Abstract
The multicomponent Petasis borono–Mannich (PBM) reaction is a useful tool for the preparation of complex molecules in a single step from boronic acids, aldehydes/ketones, and amines. Here, we describe the use of glycerol in the PBM reaction of salicylaldehydes or 2-pyridinecarbaldehyde with several boronic acids and secondary amines. From these readily available starting materials, alkylaminophenols, 2-substituted pyridines, and 2H-chromenes were prepared in reasonable to good yields. Glycerol was compared with other solvents, and in some cases, it provided the reaction product in higher yield. Crude glycerol, as generated by the biodiesel industry, was evaluated and found to be a suitable solvent for the PBM reaction, successfully expanding the potential use of this industry by-product. Based on density functional theory (DFT) calculations and the obtained experimental results, the involvement of glycerol-derived boronic esters in the reaction mechanism is suggested to be competitive with the free boronic acid pathway. Similar Gibbs free energies for the aryl migration from the boronate species to the iminium were determined for both mechanisms.
Keywords: amines, boron, glycerol, multicomponent reactions, sustainable chemistry
Introduction
The Petasis borono–Mannich (PBM) reaction, a multicomponent reaction of boronic acids, aldehydes/ketones, and amines, is a great tool for the preparation of complex molecules in a single step from readily available starting materials.[1] The reaction has been used in the preparation of different classes of compounds, such as α-amino acids,[2] α-amino alcohols,[3] 2H-chromenes,[4] α-hydrazinocarboxylic acids,[5] 2-hydroxymorpholines and aminodiols,[6] 2-aminomorpholines,[7] imininocyclitols,[8] and 2,5-dihydrofurans.[9] Additionally, this tool has also been used in the synthesis of several natural products.[10] A feature of the PBM reaction is the mandatory presence of a coordinative group close to the reactive aldehyde or ketone carbonyl group. Such a group (usually an OH moiety) activates and directs the migration of the boronic acid or ester substituent. Salicylaldehydes have been widely explored either in the preparation of alkylaminophenols[11] from reaction with arylboronic acids or 2H-chromenes from reaction with vinyl boronic acids.[4a] Recently, the range of aromatic aldehydes suitable for this reaction was further expanded to include 2-pyridinecarbaldehydes.[12] The PBM reaction of salicylaldehyde was reported to proceed in good yields under solvent-free conditions using either microwave[13] or conventional[14] heating, and protic media such as alcohols and water are often used as solvents.[11], [15]
Glycerol is an abundant, biodegradable, cheap, nontoxic, and highly hydrophilic solvent, composed of a strong hydrogen-bond network. It has low vapour pressure, a high boiling point, a high dielectric constant, and a polarity value similar to dimethyl sulfoxide (DMSO) or N,N-dimethylformamide (DMF). Such characteristics have made it a suitable solvent for microwave and ultrasound irradiation procedures.[16] Glycerol has also been used as a solvent in biotransformations[17] and as a component of deep eutectic mixtures.[18] Being a polyol, glycerol is able to dissolve many organic and inorganic compounds, and its use as a solvent has also been explored in catalysed and noncatalysed processes.[16], [19] However, the use of glycerol as a reaction solvent poses some limitations, such as high viscosity that causes mass transfer problems and low solubility of highly hydrophobic compounds and gases. Glycerol is a side product in the production of biodiesel; it represents about 10 wt % of the total output, and its worldwide production was estimated to be around 2 million tonnes in 2010.[20] Additionally, the production of the next generation of biodiesel using algal lipid as feedstock or land plants unsuitable for food is expected to increase the quantities of biodiesel commercially produced.[20] In 10–15 years, it is expected that biodiesel production from algae will account for 37 % of the worldwide production. If so, this could result in a twenty-fold oversupply of glycerol in upcoming years.[21] Besides its widely spread use, for example in the cosmetic, pharmaceutical, food, and textile industries, new applications of glycerol are desirable in order to solve the surplus production issue. Some of the approaches explored are its transformation into other small platform chemicals with added commodity value, such as glycidol, epichlorohydrin, acrolein, and propylene glycol,[19a] and into a precursor of olefins, such as propene or ethylene.[22]
Considering the successful use of either boronic acids or boronic esters in the PBM reaction,[1a] it was envisioned that by mixing a boronic acid in glycerol, the corresponding glycerol boronic esters could be formed and subsequently react to provide the PBM product (Scheme 1). Such a process could also be favoured by the strong hydrogen-bonding network of glycerol,[23] hypothetically increasing the iminium formation rate.[24]
Scheme 1.

Hypothesised Petasis borono–Mannich (PBM) reaction in glycerol.
The use of glycerol as a solvent in reactions where a boronic acid is one of the components has been successfully explored in palladium-catalysed Suzuki[25] reactions and in cross coupling of diaryl diselenides.[26]
Results and Discussion
To test our initial hypothesis, the PBM reaction was carried out in dichloroethane with a mixture of glycerol phenylboronic esters (Scheme 2), prepared according to a previously reported procedure.[27] After obtaining the desired tertiary amine (4) in 40 % yield,[28] the use of glycerol as a solvent in the PBM reaction with aromatic aldehydes and aryl boronic acids was investigated.
Scheme 2.

Petasis borono–Mannich (PBM) reaction of salicylaldehyde with glycerol phenylboronic esters. Reagents and conditions: a) 1 a (0.41 mmol), 1.2 equiv 2 a and glycerol phenylboronic esters in C2H4Cl2 (1 mL), 50 °C, 15 h, 40 %.
Using morpholine, phenylboronic acid, and salicylaldehyde as starting materials, the reaction was performed at different temperatures ranging from room temperature to 120 °C (Table 1). Despite the known mass transfer issues associated with the high viscosity of glycerol, the reaction was observed to proceed better at 50 °C (Table 1, entry 2). This result poses an advantage over the use of water as solvent, considering that in water the reaction is better performed at 80 °C.[15] The modification of the stoichiometric amounts of the reactants and an increase in the reaction time allowed the isolation of the desired tertiary morpholine derivative (4) up to 80 % yield (Table 1, entry 7) after aqueous basic work-up and extraction with diethyl ether followed by chromatography.
Table 1.
Optimization of the Petasis borono–Mannich (PBM) reaction conditions in glycerol.[a]
 | |||
|---|---|---|---|
| Entry | Temp. [°C] | Equiv of2 a/3 a | Yield of4[%][b] |
| 1 | 25 | 1.2:1.2 | 38 |
| 2 | 50 | 1.2:1.2 | 56 |
| 3 | 80 | 1.2:1.2 | 42 |
| 4 | 100 | 1.2:1.2 | 44 |
| 5 | 120 | 1.2:1.2 | 44 |
| 6 | 50 | 1.5:1.5 | 66 |
| 7 | 50 | 1.5:1.5 | 77[c,d] |
Reagents and conditions: salicylaldehyde (0.41 mmol) in bidistilled glycerol (99.5 % w/v; 1 mL), open atmosphere, 24 h.
Isolated yield after column chromatography.
48 h reaction time.
Averaged yield of three runs (75 %; 76 %; 80 %).
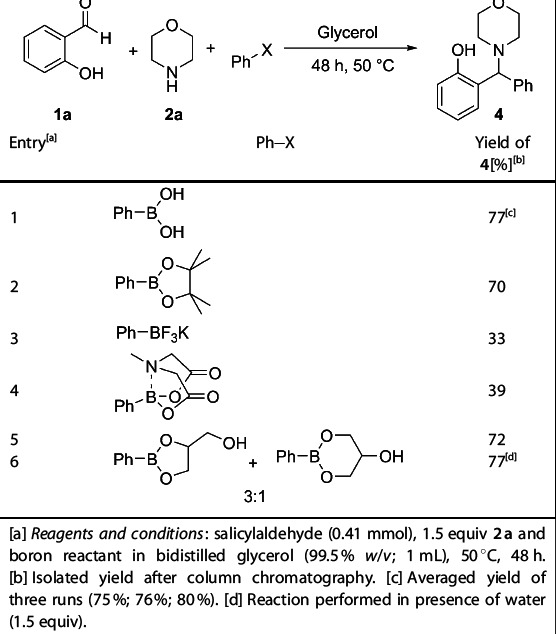
Other boron reactants were investigated and compared with phenylboronic acid (Table 2). Pinacol boronate, potassium trifluoroborate, N-methyliminodiacetic acid (MIDA)-protected boronate, and the previously prepared glycerol phenylboronic ester were tested as PBM components in glycerol. While pinacol boronate and glycerol boronic esters were able to deliver the product in comparable yields as phenylboronic acid (Table 2, entries 2 and 5), the equivalent reaction using potassium trifluoroborate salts or MIDA-protected boronate ester led to product formation in low yields (Table 2, entries 3 and 4), which can be attributed to their higher stability.[29] When performing the reaction with glycerol phenylboronic ester in presence of 1.5 equivalents of water, the yield slightly increased, delivering the product in the same yield as for phenylboronic acid (Table 2, entry 6).
Table 2.
Petasis borono–Mannich (PBM) reaction between salicylaldehyde, morpholine, and several boron reactants[a]
Keeping morpholine as the amine component, other boronic acids and salicylaldehydes were tested, and several tertiary morpholine derivatives were successfully obtained (Table 3). In glycerol, the reaction is sensitive to the substitution pattern of the boronic acid. The 2- and 4-methyl-substituted phenyl boronic acids provided the tertiary amine in good yields (Table 3, entries 1 and 2). On the other hand, 2,6-dimethyl-substituted phenylboronic acids failed to provide the reaction product in reasonable yields, most likely due to stereochemical constraints (Table 3, entry 3). Regarding the electronic nature of the phenylboronic acid, electron-rich arylboronic acids decorated with MeO or an allyl group resulted in the corresponding product formation in reasonable yields (Table 3, entries 4–7). The presence of electron-withdrawing substituents in the arylboronic acid clearly deactivates the migratory aptitude of the aryl moiety, and higher temperatures (80 °C) are needed to induce product formation in reasonable yields (Table 3, entries 7 and 8). The preparation of N-substituted morpholines was extended to other salicylaldehydes (Table 3, entries 9 and 10), and the products were obtained in up to 58 % yield for the 4-MeO-substituted aldehyde.
Table 3.
Reaction scope of phenyl boronic acids and salicylaldehydes [a]
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | R4 | R5 | Product | Yield [%][b] |
| 1[c] | H | H | H | Me | H | 5 | 86 |
| 2 | H | Me | H | H | H | 6 | 75 |
| 3 | H | Me | H | H | Me | 7 | 11 |
| 4 | H | H | H | OMe | H | 8 | 77 |
| 5 | H | H | OMe | H | H | 9 | 70 |
| 6 | H | H | H | CH=CH2 | H | 10 | 76 |
| 7[d] | H | H | H | Cl | H | 11 | 72 |
| 8[d] | H | H | H | NO2 | H | 12 | 34 |
| 9 | 4-MeO | H | H | H | H | 13 | 58 |
| 10 | 5-NO2 | H | H | H | H | 14 | 55 |
[a] Reagents and conditions: 1 (0.41 mmol), 1.5 equiv 2 a and 3 in bidistilled glycerol (99.5 % w/v; 1 mL), open atmosphere, 50 °C, 48 h. [b] Isolated yield after column chromatography. [c] 24 h reaction time. [d] Reaction performed at 80 °C.
Different secondary amines as partners in the PBM reaction in glycerol were also evaluated (Table 4). The corresponding products were obtained in comparable yields as in the case of morpholine (Table 2, entry 1), and indoline 2 e proved to be the best amine amongst those tested leading to product formation in 92 % yield (Table 3, entry 4).
Table 4.
Petasis borono–Mannich (PBM) reaction with different amines[a]

[a] Reagents and conditions: 1 a (0.41 mmol), 1.5 equiv 2 and 3 b in bidistilled glycerol (99.5% w/v; 1 mL), open atmosphere, 50°C, 48 h. [b] Isolated yield after column chromatography
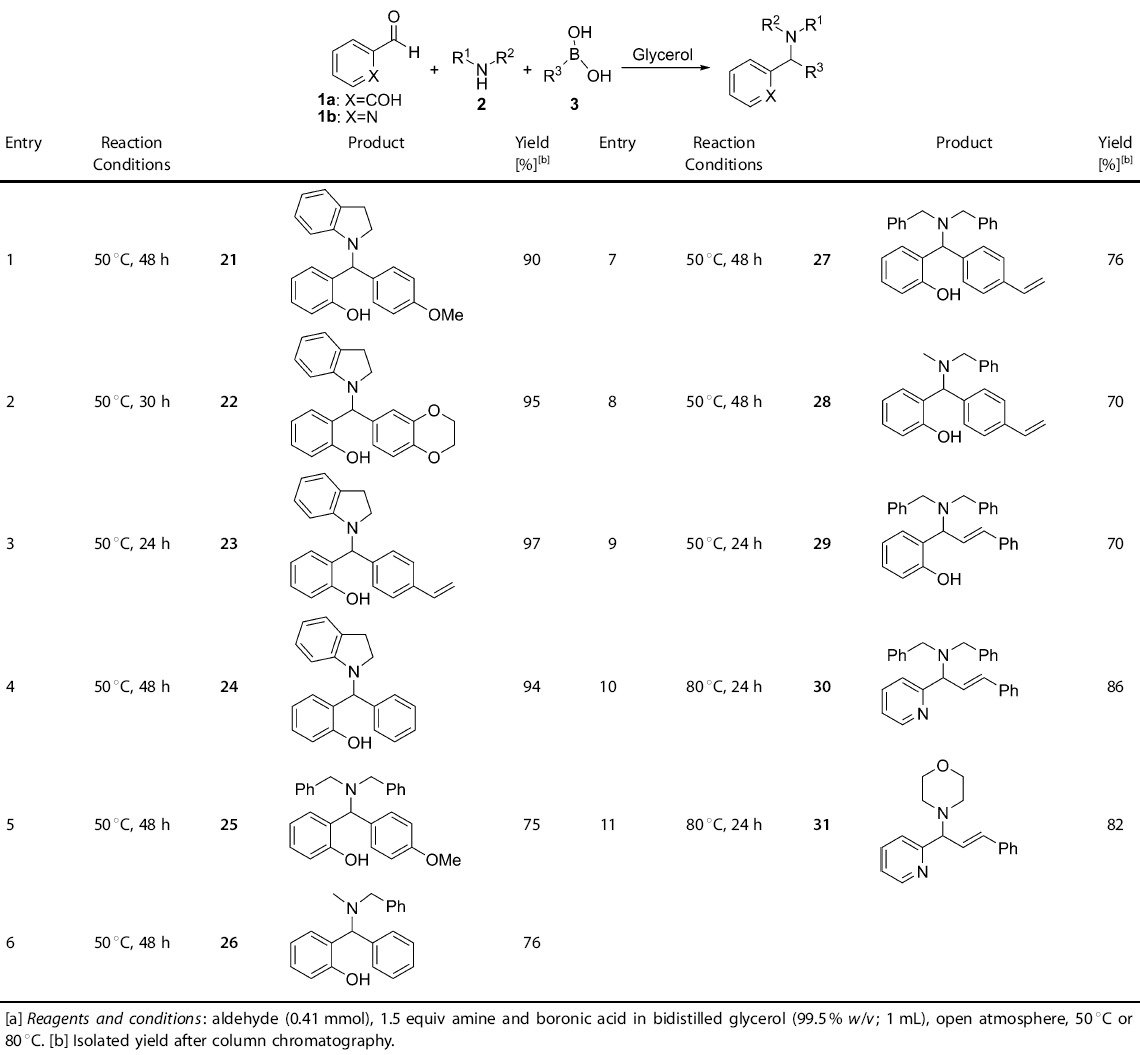
Using glycerol as solvent in the PBM reaction, the reaction scope was extended to the combination of several secondary amines and other boronic acids, providing the resultant tertiary amines in good to excellent yields (Table 5). Besides aryl boronic acids, phenylvinyl boronic acid was also used as the boronic component, and the corresponding allyl amine (29) could be obtained in 70 % yield. By increasing the reaction temperature to 80 °C, it was also possible to expand this protocol to 2-pyridinecarbaldehyde (1 b), and tertiary allyl amines 30 and 31 were obtained in up to 86 % yield (Table 5, entries 10 and 11).
Table 5.
Petasis borono–Mannich (PBM) reaction in glycerol—scope of the reaction.[a]
Motivated by the good yields obtained with the use of 2-pyridinecarbaldehyde, glycerol was compared with other solvents for this reaction (Table 6). Glycerol proved to be a good solvent for this reaction, surpassing ethanol and water, and the results were comparable with the best solvent identified in previous studies (acetonitrile).[12a], [12b]
Table 6.
Comparison of glycerol with other solvents for Petasis borono–Mannich (PBM) reaction of 2-pyridinecarbaldehyde[a]
 | ||
|---|---|---|
| Entry | Solvent | Yield of31[%][b] |
| 1 | Ethanol | 4 |
| 2 | Water | 11 |
| 3 | C2H4Cl2 | 57 |
| 4 | CH3CN | 68 |
| 5 | Bidistilled glycerol (99.5 % w/v) | 56 |
[a] Reagents and conditions: 1 b (0.41 mmol), 1.5 equiv 2 f and 3 c in solvent (1 mL), 80 °C, 2 h. [b] Isolated yield after column chromatography.
As previously pointed out, glycerol is a major by-product in the transesterification process for biodiesel production. The glycerol formed in those processes, usually called glycerine, is a mixture of variable purity and is difficult to refine due to the high-energy-demand processes required. The typical composition of crude glycerol is 40–70 % glycerol, 10 % water, 4 % salt, less than 0.5 % methanol, and 0.5 % free fatty acids.[20] Hence, it is desirable to find processes in which crude glycerol can be used without further purification. The PBM reaction was carried out in different protic media (Table 7). For the formation of 4, the use of glycerol as a solvent outperformed the use of ethanol, whilst the presence of water and ethanol in the glycerol had a detrimental effect in the reaction yield. We tested our protocol in the preparation of products 22–24 in crude glycerol (Table 8).[30] When comparing the use of crude glycerol with the use of pure glycerol as reaction media, we were pleased to observe only a slight decrease in the reaction yield for the former. Hence, the desired products were isolated in good to excellent yields (84–95 %), demonstrating the effectiveness of crude glycerol as a medium for the PBM reaction.
Table 7.
Comparison of glycerol with other media for Petasis borono–Mannich (PBM) reaction of salicylaldehyde.[a]
 | ||
|---|---|---|
| Entry | Solvent | Yield of4[%][b] |
| 1 | Ethanol | 47 |
| 2 | Ethanol/Glycerol (1:1 v/v) | 56 |
| 3 | Water/Glycerol (1:1 v/v) | 53 |
| 4 | Glycerol | 64 |
[a] Reagents and conditions: 1 a (0.41 mmol), 1.5 equiv 2 a and 3 a in solvent (1 mL), 50 °C, 24 h. Bidistilled glycerol (99.5 % w/v) was used. [b] Isolated yield after column chromatography.
Table 8.
Petasis borono–Mannich (PBM) reaction in crude glycerol.[a]

The developed procedure was also applied to the preparation of 2H-chromenes. Dibenzyl amine was successfully used as a catalyst (20–40 mol %), providing the 2H-chromenes in good to excellent yields at 90 °C for several salicylaldehyde derivatives (Table 9). Considering the previous report on the use of tertiary amines as mediator of this process,[4b] triethyl amine was also tested. The use of 1.5 equivalents of that amine failed to provide more than just traces of the product. Interestingly, when performing the same reaction at 50 °C, even in the presence of 1.5 equivalents of dibenzyl amine, the corresponding alkylaminophenol (29) could be obtained in 70 % yield (Table 5, entry 9).
Table 9.
Preparation of 2H-chromenes in glycerol[a]
 | ||||
|---|---|---|---|---|
| Entry | R | Reaction time [h][b] | Product | Yield [%][c] |
| 1 | H | 7 (20 mol %) | 32 | 94 |
| 2 | 4-MeO | 3 (40 mol %) | 33 | 84 |
| 3 | 5-NO2 | 3 (40 mol %) | 34 | 85 |
[a] Reagents and conditions: 1 (0.41 mmol), 1.2 equiv 3 c and 2 f in bidistilled glycerol (99.5 % w/v; 1 mL), open atmosphere, 90 °C. [b] Catalyst loading is given in parenthesis. [c] Isolated yield after column chromatography.
Density functional theory (DFT) calculations
Further insight into the reaction mechanism was achieved by performing a DFT study.[31] The calculations were performed at the M062X/6-311+G(d,p)//PBE1PBE/6-31G(d,p) level. Ethylene glycol was considered as reaction solvent due to its similar dielectric constant value with glycerol (42.5 ɛ for glycerol and 37.3 ɛ for ethylene glycol at 25 °C).[32] Considering the possibility of the cyclic glycerol esters formation in the reaction medium, the reaction free energy was computed to be favoured by 7.5 and 8.6 kcal mol−1 for the five-membered cyclic ester glycerol 1,2-phenylboronate (gbe1) and six-membered cyclic ester glycerol 1,3-phenylboronate (gbe2), respectively (Scheme 3). The formation of the monoester species by reaction of the phenylboronic acid with the primary hydroxy group of the glycerol is favoured by 0.2 kcal mol−1. It may be, therefore, that the glycerol 1-phenylboronate monoester (me) is present at a very low concentration and subsequently leads to formation of gbe1 and gbe2.
Scheme 3.

Glycerol ester formation from phenylboronic acid and changes in free energy (in kcal mol−1).
A comparative study including the reactions of gbe1 and gbe2 with salicylaldehyde and dimethylamine as model reactants was performed. The reaction of phenylboronic acid 3 a with salicylaldehyde and dimethylamine was also performed and can be found in the Supporting Information.
Considering gbe1 and gbe2 as the reacting boron species, the PBM mechanism was elucidated. The free-energy profiles obtained are represented in Figure 1, path 1 (solid lines) for the mechanism derived from gbe1 and path 2 (dashed lines) for gbe2. A general working model mechanism is represented in Scheme 4. The study reported here considers the starting material to be a zwitterionic iminium formed after condensation of dimethylamine with salicylaldehyde. It was previously reported that coordination of the boron species to the phenoxide was energetically more favoured than coordination with the phenol moiety.[15], [33] Hence, the activation of the glycerol boronic ester by the phenoxy group to form the “ate complex” was also considered presumed? to be the earliest step of this reaction. A decrease in both electronic and free energies for the ate complex formation was determined, demonstrating the stabilisation of the species by interaction of the zwitterionic iminium and the boron species through B−O bond formation, and corroborated by the 0.5–0.6 Wiberg indices (WI) determined for the B−O bond in ATE1 and ATE2. The stabilisation of the six-membered ring ate complex ATE1 is higher than the stabilisation of ATE2 by 4 kcal mol−1 when compared with the initial set of reagents. Inspection of the conformations of the ate complexes reveals the tetrahedral character of the boron atom, forcing the five-membered cyclic boronate ester moiety to adopt a puckered conformation in ATE2 and a chair conformation in ATE1.
Figure 1.

Free-energy profiles calculated for the Petasis reaction between dimethylamine, salicylaldehyde, and glycerol 1,2-phenylboronate (solid lines) or glycerol 1,3-phenylboronate (dashed lines). The geometries optimised for the reactions are presented. The relevant bond lengths (Å) and the respective Wiberg indices (WI, italics) are indicated. The minimum and the transition states were optimised, and the energy values (kcal mol−1) refer to the optimised, zwitterionic 2-[dimethyliminio)methyl]phenolate (Im) and glycerol 1,2-phenylboronate (gbe1) set of reagents, and include the thermal correction to the Gibbs free energy in 1,2-ethanediol. Hydrogen atoms are omitted for clarity.
Scheme 4.

Petasis borono–Mannich (PBM) reaction with glycerol-derived boronic esters. Relative energies given in parenthesis (in kcal mol−1) refer to the optimised, zwitterionic 2-[dimethyliminio)methyl]phenolate (Im) and glycerol 1,2-phenylboronate (gbe1) set of reagents, and include the thermal correction to the Gibbs free energy in 1,2-ethanediol.
In the determined energy profile, the ate complex needs to adopt a different conformation, placing the migratory substituent closer to the sp2 carbon of the iminium. Such conformations (ATE1′ and ATE2′) are only <1 kcal mol−1 higher in energy than the previous ATE1 and ATE2 conformations. The energy required to achieve product formation starting from either the five-membered or the six-membered boronate ester was shown to be very similar. Indeed, there is no energetic difference between TS1 and TS2, and the energy barrier totals 20.2 kcal mol−1 for TS1 and 18.1 kcal mol−1 for TS2.
The transition states obtained have similar features, with intermediate geometries between the second conformation of the ate complex (ATE1′ and ATE2′) and the tertiary amines (TA1 and TA2). In both cases, it is clear that the B−C(Ph) boron bond is being broken (d=1.71 Å, WI=0.54 for TS1 and d=1.47 Å, WI=0.65 for TS2), whilst a new C−C(Ph) bond is starting to form (d=1.97 Å, WI=0.49 for TS1 and d=2.00 Å, WI=0.46 for TS2). The long distance and small WI value associated with the recently formed C−C bond suggest an early transition state, which is even more pronounced in the five-membered cyclic boronate ester pathway (TS2).
A mechanism where phenylboronic acid was considered as the boron-reacting species was also calculated (see Supporting Information). A Gibbs energy barrier of only 3.1 kcal mol−1 higher than the one determined for path 2 was calculated.
Continuum models are limited to account for electrostatic interactions. However, solute–environment interactions,[34] such as hydrogen bonding between the intervenient species and glycerol, can be anticipated to have a determinant role in these mechanisms. Assuming that such interactions have the same effect in both mechanisms (i.e., involving the boronic ester or the boronic acid), and considering the small difference in their energetic barriers and the similar yields obtained for the reaction of glycerol phenylboronic esters in presence and absence of water (Table 2, entries 5 and 6), it is likely that both mechanisms are competitive in glycerol.
Conclusions
We have demonstrated that glycerol is an effective medium for the Petasis borono–Mannich (PBM) reaction. Alkylaminophenols containing tertiary amines, allyl derivatives, and 2-substituted pyridines, as well as 2H-chromenes, can be prepared in glycerol in good yields. In some cases, glycerol outperformed ethanol as a solvent for this reaction, and with 2-pyridinecarbaldehyde as the carbonyl component, glycerol was found to be comparable with acetonitrile. The results of a comparative mechanistic study of the reaction suggested that the participation of glycerol-derived boronic esters is competitive with the mechanism via free boronic acid.. Overall, the reported results show for the first time the possibility of using glycerol in the PBM reaction, and the examples described here can be considered to be new entries in the compendium of reactions that revalorise waste materials generated by the biodiesel industry.
Experimental Section
General Procedure: A long, capped test tube containing a magnetic stirrer was charged with the appropriate boronic acid (1.5 equiv) and pure glycerol (1.0 mL). The boronic acid was left to dissolve for 5 min at 50, 80, or 90 °C. The aldehyde (0.41 mmol) was then added, and the reaction was stirred for 2 min at the same temperature, followed by addition of amine (1.5 equiv). The reaction was stirred at that temperature until complete consumption of the aldehyde as monitored by thin-layer chromatography (TLC) or for 48 h at the longest. After cooling to RT, the reaction was quenched by the addition of water (1.0 mL) and saturated aq NaHCO3 (1.0 mL), and then extracted with Et2O (3–5×5 mL) until the extract contained no further product as determined by TLC. The combined organic layers were concentrated in vacuo, and the crude product was purified by flash chromatography on silica gel (EtOAc/hexane).
Supporting Information: Detailed experimental procedures, spectroscopy data for the synthesized compounds, computational methods, and the energy profile for the Petasis borono–Mannich (PBM) reaction with phenylboronic acid.
Acknowledgments
The authors acknowledge CSC–IT Center for Science Ltd, Finland for the allocation of computational resources, the support of Prof. Luis F. Veiros (Universidade Técnica de Lisboa, Portugal) in the DFT study, and Mrs. Päivi Joensuu (University of Oulu, Finland) for analysing the HRMS data.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
References
- 1.Candeias NR. Montalbano F. Cal PMSD. Gois PMP. Chem. Rev. 2010;110:6169–6193. doi: 10.1021/cr100108k. [DOI] [PubMed] [Google Scholar]
- Yu T. Li H. Wu XY. Yang J. Chin. J. Org. Chem. 2012;32:1836–1845. [Google Scholar]
- 2.Petasis NA. Zavialov IA. J. Am. Chem. Soc. 1997;119:445–446. [Google Scholar]
- Kaiser PF. Churches QI. Hutton CA. Aust. J. Chem. 2007;60:799–810. [Google Scholar]
- McLean NJ. Tye H. Whittaker M. Tetrahedron Lett. 2004;45:993–995. [Google Scholar]
- Jourdan H. Gouhier G. Van Hijfte L. Angibaud P. Piettre SR. Tetrahedron Lett. 2005;46:8027–8031. [Google Scholar]
- 3.Petasis NA. Zavialov IA. J. Am. Chem. Soc. 1998;120:11798–11799. [Google Scholar]
- Prakash GKS. Mandal M. Schweizer S. Petasis NA. Olah GA. Org. Lett. 2000;2:3173–3176. doi: 10.1021/ol000195f. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q. Finn MG. Org. Lett. 2000. pp. 4063–4065. [DOI] [PubMed]
- Petasis NA. Butkevich AN. J. Organomet. Chem. 2009;694:1747–1753. doi: 10.1016/j.jorganchem.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portlock DE, Naskar D, West L, Li M. Tetrahedron Lett. 2002;43:6845–6847. [Google Scholar]
- 6.Berrée F, Debache A, Marsac Y, Collet B, Girard-Le Bleiz P, Carboni B. Tetrahedron. 2006;62:4027–4037. [Google Scholar]
- 7.Régnier T, Berrée F, Lavastre O, Carboni B. Green Chem. 2007;9:125–126. [Google Scholar]
- 8.Hong Z. Liu L. Hsu C-C. Wong C-H. Angew. Chem. Int. Ed. 2006;45:7417–7421. doi: 10.1002/anie.200601555. Angew. Chem. 2006, 118, 7577 –7581. [DOI] [PubMed] [Google Scholar]
- Hong Z. Liu L. Sugiyama M. Fu Y. Wong C-H. J. Am. Chem. Soc. 2009;131:8352–8353. doi: 10.1021/ja901656e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui C-X, Li H, Yang X-J, Yang J, Li X-Q. Org. Lett. 2013;15:5944–5947. doi: 10.1021/ol402782f. [DOI] [PubMed] [Google Scholar]
- 10.Ritthiwigrom T. Pyne SG. Org. Lett. 2008. pp. 2769–2771. [DOI] [PubMed]
- Au CWG. Nash RJ. Pyne SG. Chem. Commun. 2010;46:713–715. doi: 10.1039/b918233k. [DOI] [PubMed] [Google Scholar]
- Moosophon P. Baird MC. Kanokmedhakul S. Pyne SG. Eur. J. Org. Chem. 2010:3337–3344. [Google Scholar]
- Ritthiwigrom T. Willis AC. Pyne SG. J. Org. Chem. 2010;75:815–824. doi: 10.1021/jo902355p. [DOI] [PubMed] [Google Scholar]
- Bouillon ME. Pyne SG. Tetrahedron Lett. 2014;55:475–478. [Google Scholar]
- 11.Petasis NA, Boral S. Tetrahedron Lett. 2001;42:539–542. [Google Scholar]
- 12.Mandai H. Murota K. Sakai T. Tetrahedron Lett. 2010;51:4779–4782. [Google Scholar]
- Mandai H. Murota K. Suga S. Heterocycles. 2012;85:1655–1669. [Google Scholar]
- Schlienger N. Bryce MR. Hansen TK. Tetrahedron Lett. 2000;41:1303–1305. [Google Scholar]
- 13.Nun P, Martinez J, Lamaty F. Synthesis. 2010:2063–2068. [Google Scholar]
- 14.Liu Y, Wang L, Sui Y, Yu J. Chin. J. Chem. 2010;28:2039–2044. [Google Scholar]
- 15.Candeias NR, Veiros LF, Afonso CAM, Gois PMP. Eur. J. Org. Chem. 2009:1859–1863. [Google Scholar]
- 16.Cintas P, Tagliapietra S, Gaudino EC, Palmisano G, Cravotto G. Green Chem. 2014;16:1056–1065. [Google Scholar]
- 17.Hernáiz MJ, Alcántara AR, García JI, Sinisterra JV. Chem. Eur. J. 2010;16:9422–9437. doi: 10.1002/chem.201000798. [DOI] [PubMed] [Google Scholar]
- 18.Abbott AP, Harris RC, Ryder KS, D′Agostino C, Gladden LF, Mantle MD. Green Chem. 2011;13:82–90. [Google Scholar]
- 19.Garcáa JI. Garcáa-Marán H. Pires E. Green Chem. 2014;16:1007–1033. [Google Scholar]
- Dáaz-Álvarez A. Cadierno V. Appl. Sci. 2013;3:55–69. [Google Scholar]
- 20.Wijffels RH. Barbosa MJ. Science. 2010;329:796–799. doi: 10.1126/science.1189003. [DOI] [PubMed] [Google Scholar]
- Quispe CAG. Coronado CJR. Carvalho JA., Jr Renewable Sustainable Energy Rev. 2013;27:475–493. [Google Scholar]
- 21.Tran NH, Kannangara GSK. Chem. Soc. Rev. 2013;42:9454–9479. doi: 10.1039/c3cs60227c. [DOI] [PubMed] [Google Scholar]
- 22.Zakaria ZY, Amin NAS, Linnekoski J. Biomass Bioenergy. 2013;55:370–385. [Google Scholar]
- 23.Pagliaro M, Rossi M. The Future of Glycerol: New Uses of a Versatile Raw Material. Cambridge: Royal Society of Chemistry; 2008. [Google Scholar]
- 24. For reviews on the acceleration of organic transformations through H-bonding catalysis see:
- 25.Wolfson A. Dlugy C. Chem. Pap. 2007. pp. 228–232.
- Wolfson A. Dlugy C. Shotland Y. Environ. Chem. Lett. 2007;5:67–71. [Google Scholar]
- Wolfson A. Snezhko A. Meyouhas T. Tavor D. Green Chem. Let. Rev. 2012;5:7–12. [Google Scholar]
- Cravotto G. Orio L. Gaudino EC. Martina K. Tavor D. Wolfson A. ChemSusChem. 2011;4:1130–1134. doi: 10.1002/cssc.201100106. [DOI] [PubMed] [Google Scholar]
- Azua A. Mata JA. Heymes P. Peris E. Lamaty F. Martinez J. Colacino E. Adv. Synth. Catal. 2013;355:1107–1116. [Google Scholar]
- 26.Ricordi VG. Freitas CS. Perin G. Lenardão ã. Jacob RG. Savegnago L. Alves D. Green Chem. 2012;14:1030–1034. [Google Scholar]
- Gonçalves LC. Fiss GF. Perin G. Alves D. Jacob RG. Lenardão EJ. Tetrahedron Lett. 2010;51:6772–6775. [Google Scholar]
- 27.Nichele TZ, Favero C, Monteiro AL. Catal. Commun. 2009;10:693–696. [Google Scholar]
- 28. Product 4 was obtained in 82 % yield, under the same reaction conditions using phenylboronic acid instead of the glycerol ester.
- 29.Stefani HA. Cella R. Vieira AS. Tetrahedron. 2007;63:3623–3658. [Google Scholar]
- Darses S. Genet J-P. Chem. Rev. 2008;108:288–325. doi: 10.1021/cr0509758. [DOI] [PubMed] [Google Scholar]
- Knapp DM. Gillis EP. Burke MD. J. Am. Chem. Soc. 2009;131:6961–6963. doi: 10.1021/ja901416p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangayil R, Karp M, Santala V. Int. J. Hydrogen Energy. 2012;37:12198–12204. Crude glycerol used was obtained from same batch as described in. [Google Scholar]
- 31.Parr RG. Yang W. Density Functional Theory of Atoms and Molecules. New York: Oxford University Press; 1989. [Google Scholar]
- Frisch Mj. Trucks Gw. Schlegel Hb. Scuseria Ge. Robb Ma. Cheeseman Jr. Scalmani G. Barone V. Mennucci B. Petersson Ga. Nakatsuji H. Caricato M. Li X. Hratchian Hp. Izmaylov Af. Bloino J. Zheng G. Sonnenberg Jl. Hada M. Ehara M. Toyota K. Fukuda R. Hasegawa J. Ishida M. Nakajima T. Honda Y. Kitao O. Nakai H. Vreven TJ. Montgomery Ja. Peralta Je. Ogliaro F. Bearpark M. Heyd Jj. Brothers E. Kudin Kn. Staroverov Vn. Kobayashi R. Normand J. Raghavachari K. Rendell A. Burant Jc. Iyengar Ss. Tomasi J. Cossi M. Rega N. Millam Jm. Klene M. Knox Je. Cross Jb. Bakken V. Adamo C. Jaramillo J. Gomperts R. Stratmann Re. Yazyev O. Austin Aj. Cammi R. Pomelli C. Ochterski Jw. Martin Rl. Morokuma K. Zakrzewski Vg. Voth Ga. Salvador P. Dannenberg Jj. Dapprich S. Daniels Ad. Farkas Ö. Foresman Jb. Ortiz Jv. Cioslowski J. Fox Dj. Gaussian 09 (Revision D.01) Wallingford CT: Gaussian, Inc.; 2009. Calculations performed at the PBE1PBE/6-311+G**//PBE1PBE/6-31G** level with the use of the Gaussian 09 package. The xenergies reported were calculated with a polarizable continuum model (PCM) with 1,2-ethanediol as solvent. A full account of the computational details is presented as Supporting Information. [Google Scholar]
- 32.Lange NA, Dean JA. Langes Handbook of Chemistry. New York: McGraw Hill; 1999. [Google Scholar]
- 33.Candeias NR, Cal PMSD, André V, Duarte MT, Veiros LF, Gois PMP. Tetrahedron. 2010;66:2736–2745. [Google Scholar]
- 34.Mennucci B. J. Phys. Chem. Lett. 2010;1:1666–1674. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary


