respectively.60 The risk of recurrent stroke in the week after a TIA or minor stroke is up to 10%,60–62 and generally, 10–20% will have a stroke within 1 month.63 The high stress level in acutely and recently symptomatic patients as quantified by these studies may shed light on the extremely high recurrent rate for TIA patients in the early stage. Following the acute event, plaque healing64,65 may occur with a smoother lumen surface66 or a thicker and stronger FC67,68 that may reduce the stress level and stabilize the lesion. However, such remodelling may promote plaque progression and eventually turn into a vulnerable lesion.
Although biomechanical analysis has shown its potential in explaining clinical observations and refining patient stratification, its benefit in patient management has been least assessed. As the first step towards this goal, direct evidence of the association of critical mechanical stress with subsequent cerebrovascular ischaemic events was reported by Sadat et al.48 61 TIA patients were recruited in that study, and the critical mechanical condition was obtained based on the in vivo baseline MR images. During a 2-year follow-up period, 20% patients (n = 12) experienced recurrent events, and Cox regression analysis indicated that high stress condition had a close relationship with the recurrent event (hazards ratio = 12.98). Apart from the high stress concentration within FC, intraplaque deformation quantified by stretch ratio may be an effective biomarker in predicting subsequent ischaemic events;69 as such, large deformations may damage the fragile neovessel's wall promoting the formation of IPH.70
ADVANCES IN INVESTIGATIONAL MR MOLECULAR IMAGING
Currently, there are only animal-based studies evaluating the effect of molecular imaging on carotid plaque characterization; however, in the past decade, there has been an increasing interest from various research groups.
P947 (DOTA-Gd-peptide) was recently identified as an MR contrast agent for the detection of the atherosclerotic plaques that are rich in matrix metalloproteinases (MMP). In an in vivo pre-clinical study by Klink et al,71 P975 showed good affinity for activated platelets. In thrombosed animals, P975 produced an immediate and sustained increase in MR signal, whereas none of the control groups revealed similar enhancement.
Another promising molecule is neutrophil gelatinase-associated lipocalin (NGAL), which is an effector molecule of the innate immune system. This molecule is highly expressed in atheromatous human plaques and associated with increased MMP-9 activity. In a study, increased levels of NGAL and the NGAL/MMP-9 complex were associated with high lipid content, high number of macrophages, high interleukin-6 (IL-6) and IL-8 levels, and low smooth muscle cell content in human atherosclerotic plaques lesions, which where obtained post endarterectomy.72 Moreover, plaque levels of NGAL tended to be higher when intraplaque haemorrhage or luminal thrombus was present than without the presence of IPH or thrombus. Additionally, MMP-9 and MMP-8 activities were strongly related to NGAL levels. This study showed that NGAL could be detected in murine atherosclerotic arteries using targeted high-resolution MRI.
Another possible target is the intraplaque and endothelial fibrin, which has recently been recognized to play an important role in the progression of atherosclerosis. The study by Makowski et al,73 aimed to investigate the feasibility of intraplaque and endothelial fibrin detection using a fibrin-targeted contrast agent (FTCA) (EPIX Pharmaceuticals, Lexington, MA), in a mouse model of atherosclerosis. Molecular MRI (mMRI) after FTCA administration demonstrated a significant increase in contrast uptake in the atherosclerotic plaques. This study demonstrated the feasibility of intraplaque and endothelial fibrin imaging using FTCA.
Oxidized LDL (OxLDL) is an agent that plays an important role in the formation, rupture and thrombus formation in atherosclerotic plaques. In a study by Teng et al, an antimouse OxLDL polyclonal antibody and non-specific immunoglobinG antibody were conjugated to polyethylene glycol-coated USPIO nanoparticles, and a carotid perivascular collar model in apolipoprotein E-deficient mice. Imaging was conducted at 7 T.74 It was shown that antiOxLDL-USPIO nanoparticles can detect OxLDL and image atherosclerotic lesions within 24 h of nanoparticle administration, suggesting a possible strategy for the therapeutic evaluation of atherosclerotic plaques in vivo.
mMRI of activated platelets as early markers of plaque instability and rupture using targeted contrast agents is a promising strategy. In a study by von Elverfeldt et al,75 the activated platelets in murine atherothrombosis were imaged by in vivo mMRI, using a dedicated animal model of plaque rupture. An antibody-targeting ligand-induced binding site (LIBS) on the glycoprotein IIb/IIIa receptor of activated platelets was conjugated to microparticles of iron oxide (MPIO) to form the LIBS-MPIO contrast agent causing a signal-extinction in T2* weighted MRILIBS-MPIO. This allows the detection of activated platelets on the surface of symptomatic atherosclerotic human plaques using mMRI. The findings from this study were that LIBS-MPIO-injected animals demonstrated significant signal extinction in MRI, which corresponded to the site of plaque rupture and atherothrombosis in histology. The signal attenuation was effective for atherothrombosis occupying ≥2% of the vascular lumen. The histological analysis that followed further confirmed that significant binding of LIBS-MPIO compared with control MPIO on the thrombus developing on the surface of ruptured plaques. So far despite the many promising experimental agents, there is not a specific molecular agent with clear advantage over the others, and there are no specific indications about the timeline for the transition from in vitro to in vivo research.
Finally, intracranial atherosclerotic disease (ICAD) has been associated with a significant number (approximately 10%) of all ischaemic cerebrovascular events and is another area with possible MR applications. As with extracranial atherosclerosis, some of the most important predictors of atherosclerotic plaque vulnerability include the degree of lumen stenosis and the underlying plaque morphology. Vascular MRI can again provide useful information about wall components and overall morphology.76 Some of the challenges in high-resolution MRI in ICAD are the necessary high in-plane resolution and the high SNR that must be achieved. So far, research studies have focused on assessing the presence of a plaque and evaluating the plaque load. However, there is limited evidence regarding the evaluation of plaque vulnerability through analysis of imaging characteristics and further research is warranted to clarity the potential of MRI in this field.76
CONCLUSION
The identification of the vulnerable carotid plaque has proven to be a particularly complicated problem with many parameters and limitations. Ultrasound has been for years the mainstay of diagnosis and is still the basis of most management plans; however, there is mounting evidence to suggest that it is not adequate. At the moment, there are many promising approaches based on MRI, although most of them are currently in the research domain. Further clinical research is needed to determine clinical outcomes and cost effectiveness.
FUNDING
This research is supported by British Heart Foundation PG/11/74/29100 and the NIHR Cambridge Biomedical Research Centre.
Contributor Information
G C Makris, Email: gm482@cam.ac.uk.
Z Teng, Email: zt215@cam.ac.uk.
A J Patterson, Email: andrew.patterson@addenbrookes.nhs.uk.
J-M Lin, Email: jy338@cam.ac.uk.
V Young, Email: viccyyoung@gmail.com.
M J Graves, Email: mjg40@radiol.cam.ac.uk.
J H Gillard, Email: jhg21@cam.ac.uk.
REFERENCES
- 1.Levy EI, Mocco J, Samuelson RM, Ecker RD, Jahromi BS, Hopkins LN. Optimal treatment of carotid artery disease. J Am Coll Cardiol 2008; 51: 979–85. doi: 10.1016/j.jacc.2007.10.052 [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123: e18–209. doi: 10.1161/CIR.0b013e3182009701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339: 1415–25. doi: 10.1056/NEJM199811123392002 [DOI] [PubMed] [Google Scholar]
- 4.Rothwell PM, Gutnikov SA, Warlow CP; European Carotid Surgery Trialist's Collaboration. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke 2003; 34: 514–23. doi: 10.1161/01.str.0000054671.71777.c7 [DOI] [PubMed] [Google Scholar]
- 5.Toussaint JF, LaMuraglia GM, Southern JF, Fuster V, Kantor HL. Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation 1996; 94: 932–8. doi: 10.1161/01.cir.94.5.932 [DOI] [PubMed] [Google Scholar]
- 6.Görtler M, Goldmann A, Mohr W, Widder B. Tissue characterisation of atherosclerotic carotid plaques by MRI. Neuroradiology 1995; 37: 631–5. [DOI] [PubMed] [Google Scholar]
- 7.Underhill HR, Hatsukami TS, Fayad ZA, Fuster V, Yuan C. MRI of carotid atherosclerosis: clinical implications and future directions. Nat Rev Cardiol 2010; 7: 165–73. doi: 10.1038/nrcardio.2009.246 [DOI] [PubMed] [Google Scholar]
- 8.Choudhury RP, Fuster V, Fayad ZA. Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov 2004; 3: 913–25. doi: 10.1038/nrd1548 [DOI] [PubMed] [Google Scholar]
- 9.Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol 2005; 25: 234–9. doi: 10.1161/01.ATV.0000149867.61851.31 [DOI] [PubMed] [Google Scholar]
- 10.Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 2005; 112: 3437–44. doi: 10.1161/circulationaha.104.528174 [DOI] [PubMed] [Google Scholar]
- 11.Trivedi RA, U-King-Im J, Graves MJ, Horsley J, Goddard M, Kirkpatrick PJ, et al. Multi-sequence in vivo MRI can quantify fibrous cap and lipid core components in human carotid atherosclerotic plaques. Eur J Vasc Endovasc Surg 2004; 28: 207–13. doi: 10.1016/j.ejvs.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 12.Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001; 104: 2051–6. doi: 10.1161/hc4201.097839 [DOI] [PubMed] [Google Scholar]
- 13.Moody AR, Murphy RE, Morgan PS, Martel AL, Delay GS, Allder S, et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation 2003; 107: 3047–52. doi: 10.1161/01.cir.0000074222.61572.44 [DOI] [PubMed] [Google Scholar]
- 14.Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, O'Brien KD, et al. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 2004; 35: 1079–84. doi: 10.1161/01.STR.0000125856.25309.86 [DOI] [PubMed] [Google Scholar]
- 15.Kampschulte A, Ferguson MS, Kerwin WS, Polissar NL, Chu B, Saam T, et al. Differentiation of intraplaque versus juxtaluminal hemorrhage/thrombus in advanced human carotid atherosclerotic lesions by in vivo magnetic resonance imaging. Circulation 2004; 110: 3239–44. doi: 10.1161/01.CIR.0000147287.23741.9A [DOI] [PubMed] [Google Scholar]
- 16.Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000; 102: 959–64. doi: 10.1161/01.cir.102.9.959 [DOI] [PubMed] [Google Scholar]
- 17.Mitsumori LM, Hatsukami TS, Ferguson MS, Kerwin WS, Cai J, Yuan C. In vivo accuracy of multisequence MR imaging for identifying unstable fibrous caps in advanced human carotid plaques. J Magn Reson Imaging 2003; 17: 410–20. doi: 10.1002/jmri.10264 [DOI] [PubMed] [Google Scholar]
- 18.Yu W, Underhill HR, Ferguson MS, Hippe DS, Hatsukami TS, Yuan C, et al. The added value of longitudinal black-blood cardiovascular magnetic resonance angiography in the cross sectional identification of carotid atherosclerotic ulceration. J Cardiovasc Magn Reson 2009; 11: 31. doi: 10.1186/1532-429x-11-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saam T, Cai JM, Cai YQ, An NY, Kampschulte A, Xu D, et al. Carotid plaque composition differs between ethno-racial groups: an MRI pilot study comparing mainland Chinese and American Caucasian patients. Arterioscler Thromb Vasc Biol 2005; 25: 611–16. doi: 10.1161/01.atv.0000155965.54679.79 [DOI] [PubMed] [Google Scholar]
- 20.Saam T, Hatsukami TS, Yarnykh VL, Hayes CE, Underhill H, Chu B, et al. Reader and platform reproducibility for quantitative assessment of carotid atherosclerotic plaque using 1.5T Siemens, Philips, and General Electric scanners. J Magn Reson Imaging 2007; 26: 344–52. doi: 10.1002/jmri.21004 [DOI] [PubMed] [Google Scholar]
- 21.Takaya N, Cai J, Ferguson MS, Yarnykh VL, Chu B, Saam T, et al. Intra- and interreader reproducibility of magnetic resonance imaging for quantifying the lipid-rich necrotic core is improved with gadolinium contrast enhancement. J Magn Reson Imaging 2006; 24: 203–10. doi: 10.1002/jmri.20599 [DOI] [PubMed] [Google Scholar]
- 22.Underhill HR, Yarnykh VL, Hatsukami TS, Wang J, Balu N, Hayes CE, et al. Carotid plaque morphology and composition: initial comparison between 1.5- and 3.0-T magnetic field strengths. Radiology 2008; 248: 550–60. doi: 10.1148/radiol.2482071114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Underhill HR, Yuan C, Zhao XQ, Kraiss LW, Parker DL, Saam T, et al. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: a high-resolution magnetic resonance imaging trial. Am Heart J 2008; 155: 584.e1–8. doi: 10.1016/j.ahj.2007.11.018 [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke 2013; 44: 3071–7. doi: 10.1161/STROKEAHA.113.002551 [DOI] [PubMed] [Google Scholar]
- 25.Saam T, Hetterich H, Hoffmann V, Yuan C, Dichgans M, Poppert H, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol 2013; 62: 1081–91. doi: 10.1016/j.jacc.2013.06.015 [DOI] [PubMed] [Google Scholar]
- 26.den Hartog AG, Bovens SM, Koning W, Hendrikse J, Luijten PR, Moll FL, et al. Current status of clinical magnetic resonance imaging for plaque characterisation in patients with carotid artery stenosis. Eur J Vasc Endovasc Surg 2013; 45: 7–21. doi: 10.1016/j.ejvs.2012.10.022 [DOI] [PubMed] [Google Scholar]
- 27.Yarnykh VL, Yuan C. Multislice double inversion-recovery black-blood imaging with simultaneous slice reinversion. J Magn Reson Imaging 2003; 17: 478–83. doi: 10.1002/jmri.10278 [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Yarnykh VL, Yuan C. Enhanced image quality in black-blood MRI using the improved motion-sensitized driven-equilibrium (iMSDE) sequence. J Magn Reson Imaging 2010; 31: 1256–63. doi: 10.1002/jmri.22149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Miller KL, Jezzard P. DANTE-prepared pulse trains: a novel approach to motion-sensitized and motion-suppressed quantitative magnetic resonance imaging. Magn Reson Med 2012; 68: 1423–38. doi: 10.1002/mrm.24142 [DOI] [PubMed] [Google Scholar]
- 30.Young VE, Patterson AJ, Tunnicliffe EM, Sadat U, Graves MJ, Tang TY, et al. Signal-to-noise ratio increase in carotid atheroma MRI: a comparison of 1.5 and 3 T. Br J Radiol 2012; 85: 937–44. doi: 10.1259/bjr/70496948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demarco JK, Ota H, Underhill HR, Zhu DC, Reeves MJ, Potchen MJ, et al. MR carotid plaque imaging and contrast-enhanced MR angiography identifies lesions associated with recent ipsilateral thromboembolic symptoms: an in vivo study at 3T. AJNR Am J Neuroradiol 2010; 31: 1395–402. doi: 10.3174/ajnr.a2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balu N, Chu B, Hatsukami TS, Yuan C, Yarnykh VL. Comparison between 2D and 3D high-resolution black-blood techniques for carotid artery wall imaging in clinically significant atherosclerosis. J Magn Reson Imaging 2008; 27: 918–24. doi: 10.1002/jmri.21282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takano K, Yamashita S, Takemoto K, Inoue T, Sakata N, Kuwabara Y, et al. Characterization of carotid atherosclerosis with black-blood carotid plaque imaging using variable flip-angle 3D turbo spin-echo: comparison with 2D turbo spin-echo sequences. Eur J Radiol 2012; 81: e304–9. doi: 10.1016/j.ejrad.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 34.Balu N, Yarnykh VL, Chu B, Wang J, Hatsukami T, Yuan C. Carotid plaque assessment using fast 3D isotropic resolution black-blood MRI. Magn Reson Med 2011; 65: 627–37. doi: 10.1002/mrm.22642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mani V, Itskovich VV, Aguiar SH, Mizsei G, Aguinaldo JG, Samber DD, et al. Comparison of gated and non-gated fast multislice black-blood carotid imaging using rapid extended coverage and inflow/outflow saturation techniques. J Magn Reson Imaging 2005; 22: 628–33. doi: 10.1002/jmri.20428 [DOI] [PubMed] [Google Scholar]
- 36.Koktzoglou I, Li D. Diffusion-prepared segmented steady-state free precession: application to 3D black-blood cardiovascular magnetic resonance of the thoracic aorta and carotid artery walls. J Cardiovasc Magn Reson 2007; 9: 33–42. doi: 10.1080/10976640600843413 [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Yarnykh VL, Hatsukami T, Chu B, Balu N, Yuan C. Improved suppression of plaque-mimicking artifacts in black-blood carotid atherosclerosis imaging using a multislice motion-sensitized driven-equilibrium (MSDE) turbo spin-echo (TSE) sequence. Magn Reson Med 2007; 58: 973–81. doi: 10.1002/mrm.21385 [DOI] [PubMed] [Google Scholar]
- 38.Zhu DC, Vu AT, Ota H, DeMarco JK. An optimized 3D spoiled gradient recalled echo pulse sequence for hemorrhage assessment using inversion recovery and multiple echoes (3D SHINE) for carotid plaque imaging. Magn Reson Med 2010; 64: 1341–51. doi: 10.1002/mrm.22517 [DOI] [PubMed] [Google Scholar]
- 39.Kwee RM, Teule GJ, van Oostenbrugge RJ, Mess WH, Prins MH, van der Geest RJ, et al. Multimodality imaging of carotid artery plaques: 18F-fluoro-2-deoxyglucose positron emission tomography, computed tomography, and magnetic resonance imaging. Stroke 2009; 40: 3718–24. doi: 10.1161/strokeaha.109.564088 [DOI] [PubMed] [Google Scholar]
- 40.Izquierdo-Garcia D, Davies JR, Graves MJ, Rudd JH, Gillard JH, Weissberg PL, et al. Comparison of methods for magnetic resonance-guided [18-F]fluorodeoxyglucose positron emission tomography in human carotid arteries: reproducibility, partial volume correction, and correlation between methods. Stroke 2009; 40: 86–93. doi: 10.1161/strokeaha.108.521393 [DOI] [PubMed] [Google Scholar]
- 41.Davies JR, Rudd JH, Fryer TD, Graves MJ, Clark JC, Kirkpatrick PJ, et al. Identification of culprit lesions after transient ischemic attack by combined 18F fluorodeoxyglucose positron-emission tomography and high-resolution magnetic resonance imaging. Stroke 2005; 36: 2642–7. doi: 10.1161/01.STR.0000190896.67743.b1 [DOI] [PubMed] [Google Scholar]
- 42.Kerwin WS, O'Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology 2006; 241: 459–68. doi: 10.1148/radiol.2412051336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calcagno C, Mani V, Ramachandran S, Fayad ZA. Dynamic contrast enhanced (DCE) magnetic resonance imaging (MRI) of atherosclerotic plaque angiogenesis. Angiogenesis 2010; 13: 87–99. doi: 10.1007/s10456-010-9172-2 [DOI] [PubMed] [Google Scholar]
- 44.Gao P, Chen ZQ, Bao YH, Jiao LQ, Ling F. Correlation between carotid intraplaque hemorrhage and clinical symptoms: systematic review of observational studies. Stroke 2007; 38: 2382–90. doi: 10.1161/STROKEAHA.107.482760 [DOI] [PubMed] [Google Scholar]
- 45.Milei J, Parodi JC, Ferreira M, Barrone A, Grana DR, Matturri L. Atherosclerotic plaque rupture and intraplaque hemorrhage do not correlate with symptoms in carotid artery stenosis. J Vasc Surg 2003; 38: 1241–7. doi: 10.1016/S0741 [DOI] [PubMed] [Google Scholar]
- 46.Teng Z, Sadat U, Brown AJ, Gillard JH. Plaque haemorrhage in carotid artery disease: pathogenesis, clinical and biomechanical considerations. J Biomech 2014; 47: 847–58. doi: 10.1016/j.jbiomech.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eliasziw M, Streifler JY, Fox AJ, Hachinski VC, Ferguson GG, Barnett HJ. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke 1994; 25: 304–8. doi: 10.1161/01.str.25.2.304 [DOI] [PubMed] [Google Scholar]
- 48.Sadat U, Teng Z, Young VE, Walsh SR, Li ZY, Graves MJ, et al. Association between biomechanical structural stresses of atherosclerotic carotid plaques and subsequent ischaemic cerebrovascular events—a longitudinal in vivo magnetic resonance imaging-based finite element study. Eur J Vasc Endovasc Surg 2010; 40: 485–91. doi: 10.1016/j.ejvs.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 49.Teng Z, Sadat U, Huang Y, Young VE, Graves MJ, Lu J, et al. In vivo MRI-based 3D mechanical stress-strain profiles of carotid plaques with juxtaluminal plaque haemorrhage: an exploratory study for the mechanism of subsequent cerebrovascular events. Eur J Vasc Endovasc Surg 2011; 42: 427–33. doi: 10.1016/j.ejvs.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 50.U-King-Im JM, Young V, Gillard JH. Carotid-artery imaging in the diagnosis and management of patients at risk of stroke. Lancet Neurol 2009; 8: 569–80. doi: 10.1016/S1474-4422(09)70092-4 [DOI] [PubMed] [Google Scholar]
- 51.Richardson PD, Davies MJ, Born GV. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet 1989; 2: 941–4. doi: 10.1016/s0140-6736(89)90953-7 [DOI] [PubMed] [Google Scholar]
- 52.Tang D, Teng Z, Canton G, Yang C, Ferguson M, Huang X, et al. Sites of rupture in human atherosclerotic carotid plaques are associated with high structural stresses: an in vivo MRI-based 3D fluid-structure interaction study. Stroke 2009; 40: 3258–63. doi: 10.1161/strokeaha.109.558676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teng Z, Canton G, Yuan C, Ferguson M, Yang C, Huang X, et al. 3D critical plaque wall stress is a better predictor of carotid plaque rupture sites than flow shear stress: an in vivo MRI-based 3D FSI study. J Biomech Eng 2010; 132: 031007. doi: 10.1115/1.4001028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li ZY, Howarth SP, Tang T, Graves MJ, U-King-Im J, Trivedi RA, et al. Structural analysis and magnetic resonance imaging predict plaque vulnerability: a study comparing symptomatic and asymptomatic individuals. J Vasc Surg 2007; 45: 768–75. doi: 10.1016/j.jvs.2006.12.065 [DOI] [PubMed] [Google Scholar]
- 55.Li ZY, Tang T, U-King-Im J, Graves M, Sutcliffe M, Gillard JH. Assessment of carotid plaque vulnerability using structural and geometrical determinants. Circ J 2008; 72: 1092–9. doi: 10.1253/circj.72.1092 [DOI] [PubMed] [Google Scholar]
- 56.Sadat U, Li ZY, Young VE, Graves MJ, Boyle JR, Warburton EA, et al. Finite element analysis of vulnerable atherosclerotic plaques: a comparison of mechanical stresses within carotid plaques of acute and recently symptomatic patients with carotid artery disease. J Neurol Neurosurg Psychiatry 2010; 81: 286–9. doi: 10.1136/jnnp.2009.190363 [DOI] [PubMed] [Google Scholar]
- 57.Sadat U, Teng Z, Young VE, Graves MJ, Gaunt ME, Gillard JH. High-resolution magnetic resonance imaging-based biomechanical stress analysis of carotid atheroma: a comparison of single transient ischaemic attack, recurrent transient ischaemic attacks, non-disabling stroke and asymptomatic patient groups. Eur J Vasc Endovasc Surg 2011; 41: 83–90. doi: 10.1016/j.ejvs.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 58.Zhu C, Teng Z, Sadat U, Young VE, Graves MJ, Li ZY, et al. Normalized wall index specific and MRI-based stress analysis of atherosclerotic carotid plaques: a study comparing acutely symptomatic and asymptomatic patients. Circ J 2010; 74: 2360–4. doi: 10.1253/circj.CJ-10-0305 [DOI] [PubMed] [Google Scholar]
- 59.Gao H, Long Q, Kumar Das S, Halls J, Graves M, Gillard JH, et al. Study of carotid arterial plaque stress for symptomatic and asymptomatic patients. J Biomech 2011; 44: 2551–7. doi: 10.1016/j.jbiomech.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 60.Chandratheva A, Mehta Z, Geraghty OC, Marquardt L, Rothwell PM; Oxford Vascular Study. Population-based study of risk and predictors of stroke in the first few hours after a TIA. Neurology 2009; 72: 1941–7. doi: 10.1212/WNL.0b013e3181a826ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000; 284: 2901–6. doi: 10.1001/jama.284.22.2901 [DOI] [PubMed] [Google Scholar]
- 62.Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369: 283–92. doi: 10.1016/S0140-6736(07)60150-0 [DOI] [PubMed] [Google Scholar]
- 63.Coull AJ, Lovett JK, Rothwell PM; Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004; 328: 326. doi: 10.1136/bmj.37991.635266.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stroke Association (Great Britain), Intercollegiate Stroke Working Party, Royal College of Physicians. National clinical guidelines for stroke. London, UK: Royal College of Physicians; 2004. [Google Scholar]
- 65.Qiao Y, Farber A, Semaan E, Hamilton JA. Images in cardiovascular medicine. Healing of an asymptomatic carotid plaque ulceration. Circulation 2008; 118: e147–8. doi: 10.1161/CIRCULATIONAHA.108.764779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Gils MJ, Homburg PJ, Rozie S, de Weert TT, Dippel DW, van der Lugt A. Evolution of atherosclerotic carotid plaque morphology: do ulcerated plaques heal? A serial multidetector CT angiography study. Cerebrovasc Dis 2011; 31: 263–70. doi: 10.1159/000322152 [DOI] [PubMed] [Google Scholar]
- 67.Teng Z, Degnan AJ, Sadat U, Wang F, Young VE, Graves MJ, et al. Characterization of healing following atherosclerotic carotid plaque rupture in acutely symptomatic patients: an exploratory study using in vivo cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2011; 13: 64. doi: 10.1186/1532-429x-13-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X, Kramer MC, Van der Loos CM, Ploegmakers HJ, DE Boer OJ, Koch KT, et al. Early onset of endothelial cell proliferation in coronary thrombi of patients with an acute myocardial infarction: implications for plaque healing. J Thromb Haemost 2012; 10: 466–73. doi: 10.1111/j.1538-7836.2012.04620.x [DOI] [PubMed] [Google Scholar]
- 69.Teng Z, Sadat U, Wang W, Bahaei NS, Chen S, Young VE, et al. Intraplaque stretch in carotid atherosclerotic plaque—an effective biomechanical predictor for subsequent cerebrovascular ischemic events. PLoS One 2013; 8: e61522. doi: 10.1371/journal.pone.0061522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teng Z, He J, Degnan AJ, Chen S, Sadat U, Bahaei NS, et al. Critical mechanical conditions around neovessels in carotid atherosclerotic plaque may promote intraplaque hemorrhage. Atherosclerosis 2012; 223: 321–6. doi: 10.1016/j.atherosclerosis.2012.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klink A, Lancelot E, Ballet S, Vucic E, Fabre JE, Gonzalez W, et al. Magnetic resonance molecular imaging of thrombosis in an arachidonic acid mouse model using an activated platelet targeted probe. Arterioscler Thromb Vasc Biol 2010; 30: 403–10. doi: 10.1161/atvbaha.109.198556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.te Boekhorst BC, Bovens SM, Hellings WE, van der Kraak PH, van de Kolk KW, Vink A, et al. Molecular MRI of murine atherosclerotic plaque targeting NGAL: a protein associated with unstable human plaque characteristics. Cardiovasc Res 2011; 89: 680–8. doi: 10.1093/cvr/cvq340 [DOI] [PubMed] [Google Scholar]
- 73.Makowski MR, Forbes SC, Blume U, Warley A, Jansen CH, Schuster A, et al. In vivo assessment of intraplaque and endothelial fibrin in ApoE(-/-) mice by molecular MRI. Atherosclerosis 2012; 222: 43–9. doi: 10.1016/j.atherosclerosis.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 74.Wen S, Liu DF, Liu Z, Harris S, Yao YY, Ding Q, et al. OxLDL-targeted iron oxide nanoparticles for in vivo MRI detection of perivascular carotid collar induced atherosclerotic lesions in ApoE-deficient mice. J Lipid Res 2012; 53: 829–38. doi: 10.1194/jlr.M018895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.von Elverfeldt D, von zur Muhlen C, Wiens K, Neudorfer I, Zirlik A, Meissner M, et al. In vivo detection of activated platelets allows characterizing rupture of atherosclerotic plaques with molecular magnetic resonance imaging in mice. PLoS One 2012; 7: e45008. doi: 10.1371/journal.pone.0045008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ryu CW, Kwak HS, Jahng GH, Lee HN. High-resolution MRI of intracranial atherosclerotic disease. Neurointervention 2014; 9: 9–20. doi: 10.5469/neuroint.2014.9.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]