Significance
Division of bacteria is executed by a contractile ring whose cytoskeletal framework is FtsZ (filamentation temperature-sensitive Z), a protein evolutionarily related to eukaryotic tubulin. The FtsZ ring is made of filaments of head-to-tail FtsZ subunits but its architecture and the rules governing its assembly are still poorly known. Here we show that MciZ, an inhibitor of FtsZ ring formation, functions by capping the minus end of FtsZ filaments. Capping by MciZ makes FtsZ filaments shorter than normal, likely by blocking filament annealing; this represents fundamental information to understand how FtsZ filaments grow and shrink, and attain their normal size. The powerful inhibition of Z-ring assembly by MciZ also suggests that an FtsZ ring cannot form from filaments smaller than a certain size.
Keywords: FtsZ, cell division, cytokinesis, filament capping, bacterial cytoskeleton
Abstract
Cytoskeletal structures are dynamically remodeled with the aid of regulatory proteins. FtsZ (filamentation temperature-sensitive Z) is the bacterial homolog of tubulin that polymerizes into rings localized to cell-division sites, and the constriction of these rings drives cytokinesis. Here we investigate the mechanism by which the Bacillus subtilis cell-division inhibitor, MciZ (mother cell inhibitor of FtsZ), blocks assembly of FtsZ. The X-ray crystal structure reveals that MciZ binds to the C-terminal polymerization interface of FtsZ, the equivalent of the minus end of tubulin. Using in vivo and in vitro assays and microscopy, we show that MciZ, at substoichiometric levels to FtsZ, causes shortening of protofilaments and blocks the assembly of higher-order FtsZ structures. The findings demonstrate an unanticipated capping-based regulatory mechanism for FtsZ.
The discovery that bacteria have actin-, tubulin-, and intermediate filament-like proteins demonstrated that the cytoskeleton is an ancient invention, predating the divergence between prokaryotes and eukaryotes (1). The GTPase FtsZ (filamentation temperature-sensitive Z) was the first prokaryotic protein to be recognized as a cytoskeletal element (2, 3). FtsZ is a tubulin-like protein, which is widely conserved in bacteria and the main component of the bacterial cytokinesis machine, or “divisome.” FtsZ self-assembles into single-stranded protofilaments and these associate further inside cells to form a superstructure known as the Z ring (4, 5). FtsZ alone can generate a constriction force to initiate division (6). The Z ring also provides a scaffold onto which several other components of the divisome—mostly cell wall synthesizing enzymes—are recruited and oriented so as to build the division septum, a cross-wall separating a progenitor cell into two isogenic daughter cells (7).
FtsZ and tubulin share several essential properties: their assembly is cooperative, stimulated by GTP, and leads to GTP hydrolysis; they form dynamic polymers whose turnover is dependent on nucleotide hydrolysis (8); they use essentially the same bond for polymer formation (9); and recent evidence indicates that they undergo similar allosteric transitions upon polymerization (10, 11). Not surprisingly, however, the functional specialization of these proteins led to some significant differences between them, the most prominent being that FtsZ exists as single protofilaments, whereas tubulin always adopts a multifilament tubular structure. This difference in their higher-order structure implies that the reactions that lead to cooperativity and subunit turnover are likely different. It has also represented a significant technical challenge for the study of FtsZ. Because FtsZ filaments are smaller than the resolution of optical microscopy, so far it has been impossible to determine essential properties associated with its dynamic behavior.
Similarly to actin filaments and microtubules, the assembly of FtsZ protofilaments into a Z ring is regulated by a number of proteins that bind directly to FtsZ and modulate its polymerization (4). Among these proteins, negative modulators have attracted the most attention because of their crucial role in determining when and where a Z ring should form. The strikingly precise positioning of the division site in rod-shaped bacteria, such as Escherichia coli and Bacillus subtilis, is because of the combined action of Min and nucleoid occlusion systems, two negative modulators that work together to ensure that the Z ring will be formed only at midcell. The Min system, whose core component is the polarly localized MinCD complex, prevents the Z ring from forming at the cell poles, reducing the chances of minicell formation (12, 13). The nucleoid occlusion system, in turn, is based on DNA binding proteins (Noc in B. subtilis and SlmA in E. coli) that recognize specific sequence signatures and inhibit Z ring formation around the bacterial chromosomes (14–16). The combination of Min and nucleoid occlusion inhibition prevent division from happening at the cell poles and over the chromosomes, leaving only the central region of the cell free for Z-ring formation. Min/nucleoid occlusion also provide a means to regulate the cell-cycle timing of Z-ring formation because the creation of an inhibitor-free zone at midcell depends on proper DNA replication and segregation. Another well-studied FtsZ modulator is the checkpoint protein SulA (suppressor of Lon A), which makes bacterial cytokinesis responsive to environmental stresses. SulA is expressed in response to DNA damage as part of the SOS system in E. coli and halts Z-ring formation and cell division until DNA damage is repaired by the cell (17, 18). More recently, other negative modulators of FtsZ have been reported whose function is to control cytokinesis in response to the nutritional [UgtP (19), KidO (20), and OpgH (21)] and developmental [MciZ (mother cell inhibitor of FtsZ) (22) and Maf (23)] state of the cell.
Eukaryotic cytoskeletal modulators use a variety of strategies to achieve their effect, including nucleation, monomer sequestration, filament capping, and severing (24, 25). The conservation of the structure and principles that govern cytoskeletal filament formation suggest that the general mechanisms operating in eukaryotes should also be present in prokaryotes. However, little is known about the molecular details of how modulators affect FtsZ assembly. SulA is one of the few inhibitors whose mechanism has been studied in detail. The crystal structure of SulA in complex with FtsZ showed that it binds to the C-terminal polymerization interface of FtsZ (26). In addition, in vitro experiments demonstrated that SulA inhibits FtsZ polymerization by a simple sequestration mechanism (27, 28).
Here we focused on MciZ (mother cell inhibitor of FtsZ), a poorly understood 40-aa peptide discovered in a yeast two-hybrid screen for FtsZ binding partners (22). MciZ is an intriguing example of a developmentally regulated division inhibitor. It is expressed during sporulation in B. subtilis and blocks Z-ring formation after cells commit to the terminally differentiated spore fate. Although it has been shown that MciZ directly inhibits FtsZ polymerization in vitro (22, 29), the nature of its interaction with FtsZ and its inhibition mechanism are still unknown. We determined the crystal structure of MciZ in complex with FtsZ from B. subtilis and, by using fluorescence and electron microscopy as well as biochemical experiments, showed that MciZ binds to the C-terminal subdomain of FtsZ and acts as a capper of the minus end of FtsZ filaments. Minus-end capping is an unusual way to inhibit filament formation and indicates that annealing plays an important role in FtsZ filament dynamics.
Results
Crystal Structure of the FtsZ:MciZ Complex.
To investigate the mechanism of MciZ inhibition, we first determined the crystal structure of the FtsZ:MciZ complex to a resolution of 3.2 Å. We coexpressed his-tagged MciZ (His6-MciZ) and a truncated version of FtsZ that included only the globular portion of the protein (FtsZ12-315) in E. coli and purified the complex by metal affinity and gel-filtration chromatographies. As expected from the high affinity of MciZ for FtsZ (Kd of 150 ± 10 nM, measured by tryptophan fluorescence) (Fig. S1A), the complex was very stable; it could be produced with high purity in milligram quantities and it crystallized under standard conditions. Crystals belonged to space group P6522 and displayed nine FtsZ:MciZ complexes in the asymmetric unit (only one will be described for clarity, FtsZ:A and MciZ:J). In FtsZ, residues 203–208 and 219–220 could not be modeled in the map potentially because of inherent flexibility. Our FtsZ structure has no guanine nucleotide bound. MciZ (residues 2–37) folds into a β-hairpin followed by a loop, an α-helix, and a helical turn. It binds to the C-terminal subdomain of FtsZ by aligning with FtsZ’s C-terminal β-sheet through hydrogen bonds established between β9 of FtsZ and β2 of MciZ, thus generating a six-stranded β-sheet (Fig. 1A). This mode of protein–protein interaction, generally termed β-strand addition, is found in diverse protein complexes (30).
Fig. 1.
MciZ binds to the minus end of FtsZ and displaces the T7 loop. (A) Cartoon representation of the FtsZ:MciZ complex structure (PDB ID code 4U39). The N-terminal domain of FtsZ (residues 13–178) is represented in blue, the C-terminal domain (residues 209–314) in green, the H7 α-helix (residues 179–202) in yellow, and MciZ (residues 2–37) in red. Cocrystallized phosphates are represented as orange sticks. (B) Superposition of 20 representative low-energy structures of MciZ (red) solved by 1H NMR (PDB ID code 2MRW). Only a segment of the α-helix H1 appears to get structured in absence of FtsZ. (C) Alignment between the FtsZ:MciZ complex (FtsZ in green/blue/yellow and MciZ in red) and the FtsZ monomer of B. subtilis (gray, PDB ID code 2VAM) showing the overlap between MciZ and the T7 loop of the monomer structure, and the absence of the T7 loop in the FtsZ:MciZ structure (blue dotted line). (D) Superposition of MciZ (red, surface rendering) onto the FtsZ dimer structure of S. aureus (PDB 3VOA). The B. subtilis FtsZ:MciZ complex structure was aligned with the upper subunit of the dimer (gray FtsZ). Note the steric clash between MciZ and the bottom subunit of the dimer (orange FtsZ).
Binding of MciZ to FtsZ leads to significant restructuring of MciZ’s N-terminal region, as seen by comparing its structure in the crystallized complex with the structure of the free peptide in solution, determined by 1H NMR (Fig. 1B). Free MciZ displays the same segments of α-helix as the complexed peptide (amino acids 16–27 and 32–35) but its N terminus is unstructured (Fig. 1B). This N terminus thus becomes structured into two β-segments upon interaction with β9 of FtsZ, as noted above. The C terminus of MciZ is unstructured in both the free and bound forms.
Binding of MciZ buries a solvent-accessible area on FtsZ of 188 Å2. In addition to the backbone hydrogen bonds involved in the β-sheet extension, hydrophobic interactions between helices H1 of MciZ and H10 of FtsZ, and between H1/β2 of MciZ with H10/β9 of FtsZ could stabilize the interaction (Fig. S1B). Recognition between FtsZ and MciZ is further strengthened by a salt bridge between the carboxylate of Asp280 of FtsZ and the guanidinium group of Arg20 of MciZ (Fig. S1B).
To further analyze the FtsZ:MciZ interaction, we introduced site-directed modifications into FtsZ. FtsZ carrying an Asp to Arg mutation at position 280 (Asp280Arg) could not stably bind MciZ (Fig. S1C), probably because of disruption of the aforementioned salt bridge with Arg20. The reciprocal mutation in MciZ, in which we substituted Arg20 to an aspartate (Arg20Asp), also disrupted the FtsZ:MciZ interaction (Fig. S1C). Thus, the Asp280-Arg20 salt bridge is critical for complex stability.
MciZ Inhibits FtsZ Polymerization by Steric Hindrance.
The binding site of MciZ is at one of the polymerization surfaces of FtsZ, which suggests that the inhibitory effect of MciZ is simply caused by steric hindrance. Indeed, modeling of MciZ onto the structure of the Staphylococcus aureus FtsZ filament (PDB ID code 3VOA) (10) showed that the presence of MciZ would completely prevent the association of FtsZ monomers (Fig. 1D). Alignment of our structure with five other B. subtilis FtsZ structures (PDB ID codes 2VAM, 2VXY, 2RHL, 2RHO, and 2RHH) showed that binding of MciZ causes no major conformational changes (rmsd of 0.610 Å) (Fig. S1D), other than the displacement the T7 loop from its usual position. In fact, we cannot observe the density corresponding to the T7 loop (residues 203–208) in our structure (Fig. 1C), suggesting that it becomes more flexible or unstructured upon binding of MciZ. The T7 loop is critical for FtsZ function, contributing contacts to filament formation and the highly conserved 208NLDFAD213 for GTP hydrolysis (3, 31). Despite this fact, T7 loop displacement is unlikely to be the direct cause of the inhibition of FtsZ polymerization by MciZ; it could be simply a consequence of steric hindrance by the latter molecule.
MciZ Does Not Compete with GTP Binding.
Ray et al. (29) recently suggested that MciZ competes with GTP for binding to FtsZ. Because GTP binds to the N-terminal subdomain of FtsZ; this is at odds with the binding site for MciZ identified in our crystal structure. We revisited the possibility of competition between MciZ and GTP by comparing the binding of GTP to free FtsZ and to the FtsZ:MciZ complex using the fluorescent analog mant-GTP. This analysis showed that mant-GTP bound equally well to free and MciZ-bound FtsZ (Kd of 1.7 ± 0.2 µM and 1.4 ± 0.3 µM, respectively) (Fig. S1E). Thus, we cannot detect the competition between MciZ and GTP reported by Ray et al., a finding that is consistent with our structural data that indicates that MciZ and GTP bind at opposite surfaces of the FtsZ molecule.
Substoichimetric MciZ Shortens FtsZ Protofilaments.
There are a variety of mechanisms by which modulatory proteins can inhibit cytoskeletal filament formation. Binding of MciZ to the polymerization interface of FtsZ is compatible with MciZ acting by either monomer sequestration or filament capping. Monomer sequesterers inhibit assembly by reducing the effective concentration of monomer and, consequently, shifting the equilibrium toward depolymerization. A sequestered subunit is incapable of binding at either end. Thus, sequesterers tend to be effective only when present at close to stoichiometric concentrations relative to the filament-forming protein (28). In contrast, cappers block the ability of a subunit to bind at one end but leave the other end available to bind to a filament and block further assembly. Cappers thus tend to exhibit effects even at substoichiometric concentrations (32, 33). To study the mechanism of MciZ inhibition, we monitored the effect of various concentrations of MciZ on the polymerization of FtsZ by light scattering. This showed that as little as a 1:10 ratio of MciZ to FtsZ caused a marked inhibition (about 50%) of the light-scattering signal (Fig. 2A). In contrast, treatment of FtsZ with the MciZ-R20D mutant did not affect the levels of light scattering, suggesting that the MciZ effect is specific and dependent on the interaction with FtsZ (Fig. S2A). Because the light-scattering signal is disproportionately sensitive to polymer size, the 50% inhibition of light scattering by 1:10 MciZ does not necessarily mean that there was a reduction by half in the polymer mass (or polymer bonds). More likely, this substoichiometric inhibition of the light-scattering signal reflects a decrease in length or width (bundling) of the polymers.
Fig. 2.
MciZ inhibits FtsZ polymerization substoichiometrically in vitro. All assembly experiments were done in HMK buffer (50 mM Hepes, 5 mM MgAc, 100 mM KAc, pH 7.7). (A and B) Effect of various concentrations (0, 0.2, 0.5, 1, and 2.5 µM) of MciZ (A) or the FtsZ-MciZ fusion (B) on the polymerization of FtsZ (5 µM) reported by right-angle light scattering. (C–E) Effect of MciZ or FtsZ-MciZ on FtsZ filament length visualized by negative-staining EM. For this, 3 µM FtsZ was polymerized without (C) and with 0.3 µM MciZ (D) or 0.3 µM FtsZ-MciZ (E). The filament length distribution in each situation was measured and is plotted (Right). (Scale bar, 100 nm.)
To investigate further this substoichiometric poisoning, we carried out electron microscopy (EM) of negatively stained FtsZ polymers formed in the presence of MciZ. Polymerization of 3 µM FtsZ in HMK buffer produced filaments with a length of 200 ± 75 nm (Fig. 2C). In contrast, reactions with 3 µM FtsZ and 0.3 µM MciZ, the same 1:10 ratio that inhibited light scattering by 50%, showed a reduction in protofilament length to 120 ± 45 nm (Fig. 2D). This reduction in length caused by 0.3 µM MciZ cannot be explained by a sequestration mechanism, in which the effect of MciZ would be to reduce the concentration of active FtsZ monomers from 3.0 to 2.7 µM. Some filaments without MciZ seem to have higher contrast than those with MciZ, suggesting that they may be small bundles. Thus, the inhibition of light scattering by MciZ may also involve a reduction of bundling, probably as a result of shortening of the protofilaments. Nevertheless, to confirm that the primary effect of MciZ is to reduce the length of protofilaments rather than inhibiting bundling, we tested the effect of higher concentrations of MciZ (0.6 and 1.0 µM) on the polymerization of 3 µM of FtsZ (Fig. S2B). The results showed shorter protofilaments as MciZ concentration was increased, and no visible structures could be identified at 1.0 µM of MciZ. Because the critical concentration (Cc) of FtsZ in the conditions of our experiments is between 1 and 1.5 µM (see Fig. 5 A and B), it is conceivable that the inhibitory effect of 1.0 µM MciZ added to 3 µM FtsZ could be explained by MciZ sequestering FtsZ to a concentration of active monomers too close to the Cc to allow for assembly. To rule out this possibility, we also did EM experiments with FtsZ-L69W, a mutant protein that has a significantly lower Cc than the wild-type protein (0.5 µM). MciZ shortened FtsZ-L69W filaments as well as affecting wild-type FtsZ (Fig. S2C), suggesting that it is not acting by sequestration.
Fig. 5.
MciZ enhances the turnover of FtsZ filaments. (A) FtsZ polymerization reported by fluorescence quenching with BODIPY-conjugated FtsZ-S152C+S223W. Assembly curves with different FtsZ and MciZ concentrations were run to steady state and the ΔFluorescence was plotted. MciZ concentrations are indicated above the lines. (B) Effect of MciZ on the GTPase activity of FtsZ. (C and D) Kinetics of disassembly of FtsZ protofilaments reported by BODIPY-quench system: (C) in 5 mM Mg2+ (HMK buffer, which supports GTPase on), (D) in 1 mM EDTA (MEK buffer, GTPase blocked). Five micromolars of FtsZ was preassembled in the absence (gray) or presence of 0.5 µM of MciZ (red) and the rate of disassembly was measured after dilution in the appropriate buffer.
A FtsZ–MciZ Fusion Protein Inhibits Assembly Similarly to MciZ.
The substoichiometric effect of MciZ suggests that it is acting as a capper. However, to decisively rule out sequestration of monomers as the mechanism of MciZ inhibition, we tested the effect of adding MciZ in the form of an FtsZ:MciZ complex on the assembly of FtsZ. If MciZ works as a sequesterer, adding MciZ that is already complexed with FtsZ should have no effect on FtsZ assembly. We tested both the noncovalent FtsZ:MciZ complex produced by copurification, as well as a covalent complex, in which we fused MciZ to the C-terminal end of FtsZ (FtsZ–MciZ). The C-terminal end of FtsZ is a mostly unstructured, flexible chain with a contour length of 23 nm (34, 35), more than enough to allow MciZ to bind to the same FtsZ molecule that it is covalently connected to. Strikingly, both the FtsZ:MciZ complex (Fig. S3A) and the FtsZ–MciZ fusion were highly effective at inhibiting FtsZ assembly (Fig. 2B). Plotting the data from all experiments together (free MciZ, FtsZ:MciZ complex, and FtsZ–MciZ fusion) indicates that the complexes are as capable as free MciZ to promote inhibition (Fig. S3B). Similarly, EM determination of filament lengths demonstrated that the FtsZ–MciZ fusion shortened FtsZ filaments somewhat more than MciZ alone (100 ± 35 nm vs. 120 ± 45 nm) (Fig. 2E). These results demonstrate that MciZ does not act as a simple monomer sequesterer and suggest that MciZ-bound FtsZ monomers can interact with FtsZ filaments to block their growth. In the simplest model, the N-terminal polymerization face of MciZ-poisoned FtsZ should be held in a polymerization-competent conformation that can bind to and cap the minus end of protofilaments.
Substoichiometric MciZ Inhibits Z-Ring Formation in Vivo.
MciZ is a very potent inhibitor of cell division in vivo, being capable of causing a complete block of Z-ring formation and robust filamentation when induced with as little as 0.1% xylose from a single integrated chromosomal copy (Fig. 3 A and B). This finding suggested that MciZ also works substoichiometrically in vivo. To ascertain that theory, we carried out quantitative Western blots of cells expressing GFP-MciZ, using anti-GFP serum and purified GFP as a standard (Fig. 3C). This process revealed that induction with 0.1% xylose leads to the accumulation of 350 ± 150 molecules of GFP-MciZ per cell (Fig. S3D). We also quantified the levels of endogenous FtsZ in the same extract (Fig. S3 C and E), and found 4,200 molecules per cell, similarly to published data that indicates that B. subtilis contains at least 5,000 molecules of FtsZ per cell when grown in rich medium (36, 37). This result suggests that a ratio of MciZ to FtsZ of less than 1:10 is enough to completely block Z-ring formation and cell division in vivo.
Fig. 3.
MciZ blocks cell division substoichiometrically in vivo. (A and B) B. subtilis cells without (A) and with (B) GFP-MciZ expression induced by 0.1% xylose for 2 h. The cells were stained with FM 5-95 membrane dye to reveal septa. (Scale bar, 3 µm.) (C) Quantification of GFP-MciZ from cell extracts. GFP-MciZ was induced with 0.1% xylose for 2 h. GFP-MciZ was detected by immunoblotting using anti-GFP antibody and quantification was carried out by comparing the intensities of the bands with that of purified GFP standards.
MciZ Binds to FtsZ Filaments in Vitro and to FtsZ Polymers in Vivo.
A capping protein should be able bind to the polymeric form of its target. This ability of MciZ is implied by the results above, but we also aimed to demonstrate the binding of MciZ to FtsZ filaments directly, both in vitro and in vivo. To show binding of MciZ to FtsZ filaments in vitro, we carried out sedimentation experiments using rhodamine-labeled MciZ and FITC-labeled FtsZ (Fig. 4 A and B and Fig. S4). Reactions with 10 µM FtsZ and no MciZ resulted in ∼50% of FtsZ in the pellet. The addition of 250 nM MciZ produced a clear reduction in pelleted FtsZ to 25%. Importantly, MciZ efficiently cosedimented with the FtsZ polymers in these reactions, being found almost equally partitioned between supernatant and pellet fractions. As a control for the specificity of the cosedimentation, we also tested the MciZ-R20D mutant and found that it neither inhibited FtsZ sedimentation nor cosedimented with FtsZ. Even though sedimentation experiments are not fully quantitative (they underestimate polymer because individual protofilaments do not pellet well), the distribution of MciZ between pellet and supernatant fractions suggests it binds preferentially to FtsZ filaments.
Fig. 4.
MciZ binds to FtsZ filaments. (A and B) Cosedimentation of FtsZ polymers and MciZ. FtsZ was added to the reaction to a final concentration of 10 µM (9 µM of unlabeled wild type FtsZ and 1 µM of FtsZ-S152C labeled with FITC). MciZ-W36C and MciZ-R20D+W36C were labeled with TMR and added to a final concentration of 250 nM (when indicated). Fluorescent measurements from supernatant (light gray) and pellet (dark gray) fractions were obtained by excitation at 495 nm (FITC) and 550 nm (TMR). NF, normalized fluorescence; P, pellet; S, supernatant. (C) Colocalization of Ftsz-mCherry and GFP-MciZ in B. subtilis live cells. (Upper) Cells expressing FtsZ-mCherry (Left) and GFP-MciZ (Right). (Lower) The mislocalization of GFP-MciZ(R20D) mutant compared with the wild-type MciZ. (Scale bar, 2 µm.)
To demonstrate binding of MciZ to FtsZ polymers in vivo, we imaged GFP-MciZ and attempted to detect decoration of Z rings with GFP-MciZ. Induction of even low levels of GFP-MciZ in a wild-type strain led to very fast disassembly of Z rings and only rarely could we observe fluorescent Z rings. To improve our observation window, we repeated the experiment in a strain bearing a mutation in FtsZ (T45M) that makes it partially resistant to MciZ. This strain also expressed a low level of FtsZ-mCherry, to allow for colocalization of MciZ and FtsZ. Induction of GFP-MciZ for short times (15 min) allowed us to detect fluorescent Z rings in a high proportion of the cells (Fig. 4C). The FtsZ-mCherry rings in Fig. 4C are dimmer than typical Z rings (compare Fig. 4C, Upper and Lower) and tended to localize at division sites that already started to constrict. This result was also observed for the GFP-MciZ rings in the same cells and suggests that the inhibitory effect of MciZ is probably stronger at newer Z rings than in mature rings, in which FtsZ is associated with other divisome proteins that could stabilize it. Despite the atypical appearance, 100% of the GFP-MciZ rings colocalized with FtsZ-mCherry rings (Fig. 4C, Upper). As a control for the specificity of the GFP-MciZ localization, we also imaged a strain expressing GFP-MciZ-R20D, which exhibited no inhibitory effect on Z-ring formation and was found to be completely dispersed in the cytosol, despite the presence of robust FtsZ-mCherry rings in this strain (Fig. 4C, Lower). These results indicate that MciZ associates with FtsZ polymers in vivo. Because detection of GFP-MciZ rings requires that more than a few GFP-MciZ molecules decorate each Z ring, these results are further support for the idea that the Z ring is formed by multiple short protofilaments with exposed minus ends.
MciZ Does Not Promote Extensive Depolymerization of FtsZ Filaments.
Substoichiometric effects have been reported before for cappers of actin filaments and microtubules (32, 38). These cappers usually bind to the growing (plus) end of filaments and restrict exchange of subunits to the minus end. Because the minus end has a higher Cc than the plus end, blocking the plus end leads to extensive depolymerization of filaments, until the free monomer concentration becomes equal to the minus-end Cc (39, 40). Such depolymerization induced by eukaryotic capping proteins is dependent on, and a hallmark of, treadmilling (41). To determine if the shortening of FtsZ protofilaments by MciZ involved substoichiometric depolymerization of FtsZ, we used a fluorescence-quenching assay capable of accurately quantifying polymerization (42). For this assay we constructed the double-mutant FtsZ(S152C/S223W) and labeled the Cys with BODIPY. As with the equivalent construct for E. coli FtsZ, the Trp quenching of the BODIPY fluorescence is decreased by the conformational change accompanying assembly, and the fluorescence increase reports the total protofilament polymer. We used the BODIPY-labeled FtsZ mixed with a ninefold excess of wild-type FtsZ to ensure that the assembly is dominated by the wild-type FtsZ and avoid the bundling observed previously for fully labeled FtsZ (42).
Fig. S5 shows that assembly kinetics reached the overshoot peak in 12 s, a short time consistent with protofilament assembly as opposed to slower bundle formation (40 s to overshoot peak in Fig. 2 A and B) (42). Fig. 5A plots the plateau assembly for a range of FtsZ up to 9 µM, and for MciZ added at 0, 1, 2, and 3 µM. Similarly to the previously reported inhibition of FtsZ by SulA (27, 28), MciZ caused an increase in the apparent critical concentration, Ccapp, of FtsZ from 1.5 to 2.4, 3.1, and 4.2 µM, respectively. The increase in Ccapp was almost equal to the amount of added MciZ, consistent with MciZ binding FtsZ with higher affinity than the ∼1.5 µM Cc. This finding means that each MciZ molecule is roughly preventing the formation of only one FtsZ–FtsZ bond and indicates that capping of the minus end does not induce extensive depolymerization of the plus end.
MciZ Increases the Dynamics of FtsZ Filaments.
We measured the GTPase activity of FtsZ in the presence of MciZ to assess whether capping by MciZ affected the dynamics of FtsZ filaments. Reactions were set up with a similar range of FtsZ concentrations and with MciZ concentrations of 0, 2, 4, and 6 µM. As expected, MciZ decreased the absolute GTPase activity of FtsZ and increased the apparent Cc in proportion to its concentration (Fig. 5B), corroborating the BODIPY results. Strikingly, however, the specific activity (the slope of the line in GTP per minute per FtsZ) was increased from 2.8 in the absence of MciZ, to 5.4 at all concentrations of MciZ. The converse experiment, in which we assayed a fixed FtsZ concentration (10 µM) in the presence of varying MciZ concentrations, also revealed a twofold increase in the GTPase activity of FtsZ in the presence of low MciZ concentrations (Fig. S6). However, at a ratio of MciZ to FtsZ higher than 1:10, the GTPase activity started to decrease (Fig. S6), probably because at high MciZ the reduction in the amount of polymerized FtsZ begins to offset the stimulatory effect of MciZ.
The increase in GTPase specific activity indicates that polymers assembled in the presence of MciZ are more dynamic. The faster dynamics could be because of MciZ increasing the rate of GTP hydrolysis, or the rate of subunit exchange, as both limit the overall GTP turnover by FtsZ polymers (43, 44). To further investigate the effect of MciZ on polymer dynamics, we applied again the BODIPY fluorescence-quenching assay, but this time to follow the disassembly kinetics of FtsZ filaments after dilution to below the Cc. Disassembly curves were fit to a single exponential with decay time τ. The disassembly of FtsZ polymers in HMK buffer (Fig. 5C) was much faster in reactions with 1:10 MciZ (τ = 3 ± 2 s), compared with those with FtsZ alone (τ = 10 ± 2 s). A similar effect was observed in reactions in which GTP hydrolysis was blocked by the addition of EDTA (Fig. 5D). Disassembly in EDTA was slower than with GTP hydrolysis, as reported previously (43). However, polymers poisoned with MciZ again exhibited faster disassembly than FtsZ alone (τ = 9 ± 2 and 20 ± 3 s, respectively). Because the effect of MciZ is independent of GTP hydrolysis, we conclude that MciZ increases the turnover of FtsZ subunits in filaments, as we explain in the discussion.
Discussion
We applied a combination of structural, biochemical, and in vivo approaches to elucidate the mechanism of MciZ, a developmentally regulated inhibitor of FtsZ polymerization of B. subtilis. Clarifying the molecular effects of the growing roster of FtsZ modulators will be key for a better understanding of Z-ring formation. Our data show that MciZ is a capping protein, and that the inhibition of FtsZ assembly at low MciZ stoichiometries is primarily a result of capping. At high stoichiometries, MciZ is capable of both capping and sequestration of FtsZ. Thus, the identification of the first FtsZ capping protein indicates that the palette of biochemical activities available for the regulation of the bacterial cytoskeleton could be as diverse as the one known to operate on actin and tubulin.
We also exploited MciZ as a probe to try to answer some longstanding questions about FtsZ. The small size of FtsZ polymers has posed a formidable challenge to the understanding of its dynamic behavior. Simple questions, such as whether the polymerization of filaments is asymmetric, or if FtsZ undergoes treadmilling or dynamic instability, have remained unanswered largely because individual FtsZ filaments are shorter than the resolution of the light microscope. By carefully analyzing the effect of MciZ in bulk measurements of FtsZ polymerization, we provide evidence suggesting that fragmentation and annealing are important determinants of FtsZ filament size and that, similarly to tubulin, the plus end is more dynamic than the minus end of FtsZ filaments. We anticipate that the use of MciZ as a reagent in further studies, together with the application of sophisticated imaging methods to visualize filaments, will soon allow the construction of a realistic model of FtsZ polymerization dynamics.
MciZ Binds to the Minus End of FtsZ and Functions as a Filament-Capping Protein.
Our crystal structure of the FtsZ:MciZ complex showed that MciZ binds to the C-terminal polymerization interface of FtsZ—the minus end of the molecule—and impedes further polymerization by steric hindrance. Because binding of MciZ to the minus end does not affect the plus end of FtsZ (Fig. 1 and Fig. S1D), MciZ should be able to function as a capper and our data show that this is indeed the case.
The evidence that MciZ is a capper is manifold. First, it binds to filaments in addition to FtsZ monomers (Fig. 4). Second, it acts at substoichiometric concentrations. At 1:10 MciZ:FtsZ, the light-scattering signal was substantially reduced, and EM showed a reduction in the length of filaments (Fig. 2). These effects were approximately the same when MciZ was added alone, in a 1:1 complex with FtsZ, and when MciZ was fused to the C terminus of FtsZ (Fig. 2 and Fig. S3). The similar effects of free and prebound MciZ can only be explained if the FtsZ:MciZ complex caps the minus end of FtsZ filaments. Third, MciZ increases the dynamics of FtsZ polymers (Fig. 5 B–D). Above the Cc, FtsZ filaments are thought to be in a steady-state on-off reaction with the pool of subunits. When diluted below the Cc, the on-reaction is eliminated, leaving only the off-reaction. If disassembly occurs primarily from the filament ends, its rate will be proportional to filament length. If the filaments are half as long, and therefore presenting twice as many ends, they should take half as long to disassemble. Because the 1:10 MciZ:FtsZ filaments disassembled two- to three-times faster than FtsZ without MciZ, this suggests that they are two- to three-times shorter. The twofold increase in GTPase promoted by MciZ is also consistent with shorter filaments and increased number of ends. Both results are in good agreement with the EM data, and represent independent demonstrations of filament shortening by MciZ.
SulA, the only other FtsZ inhibitor whose mechanism is well established, also binds to the minus end of FtsZ (26). Interestingly, however, SulA functions as a monomer sequesterer instead of a capper (27, 28). Because SulA does not directly occlude the plus end of FtsZ, a monomer bound to SulA could still polymerize using its free plus end, but this does not seem to occur. The reason for the difference in the mechanisms of MciZ and SulA may reside in the details of how these inhibitors interact with the minus end of FtsZ, or may be related to the different affinities that these inhibitors display for FtsZ. SulA displays 10-fold lower affinity for FtsZ than MciZ and may not be able to compete effectively with monomeric FtsZ for binding to filament ends.
How Does MciZ Shorten FtsZ Filaments?
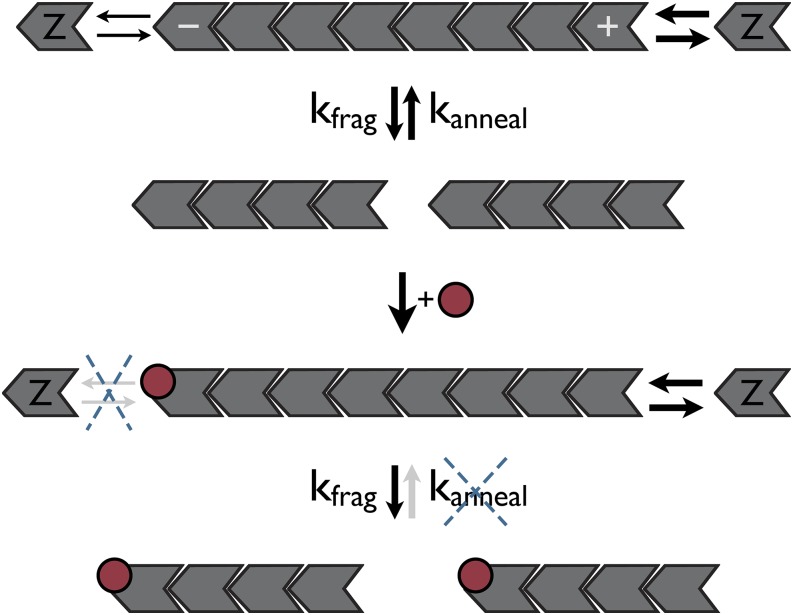
Minus-end cappers (γ-tubulin, Tropomodulin, Arp2/3) generally exert a stabilizing or nucleating role for actin and microtubules (45–47). Because the minus end is usually the site of subunit loss (40, 48), capping of the minus end prevents disassembly and stabilizes filaments. In contrast to the eukaryotic paradigm, MciZ is a minus-end capper that shortens FtsZ filaments and destabilizes the Z ring. To explain this apparent paradox we recall that in addition to affecting the exchange of subunits at filament ends, cappers will also affect annealing, the reaction by which two filaments become joined by their ends. Capping of either end of a filament will block annealing and, depending on how frequent annealing is, it could have a marked effect on filament sizes. Thus, to explain the effect of MciZ on FtsZ filaments we propose a model in which filament length at steady state will depend on the gain and loss of subunits at filament ends and on the balance between filament fragmentation and annealing (Fig. 6). Because capping of the minus end does not induce extensive depolymerization from the plus end (Fig. 5A), we postulate that blocking annealing may be an important pathway by which MciZ reduces filament size. Fragmentation and annealing of FtsZ filaments have been observed for filaments adsorbed to mica or supported lipid bilayers (49–51), and also in solution (43), although the rates of these reactions are yet to be precisely determined. Annealing may be particularly important in vivo, when the filaments are concentrated on the membrane at the center of the cell (5, 52). Thus, blocking annealing in vivo would be a powerful way to block the overall assembly of the Z ring.
Fig. 6.
Mechanism of FtsZ polymerization inhibition by MciZ. FtsZ filament length is determined by the rates of subunit gain and loss at filament ends, and the balance between filament fragmentation (kfrag) and annealing (kanneal). Capping of filament minus ends by MciZ (red circles) will inhibit subunit addition at these ends, but in principle, growth at the free plus ends could compensate that. In contrast, inhibition of annealing by MciZ turns fragmentation into an irreversible reaction, leading to shortening of FtsZ filaments. At close to stoichiometric concentrations, MciZ should also promote subunit sequestration in addition to capping.
Our observation that a minus-end capper destabilizes FtsZ filaments is also supported by recent work by Arumugam et al. (51), who produced a truncated FtsZ termed “NZ” that included only the N-terminal subdomain, and found that it also worked as a capper and caused the disassembly of FtsZ bundles on a supported lipid bilayer. This finding indicates that FtsZ filaments are susceptible to capping when assembled in higher-order structures tethered to the membrane, a more physiological situation than our in vitro system, and supports the conclusion that the powerful inhibition of Z-ring formation by MciZ in vivo is a consequence of capping.
MciZ Acts as a Sequesterer at High Concentrations.
Cytoskeletal modulatory proteins rarely have a single effect on their targets and it is not unexpected that a capper may also exhibit sequestration effects. For example, tropomodulin, which is a minus-end capper like MciZ, has been shown to bind to actin monomers and promote sequestration at high concentrations (53). MciZ behaves as a sequesterer at high concentrations, as demonstrated by the increase in Ccapp that is approximately equal to the concentration of added MciZ (Fig. 5 A and B). SulA produces an increase in Ccapp somewhat lower than its concentration, because its 0.7 µM Kd is comparable to the Kd for FtsZ filament formation [which is equal to the Cc (28)]. MciZ binds with a much higher affinity, Kd ∼0.1 µM, measured from trp fluorescence (Fig. S1A), so the sequestration is approximately equal to the concentration of MciZ. Sequestering activity may be explained as follows. The FtsZ:MciZ complex is sterically blocked at the minus end, making it impossible to bind to the plus end of a filament. The complex can bind to the minus end of a filament but at high MciZ concentration all filament minus ends should already be capped. Thus, the bound subunit is effectively sequestered.
The Polarity of FtsZ Filaments.
Some of our results are consistent with FtsZ exhibiting the same kinetic polarity as tubulin. A significant observation is that the capped filaments apparently do not disassemble at the plus end (Fig. 5A), indicating that the Cc of the plus end is similar or lower than the Cc of the minus end. In addition, because the rate of polymerization in the presence of MciZ is not markedly changed (Fig. S5), this suggests that the plus end dominates the kinetics of assembly. A more dynamic plus end means that FtsZ filaments should be inherently capable of treadmilling and, in fact, treadmilling has recently been reported for FtsZ filaments tethered to planar lipid monolayers by FtsA (54). However, our conclusion that assembly of FtsZ is maintained primarily at the plus end is the opposite of a previous treadmilling interpretation. Redick et al. made an effort to create FtsZ cappers by making debilitating point mutants of the top and bottom interfaces of E. coli FtsZ (55). Surprisingly, top-cap mutants, with a debilitating mutation on the upper GTP binding surface were completely inactive. Bottom-cap mutants showed dominant-negative effects in vivo, and blocked GTPase activity when mixed with wild-type FtsZ in vitro; however, these were extremely weak, requiring approximately 10-times excess of the cap mutant relative to wild-type. The differential effect of the top and bottom caps suggested treadmilling, but with reversed polarity relative to tubulin (subunits added primarily to the minus end, and dissociated primarily at the plus end). It is possible that the differential effect of top- and bottom-cap mutants is simply a result of a poorer ability of the top-mutant subunits to associate with filaments. Nevertheless, we will need more information, preferably the direct observation of filaments, before we can resolve the issue of FtsZ polarity.
A Threshold Size for FtsZ Filaments in Vivo?
The substoichiometric inhibition and the high affinity for FtsZ means that MciZ is the most powerful inhibitor of Z-ring formation described to date. This finding makes sense in light of MciZ’s function. In contrast with other negative modulators, such as SulA and MinC, whose activity needs to be restricted in space/time or reversed, MciZ acts on a terminally differentiating cell that is going to die. Thus, MciZ effects do not need to be reversed. The substoichiometric effect of MciZ in vivo also suggests that FtsZ filaments have a minimum size, below which a Z ring does not form. Assuming similar effects in vitro and in vivo, our data suggest that halving filament length suffices to block Z-ring formation in vivo. Why do shorter filaments not work? Shorter filaments seem less capable of interacting with FtsA and getting recruited to the membrane (54). In addition, they may have a lower tendency to make the lateral interactions necessary to form a coherent ring. Further investigation of this hypothesis may produce new insights on the structure and assembly of the Z ring.
Materials and Methods
General Methods.
All B. subtilis strains were derived from the wild-type PY79 strain. Final data collection, structure solution, and refinement statistics are in Tables S1 and S2. Oligonucleotides are listed in Table S3 and plasmids in Table S4. Purification of FtsZ and the FtsZ:MciZ complex is described in SI Materials and Methods. Immunoblots were performed using anti-GFP and anti-FtsZ antisera. The detailed protocol and procedure to estimate cellular protein concentration can be found in SI Materials and Methods.
Structure Determination.
The crystal structure of the FtsZ:MciZ complex was solved to 3.2 Å using molecular replacement techniques, and the solution structure of free MciZ was solved by 2D 1H-NMR. Both methodologies are described in SI Materials and Methods.
Biochemical Experiments.
The affinity of MciZ for FtsZ was measured by tryptophan fluorescence. The affinity of the FtsZ:MciZ complex for GTP was measured using the fluorescent analog mant-GTP. FtsZ polymerization was measured by right angle light scattering or by a BODIPY-based fluorescence quenching system. The GTPase activity of FtsZ was determined using the malachite green method. Detailed protocols for each procedure are in SI Materials and Methods.
Microscopy.
FtsZ protofilaments were imaged by negative stain EM. Cell filamentation and GFP-MciZ localization were determined by live-cell fluorescence microscopy. Detailed protocols for microscopy procedures are in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ethan Garner, Jessica Polka, Masaki Osawa, and José Manuel Andreu for discussions; the Brazilian Biosciences National Laboratory for access to their facilities; J. Marquez and the High-Throughput Crystallization Laboratory team (Grenoble Partnership for Structural Biology) for access to and help with high-throughput crystallization; and the European Synchrotron Radiation Facility for access to beamlines. This work used the platforms of the Grenoble Instruct Center (UMS 3518 Centre National de la Recherche Scientifique-Commissariat à l’Energie Atomique et aux Energies Alternatives-Université Joseph Fourier-European Molecular Biology Laboratory) with support from the French Infrastructure for Structural Biology (ANR-10-INSB-05-02) and the Genoble Alliance for Integrated Structural Cell Biology (ANR-10-LABX-49-01) within the Grenoble Partnership for Structural Biology. This work was supported by Grants 10/51866-0 (Smolbnet 2.0) from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and 1169/2013 from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (to F.J.G.-F.); National Institute of Health Grant GM66014 (to H.P.E.); a doctoral fellowship from FAPESP and a doctoral sandwich fellowship from the Science without Borders program (to A.W.B.-F.); a doctoral fellowship from FAPESP (to V.B.); a postdoctoral fellowship from FAPESP (to P.C.); and a PQ-2 fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (to F.J.G.-F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 4U39 (FtsZ:MciZ complex), and 2MRW (free MciZ)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414242112/-/DCSupplemental.
References
- 1.Theriot JA. Why are bacteria different from eukaryotes? BMC Biol. 2013;11:119. doi: 10.1186/1741-7007-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bi EF, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354(6349):161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 3.Nogales E, Downing KH, Amos LA, Löwe J. Tubulin and FtsZ form a distinct family of GTPases. Nat Struct Biol. 1998;5(6):451–458. doi: 10.1038/nsb0698-451. [DOI] [PubMed] [Google Scholar]
- 4.Adams DW, Errington J. Bacterial cell division: Assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol. 2009;7(9):642–653. doi: 10.1038/nrmicro2198. [DOI] [PubMed] [Google Scholar]
- 5.Erickson HP, Anderson DE, Osawa M. FtsZ in bacterial cytokinesis: Cytoskeleton and force generator all in one. Microbiol Mol Biol Rev. 2010;74(4):504–528. doi: 10.1128/MMBR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osawa M, Anderson DE, Erickson HP. Reconstitution of contractile FtsZ rings in liposomes. Science. 2008;320(5877):792–794. doi: 10.1126/science.1154520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Boer PA. Advances in understanding E. coli cell fission. Curr Opin Microbiol. 2010;13(6):730–737. doi: 10.1016/j.mib.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee A, Lutkenhaus J. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 1998;17(2):462–469. doi: 10.1093/emboj/17.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aylett CHS, Löwe J, Amos LA. New insights into the mechanisms of cytomotive actin and tubulin filaments. Int Rev Cell Mol Biol. 2011;292(40):1–71. doi: 10.1016/B978-0-12-386033-0.00001-3. [DOI] [PubMed] [Google Scholar]
- 10.Matsui T, et al. Structural reorganization of the bacterial cell-division protein FtsZ from Staphylococcus aureus. Acta Crystallogr D Biol Crystallogr. 2012;68(Pt 9):1175–1188. doi: 10.1107/S0907444912022640. [DOI] [PubMed] [Google Scholar]
- 11.Ravelli RBG, et al. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428(6979):198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 12.Rothfield L, Taghbalout A, Shih YL. Spatial control of bacterial division-site placement. Nat Rev Microbiol. 2005;3(12):959–968. doi: 10.1038/nrmicro1290. [DOI] [PubMed] [Google Scholar]
- 13.Lutkenhaus J. Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annu Rev Biochem. 2007;76:539–562. doi: 10.1146/annurev.biochem.75.103004.142652. [DOI] [PubMed] [Google Scholar]
- 14.Wu LJ, et al. Noc protein binds to specific DNA sequences to coordinate cell division with chromosome segregation. EMBO J. 2009;28(13):1940–1952. doi: 10.1038/emboj.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho H, McManus HR, Dove SL, Bernhardt TG. Nucleoid occlusion factor SlmA is a DNA-activated FtsZ polymerization antagonist. Proc Natl Acad Sci USA. 2011;108(9):3773–3778. doi: 10.1073/pnas.1018674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonthat NK, et al. Molecular mechanism by which the nucleoid occlusion factor, SlmA, keeps cytokinesis in check. EMBO J. 2011;30(1):154–164. doi: 10.1038/emboj.2010.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huisman O, D’Ari R, Gottesman S. Cell-division control in Escherichia coli: Specific induction of the SOS function SfiA protein is sufficient to block septation. Proc Natl Acad Sci USA. 1984;81(14):4490–4494. doi: 10.1073/pnas.81.14.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi E, Lutkenhaus J. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol. 1993;175(4):1118–1125. doi: 10.1128/jb.175.4.1118-1125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weart RB, et al. A metabolic sensor governing cell size in bacteria. Cell. 2007;130(2):335–347. doi: 10.1016/j.cell.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radhakrishnan SK, Pritchard S, Viollier PH. Coupling prokaryotic cell fate and division control with a bifunctional and oscillating oxidoreductase homolog. Dev Cell. 2010;18(1):90–101. doi: 10.1016/j.devcel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Hill NS, Buske PJ, Shi Y, Levin PA. A moonlighting enzyme links Escherichia coli cell size with central metabolism. PLoS Genet. 2013;9(7):e1003663. doi: 10.1371/journal.pgen.1003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handler AA, Lim JE, Losick R. Peptide inhibitor of cytokinesis during sporulation in Bacillus subtilis. Mol Microbiol. 2008;68(3):588–599. doi: 10.1111/j.1365-2958.2008.06173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briley K, Jr, Prepiak P, Dias MJ, Hahn J, Dubnau D. Maf acts downstream of ComGA to arrest cell division in competent cells of B. subtilis. Mol Microbiol. 2011;81(1):23–39. doi: 10.1111/j.1365-2958.2011.07695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollard TD, Cooper JA. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- 25.Winder SJ, Ayscough KR. Actin-binding proteins. J Cell Sci. 2005;118(Pt 4):651–654. doi: 10.1242/jcs.01670. [DOI] [PubMed] [Google Scholar]
- 26.Cordell SC, Robinson EJ, Lowe J. Crystal structure of the SOS cell division inhibitor SulA and in complex with FtsZ. Proc Natl Acad Sci USA. 2003;100(13):7889–7894. doi: 10.1073/pnas.1330742100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dajkovic A, Mukherjee A, Lutkenhaus J. Investigation of regulation of FtsZ assembly by SulA and development of a model for FtsZ polymerization. J Bacteriol. 2008;190(7):2513–2526. doi: 10.1128/JB.01612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Milam SL, Erickson HP. SulA inhibits assembly of FtsZ by a simple sequestration mechanism. Biochemistry. 2012;51(14):3100–3109. doi: 10.1021/bi201669d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray S, Kumar A, Panda D. GTP regulates the interaction between MciZ and FtsZ: A possible role of MciZ in bacterial cell division. Biochemistry. 2013;52(2):392–401. doi: 10.1021/bi301237m. [DOI] [PubMed] [Google Scholar]
- 30.Remaut H, Waksman G. Protein–protein interaction through beta-strand addition. Trends Biochem Sci. 2006;31(8):436–444. doi: 10.1016/j.tibs.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Oliva MA, Cordell SC, Löwe J. Structural insights into FtsZ protofilament formation. Nat Struct Mol Biol. 2004;11(12):1243–1250. doi: 10.1038/nsmb855. [DOI] [PubMed] [Google Scholar]
- 32.MacLean-Fletcher S, Pollard TD. Mechanism of action of cytochalasin B on actin. Cell. 1980;20(2):329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- 33.Kilimann MW, Isenberg G. Actin filament capping protein from bovine brain. EMBO J. 1982;1(7):889–894. doi: 10.1002/j.1460-2075.1982.tb01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buske PJ, Levin PA. A flexible C-terminal linker is required for proper FtsZ assembly in vitro and cytokinetic ring formation in vivo. Mol Microbiol. 2013;89(2):249–263. doi: 10.1111/mmi.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner KAJA, Moore DA, Erickson HP. The C-terminal linker of Escherichia coli FtsZ functions as an intrinsically disordered peptide. Mol Microbiol. 2013;89(2):264–275. doi: 10.1111/mmi.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feucht A, Lucet I, Yudkin MD, Errington J. Cytological and biochemical characterization of the FtsA cell division protein of Bacillus subtilis. Mol Microbiol. 2001;40(1):115–125. doi: 10.1046/j.1365-2958.2001.02356.x. [DOI] [PubMed] [Google Scholar]
- 37.Haeusser DP, Schwartz RL, Smith AM, Oates ME, Levin PA. EzrA prevents aberrant cell division by modulating assembly of the cytoskeletal protein FtsZ. Mol Microbiol. 2004;52(3):801–814. doi: 10.1111/j.1365-2958.2004.04016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pecqueur L, et al. A designed ankyrin repeat protein selected to bind to tubulin caps the microtubule plus end. Proc Natl Acad Sci USA. 2012;109(30):12011–12016. doi: 10.1073/pnas.1204129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wegner A, Isenberg G. 12-fold difference between the critical monomer concentrations of the two ends of actin filaments in physiological salt conditions. Proc Natl Acad Sci USA. 1983;80(16):4922–4925. doi: 10.1073/pnas.80.16.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollard TD, Mooseker MS. Direct measurement of actin polymerization rate constants by electron microscopy of actin filaments nucleated by isolated microvillus cores. J Cell Biol. 1981;88(3):654–659. doi: 10.1083/jcb.88.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neuhaus JM, Wanger M, Keiser T, Wegner A. Treadmilling of actin. J Muscle Res Cell Motil. 1983;4(5):507–527. doi: 10.1007/BF00712112. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Erickson HP. Conformational changes of FtsZ reported by tryptophan mutants. Biochemistry. 2011;50(21):4675–4684. doi: 10.1021/bi200106d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Erickson HP. FtsZ filament dynamics at steady state: Subunit exchange with and without nucleotide hydrolysis. Biochemistry. 2009;48(28):6664–6673. doi: 10.1021/bi8022653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romberg L, Mitchison TJ. Rate-limiting guanosine 5′-triphosphate hydrolysis during nucleotide turnover by FtsZ, a prokaryotic tubulin homologue involved in bacterial cell division. Biochemistry. 2004;43(1):282–288. doi: 10.1021/bi035465r. [DOI] [PubMed] [Google Scholar]
- 45.Li Q, Joshi HC. gamma-tubulin is a minus end-specific microtubule binding protein. J Cell Biol. 1995;131(1):207–214. doi: 10.1083/jcb.131.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer RS, Fowler VM. Tropomodulins: Life at the slow end. Trends Cell Biol. 2003;13(11):593–601. doi: 10.1016/j.tcb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: Nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci USA. 1998;95(11):6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Margolis RL, Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- 49.Mingorance J, et al. Visualization of single Escherichia coli FtsZ filament dynamics with atomic force microscopy. J Biol Chem. 2005;280(21):20909–20914. doi: 10.1074/jbc.M503059200. [DOI] [PubMed] [Google Scholar]
- 50.Mateos-Gil P, et al. Depolymerization dynamics of individual filaments of bacterial cytoskeletal protein FtsZ. Proc Natl Acad Sci USA. 2012;109(21):8133–8138. doi: 10.1073/pnas.1204844109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arumugam S, Petrašek Z, Schwille P. MinCDE exploits the dynamic nature of FtsZ filaments for its spatial regulation. Proc Natl Acad Sci USA. 2014;111(13):E1192–E1200. doi: 10.1073/pnas.1317764111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Surovtsev IV, Morgan JJ, Lindahl PA. Kinetic modeling of the assembly, dynamic steady state, and contraction of the FtsZ ring in prokaryotic cytokinesis. PLOS Comput Biol. 2008;4(7):e1000102. doi: 10.1371/journal.pcbi.1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer RS, et al. Tropomodulin 3 binds to actin monomers. J Biol Chem. 2006;281(47):36454–36465. doi: 10.1074/jbc.M606315200. [DOI] [PubMed] [Google Scholar]
- 54.Loose M, Mitchison TJ. The bacterial cell division proteins FtsA and FtsZ self-organize into dynamic cytoskeletal patterns. Nat Cell Biol. 2014;16(1):38–46. doi: 10.1038/ncb2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Redick SD, Stricker J, Briscoe G, Erickson HP. Mutants of FtsZ targeting the protofilament interface: Effects on cell division and GTPase activity. J Bacteriol. 2005;187(8):2727–2736. doi: 10.1128/JB.187.8.2727-2736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.