Highlight:
ICE1 of Poncirus trifoliata (L.) Raf. plays a positive role in cold tolerance and modulates polyamine synthesis by interacting with arginine decarboxylase.
Key words: bHLH, cold tolerance, polyamine, Poncirus trifoliata (L.) Raf., protein-interacting protein, ROS, transcription factor.
Abstract
ICE1 (Inducer of CBF Expression 1) encodes a MYC-like basic helix–loop–helix transcription factor that acts as a central regulator of cold response. In this study, we elucidated the function and underlying mechanisms of PtrICE1 from trifoliate orange [Poncirus trifoliata (L.) Raf.]. PtrICE1 was upregulated by cold, dehydration, and salt, with the greatest induction under cold conditions. PtrICE1 was localized in the nucleus and could bind to a MYC-recognizing sequence. Ectopic expression of PtrICE1 in tobacco and lemon conferred enhanced tolerance to cold stresses at either chilling or freezing temperatures. Yeast two-hybrid screening revealed that 21 proteins belonged to the PtrICE1 interactome, in which PtADC (arginine decarboxylase) was confirmed as a bona fide protein interacting with PtrICE1. Transcript levels of ADC genes in the transgenic lines were slightly elevated under normal growth condition but substantially increased under cold conditions, consistent with changes in free polyamine levels. By contrast, accumulation of the reactive oxygen species, H2O2 and O2 –, was appreciably alleviated in the transgenic lines under cold stress. Higher activities of antioxidant enzymes, such as superoxide dismutase and catalase, were detected in the transgenic lines under cold conditions. Taken together, these results demonstrated that PtrICE1 plays a positive role in cold tolerance, which may be due to modulation of polyamine levels through interacting with the ADC gene.
Introduction
Cold is one of the most devastating abiotic stresses that impair plant growth and development, reduce productivity, and limit geographical distribution of natural populations. As most citrus commercial cultivars are not cold hardy, cold has been documented to cause great economic loss in several important citrus-producing regions globally (Sahin-Çevik and Moore, 2006). Therefore, improvement of cold tolerance is a major task of citrus breeding programmes. However, due to special reproductive natures, such as polyembryony, a long juvenile period, and a high degree of heterozygosity (Talon and Gmitter, 2008), limited achievements have been made so far to improve citrus cold tolerance by means of traditional breeding approaches. A vast number of elegant studies have provided evidence showing that genetic engineering is a powerful strategy for creating germplasms with enhanced cold tolerance (Lata and Prasad, 2011; Krasensky and Jonak, 2012). The underlying prerequisite for this strategy is to characterize valuable genes that can be genetically engineered.
Being sessile organisms, plants have evolved to interpret and respond to adverse environmental cues by molecular, physiological, and biochemical alterations, enabling them to survive under harsh situations (Nakashima et al., 2009; Thomashow, 2010; Theocharis et al., 2012). Over the last decades, enormous progress has been made in deciphering significant components implicated in the cold signalling network (Wisniewski et al., 2014; Shi et al., 2015). One of the most important milestones has been the identification of C-repeat-binding factor (CBF) genes, including CBF1, CBF2, and CBF3 (Liu et al., 1998; Thomashow 2001; Chinnusamy et al., 2006; Medina et al., 2011). The CBFs, conserved among monocots and dicots, play crucial roles in regulating a large spectrum of cold-regulated (COR) genes, collectively called the CBF regulon, implying that they act as central hub in the cold signalling network (Yamaguchi-Shinozaki and Shinozaki, 2005; Thomashow, 2010; J. H. Liu et al., 2014). Another important breakthrough has been characterization of Inducer of CBF Expression 1 in Arabidopsis thaliana (AtICE1).
ICE1 encodes a MYC-type basic helix–loop–helix (bHLH) transcription factor (Chinnusamy et al., 2003). Overexpression of ICE1 led to stronger induction of CBF3 and downstream COR genes, but induction of these genes was impaired in an ice1 mutant. Further in-depth work showed that ICE1 regulated the expression of CBF3 by binding to a cis-acting element containing the MYC-recognizing (MYCR) core sequence (CANNTG) in the CBF3 promoter region (Chinnusamy et al., 2003; Lee et al., 2005). In addition, ICE2, an ICE1 homologue in Arabidopsis thaliana, was identified as functioning in regulating cold tolerance via modulation of CBF1 expression (Fursova et al., 2009). These findings suggest that ICE1 or ICE2 acts as a master regulator of the cold signalling pathway and plays a pivotal role in mediating plant responses to cold, thus establishing ICE1–CBF–COR as the most significant signalling cascade. To date, ICE1 and ICE2 homologues have been unravelled in various plants, such as Calellia sinensis (Wang et al., 2012), apple (Feng et al., 2012), tomato (Feng et al., 2013), Chrysanthemum dichrum (Chen et al., 2013), trifoliate orange (Huang et al., 2013), Phalaenopsis aphrodite (Peng et al., 2014), and banana (Shan et al., 2014). Recently, Hu et al. (2013) reported that jasmonate functions positively as a critical upstream signal regulating the ICE1-mediated cold response, which adds new vision to our understanding of the cold stress response.
It is generally accepted that ICE1 functions in cold tolerance due to its essential role in governing the expression of CBFs, which in turn regulate the downstream target genes (Chinnusamy et al., 2003; Van Buskirk and Thomashow, 2006; Thomashow, 2010). However, the possibility of the existence of other unexplored mechanisms underlying the role of ICE1 cannot be fully ruled out. As a matter of fact, recent work by Chen et al. (2013) demonstrated that, in addition to the established ICE1–CBF cascade, CdICE1 of Chrysanthemum dichrum could mediate freezing tolerance by regulating expression of the microRNA miR398, which in turn modified transcript levels of superoxide dismutase (SOD) associated with reactive oxygen species (ROS) scavenging. Therefore, it is reasonable to hypothesize that some undetermined molecular mechanisms might also contribute to the cold tolerance imparted by ICE1 and its homologues.
Trifoliate orange [Poncirus trifoliata (L.) Raf.] has deciduous leaves and undergoes winter dormancy. It is widely used as a citrus rootstock to boost cold tolerance in citrus cultivars grafted onto it due to its striking tolerance to extremely low temperatures after thorough cold acclimation. This unusual property makes trifoliate orange a major source of candidate genes for cold tolerance improvement in citrus. In this study, we report that overexpression of P. trifoliata ICE1 (PtrICE1) led to enhanced cold tolerance in tobacco and lemon transgenic lines. Yeast two-hybrid (Y2H) screening gave rise to 21 potential PtrICE1-interacting proteins, in which P. trifoliata arginine decarboxylase (PtADC) was experimentally confirmed. We also demonstrated that transcript levels of ADC genes were elevated in the transgenic plants of tobacco and lemon, in particular under cold treatment, concomitant with an increase in free polyamine levels compared with the wild type (WT). All of these findings indicate that PtrICE1 is involved in the regulation of cold tolerance via modulating polyamine synthesis by interacting with ADC.
Materials and methods
Plant materials and treatments
Young shoots collected from 2-year-old trifoliate orange plants grown in a greenhouse under natural illumination were used to analyse expression of PtrICE1. The shoots were first incubated in distilled water for 48h at room temperature before being treated with various abiotic stresses, including dehydration, cold, and salt. For dehydration treatment, the shoots were placed in empty flasks for 6h; leaves were collected at 0, 0.5, 1, 3, and 6h after dehydration treatment. For cold stress, the shoots were kept in a growth chamber set at 4 °C for 72h; the leaves were harvested at 0, 5, 24, 48, and 72h after initiation of the treatment. For salt stress, the shoots were inserted into flasks containing 200mM NaCl; the leaves were collected at the same time points as for cold treatment. The leaf samples were frozen immediately in liquid nitrogen and stored at –80 °C until use for expression analysis.
Isolation and bioinformatics analysis of PtrICE1
A database search against Harvest (http://harvest.ucr.edu) was carried out using ‘ICE1’ as an entry keyword, as performed previously (Huang et al., 2013). Sequence analysis of the outputs showed that one (782bp) was annotated as an ICE1 homologue. As the 5’ terminus of this sequence was missing, 5’ rapid amplification of cDNA ends (5’-RACE) PCR with a gene-specific primer (GSP, 5’-CTGATCTCCACCAGTGATAGTGCT-3’) was performed using a SMART RACE cDNA Amplification kit (Clontech, USA) based on the manufacturer’s instructions. The 5’-RACE sequence and the initial one were combined into a contig containing an open reading frame (ORF), which was validated by reverse transcription (RT)-PCR with a pair of primers (GSP1; see Supplementary Table S1 at JXB online, in which primers used for this study are listed unless otherwise stated). The sequence was subjected to bioinformatics analysis, as described by Liu et al. (2009). In addition, a phylogenetic tree was reconstructed by the neighbour-joining method using MEGA 4.0.
Subcellular localization of PtrICE1
The full-length cDNA of PtrICE1 without a stop codon was amplified by RT-PCR with primer pair GSP2 (Supplementary Table S1), and fused to the N terminus of the green fluorescent protein (GFP) gene of the pBI121 vector, driven by the cauliflower mosaic virus (CaMV) 35S promoter. The subcellular localization of PtrICE1 was examined by Agrobacterium tumefaciens-mediated transient expression of the fusion vector (PtrICE1–GFP) and control vector (GFP) in onion epidermal cells (Huang et al., 2011). The transformed cells were cultured in the dark on MS medium for 1–2 d before visual observation under a universal fluorescence microscope (BX61; Olympus, Tokyo, Japan).
Transcriptional activation and MYCR-binding assay of PtrICE1
The ORF of PtrICE1 generated by RT-PCR using primer pair GSP3 (Supplementary Table S1) was digested with BamHI and XhoI and inserted into pENTR3C (Invitrogen). The recombinant vector (pENTR3C-PtrICE1) was fused in frame downstream of the yeast GAL4 DNA-binding domain (BD) of vector pDEST32 (Invitrogen). The fusion vector and negative control (pDEST32) were transformed independently into yeast MaV203 strain (Invitrogen) according to the manufacturer’s instructions. The transformed yeast cells were plated for 3 d on SD/–Leu/–Trp or SD/–Leu/–Trp/–His medium added with or without 5 and 15mM 3-aminotriazole (3-AT), followed by growth observation.
A yeast one-hybrid assay (Clontech) was performed to investigate whether PtrICE1 could bind to the MYCR sequence. The PtrICE1 ORF was amplified using the primer pair GSP4 (Supplementary Table S1) and fused to the GAL4 activation domain (AD) in the pGADT7 vector to create pGADT7-PtrICE1. A 66bp DNA fragment composed of triple tandem repeats of a sequence containing MYCR (CACATG) was inserted into the pHIS2 vector, generating a recombinant construct of pHIS2-MYCR. Thereafter, pGADT7-PtrICE1 and pHIS2-MYCR were co-transformed into yeast cells (strain Y187), which were grown for 3 d on SD/–Leu/–Trp/–His medium with or without 15mM 3-AT.
Expression analysis by quantitative real-time RT-PCR (qPCR)
Total RNA was isolated using Trizol reagent (Invitrogen) and digested with RNase-free DNaseI (TaKaRa) to remove contaminating DNA. About 1 μg of total RNA was used to synthesize the first-strand cDNA with Moloney murine leukemia virus reverse transcriptase (Promega) in a 20 µl reaction mixture. To investigate gene expression (PtrICE1 and ADC), qPCR analysis with a SYBR Green PCR kit (SYBR Green; Applied Biosystems) was carried out in an ABI 7500 Real-Time System (PE Applied Biosystems, Foster City, CA, USA). The PCR mixture (10 µl) contained 5 µl of 2× SYBR Green RealMasterMix, 50ng of cDNA, and 0.2 µM of each primer (GSP5 pair; Supplementary Table S1). Thermal conditions were 50 °C for 2min and 95 °C for 10min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1min. Each sample was analysed in four replicates, and the 2–ΔΔCt method was applied to calculate the relative expression levels of each gene. Actin and Ubiquitin were analysed in parallel as internal reference controls for trifoliate orange/lemon and tobacco, respectively, to normalize expression levels.
Transformation and characterization of transgenic plants
The full-length PtrICE1 gene was inserted into binary vector pBI121 linearized with XhoI and KpnI, under the control of the CaMV 35S promoter. The recombinant plasmid was introduced into Agrobacterium tumefaciens strain EHA105 by heat shock. Agrobacterium-mediated transformation of tobacco (Nicotiana tabacum) and lemon (Citrus limon) was carried out (Horsch et al., 1985; Huang et al., 2010; Fu et al., 2011). Kanamycin-resistant plants derived from independent transformation events were verified by genomic PCR using two pairs of primers (NPTII and CaMV 35S-PtrICE1; Supplementary Table S1). In addition, overexpression of PtrICE1 was assayed by semi-quantitative PCR or qPCR in transgenic lines of lemon and tobacco, respectively. Homozygous tobacco plants at the T2 generation and vegetatively multiplied lemon plants were used for a stress tolerance assay and physiological measurement.
Assessment of cold tolerance in transgenic lines
Tobacco plants were treated with cold at either chilling (4 °C) or freezing (0 °C) temperatures. For chilling treatment, tobacco seedlings planted at 25 °C were kept at 4 °C for 48h or 5 d. Electrolyte leakage and ROS levels were assessed after the chilling treatment. In another experiment, 35-d-old plants were first treated at 0 °C for 24h and then moved to an ambient environment for further growth for 5 d (recovery), followed by assessment of survival rate. Leaves collected before and after the freezing treatment were used for analysis of polyamine levels, ROS accumulation, and expression of ADC (NtADC1 and NtADC2) genes.
For lemon, two transgenic lines (#17 and #21) and WT plants were treated for 4h at –3 °C and then moved to an ambient environment for further growth for 5 d (recovery). The leaves were sampled before and/or after the freezing treatment for analysis of electrolyte leakage, chlorophyll content, cell death, ROS accumulation, polyamine levels, and ADC gene (ClADC) expression. In addition, they were treated at –6 ºC for 1.5h, followed by recovery for 4 d to further compare the freezing tolerance.
Physiological measurements and histochemical staining
Electrolyte leakage was measured according to previous studies (Huang et al., 2010; Wang et al., 2011). Chlorophyll contents [chlorophyll a (Ca); chlorophyll b (Cb), and total chlorophyll (Ct)] were quantitatively measured based on the method of Liu et al. (2008). Histochemical staining with 3,3’-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) was used to analyse the in situ accumulation of H2O2 and O2 –, respectively, as depicted by Huang et al. (2010) and Shi et al. (2010). Quantitative measurement of H2O2 and O2 – was performed using specific detection kits (Nanjing Jiancheng Bioengineering Institute, China), and protein concentration was determined colorimetrically (Bradford, 1976). Cell death was examined with trypan blue staining based on the method of Pogány et al. (2009).
Quantification of polyamine levels by high-performance liquid chromatography (HPLC)
Free polyamines were extracted with 5% cold perchloric acid containing 500mg l–1 of dithiothreitol as described by Liu and Moriguchi (2007) and then derivatized with benzoyl chloride. Polyamines were separated at room temperature using an Agilent HPLC system (USA) equipped with a C18 reversed-phase column (4.6×150mm, particle size 5 μm) and UV detector (230nm), using hexamethylene diamine as an internal control. The solvent (HPLC-grade methanol and water) changed from 50:50 (v/v) to 95:5 in 10min at a flow rate of 0.7ml min–1. Quantification of the polyamine levels, expressed as nmol g–1 of fresh weight, was performed in triplicate.
Y2H-based screening of PtrICE1-interacting proteins
Full-length and truncated PtrICE1 (deletion of the transactivation region at aa 49–98) were amplified with primer pairs GSP3 and GSP6 (Supplementary Table S1), respectively, and inserted into pENTRTM3C (Invitrogen). The recombinant constructs were then fused in frame with the GAL4 BD of pDEST32 vector using a homologous recombination system (ProQuest Two-Hybrid System; Invitrogen). The fused proteins were expressed in yeast strain MaV203 cells to examine autoactivation. For Y2H analysis, a cDNA library was constructed with trifoliate orange treated at 4 °C for 72h, and then screened using the truncated PtrICE1 as bait following the manufacturer’s instructions (Invitrogen). The yeast cells were cultured for 7 d at 30 °C on selection medium (SD/–Leu/–Trp/–His) supplemented with 50mM 3-AT. Positive clones were sequenced and analysed bioinformatically.
Protein–protein interaction analysis
To confirm an interaction between PtrICE1 and PtADC, full-length cDNA of PtADC was amplified using primer pair GSP7 (Supplementary Table S1) and cloned into the XhoI and KpnI sites of pDEST22 vector to get AD–PtADC (prey), while the truncated PtrICE1 was inserted into XhoI and KpnI sites of pDEST32 vector to generate BD–PtrICE1 (bait). The two constructs were co-transformed into yeast cells, which were cultured as described above. For bimolecular fluorescence complementation (BiFC) analysis (Walter et al., 2004), the PtrICE1 ORF without a stop codon was PCR amplified with primer pair GSP9 (Supplementary Table S1) and then subcloned into pSPYNE-35S containing the N-terminal fragment of yellow fluorescent protein (nYFP) to get PtrICE1–nYFP. Meanwhile, full-length PtADC without a stop codon was amplified using primer pair GSP10 (Supplementary Table S1) and inserted into pSPYCE-35S containing the C-terminal fragment of YFP (cYFP) to generate PtrADC–cYFP. The recombinant vectors were introduced into Agrobacterium tumefaciens strain EHA105 and used for transient expression in tobacco (Nicotiana benthamiana) via leaf infiltration (Walter et al., 2004). YFP fluorescence in the epidermis was monitored via a universal fluorescence microscope (ECLIPSE 90i). Positive (AtbZIP63–nYFP and AtbZIP63–cYFP) and negative (PtrICE1–nYFP and cYFP) controls were processed in the same way.
Statistical analysis
The data were statistically processed using the SAS software package (SAS Institute); statistic difference was compared using one-way analysis of variance based on a t-test, at the significance levels of P<0.05, P<0.01, and P<0.001.
Results
Cloning and bioinformatics analysis of PtrICE1
To obtain the trifoliate orange PtrICE1 gene, we first searched the Harvest database using ICE1 as an entry, which yielded different outputs (Huang et al., 2013), including an expressed sequence tag (EST) of 782bp. Homology comparison in the NCBI database showed that the EST exhibited high sequence homology to ICE1. As the 5’ terminus was missing, 5’-RACE PCR was performed to obtain the 5’-terminus sequence, leading to amplification of a sequence of 1246bp. Merging of the 5’-RACE sequence and the original EST generated a sequence of 1869bp. Analysis in ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) indicated that it contained a complete ORF of 1680bp, along with a 160bp 5’-untranslated region and a 29bp 3’-untranslated region. For convenience of description, the full-length cDNA was designated PtrICE1 and has been deposited in GenBank under accession number KJ812152. PtrICE1 was predicted to encode a protein of 559 aa, with an estimated molecular mass of 61.2kDa and a pI of 5.27. Multiple alignments indicated that PtrICE1 shared a high degree of sequence identity with ICE1 proteins of eight other plants at the C terminus, whereas they varied extensively among each other at the N terminus (Fig. 1). Motif scanning revealed that PtrICE1 contained a MYC-like bHLH domain of 48 aa (from aa 371 to 418) composed of a 15 aa basic region and two helices (14 aa each) that were connected by a 5 aa loop. In addition, an acidic region existed at the N terminus (aa 49–98) of PtrICE1. An unrooted phylogenetic tree was reconstructed using complete amino acid sequences of PtrICE1 and ICE1s of other plants, in which PtrICE1 was most closely related to RcICE1 of Ricinus communis (Supplementary Fig. S1 at JXB online). In addition, at the amino acid level, PtrICE1 displayed 46.4% sequence identity to PtrbHLH, an ICE1-like bHLH protein in P. trifoliata (Huang et al., 2013; Supplementary Fig. S2 at JXB online).
Fig. 1.
Amino acid sequence alignment of PtrICE1 and ICE1 or ICE2 of other plants, comprising Populus trichocarpa (NCBI Protein no. ABN58427), PsICE1 of Populus suaveolens (ABF48720), RcICE1 of Ricinus communis (EEF51703), MdICE1 of apple (ABS50251), GmICE1 of soybean (ACJ39211), AtICE1 and AtICE2 of Arabidopsis thaliana (AAP14668 and BAC42644, respectively), and CbICE1 of Capsella bursa-pastoris (AAS79350). Identical and similar residues are shown in black and grey background, respectively. The single bold line below the sequence indicates the basic region, while double lines represent the helix regions, which are connected by a loop, indicated by the dotted line. The dashed line shows the leucine-zipper region. The asterisk and square indicate glutamate and arginine, respectively.
PtrICE1 is localized in the nucleus and has transactivation activity in yeast
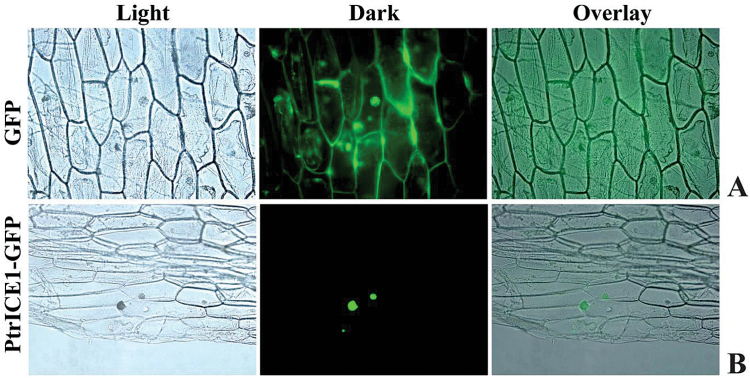
Sequence analysis showed that PtrICE1 contained two nuclear localization signals (aa 298–312 and 366–383), implying that it may be localized in the nucleus. To verify this, the subcellular localization pattern of PtrICE1 was examined using a fusion protein of PtrICE1 and GFP, under the control of the CaMV 35S promoter. Microscopic visualization showed that the control GFP was uniformly distributed throughout the whole cell (Fig. 2A), while the PtrICE1–GFP fusion protein was observed exclusively in the nucleus (Fig. 2B), indicating that PtrICE1 was a nuclear protein.
Fig. 2.
Nuclear localization of PtrICE1. Onion epidermis was transformed with vectors containing GFP (A), used as a control, or PtrICE1–GFP fusion protein (B). Subcellular localization was visualized by fluorescence microscopy. Representative images show cells expressing GFP or PtrICE1–GFP fusion protein under bright field (light) or UV field (dark), together with corresponding overlaid images.
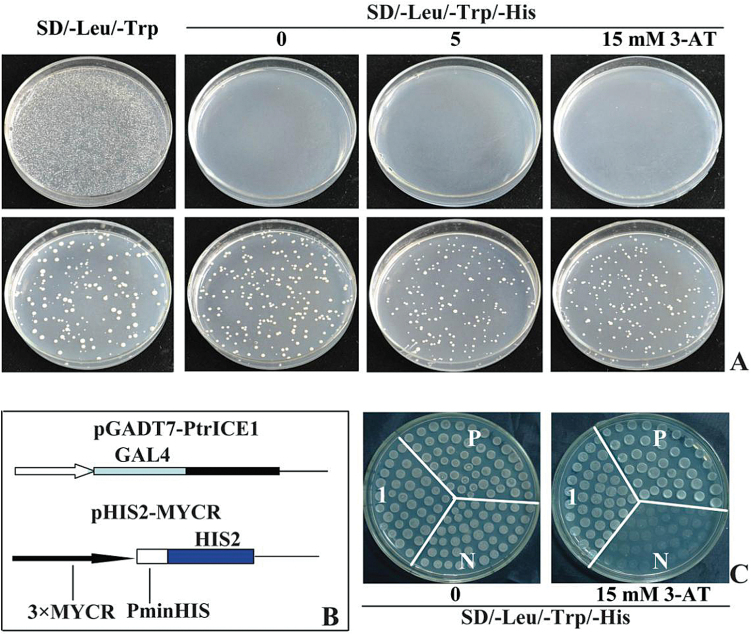
In addition, the Y2H system was used to investigate whether PtrICE1 functioned as a transcriptional activator. The yeast cells carrying the control plasmid grew well on SD/-Leu/-Trp medium, but failed to grow on SD/-Leu/-Trp/-His medium added with or without 3-AT. By contrast, the yeast cells transformed with the fusion plasmid containing PtrICE1 grew healthily on all tested media (Fig. 3A), indicating that PtrICE1 had transcriptional activation activity.
Fig. 3.
Transcriptional activation assay of PtrICE1. (A) Growth of yeast cells (strain MaV203) transformed with either control vector (upper panels) or fusion vector harbouring PtrICE1 (bottom panels) on SD/–Leu/–Trp or SD/–Leu/–Trp/–His with or without 3-AT. (B) Schematic illustration of the vectors (pGADT7-PtrICE1 and pHIS2-MYCR) used for the transactivation assay. (C) Growth of yeast cells co-transformed with vectors of the positive control (P), negative control (N), and pGADT7-PtrICE1 with pHIS2-MYCR (1) on SD/–Leu/–Trp/–His with or without 15mM 3-AT.
ICE1 of Arabidopsis thaliana can bind to the MYCR cis-acting element (Chinnusamy et al., 2003), which compelled us to examine whether PtrICE1 could also bind to a sequence containing the MYCR sequence. For this purpose, the PtrICE1 ORF was fused to the GAL4 AD in the pGADT7 vector to generate an effector, while a reporter construct (pHIS2-MYCR) was created by ligating a sequence containing three repeats of MYCR in the pHIS2 vector. The yeast (strain Y187) cells co-transformed with the effector and reporter grew normally, whereas those of control cells died on the selection medium (Fig. 3B), suggesting that PtrICE1 binds to MYCR and activates the reporter genes in yeast.
Expression patterns of PtrICE1 under various treatments
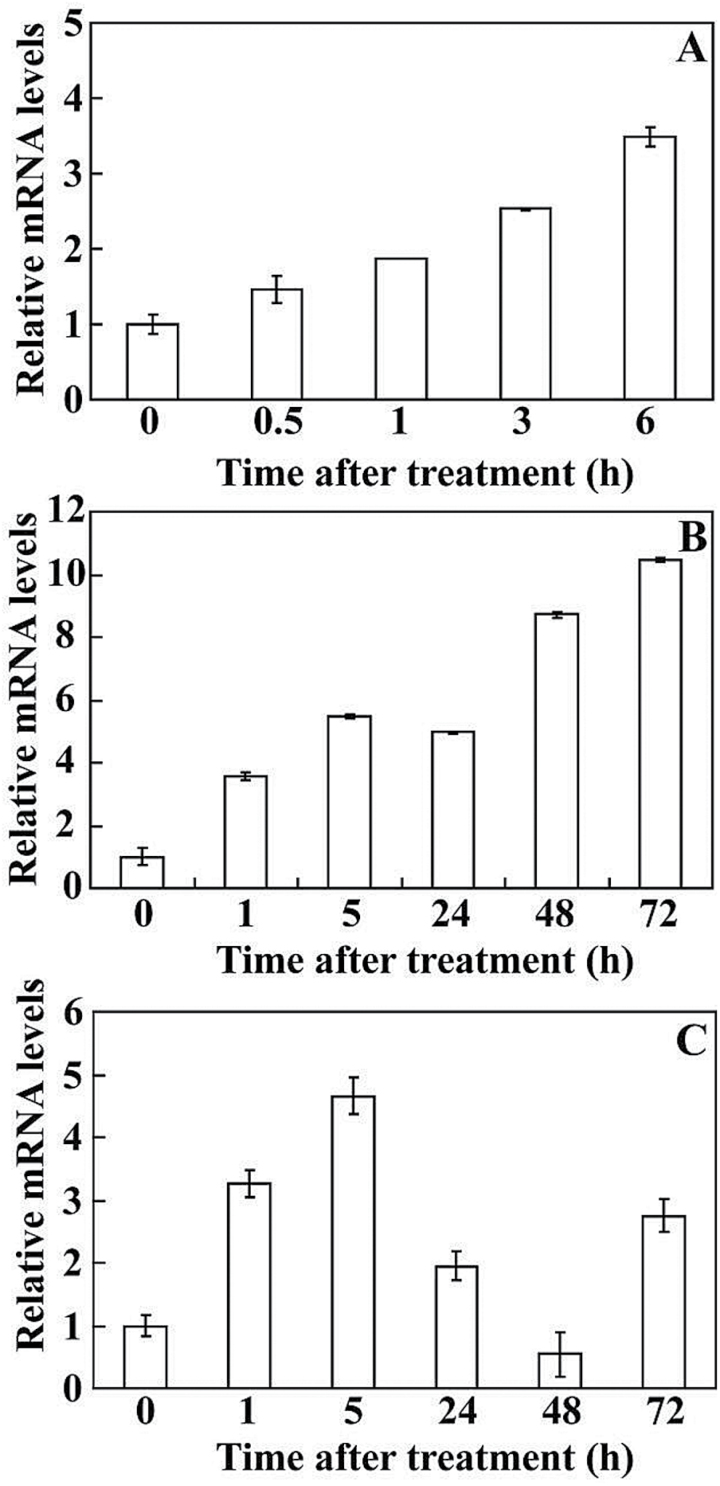
In order to illustrate the potential function of PtrICE1, expression patterns of PtrICE1 under various abiotic stresses were analysed by qPCR. PtrICE1 mRNA accumulated at 0.5h after dehydration, and continued to increase, reaching a maximum at 6h (Fig. 4A). Under cold treatment, the transcript level of PtrICE1 was pronouncedly induced by nearly 4 folds, and then increased progressively to its highest level at 72h (more than 10-fold the initial level) (Fig. 4B). PtrICE1 transcripts exhibited steady elevation within 5h of salt stress initiation, but gradually decreased to their lowest value at 48h, followed by an increase at the last time point (Fig. 4C).
Fig. 4.
Expression of PtrICE1 under abiotic stresses, analysed by qPCR. Time-course expression patterns of PtrICE1 in response to dehydration (A), cold (B), and salt stress (C). Error bars show the standard deviation based on four replicates.
Overexpression of PtrICE1 confers cold tolerance in transgenic tobacco and lemon
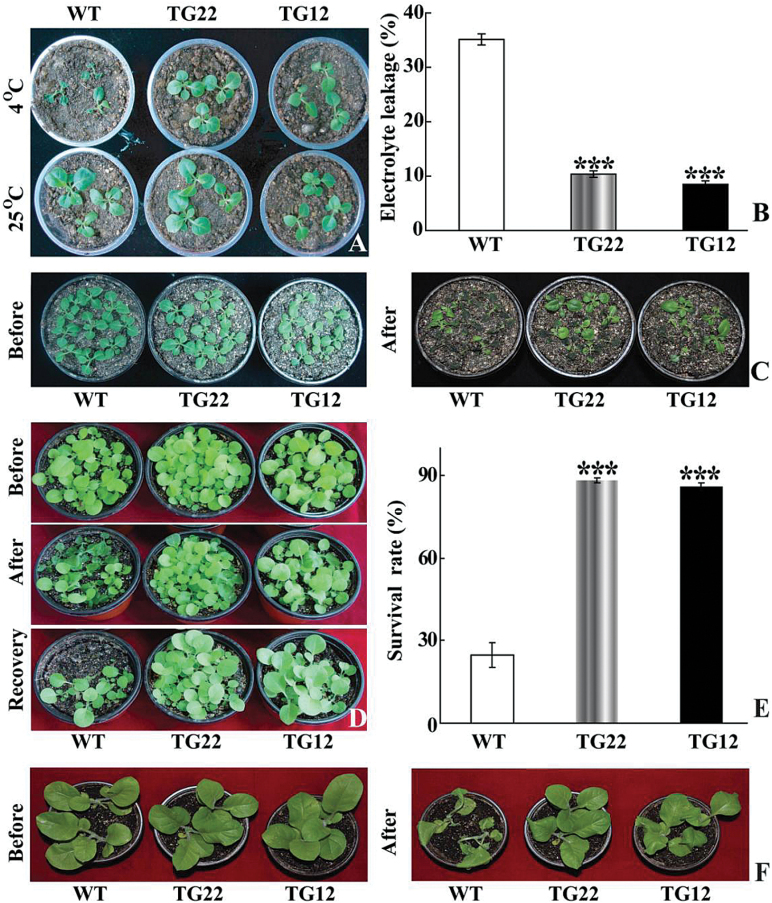
As PtrICE1 transcripts were induced to their highest level by cold, efforts were then made to illustrate the role of this gene in cold tolerance. To this end, we first generated transgenic tobacco plants overexpressing PtrICE1 via Agrobacterium-mediated transformation. Two independent lines (hereafter designated as TG22 and TG12) with higher transcript levels of PtrICE1 were used for cold tolerance analysis. To assess cold tolerance, tobacco plants of different ages were subjected to cold treatment at either chilling (4 °C) or freezing (0 °C) temperature. The transgenic plants were morphologically indistinguishable from the WT under normal growth conditions. When 30-d-old seedlings were treated for 48h at 4 °C, the WT plants suffered more serious cold damage compared with the transgenic lines (Fig. 5A). Electrolyte leakage, a reliable indicator of cell membrane damage caused by abiotic stresses, was used to indicate the stress tolerance capacity. At the end of cold stress, electrolyte leakage of TG12 (9.8%) and TG22 (10.0%) plants was significantly lower than that of the WT (35.0%) (Fig. 5B). When the plants were exposed to cold stress (4 °C for 5 d), the transgenic lines displayed less serious damage in comparison with the WT (Fig. C). In another experiment, exposure of 35-d-old plants to 0 °C for 24h led to severe injury to the WT plants, whereas the transgenic plants were affected to a less serious degree (Fig. 5D). After recovery growth for 12 d in an ambient environment, the survival rate of WT plants was 24.7%, significantly lower than that of the transgenic lines: 88.2% for TG22 and 86.0% for TG12 (Fig. 5E). When larger plants were treated at 0 °C for 24h, the transgenic plants also displayed a better tolerance relative to the WT (Fig. 5F).
Fig. 5.
Overexpression of PtrICE1 confers enhanced cold tolerance in tobacco. (A) Plant phenotype of tobacco WT and transgenic (TG22 and TG12) plants before and after cold treatment for 2 d at 4 °C. (B) Electrolyte leakage of WT and transgenic plants after cold treatment. (C) Plant phenotypes of WT and transgenic lines before and after cold treatment at 4 °C for 5 d. (D) Plant phenotypes of WT and transgenic lines before and after cold treatment at 0 °C for 1 d, followed by recovery growth for 5 d in an ambient environment. (F) Survival rates of WT and transgenic plants. (G) Plant phenotype of larger WT and transgenic plants before and after cold treatment at 4 °C for 1 d. Asterisks indicate that the transgenic lines and WT are significantly different from each other (***P<0.01). (This figure is available in colour at JXB online.)
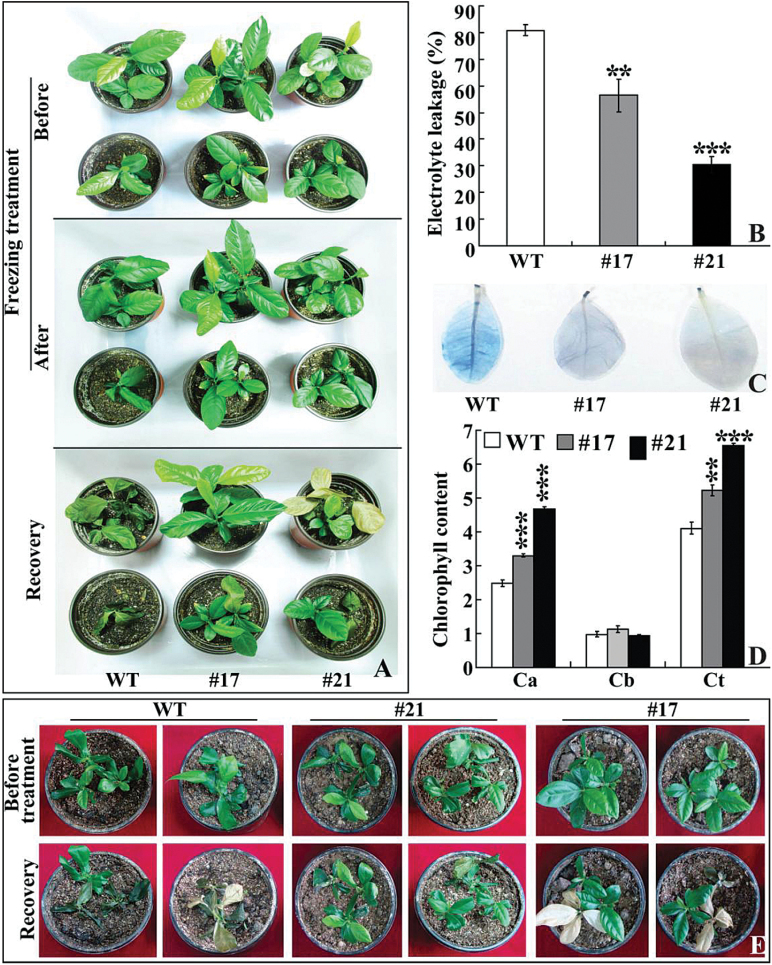
PtrICE1 was also transferred into lemon to further characterize its role in cold tolerance. Two transgenic lemon lines (#17 and #21) exhibiting a higher abundance of PtrICE1 mRNA, along with untransformed plants (WT), were subjected to cold treatment at freezing temperature. They were similar to each other in plant morphology in the absence of cold stress. Treatment at –3 °C for 4h led to conspicuous damage on the WT plants, but the damage on transgenic lines was less serious. After recovery growth for 5 d in an ambient environment, the WT plants failed to survive, whereas most of the transgenic plants grew healthily (Fig. 6A). WT had an electrolyte leakage of 80.9%, while electrolyte leakage of the two transgenic lines was significantly lower, 56.4% for #17 and 30.4% for #21 (Fig. 6B). Trypan blue staining was used to reveal cell death after the freezing treatment. As shown in Fig. 6C, the WT was more strongly stained in comparison with lines #17 and #21, suggesting that it experienced more detrimental damage. Chlorophyll level, a parameter for indicating leaf damage associated with cold stress, was also quantitatively measured. After the freezing treatment, chlorophyll a and total chlorophyll levels in the transgenic lines were considerably higher than those in the WT, but no significant difference in chlorophyll b was observed (Fig. 6D). These data indicated that overexpression of PtrICE1 in both tobacco and lemon conferred enhanced cold tolerance of the transgenic plants.
Fig. 6.
Overexpression of PtrICE1 confers enhanced cold tolerance in lemon. (A) Plant phenotypes of lemon WT and transgenic lines (#17 and #21) before and after freezing treatment (–3 °C for 4h), followed by recovery growth for 5 d in an ambient environment. (B–D) Electrolyte leakage (B), cell death (C), and chlorophyll content (D) in WT and transgenic lines after freezing treatment. Asterisks indicate a significant difference between transgenic lines and WT (**P<0.01; ***P<0.001). Ca, chlorophyll a; Cb, chlorophyll b; Ct, total chlorophyll. (E) Tolerance assay of WT and transgenic lines subjected to freezing treatment (–6 °C for 1.5h), followed by 4 d recovery in an ambient environment. The upper panels are plants before treatment, while the bottom panels are plants after recovery. (This figure is available in colour at JXB online.)
PtrICE1 interacts with PtADC
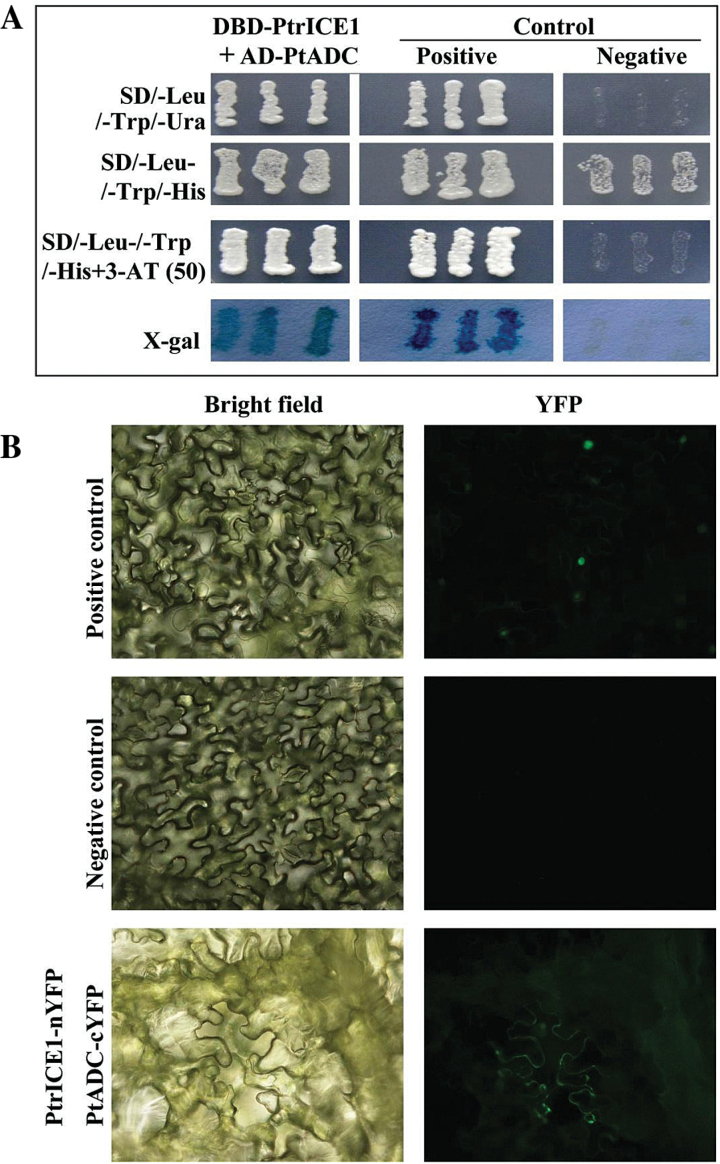
To elucidate the molecular mechanisms underlying the enhanced cold tolerance of transgenic plants overexpressing PtrICE1, a Y2H assay was carried out to identify proteins that may function in a complex with PtrICE1. Autoactivation was examined initially on the full-length or truncated PtrICE1; the assay showed that only full-length PtrICE1 could activate the reporter gene in the absence of prey (data not shown), suggesting that PtrICE1 could autoactivate itself. Therefore, truncated PtrICE1 was used to generate the bait for screening of a cDNA library. A total of 44 putative PtrICE1-interacting candidates were obtained. After sequencing, 21 positive proteins were identified (Table 1), due to the presence of more than one copy for some candidates. The PtrICE1-interacting proteins included transcription factors (bHLH122, MYB, and YABBY3), an abscisic acid receptor (PYR1), kinases (casein kinase and serine/threonine protein kinase), stress-associated proteins (arginine decarboxylase, Cu/Zn superoxide dismutase, and LEA5), and others. Interestingly, PtADC of trifoliate orange has been shown previously to play a role in cold tolerance (Wang et al., 2011), which prompted us to focus on this protein. First, the interaction between PtrICE1 and PtADC was confirmed by a point-to-point Y2H assay. As shown in Fig. 7A, the yeast cells could grow on SD/-Leu/-Trp/-His medium. When 3-AT was added to the medium, only yeast cells of the positive control and co-transformant with bait and prey grew normally, whereas the negative control did not survive, consistent with the growth on SD/–Leu/–Trp/–Ura medium. The yeast growth was further supported by the assay of α-galactosidase activity. A BiFC assay was then performed to verify the Y2H analysis. Green fluorescence was observed in tobacco epidermis transformed with vectors containing PtrICE1–nYFP and PtADC–cYFP or with vectors of the positive control. In contrast, there was no fluorescence in the cells transformed with vectors of the negative control (Fig. 7B). Both the Y2H and BiFC assays indicated that PtrICE1 could interact with PtADC.
Table 1.
Information about the PtrICE1-interacting proteins
| No. | Accession no. | Details | Clones |
|---|---|---|---|
| 1 | Cs5g13930.1 | 21kDa seed protein; Miraculin | 10 |
| 2 | orange1.1t00168.2 | 40S ribosomal protein S3-3; 30S ribosomal protein S3P | 2 |
| 3 | Cs8g14970.1 | Putative 60S ribosomal protein L10A;60S ribosomal protein L10a-3 | 1 |
| 4 | orange1.1t01026.1 | Abscisic acid receptor PYR1 | 1 |
| 5 | Cs8g07560.2 | Arginine decarboxylase | 2 |
| 6 | orange1.1t03173.1 | Transcription factor bHLH122 | 1 |
| 7 | Cs4g16350.1 | Casein kinase I isoform delta-like; casein kinase I isoform delta-A | 2 |
| 8 | Cs3g27290.1 | Catalase | 3 |
| 9 | Cs3g12080.1 | Cu/Zn superoxide dismutase | 1 |
| 10 | Cs5g07190 | Elongation factor 1-delta 2 | 3 |
| 11 | Cs4g18520.1 | Glycine dehydrogenase A | 2 |
| 12 | Cs2g20230.2 | Glycine-rich protein | 1 |
| 13 | Cs9g04210.1 | Late embryogenesis abundant protein Lea5 | 2 |
| 14 | Cs1g24390 | Mannan endo-1,4-β- mannosidase 7 | 1 |
| 15 | orange1.1t05358.1 | Putative enoyl-CoA hydratase/isomerase yngF | 2 |
| 16 | Cs2g06140.2 | Monothiol glutaredoxin-S17; glutaredoxin-3 | 1 |
| 17 | Cs5g04740.1 | Transcription factor MYB1R1 | 1 |
| 18 | Cs1g21570.2 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 4 | 1 |
| 19 | Cs1g21570.2 | NADH-ubiquinone oxidoreductase | 4 |
| 20 | Cs4g04760.1 | Serine/threonine protein kinase | 1 |
| 21 | Cs7g19090.1 | Tubulin-binding cofactor C domain-containing protein 1 | 2 |
Fig. 7.
Analysis of the interaction between PtrICE1 and PtADC by Y2H and BiFC assays. (A) Growth of yeast cells of the positive control, negative control, and co-transformants of PtrICE1 and PtADC on SD/–Leu/–Trp/–Ura or SD/–Leu/–Trp/–His added with or without 3-AT. The blue colour indicates X-gal activity on SD/–Leu/–Trp medium. (B) BiFC assay using tobacco leaf epidermis. Representative images of the epidermal cells under bright field and fluorescence microscopy are shown. Positive and negative controls were bZIP63–cYFP+bZIP–nYFP and PtrICE1–nYFP+cYFP, respectively.
Transgenic plants have higher levels of ADC transcripts and free polyamines
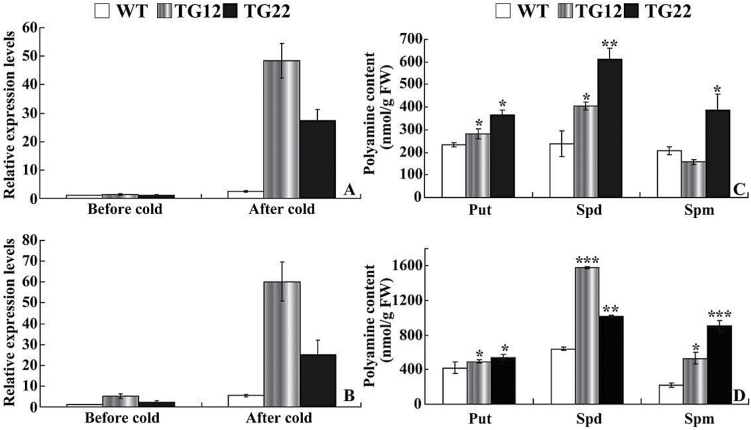
ADC is a key enzyme responsible for the synthesis of putrescine, which is then converted into spermidine and spermine. Efforts were thus made to compare the levels of ADC transcripts and free polyamines between transgenic lines and the WT. Under non-stressful conditions, mRNA levels of NtADC1 and NtADC2 in the transgenic tobacco lines (TG12 and TG22) were slightly higher than those of the WT. Exposure to cold stress caused minor upregulation of NtADC1 and NtADC2 in the WT, but greater induction was observed in the transgenic lines. The transcript levels of NtADC1 in TG12 and TG22 were 19.8 and 11.2 times that in the WT, respectively, while NtADC2 levels in the two transgenic lines were 4.1–11.1 times that in the WT (Fig. 8A, B).
Fig. 8.
Analysis of ADC expression and free polyamines in tobacco. (A, B) Expression patterns of NtADC1 (A) and NtADC2 (B) in tobacco WT and transgenic lines before and after cold stress. (C, D) Free polyamine contents in WT and transgenic lines before (C) and after (D) cold stress. Put, putrescine; Spd, spermidine; Spm, spermine. Asterisks indicate a significant difference between transgenic lines and the WT at the same time point (*P<0.05; **P<0.01; ***P<0.001).
HPLC measurement showed that, in the absence of cold stress, the putrescine content of the WT was 233.5 nmol per g of fresh weight, while TG12 and TG22 had putrescine levels of 282.3 and 363.4 nmol per g of fresh weight, respectively (Fig. 8C). The contents of spermidine and spermine in the two transgenic lines were significantly higher than those of the WT except spermine of TG12. Cold treatment elevated the three polyamines in all tested lines, but it was noticeable that the polyamine levels in TG12 and TG22 were higher than those of the WT (Fig. 8D).
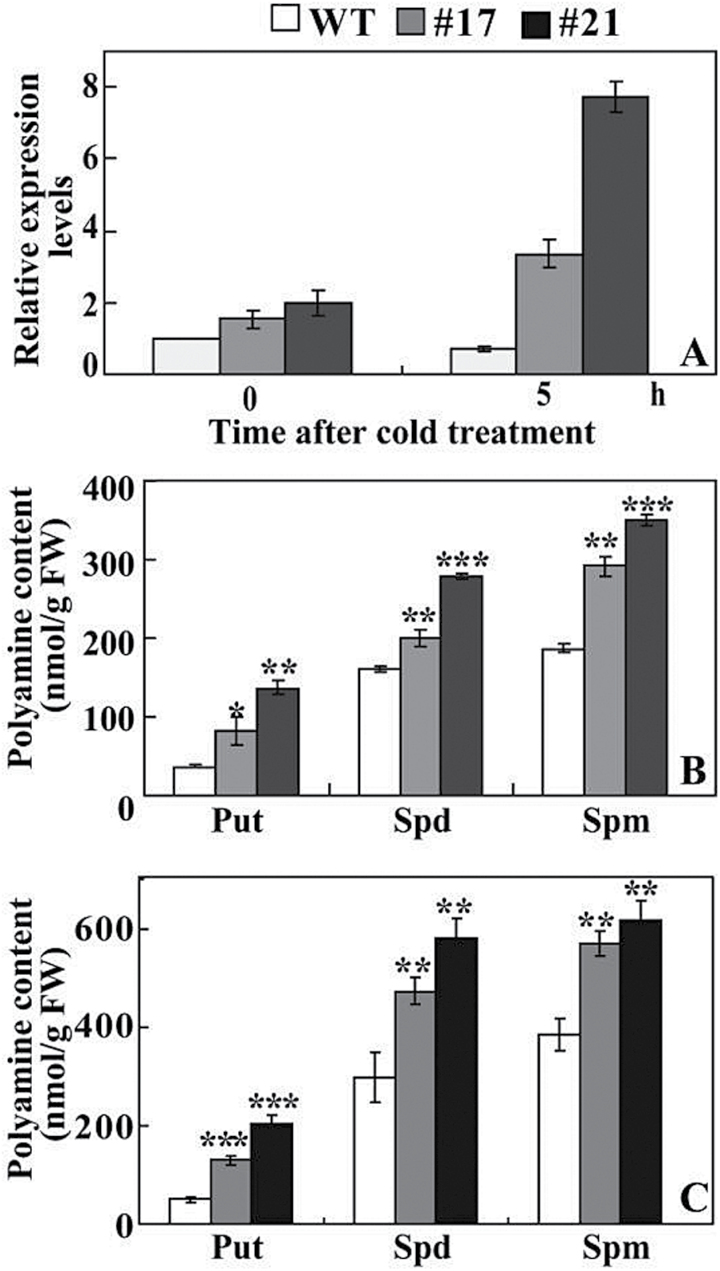
Expression levels of ClADC in lemon were increased in the two transgenic lines when compared with the WT without cold stress, although the difference was not dramatic. After cold treatment for 5h, ClADC transcripts of #17 and #21 were remarkably enhanced, in contrast to a slight induction in the WT (Fig. 9A). The polyamine contents of the two transgenic lines were significantly higher than those of the WT without cold stress (Fig. 9B). Cold treatment increased the levels of putrescine, spermidine, and spermine, whereas the elevation in the transgenic lines was more substantial than in the WT. As a result, #17 and #21 displayed higher levels of the three types of polyamines than the WT did (Fig. 9C).
Fig. 9.
Analysis of ADC expression and free polyamines in lemon. (A) Expression patterns of ClADC in lemon WT and transgenic lines before and after 5h of cold stress. (B) Free polyamine contents in WT and transgenic lines before and after cold stress. Put, putrescine; Spd, spermidine; Spm, spermine. Asterisks indicate a significant difference between transgenic lines and the WT at the same time point (*P<0.05; **P<0.01; ***P<0.001).
Transgenic lines accumulate less ROS and exhibit higher antioxidant enzyme activities under cold stress
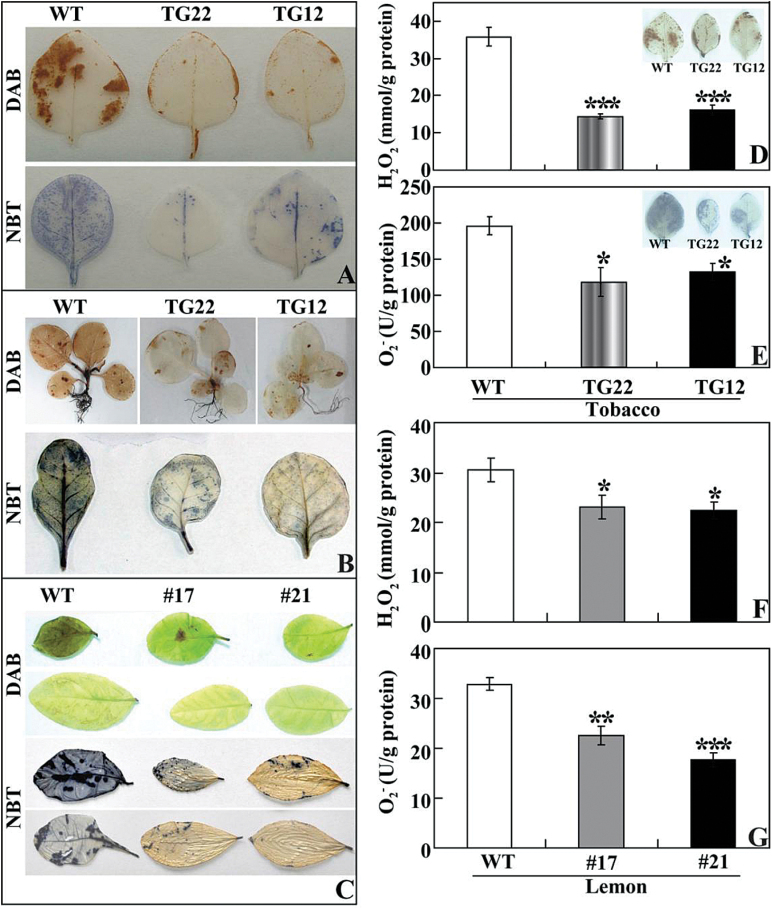
It has been suggested that polyamines are involved in the modulation of ROS homeostasis under abiotic stresses (Liu et al., 2007; Moschou et al., 2008; Tiburcio et al., 2014), so we determined ROS levels in the tested samples. We first examined in situ accumulation of H2O2 and O2 – by histochemical staining with DAB and NBT, respectively. After cold stress at chilling or freezing temperatures, the leaves of transgenic tobacco (Fig. 10A, B) and lemon (Fig. 10C) were stained to a lighter extent compared with those of the corresponding WT. Quantitative measurement further demonstrated that H2O2 and O2 – contents in the transgenic lines of tobacco (Fig. 10D, E) and lemon (Fig. 10F, G) were lower than in the WT. Both histochemical staining and quantitative measurement indicated that the transgenic lines accumulated lower levels of ROS under the cold stresses.
Fig. 10.
Analysis of H2O2 and O2 – in tobacco and lemon under cold stress. (A, B) Representative images indicating in situ accumulation of H2O2 and O2 – in tobacco WT and transgenic lines (TG22 and TG12) after cold stress at chilling (A) and freezing (B) temperatures. (C) Representative images indicating in situ accumulation of H2O2 and O2 – in lemon WT and transgenic lines (#17 and #21) after cold stress. (D, E) Levels of H2O2 (D) and O2 – (E) in tobacco WT and transgenic lines (TG22 and TG12) after cold treatment. The insets in (D) and (E) are histochemical staining patterns using DAB and NBT, respectively. (F, G) Levels of H2O2 (F) and O2 – (G) in lemon WT and transgenic lines (#17 and #21) after cold stress at freezing temperatures. Asterisks indicate significant difference between transgenic lines and the WT (*P<0.05; **P<0.01; ***P<0.001).
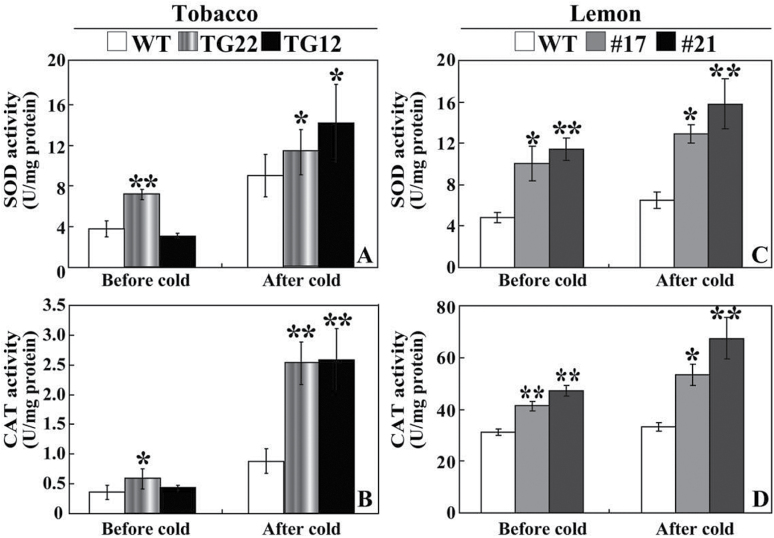
The fact that antioxidant enzymes, such as SOD and catalase (CAT), play crucial roles in ROS detoxification (Gill and Tuteja, 2010) and that they were isolated by Y2H (Table 1) prompted us to assess the enzyme activities of SOD and CAT. Without cold tolerance, the enzyme activities of SOD and CAT in tobacco transgenic lines were higher than (TG22) or similar to (TG12) to those of the WT. Exposure to cold increased the activities of both enzymes, but the activities of the transgenic lines were significantly higher than in the WT (Fig. 11 A, B). Likewise, the enzyme activities of SOD and CAT in the two lemon transgenic lines (#17 and #21) were significantly higher than those of the WT before and after cold treatment (Fig. 11 C, D).
Fig. 11.
Analysis of SOD and CAT activities in tobacco and lemon. (A, B) Activity of SOD (A) and CAT (B) in tobacco WT and transgenic lines (TG22 and TG12) before and after cold treatment. (C, D) Activity of SOD (C) and CAT (D) in lemon WT and transgenic lines (#17 and #21) before and after cold stress. Asterisks indicate a significant difference between transgenic lines and the WT (*P<0.05; **P<0.01).
Discussion
bHLH proteins constitute one of the largest transcription factor families in plants; 177 and 167 bHLHs have been unravelled in the genomes of rice and Arabidopsis thaliana (Bailey et al., 2003; Toledo-Ortiz et al., 2003; Li et al., 2006; Carretero-Paulet et al., 2010). Recently, 117 transcript models encoding canonical bHLHs were identified in the lotus genome (Hudson and Hudson, 2014). Numerous studies indicate that plant bHLH proteins are tightly implicated in a variety of biological processes, such as cell specification (Bernhardt et al., 2003), cell elongation (Bai et al., 2012), flowering (Ito et al., 2012), pollen development (Ko et al., 2014), hormone signalling response (Sasaki-Sekimoto et al., 2014), and secondary metabolism (Xie et al., 2012; Ji et al., 2014). In addition, accumulating evidence shows that bHLHs play pivotal roles in plant responses to various abiotic stresses, including iron deficiency (Sivitz et al., 2012), drought (W. Liu et al., 2014), and cold (Chinnusamy et al., 2003; Chen et al., 2013; Huang et al., 2013; Peng et al., 2014). ICE1 is a well-characterized bHLH protein that acts as an upstream regulator of the transcriptional regulation cascade of the cold response in Arabidopsis (Toledo-Ortiz et al., 2003). However, little is known about the roles of ICE1 homologues in trifoliate orange, a very cold-hardy plant. Thus, characterization of an ICE1 gene of trifoliate orange is crucial to decipher the cold signalling pathway pertinent to freezing tolerance and to provide valuable gene candidates for genetic manipulation.
The bHLH proteins are defined by the presence of conserved bHLH signature domains consisting of two distinct segments, a basic region at the N-terminal end and an HLH region at the C-terminal end (Buck and Atchley, 2003; Toledo-Ortiz et al., 2003; Li et al., 2006). The HLH region contains two amphipathic helices, composed predominantly of hydrophobic residues, which are separated by a loop region of variable length. The basic region, typically rich in basic amino acids, has been shown to be critical for DNA binding, while the HLH domain is responsible for protein–protein interactions to form either homo- or heterodimeric complexes (Toledo-Ortiz et al., 2003). As well as these key signatures, bHLHs contain a leucine-zipper motif characterized by heptad repeats of leucine residues, adjacent to the second helix in the bHLH region (Hudson and Hudson, 2014). In this study, PtrICE1 has the entire set of above-mentioned signature motifs required for defining a typical bHLH transcription factor, despite a low degree of sequence conservation outside the bHLH domain. According to the criterion proposed by Toledo-Ortiz et al. (2003), PtrICE1 should be classified into the category of E-box binders as it contains two specific residues, glutamate (E) and arginine (R), in the basic region. This was supported by a yeast one-hybrid assay, in which PtrICE1 was revealed to recognize a DNA sequence harbouring the E-box element. Multiple alignments revealed that PtrICE1 shared a striking sequence similarity with AtICE1 of Arabidopsis and other plants, indicating that PtrICE1 is a putative ICE1 homologue of trifoliate orange.
PtrICE1 was induced by abiotic stresses, including cold, salt, and dehydration. Upregulation of PtrICE1 by salt and cold was consistent with the expression profiles of AtICE1, but they differed in the response to dehydration, as AtICE1 was not induced under dehydration (Chinnusamy et al., 2003). The disparity of expression patterns between PtrICE1 and AtICE1 in response to dehydration might be presumably ascribed to the inherent difference in plant species. However, durations of dehydration treatment in these studies may also account for the difference, as shorter time frame (30min) was used for Arabidopsis thaliana in comparison with our work. The strongest induction of PtrICE1 transcripts by cold stress forced us to elucidate its function in cold tolerance. The assays demonstrated that overexpression of PtrICE1 in either annual (tobacco) or perennial (lemon) plants resulted in pronouncedly enhanced tolerance to cold stresses, indicating that PtrICE1 acts as a positive regulator of cold signalling cascade. Meanwhile, overexpression of PtrICE1 did not cause negative impacts on plant growth of the transgenic lines under normal growth conditions, suggesting that PtrICE1 might hold great potential for genetic engineering to improve cold tolerance.
ICE1 plays a critical role in cold response by positively regulating CBF3 through binding specifically to the MYCR element in the promoter region (Chinnusamy et al., 2003). This regulation is considered as a classical mode of action on ICE1, which is also reasonable as ICE1, encoding a bHLH transcription factor, might function in cold signalling via transcriptional regulation of its target genes. However, it is worth mentioning that, as protein–protein interactions are important for executing gene function, exploration of PtrICE1-interacting protein may shed new light on the mechanisms underlying enhanced cold tolerance from a different aspect. As a matter of fact, bHLH proteins have been revealed to interact with other non-bHLH transcription factors or functional proteins, forming protein complexes, to participate in various cellular processes. For example, an interaction between MYBs and bHLHs has been shown to play an essential role in governing the synthesis of secondary metabolites, such as glucosinolate and anthocyanin (Xie et al., 2012; Frerigmann and Gigolashvili, 2014). In another work, a dehydrin protein of Medicago truncatula, MtCAS (cold-acclimation specific protein 31), was reported to interact with MtICE1 and influenced stomatal development (Xie et al., 2012). In the current study, Y2H analysis revealed that a total of 21 proteins might constitute the PtrICE1 interactome, among which two proteins (MYB1R1 and bHLH122) belonged to MYB and bHLH families that are capable of interacting with bHLH protein (Xie et al., 2012; Ko et al., 2014). In the future, more work is required to experimentally clarify the interaction between these two proteins and PtrICE1, and to decipher their role in cold tolerance. Interestingly, the interactome included several functional proteins associated with stress tolerance, such as SOD, CAT, late embryogenesis abundant protein (LEA), and ADC. These proteins function, directly or indirectly, in counteracting the abiotic stresses. For instance, SOD and CAT are important antioxidant enzymes for scavenging ROS produced under abiotic stresses (McKersie et al., 1993; Chen et al., 2013). LEAs are hydrophilic proteins involved in the stabilization of proteins and membrane, and the protection of enzymes (Olvera-Carrillo et al., 2011). ADC is a key enzyme responsible for synthesis of polyamines that has been suggested to play a protective role in combating abiotic stresses (Shi and Chan, 2013; Tiburcio et al., 2014). Based on these illustrations, it is tempting to hypothesize that the interaction between PtrICE1 and the stress-associated proteins may work in concert with the well-defined ICE1–CBF3 signalling pathway to fight against cold stress.
PtADC was validated as the bona fide protein interacting with PtrICE1, which led us to examine the expression of ADC genes and endogenous polyamine levels in the transgenic plants. In the absence of cold stress, expression levels of ADC genes in tobacco and lemon transgenic lines were only slightly higher than in the WT. However, exposure to the cold stress resulted in more pronounced induction of the ADC genes in the transgenic lines compared with the WT, leading to more noticeable differences in the transcript levels between them. Whether the interaction accounts for this increase remains to be investigated. Changes in the ADC expression patterns agreed with that of CBF3, which was not strongly activated at warm temperatures but was dramatically induced by cold, in the transgenic lines overexpressing ICE1 (Chinnusamy et al., 2003). It is now clear that post-transcriptional modification, such as sumoylation and ubiquitination (Dong et al., 2006; Miura et al., 2007, 2011), influence the regulation of ICE1 on CBF3 under cold conditions. However, it remains to be investigated whether greater activation of ADC genes in the transgenic lines results from cold-induced modification of PtrICE1. The greater abundance of ADC mRNA was concurrent with higher putrescine levels in the transgenic lines in comparison with the WT. This, in turn, was accompanied by significantly higher levels of spermidine and spermine in the transgenic lines, except for spermine levels in TG12 before cold stress. As putrescine is the precursor of spermidine and spermine, active synthesis of putrescine may cause a feed-forward stimulation of downstream metabolism, leading to the promotion of spermidine and spermine synthesis. It was noticed that higher polyamine levels in the transgenic lines were consistent with enhanced cold tolerance, whereas pre-treatment of the tobacco transgenic lines with d-arginine, an ADC inhibitor, drastically compromised the cold tolerance (data not shown). These results suggest that elevation of polyamine synthesis might contribute to the improvement of cold tolerance in the transgenic lines overexpressing PtrICE1. Our data corroborate previous studies indicating the important roles of polyamines in cold stress tolerance (Shen et al., 2000; Alcázar et al., 2011). In an earlier work, accumulation of putrescine was essential for cold acclimation and plant survival at freezing temperature in Arabidopsis thaliana (Cuevas et al., 2008). In addition, an increase in endogenous polyamine levels by overexpression of polyamine biosynthetic genes or exogenous application of polyamines has been demonstrated to enhance cold tolerance in various plants (Wang et al., 2011; Xu et al., 2011; Song et al., 2014). Polyamines have been suggested to function in elevating stress tolerance due to maintenance of ROS homeostasis by modulating the antioxidant system (Liu et al., 2007; Alcázar et al., 2010; Shi et al., 2010; Wang et al., 2011; Shi and Chan, 2014; Song et al., 2014). Therefore, elevation of the polyamine pool may, along with activated antioxidant enzymes like SOD and CAT, provide a robust ROS detoxification system in the transgenic lines. As a consequence, ROS produced in these lines under cold stress may be detoxified in a more efficient manner, leading to alleviation of oxidative stresses and cold damage. This notion is supported, at least in part, by accumulation of less ROS in the transgenic lines relative to the WT under cold stress conditions.
Taken together, PtrICE1 of trifoliate orange was upregulated by various abiotic stresses, including cold, salt, and dehydration, with the strongest induction under cold conditions. Overexpression of PtrICE1 in tobacco and lemon conferred enhanced tolerance to cold at either chilling or freezing temperatures. A total of 21 proteins were identified as potential candidates interacting with PtrICE1, among which PtADC was confirmed as a true member in the interactome. In addition, higher levels of ADC transcripts and free polyamines were detected in the transgenic lines, concomitant with accumulation of less ROS. All of these results demonstrate that PtrICE1 functions positively in cold tolerance by promoting polyamine accumulation by interacting with ADC. The current study provides new knowledge of the function and underlying mechanism of ICE1 and expands our understanding of the complex cold signalling network.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Phylogenetic comparison of PtrICE1 (shown by a red circle) and 16 ICE1 proteins of other plants.
Supplementary Fig. S2. Alignment between PtrICE1 and PtrbHLH, an ICE1-like protein.
Supplementary Table S1. Primers used in this study.
Acknowledgements
This work was financially supported by the National Science Foundation of China (31272147, 20130146110019), the Ministry of Agriculture, and the National High Technology Research and Development Program of China (2011AA100205).
Glossary
Abbreviations:
- AD
activation domain
- BD
DNA-binding domain
- BiFC
bimolecular fluorescence complementation
- bHLH
basic helix–loop–helix
- CaMV
cauliflower mosaic virus
- DAB
3,3’-diaminobenzidine
- EST
expressed sequence tag
- HPLC
high-performance liquid chromatography
- NBT
nitroblue tetrazolium
- ORF
open reading frame
- qPCR
quantitative real-time RT-PCR
- RACE
rapid amplification of cDNA ends
- ROS
reactive oxygen species
- RT-PCR
reverse transcription-PCR
- Y2H
yeast two-hybrid
- YFP
yellow fluorescent protein.
References
- Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF. 2010. Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231, 1237–1249. [DOI] [PubMed] [Google Scholar]
- Alcázar R, Cuevas JC, Planas J, Zarza X, Bortolotti C, Carrasco P, Salinas J, Tiburcio AF, Altabella T. 2011. Integration of polyamines in the cold acclimation response. Plant Science 180, 31–38. [DOI] [PubMed] [Google Scholar]
- Bai MY, Fan M, Oh E, Wang ZY. 2012. A triple helix-loop-helix/basic helix-loop-helix cascade controls cell elongation downstream of multiple hormonal and environmental signaling pathways in Arabidopsis . The Plant Cell 24, 4917–4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey PC, Martin C, Toledo-Ortiz G, Quail PH, Huq E, Heim MA, Jakoby M, Werber M, Weisshaar B. 2003. Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana . The Plant Cell 15, 2497–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt C, Lee MM, Gonzalez A, Zhang F, Lloyd A, Schiefelbein J. 2003. The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development 130, 6431–6439. [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Buck MJ, Atchley WR. 2003. Phylogenetic analysis of plant basic helix-loop-helix proteins. Journal of Molecular Evolution 56, 742–750. [DOI] [PubMed] [Google Scholar]
- Carretero-Paulet L, Galstyan A, Roig-Villanova I, Martinez-Garcia JF, Bilbao-Castro JR, Robertson DL. 2010. Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis, poplar, rice, moss, and algae. Plant Physiology 153, 1398–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Jiang JF, Song AP, et al. 2013. Ambient temperature enhanced freezing tolerance of Chrysanthemum dichrum CdICE1 Arabidopsis via miR398. BMC Biology 11, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong XH, Agarwal M, Zhu JK. 2003. ICE1, a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis . Genes & Development 17, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu J, Zhu JK. 2006. Gene regulation during cold acclimation in plants. Physiologia Plantarum 126, 52–61. [Google Scholar]
- Cuevas JC, Lopez-Cobollo R, Alcazar R, Zarza X, Koncz C, Altabella T, Salinas J, Tiburcio AF, Ferrando A. 2008. Putrescine is involved in Arabidopsis freezing tolerance and cold acclimation by regulating abscisic acid levels in response to low temperature. Plant Physiology 148, 1094–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CH, Agarwal M, Zhang YY, Xie Q, Zhu JK. 2006. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proceedings of the National Academy of Sciences, USA 103, 8281–8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HL, Ma NN, Meng X, Zhang S, Wang JR, Chai S, Meng QW. 2013. A novel tomato MYC-type ICE1-like transcription factor, SlICE1a, confers cold, osmotic and salt tolerance in transgenic tobacco. Plant Physiology and Biochemistry 73, 309–320. [DOI] [PubMed] [Google Scholar]
- Feng XM, Zhao Q, Zhao LL, Qiao Y, Xie XB, Li HF, Yao YX, You CX, Hao YJ. 2012. The cold-induced basic helix-loop-helix transcription factor gene MdCIbHLH1 encodes an ICE-like protein in apple. BMC Plant Biology 12, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerigmann H, Gigolashvili T. 2014. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana . Molecular Plant 7, 814–828. [DOI] [PubMed] [Google Scholar]
- Fu XZ, Khan EU, Hu SS, Fan QJ, Liu JH. 2011. Overexpression of the betaine aldehyde dehydrogenase gene from Atriplex hortensis enhances salt tolerance in the transgenic trifoliate orange (Poncirus trifoliata L. Raf.). Environmental and Experimental Botany 74, 106–113. [Google Scholar]
- Fursova OV, Pogorelko GV, Tarasov VA. 2009. Identification of ICE2, a gene involved in cold acclimation which determines freezing tolerance in Arabidopsis thaliana . Gene 429, 98–103. [DOI] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48, 909–930. [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. 1985. A simple and general-method for transferring genes into plants. Science 227, 1229–1231. [DOI] [PubMed] [Google Scholar]
- Hu YR, Jiang LQ, Wang F, Yu DQ. 2013. Jasmonate regulates the INDUCER OF CBF EXPRESSION-C-REPEAT BINDING FACTOR/DRE BINDING FACTOR1 cascade and freezing tolerance in Arabidopsis. The Plant Cell 25, 2907–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XS, Liu JH, Chen XJ. 2010. Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Biology 10, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XS, Luo T, Fu XZ, Fan QJ, Liu JH. 2011. Cloning and molecular characterization of a mitogen-activated protein kinase gene from Poncirus trifoliata whose ectopic expression confers dehydration/drought tolerance in transgenic tobacco. Journal of Experimental Botany 62, 5191–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XS, Wang W, Zhang Q, Liu JH. 2013. A basic helix-loop-helix transcription factor, PtrbHLH, of Poncirus trifoliata confers cold tolerance and modulates peroxidase-mediated scavenging of hydrogen peroxide. Plant Physiology 162, 1178–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson KA, Hudson ME. 2014. The basic helix-loop-helix transcription factor family in the sacred lotus, Nelumbo nucifera . Tropical Plant Biology 7, 65–70. [Google Scholar]
- Ito S, Song YH, Josephson-Day AR, Miller RJ, Breton G, Olmstead RG, Imaizumi T. 2012. FLOWERING BHLH transcriptional activators control expression of the photoperiodic flowering regulator CONSTANS in Arabidopsis . Proceedings of the National Academy of Sciences, USA 109, 3582–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Xiao J, Shen Y, Ma D, Li Z, Pu G, Li X, Huang L, Liu B, Ye H, Wang H. 2014. Cloning and characterization of AabHLH1, a bHLH transcription factor that positively regulates artemisinin biosynthesis in Artemisia annua . Plant & Cell Physiology 55, 1592–1604. [DOI] [PubMed] [Google Scholar]
- Ko SS, Li MJ, Sun-Ben Ku M, et al. 2014. The bHLH142 transcription factor coordinates with TDR1 to modulate the expression of EAT1 and regulate pollen development in rice. The Plant Cell 26, 2486–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasensky J, Jonak C. 2012. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. Journal of Experimental Botany 63, 1593–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata C, Prasad M. 2011. Role of DREBs in regulation of abiotic stress responses in plants. Journal of Experimental Botany 62, 4731–4748. [DOI] [PubMed] [Google Scholar]
- Lee BH, Henderson DA, Zhu JK. 2005. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. The Plant Cell 17, 3155–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XX, Duan XP, Jiang HX, et al. 2006. Genome-wide analysis of basic/helix-loop-helix transcription factor family in rice and Arabidopsis . Plant Physiology 141, 1167–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JH, Ban Y, Wen XP, Nakajima I, Moriguchi T. 2009. Molecular cloning and expression analysis of an arginine decarboxylase gene from peach (Prunus persica). Gene 429, 10–17. [DOI] [PubMed] [Google Scholar]
- Liu JH, Inoue H, Moriguchi T. 2008. Salt stress-mediated changes in free polyamine titers and expression of genes responsible for polyamine biosynthesis of apple in vitro shoots. Environmental and Experimental Botany 62, 28–35. [Google Scholar]
- Liu JH, Kitashiba H, Wang J, Ban Y, Moriguchi T. 2007. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnology 24, 117–126. [Google Scholar]
- Liu JH, Moriguchi T. 2007. Changes in free polyamine titers and expression of polyamine biosynthetic genes during growth of peach in vitro callus. Plant Cell Reports 26, 125–131. [DOI] [PubMed] [Google Scholar]
- Liu JH, Peng T, Dai WS. 2014. Critical cis-acting elements and interacting transcription factors, key players associated with abiotic stress responses in plants. Plant Molecular Biology Reporter 32, 303–317. [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. 1998. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought-and low-temperature-responsive gene expression, respectively, in Arabidopsis . The Plant Cell 10, 1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Tai H, Li S, Gao W, Zhang M, Xie C, Li WX. 2014. bHLH122 is important for drought and osmotic stress resistance in Arabidopsis and in the repression of ABA catabolism. New Phytologist 201, 1192–1204. [DOI] [PubMed] [Google Scholar]
- McKersie BD, Chen YR, Debeus M, Bowley SR, Bowler C, Inze D, Dhalluin K, Botterman J. 1993. Superoxide-dismutase enhances tolerance of freezing stress in transgenic alfalfa (Medicago sativa L). Plant Physiology 102, 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J, Catala R, Salinas J. 2011. The CBFs, three Arabidopsis transcription factors to cold acclimate. Plant Science 180, 3–11. [DOI] [PubMed] [Google Scholar]
- Miura K, Jin JB, Lee J, Yoo CY, Stirm V, Miura T, Ashworth EN, Bressan RA, Yun DJ, Hasegawa PM. 2007. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis . The Plant Cell 19, 1403–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Ohta M, Nakazawa M, Ono M, Hasegawa PM. 2011. ICE1 Ser403 is necessary for protein stabilization and regulation of cold signaling and tolerance. The Plant Journal 67, 269–279. [DOI] [PubMed] [Google Scholar]
- Moschou PN, Paschalidis KA, Delis ID, Andriopoulou AH, Lagiotis GD, Yakoumakis DI, Roubelakis-Angelakis KA. 2008. Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. The Plant Cell 20, 1708–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Ito Y, Yamaguchi-Shinozaki K. 2009. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiology 149, 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvera-Carrillo Y, Luis Reyes J, Covarrubias AA. 2011. Late embryogenesis abundant proteins: versatile players in the plant adaptation to water limiting environments. Plant Signaling & Behavior 6, 586–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng PH, Lin CH, Tsai HW, Lin TY. 2014. Cold Response in Phalaenopsis aphrodite and characterization of PaCBF1 and PaICE1 . Plant & Cell Physiology 55, 1623–1635. [DOI] [PubMed] [Google Scholar]
- Pogány M, von Rad U, Grun S, Dongo A, Pintye A, Simoneau P, Bahnweg G, Kiss L, Barna B, Durner J. 2009. Dual roles of reactive oxygen species and NADPH oxidase RBOHD in an Arabidopsis-Alternaria pathosystem. Plant Physiology 151, 1459–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin-Çevik M, Moore GA. 2006. Identification and expression analysis of cold regulated genes from the cold-hardy citrus relative Poncirus trifoliata (L.) Raf. Plant Molecular Biology 62, 83–97. [DOI] [PubMed] [Google Scholar]
- Sasaki-Sekimoto Y, Jikumaru Y, Obayashi T, Saito H, Masuda S, Kamiya Y, Ohta H, Shirasu K. 2014. Basic helix-loop-helix transcription factors JASMONATE- ASSOCIATED MYC2-LIKE1 (JAM1), JAM2, and JAM3 are negative regulators of jasmonate responses in Arabidopsis . Plant Physiology 163, 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan W, Kuang JF, Lu WJ, Chen JY. 2014. Banana fruit NAC transcription factor MaNAC1 is a direct target of MaICE1 and involved in cold stress through interacting with MaCBF1. Plant, Cell & Environment 37, 2116–2127. [DOI] [PubMed] [Google Scholar]
- Shen W, Nada K, Tachibana S. 2000. Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiology 124, 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Chan Z. 2014. Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. Journal of Integrative Plant Biology 56, 114–121. [DOI] [PubMed] [Google Scholar]
- Shi HT, Chan ZL. 2013. In vivo role of Arabidopsis arginase in arginine metabolism and abiotic stress response. Plant Signaling & Behavior 8, e24138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Fu XZ, Peng T, Huang XS, Fan QJ, Liu JH. 2010. Spermine pretreatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiology 30, 914–922. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ding Y, Yang S. 2015. Cold signal transduction and its interplay with phytohormones during cold acclimation. Plant & Cell Physiology 56, 7–15. [DOI] [PubMed] [Google Scholar]
- Sivitz AB, Hermand V, Curie C, Vert G. 2012. Arabidopsis bHLH100 and bHLH101 control iron homeostasis via a FIT-independent pathway. PLoS One 7, e44843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Diao Q, Qi H. 2014. Putrescine enhances chilling tolerance of tomato (Lycopersicon esculentum Mill.) through modulating antioxidant systems. Acta Physiologiae Plantarum 36, 3013–3027. [Google Scholar]
- Talon M, Gmitter FG., Jr 2008. Citrus genomics. International Journal of Plant Genomics 2008, 528361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharis A, Clément C, Barka EA. 2012. Physiological and molecular changes in plants grown at low temperatures. Planta 235, 1091–1105. [DOI] [PubMed] [Google Scholar]
- Thomashow MF. 2001. So what’s new in the field of plant cold acclimation? Lots! Plant Physiology 125, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF. 2010. Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiology 154, 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio AF, Altabella T, Bitrian M, Alcázar R. 2014. The roles of polyamines during the lifespan of plants: from development to stress. Planta 240, 1–18. [DOI] [PubMed] [Google Scholar]
- Toledo-Ortiz G, Huq E, Quail PH. 2003. The Arabidopsis basic/helix-loop-helix transcription factor family. The Plant Cell 15, 1749–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk HA, Thomashow MF. 2006. Arabidopsis transcription factors regulating cold acclimation. Physiologia Plantarum 126, 72–80. [Google Scholar]
- Walter M, Chaban C, Schutze K, et al. 2004. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. The Plant Journal 40, 428–438. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun PP, Chen CL, Wang Y, Fu XZ, Liu JH. 2011. An arginine decarboxylase gene PtADC from Poncirus trifoliata confers abiotic stress tolerance and promotes primary root growth in Arabidopsis . Journal of Experimental Botany 62, 2899–2914. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jiang CJ, Li YY, Wei CL, Deng WW. 2012. CsICE1 and CsCBF1: two transcription factors involved in cold responses in Camellia sinensis . Plant Cell Reports 31, 27–34. [DOI] [PubMed] [Google Scholar]
- Wisniewski M, Nassuth N, Teulières C, Marque C, Rowland J, Cao PB, Brown A. 2014. Genomics of cold hardiness in woody plants. Critical Reviews in Plant Science 33, 92–124. [Google Scholar]
- Xie XB, Li S, Zhang RF, Zhao J, Chen YC, Zhao Q, Yao YX, You CX, Zhang XS, Hao YJ. 2012. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell & Environment 35, 1884–1897. [DOI] [PubMed] [Google Scholar]
- Xu S, Hu J, Li Y, Ma W, Zheng Y, Zhu S. 2011. Chilling tolerance in Nicotiana tabacum induced by seed priming with putrescine. Plant Growth Regulation 63, 279–290. [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. 2005. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends in Plant Science 10, 88–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.