Abstract
How extraintestinal pathogenic Escherichia coli (ExPEC) and antimicrobial-resistant E. coli disseminate through the population is undefined. We studied public restrooms for contamination with E. coli and ExPEC in relation to source and extensively characterized the E. coli isolates. For this, we cultured 1,120 environmental samples from 56 public restrooms in 33 establishments (obtained from 10 cities in the greater Minneapolis-St. Paul, MN, metropolitan area in 2003) for E. coli and compared ecological data with culture results. Isolates underwent virulence genotyping, phylotyping, clonal typing, pulsed-field gel electrophoresis (PFGE), and disk diffusion antimicrobial susceptibility testing. Overall, 168 samples (15% from 89% of restrooms) fluoresced, indicating presumptive E. coli: 25 samples (2.2% from 32% of restrooms) yielded E. coli isolates, and 10 samples (0.9% from 16% of restrooms) contained ExPEC. Restroom category and cleanliness level significantly predicted only fluorescence, gender predicted fluorescence and E. coli, and feces-like material and toilet-associated sites predicted all three endpoints. Of the 25 E. coli isolates, 7 (28%) were from phylogenetic group B2(virulence-associated), and 8 (32%) were ExPEC. ExPEC isolates more commonly represented group B2 (50% versus 18%) and had significantly higher virulence gene scores than non-ExPEC isolates. Six isolates (24%) exhibited ≥3-class antibiotic resistance, 10 (40%) represented classic human-associated sequence types, and one closely resembled reference human clinical isolates by pulsed-field gel electrophoresis. Thus, E. coli, ExPEC, and antimicrobial-resistant E. coli sporadically contaminate public restrooms, in ways corresponding with restroom characteristics and within-restroom sites. Such restroom-source E. coli strains likely reflect human fecal contamination, may pose a health threat, and may contribute to population-wide dissemination of such strains.
INTRODUCTION
Escherichia coli is a major cause of urinary tract and other extraintestinal infections, resulting in considerable morbidity, mortality, and costs (1). Most such infections are caused by distinctive E. coli strains called extraintestinal pathogenic E. coli (ExPEC) because of their enhanced ability to invade and cause disease at extraintestinal sites (2). Such strains' main reservoir is the human intestinal tract, where they usually reside harmlessly as long-term colonizers (3). ExPEC strains can be distinguished from other E. coli strains by their extensive virulence gene repertoire and primarily group B2 phylogenetic background (2).
Dissemination of virulent and antimicrobial-resistant E. coli clones through the human population has been recognized recently as an important contributor to the overall burden of ExPEC-associated disease (4–7). However, the mechanisms of such clonal dissemination remain undefined. Flush toilets create microdroplets containing viable bacteria (8, 9), and both public and private restrooms have been shown to be variably contaminated with human-source bacteria (10–13). However, specific data are scant for E. coli and are absent for ExPEC and antimicrobial-resistant E. coli. Thus, although public restrooms conceivably could represent an environmental reservoir and dissemination point for human gut-source E. coli (14), including ExPEC and antimicrobial-resistant strains, leading to transmission among healthy individuals, further study is needed.
Accordingly, we surveyed public restrooms for environmental E. coli and ExPEC. Specifically, we sought to define (i) the overall prevalence of E. coli and ExPEC in public restrooms, (ii) restroom characteristics associated with positive cultures, (iii) the specific sites within the restroom at highest risk for E. coli, and (iv) the likely origins and potential human health implications of such organisms.
MATERIALS AND METHODS
Epidemiological data.
During 2003, one-time culture surveillance was done in 56 public restrooms located in 33 different establishments in 10 different municipalities situated within 3 counties (Hennepin, Ramsey, and Dakota) in the greater Minneapolis-St. Paul, MN, metropolitan area. These counties are the three most populous in Minnesota, accounting for more than 30% of the state's total population and collectively covering a surface area of 1,362 square miles.
The restrooms were selected as 10 each from 5 different types of establishments (fast-food outlets, gas stations, public parks, supermarkets, and the Minneapolis Veterans Affairs Medical Center [MVAMC]) and 6 from malls/stores. For each restroom, in addition to geographic and institutional location (category of establishment), the data recorded included the restroom's gender status (male, female, or unisex) and overall cleanliness level, which was scored subjectively as 1 to 4 (where 1 is “excellent” and 4 is “poor”), based on visual inspection according to specific criteria (see Table S1 in the supplemental material). Additionally, a traffic score ranging from 0 to 6 was assigned depending on how many people used the restroom during the sampling visit. For each site sampled within the restroom, the presence or absence of presumptive fecal material was recorded.
Sampling approach.
Within each restroom, 20 swab samples were collected from diverse prespecified sites that a priori were considered likely to have fecal contamination or to be touched with bare hands (15). Study personnel were allowed some flexibility in selection of sampling sites, depending on local conditions. Sites were classified as toilet associated (e.g., floor surfaces within 2 ft of the toilet, toilet seat, toilet surfaces, toilet water, and in-stall sanitary napkin receptacle) versus non-toilet associated (e.g., stall latch, sink drain, cold water tap, and handle of the diaper changing table) (see Table S2 in the supplemental material). A checklist was used in each restroom to guide sample collection from appropriate sites.
Swabbing was done systematically by using sterile 6-in., wooden-shaft, cotton-tipped applicators (Fisher Scientific Company, LLC). Swabs were moistened with sterile saline for dry surfaces and were used dry for toilet bowls (water and internal surfaces) and sink drains. The swabbing technique was adjusted for the nature of the surface (e.g., knobs, drains, faucets, floor, toilet bowl, or seat) but was consistent for each type of surface. For flat surfaces, an approximately 100-cm2 area was swabbed. Firm pressure was applied during swabbing. For each site, any substance resembling feces was specifically targeted.
Cultures of primary samples.
After sample collection, swabs were placed immediately in culture tubes containing 10 ml tryptic soy broth supplemented with 20 mg/ml vancomycin and 0.35 mg/ml 4-methyl-umbelliferyl-β-d-glucuronide (MUG), a substrate hydrolyzed by β-glucuronidase (an enzyme characteristic of E. coli) to produce a fluorescent metabolite (16). Samples were kept at ambient temperature during transport to the laboratory (2 h maximum) and then were incubated at 37°C overnight. After incubation, turbid broths were checked with UV light, and 10 μl from each tube that fluoresced was streaked onto eosin-methylene blue agar plates, which were incubated at 37°C overnight. For plates that yielded presumptive E. coli (i.e., lactose- and indole-positive, citrate-negative Gram-negative bacilli with a characteristic E. coli colonial morphology), one such colony per sample, plus mixed Gram-negative growth harvested from the inoculum area of the plate, was saved for PCR-based genotyping.
Virulence genotyping.
To assess the extraintestinal virulence potential of any E. coli strains present, lysates were tested for 38 virulence genes of ExPEC, using established multiplex PCR-based assays (17, 18). The presence of ExPEC was inferred from detection of ≥2 of the following: papAH and/or papC (P fimbriae), sfa/focDE (S and F1C fimbriae), afa/draBC (Dr family adhesins), iutA (aerobactin receptor), and kpsMT II (group 2 capsule synthesis) (19–21). The virulence score was the total number of virulence genes detected, adjusted for multiple detection of the pap, sfa/foc, and kps operons.
Phylogenetic analysis.
For individual E. coli isolates, extended virulence genotype and ExPEC status were determined as described above for lysates. Phylogenetic background (for groups A, B1, B2, C, D, E, and F and Escherichia cryptic clade I) was determined by multiplex PCR (22). Clonal lineage, as indicated by sequence type (ST), was determined using a combination of established ST-specific PCR assays (23), sequence analysis of fumC and fimH (i.e., CH typing) (24), and multilocus sequence typing (MLST) according to the Achtman system (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli).
Antimicrobial susceptibility testing.
Susceptibility to 19 antimicrobial agents was determined by disk diffusion, using Clinical and Laboratory Standards Institute-specified methods, reference strains, and interpretive criteria (25). The agents tested included amikacin, amoxicillin-clavulanate, ampicillin, aztreonam, cefepime, cefoxitin, ceftriaxone, chloramphenicol, ciprofloxacin, ertapenem, gentamicin, imipenem, nalidixic acid, nitrofurantoin, piperacillin-tazobactam, streptomycin, tetracycline, trimethoprim, and trimethoprim-sulfamethoxazole. Intermediate interpretations were analyzed as resistant. The resistance score was the number of agents to which an isolate exhibited resistance. Multidrug resistance status was defined as resistance to at least one representative each of ≥3 drug classes, counting penicillins and cephalosporins separately (26).
PFGE analysis.
XbaI pulsed-field gel electrophoresis (PFGE) analysis was used to assign isolates to pulsotypes based on 94% profile similarity to reference strains (27). Dendrograms were inferred within BioNumerics, version 6.6 (Applied Maths, Austin, TX) according to the unweighted-pair group method based on Dice coefficients. Profiles also were compared with a large private PFGE profile reference library containing 1,925 pulsotypes and representing 4,916 E. coli isolates, as collected from diverse locales, specimen types, hosts, clinical syndromes, and time periods (28).
Statistical analysis.
Comparisons of proportions were assessed using a chi-square test [with (N−1)/N correction] (29). When multiple subgroups were present (i.e., restroom category and gender), pairwise comparisons between subgroups were tested statistically only if the overall comparison was significant. Comparisons of scores were assessed using the Mann-Whitney U test (two-tailed). The significance criterion was P < 0.05.
RESULTS
Screening for E. coli and ExPEC.
In total, 1,120 environmental surveillance samples were collected systematically during 2003 from 56 diverse public restrooms in the greater Minneapolis-St. Paul metropolitan area. Of the 1,120 corresponding MUG-supplemented broth cultures, 168 (15%) from 50 different restrooms (89% of 56) fluoresced, suggesting the presence of E. coli (Fig. 1). Of these, 25 (2.2% overall; 14.9% of fluorescent cultures) from 18 different restrooms (32% of 56) yielded a confirmed E. coli isolate. Of these, 10 (40% of 25; 0.9% overall) from 9 different restrooms (16% of 56) presumptively contained ExPEC according to population DNA PCR, whereas 8 yielded a confirmed ExPEC isolate.
FIG 1.
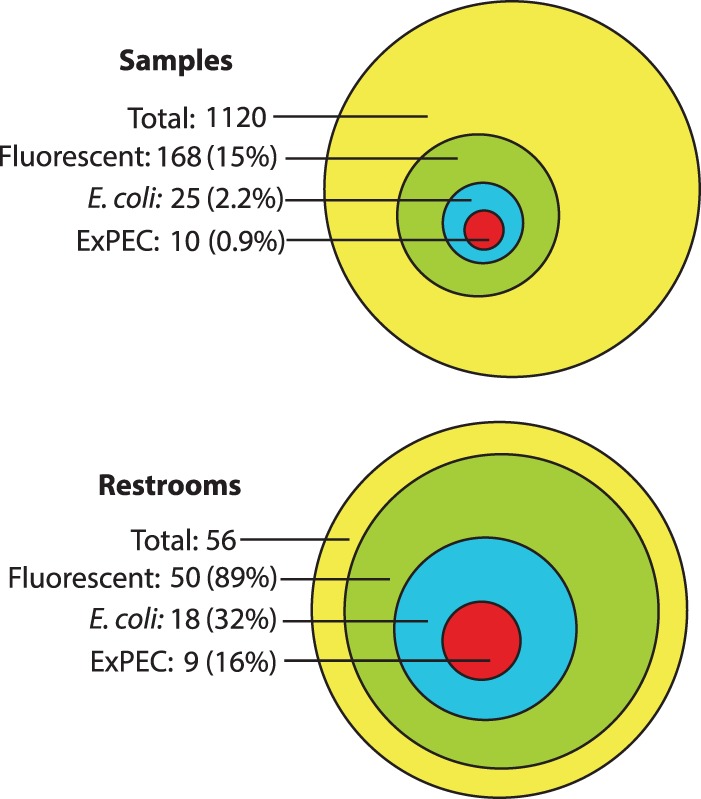
Overall prevalence of positive culture results among environmental samples from public restrooms. (Top) Prevalence by sample (n = 1,120). (Bottom) Prevalence by restroom (n = 56). Culture endpoints of interest included fluorescence (suggesting the presence of β-glucuronidase, hence Escherichia coli), E. coli (i.e., isolated E. coli colonies), and extraintestinal pathogenic E. coli (ExPEC).
Epidemiological associations.
The characteristics of the restrooms and the sites sampled were compared statistically with culture results to identify epidemiological correlates of the microbiological outcomes of interest: i.e., fluorescence, E. coli, and ExPEC. At the restroom level, restroom category was significantly associated only with the prevalence of fluorescence, which ranged from 24% (public parks) to 10% (supermarkets) (6-group comparison, P < 0.001) (Fig. 2). In pairwise comparisons by restroom category, fluorescence was significantly more prevalent for public parks than gas stations (P = 0.005), the MVAMC (P < 0.001), or supermarkets (P < 0.001) and for fast food outlets than the MVAMC (P = 0.04) or supermarkets (P = 0.03) (Fig. 2).
FIG 2.
Prevalence of positive culture results in relation to restroom category among 1,120 environmental samples from public restrooms. Culture endpoints of interest included fluorescence (suggesting the presence of Escherichia coli), E. coli (i.e., isolated colonies), and extraintestinal pathogenic E. coli (ExPEC). The number of restrooms screened per restroom category was 10 for all but malls/stores, for which the number was 6. The number of specimens per restroom was 20. P values are shown for pairwise restroom category comparisons when P is <0.05, as determined by chi-square test [with (N − 1)/N correction]. Significant pairwise comparisons included those between samples from public parks versus gas stations, the Veterans Affairs Medical Center, and supermarkets (top arrows) and those between samples from fast food outlets versus the Veterans Affairs Medical Center and supermarkets (bottom arrows).
Restroom gender also was associated significantly with culture results, for both fluorescence and E. coli but not for ExPEC (Fig. 3). For fluorescence, female and unisex restrooms exhibited a significantly higher prevalence than male restrooms (P = 0.02 and 0.01, respectively) but did not differ from one another. For E. coli, female restrooms exhibited a significantly higher prevalence than unisex restrooms, with male restrooms not significantly different from the others (Fig. 3).
FIG 3.
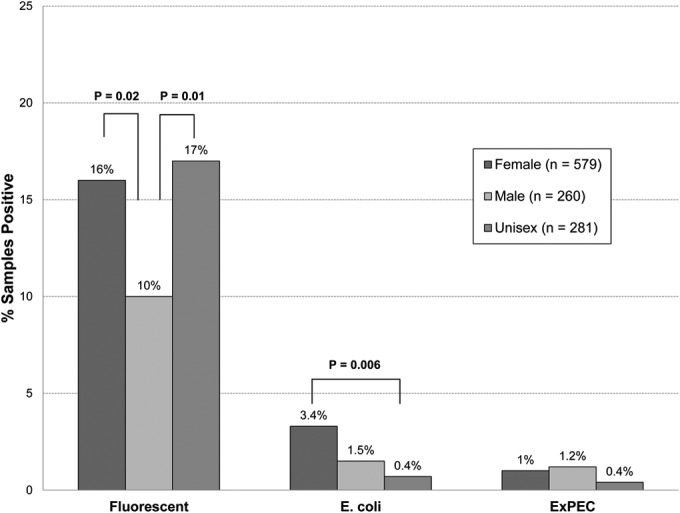
Prevalence of positive culture results in relation to restroom gender among 1,120 environmental samples from public restrooms. Culture endpoints of interest included fluorescence (suggesting the presence of Escherichia coli), E. coli (i.e., isolated colonies), and extraintestinal pathogenic E. coli (ExPEC). P values are shown for comparisons of the three culture endpoints in relation to restroom gender when P is <0.05, as determined by chi-square test [with (N−1)/N correction].
Restroom cleanliness level, which spanned the full range from 1 to 4 (median, 2), was associated weakly with fluorescence (P = 0.04) but not with E. coli or ExPEC (data not shown). In contrast, restroom traffic score, which spanned the full range from 0 to 6 (median, 2), was not associated with any microbiological endpoint (data not shown).
Regarding characteristics of individual samples, the presence of visible feces-like material was significantly associated with all three endpoints, i.e., fluorescence (50% versus 14.6%: P < 0.001), E. coli (33% versus 2%; P < 0.001), and ExPEC (8% versus 0.8%; P = 0.006) (Fig. 4). Toilet-associated sites likewise were significantly associated with all three endpoints: i.e., fluorescence (18.2% versus 12%; P = 0.004), E. coli (4% versus 0.5%; P < 0.001), and ExPEC (1.6% versus 0.2%; P = 0.01) (Fig. 5). Nonetheless, E. coli and/or ExPEC strains were recovered from multiple non-toilet-associated sites and from sites without visible fecal material, including some likely to be touched by bare hands, such as a sink drain, stall lock, and cold water tap (see Table S2 in the supplemental material).
FIG 4.

Prevalence of positive culture results in relation to presence of feces-like material among 1,120 environmental samples from public restrooms. Culture endpoints of interest included fluorescence (suggesting the presence of Escherichia coli), E. coli (i.e., isolated colonies), and extraintestinal pathogenic E. coli (ExPEC). P values are shown for comparisons of the three culture endpoints in relation to presence of feces-like material, as determined by chi-square test [with (N−1)/N correction].
FIG 5.
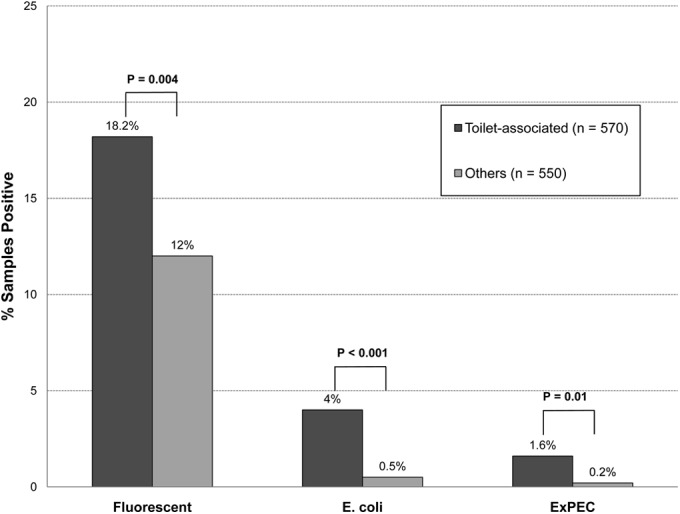
Prevalence of positive culture results in relation to proximity to the toilet among 1,120 environmental samples from public restrooms. Culture endpoints of interest included fluorescence (suggesting the presence of Escherichia coli), E. coli (i.e., isolated colonies), and extraintestinal pathogenic E. coli (ExPEC). P values are shown for comparisons of the three culture endpoints in relation to proximity to the toilet, as determined by chi-square test [with (N−1)/N correction]. Sites in close proximity to the toilet were classified as toilet associated.
Phylogenetic group distribution.
To infer their likely origins and pathogenic potential, the 25 individual E. coli isolates were characterized further as to phylogenetic group, extended virulence genotype, antimicrobial susceptibility profile, and sequence type. Of the eight recognized E. coli phylogenetic groups, five (A, B1, B2, D, and F) were represented, accounting for from 4% to 32% of isolates each, with groups A (commensal-associated, 32%) and B2 (virulence-associated, 28%) predominating (Table 1). Phylogenetic group distribution varied in relation to ExPEC status, with ExPEC isolates being predominantly from group B2 (50% versus 18% for non-ExPEC) and never from group B1 (0% versus 35% for non-ExPEC; P = 0.03).
TABLE 1.
Phylogenetic group distribution by ExPEC status among 25 Escherichia coli isolates from public restrooms
| Phylogenetic group | Prevalence of phylogenetic group, no. of isolates (% of n)a |
||
|---|---|---|---|
| Total (n = 25) | ExPEC (n = 8) | Non-ExPEC (n = 17) | |
| A | 8 (32) | 2 (25) | 6 (35) |
| B1 | 6 (24) | 0 (0) | 6 (35) |
| B2 | 7 (28) | 4 (50) | 3 (18) |
| D | 3 (12) | 1 (12.5) | 2 (12) |
| F | 1 (4) | 1 (12.5) | 0 (0) |
For each phylogenetic group, for extraintestinal pathogenic E. coli (ExPEC) versus non-ExPEC, P >0.10 [by chi-square test, with (N−1)/N correction]. For prevalence of ExPEC among group B2 versus B1, P = 0.03.
Extended virulence genotypes.
Of the 38 studied virulence genes, 32 (84%) were detected in ≥1 of the 25 E. coli isolates, in prevalences ranging from 4% to 100% (Table 2). Multiple virulence genes were detected within each functional category.
TABLE 2.
Virulence-associated traits of 25 restroom-source Escherichia coli isolates
| Traita | Description | Total (% of 25) |
|---|---|---|
| papA, -EF, C, or -G alleles I to IIIb | Pilus associated with pyelonephritis (P fimbriae) | 4 (16) |
| sfa/focDEb | S and F1C fimbriae | 1 (4) |
| sfaS | S fimbriae (sialic acid specific) | 1 (4) |
| afa/draBCb | Dr antigen-specific adhesin operons | 3 (12) |
| iha | Iron-regulated gene homologue adhesin | 6 (24) |
| fimH | d-Mannose-specific adhesion, type 1 fimbriae | 25 (100) |
| hlyD | Alpha-hemolysin | 1 (4) |
| cnf1 | Cytotoxic necrotizing factor 1 | 1 (4) |
| cdtB | Cytolethal distending toxin | 2 (8) |
| ireA | Iron-regulated element | 2 (8) |
| sat | Secreted autotransporter toxin | 5 (20) |
| astA | Enteroaggregative E. coli heat-stable cytotoxin | 1 (4) |
| iroN | Catecholate siderophore receptor | 5 (20) |
| fyuA | Yersinia siderophore receptor | 14 (56) |
| iutAb | Ferric aerobactin receptor (iron uptake: transport) | 7 (28) |
| kpsMT IIb | Group II capsule polysaccharide synthesis (e.g., K1, K5, or K12) | 12 (48) |
| kpsMT K1b | K1 capsule | 5 (20) |
| kpsM K2/K100b | K2 or K100 capsule | 3 (12) |
| cvaC | Microcin (colicin) V | 4 (16) |
| traT | Surface exclusion, serum survival associated | 14 (56) |
| ibeA | Invasion of brain endothelium | 4 (16) |
| ompT | Outer membrane protein T (protease) | 7 (28) |
| iss | Increased serum survival | 3 (12) |
| usp | Uropathogenic-specific protein (bacteriocin) | 7 (28) |
| malX | Pathogenicity-associated island marker | 7 (28) |
| H7 fliC | H7 flagellin variant | 1 (4) |
Six additional traits were sought but were not found, including focG (F1C fimbriae), bmaE, (M fimbriae), gafD (G and F17c fimbriae), clpG (K88-related CS31A adhesin), afaE8 (variant Dr-binding afimbrial adhesin), and rfc (O4 lipopolysaccharide synthesis).
Traits contributing to molecular definition of ExPEC.
Compared with 17 non-ExPEC isolates, the 8 ExPEC isolates had a significantly higher prevalence of 8 virulence genes apart from those used to determine ExPEC status and a significantly lower prevalence of none (Fig. 6). Genes encoding adhesins or toxins were found only among ExPEC isolates (Fig. 6). Similarly, multiple virulence genes were significantly more prevalent among group B2 isolates (i.e., 14 to 100%) than non-group B2 isolates (i.e., 0 to 34%), whereas none were significantly more prevalent among non-B2 isolates (Fig. 6). These patterns mirror those commonly seen among human-source isolates (19).
FIG 6.
Virulence genotypes of 25 Escherichia coli isolates from public restrooms. (Left) Extraintestinal pathogenic E. coli (ExPEC) versus non-ExPEC; right, phylogenetic group B2 versus non-B2. The traits shown are those (among 38 total) that yielded P values of <0.05 for the comparisons of ExPEC isolates (pink bars) versus non-ExPEC isolates (blue bars) and/or for group B2 isolates (pink bars) versus non-B2 isolates (blue bars). Traits are arranged from top to bottom in order of descending prevalence among ExPEC isolates. P value symbols are shown adjacent to the higher-prevalence group when P is < 0.05 as follows: *, P < 0.05, **, P < 0.01, and ***, P < 0.001, as determined by chi-square test [with (N-1)/N correction]. Rectangles enclose traits contributing to molecular definition of ExPEC. Trait definitions: afa/draBC, Dr antigen-specific adhesion operons; cdtB, cytolethal distending toxin; fyuA, Yersinia siderophore receptor; ibeA, invasion of brain endothelium; iha, iron-regulated gene homologue adhesin; ireA, iron-regulated element; iroN, catecholate siderophore receptor; iutA, ferric aerobactin receptor; kpsM II, group 2 capsule polysaccharide synthesis (e.g., K1, K5, and K12); kpsM K1 and K2/K100, group 2 capsule variants; malX, pathogenicity-associated island marker; ompT, outer membrane protein T (protease); papA, P fimbrial structural subunit (with papC, papEF, papG, and papG allele II giving the same result as papA); sat, secreted autotransporter toxin; traT, surface exclusion, serum survival-associated; usp, uropathogenic-specific protein (bacteriocin).
Overall, virulence gene scores ranged from 1 to 13 (median, 5). Collectively, the 8 ExPEC isolates exhibited much higher virulence gene scores (median, 9.5; range, 6 to 13) than the 17 non-ExPEC isolates (median, 2; range, 1 to 10; P < 0.001). Virulence scores also varied significantly by phylogenetic group, along a descending gradient by group median as follows: group B2, 11 (range, 7 to 13); group F, 7 (no range [1 isolate]); group D, 4 (range, 1 to 10); group A, 3 (range, 1 to 8); and group B1, 2 (range, 1 to 5) (P < 0.001). Group B2 isolates had significantly higher scores (median, 11; range, 7 to 13) than did non-B2 isolates (median, 3; range, 1 to 10; P < 0.001) (Fig. 7). These patterns likewise mirror those commonly seen among human-source isolates (19).
FIG 7.

Virulence scores among 25 Escherichia coli isolates from public restrooms. (Left) Extraintestinal pathogenic E. coli (ExPEC) (solid squares) versus non-ExPEC (squares) isolates. (Right) Phylogenetic group B2 (solid circles) versus non-B2 (circles) isolates. Horizontal lines, group medians. P values, as determined by the Mann-Whitney U test (two tailed), are for ExPEC versus non-ExPEC and group B2 versus non-B2.
Antimicrobial resistance.
Resistance was detected in at least one E. coli isolate each for 8 of the 19 study antibiotics, ranging in prevalence from 4% (cefoxitin) to 48% (streptomycin) (Table 3). Resistance scores ranged from 0 to 6 (median, 1) and were as high among ExPEC and group B2 isolates as among non-ExPEC and non-B2 isolates (data not shown).
TABLE 3.
Prevalence of antimicrobial resistance among 25 Escherichia coli isolates from public restrooms

No resistance was detected to amikacin, aztreonam, cefepime, ceftriaxone, chloramphenicol, ciprofloxacin, ertapenem, gentamicin, imipenem, nitrofurantoin, and piperacillin-tazobactam.
Extraintestinal pathogenic Escherichia coli (ExPEC) and non-ExPEC isolates did not differ significantly for the prevalence of any single antimicrobial agent or multidrug resistance status [P > 0.10, by chi-square test, with (N−1)/N correction].
Sequence types.
Clonal typing showed that the 25 E. coli isolates derived from 17 STs. Six of these STs, which accounted for 10 (40%) of the 25 isolates, represented familiar human-associated STs that are known for causing endemic or epidemic ExPEC infections, with or without associated antimicrobial resistance, and/or for human gut colonization (5, 7). These included O1/O2/O18:K1:H7-group B2-ST95 (1 isolate), variable serotype-group A-ST10 (5 isolates), variable serotype-group D-ST405 (1 isolate), O6:K+:H1-group B2-ST127 (1 isolate), O11/O17/O77:K52:H18-group D-ST69 (1 isolate), and O75:K+:H5-group B2-ST14 (1 isolate). Compared with other isolates, a greater proportion of isolates from these familiar STs qualified as ExPEC (6/10 [60%] versus 2/15 [13%]; P = 0.03).
PFGE analysis.
Of the 25 PFGE profiles, 21 (84%) were ≤80% similar to another profile within this study, indicating the independence of most isolates (Fig. 8). The four exceptions were two pairs of indistinguishable profiles, each of which comprised two isolates from the same or adjacent (men's versus women's) restrooms, suggesting local cross-contamination. Comparison of the present PFGE profiles with those in a large private PFGE database (4,916 total profiles, 1,925 total pulsotypes) identified a close match between the ST95 study isolate (FF5-2) and multiple reference ST95 human clinical isolates from diverse locales and clinical syndromes (Fig. 9).
FIG 8.
XbaI pulsed-field gel electrophoresis (PFGE)-based dendrogram for 25 Escherichia coli isolates from public restrooms. The dendrogram was inferred within BioNumerics according to the unweighted-pair group method, based on Dice similarity coefficients. Rectangles enclose two isolate pairs with indistinguishable profiles. ExPEC, extraintestinal pathogenic E. coli; STc, sequence type complex (group of closely related STs).
FIG 9.

XbaI pulsed-field gel electrophoresis (PFGE)-based dendrogram for 11 Escherichia coli isolates from pulsotype 857, sequence type (ST) 95. Isolate FF5-2 (rectangle) is a public restroom isolate from ST95. The 10 reference isolates, all of which likewise represent ST95, were selected from a large private PFGE database based on their PFGE profile similarity to isolate FF5-2.
DISCUSSION
This point-prevalence survey of 56 diverse public restrooms from 10 municipalities in three counties in the greater Minneapolis-St. Paul metropolitan area (2003) yielded four main findings, which have potentially significant public health implications. First, public restrooms were sporadically contaminated with E. coli and ExPEC, many strains of which were antimicrobial resistant. Second, this contamination occurred in somewhat predictable ways in relation to the characteristics of the restrooms, providing some guidance for avoidance and remediation. Third, although the presence of E. coli and ExPEC corresponded significantly with toilet-associated sites and gross fecal material, consistent with a human fecal source, several other sites also yielded E. coli and/or ExPEC, indicating more widespread contamination and, therefore, risk areas. Fourth, some of the restroom-derived ExPEC strains exhibited extensive virulence gene profiles, were multiply antimicrobial resistant, represented classic human-associated STs, and/or resembled known human pathogens by PFGE, implying a potential human health threat. Collectively, these findings provide novel evidence that public restroom-source E. coli strains, many of which represent ExPEC and are antimicrobial resistant, likely reflect human fecal contamination and conceivably could contribute to the population-wide dissemination of such strains.
Regarding the overall prevalence of contamination, although 15% of samples (including at least one sample from 89% of restrooms) fluoresced, suggesting the presence of (E. coli-specific) β-glucuronidase, barely 14% of fluorescing samples (i.e., 2.2% of all samples) yielded confirmed E. coli colonies. This could represent either false-positive fluorescence screens (e.g., from non-E. coli organisms producing β-glucuronidase) or false-negative plate cultures (e.g., from a small E. coli subpopulation dominated on the plates by a majority population of other Gram-negative bacilli). Nonetheless, the confirmed E. coli-positive samples represented 32% of the screened restrooms, indicating fairly widespread contamination at the by-restroom level.
Notably, this is the first reported study to assess restroom-source E. coli for virulence-related traits. We anticipated that we would find E. coli in public restrooms, as humans are known carriers of E. coli and feces are a likely source of environmental E. coli in restrooms. However, a study was needed to empirically verify this hypothesis. Although a fairly small proportion of the present E. coli isolates, as well as the corresponding mixed Gram-negative growth, fulfilled the molecular criteria for ExPEC, the resulting prevalence estimates (0.9% of samples, 18% of restrooms) doubtless understate the true prevalence of ExPEC. This is because we screened only a small fraction of the total surface area of each restroom, albeit focusing on areas that we considered most likely to be contaminated with E. coli, and did not screen for ExPEC in samples that yielded fluorescent broths but no visible E. coli colonies. As such, our findings provide at best a minimum estimate of the true prevalence of E. coli and ExPEC contamination.
Most of the studied restroom characteristics predicted contamination. For example, samples from restrooms in public parks and fast food outlets, as well as visibly unclean restrooms, were more likely to yield fluorescence (although not E. coli isolates or ExPEC) than those from the MVAMC or supermarkets or that appeared clean. Thus, a restroom's location and appearance may predict its likelihood of low-level E. coli contamination, if “fluorescence-only” cultures signify this. Likewise, unisex and female restrooms were significantly associated with fluorescence, and female restrooms were significantly associated with E. coli. Since females are especially vulnerable to urinary tract infections (30), this finding suggests that females might benefit from using extra caution when frequenting public restrooms, e.g., by practicing fastidious hand hygiene and perhaps using barriers on toilet seats.
The finding that the highest-risk areas for contamination were toilet-associated sites and those with feces-like material suggests that human feces are the main source for the recovered E. coli and ExPEC strains, as opposed to the organisms being free-living environmental strains (31, 32). In turn, this suggests that these organisms likely are well adapted for colonization of new human hosts, if acquired by restroom users. Notably, however, E. coli and ExPEC were recovered from certain other areas likely to be touched by hands (sink drain, stall lock, and cold water tap), possibly even after hand washing. Thus, the risk for transmission is not confined to toilet-associated sites or those with visible fecal contamination, so it could not be fully eliminated by careful hand washing or avoidance of fecal-appearing debris. Individuals conceivably could reduce their risk of acquiring E. coli from touchable sites by using hand sanitizers after exiting the restroom. Likewise, the community conceivably could benefit if disinfectant products were used to clean public restrooms for greater bio-burden reduction; however, personal hand hygiene remains crucial (33).
Regarding the isolates' characteristics, many of these environmental E. coli strains were from phylogenetic group B2, contained multiple virulence genes, qualified molecularly as ExPEC, and/or represented classic human-associated lineages, such as STs 10, 14, 69, 95, 127, and 405 (34). Some of these lineages have recently spread widely, via as-yet-undefined mechanisms, to emerge as prominent antibiotic-resistant human pathogens (35–40). Moreover, the ST95 study isolate closely resembled multiple known human clinical ST95 isolates according to PFGE, a more discriminating genomic typing method than MLST (28). These findings further support that the isolates likely originated from human feces, have human virulence potential, and so presumably pose a threat of transmission to restroom users and the possibility of a subsequent infection.
Since several of the restroom-source ExPEC isolates were antimicrobial resistant, transmission to a restroom user conceivably could result in an antimicrobial-resistant infection (41). Additionally, to the extent that non-ExPEC antimicrobial-resistant isolates contain transmissible resistance elements, they also may pose a threat, since if they were to be acquired during restroom use, they conceivably could transfer their resistance elements to a restroom user's (antibiotic-susceptible) endogenous intestinal ExPEC strains (42, 43).
Our study can be contrasted with prior work in the field, which includes three studies that surveyed public restrooms (10–12) and one that surveyed household restrooms (13). For geographic diversity and number of restrooms surveyed, only one prior study exceeded ours (Mendes et al.), and it did not report on E. coli distribution in relation to restroom category (10). Mkrtchyan et al. and Flores et al. surveyed 18 and 12 public restrooms, respectively (11, 12). They reported data mainly for Staphylococcus species and for general gut- and skin-associated taxa, without providing data specifically for E. coli (or did not even study E. coli). For prevalence of contamination in relation to site within restroom, these two studies identified toilet-related sites as highest risk and identified contamination also of some non-toilet sites (hand dryer systems, inner door surfaces, taps, and soap dispensers) but provided no specific details for E. coli. Thus, our study provides novel data relevant to dissemination specifically of pathogenic and antimicrobial-resistant E. coli.
The study limitations include the limited number of restrooms per category, the single-region study design (which may reduce generalizability), reliance on molecular typing to infer virulence potential, and the remote sampling period (2003). Repetition of such a study today to screen for current epidemic clonal groups such as ST131 (39) would be of interest. The study strengths include the diverse and systematic sampling, extensive molecular typing (which allowed comparisons with reference human isolates), and attention to multiple ecological variables as correlates of the microbiological endpoints.
In summary, we have documented the sporadic but widespread presence of ExPEC and antimicrobial-resistant E. coli in public restrooms in a major Midwestern U.S. metropolitan area. These environmental organisms' ecological correlates (i.e., toilet-associated sites and feces-like material) and bacterial characteristics implicate human fecal contamination as their source, implying that they may be adapted for acquisition by and colonization or infection of restroom users. More attention to personal and/or environmental hygiene in public restrooms conceivably could help to protect individual restroom users and also prevent population-wide dissemination of ExPEC and antimicrobial-resistant E. coli.
Supplementary Material
ACKNOWLEDGMENTS
This material is based upon work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, grants 1 I01 CX000192-01 and 1 I01 CX000920-01 (J.R.J.).
Jack Erickson (Minneapolis VAMC) helped prepare the figures.
As a potential conflict of interest, J. R. Johnson has received research grants or consultancies from Crucell, ICET, Merck, Syntiron, and Tetraphase and has patent applications for tests to detect specific E. coli clones. The other authors report no financial conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00638-15.
REFERENCES
- 1.Russo TA, Johnson JR. 2003. Medical and economic impact of extraintestinal infections due to Escherichia coli: an overlooked epidemic. Microbes Infect 5:449–456. doi: 10.1016/S1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 2.Russo TA, Johnson JR. 2000. A proposal for an inclusive designation for extraintestinal pathogenic Escherichia coli: ExPEC. J Infect Dis 181:1753–1754. doi: 10.1086/315418. [DOI] [PubMed] [Google Scholar]
- 3.Nowrouzian FL, Oswald E. 2012. Escherichia coli strains with the capacity for long-term persistence in the bowel microbiota carry the potentially genotoxic pks island. Microb Pathog 53:180–182. doi: 10.1016/j.micpath.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Karlowsky JA, Hoban DJ, Decorby MR, Laing NM, Zhanel GG. 2006. Fluoroquinolone-resistant urinary isolates of Escherichia coli from outpatients are frequently multidrug resistant: results from the North American Urinary Tract Infection Collaborative Alliance-Quinolone Resistance study. Antimicrob Agents Chemother 50:2251–2254. doi: 10.1128/AAC.00123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manges AR, Johnson JR, Foxman B, O'Bryan TT, Fullerton KE, Riley LW. 2001. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N Engl J Med 345:1007–1013. doi: 10.1056/NEJMoa011265. [DOI] [PubMed] [Google Scholar]
- 6.Colpan AJB, Porter S, Clabots C, Anway R, Thao L, Kuskowski MA, Tchesnokova V, Sokurenko EV, Johnson JR, VICTORY (Veterans Influence of Clonal Types on Resistance: Year 2011) Investigators. 2013. Escherichia coli sequence type 131 (ST131) as an emergent multidrug-resistant pathogen among U.S. veterans. Clin Infect Dis 57:1256–1265. doi: 10.1093/cid/cit503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley LW. 2014. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin Microbiol Infect 20:380–390. doi: 10.1111/1469-0691.12646. [DOI] [PubMed] [Google Scholar]
- 8.Gerba CP, Wallis C, Melnick JL. 1975. Microbiological hazards of household toilets: droplet production and the fate of residual organisms. Appl Microbiol 30:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker J, Jones MV. 2005. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J Appl Microbiol 99:339–347. doi: 10.1111/j.1365-2672.2005.02610.x. [DOI] [PubMed] [Google Scholar]
- 10.Mendes MF, Lynch DJ. 1976. A bacteriological survey of washrooms and toilets. J Hyg (Lond) 76:183–190. doi: 10.1017/S002217240005508X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flores GE, Bates ST, Knights D, Lauber CL, Stombaugh J, Knight R, Fierer N. 2011. Microbial biogeography of public restroom surfaces. PLoS One 6:e28132. doi: 10.1371/journal.pone.0028132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mkrtchyan HV, Russell CA, Wang N, Cutler RR. 2013. Could public restrooms be an environment for bacterial resistomes? PLoS One 8:e54223. doi: 10.1371/journal.pone.0054223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott E, Bloomfield SF, Barlow CG. 1982. An investigation of microbial contamination in the home. J Hyg (Lond) 89:279–293. doi: 10.1017/S0022172400070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerhardts A, Hammer TR, Balluff C, Mucha H, Hoefer D. 2012. A model of the transmission of micro-organisms in a public setting and its correlation to pathogen infection risks. J Appl Microbiol 112:614–621. doi: 10.1111/j.1365-2672.2012.05234.x. [DOI] [PubMed] [Google Scholar]
- 15.Rusin P, Orosz-Coughlin P, Gerba C. 1998. Reduction of faecal coliform, coliform and heterotrophic plate count bacteria in the household kitchen and bathroom by disinfection with hypochlorite cleaners. J Appl Microbiol 85:819–828. doi: 10.1046/j.1365-2672.1998.00598.x. [DOI] [PubMed] [Google Scholar]
- 16.Shadix LC, Dunnigan ME, Rice EW. 1993. Detection of Escherichia coli by the nutrient agar plus 4-methylumbelliferyl beta-d-glucuronide (MUG) membrane filter method. Can J Microbiol 39:1066–1070. doi: 10.1139/m93-161. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JR, Stell AL. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis 181:261–272. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JR, Kuskowski MA, Owens K, Soto S, Horcajada JP, Jimenez de Anta MT, Vila J. 2005. Extended virulence genotypes of Escherichia coli isolates from patients with cystitis, pyelonephritis, or prostatitis. J Infect Dis 191:46–50. doi: 10.1086/426450. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JR, Delavari P, Kuskowski M, Stell AL. 2001. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J Infect Dis 183:78–88. doi: 10.1086/317656. [DOI] [PubMed] [Google Scholar]
- 20.Picard B, Sevali Garcia J, Gouriou S, Duriez P, Brahimi N, Bingen E, Elion J, Denamur E. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect Immun 67:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson JR, Murray AC, Gajewski A, Sullivan M, Snippes P, Kuskowski MA, Smith KE. 2003. Isolation and molecular characterization of nalidixic acid-resistant extraintestinal pathogenic Escherichia coli from retail chicken products. Antimicrob Agents Chemother 47:2161–2168. doi: 10.1128/AAC.47.7.2161-2168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clermont O, Christenson JK, Denamur E, Gordon DM. 2013. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep 5:58–65. doi: 10.1111/1758-2229.12019. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JR, Menard M, Johnston B, Kuskowski MA, Nichol K, Zhanel GG. 2009. Epidemic clonal groups of Escherichia coli as a cause of antimicrobial-resistant urinary tract infections in Canada, 2002-2004. Antimicrob Agents Chemother 53:2733–2739. doi: 10.1128/AAC.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weissman SJ, Johnson J, Tchesnokova V, Billig M, Dykhuizen D, Scholes D, Riddel K, Rogers P, Qin X, Fang F, Cookson B, Chattopadhyay S, Sokurenko E. 2012. High-resolution two-locus clonal typing of extraintestinal Escherichia coli. Appl Environ Microbiol 78:1353–1360. doi: 10.1128/AEM.06663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2011. M100-S21 Performance standards for antimicrobial susceptibility testing; 21st informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.Sahm DF, Thornsberry C, Mayfield DC, Jones ME, Karlowsky JA. 2001. Multidrug-resistant urinary tract isolates of Escherichia coli: prevalence and patient demographics in the United States in 2000. Antimicrob Agents Chemother 45:1402–1406. doi: 10.1128/AAC.45.5.1402-1406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis 3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JR, Nicolas-Chanoine MH, DebRoy C, Castanheira M, Robicsek A, Hansen G, Weissman S, Urban C, Platell J, Trott D, Zhanel G, Clabots C, Johnston BD, Kuskowski MA, MASTER Investigators. 2012. Comparison of Escherichia coli ST131 pulsotypes, by epidemiologic traits, 1967-2009. Emerg Infect Dis 18:598–607. doi: 10.3201/eid1804.111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell I. 2007. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Statist Med 26:3661–3675. doi: 10.1002/sim.2832. [DOI] [PubMed] [Google Scholar]
- 30.Foxman B. 2003. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon 49:53–70. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 31.Ishii S, Sadowsky MJ. 2008. Escherichia coli in the environment: implications for water quality and human health. Microbes Environ 23:101–108. doi: 10.1264/jsme2.23.101. [DOI] [PubMed] [Google Scholar]
- 32.Power ML, Littlefield-Wyer J, Gordon DM, Veal DA, Slade MB. 2005. Phenotypic and genotypic characterization of encapsulated Escherichia coli isolated from blooms in two Australian lakes. Environ Microbiol 7:631–640. doi: 10.1111/j.1462-2920.2005.00729.x. [DOI] [PubMed] [Google Scholar]
- 33.Medrano-Felix A, Martinez C, Castro-del Campo N, Leon-Felix J, Peraza-Garay F, Gerba CP, Chaidez C. 2011. Impact of prescribed cleaning and disinfectant use on microbial contamination in the home. J Appl Microbiol 110:463–471. doi: 10.1111/j.1365-2672.2010.04901.x. [DOI] [PubMed] [Google Scholar]
- 34.Manges AR, Johnson JR. 2012. Food-borne origins of Escherichia coli causing extraintestinal infections. Clin Infect Dis 55:712–719. doi: 10.1093/cid/cis502. [DOI] [PubMed] [Google Scholar]
- 35.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. 2010. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis 51:286–294. doi: 10.1086/653932. [DOI] [PubMed] [Google Scholar]
- 36.Cagnacci S, Gualco L, Debbia E, Schito GC, Marchese A. 2008. European emergence of ciprofloxacin-resistant Escherichia coli clonal groups O25:H4-ST 131 and O15:K52:H1 causing community-acquired uncomplicated cystitis. J Clin Microbiol 46:2605–2612. doi: 10.1128/JCM.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cerquetti M, Guifre M, Garcia-Fernandez A, Accogli M, Fortini D, Luzzi I, Carottoli A. 2010. Ciprofloxacin-resistant, CTX-M-15-producing Escherichia coli ST131 clone in extraintestinal infections in Italy. Clin Microbiol Infect 16:1555–1558. doi: 10.1111/j.1469-0691.2010.03162.x. [DOI] [PubMed] [Google Scholar]
- 38.Clermont O, Lavollay M, Vimont S, Deschamps C, Forestier C, Branger C, Denamur E, Arlet G. 2008. The CTX-M-15-producing Escherichia coli diffusing clone belongs to a highly virulent B2 phylogenetic subgroup. J Antimicrob Chemother 61:1024–1028. doi: 10.1093/jac/dkn084. [DOI] [PubMed] [Google Scholar]
- 39.Lee MY, Choi HJ, Choi JY, Song M, Song YG, Kim SW, Chang JJ, Jung SI, Kim YS, Ki HK, Son JS, Kwon KT, Heo ST, Yeom JS, Shin SY, Chung DR, Peck KR, Song JH, Ko SS. 2010. Dissemination of ST131 and ST393 community-onset, ciprofloxacin-resistant Escherichia coli clones causing urinary tract infections in Korea. J Infect 60:146–153. doi: 10.1016/j.jinf.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Nicolas-Chanoine M-H, Blanco J, Leflon-Guibout V, Demarty R, Alonso MP, Caniça MM, Park YJ, Lavigne JP, Pitout J, Johnson JR. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother 61:273–281. [DOI] [PubMed] [Google Scholar]
- 41.Pitout JD. 2012. Extraintestinal pathogenic Escherichia coli: a combination of virulence with antibiotic resistance. Front Microbiol 3:9. doi: 10.3389/fmicb.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linton AH. 1988. Plasmids in the environment. Schriftenr Ver Wasser Boden Lufthyg 78:197–224. [PubMed] [Google Scholar]
- 43.Laroche-Ajzenberg E, Flores Ribeiro A, Bodilis J, Riah W, Buquet S, Chaftar N, Pawlak B. 2015. Conjugative multiple-antibiotic resistance plasmids in Escherichia coli isolated from environmental waters contaminated by human faecal wastes. J Appl Microbiol 118:399–411. doi: 10.1111/jam.12691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





