Abstract
Nitrogen (N) cycling is a fundamental process central to numerous ecosystem functions and services. Accumulating evidence suggests that species within detritus- and plant-based food chains can play an instrumental role in regulating this process. However, the effects of each food chain are usually examined in isolation of each other, so it remains uncertain if their effects are equally important or if one chain exerts predominant control. We experimentally manipulated the species composition of detritus-based (isopods and spiders) and plant-based (grasshoppers and spiders) food chains individually and in combination within mesocosms containing plants and microbes from an old-field ecosystem. We tested: (i) their relative impact on N cycling, and (ii) whether interactions between them moderated the influence of one group or the other. We found that spiders in plant-based food chains exerted the only positive effect on N cycling. Detritus-based food chains had no net effects on N cycling but, when combined with plant-based food chains, ameliorated the positive effects of plant-based species. Our results suggest that detritus-based food chains may ultimately limit rates of N cycling by eroding the enhancing effects of plant-based food chains when antagonistic interactions between detritus- and plant-based species exist.
Keywords: trophic cascade, nitrogen mineralization, above-ground–below-ground interactions
1. Introduction
Nitrogen (N) cycling is an important ecosystem process that determines production rates, levels of biodiversity and the trophic structure of ecosystems [1]. Classical theory holds that in terrestrial ecosystems soil macrofauna within detritus-based food chains play a key role in determining N cycling rates, owing to their critical role in breaking down detrital inputs and priming that detritus for microbial decomposition [2,3]. These indirect effects may in turn feed back up the plant-based food chain to control the level of primary and secondary productivity and community structure [2–5]. Theory and emerging evidence shows that carnivores and herbivores within plant-based chains can also control N cycling. These effects can be propagated through predator-driven changes in the feeding behaviour of herbivores, which influences the composition of the plant community and hence plant inputs to the soil through litter fall or root exudation [6–9]. Furthermore, predators can alter herbivore physiology by causing chronic stress and increasing the N excreted by herbivores in their faeces [10]. It follows that net N cycling rate should reflect the combined effects of interactions among species in plant- and detritus-based food chains [6,11]. Yet, research examining trophic effects on N cycling typically considers the effects of these trophic chains in isolation [6]. It therefore remains uncertain if trophic effects propagating along each chain will be maintained if both chains are present. Understanding interactions and feedbacks within and between plant- and detritus-based food chains is needed to offer a complete picture of the impact of species composition on N cycling in ecosystems [12]. To this end, we report here on an experimental evaluation of the relative importance of species interactions within and between plant- and detritus-based food chains for controlling N cycling over a single season.
2. Material and methods
Methodology is explained in detail in the electronic supplementary material.
(a). Experimental design
The experiment was conducted using mesocosms designed to explore the mechanisms of interaction among a circumscribed set of species (figure 1) that are dominant members of plant- and detritus-based food chains of a New England old-field ecosystem [6]. Our explicit goal in using this particular set of species was to systematically resolve mechanisms governing plant- and detritus-based feedbacks on N cycling using a factorial experimental design (figure 1). This mechanistic, factorial approach would not be feasible if we used a broader complement of animal species that normally comprise the old-field system. Hence, our approach reflects a compromise in which we use functionally representative species to capture some biological realism, yet allow for experimental tractability in order to resolve mechanisms [6,13–15]. The soil was hand sorted and mixed with sand in order to exclude soil macrofauna, so our plant communities were grown from rhizomes and seed before the start of the experiment. Natural history observations of plant- and detritus-based food chains in the field offer a working hypothesis of direct interactions among the species, as portrayed in figure 2a.
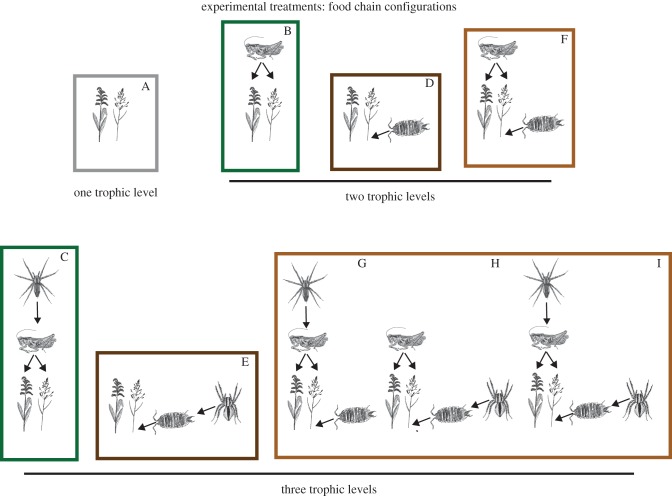
Figure 1.
Illustration of the experimental treatments in which the number of trophic levels and the species composition of the trophic levels were systematically manipulated to test for the effects of species interactions and feedbacks on N cycling. The treatments include a plant-only control (treatment A), plant-based chains (treatments B and C), detritus-based chains (treatments D and E) and mixtures of plant- and detritus-based chains (treatments F–I). Each was replicated five times. The plant species are the grass Poa pratensis and the goldenrod herb Solidago rugosa. The animal species are listed in figure 2. (Online version in colour.)
Figure 2.
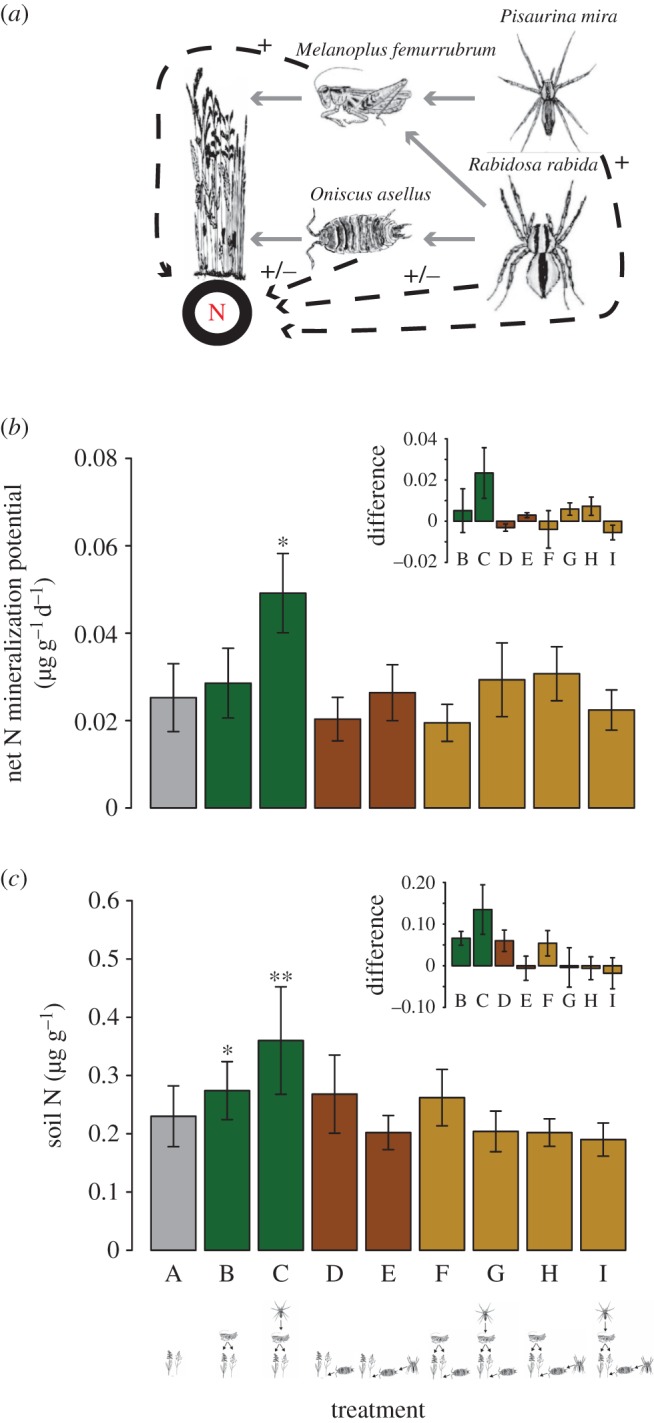
(a) Hypothesized interactions among species used in our experiment. Solid lines represent direct interactions, dashed lines represent positive (+) or context-dependent (±) indirect effects on soil N. (b) Effects of experimental treatment on net N mineralization potential and (c) soil N. Symbols indicate treatments that were significant predictors of variation in net N mineralization potential or soil N (*p < 0.08, **p < 0.01). Insets show the net directional change in treatment effect. Grey, control; green, plant-based chain; brown, detritus-based chain; gold, mixed treatments. (Online version in colour.)
The experiment involved nine treatments across different degrees of trophic complexity (figure 1). The treatments were replicated five times using a randomized block design and isolated the effects of each animal species within each food chain, with the proviso that predators were not added without their primary prey (figure 1).
(b). Data collection
Forty-six days after adding the animals to experimental mesocosms, we captured all the surviving animals, collected plant biomass (roots and shoots) and took soil samples to measure soil N content and to conduct laboratory incubations for net N mineralization potential and microbial biomass.
(c). Data analysis
We analysed net N mineralization potential and soil N using linear mixed-effects models with treatment as the fixed effect and block as the random effect ([16], R v. 3.1.1). We used a linear mixed model analysis with a categorical independent variable and a control treatment. In such an analysis, a significant treatment effect indicates that the treatment deviated significantly from the control after block effects are taken into account. Net N mineralization potential and soil N data were log transformed to meet normality requirements and we removed two replicates from our analyses in which the grass did not successfully seed (final cover less than 10%).
3. Results
(a). Net nitrogen mineralization potential and soil nitrogen
The fully intact plant-based chain treatment (treatment C) composed of plants, grasshoppers and sit-and-wait spiders was the only significant predictor of differences in net N mineralization potential or soil N (p = 0.0314, d.f. = 29, figure 2b; p = 0.0082, d.f. = 30, figure 2c) by enhancing the process (figure 2, insets). The plant-based chain with grasshoppers and plants (treatment B) had a marginally significant effect on soil N (p = 0.074, d.f. = 30, figure 2c). None of the other treatments differed significantly from the plant-only control treatment (p > 0.4).
(b). Plant and microbial biomass
Although grasshoppers and isopods typically prefer N-rich grass in the absence of stress [6,17], we saw no differences in plant community composition or plant biomass across our treatments (electronic supplementary material, figures S2 and S3).
(c). Block effects
Block effects accounted for a large portion of the within-treatment variability in our data and were largely driven by a single block, wherein net N mineralization potential (p = 0.009, d.f. = 37) and soil N (p < 0.001, d.f. = 38) were significantly higher for all treatments.
4. Discussion
We found that the sit-and-wait spiders in the three trophic-level plant-based chain had the only significant impact on net N mineralization potential and soil N. The effect of this particular species on N cycling is consistent with previous work conducted in field plots within our old-field ecosystem [6]. The presence of sit-and-wait spiders is known to alter grasshopper habitat use and feeding behaviour in a manner that increases the grasshopper feeding on goldenrod [6] and causes the grasshoppers to shed extra N in their faeces [10]. This spider species generally does not [6,10,18] and was not found here (electronic supplementary material, figure S5) to increase grasshopper mortality (i.e. mortality was compensatory to natural mortality). The stress-induced changes in grasshopper physiology are likely to have increased soil N and net N mineralization potential, because goldenrod dominance slows N mineralization [18] and grasshopper fecal inputs increase soil N stocks [19].
The detritus-based species alone had no net effect on net N mineralization potential or soil N. One potential explanation is that the soils we used had 26% less soil N (x ± s.e.; 0.24 ± 0.016 µg g dry mass equivalent soil−1) than soils where top-down control on N cycling in detritus-based food chains has been documented (90 µg g dry soil−1 [20]; 25.5 µg g dry soil−1 [21]). Thus, in our experiment, microbes may have been strongly limited by N so that grazing by isopods had little impact on their biomass or functional capacity (electronic supplementary material, figure S4; [22]). Furthermore, isopod survival to the end of the experiment was low, most probably in response to predation and moisture stress (electronic supplementary material, table S3). Low isopod survival and a microbial community limited by low soil N means that the conditions in our experiment probably were outside of the range where top-down feedbacks in the detritus-based chain might predominate [22].
Classic approaches typically consider how plant-based food chains might influence belowground species and associated processes through biomass inputs of detritus to soil [8,19]. The recent appreciation for interactions between detritus- and plant-based species [6,11] motivated our experiment to quantify the interactions and feedbacks concurrently. We show that top-down effects from the plant-based food chain on soil N cycling disappeared when those species were combined with detritus-based species. Our explanation is that between-chain interactions mitigated the net effects of grasshoppers on goldenrod, thereby lowering the N mineralization potential and soil N stocks. This antagonistic interaction was probably density-mediated, because we saw consistently lower grasshopper survival in cages containing both isopods and either or both spiders (electronic supplementary material, figure S5). One explanation is that isopod activity at the soil surface caused a shift in the grasshopper habitat use, most likely early in the experiment when grasshoppers were of a similar size to isopods, that made the grasshoppers more vulnerable to predation [6]. The body mass of the sit-and-wait spiders at the end of the experiment was higher in treatments with isopods, suggesting that the presence of isopods increased their predation success (electronic supplementary material, table S3). Wolf spider predation in treatments H and I may have contributed to the lower grasshopper survival. Therefore, species in the detritus-based chain appear to have disrupted the plant-based trophic cascade by influencing grasshopper survival. Other possible explanations including an isopod effect mediated by the soil microbial community or isopod coprophagy on grasshopper faeces cannot be ruled out using our data, but appear less likely given that isopods had no impact in the absence of spiders. Hence, rate limitation of N cycling in our study appears to have arisen because detritus-based species eroded the positive effects of the plant-based food chain, as opposed to the detritus-based species being exclusive drivers of the process.
Our study was carried out in mesocosms within a specific set of biophysical conditions (e.g. low soil N content, simplified food webs). Even in our highly controlled experimental setting, variation in blocks was driven in part by goldenrod genotype with one genotype causing consistent increases in N mineralization and soil N across all treatments [23]. An important next step then is to quantify how such plant- and detritus-based chain interactions vary across relevant biotic or abiotic (soil N or moisture) gradients. Replicating factorial experiments such as ours across gradients could lead to a more general, systematic understanding of how plant- and detritus-based food chain feedbacks vary with abiotic context or site characteristics, factors which now only confound prediction and management [12]. Considering the impacts of plant- and detritus-based species across nutrient gradients may be particularly informative given the role of nutrient availability in moderating the strength of trophic cascades in both plant- and detritus-based food chains [11,17].
Supplementary Material
Acknowledgements
We thank B. Crowley, T. Crowther, M. Bradford, K. Burghardt, M. Lambert, E. Lazo-Wasem, D. Lopes, J. Smith, A. Trainor and K. Urban-Mead for help and advice and three anonymous referees for helpful comments.
Ethics statement
All methods adhered to Yale University standards and required no permits.
Data accessibility
All raw data are available in the electronic supplemental material, table S3.
Funding statement
Funding was provided by the Schiff Fund of Yale University and the Edna Bailey Sussman Fund and a Natural Science and Engineering Research Council of Canada grant to R.W.B. and NSF grant no. DEB-0816504 to O.J.S.
Author contributions
R.W.B. conceived of the idea and conducted the experiments. R.W.B. and O.J.S. designed the experiments and wrote the manuscript.
Conflict of interests
We declare no competing interests.
References
- 1.Schimel JP, Bennett J. 2004. Nitrogen mineralization: challenges of a changing paradigm. Ecology 85, 591–602. ( 10.1890/03-8002) [DOI] [Google Scholar]
- 2.Seastedt TR. 1984. The role of microarthropods in decomposition and mineralization processes. Annu. Rev. Entomol. 29, 25–46. ( 10.1146/annurev.ento.29.1.25) [DOI] [Google Scholar]
- 3.Moore JC, et al. 2004. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 7, 584–600. ( 10.1111/j.1461-0248.2004.00606.x) [DOI] [Google Scholar]
- 4.Clarholm M. 1985. Interactions of bacteria, protozoa and plants leading to mineralization of soil-nitrogen. Soil Biol. Biochem. 17, 181–187. ( 10.1016/0038-0717(85)90113-0) [DOI] [Google Scholar]
- 5.Bradford MA, et al. 2014. Discontinuity in the responses of ecosystem processes and multifunctionality to altered soil community composition. Proc. Natl Acad. Sci. USA 111, 14 478–14 483. ( 10.1073/pnas.1413707111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz OJ. 2010. Resolving ecosystem complexity, p. 173 Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Strickland MS, Hawlena D, Reese A, Bradford MA, Schmitz OJ. 2013. Trophic cascade alters ecosystem carbon exchange. Proc. Natl Acad. Sci. USA 110, 11 035–11 038. ( 10.1073/pnas.1305191110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank DA. 2008. Evidence for top predator control of a grazing ecosystem. Oikos 117, 1718–1724. ( 10.1111/j.1600-0706.2008.16846.x) [DOI] [Google Scholar]
- 9.Yang LH, Gratton C. 2014. Insects as drivers of ecosystem function. Curr. Opin. Insect Sci. 2, 26–32. ( 10.1016/j.cois.2014.06.004) [DOI] [PubMed] [Google Scholar]
- 10.Hawlena D, Schmitz OJ. 2010. Herbivore physiological response to predation risk and implications for ecosystem nutrient dynamics. Proc. Natl Acad. Sci. USA 107, 15 503–15 507. ( 10.1073/pnas.1009300107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wardle DA, Bardgett RD, Klironomos JN, Setala H, van der Putten WH, Wall DH. 2004. Ecological linkages between aboveground and belowground biota. Science 304, 1629–1633. ( 10.1126/science.1094875) [DOI] [PubMed] [Google Scholar]
- 12.Bardgett RD, Wardle DA. 2010. Aboveground–belowground linkages: biotic interactions, ecosystem processes, and global change, p. 301 New York, NY: Oxford University Press. [Google Scholar]
- 13.Sitvarin MI, Rypstra AL. 2014. Fear of predation alters soil carbon dioxide flux and nitrogen content. Biol. Lett. 10, 20140366 ( 10.1098/rsbl.2014.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowther TW, Boddy L, Jones TH. 2011. Outcomes of fungal interactions are determined by soil invertebrate grazers. Ecol. Lett. 14, 1134–1142. ( 10.1111/j.1461-0248.2011.01682.x) [DOI] [PubMed] [Google Scholar]
- 15.Petersen H, Luxton M. 1982. A comparative analysis of soil fauna populations and their role in decomposition processes. Oikos 39, 287–388. ( 10.2307/3544689) [DOI] [Google Scholar]
- 16.Pinheiro J, Bates D, DebRoy S, Sarkar D, Team ATRDC. 2011. nlme: linear and nonlinear mixed effects models. R package v. 3.1–102. See http://cran.r-project.org/web/packages/nlme/index.html. [Google Scholar]
- 17.Zimmer M, Kautz G, Topp W. 2005. Do woodlice and earthworms interact synergistically in leaf litter decomposition? Funct. Ecol. 19, 7–16. ( 10.1111/j.0269-8463.2005.00926.x) [DOI] [Google Scholar]
- 18.Schmitz OJ. 2006. Predators have large effects on ecosystem properties by changing plant diversity, not plant biomass. Ecology 87, 1432–1437. ( 10.1890/0012-9658(2006)87[1432:phleoe]2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 19.Bardgett RD, Wardle DA. 2003. Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84, 2258–2268. ( 10.1890/02-0274) [DOI] [Google Scholar]
- 20.Mikola J, Setälä H. 1998. No evidence of trophic cascades in an experimental microbial-based soil food web. Ecology 79, 153–164. ( 10.1890/0012-9658(1998)079[0153:neotci]2.0.co;2) [DOI] [Google Scholar]
- 21.Lenoir L, Persson T, Bengtsson J, Wallander H, Wiren A. 2007. Bottom-up or top-down control in forest soil microcosms? Effects of soil fauna on fungal biomass and C/N mineralisation. Biol. Fertil. Soils 43, 281–294. ( 10.1007/s00374-006-0103-8) [DOI] [Google Scholar]
- 22.Crowther TW, Grossart H. 2015. The role of bottom-up and top-down interactions in determining microbial and fungal diversity and function. In Trophic ecology: bottom-up and top-down interactions across aquatic and terrestrial systems (eds Hanley T, Pierre KL.), pp. 260–287. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Crutsinger GM, Collins MD, Fordyce JA, Gompert Z, Nice CC, Sanders NJ. 2006. Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 313, 966–968. ( 10.1126/science.1128326) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data are available in the electronic supplemental material, table S3.