Abstract
Comprehensive and precise assessment of left ventricular (LV) systolic and diastolic function is necessary to establish, or exclude, heart failure as a cause or component of dyspnea. Echocardiography with Doppler readily assesses LV diastolic function; advantages include that echocardiography is non-invasive, does not require radiation, is portable, rapid, readily available, and in competent hands, can provide an accurate and comprehensive assessment of LV systolic and diastolic function. Correct assessment of LV diastolic function is relevant in patients with both depressed and preserved LV ejection fraction (EF ≥ 50%, and < 50%, respectively). Tissue Doppler (TD) imaging has been useful in demonstrating impaired LV relaxation in the setting of preserved LVEF, which, in the setting of increased cardiac volume, can result in elevated LV filling pressures, and dyspnea due to diastolic heart failure. TD imaging is not always critical in patients with depressed LVEF, since such patients by definition have impaired LV relaxation, and thus significant increases in volume will result in increases in LV filling pressure due to impaired LV compliance. Thus, in depressed LVEF, transmitral flow velocities (E and A, and E/A) and deceleration time, pulmonary venous Doppler, left atrial volume, and pulmonary artery (PA) pressures suffice for the accurate assessment of LV filling pressures. Overall, diastolic assessment by echo-Doppler can be readily achieved in by using a comprehensive diastolic assessment—incorporating many 2-dimensional, conventional and tissue Doppler variables—as opposed to relying on any single, diastolic parameter, which can lead to errors.
Background
Echocardiography with Doppler readily assesses LV diastolic function; advantages include that echocardiography is non-invasive, does not require radiation, is portable, rapid, readily available, and in competent hands, can provide an accurate and comprehensive assessment of LV systolic and diastolic function. Correct assessment of LV diastolic function is relevant in patients with both depressed and preserved LV ejection fraction (EF ≥ 50%, and < 50%, respectively). Tissue Doppler (TD) imaging has been useful in demonstrating impaired LV relaxation in the setting of preserved LVEF, which, in the setting of increased cardiac volume, can result in elevated LV filling pressures, and dyspnea due to diastolic heart failure. TD imaging is not always critical in patients with depressed LVEF, since such patients by definition have impaired LV relaxation, and thus significant increases in volume will result in increases in LV filling pressure due to impaired LV compliance. Thus, in depressed LVEF, transmitral flow velocities (E and A, and E/A) and deceleration time, pulmonary venous Doppler, left atrial volume, and pulmonary artery (PA) pressures suffice for the accurate assessment of LV filling pressures. Overall, diastolic assessment by echo-Doppler can be readily achieved in by using a comprehensive diastolic assessment—incorporating many 2-dimensional, conventional and tissue Doppler variables—as opposed to relying on any single, diastolic parameter, which can lead to errors.
Two-dimensional echocardiography: Left ventricular mass and wall motion, and left atrial size
The following criteria are needed for the diagnosis of diastolic heart failure (DHF): clinical picture consistent with HF, demonstration of preserved left ventricular ejection fraction (LVEF), and demonstration of diastolic dysfunction.1 Clinically, diastolic dysfunction, secondary to impaired LV relaxation and increased LV stiffness, is usually demonstrated by echocardiography and Doppler.2–6 The best correlate of symptoms and survival in DHF is elevation of left atrial (or left ventricular filling) pressure, readily estimated using comprehensive echocardiography with Doppler.1,2 Demonstration of preserved LVEF is readily demonstrated with 2-dimensional echocardiography.7 It should be noted that DHF is a term used relatively interchangeably with “HF with preserved LVEF” and “HF with normal LVEF”.
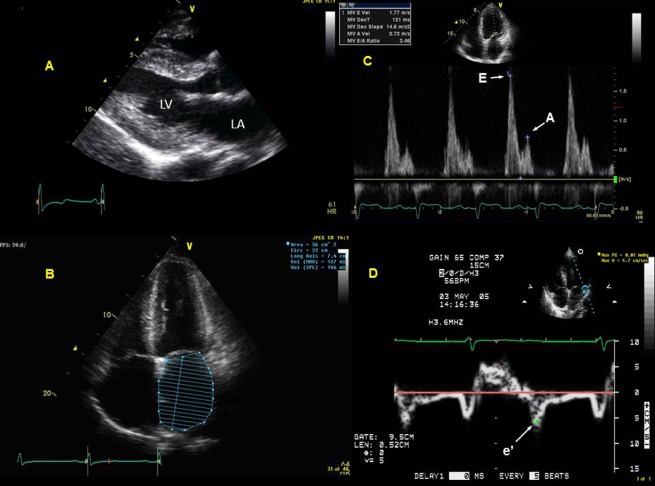
In patients with heart failure and preserved LVEF, LVEF is preserved ≥ 50%, yet, left atrial pressures—synonymous with LV filling pressures in the absence of obstructive MV disease—are elevated, causing increased pulmonary venous pressures and dyspnea at rest or during exertion.1–6 In order for LA pressures to be elevated in the absence of significantly depressed LVEF, LV relaxation and compliance generally are depressed, most often occurring in hypertensive or ischemic heart disease.2–5 2-dimensional echocardiography, therefore, identifies LV abnormalities that create the substrate for LV diastolic dysfunction: LV hypertrophy and LV wall motion abnormalities. Increased LV mass ( ≥ 90 g/m2 for women and ≥ 115 g/m2 for men, i.e. LV hypertrophy) is common in patients with DHF5 (Figure 1). Previous studies have correlated increasing degrees of LV mass with increasing LV diastolic dysfunction and filling pressures.8 In addition, since LVEF can be preserved even in presence of significant coronary artery disease, LV wall motion abnormalities create the substrate for significant LV diastolic dysfunction even in the patient with preserved LVEF who may have a diagnosis of DHF. Therefore, accurate identification of LV wall motion abnormalities is of great importance in the assessment of the patient with potential diastolic dysfunction. Since LA pressures are elevated in patients with significant diastolic dysfunction in the presence of increased preload, and since the LA cannot adequately empty in to the LV during diastole in this hemodynamic scenario, LA enlargement ( ≥ 30 ml/m2) is usually seen5 (Figure 1).
Figure 1.
Comprehensive diastolic assessment with echocardiography in a patient with diastolic heart failure: Chronic hypertension is a common scenario for the development of diastolic dysfunction, and the hypertrophied left ventricle (LV) develops impaired relaxation, and in the right loading conditions, can result in elevated left atrial (LA) pressure. This patient with chronic hypertension presented with dyspnea. Echocardiography revealed concentric LV hypertrophy (LV mass index was 119 g/m2, Panel A) with preserved LV ejection fraction of 62%. The patient also has severely dilated left atrium (LA) from chronic elevation in LA pressures in the setting of left ventricular hypertrophy from chronic hypertension (Panel B). This patient had severe LA enlargement, with an unindexed LA volume of 137 ml and an indexed volume of 72 ml/m2. Panel C shows restrictive diastolic filling pattern in the transmitral spectral Doppler with E/A = 2.46 and deceleration time = 121 ms, reflective of significantly elevated LV filling pressures. Panel D shows tissue Doppler at the lateral LV mitral annulus with a depressed e′ velocity of 5.7 cm/s, indicating impaired LV relaxation; when e′ is divided into the transmitral E velocity of 177 cm/s, this results in an elevated E/e′ of 31, confirming significantly elevated LV filling pressures.
Increasing LA size correlates with increasing LV filling pressures and worse outcome in patients with diastolic HF.9 In addition, LA size has been called the barometer of LV diastolic dysfunction or LV filling pressures, although certainly other entities, such as atrial fibrillation or chronic hypertension, can result in LA enlargement in absence of significant elevation of LA pressure.6 It has therefore been said that LA volume has a better negative—as opposed to positive—predictive value for significant diastolic dysfunction and heart failure; that is, a normal or small LA largely excludes significantly elevated LA pressure, while a large LA volume may occur in the absence of significant LA dilation.10 Studies have also shown that LA volume is a much better measurement of LA enlargement than a simple antero-posterior diameter, and therefore is the recommended way to measure LA size by echocardiography.6 It is also important to integrate 2-dimensional echocardiographic variables in the assessment of diastolic function; for instance, in cases of ischemic or infiltrative heart disease, significant LV hypertrophy may be absent, yet LA volumes are often enlarged.5
Diastolic dysfunction and demonstration of elevated LV filling pressures
Transmitral Doppler
Transmitral Doppler (“mitral inflow pattern”) is essential for the assessment of LV filling pressures. Early mitral filling depends on intrinsic LV relaxation, and the difference between LA and LV early (or “opening”) diastolic pressure.5 In a healthy, young heart with normal, rapid diastolic suction, the LV literally “sucks” blood into the LV, resulting in rapid LA emptying. In this scenario, there is a relatively tall E wave, and a shorter A (late diastolic or “atrial contraction” wave) (Figure 3). In an LV with impaired relaxation but normal LV filling pressures, there is no rapid LV diastolic suction, thus LA emptying is more gradual, and results in a relatively low velocity E wave; LA emptying is therefore dependent on LA contraction, and results in a relatively high amplitude A wave. In the setting of impaired LV relaxation and mildly elevated LA pressure, high LA pressure that “drives” open the MV, resulting in a large E wave and smaller A wave; this pattern is termed “pseudonormalization”. In markedly elevated LV filling pressure in which LV stiffness is high, the MV is forced open early due to high LA pressure, but there is rapid equilibration with the high resting LV diastolic pressure resulting in a rapid deceleration time of E. This pattern is termed “restrictive filling” (Figure 1).3–5 The grades of diastolic function, as assessed using comprehensive echo-Doppler examination, are shown in Figure 2.
Figure 3.
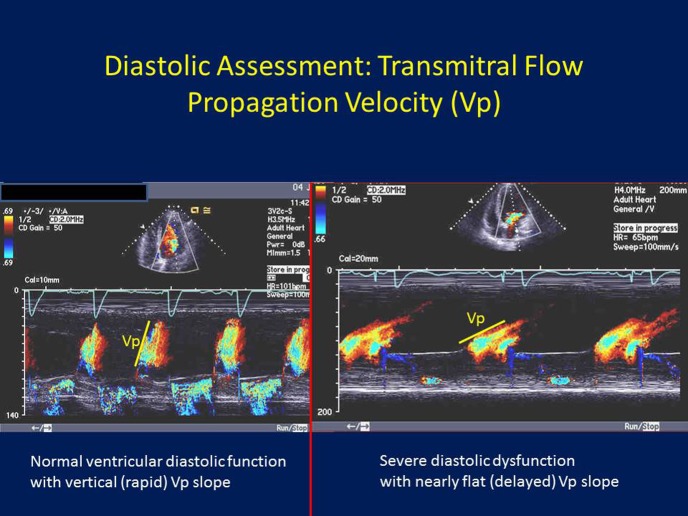
Diastolic assessment: Transmitral flow propagation velocity (Vp): Placing the color Doppler sample volume from the mitral annular level to the left ventricular (LV) apex, the more rapid the LV relaxation, the faster blood travels from the mitral annular level to the LV apex, and hence the more vertical the color Doppler mitral inflow m-mode and the more rapid the Vp slope (left panel). On the other hand, the more impaired the LV relaxation, the slower it take for blood to go from the mitral annular to the LV apex, hence a “flatter” Vp slope (right panel).
Figure 2.
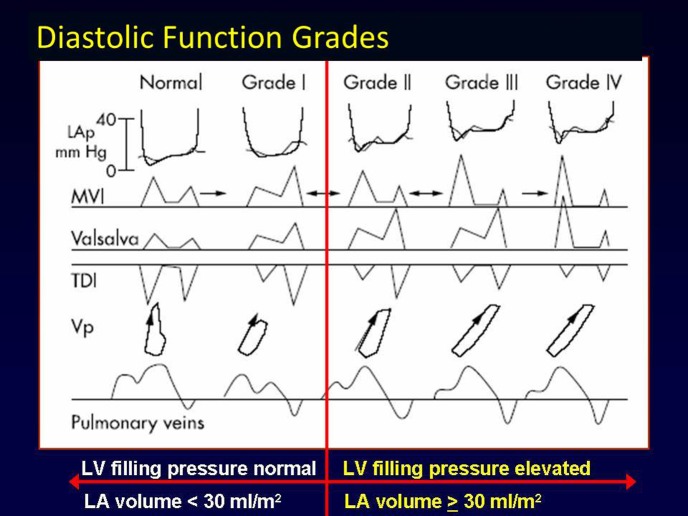
Diastolic function grades: Left ventricular (LV) diastolic function ranges from normal (Grade 0) to impaired relaxation (Grade I), to pseudonormal (Grade II), to restrictive (Grade III), and irreversibly restrictive (Grade IV). LV relaxation and left atrial pressures (LAp) increase from Grades 0 to IV, as does LA volume. Mitral valve inflow (MVI), tissue Doppler imaging, Valsalva manoeuver, flow propagation velocity (Vp) and pulmonary venous flow are all helpful in distinguishing Grades of LV diastolic function, and should be used together for an integrated approach to the assessment of diastolic function as recommended in current guidelines (Figure adapted from Reference [31]).
Valsalva manoeuver
The Valsalva manoeuver, in which the patient forces expiration against a closed glottis, there is increased intrathoracic pressure which results in decrease in right heart filling which by definition, results in decreased LV filling (decreased preload). Since a pseudonormal filling pattern exists in the setting of elevated LA pressure in the presence of impaired LV relaxation, this decrease in preload lowers LA pressure, which then “unmasks” the underlying impaired relaxation pattern (that is, E>A in the setting of impaired relaxation and with Valsalva manoeuver changes the transmitral pattern to E < A) (Figure 5). On the other hand, in the setting of normal diastolic function, the decrease in preload resulting from the Valsalva manoeuver preserves the E>A pattern, without changing it to E < A. Therefore, one of the main uses of the Valsalva manoeuver—similar to tissue Doppler e′—is to help distinguish normal from pseudonormal filling pattern. In the presence of a restrictive filling pattern, the Valsalva manoeuver will decrease preload and therefore help distinguish irreversible restrictive filling pattern (in which E≫A will not change) from reversible restrictive filling, where decreased preload changes the restrictive filling pattern to either pseudonormal (E>A) or impaired relaxation pattern (E < A), since the increased intrathoracic pressure resulting from Valsalva decreases LA pressure.
Figure 5.
An integrated approach to the assessment of left ventricular diastolic function: Normal LV ejection fraction: As recommended in current guidelines, use of multiple echo-Doppler parameters results in a more accurate assessment of left ventricular (LV) diastolic function than using any single echo-Doppler parameter in isolation. In the patient with normal LV ejection fraction (EF), it is reasonable to start with early transmitral diastolic velocity/tissue Doppler early diastolic velocity (E/e′), as it can be difficult to discern whether a patient with preserved LVEF has impaired or normal LV relaxation. Following E/e′, other echo-Doppler variables are added to result in an accurate assessment of LV diastolic function (From Reference [5]).
Tissue Doppler imaging
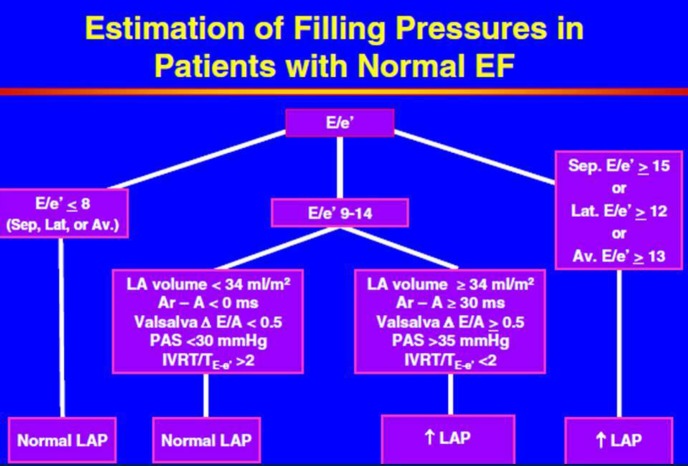
Tissue Doppler early diastolic mitral annular velocity (e′), which is relatively non-load dependent in patients with cardiac disease, is generally thought to be the best non-invasive estimate of LV relaxation.11–14 The longer it takes for the LV to relax, the lower is the e′ velocity. The resulting E/e′ ratio, has been validated as a reasonably reliable non-invasive indicator of LV filling pressure in patients with preserved or depressed LVEF (Figure 1).11–14 It should be mentioned that one study, performed in the intensive care unit in patients in decompensated heart failure, questioned the correlation of E/e′ and LV filling pressures.15 In patients with normal hearts, E/e′ does not accurately predict LV filling pressure due to correlation of e′ with LV filling pressures in such subjects, as opposed to lack of such a correlation in patients with cardiac disease.16 The E/e′ ratio has since been demonstrated to be useful in estimating LV filling pressures in hypertrophic cardiomyopathy,17 sinus tachycardia,18 atrial fibrillation,19 and post-cardiac transplantation.20 An important consideration when using E/e′ is the “grey zone” of indeterminate values: in general, in patients with preserved LVEF and using e′ averaged from the mitral septal and lateral annuli, if E/e′ ≤ 8, LV filling pressures are likely normal; if E/e′ ≥ 13, LV filling pressures are likely elevated;5 however, between 9 and 12, E/e′ can be indeterminate. In such cases—in addition to incorporating other echo-Doppler parameters (LA volume, pulmonary venous Doppler, PA pressure)—use of B-type natriuretic peptide (BNP) and N-terminal pro-BNP can be useful to help decide if LV filling pressures are indeed elevated or not.21,22 The E/A ratio, mitral deceleration time, and E/e′ ratio have all been shown to be useful echocardiographic indicator of LV diastolic dysfunction, and more particularly, of elevated LV filling pressures.5 These variables have also been shown to be markers of outcome in patients with HF.9,23
Transmitral color Doppler flow propagation velocity
Color Doppler m-mode imaging can be applied in the apical views to semi-quantitate the velocity and rate of blood flow from the mitral valve annulus to the LV apex (Figure 3). In this way, the early diastolic filling wave by color m-mode, which appears in red color as blood flow from the mitral valve level to the LV apex can be identified. The slope of this early diastolic color m-mode wave (Vp), is rapid (vertical) in patients with normal diastolic function due to rapid diastolic suction in which blood quickly flow from MV to LV apex. However, in the presence of increasingly impaired relaxation, this slope become flatter and flatter, reflecting increasingly impaired LV relaxation. A ratio, E/Vp, similar to E/e, has therefore been developed and validated, and correlates to mean LA pressure.24 An E/Vp > 15 reasonably correlates with PCWP >15 mmHg, although there are many hemodynamic, rhythm and myocardial motion variables which can impact this relationship. Furthermore, some studies have shown that, in comparison to invasive measurement of LV filling pressures, E/e′ appears more accurate than E/Vp.25
Spectral Doppler pulmonary venous flow
In the normal heart with rapid ventricular suction, the diastolic pulmonary venous wave is augmented, due to rapid flow through the pulmonary veins into the LA, through the mitral valve, and into the LV. Therefore, in the completely normal heart, the dominant PV diastolic wave corresponds to the dominant transmitral E wave, and is a sign of normal LV lussotropic function; the completely normal heart therefore has PV S < D. However, when LV relaxation becomes impaired, PV flow during LV diastole becomes truncated, and therefore most filling occurs during LV systole, resulting in S>D, which corroborates to transmitral E < A (Grade I diastolic dysfunction).26 When LA pressure becomes elevated, PV flow during LV systole decreases, as LA pressure in the setting of a closed MV, prevents normal PV flow, and PV flow becomes higher in diastolic when the mitral valve opens, relieving elevated LA pressure; this results in PV S < D, corresponding to E>A (Grade II diastolic dysfunction). Therefore, PV flow is subject to pseudonormalization in the same way as transmitral flow. In restrictive filling, the PV pseudonormal pattern becomes more exaggerated, with S≪D, with a rapid deceleration time of D. While the PV S/D ratio reflects mean LA pressure, the PV atrial reversal wave reflects LV end-diastolic pressure, as, if the LA contracts against the high diastolic pressure in the LV, blood will preferentially flow backwards into the PV, as opposed to transmitrally into the LV. Therefore, PV Ar wave is higher and longer than transmitral A, rendering PV Ar-A a measure of LV end-diastolic pressure.
Tricuspid regurgitation velocity for pulmonary artery pressure estimation
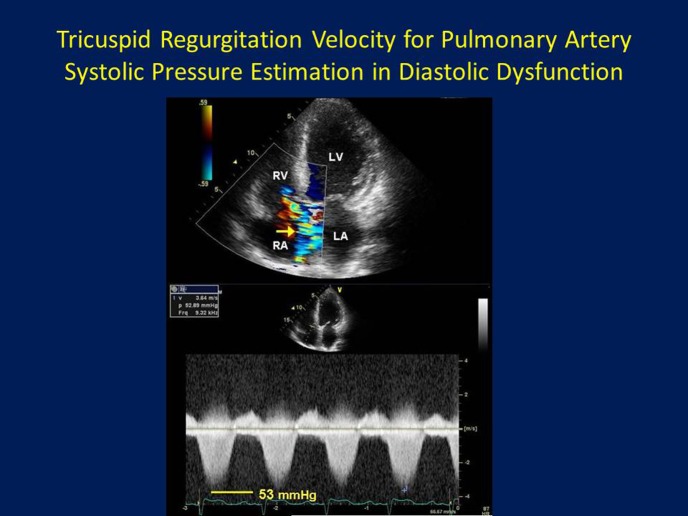
In the setting of high LA pressure in the patient with diastolic heart failure, pressure is transmitted back into the pulmonary veins, and across the pulmonary venous-capillary bed into the pulmonary arterioles and into the pulmonary arteries; therefore, pulmonary arterial hypertension (PAH) can result.27 In this way, PA pressure estimated by addition of an estimate of RA pressure (by assessing IVC size and response to respiration), is a good surrogate marker of significant, and often chronic, LA pressure elevation (Figure 4). As recommended by guidelines, PASP > 35 mmHg often accompanies advanced or significant LV diastolic dysfunction with elevated LA pressures.5 However, as with LA volume, the presence of significant PAH does not necessarily mean significant diastolic dysfunction, as significant elevations in pulmonary vascular resistance (PVR) due to intrinsic lung disease must first be excluded. Likewise, normal PASP can be helpful in excluding significant and long standing LA pressure elevation, provided a complete TR jet is obtained with correct Doppler sample volume angulation in respect of the direction of TR, and with correct RA pressure estimation. PA end-diastolic pressure, which in the absence of significant elevations in PVR can be a good estimate of mean LA pressure, can be estimated from the pulmonary regurgitation diastolic wave, with RA pressure then added to it as is done with PASP.28
Figure 4.
Tricuspid regurgitation velocity for pulmonary artery systolic pressure estimation in diastolic dysfunction: In patients with significant left ventricular (LV) diastolic dysfunction with chronically elevated LV filling pressures, back-pressure through the left atrium (LA), into the pulmonary veins, and across the pulmonary venous-capillary bed into the pulmonary arterioles and pulmonary arteries (PA), results in elevation of PA pressure. Thus, PA systolic pressure elevation, in the absence of significant intrinsic lung disease and resultant elevated pulmonary vascular resistance (PVR), is a reasonable correlate of elevated LA pressures. PA systolic pressure can be estimated by Doppler using the tricuspid regurgitation peak systolic velocity and adding to it an estimate of right atrial (RA) pressure. This image shows a TR velocity of 3.64 m/s, equivalent to a TR systolic pressure of 53 mmHg, which indicates at least moderate PA hypertension in patient with chronically elevated LA pressure due to ischemic cardiomyopathy and diastolic dysfunction. RV = right ventricular.
The patient with atrial fibrillation and other special situations: Diastolic assessment
It may be challenging to accurately assess LV diastolic function by echocardiography in patients with atrial fibrillation. Owing to elevated heart rate, irregular R-R intervals, and loss of atrial contraction, echo-Doppler assessment can at times be problematic. However, mitral DT < 150 ms, lack of variation in E wave velocity despite varying R-R intervals (as there remains an elevated opening gradient between the LA and LV at mitral opening—early diastolic filling E wave—despite longer diastolic filling periods when the LA pressure should decrease), IVRT < 65 ms, elevation of E/e′ (>11), and presence of pulmonary hypertension in the absence of lung disease, are all clues to the presence of elevated LA pressure in the setting of AF.5,29,30 The E/e′ ratio can also be used in patients with AF, although with somewhat lower accuracy than in patients in sinus rhythm, for the estimation of LV filling pressures, as long as >5 cardiac cycles are used and averaged (which holds for any Doppler parameter in AF).19 In patients who are in supraventricular tachycardia, atrial flutter, paced rhythm or heart block, LV diastolic assessment can be very difficult, although presence of both significant LA enlargement and pulmonary hypertension in the absence of lung disease can be an important clue to elevated LA pressures in these scenarios. Another unclear scenario is the effect of significant mitral regurgitation (MR) on e′ and the E/e′ ratio in estimating LV filling pressures. It has been shown that in patients with secondary MR (due to LV disease), E/e′ accurately predicted PCWP; however, in patients with primary MR (due to a primary mitral valve abnormality), E/e′ was not reliably predictive of PCWP.31
Diastolic stress echocardiography
Patients with LVEF ( ≥ 50%) who present with dyspnea—particularly exertional dyspnea—may not have significant LV diastolic dysfunction at rest. Yet, during exercise, increased heart rate, and peripheral muscle and central organ oxygen demand may cause LV diastolic decompensation, unmasking significant LV diastolic dysfunction with exertion not detectable at rest.32,33 In this way, transmitral Doppler parameters (E, A, E/A), E/e′, and Doppler-estimated pulmonary artery pressures, and their response to exertion, are valuables tools to diagnose exertional diastolic heart failure.34,35 Clinically speaking, elderly, hypertensive, diabetic, obese, and female patients are at highest risk of having exercise induced diastolic dysfunction, and are often candidates for diastolic stress echocardiography (DSE). Tables 1 and 2 illustrate, respectively, a suggested protocol for performance of DSE, and the changes in echo-Doppler variables that connote a positive test. It is important to note that patients with ≥ grade II diastolic dysfunction at rest (pseudonormal filling or greater) already have findings consistent with elevated LV filling pressures, and generally do not need to undergo diastolic stress echocardiography; therefore, patients with exertional dyspnea and normal or minimally elevated LV filling pressures are rest are those best considered for DSE.
Table 1.
Protocol for diastolic stress echocardiography
| 1. | Perform baseline echocardiogram to rule out other causes of exertional dyspnea (resting advanced diastolic dysfunction, depressed left ventricular ejection fraction (LVEF) and significant ( ≥ moderate) aortic or mitral valve disease) as these conditions can be cause on their own for dyspnea, and such patients generally do not undergo diastolic stress echocardiography. |
| 2. | At rest, obtain echocardiographic images of the LV and transmitral Doppler parameters (E, A, E/A), tissue Doppler early diastolic velocity (e′ and E/e′), and peak TR velocity. |
| 3. | Attach ECG electrodes to patient's chest for continuous ECG monitoring. |
| 4. | Obtain baseline blood pressure and heart rate. |
| 5. | Calculate target heart rate: 85% of (220 – patient's age). |
| 6. | Start exercise (either supine bicycle or treadmill). |
| 7. | Measure blood pressure at 3 minute intervals. |
| 8. | At target heart rate, obtain echocardiographic images of the LV and transmitral Doppler parameters (E, A, E/A), tissue Doppler early diastolic velocity (e′ and E/e′) and pulmonary artery pressure (by peak TR velocity) to assess for significant changes (see Table 2) |
A = late transmitral diastolic velocity; E = early transmitral diastolic velocity; TR = tricuspid regurgitation.
Table 2.
Echo-Doppler findings indicating a positive diastolic stress echocardiogram
| 1. | Increase (from rest) in the E velocity with exercise (to ≥ 80 m/s) |
| 2. | Increase in the E/A ratio with exercise (to ≥ 1) |
| 3. | Increase in E/e′ (to ≥ 12) |
| 4. | Increase in peak TR velocity (to ≥ 3.5 m/s). This finding must accompany one of the 3 above, to distinguish from exercise induced isolated pulmonary hypertension. |
A = late transmitral diastolic velocity; E = early transmitral diastolic velocity; TR = tricuspid regurgitation.
Multiple echocardiographic variables are needed for accurate diastolic assessment
It is critical to integrate several variables—2-dimensional, conventional and tissue Doppler—in order to arrive at a correct diastolic assessment, as opposed to relying on a single variable (such as LA size or E/e′) alone which can lead to errors (Table 3). Indeed, current guideline recommend and integrated approach of many diastolic variables (Figures 5 and 6), and data has shown that additional echocardiographic variables, when added to E/e′ can result in more accurate diastolic determination, compared to invasively measured LV filling pressures, than E/e′ alone.36 Not infrequently, echo-Doppler parameters appear to conflict: for instance, in a patient with normal LVEF, E/e′ = 13, but LA volume is not enlarged, E < A, and there are normal pulmonary pressures by Doppler. In such cases, the E/e′ ratio should likely be dropped, since all other variables point toward normal LV filling pressures. Therefore, as rule, multiple echo-Doppler parameters of LV diastolic function should be assessed in every patient,37 and the conclusion to which most parameters point, should be the overall diastolic assessment, with “outlying” parameters discarded. In most cases of conflicting echo-Doppler diastolic parameters, a cogent conclusion can be reached, although in some cases, the diastolic assessment may remain equivocal. Above all, no single diastolic parameter should be used in isolation to arrive at a diastolic conclusion in a given patient.5
Table 3.
Strengths and limitations of individual echo-Doppler variables for diastolic assessment
| Variable | Strength | Limitations |
| Left ventricular (LV) hypertrophy | A good morphologic marker of impaired relaxation | Prone to measurement error; absence of LVH does not guarantee normal relaxation |
| LV ejection fraction | If LVEF is depressed ( < 50%), there is, by definition, impaired LV relaxation present | For patients with preserved LVEF ( ≥ 50%), other markers of LV relaxation are required: e′, LV strain |
| Left atrial volume | Can be used as a “barometer of LV filling pressure” | Can be enlarged in situations where LV filling pressures are normal: atrial fibrillation |
| Mitral inflow (E, A, E/A, DT) | Very flow dependent, so provide “real-time” assessment of cardiac load | Prone to pseudo-normalization; thus, a load-independent measure of LV relaxation (e′, strain) is needed to interpret |
| Tissue Doppler early diastolic relaxation (e′ and E/e′) | Load-independent assessment of LV relaxation (e′); robust data on estimation of LV filling pressure and prognosis (E/e′) | Can be difficult in non-sinus rhythm, or in BBB; prone to spectral broadening; “grey zone” for E/e′ (9–13) |
| Flow Propagation Velocity (Vp) | Can provided reasonable estimated of LV relaxation (Vp) and LV filling pressure (E/Vp) | Not as dependable in patients with preserved LVEF |
| Pulmonary venous Doppler | Very flow dependent, so provide “real-time” assessment of cardiac load; S/D related to mean LA pressure; Ar-A duration to LVEDP | Prone to pseudo-normalization; thus, a load-independent measure of LV relaxation (e′, strain) is needed to interpret; difficult in non-sinus rhythm |
| Pulmonary artery pressure (by peak TR velocity) | Correlates relatively well with elevated LV filling pressure; good negative predictive value | Can be elevated in absence of elevated LV filling pressure: (primary pulmonary hypertension, cor pulmonale) |
| LV speckle strain | Can provide a non-Doppler assessment of LV relaxation; measurable in multiple vectors | Requires high 2D frame rate (40–70 fps), and specific echo equipment and expertise; inter-reader variability can be an issue |
A = late transmitral diastolic velocity; Ar = pulmonary venous atrial reversal; DT = mitral deceleration time; E = early transmitral diastolic velocity; LA = left atrial; TR = tricuspid regurgitation.
Figure 6.
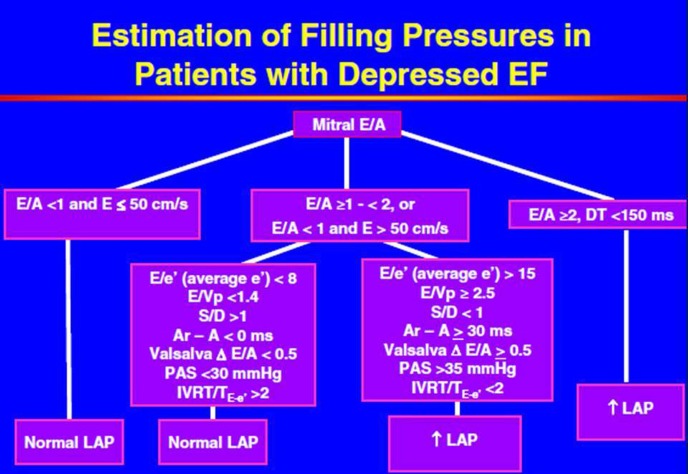
An integrated approach to the assessment of left ventricular diastolic function: Depressed ejection fraction: As recommended in current guidelines, use of multiple echo-Doppler parameters results in a more accurate assessment of left ventricular (LV) diastolic function than using any single echo-Doppler parameter in isolation. In the patient with depressed LV ejection fraction (EF), it is reasonable to start with early and late transmitral diastolic inflow velocities and deceleration time (E, A and DT, respectively), as it can assumed that patients with depressed LVEF ( < 50%) have, by definition, impaired LV relaxation. Following transmitral diastolic flow, other echo-Doppler variables are added to result in an accurate assessment of LV diastolic function (From Reference [5]).
Future directions
Speckle tracking echocardiography tracks signature gray-scale characteristics of points in the LV myocardium, thus providing information on displacement, velocity, deformation and deformation rate (strain and strain rate, respectively), independent of angulation and cardiac translational motion.38 Such non-Doppler-based 2D imaging variables have provided detailed information on myocardial mechanics in hypertensive heart disease, hypertrophic cardiomyopathy, diastolic and systolic LV failure, and well as in cases of pulmonary hypertension.39–41 Currently, such speckle-based measures are being studied to assess their role in identifying patient outcome in various heart failure states. One of the most attractive features of speckle tracking is that it can demonstrate the presence of systolic abnormalities, especially regional ones, in the presence of preserved LVEF in patients with cardiac disease.42 Speckle tracking can also demonstrate systolic and diastolic abnormalities in multiple vectors (longitudinal, radial, circumferential and rotational) as characteristic myocardial markers are tracked throughout the cardiac cycle and in space as the heart translates in the thoracic cavity. In particular, patients with diastolic dysfunction and DHF have been shown to have preserved LV twist (systole) and untwist (diastole) but impaired longitudinal strain, whereas patients with HF with depressed EF have impaired twist/untwist as well as depressed longitudinal and circumferential strain.38–42 Newer echocardiographic variables such as flow velocity fields derived from color sequences can measure intraventricular fluid mechanics, an important determinant of global chamber LV operative stiffness; in this way, impaired LV vortex generation can be identified, which has been shown to be a mechanism of LV diastolic dysfunction.43 Finally, LA strain and strain rate, which are measures of LA deformation and deformation rate, respectively, have also been shown to be markers of early diastolic dysfunction, in both patients with heart failure with preserved LVEF44–46 and in hypertrophic cardiomyopathy.47
Conclusions
To arrive at an accurate assessment of cardiac diastolic function, comprehensive echocardiography with 2-dimensional imaging, spectral and color Doppler—as well as newer techniques speckle strain echocardiography—are utilized. This assessment, which includes LV mass and regional wall motion assessment, LA volume, transmitral, pulmonary venous, and tissue Doppler as well as estimation of PA systolic and diastolic pressures, can provide accurate assessments of diastolic function in the majority of patients. It is important to note that, as recommended in current guidelines, use of any single echo-Doppler diastolic variable (for example only E/e′) in isolation, can lead to errors. Therefore, it is of utmost importance that a comprehensive assessment of LV diastolic function include integration of all available 2D and Doppler, and tissue Doppler variables to arrive at the most accurate diastolic assessment.
References
- 1.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 2.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure – abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 3.Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol. 2006;47:500–506. doi: 10.1016/j.jacc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol. 2008;51:679–689. doi: 10.1016/j.jacc.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 5.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 6.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 7.Lang RM, Bierig M, Devereaux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Dokainish H, Sengupta R, Pillai M, Bobek J, Lakkis N. Assessment of left ventricular systolic function using echocardiography in patients with preserved ejection fraction and elevated diastolic pressures. Am J Cardiol. 2008;101:1766–1771. doi: 10.1016/j.amjcard.2008.02.070. [DOI] [PubMed] [Google Scholar]
- 9.Dokainish H, Zoghbi WA, Lakkis NM, Ambriz E, Patel R, Quinones MA, Nagueh SF. Incremental predictive power of B-type natriuretic peptide and tissue Doppler echocardiography in the prognosis of patients with congestive heart failure. J Am Coll Cardiol. 2005;45:1223–1226. doi: 10.1016/j.jacc.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Dokainish H, Zoghbi WA, Lakkis NM, Al-Bakshy F, Dhir M, Quinones MA, Nagueh SF. Optimal noninvasive assessment of left ventricular filling pressures: a comparison of tissue Doppler echocardiography and B-type natriuretic peptide in patients with pulmonary artery catheters. Circulation. 2004;109:2432–2439. doi: 10.1161/01.CIR.0000127882.58426.7A. [DOI] [PubMed] [Google Scholar]
- 11.Sohn D, Chai L, Lee D, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 12.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 13.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 14.Kasner M, Westermann D, Steendijk P. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 15.Mullens W, Borowski AG, Curtin RJ, Thomas JD, Tang WH. Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation. 2009;119:62–70. doi: 10.1161/CIRCULATIONAHA.108.779223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firstenberg MS, Levine BD, Garcia MJ, Greenberg NL, Cardon L, Morehead AJ, Zuckerman J, Thomas JD. Relationship of echocardiographic indices to pulmonary capillary wedge pressures in healthy volunteers. J Am Coll Cardiol. 2000;36:1664–1669. doi: 10.1016/s0735-1097(00)00909-8. [DOI] [PubMed] [Google Scholar]
- 17.Nagueh SF, Lakkis NM, Middleton KJ, Spencer WH, 3rd, Zoghbi WA, Quinones MA. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation. 1999;99:254–261. doi: 10.1161/01.cir.99.2.254. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quinones MA, Zoghbi WA. Middleton KJ, Quinones MA, Zoghbi WA. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue Doppler imaging. Circulation. 1998;98:1644–1650. doi: 10.1161/01.cir.98.16.1644. [DOI] [PubMed] [Google Scholar]
- 19.Sohn DW, Song JM, Zo JH, Chai IH, Kim HS, Chun HG, Kim HC. Mitral annulus velocity in the evaluation of left ventricular diastolic function in atrial fibrillation. J Am Soc Echocardiogr. 1999;12:927–931. doi: 10.1016/s0894-7317(99)70145-8. [DOI] [PubMed] [Google Scholar]
- 20.Sundereswaran L, Nagueh SF, Vardan S, Middleton KJ, Zoghbi WA, Quinones MA, Torre-Amione G. Estimation of left and right ventricular filling pressures after heart transplantation by tissue Doppler imaging. Am J Cardiol. 1998;82:352–357. doi: 10.1016/s0002-9149(98)00346-4. [DOI] [PubMed] [Google Scholar]
- 21.Grewal J, McKelvie R, Lonn E, Tait P, Carlsson J, Gianni M, Jarnert C, Persson H. BNP and NT-proBNP predict echocardiographic severity of diastolic dysfunction. Eur J Heart Fail. 2008;10:252–259. doi: 10.1016/j.ejheart.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Dokainish H, Zoghbi WA, Lakkis NM, Ambriz E, Patel R, Quinones MA, Nagueh SF. Incremental predictive power of B-type natriuretic peptide and tissue Doppler echocardiography in the prognosis of patients with congestive heart failure. J Am Coll Cardiol. 2005;45:1223–1226. doi: 10.1016/j.jacc.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007;49:1903–1914. doi: 10.1016/j.jacc.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 24.Garcia MJ, Ares MA, Asher C, Rodriguez L, Vandervoort P, Thomas JD. An index of early left ventricular filling that combined with pulsed Doppler peak E velocity may estimate capillary wedge pressure. J Am Coll Cardiol. 1997;29:448–454. doi: 10.1016/s0735-1097(96)00496-2. [DOI] [PubMed] [Google Scholar]
- 25.Rivas-Gotz C, Manolios M, Thohan V, Nagueh SF. Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. Am J Cardiol. 2003;91:780–784. doi: 10.1016/s0002-9149(02)03433-1. [DOI] [PubMed] [Google Scholar]
- 26.Appleton CP, Galloway JM, Gonzalez MS, Gaballa M, Basnight MA. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol. 1993;22:1972–1982. doi: 10.1016/0735-1097(93)90787-2. [DOI] [PubMed] [Google Scholar]
- 27.Neuman Y, Kotliroff A, Bental T, Siegel RJ, David D, Lishner M. Pulmonary artery pressure and diastolic dysfunction in normal left ventricular systolic function. Int J Cardiol. 2008;127:174–178. doi: 10.1016/j.ijcard.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Paraskevaidis IA, Tsiapras DP, Karavolias GK, Cokkinos P, Kremastinos DT. Doppler-derived left ventricular end-diastolic pressure prediction model using the combined analysis of mitral and pulmonary A waves in patients with coronary artery disease and preserved left ventricular systolic function. Am J Cardiol. 2002;90:720–724. doi: 10.1016/s0002-9149(02)02596-1. [DOI] [PubMed] [Google Scholar]
- 29.Nagueh SF, Kopelen HA, Quiñones MA. Assessment of left ventricular filling pressures by Doppler in the presence of atrial fibrillation. Circulation. 1996;94:2138–2145. doi: 10.1161/01.cir.94.9.2138. [DOI] [PubMed] [Google Scholar]
- 30.Al-Omari MA, Finstuen J, Appleton CP, Barnes ME, Tsang TS. Echocardiographic assessment of left ventricular diastolic function and filling pressure in atrial fibrillation. Am J Cardiol. 2008;101:1759–1765. doi: 10.1016/j.amjcard.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 31.Bruch C, Stypmann J, Gradaus R, Breithardt G, Wichter T. Usefulness of tissue Doppler imaging for estimation of filling pressures in patients with primary or secondary pure mitral regurgitation. Am J Cardiol. 2004;93:324–328. doi: 10.1016/j.amjcard.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Talreja DR, Nishimura RA, Oh JK. Estimation of left ventricular filling pressure with exercise by Doppler echocardiography in patients with normal systolic function: a simultaneous echocardiographic-cardiac catheterization study. J Am Soc Echocardiogr. 2007;20:477–479. doi: 10.1016/j.echo.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Grewal J, McCully RB, Kane GC, Lam C, Pellikka PA. Left ventricular function and exercise capacity. JAMA. 2009;301:286–294. doi: 10.1001/jama.2008.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ha JW, Oh JK, Pellikka PA, Ommen SR, Stussy VL, Bailey KR, Seward JB, Tajik AJ. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr. 2005;18:63–68. doi: 10.1016/j.echo.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 35.Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47:1891–1900. doi: 10.1016/j.jacc.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 36.Dokainish H, Nguyen JS, Sengupta R, Pillai M, Alam M, Bobek J, Lakkis N. Do additional echocardiographic variables increase the accuracy of E/e′ for predicting left ventricular filling pressure in normal ejection fraction? An echocardiographic and invasive hemodynamic study. J Am Soc Echocardiogr. 2010;23:156–161. doi: 10.1016/j.echo.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Ommen SR, Nishimura RA. A clinical approach to the assessment of left ventricular diastolic function by Doppler echocardiography: update 2003. Heart. 2003;89(Suppl 3):iii18–iii23. doi: 10.1136/heart.89.suppl_3.iii18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perk G, Tunick PA, Kronzon I. Non-Doppler two-dimensional strain imaging by echocardiography – from technical considerations to clinical applications. J Am Soc Echocardiogr. 2007;20:234–243. doi: 10.1016/j.echo.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008;29:1283–1289. doi: 10.1093/eurheartj/ehn141. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Left ventricular untwisting rate by speckle tracking echocardiography. Circulation. 2007;116:2580–2586. doi: 10.1161/CIRCULATIONAHA.107.706770. [DOI] [PubMed] [Google Scholar]
- 41.Dokainish H, Sengupta R, Pillai M, Bobek J, Lakkis N. Usefulness of new diastolic strain and strain rate indexes for the estimation of left ventricular filling pressure. Am J Cardiol. 2008;101:1504–1509. doi: 10.1016/j.amjcard.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen JS, Lakkis NM, Bobek J, Goswami R, Dokainish H. Systolic and diastolic myocardial mechanics in patients with cardiac disease and preserved ejection fraction: impact of left ventricular filling pressure. J Am Soc Echocardiogr. 2010;23:1273–1280. doi: 10.1016/j.echo.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Martínez-Legazpi P, Bermejo J, Benito Y, Yotti R, Pérez Del Villar C, González-Mansilla A, Barrio A, Villacorta E, Sánchez PL, Fernández-Avilés F, del Álamo JC. Contribution of the diastolic vortex ring to left ventricular filling. J Am Coll Cardiol. 2014;64:1711–1721. doi: 10.1016/j.jacc.2014.06.1205. [DOI] [PubMed] [Google Scholar]
- 44.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJ, Solomon SD. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–456. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurt M, Tanboga IH, Aksakal E, Kaya A, Isik T, Ekinci M, Bilen E. Relation of left ventricular end-diastolic pressure and N-terminal pro-brain natriuretic peptide level with left atrial deformation parameters. Eur Heart J Cardiovasc Imaging. 2012;13:524–530. doi: 10.1093/ejechocard/jer283. [DOI] [PubMed] [Google Scholar]
- 46.Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Tsioulpas C, Bernazzali S, Maccherini M. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound. 2010:8–14. doi: 10.1186/1476-7120-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badran HM, Soltan G, Hassan H, Nazmy A, Faheem N, Saadan H, Yacoub MH. Changes in left atrial deformation in hypertrophic cardiomyopathy: Evaluation by vector velocity imaging. Glob Cardiol Sci Pract. 2013;2012:67–80. doi: 10.5339/gcsp.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]