Abstract
A variety of cell intrinsic or extrinsic stresses evoke perturbations in the folding environment of the endoplasmic reticulum (ER), collectively known as ER stress. Adaptation to stress and reestablishment of ER homeostasis is achieved by activation of an integrated signal transduction pathway called the unfolded protein response (UPR). Both ER stress and UPR activation have been implicated in a variety of human cancers. Although at early stages, or physiological conditions of ER stress, the UPR generally promotes survival, when the stress becomes more stringent or prolonged, its role can switch to a pro-cell death one. Here, we discuss historical and recent evidence supporting an involvement of the UPR in malignancy, describe the main mechanisms by which how tumor cells overcome ER stress to promote their survival, tumor progression and metastasis and discuss the current state of efforts to develop therapeutic approaches of targeting the UPR.
Keywords: ER stress, UPR, cancer, tumorigenesis, therapeutic approaches
1. Introduction
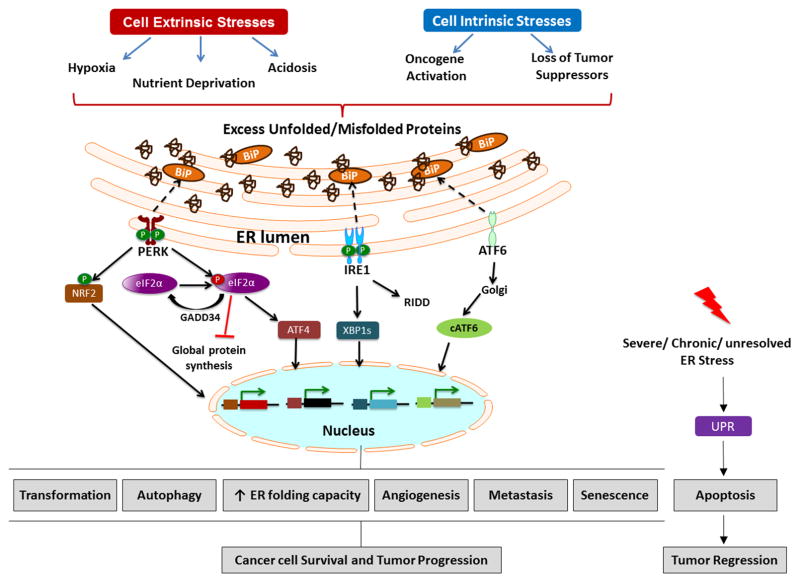
The endoplasmic reticulum (ER) is an extensive membranous network found in all eukaryotic cells. The ER regulates calcium (Ca2+) homeostasis, lipid biogenesis and folding of secretory and membrane bound proteins. The complexity of the ER depends on the predominant functions of the cell type. For example, highly secretory cells, such as pancreatic islets, immune B cells and endothe-lial cells demand a well-developed ER to perform their functions. Proper protein folding and post-translational modifications (glycosylation and lipidation) require both an oxidizing and a Ca2+-rich environment, which is accomplished by the high concentrations of ER chaperone proteins, such as the glucose-regulated protein 78 (GRP78, also known as BiP), calnexin, calreticulin and protein disulfide isomerases (PDI). Many of these chaperones are Ca2+ dependent, underscoring the significance of maintaining the ER Ca2+ concentrations at high levels [1, 2]. Depletion of Ca2+ levels, oxidative stress caused by reactive oxygen species (ROS), low oxygen (hypoxia) or glucose deprivation encountered in pathological conditions (malignancy, neurodegenerative diseases, viral infections), affect ER ho-meostasis, leading to the accumulation of unfolded/misfolded proteins, known as “ER stress” [3]. To overcome these perturbations, a set of signal transduction pathways are activated, which are collectively named the Unfolded Protein Response (UPR) [4]. (Fig. 1)
Figure 1.
Cell extrinsic stresses such as hypoxia, nutrient deprivation and acidosis as well as cell intrinsic stresses that result from oncogene activation and loss of tumor suppressors lead to accumulation of unfolded/misfolded proteins in the ER creating an imbalance between nascent polypeptides and chaperones. Upon ER stress, GRP78 (BiP) is titrated away from ER resident transmembrane proteins to help fold nascent polypeptides and misfolded proteins. Activation of PERK, IRE1 and ATF6 is often seen in tumors and found to be important in regulating processes such as transformation, autophagy, ER folding capacity, angiogenesis, metastasis and senescence, thus promoting tumor initiation and progression. However during chronic or severe ER stress that cannot be mitigated, the UPR can also elicit apoptosis which promotes tumor regression. Thus, the UPR can be a double-edged sword during tumorigenesis.
In mammalian cells, there are three major ER stress sensors, pancreatic ER kinase (PKR)-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1) and activating transcription factor-6 (ATF6), whose main role is to convey the signal from the ER lumen to cytoplasm and nucleus in order to initiate mechanisms to alleviate ER stress [4]. Primarily, cells aim to restore ER homeostasis by increasing the ER capacity, reducing the load of newly synthesized proteins in the ER lumen through inhibition of global protein synthesis and by enhancing ER associated degradation of misfolded proteins (ERAD) [5, 6]. However, if the ER stress persists or ER homeostasis cannot be restored, the role of the UPR tilts towards cell death primarily by initiating apoptosis [7, 8]. The UPR pathway integrates transcriptional and translational responses that enable cells adapt to both cell autonomous and non-cell autonomous stresses. This pathway is often co-opted by cancer cells to promote growth and survival in unfavorable conditions. In this review, we will highlight the role of UPR signaling in cancer and discuss new developments in the field with an emphasis on new therapeutic opportunities targeting ER stress pathways.
2. Evidence of UPR involvement in cancer
Activation of all arms of the UPR has been widely reported in a variety of human tumors including glioblastoma, lymphoma, myeloma and carcinoma of the cervix and breast [9–12]. Tumor cells are often characterized by increased rates of protein synthesis and also face conditions of glucose and oxygen deprivation in the tumor microenvironment [13, 14]. Adaptation to such adverse conditions requires an ER with enhanced folding capacity achieved by increased presence of chaperones and folding enzymes. Indeed, elevated levels of ER chaperones such as GRP78 and GRP94 have been widely reported in tumors and associate with poor outcome and recurrence [15]. Historically, the glucose-regulated proteins were found to be induced during glucose starvation and subsequently their expression was shown to also be increased during ER stress [16, 17]. Specifically, GRP78 was shown to promote tumorigenesis through the regulation of proliferation, invasion and metastasis, an-giogenesis as well as therapy resistance through extensive studies in cell culture and transgenic mouse models of cancer [18–21].
GRP78 has been firmly established as a major regulator of the ER stress sensors PERK, IRE1 and ATF6 [22]. According to the current models, in non-stressed conditions, GRP78 associates with PERK, IRE1 and ATF6 keeping them in an inactive state. During ER stress, GRP78 dissociates from the ER stress sensors to aid in the folding of nascent polypeptides, resulting in activation of the UPR transducers [23]. Upon GRP78 dissociation, PERK is activated through oligomerization and tran-sautophosphorylation [24]. Active PERK regulates translation through phosphorylation of the alpha subunit of eukaryotic initiation factor (eIF2α) at serine 51 [25]. This phosphorylation prevents the exchange of guanidine diphosphate (GDP) to guanidine triphosphate (GTP) on eIF2α [26]. Thus, by decreasing the pools of GTP-bound eIF2α, PERK transiently inhibits global translation, reducing the influx of nascent polypeptides to the ER and allowing time for recovery. Concomitant with suppression of translation, phosphorylation of eIF2α enhances the preferential translation of select mRNAs such as the transcription factor ATF4. ATF4 induces the transcription of chaperones, antioxidants and autophagy promoting genes [27, 28]. Moreover, ATF4 relieves translation inhibition by indirectly upregulating growth arrest and DNA damage gene 34 (GADD34), a protein phosphatase 1 (PP1) cofactor, responsible for eIF2α dephosphorylation, completing a negative feedback loop [29]. In addition to eIF2α, PERK phosphorylates the transcription factor NF-E2-related factor-2 (NRF2), which promotes redox homeostasis [30].
The PERK arm of the UPR has been implicated in tumor initiation and progression in both solid and hematological cancers [9, 10, 31]. PERK was shown to play a major role in tumor growth in vivo in a xenograft model of RASV12-transformed mouse embryonic fibroblasts (MEFs). PERK deficiency resulted in significantly reduced tumor size compared to WT tumors. Similar results were observed with colon carcinoma cells expressing a dominant-negative PERK construct [9]. PERK deficiency significantly reduced tumor proliferation, growth and vascularity in a transgenic mouse model of insuli-noma (pancreatic beta cell tumor), demonstrating the role of PERK in tumor growth in vivo through promoting cell cycle progression and angiogenesis [32]. In a mouse breast cancer model of tumori-genesis, loss of PERK also led to a reduction in the size of growing tumors [31]. Mechanistically, in this model, the PERK/NRF2 arm was shown to regulate proliferation through reduction of oxidative stress. As a result, loss of PERK in breast cancer cells led to G2/M cell cycle arrest through an increase of oxidative stress that activated DNA double strand break checkpoint. Restoration of NRF2 rescues this phenotype. On the other hand, long-term loss of PERK in mammary epithelium modestly increased incidence of adenocarcinomas in aged mice, indicating that PERK/NRF2-mediated suppression of oxidative damage prevents accumulation of DNA damage and suppresses genomic instability that ultimately prevents spontaneous tumor formation [31]. Collectively, these studies provide evidence that PERK is involved in regulating tumor proliferation and growth, yet through suppressing oxidative stress, PERK may also protect normal, untransformed cells from oxidative insults, preventing initial tumor formation.
In other settings, PERK was shown to delay cell cycle progression and suppress tumor formation. PERK promotes cell cycle arrest by suppressing translation of cell cycle regulators, such as Cyclin D1, thus attenuating proliferation during times of ER stress [33, 34]. Expression of dominant negative PERK in mammary epithelial cells enhanced mammary acinar proliferation when cultured in 3D ma-trigel and resulted in disrupted acinar structure with filled lumen. The same cells also displayed increased tumor formation in vivo [35]. On the other hand, PERK has been shown by several groups to be required for prevention anoikis, a type of cell death that occurs after extracellular matrix detachment. Acinar cells that detach from the basement membrane undergo anoikis, resulting in a hollow lumen in 3D cultures. In this study, inducible activation of PERK in mammary epithelial cells resulted in increased survival of cells undergoing anoikis through activation of autophagy and antioxidant responses [36]. These studies indicate that PERK can have both anti-proliferative and pro-survival effects during tumor initiation and tumor progression. However, loss of PERK from normal epithelium prior to tumor initiation can, in certain cases, tip the balance towards delaying tumorigenesis [31]. Interestingly, the level of active PERK that regulates proliferation may be cell type and context-dependent. One example is the finding that basal activation of PERK present in dormant human squamous carcinoma cells supports proliferation, but increased pharmacological activation of PERK in these cells arrests growth in vitro [37]. The activity of PERK could thus be fine-tuned to promote tumor cell survival and the anti-tumorigenic arms could be inactivated through other mechanisms, such as expression of microRNAs that modulate apoptosis [38, 39]. For instance, Chitnis and colleagues reported that PERK/eIF2α/ATF4-mediated expression of miR-211 promoted survival during ER stress by repressing pro-apoptotic CHOP (C/EBP homologous protein) expression. Expression of mir-211 was found to be elevated in transgenic mouse models of mammary tumors compared to control tissue in a PERK-dependent manner. Furthermore, elevated expression of mir-211 was also observed in human B-cell lymphomas, suggesting that microRNAs can suppress anti-tumor effects of PERK that would otherwise negatively impact tumor initiation and progression.
IRE1 is the only branch of the UPR that is conserved in all eukaryotic cells. Similar to PERK, activation of IRE1 during ER stress depends on dissociation of GRP78 [23]. Unlike PERK however, under stress conditions, IRE1 uses its kinase domain for auto-transactivation and its RNase domain to specifically remove an intron from unspliced XBP1 to form a spliced XBP1 (XBP1s). The RNase domain also leads to decay of other mRNAs, which further contributes to decreased protein translation during ER stress [40]. XBP1s is a potent transcription factor responsible for the activation of genes that regulate ER quality control, chaperones and degradation of misfolded proteins [41]. High levels of XBP1s is observed in breast cancer, hepatocellular carcinoma, lymphoma and multiple myeloma (MM) [42–44]. MM is a malignant hematologic cancer characterized by highly secretory plasma cells [45] The IRE1-XBP1 pathway is required for antibody-secreting plasma cell differentiation and function [46]. It is therefore not surprising that XBP1 is highly expressed in multiple myeloma patient samples and correlates with poor survival and clinical outcome to thalidomide based treatment [12]. Although these findings implicate XBP1 in MM, they do not demonstrate a direct role of XBP-1 in the pathogenesis of MM. To address this, Carrasco et al., developed a transgenic mouse model of MM that overexpresses XPB1s in B cells and showed hallmarks of the human disease, suggesting a causative role of XBP-1 in tumorigenesis [47]. In another study, preventing XBP1 splicing by a small molecule that inhibits the endoribonuclease domain of IRE1 significantly decreased MM tumor growth in vivo and increased cytotoxicity in combination with a small molecule inhibitor of the proteasome, bortezomib [48]. Furthermore, a recent study reported XBP1s promoting tumorigenicity and tumor relapse in triple negative breast cancer (TNBC) [49]. Together, these studies provide ample evidence that implicates XBP1 in tumor progression and response to treatment.
Activation of ATF6 during ER stress involves dissociation of GRP78 from the luminal domain and translocation to the Golgi apparatus where it is cleaved by two resident proteases, S1P and S2P [50]. This process releases the ATF6 domain, which serves as a potent transcription factor, targeting a set of genes encoding ER chaperones, proteins involved in quality control and ER associated degradation (ERAD) [51]. Although the role of ATF6 in tumorigenesis has not been studied as extensively as other arms of the UPR, elevated expression of active ATF6 is seen in patient tissues of hepatocellular carcinoma (HCC) as well as Hodgkin lymphoma [44, 52]. Similarly, enhanced expression of active ATF6 is observed in a chemically-induced mouse model of HCC as well as in various human adeno-carcinomas [53]. The consequence of ATF6 activation in tumors is not well understood. Indeed, active ATF6 transfected HCC cells upregulate chaperons such as GRP78 as well as genes involved in regulation of cell cycle, signal transduction, apoptosis, transport and cell adhesion. Enhanced expression of these genes was also observed in human HCC samples suggesting perhaps ATF6 may be important for growth in vivo through regulation of proliferation [54]. In contrast to ATF6 regulating proliferation, ATF6 is also involved in promoting survival of dormant squamous carcinoma cells through activation of Rheb and mTOR [55]. Furthermore, ATF6 could play a role in tumor progression through its effect in cells found in the tumor-microenvironment, such as endothelial cells. Karali et al recently showed that vascular endothelial growth factor (VEGF) activates the UPR in endothelial cells in the absence of unfolded proteins through the mTORC1 pathway. ATF6 and eIF2α were shown to be critical for activation of mTORC2, thus ensuring survival and promoting angiogenesis in human endothelial cells [56]. Taken together, these studies implicate the involvement of ATF6 in tumor progression. Loss of function studies in the future would elucidate the role of ATF6 in tumorigenesis.
The mechanisms of UPR activation during tumor initiation and progression can be grouped into two categories: cell extrinsic or non-cell autonomous stresses, such as hypoxia, acidosis, and nutrient deprivation and cell intrinsic or cell autonomous stresses that result from overactivation of oncogenes or loss of tumor suppressors. (Fig. 1)
3. Cell extrinsic factors that activate the UPR
3.1 Hypoxia and acidosis
Inadequate oxygen availability is a common feature of tumors as cancer cells outgrow their blood supply due to accelerated proliferation and is associated with a more aggressive phenotype and therapy resistance. Oxygen levels in tumors tend to have a very heterogeneous distribution, ranging from moderate to severe hypoxia followed by intermittent reoxygenation [57]. Hypoxic stress elicits response pathways that enable adaptation, thus promoting survival. The most studied pathway involves stabilization of the oxygen sensitive hypoxia-inducible factors, HIF1α and HIF2α. The HIFα form a heterodimer with the constitutively expressed HIF1β to induce expression of a wide range of genes that support adaptation, metabolism and migration of tumor cells enabling escape to a secondary site. [58]. Several reports also show that severe hypoxia results in ER stress triggering the UPR [59, 60]. The mechanism of induction of ER stress during hypoxia is still an active area of research. So far, studies indicate that hypoxia-induced ER stress involves disruption of redox homeostasis in the ER. Protein folding requires disulfide bond formation mediated by PDI, which oxidizes cysteine residues on nascent polypetides and thus itself becomes reduced. Oxidation of PDI is mediated by the thiol reductase ER oxidoreductin 1 (ERO1) and requires oxygen as a final electron acceptor, although there is evidence that indicates a yet unknown electron acceptor besides oxygen in the ER [61, 62]. Thus, during hypoxia, disulfide bond formation is compromised for folding of specific proteins. More-over, increased ROS (H2O2) during hypoxia is also involved in activating the UPR as pretreatment with an antioxidant suppressed hypoxia induced phosphorylation of eIF2α [63].
Protein translation is an energy demanding process and during hypoxia, when cellular energy is limiting, the accumulation of unfolded proteins results in the attenuation of protein translation. The HIFs inhibit mTORC1 which decreases cap-dependent protein translation, whereas, PERK exerts an inhibitory effect on protein synthesis via phosphorylation of eIF2α [60, 64]. As a result, cells with a compromised PERK/eIF2α arm of the UPR fail to block protein synthesis and exhibit reduced survival during hypoxia [9, 59, 65]. PERK-mediated protection of tumors from apoptosis during hypoxia is also observed in xenograft models. Apoptotic cells colocalize with EF-5, a hypoxia marker, in PERK−/− but not in PERK +/+ tumors, suggesting that PERK promotes survival of tumor cells experiencing hy-poxia. ATF4 also localizes to hypoxic regions of solid tumors, such as cervical cancer, and enhanced ATF4 expression is also seen in necrotic areas of metastatic breast tumors known to be severely hy-poxic. This suggests a role for ATF4 in tumor progression and hypoxia tolerance [9, 66, 67]. PERK enhances tolerance to severe hypoxia by activating cytoprotective autophagy through ATF4 mediated upregulation of autophagy genes [68, 69]. Cycling hypoxia that is characterized by oxygen deprivation and re-oxygenation, creates increased ROS levels in hypoxic cells [70]. Furthermore, PERK was shown to be required for suppression of ROS levels through promoting antioxidant responses, thus supporting survival during cycling hypoxia [71]. Importantly, the PERK arm of the UPR also promotes invasion, migration and metastasis of hypoxic breast cancer cells through ATF4 mediated activation of lysosomal-associated membrane protein 3(LAMP3) [72]. The IRE1-XBP1 arm is also important for survival during hypoxia, as cells lacking XBP-1 have decreased viability when exposed to hypoxia compared to their WT counterparts [73].
Hypoxic cells are characterized by a high glycolytic rate mediated by the HIF transcription factors, which promote higher glucose uptake and excess synthesis of lactate. Increased accumulation of acidic metabolites, such as lactate, carbon dioxide and protons, reduces intracellular pH resulting in lactic acidosis, which is detrimental to cell viability. Tumor cells adapt to acidosis by activating transporters that efflux lactate to the extracellular environment [74]. Acidosis also activates the UPR although the molecular mechanisms of how acidosis results in ER stress are not clear. Increased p-eIF2α and ATF4 are observed in vitro when cells are exposed to lactic acidosis [75]. Interestingly, the combination of hypoxia with lactic acidosis increased ATF4 levels while it prevented HIF1α protein synthesis. Inhibition of ATF4 expression with a short hairpin RNA during combination of hypoxia and lactic acidosis significantly increased apoptosis, illustrating that ATF4 is critical for survival of cancer cells experiencing both hypoxia and acidosis [75].
Crosstalk between the HIF pathway and the UPR is beginning to emerge. Zhu et al recently demonstrated that ATF4 is a critical regulator of bone angiogenesis in response to hypoxia through directly forming a complex with and stabilizing HIF1α in osteoblasts [76]. Since the bone is one of the major sites of metastasis for primary tumors, this study raises the possible role of ATF4 in mediating survival of metastatic cells in the bone by activating angiogenesis. ATF4 and HIF also share common targets such as carbonic anhydrase 9 (CA9) and VEGF [77, 78]. This suggests that the two pathways could cooperate to regulate tumor pH and induce angiogenesis in hypoxic regions of the tumor. Indeed, Pereira et al recently reported the interaction between the UPR and HIF pathway results in the UPR potentiating activity of HIF1α mediated transactivation of target genes such as VEGFA and BNIP3 [79]. Furthermore, direct interaction of XBP1s with HIF1α was recently described to enhance HIF1α transcriptional activity in TNBC. In the same study, the XBP-1 gene signature correlated with HIF1α gene signature and associated with poor survival in patients with TNBC [49]. Collectively, these studies highlight the role of the UPR in promoting survival and migratory ability of hypoxic tumor cells, along with possibly collaborating with the HIF pathway.
Tumors initiate angiogenesis that supports their growth, albeit the blood vessels tend to be abnormal and leaky. Mounting evidence now implicates the UPR as a mediator of the angiogenic switch through regulation of a plethora of angiogenic factors including VEGF during both glucose deprivation and hypoxia [79–84]. PERK deficient cells show significant impairment of tumor growth accompanied by poor vascularization in vivo compared to their WT counterparts. Mechanistically, PERK promoted translation of angiogenic factors in response to hypoxia to support survival and maturation of blood vessels [85]. Furthermore, pharmacological inhibition of PERK in xenograft models of multiple human cancer cells impaired tumor growth and significantly reduced blood vessel density [86]. Similarly, knockdown of XBP1 in MEFs and human fibrosarcoma cells significantly reduced tumor growth and decreased blood vessel formation [80, 81]. Moreover, glucose deprivation induced multiple pro-angiogenic mediators while suppressing anti-angiogenic factors in a UPR-dependent but HIF1α-independent manner, highlighting a unique role of UPR as a responder of both hypoxia and glucose deprivation [84].
Tumor angiogenesis has been targeted therapeutically and is effective in some contexts. However most approaches involving angiogenesis inhibitors face therapy resistance through processes including but not limited to increased hypoxia, which further promotes invasive and highly metastatic tumors [87]. The current established role of the UPR in tumor angiogenesis makes it an attractive target in combination with angiogenic inhibitors, thus eliminating hypoxic cells that would otherwise survive through activation of the UPR.
3.2 Nutrient Deprivation
In addition to hypoxia, nutrient deprivation is a common feature of tumors attributed to abnormal vasculature and poor perfusion. Eukaryotic cells adapt to nutrient starvation conditions by activating the Integrated Stress Response (ISR). The ISR is mediated by four kinases, PERK, general control nonderepressible 2 (GCN2), RNA-dependent protein kinase (PKR) and heme regulated inhibitor (HRI) that converge on phosphorylation of eIF2α [88]. Each kinase is activated by specific cellular stresses, but the common target of the ISR is phosphorylation of eIF2α which culminates in the translation of ATF4. ATF4 is critical for inducing a transcriptional response tailored for each stress, but how ATF4 elicits different responses to alleviate various stresses that activate the ISR kinases is a critical question that remains to be examined. Although ATF4 is a common target, each kinase can also activate different factors which in collaboration with ATF4 influence the stress response [89]. In this review we will focus on the kinase GCN2 that is activated by amino acid deprivation (AAD). During AAD, uncharged tRNAs activate GCN2, which phosphorylates eIF2α and subsequently increases ATF4 translation [90, 91]. ATF4 regulates adaptation to AAD by augmenting amino acid metabolism through transcription of amino acid transporters (SLC3A2, SLC7A5 and GLYT1) and enzymes that regulate synthesis of amino acids, such as asparagine synthase (ASNS). Activation of ATF4 is also vital for suppressing oxidative stress through induction of gluthathione biosynthesis. Therefore, ATF4 deficient MEFs require supplementation of non-essential amino acids and antioxidants for growth in vitro and fail to form tumors in vivo [27].
The GCN2/eIF2α/ATF4 signaling is elevated in primary human liver, breast, lung and head and neck tumors [92, 93]. Tumor cells require ATF4 for proliferation and growth in vitro. This defect can be partially rescued by expression of ASNS, an enzyme that is involved in the synthesis of aspar-agine. The GCN2-ATF4 pathway is also essential for tumor growth in vivo, suggesting that this pathway promotes tumor cell survival through eliciting an adaptation response that promotes amino acid import and synthesis [92]. L-asparaginase (ASNase), which depletes plasma asparagine, is widely used as part of a multidrug regimen for the treatment of childhood acute lymphoblastic leukemia (ALL). Although this treatment leads to remission in most patients, development of resistance is reported in about 15% of ALL patients [94]. Cell culture studies show ASNase resistant cells have elevated ASNS expression as well as amino acid transporters to enhance asparagine synthesis [95].
While increasing amino acid metabolism certainly is critical for survival during nutrient deprivation, tumor cells have to ultimately acquire a new blood supply to ensure nutrient availability and metastasize. Wang et al., showed that the GCN2-ATF4 pathway also regulates angiogenesis in vivo. Impairing GCN2 expression in human head and neck squamous cell carcinoma cells resulted in small tumors with decreased blood vessel density. GCN2 knockdown tumors had decreased expression of ATF4 and VEGF compared to GCN2 WT tumors, indicating that ATF4-mediated expression of VEGF is vital for tumor angiogenesis [93].
4. Cell Intrinsic Stresses (Myc, RAS, BRAF and HER2)
The path to becoming a cancer cell is a multistep process that involves stages such as neoplastic transformation, aberrant proliferation, cell growth and eventually metastasis. Importantly, to form a tumor, cancer cells have to override multiple “check points” including apoptotic pathways that block aberrant growth and be able to sustain the energy to meet the demands of rapid proliferation. The transformation stage involves mutations that result in oncogene activation and loss of tumor suppressors. Previous study showed that loss of the tuberous sclerosis complex (TSC1 and TSC2) tumor suppressor genes activates the UPR in a cell autonomous manner [96]. Oncogenes can take advantage of normal functions they regulate and activate additional pathways to support growth and survival. Oncogene activation induces replicative and metabolic stresses in cells [97, 98]. Thus, oncogene activation is a type of intrinsic stress because of the increased burden placed on cells to augment biosynthetic pathways and rewire metabolism to meet the demand of rapid proliferation. Here, we will discuss the involvement of the UPR in oncogene induced transformation and tumor progression (Fig. 1).
4.1 MYC
The proto-oncogene c-MYC (hereafter referred to as MYC) belongs to a family of genes (MYC, MYCN, MYCL) and regulates important biological processes such as proliferation, metabolism, cell growth and apoptosis [99]. Given its pleiotropic functions that are vital for cancer cell growth and survival, it is not surprising that it is one of the highly deregulated genes in human tumors. MYC is rather unique as an oncogene, as its activation dramatically enhances protein translation via transcriptional upregulation of the translational machinery, including ribosomal proteins and initiation factors [13]. The ability of MYC to upregulate protein synthesis was observed previously in a transgenic mouse model of lymphoma, Eμ-Myc, that overexpress myc in B cells [100, 101]. Many groups have focused on targeting the translation machinery to test whether it affects lymphomagenesis in Eμ-Myc mouse [102–104]. The studies showed increased survival of animals when protein translation factors were targeted. Barna et al., demonstrated that genetically restoring protein synthesis in Eμ-Myc mice to normal levels significantly reduced lymphomagenesis through enhanced apoptosis of lymphoma cells [103].
Recently, our group, in collaboration with the Ruggero lab, demonstrated that the ability of MYC to increase rate of protein synthesis in cells can also activate the PERK arm of UPR, which is required for supporting MYC induced transformation and survival. Although MYC is a potent activator of proliferation, it also induces apoptosis, preventing transformation and tumor initiation. Hence, suppression of apoptosis through cooperation with other oncogenes and pathways are reported to promote MYC induced transformation [99]. Consistently, PERK activation was required for suppression of MYC induced apoptosis. Ablation of PERK in hematopoietic progenitor cells significantly reduced transformation by MYC and dominant negative p53 as a result of increased apoptosis. PERK was required for counteracting MYC induced apoptosis by activating cytoprotective autophagy and attenuating Ca+ release from the ER [10]. Importantly, enhanced activation of the PERK/eIF2α pathway was observed in B cells isolated from lymphoma patients compared to healthy donors suggesting that MYC can activate stress response pathways such as the UPR that sustain its tumorigenic property and further promote survival. Another study in Drosophila melanogaster has corroborated these findings. Overexpression of MYC in Drosophila fat cells resulted in PERK-mediated autophagy induction. Interestingly, PERK and autophagy were both dispensable for physiological growth of fat cells but required for MYC induced cell growth as inhibition of PERK or autophagy suppressed MYC induced cell growth [105]. Therefore, through its direct effect on eIF2α, PERK appears to act as a “brake” to fine-tune protein translation as well as activate cytoprotective autophagy. Although the molecular mechanisms of survival that are mediated by autophagy to counteract apoptosis in these conditions remain to be elucidated, an attractive hypothesis is that autophagy prevents protein toxicity through degradation of excess unfolded proteins. The involvement of the other arms during MYC induced tumorigene-sis is currently under investigation. Thus, the UPR is one such pathway activated to support MYC induced transformation and cell growth, making it an attractive therapeutic target in MYC driven cancers.
ATF4 appears to have dual roles in MYC driven cancer cells, as it has been reported to induce apoptosis in response to glutamine deprivation but also required for glutamine metabolism. Qing and colleagues reported that ATF4 mediates apoptosis upon glutamine deprivation in human neuroblas-toma cells with amplified MYCN through the transcription of pro-apoptotic NOXA, PUMA and TRIB3. Knockdown of ATF4 partially rescued viability during glutamine starvation [106]. Interestingly, in another study ATF4 was shown to regulate glutamine metabolism in neuroblastoma cells through cooperative transcriptional induction of a glutamine transporter, ASC2, with MYCN. The same study showed that relative expression of MYCN, ATF4 and ASC2 was significantly elevated in human neu-roblastomas with amplified MYCN [107]. These studies indicate the complex role of ATF4 in MYC overexpressing cells and calls for further examination of the role of ATF4 at different stages of tumor initiation and progression as well as correlation with its levels induced by physiological stress versus pharmaceutical intervention.
4.2 RAS
The role of the UPR in RAS-induced tumorigenesis is still unclear, and it appears that the UPR may again play a dual role in this process, depending on the stage of transformation/tumor progression. ATF4 has been shown to be involved in RAS induced transformation and oncogene induced senescence. Horiguchi et al., reported that unlike their wildtype counterparts, ATF4 deficient MEFs transformed with SV40 large T antigen and H-RASG12V oncogenes displayed slow growth, failed to form colonies on soft agar and formed significantly smaller tumors in vivo [108]. The mechanism of decrease in SV40 large T antigen and H-RASG12V mediated transformation was attributed to INK4a/ARF mediated senescence, enhanced apoptosis and accumulation of p53 in ATF4 knockout MEFs. However, transformation of PERK deficient cells by SV40 large T antigen and K-RASG12V did not affect growth and anchorage independent growth, suggesting that ATF4 could have some PERK-independent functions during transformation [9]. However, both PERK and ATF4-deficient MEFs show impaired tumor growth in vivo, suggesting both are required for tumor growth.
Interestingly, introduction of H-RASG12V in primary melanocytes induced senescence and activated all arms of the UPR. H-RASG12V driven senescence required the UPR and was independent of p53 and p16INK4a, as only knockdown of ATF4, XBP-1 and ATF6 reduced senescence but not p53 and INK4a. Even though the UPR was required for senescence, loss of UPR genes increased cell death in H-RASG12V transduced cells, suggesting that the UPR is required for survival during oncogene induced transformation [109]. Nevertheless, enhanced GRP78 is observed even at earlier stages of melanoma development [110] Likewise, enhanced levels of p-eIF2α, XPB1s and GRP78 were observed in Nf1/p53 mutant mouse model of malignant peripheral nerve sheath tumors (MPNSTs), suggesting that the UPR is activated in RAS driven tumors in vivo [111].
4.3 BRAF
The mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) is a signaling cascade that regulates proliferation, senescence, differentiation and survival in response to extracellular stimuli. BRAF is a serine/threonine protein kinase of the RAF family in the RAS/RAF/MEK/ERK pathway. This pathway is frequently elevated in human tumors. Activating mutations in the pathway result in aggressive tumors, such as melanoma [112]. Particularly, the BRAFV600E mutation results in increased activation of the kinase, leading to enhanced MEK/ERK signaling in the absence extracellular signals. The BRAFV600E mutation is present in 40–70% of malignant melanoma and other cancers [113]. Although the particular mechanism of activation ER stress and role is not clear in melanoma, some reports show that mutant BRAF induces ER stress. Introduction of BRAFV600, but not wildtype BRAF, increased protein synthesis and activated XBP1 and GRP78 in human melanocytes. Activation of the UPR was dependent on protein synthesis, as suppression of protein synthesis attenuates activation of XBP1s and GRP78 [114]. In addition, oncogenic BRAF has been shown to activate both basal and treatment-induced autophagy via IRE1 and PERK [115, 116]. The autophagy marker LC3 was significantly increased in BRAF inhibitor resistant melanoma patient tumor samples post-treatment and correlated with reduced progression free survival. In vitro, treatment of BRAFV600E cell line melanoma cells with BRAF inhibitor, increased activation of the PERK/eIF2α/ATF4 arm of UPR, which was required for induction of therapy-induced autophagy. Pharmacological inhibition of PERK reduced autophagy and increased cell death making the UPR a potential druggable target in combination with BRAF inhibitors [116].
4.4 HER2
The human epidermal growth factor receptor-2 (HER2) is amplified in a third of breast cancers and associates with worse clinical outcome [117]. Loss of PERK in MMTV-Neu, a HER2-mouse model of breast cancer, delayed mammary tumor onset in vivo and resulted in significant reduction in lung metastasis. In this model system, PERK activated NRF2, which suppressed oxidative stress in tumors thus preventing DNA damage induced cell cycle arrest [31]. Importantly, PERK was not required for proliferation of mammary epithelial cells, demonstrating that there is a specific requirement of PERK for cell cycle progression in HER2 driven tumors. Trastuzumab is a monoclonal antibody directed against HER2 and currently approved therapy for HER2 positive breast cancer. Trastuzmuab resistance and tumor relapse occur due to several escape mechanisms, including but not limited to loss of expression of HER2, expression of HER1 and HER3 and constitutive PI3K activity [118]. Interestingly, ATF4 was recently reported to be elevated in some trastuzumab-resistant HER2 positive breast cancer specimens compared to sensitive patient tumors suggesting involvement of ATF4 in trastuzumab resistance [119].
5. Dual role of the UPR in cell fate decisions
The role of the UPR in regulation of cell survival and death is a complex one and appears to depend on the origin, intensity and duration of the stress stimuli and on the cell or tumor type [120, 121]. Under mild or progressive ER stress (such as that induced by hypoxia, nutrient deprivation, etc.), a series of coordinated biochemical responses is engaged by PERK as a way to reduce the ER load and recover from ER stress, including inhibition of global protein translation [26] cell growth arrest [33] and up-regulation of genes involved in ER homeostasis, amino acid synthesis and antioxidant responses [27, 30, 122]. Similarly, the IRE1/XBP1 arm activates genes responsible for ER quality control, ER chaperones and degradation of misfolded proteins [41], whereas ATF6 induces the expression of XBP1 gene, which upregulates IRE1, and confers cell survival [123, 124].
The pro-survival effects of the UPR, which is elicited to restore ER homeostasis, can be converted to a pro-death signal when the stress stimuli are acute or persistent or the UPR is unable to ameliorate cells from the ER stress (such as those induced by pharmacological agents and acute pathophysiological states such as anoxia). The mechanisms behind this switch are not entirely clear. However, a possible explanation emanates from work by Rutkowski et al, proposing that differential stabilities of pro-survival and pro-death mRNAs/proteins are responsible for cell adaptation to mild or severe ER stress [125]. As, described previously, the PERK arm of the UPR can activate mechanisms leading to cell survival, but under prolonged stress, sustained PERK signaling tips the balance from cell survival to cell death [121]. A potential mechanism for this switch is the induction of an ATF4 target, CHOP, which serves as a pro-apoptotic transcription factor [25], by the induction of various genes, which are implicated in apoptosis and described in detail below. Also, under prolonged ER stress, the other UPR stress sensor, IRE1, has been shown to elicit apoptosis, independently of XBP1s. Even though the role of the ATF6 arm of the UPR under sustained ER stress is not generally clear, it is suspected that it can cause cell death by regulation of CHOP expression [126].
6. How the UPR influences tumor cell fate: autophagy, senescence and apoptosis
6.1 Autophagy
Autophagy and the ubiquitin/proteasome system (UPS) comprise the two major protein degradation systems. During ER stress, the unfolded/misfolded proteins translocate to the cytoplasm and are degraded by the ER-associated ubiquitin/proteasome system (ERAD) [127]. However, when the UPS is suppressed, autophagy is induced in order to clear the ER lumen of the misfolded proteins and ameliorate the cell from ER stress [128–130]. Autophagy is a highly conserved lysosome-dependent mechanism responsible for the degradation of mainly of long-lived proteins and other cytoplasmic components as a response to cellular stress [131].
It has been hypothesized that autophagy is inhibited at the early stages of tumor progression [132, 133], whereas it is upregulated during later stages of tumor development [134, 135] and also in aggressive and metastatic tumors as a way to protect cells from stress conditions [136, 137]. An increasing number of studies support a role of the UPR as a mediator of autophagy under ER stress [138, 139], with the PERK branch as a primary inducer [10, 140]. Polyglutamine (polyQ) aggregation in the ER induced autophagy via upregulation of ATG12 and probably via activation of the ATG5-ATG12-ATG16L1 complex, mediated by the PERK/eIF2α arm of the UPR [141]. The PERK-eIF2α-ATF4-CHOP arm induces autophagy in human hypoxic cancer cells [68] and in a tissue factor (TF) knockdown colon cancer cell line (LOVO cells) [142] via upregulation of both ATG5 and LC3. Radiation, a primary treatment modality for many aggressive tumors, can also induce autophagy in cancer cells, with either cytoprotective or lethal effects [143, 144]. For example, irradiation of caspase-3/7 double-knockout MCF-7 cells showed PERK/eIF2α mediated activation of cytotoxic autophagy [145]. Although the PERK/eIF2α/ATF4 arm plays a crucial role in the regulation of ER-stress-induced auto-phagy, the other three ISR kinases, GCN2, PKR and HRI, can also phosphorylate eIF2α and induce autophagy [146]. Thus, it is possible that eIF2α, rather than PERK constitutes the key player that links ER-stress-induced UPR to autophagy.
While there is substantial evidence supporting the involvement of PERK in enhancing autophagy in cancer cells, the role of IRE1 in autophagy is less clear. It has been shown that IRE1 prevents au-tophagy induction, which is reversed upon inhibition of both IRE1 and its target XBP1 [147, 148]. On the other hand, Ogata et al., suggested that IRE1 is required for autophagy induction under ER stress, triggered by tunicamycin or thapsigargin [138] and is mediated by c-Jun N-terminal kinase (JNK). JNK can also activate autophagy through phosphorylation of BCL-2 [149] or by c-Jun dependent up-regulation of Beclin-1 expression [150], while its pharmacological inhibition blocks autophagic flux and increases apoptosis [151]. Also, during ER stress, IRE1 binds to TRAF2, activates its downstream target JNK and regulate autophagy [128], which is inhibited by the expression of BI-1 (BAX inhibitor) [152]. Furthermore, under ER stress, both XBP1s and CHOP trigger autophagy in an IRE1-dependent manner [153, 154]. These results suggest that IRE1, which acts as a common trigger molecule in both IRE1/XBP1/CHOP and IRE1/TRAF2/JNK signaling pathways, plays a significant role in autophagy regulation. In summary, autophagy is activated by the UPR in a variety of stresses and mainly serves as a survival mechanism in response to the ER stress [155, 156].
6.2 Senescence
In addition to autophagy and apoptosis, another key mechanism causally linked to tumor suppression is premature senescence [157], which has been shown to occur in premalignant lesions [158, 159]. Cellular senescence is induced upon activation of oncogenes (i.e. K-RAS) in a wild-type p53 background and by treatment with genotoxic agents or chemotherapy (UV, irradiation, doxorubi-cin etc.) [160], and involves two major pathways, regulated by p53 and Rb tumor suppressor proteins [161]. Interestingly, the UPR is also activated under the same ER stress-induced conditions, but its connection with senescence is not well defined. PERK knockdown or its inhibition by the long-term use of 4-phenylbutyric acid, a chemical chaperone, resulted in cellular senescence in both MCF-7 and HT1080 cells [162]. Opposite responses of primary and tumor cells to intracellular levels of ROS are based on PERK/eIF2α. Specifically, primary cells with impaired PERK/eIF2α induce senescence and are sensitive to cellular ROS accumulation, whereas eIF2α deficient immortalized or tumor cells are tolerant to high ROS levels [163]. Thus, the PERK/eIF2α axis is a key player in regulating senescence in response to oxidative stress, rendering it a potential target for increasing the anti-tumor effects of pro-oxidant drugs, such as doxorubicin. IRE1 is also involved in the control of senescence via different pathways. Induction of senescence dependent on the IRE1/XBP1 pathway was observed after expression of oncogenic forms of HRAS (H-RASG12V) or in IRE1 knockdown HEK293 cells, under ER stress conditions caused by dithiothreitol or tunicamycin [109, 164]. Furthermore, it was shown that the E3 ligase CHIP antagonized senescence via regulation of the IRE1/TRAF2/JNK pathway through IRE1 ubiquitination [164]. Finally, our group recently described a small molecule compound, E235, that activates the ISR in transformed cells and causes cellular senescence in a p53-and p21 independent manner [165]. Even though the link between the UPR and senescence is still not so clear an intriguing hypothesis is that under ER stress the activation of UPR allows cells to overcome senescence and promote cell proliferation and tumorigenesis.
6.3 Apoptosis
Severe, chronic or unresolved ER stress conditions can force the UPR from a pro-survival to a pro-cell death role, primarily via apoptosis. This switch can happen if the ratio of unfolded or mis-folded proteins to the rate of their degradation exceeds a certain threshold [166]. Different pathways initiated mainly from the PERK and IRE1 UPR arms are involved in triggering apoptosis [167]. The PERK/eIF2α/ATF4 axis upregulates the pro-apoptotic transcription factor, CHOP, which is involved in ER-stress induced apoptosis.
Several targets of CHOP were found to be implicated in these apoptotic events. During prolonged periods of ER stress, overexpression of GADD34 can initiate apoptosis due to restoration of protein synthesis and overload of ER [168]. Another CHOP target, ERO1a, which contributes to the hyper-oxidation of the ER for proper disulfide bond formation [168] is involved in apoptotic cell death through activation of inositol 1,4,5 triphosphate receptor 1 (IP3R1)/calcium/calmodulin-dependent protein kinase II (CaMKII) [169]. In turn, the CHOP/ERO1a/IP3R1/CaMKII pathway upregulates the NADPH oxidase subunit 2 (NOX2), leading to the generation of ROS and ultimately to cell death [170]. Additional targets of CHOP are death receptor 5 (DR5), which, under ER stress, sensitizes cells to apoptosis [171] and tribbles 3 (TRB3), which likely exerts its apoptotic function by binding to preventing phosphorylation of AKT [172].
CHOP has been shown to suppress the pro-survival protein BCL-2 through its overexpression in both HeLa cells and a rat fibroblast cell line [173], or by binding to the liver inhibitory protein (LIP), an isoform of C/EBPβ, in thapsigargin treated MEFs [174]. CHOP-mediated suppression of BCL-2 leads to the release of BH3-only proteins, such as BAD, PUMA and NOXA, as well as BAD and BAX, to induce mitochondrial dependent apoptosis [175]. Apoptosis mediated by CHOP, can also be induced by the direct transcriptional induction of the pro-apoptotic BH3-only protein, BIM [176]. Thus, the concomitant suppression of pro-survival and upregulation of pro-apoptotic proteins of the BCL-2 family converge to increase levels of apoptosis.
Although, IRE1 arm has been characterized as a primarily pro-survival pathway, which is temporally activated following PERK and ATF6 activation, it has also been implicated in apoptosis following prolonged ER stress and independent of XBP1 splicing. This is mainly achieved through interaction of IRE1 with the tumor necrosis factor receptor associated factor 2 (TRAF2), [177]. The IRE1-TRAF2 complex can activate the MAP3K apoptosis signal regulated kinase1 (ASK1), which in turn stimulates JNK activation [178]. JNK contributes to cell death through phosphorylation and suppression of the anti-apoptotic activity of Bcl-2 and in parallel by activation (phosphorylation) of BH3-only proteins, such as BIM [179–181]. Moreover, Hetz et al., propose that there is direct interaction of IRE1 with BAX and BAK in mitochondrial induced apoptosis, revealing a new link between the BCL-2 family members and the UPR [182]. IRE1 is also implicated to ER stress-induced apoptosis by the process of IRE1-dependent decay of mRNAs (RIDD), which is independent of XBP1 splicing [183]; however the mechanism is not entirely clear [40, 184].(Fig. 1)
Under ER stress, apoptosis can also be induced by cleavage and activation of caspase-12 [185]. The latter has been shown to be regulated by either ER-localized BAX and BAK proteins [186] or by calpain [187], after intracellular calcium storage disturbances. Also, IRE1-TRAF2 complex has been reported to be part of caspase-12 activation, leading to cellular apoptosis [188]. ATF6, is also activated at early time points under ER stress. Although, it regulates the induction of apoptotic factors, such as CHOP [126], its role under prolonged ER stress is not yet clear.
In summary, under ER stress, the UPR has been shown to regulate senescence, autophagy and apoptosis in a manner that favors the tumor survival and progression. Attenuation or activation of one of these mechanisms may therefore represent an effective therapeutic approach.
7. The role of the UPR in Epithelial to Mesenchymal Transition (EMT) and metastasis
Most malignant tumor cells are able to metastasize, a process that is mainly driven by the transformation of cancer cells from an epithelial to a migratory and invasive mesenchymal phenotype by the EMT process [189, 190]. During EMT, cancer cells require high secretory protein levels, leading to a cascade of events, including induction of ER stress, UPR activation and eventually autophagy, which allows them to survive and metastasize. All arms of UPR were shown to be involved in EMT, but the PERK/eIF2α seems to have the highest impact.
Breast cancer cells undergoing EMT are characterized as highly secretory cells due enhanced synthesis and secretion of ECM proteins, which results in activation of the PERK/eIF2α/ATF4 arm of the UPR that promotes their survival and metastasis [191]. LAMP3 has been identified as a hypoxia inducible gene, which is regulated by the PERK/eIF2α/ATF4 pathway independently of HIF and confers a link between hypoxic stress and metastasis [72, 192]. Although the PERK/eIF2α UPR arm confers cell survival and promotes metastasis by the induction of autophagy, the N-myc downstream regulated gene 1 (NDRG1) can act as a negative regulator on breast cancer cell lines, leading to apoptosis [193]. On the other hand, Tian et al, suggested that depletion of TF, which highly contributes to hepatic metastasis of colorectal cancer, promotes PERK/eIF2α/ATF4-dependent autophagy and apoptosis of LOVO cells, ultimately leading to inhibition of metastasis [142]. Similarly, suppression of the enzyme N-acetylglucosaminyltransferase V (GnT-V), which is overexpressed in a variety of human tumors and connected with tumor invasion and metastasis, causes the activation of both the PERK and IRE1/XBP-1 arms of UPR signaling, possibly leading to ER stress-dependent apop-tosis [194]. Resistance to anoikis, is a hallmark feature of metastatic cancer cells to form secondary tumor sites. Recent studies have shown that loss of extracellular matrix attachment is characterized by PERK-mediated activation of the UPR [36]. The mechanism by which the UPR promotes survival following matrix detachment involves the activation of antioxidant responses and cytoprotective auto-phagy, both of which are regulated by PERK. Unpublished data from our lab also shows that such responses are mediated mainly through ATF4.
CIM (endoplasmic reticilum lectin 1), a protein involved in invasion and metastasis, was shown to positively regulate the expression of HIF1α and to protect cancer cells from apoptosis via the IRE1/XBP1 arm under ER stress [195]. Furthermore, the serine/cysteine protease inhibitor SCCA1 (SERPINB3), which is upregulated in many cancer types, promotes EMT and induces NF-kB and IL6 expression, through a low-level induction of the UPR by activation of PERK and ATF6, but not IRE1 [196]. Finally, an IFN-γ-induced enzyme, the death-associated protein kinase 1 (DAPK1), which acts as a negative regulator for tumor growth and metastasis, mainly via apoptosis or autophagy, is dependent on the IFN-γ/ATF6/C/EBP-β/DAPK1 axis [197]. Collectively, the role of the UPR in EMT and metastasis is still controversial. Further study is required to clarify whether induction of autophagy promotes or inhibits tumor invasion and metastasis.
8. Targeting the UPR as therapeutic approach
8.1 Inhibition of UPR by small molecules
Targeting the UPR for cancer treatment should be considered a promising approach, since the UPR appears to be activated in several human tumor types [198]. There are two distinct approaches which could conceivably achieve this: inhibition of the UPR to eliminate cells that are “UPR-addicted” for survival in unfavorable tumor conditions, or overactivation of ER stress by pharmacological agents.
One promising approach to inhibit the pro-survival role of the UPR is to develop small molecule inhibitors against the UPR transducers to prevent tumor cells from overcoming ER stress, leading to cell death. A highly specific, orally available PERK inhibitor, GSK2656157, developed by GlaxoS-mithKline, has potent antitumor activity against human pancreatic and multiple myeloma xenograft tumors [86]. Artigenin (ARC-G) was found to down regulate the UPR-regulated proteins GRP78, p-PERK, ATF4 and CHOP and to induce apoptosis via activation of both caspases-9 and -3 during glucose deprivation. Also, HT-29 colon xenograft tumors treated with ARC-G presented delayed tumor growth [199]. Another class of inhibitors targets the IRE1 arm of the UPR. The compound 4μ8C was found to inhibit the IRE1 endonuclease activity and subsequently Xbp1 splicing, but has no impact in defending cells against ER stress [200]. Furthermore, both the compound STF-083010 and toyoca-mycin, a product of an Actinomycete strain, can effectively inhibit the endonuclease, but not the kinase activity of IRE1 and display potent antitumor activity against human multiple myeloma xeno-grafts [201, 202]. Similarly, a class of molecules, termed hydroxy-aryl-aldehydes (HAA), which selectively inhibit IRE1α RNase activity, can potentially be used for therapeutic purposes [203].
There are also different approaches for blocking the pro-survival role of the UPR under ER stress. Chen S et al., showed that treatment of MDA-MB-231 breast cancer cells with fucoidal, a fucose-rich polysaccharide, caused cell growth inhibition and induction of apoptosis via dowregulation of GRP78 and parallel activation of eIF2α/CHOP pathway [204]. SubAB is a bacterial cytotoxin, which also inhibits GRP78 by specific cleavage [205]. Due uncontrolled ER stress and lethality in mice, the subti-lase cytotoxin catalytic subunit (SubA) has been fused with human epidermal growth factor (EGF) for more targeted actions [206] and found to cause a non-typical apoptotic cell death in combination with photodynamic therapy [207].
8.2 Causing excessive ER stress as a means of targeted anti-tumor modalities
Hyperactivation of the UPR may be an effective approach to kill tumor cells through proapoptotic UPR signaling. Proteasome inhibitors comprise a known class of compounds, which cause overacti-vation of the UPR [208], due to the accumulation of unfolded or misfolded proteins, that cannot be degraded. Experiments from our group showed that under hypoxic conditions, the use of bortezomib induced apoptosis of hypoxic cancer cells, which are more resistant to genotoxic chemotherapeutic agents [209]. Myeloma cells, which are highly secretory cells with constitutively high levels of UPR activation, underwent apoptosis after treatment with bortezomib [210]. In addition, several protea-some inhibitors, such as carfilzomib and MLN9708, are being used in ongoing clinical trials for the treatment of multiple myeloma, myeloma bone disease [211, 212] and chronic lymphocytic lymphoma [213, 214].
Autophagy, which has been shown to serve as a protective and pro-survival mechanism under ER stress, is an attractive target for the development of small molecules inhibitors, able to deteriorate ER stress causing cell death and eventually leading to tumor regression. Chloroquine is a molecule that blocks the final step of autophagy (fusion of the lysosome with the autophagosome). Recent studies have shown that chloroquine-treated radioresistant breast tumor cells and U373 MG (glioblas-toma-astrocytoma) tumors presented significant sensitivity to irradiation [68, 215]. Similarly, the use of other autophagy inhibitors, such as bafilomycin A1 and 3-methyladenine, in combination with irradiation, led to more pronounced and prolonged DNA damage in glioma and breast cancer cells, compared to those cells treated with radiation only [216, 217]. Current autophagy inhibitors tend to have off-target effects demonstrating a need to develop specific autophagy inhibitors.
Collectively, there have been many studies indicating promising antitumor effects by either inhibition or overactivation of UPR signaling. Moreover, the use of UPR inhibitors and activators in combination with established agents, such as angiogenic and proteasome inhibitors and pro-oxidant drugs, may elicit a more pronounced anticancer effect, leading to a new open window for further research.
Conclusions and Future Directions
Experimental data from various groups as well as accumulating data from patient tumors in the past couple of decades have supported a key role of UPR activation during tumorigenesis and a reliance on UPR pathways in transformed cells for adaptation to either cell autonomous or non-cell autonomous stresses. In the near future, careful analysis of the human tumor databases and data derived from gene expression studies may reveal which tumors are “addicted” to UPR pathways. Since the UPR impinges upon different process such as cell cycle progression, translation and apoptosis, it is important to understand which processes are activated and/or suppressed at different stages of tumor formation. This also calls for the development of transgenic mouse models that target each UPR pathway in a conditional manner to study their contribution to cancer initiation and progression. Furthermore, careful understanding of other factors that influence the UPR autonomously or through interaction with the ER sensors is essential for therapeutically targeting this pathway. For instance, a recent report showed a new interactor of phosphorylated PERK, transducin (beta)-like 2 (TBL2) that regulates PERK dependent activation of ATF4 as well as survival [218]. Based on this study, one can speculate the possibility of multiple factors that modulate the UPR that are yet to be discovered. Moreover, another aspect that needs additional investigation is the impact of the UPR on host cells found in the tumor microenvironment, such as myeloid and dendritic cells, as recent studies implicate the UPR to facilitate tumor progression through signaling outside of the cell [219, 220]. Collectively, the studies mentioned in this review, as well as others we could not reference due to space limitations, underscore an important role of the UPR in tumorigenesis, survival and proliferation of cancer cells, making the UPR a fertile area of research in the identification of novel druggable targets against multiple malignancies.
Acknowledgments
Our work is supported by National Cancer Institute grant 1PO1-CA165997. Feven Tameire is supported by NIH predoctoral fellowship F31CA183569-01 and Dr. Ioannis Verginadis is supported by a postdoctoral fellowship from the Bodossaki Foundation, Greece.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Healy SJ, Gorman AM, Mousavi-Shafaei P, Gupta S, Samali A. Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur J Pharmacol. 2009;625:234–46. doi: 10.1016/j.ejphar.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 2.Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem. 2011;80:71–99. doi: 10.1146/annurev-biochem-062209-093836. [DOI] [PubMed] [Google Scholar]
- 3.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–73. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 4.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 5.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–8. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Friedlander R, Jarosch E, Urban J, Volkwein C, Sommer T. A regulatory link between ER-associated protein degradation and the unfolded-protein response. Nat Cell Biol. 2000;2:379–84. doi: 10.1038/35017001. [DOI] [PubMed] [Google Scholar]
- 7.Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65:862–94. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–81. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, et al. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Invest. 2012;122:4621–34. doi: 10.1172/JCI62973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nature reviews Cancer. 2004;4:966–77. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 12.Bagratuni T, Wu P, Gonzalez de Castro D, Davenport EL, Dickens NJ, Walker BA, et al. XBP1s levels are implicated in the biology and outcome of myeloma mediating different clinical outcomes to thalidomide-based treatments. Blood. 2010;116:250–3. doi: 10.1182/blood-2010-01-263236. [DOI] [PubMed] [Google Scholar]
- 13.Ruggero D. Translational control in cancer etiology. Cold Spring Harb Perspect Biol. 2013:5. doi: 10.1101/cshperspect.a012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nature reviews Cancer. 2004;4:437–47. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 15.Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nature reviews Cancer. 2014;14:263–76. doi: 10.1038/nrc3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee AS, Delegeane A, Scharff D. Highly conserved glucose-regulated protein in hamster and chicken cells: preliminary characterization of its cDNA clone. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:4922–5. doi: 10.1073/pnas.78.8.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AS. Coordinated regulation of a set of genes by glucose and calcium ionophores in mammalian cells. Trends in Biochemical Sciences. 1987;12:20–3. [Google Scholar]
- 18.Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proceedings of the National Academy of Sciences. 1996;93:7690–4. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer research. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- 20.Fu Y, Wey S, Wang M, Ye R, Liao CP, Roy-Burman P, et al. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19444–9. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805–18. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendershot LM. The ER function BiP is a master regulator of ER function. The Mount Sinai journal of medicine, New York. 2004;71:289–97. [PubMed] [Google Scholar]
- 23.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 24.Ma K, Vattem KM, Wek RC. Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. The Journal of biological chemistry. 2002;277:18728–35. doi: 10.1074/jbc.M200903200. [DOI] [PubMed] [Google Scholar]
- 25.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Molecular cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 26.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–4. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 27.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Molecular cell. 2003;11:619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 28.B’Chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, et al. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41:7683–99. doi: 10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. The Journal of cell biology. 2001;153:1011–22. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Molecular and cellular biology. 2003;23:7198–209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, Zhang F, Ye J, Koumenis C, et al. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29:3881–95. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta S, McGrath B, Cavener DR. PERK regulates the proliferation and development of insulin-secreting beta-cell tumors in the endocrine pancreas of mice. PloS one. 2009;4:e8008. doi: 10.1371/journal.pone.0008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12625–30. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamanaka RB, Bennett BS, Cullinan SB, Diehl JA. PERK and GCN2 contribute to eIF2alpha phosphorylation and cell cycle arrest after activation of the unfolded protein response pathway. Mol Biol Cell. 2005;16:5493–501. doi: 10.1091/mbc.E05-03-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sequeira SJ, Ranganathan AC, Adam AP, Iglesias BV, Farias EF, Aguirre-Ghiso JA. Inhibition of proliferation by PERK regulates mammary acinar morphogenesis and tumor formation. PloS one. 2007;2:e615. doi: 10.1371/journal.pone.0000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, et al. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Molecular and cellular biology. 2011;31:3616–29. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranganathan AC, Ojha S, Kourtidis A, Conklin DS, Aguirre-Ghiso JA. Dual function of pancreatic endoplasmic reticulum kinase in tumor cell growth arrest and survival. Cancer research. 2008;68:3260–8. doi: 10.1158/0008-5472.CAN-07-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan Q, Wang X, Gong W, Ni L, Chen C, He X, et al. ER stress negatively modulates the expression of the miR-199a/214 cluster to regulates tumor survival and progression in human hepatocellular cancer. PloS one. 2012;7:e31518. doi: 10.1371/journal.pone.0031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chitnis NS, Pytel D, Bobrovnikova-Marjon E, Pant D, Zheng H, Maas NL, et al. miR-211 is a prosurvival microRNA that regulates chop expression in a PERK-dependent manner. Molecular cell. 2012;48:353–64. doi: 10.1016/j.molcel.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maurel M, Chevet E, Tavernier J, Gerlo S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends in Biochemical Sciences. 2014;39:245–54. doi: 10.1016/j.tibs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9946–51. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu G, Liu K, Anderson J, Patrene K, Lentzsch S, Roodman GD, et al. Expression of XBP1s in bone marrow stromal cells is critical for myeloma cell growth and osteoclast formation. Blood. 2012;119:4205–14. doi: 10.1182/blood-2011-05-353300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujimoto T, Onda M, Nagai H, Nagahata T, Ogawa K, Emi M. Upregulation and overexpression of human X-box binding protein 1 (hXBP-1) gene in primary breast cancers. Breast Cancer. 2003;10:301–6. doi: 10.1007/BF02967649. [DOI] [PubMed] [Google Scholar]
- 44.Shuda M, Kondoh N, Imazeki N, Tanaka K, Okada T, Mori K, et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38:605–14. doi: 10.1016/s0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 45.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nature reviews Cancer. 2007;7:585–98. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 46.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–7. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 47.Carrasco DR, Sukhdeo K, Protopopova M, Sinha R, Enos M, Carrasco DE, et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer cell. 2007;11:349–60. doi: 10.1016/j.ccr.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mimura N, Fulciniti M, Gorgun G, Tai Y-T, Cirstea D, Santo L, et al. Blockade of XBP1 splicing by inhibition of IRE1αis a promising therapeutic option in multiple myeloma. Blood. 2012;119:5772–81. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Iliopoulos D, Zhang Q, Tang QZ, Greenblatt MB, Hatziapostolou M, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1 alpha pathway. Nature. 2014;508:103. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Molecular cell. 2000;6:1355–64. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 51.Adachi Y, Yamamoto K, Okada T, Yoshida H, Harada A, Mori K. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell structure and function. 2008;33:75–89. doi: 10.1247/csf.07044. [DOI] [PubMed] [Google Scholar]
- 52.Chang KC, Chen PC, Chen YP, Chang Y, Su IJ. Dominant expression of survival signals of endoplasmic reticulum stress response in Hodgkin lymphoma. Cancer Sci. 2011;102:275–81. doi: 10.1111/j.1349-7006.2010.01765.x. [DOI] [PubMed] [Google Scholar]
- 53.Scaiewicz V, Nahmias A, Chung RT, Mueller T, Tirosh B, Shibolet O. CCAAT/enhancer-binding protein homologous (CHOP) protein promotes carcinogenesis in the DEN-induced hepatocellular carcinoma model. PloS one. 2013;8:e81065. doi: 10.1371/journal.pone.0081065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arai M, Kondoh N, Imazeki N, Hada A, Hatsuse K, Kimura F, et al. Transformation-associated gene regulation by ATF6alpha during hepatocarcinogenesis. FEBS Lett. 2006;580:184–90. doi: 10.1016/j.febslet.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 55.Schewe DM, Aguirre-Ghiso JA. ATF6α-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proceedings of the National Academy of Sciences. 2008;105:10519–24. doi: 10.1073/pnas.0800939105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karali E, Bellou S, Stellas D, Klinakis A, Murphy C, Fotsis T. VEGF Signals through ATF6 and PERK to promote endothelial cell survival and angiogenesis in the absence of ER stress. Molecular cell. 2014;54:559–72. doi: 10.1016/j.molcel.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 57.Sutherland RM, Ausserer WA, Murphy BJ, Laderoute KR. Tumor Hypoxia and Heterogeneity: Challenges and Opportunities for the Future. Seminars in radiation oncology. 1996;6:59–70. doi: 10.1053/SRAO0060059. [DOI] [PubMed] [Google Scholar]
- 58.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nature reviews Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Molecular and cellular biology. 2002;22:7405–16. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nature Reviews Cancer. 2008;8:851–64. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 61.Tu BP, Weissman JS. The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Molecular cell. 2002;10:983–94. doi: 10.1016/s1097-2765(02)00696-2. [DOI] [PubMed] [Google Scholar]
- 62.Koritzinsky M, Levitin F, van den Beucken T, Rumantir RA, Harding NJ, Chu KC, et al. Two phases of disulfide bond formation have differing requirements for oxygen. The Journal of cell biology. 2013;203:615–27. doi: 10.1083/jcb.201307185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu L, Wise DR, Diehl JA, Simon MC. Hypoxic Reactive Oxygen Species Regulate the Integrated Stress Response and Cell Survival. Journal of Biological Chemistry. 2008;283:31153–62. doi: 10.1074/jbc.M805056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koumenis C, Wouters BG. “Translating” tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways. Molecular cancer research : MCR. 2006;4:423–36. doi: 10.1158/1541-7786.MCR-06-0150. [DOI] [PubMed] [Google Scholar]
- 65.Fels DR, Koumenis C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5:723–8. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- 66.Blais JD, Filipenko V, Bi MX, Harding HP, Ron D, Koumenis C, et al. Activating transcription factor 4 is translationally regulated by hypoxic stress. Molecular and cellular biology. 2004;24:7469–82. doi: 10.1128/MCB.24.17.7469-7482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ameri K, Lewis CE, Raida M, Sowter H, Hai T, Harris AL. Anoxic induction of ATF-4 through HIF-1–independent pathways of protein stabilization in human cancer cells. Blood. 2004;103:1876–82. doi: 10.1182/blood-2003-06-1859. [DOI] [PubMed] [Google Scholar]
- 68.Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120:127–41. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pike LR, Singleton DC, Buffa F, Abramczyk O, Phadwal K, Li JL, et al. Transcriptional up-regulation of ULK1 by ATF4 contributes to cancer cell survival. The Biochemical journal. 2013;449:389–400. doi: 10.1042/BJ20120972. [DOI] [PubMed] [Google Scholar]
- 70.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nature reviews Cancer. 2008;8:425–37. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rouschop KM, Dubois LJ, Keulers TG, van den Beucken T, Lambin P, Bussink J, et al. PERK/eIF2alpha signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4622–7. doi: 10.1073/pnas.1210633110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mujcic H, Nagelkerke A, Rouschop KM, Chung S, Chaudary N, Span PN, et al. Hypoxic activation of the PERK/eIF2alpha arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clin Cancer Res. 2013;19:6126–37. doi: 10.1158/1078-0432.CCR-13-0526. [DOI] [PubMed] [Google Scholar]
- 73.Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee A-H, Yoshida H, et al. XBP1 Is Essential for Survival under Hypoxic Conditions and Is Required for Tumor Growth. Cancer research. 2004;64:5943–7. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- 74.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Seminars in radiation oncology. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 75.Tang X, Lucas JE, Chen JL, LaMonte G, Wu J, Wang MC, et al. Functional interaction between responses to lactic acidosis and hypoxia regulates genomic transcriptional outputs. Cancer research. 2012;72:491–502. doi: 10.1158/0008-5472.CAN-11-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu K, Jiao H, Li S, Cao H, Galson DL, Zhao Z, et al. ATF4 promotes bone angiogenesis by increasing VEGF expression and release in the bone environment. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28:1870–84. doi: 10.1002/jbmr.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van den Beucken T, Koritzinsky M, Niessen H, Dubois L, Savelkouls K, Mujcic H, et al. Hypoxia-induced expression of carbonic anhydrase 9 is dependent on the unfolded protein response. The Journal of biological chemistry. 2009;284:24204–12. doi: 10.1074/jbc.M109.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and cellular biology. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pereira ER, Liao N, Neale GA, Hendershot LM. Transcriptional and post-transcriptional regulation of proangiogenic factors by the unfolded protein response. PloS one. 2010:5. doi: 10.1371/journal.pone.0012521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drogat B, Auguste P, Nguyen DT, Bouchecareilh M, Pineau R, Nalbantoglu J, et al. IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-A expression and contributes to angiogenesis and tumor growth in vivo. Cancer research. 2007;67:6700–7. doi: 10.1158/0008-5472.CAN-06-3235. [DOI] [PubMed] [Google Scholar]
- 81.Romero-Ramirez L, Cao H, Regalado MP, Kambham N, Siemann D, Kim JJ, et al. X box-binding protein 1 regulates angiogenesis in human pancreatic adenocarcinomas. Transl Oncol. 2009;2:31–8. doi: 10.1593/tlo.08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, et al. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PloS one. 2010;5:e9575. doi: 10.1371/journal.pone.0009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Auf G, Jabouille A, Guerit S, Pineau R, Delugin M, Bouchecareilh M, et al. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15553–8. doi: 10.1073/pnas.0914072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Alam GN, Ning Y, Visioli F, Dong Z, Nor JE, et al. The unfolded protein response induces the angiogenic switch in human tumor cells through the PERK/ATF4 pathway. Cancer research. 2012;72:5396–406. doi: 10.1158/0008-5472.CAN-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blais JD, Addison CL, Edge R, Falls T, Zhao H, Wary K, et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Molecular and cellular biology. 2006;26:9517–32. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer research. 2013;73:1993–2002. doi: 10.1158/0008-5472.CAN-12-3109. [DOI] [PubMed] [Google Scholar]
- 87.De Bock K, Mazzone M, Carmeliet P. Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nature reviews Clinical oncology. 2011;8:393–404. doi: 10.1038/nrclinonc.2011.83. [DOI] [PubMed] [Google Scholar]
- 88.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 89.Shan JX, Ord D, Ord T, Kilberg MS. Elevated ATF4 Expression, in the Absence of Other Signals, Is Sufficient for Transcriptional Induction via CCAAT Enhancer-binding Protein-activating Transcription Factor Response Elements. Journal of Biological Chemistry. 2009;284:21241–8. doi: 10.1074/jbc.M109.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wek RC, Ramirez M, Jackson BM, Hinnebusch AG. Identification of positive-acting domains in GCN2 protein kinase required for translational activation of GCN4 expression. Molecular and cellular biology. 1990;10:2820–31. doi: 10.1128/mcb.10.6.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wek SA, Zhu S, Wek RC. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Molecular and cellular biology. 1995;15:4497–506. doi: 10.1128/mcb.15.8.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–96. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Y, Ning Y, Alam GN, Jankowski BM, Dong Z, Nor JE, et al. Amino acid deprivation promotes tumor angiogenesis through the GCN2/ATF4 pathway. Neoplasia. 2013;15:989–97. doi: 10.1593/neo.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Richards NG, Kilberg MS. Asparagine synthetase chemotherapy. Annu Rev Biochem. 2006;75:629–54. doi: 10.1146/annurev.biochem.75.103004.142520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aslanian AM, Kilberg MS. Multiple adaptive mechanisms affect asparagine synthetase substrate availability in asparaginase-resistant MOLT-4 human leukaemia cells. The Biochemical journal. 2001;358:59–67. doi: 10.1042/0264-6021:3580059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Molecular cell. 2008;29:541–51. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hills SA, Diffley JF. DNA replication and oncogene-induced replicative stress. Current biology : CB. 2014;24:R435–44. doi: 10.1016/j.cub.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 98.Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nature reviews Drug discovery. 2013;12:829–46. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- 99.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iritani BM, Eisenman RN. c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13180–5. doi: 10.1073/pnas.96.23.13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Langdon WY, Harris AW, Cory S, Adams JM. The c-myc oncogene perturbs B lymphocyte development in E-mu-myc transgenic mice. Cell. 1986;47:11–8. doi: 10.1016/0092-8674(86)90361-2. [DOI] [PubMed] [Google Scholar]
- 102.Lin CJ, Nasr Z, Premsrirut PK, Porco JA, Jr, Hippo Y, Lowe SW, et al. Targeting synthetic lethal interactions between Myc and the eIF4F complex impedes tumorigenesis. Cell Rep. 2012;1:325–33. doi: 10.1016/j.celrep.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barna M, Pusic A, Zollo O, Costa M, Kondrashov N, Rego E, et al. Suppression of Myc oncogenic activity by ribosomal protein haploinsufficiency. Nature. 2008;456:971–5. doi: 10.1038/nature07449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pourdehnad M, Truitt ML, Siddiqi IN, Ducker GS, Shokat KM, Ruggero D. Myc and mTOR converge on a common node in protein synthesis control that confers synthetic lethality in Myc-driven cancers. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:11988–93. doi: 10.1073/pnas.1310230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nagy P, Varga A, Pircs K, Hegedus K, Juhasz G. Myc-driven overgrowth requires unfolded protein response-mediated induction of autophagy and antioxidant responses in Drosophila melanogaster. PLoS Genet. 2013;9:e1003664. doi: 10.1371/journal.pgen.1003664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qing G, Li B, Vu A, Skuli N, Walton ZE, Liu X, et al. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer cell. 2012;22:631–44. doi: 10.1016/j.ccr.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ren P, Yue M, Xiao D, Xiu R, Gan L, Liu H, et al. ATF4 and N-Myc coordinate glutamine metabolism in MYCN-amplified neuroblastoma cells through ASCT2 activation. J Pathol. 2014 doi: 10.1002/path.4429. [DOI] [PubMed] [Google Scholar]
- 108.Horiguchi M, Koyanagi S, Okamoto A, Suzuki SO, Matsunaga N, Ohdo S. Stress-regulated transcription factor ATF4 promotes neoplastic transformation by suppressing expression of the INK4a/ARF cell senescence factors. Cancer research. 2012;72:395–401. doi: 10.1158/0008-5472.CAN-11-1891. [DOI] [PubMed] [Google Scholar]
- 109.Denoyelle C, Abou-Rjaily G, Bezrookove V, Verhaegen M, Johnson TM, Fullen DR, et al. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat Cell Biol. 2006;8:1053–63. doi: 10.1038/ncb1471. [DOI] [PubMed] [Google Scholar]
- 110.Hersey P, Zhang XD. Adaptation to ER stress as a driver of malignancy and resistance to therapy in human melanoma. Pigment Cell & Melanoma Research. 2008;21:358–67. doi: 10.1111/j.1755-148X.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- 111.De Raedt T, Walton Z, Yecies JL, Li D, Chen Y, Malone CF, et al. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer cell. 2011;20:400–13. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cantwell-Dorris ER, O’Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10:385–94. doi: 10.1158/1535-7163.MCT-10-0799. [DOI] [PubMed] [Google Scholar]
- 113.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 114.Croft A, Tay KH, Boyd SC, Guo ST, Jiang CC, Lai F, et al. Oncogenic activation of MEK/ERK primes melanoma cells for adaptation to endoplasmic reticulum stress. J Invest Dermatol. 2014;134:488–97. doi: 10.1038/jid.2013.325. [DOI] [PubMed] [Google Scholar]
- 115.Corazzari M, Rapino F, Ciccosanti F, Giglio P, Antonioli M, Conti B. Oncogenic BRAF induces chronic ER stress condition resulting in increased basal autophagy and apoptotic resistance of cutaneous melanoma. Cell Death Differ. 2014 doi: 10.1038/cdd.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ma XH, Piao SF, Dey S, McAfee Q, Karakousis G, Villanueva J, et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J Clin Invest. 2014;124:1406–17. doi: 10.1172/JCI70454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ursini-Siegel J, Schade B, Cardiff RD, Muller WJ. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nature reviews Cancer. 2007;7:389–97. doi: 10.1038/nrc2127. [DOI] [PubMed] [Google Scholar]
- 118.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nature reviews Clinical oncology. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 119.Nam S, Chang HR, Jung HR, Gim Y, Kim NY, Grailhe R, et al. A pathway-based approach for identifying biomarkers of tumor progression to trastuzumab-resistant breast cancer. Cancer Lett. 2015;356:880–90. doi: 10.1016/j.canlet.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 120.Jager R, Bertrand MJ, Gorman AM, Vandenabeele P, Samali A. The unfolded protein response at the crossroads of cellular life and death during endoplasmic reticulum stress. Biol Cell. 2012;104:259–70. doi: 10.1111/boc.201100055. [DOI] [PubMed] [Google Scholar]
- 121.Lin JH, Li H, Zhang Y, Ron D, Walter P. Divergent effects of PERK and IRE1 signaling on cell viability. PloS one. 2009;4:e4170. doi: 10.1371/journal.pone.0004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Molecular cell. 2000;6:1099–108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 123.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–76. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 124.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–91. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 125.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, et al. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Molecular and cellular biology. 2000;20:6755–67. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–4. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 128.Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, et al. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol. 2007;171:513–24. doi: 10.2353/ajpath.2007.070188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. The Journal of biological chemistry. 2006;281:30299–304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 132.Schwarze PE, Seglen PO. Reduced autophagic activity, improved protein balance and enhanced in vitro survival of hepatocytes isolated from carcinogen-treated rats. Exp Cell Res. 1985;157:15–28. doi: 10.1016/0014-4827(85)90148-x. [DOI] [PubMed] [Google Scholar]
- 133.Edinger AL, Thompson CB. Defective autophagy leads to cancer. Cancer cell. 2003;4:422–4. doi: 10.1016/s1535-6108(03)00306-4. [DOI] [PubMed] [Google Scholar]
- 134.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 135.Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14:70–7. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 136.Kenific CM, Thorburn A, Debnath J. Autophagy and metastasis: another double-edged sword. Curr Opin Cell Biol. 2010;22:241–5. doi: 10.1016/j.ceb.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nature reviews Drug discovery. 2012;11:709–30. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Molecular and cellular biology. 2006;26:9220–31. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rouschop KM, Wouters BG. Regulation of autophagy through multiple independent hypoxic signaling pathways. Curr Mol Med. 2009;9:417–24. doi: 10.2174/156652409788167131. [DOI] [PubMed] [Google Scholar]
- 141.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, et al. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–9. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 142.Tian M, Wan Y, Tang J, Li H, Yu G, Zhu J, et al. Depletion of tissue factor suppresses hepatic metastasis and tumor growth in colorectal cancer via the downregulation of MMPs and the induction of autophagy and apoptosis. Cancer Biol Ther. 2011;12:896–907. doi: 10.4161/cbt.12.10.17679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gewirtz DA, Sundaram S, Magnet KJ. Influence of topoisomerase II inhibitors and ionizing radiation on growth arrest and cell death pathways in the breast tumor cell. Cell biochemistry and biophysics. 2000;33:19–31. doi: 10.1385/CBB:33:1:19. [DOI] [PubMed] [Google Scholar]
- 144.Jo GH, Bogler O, Chwae YJ, Yoo H, Lee SH, Park JB, et al. Radiation-Induced Autophagy Contributes to Cell Death and Induces Apoptosis Partly in Malignant Glioma Cells. Cancer Res Treat. 2014 doi: 10.4143/crt.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim KW, Moretti L, Mitchell LR, Jung DK, Lu B. Endoplasmic reticulum stress mediates radiation-induced autophagy by perk-eIF2alpha in caspase-3/7-deficient cells. Oncogene. 2010;29:3241–51. doi: 10.1038/onc.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–82. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 147.Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Molecular cell. 2010;40:280–93. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Molecular cell. 2008;30:678–88. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li DD, Wang LL, Deng R, Tang J, Shen Y, Guo JF, et al. The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells. Oncogene. 2009;28:886–98. doi: 10.1038/onc.2008.441. [DOI] [PubMed] [Google Scholar]
- 151.Raciti M, Lotti LV, Valia S, Pulcinelli FM, Di Renzo L. JNK2 is activated during ER stress and promotes cell survival. Cell Death Dis. 2012;3:e429. doi: 10.1038/cddis.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Castillo K, Rojas-Rivera D, Lisbona F, Caballero B, Nassif M, Court FA, et al. BAX inhibitor-1 regulates autophagy by controlling the IRE1alpha branch of the unfolded protein response. EMBO J. 2011;30:4465–78. doi: 10.1038/emboj.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Margariti A, Li H, Chen T, Martin D, Vizcay-Barrena G, Alam S, et al. XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. The Journal of biological chemistry. 2013;288:859–72. doi: 10.1074/jbc.M112.412783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shimodaira Y, Takahashi S, Kinouchi Y, Endo K, Shiga H, Kakuta Y, et al. Modulation of endoplasmic reticulum (ER) stress-induced autophagy by C/EBP homologous protein (CHOP) and inositol-requiring enzyme 1alpha (IRE1alpha) in human colon cancer cells. Biochem Biophys Res Commun. 2014;445:524–33. doi: 10.1016/j.bbrc.2014.02.054. [DOI] [PubMed] [Google Scholar]
- 155.Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell structure and function. 2002;27:421–9. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- 156.Verfaillie T, Salazar M, Velasco G, Agostinis P. Linking ER Stress to Autophagy: Potential Implications for Cancer Therapy. Int J Cell Biol. 2010;2010:930509. doi: 10.1155/2010/930509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 158.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–4. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 159.Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–5. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 160.Dorr JR, Yu Y, Milanovic M, Beuster G, Zasada C, Dabritz JH, et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501:421–5. doi: 10.1038/nature12437. [DOI] [PubMed] [Google Scholar]
- 161.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–76. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 162.Kim HD, Jang CY, Choe JM, Sohn J, Kim J. Phenylbutyric acid induces the cellular senescence through an Akt/p21(WAF1) signaling pathway. Biochem Biophys Res Commun. 2012;422:213–8. doi: 10.1016/j.bbrc.2012.04.086. [DOI] [PubMed] [Google Scholar]
- 163.Rajesh K, Papadakis AI, Kazimierczak U, Peidis P, Wang S, Ferbeyre G, et al. eIF2alpha phosphorylation bypasses premature senescence caused by oxidative stress and pro-oxidant antitumor therapies. Aging (Albany NY) 2013;5:884–901. doi: 10.18632/aging.100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhu X, Zhang J, Sun H, Jiang C, Dong Y, Shan Q, et al. Ubiquitination of Inositol-requiring Enzyme 1 (IRE1) by the E3 Ligase CHIP Mediates the IRE1/TRAF2/JNK Pathway. The Journal of biological chemistry. 2014;289:30567–77. doi: 10.1074/jbc.M114.562868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sayers CM, Papandreou I, Guttmann DM, Maas NL, Diehl JA, Witze ES, et al. Identification and Characterization of a Potent Activator of P53-Independent Cellular Senescence Via a Small Molecule Screen for Modifiers of the Integrated Stress Response. Molecular Pharmacology. 2012 doi: 10.1124/mol.112.081810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 167.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 168.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, et al. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. The Journal of cell biology. 2009;186:783–92. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. The Journal of biological chemistry. 2004;279:45495–502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 172.Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–7. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 173.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Molecular and cellular biology. 2001;21:1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chiribau CB, Gaccioli F, Huang CC, Yuan CL, Hatzoglou M. Molecular symbiosis of CHOP and C/EBP beta isoform LIP contributes to endoplasmic reticulum stress-induced apoptosis. Molecular and cellular biology. 2010;30:3722–31. doi: 10.1128/MCB.01507-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Molecular cell. 2001;8:705–11. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 176.Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–49. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 177.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 178.Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, et al. ASK1 is essential for JNK/SAPK activation by TRAF2. Molecular cell. 1998;2:389–95. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 179.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 180.Bassik MC, Scorrano L, Oakes SA, Pozzan T, Korsmeyer SJ. Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. EMBO J. 2004;23:1207–16. doi: 10.1038/sj.emboj.7600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–51. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–6. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 183.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. The Journal of cell biology. 2009;186:323–31. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–75. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186–94. doi: 10.1196/annals.1299.032. [DOI] [PubMed] [Google Scholar]
- 186.Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, et al. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. The Journal of cell biology. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. The Journal of cell biology. 2000;150:887–94. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. The Journal of biological chemistry. 2001;276:13935–40. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 189.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 190.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Feng YX, Sokol ES, Del Vecchio CA, Sanduja S, Claessen JH, Proia TA, et al. Epithelial-to-mesenchymal transition activates PERK-eIF2alpha and sensitizes cells to endoplasmic reticulum stress. Cancer Discov. 2014;4:702–15. doi: 10.1158/2159-8290.CD-13-0945. [DOI] [PubMed] [Google Scholar]
- 192.Nagelkerke A, Bussink J, van der Kogel AJ, Sweep FC, Span PN. The PERK/ATF4/LAMP3-arm of the unfolded protein response affects radioresistance by interfering with the DNA damage response. Radiother Oncol. 2013;108:415–21. doi: 10.1016/j.radonc.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 193.Sahni S, Bae DH, Lane DJ, Kovacevic Z, Kalinowski DS, Jansson PJ, et al. The metastasis suppressor, N-myc downstream-regulated gene 1 (NDRG1), inhibits stress-induced autophagy in cancer cells. The Journal of biological chemistry. 2014;289:9692–709. doi: 10.1074/jbc.M113.529511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fang H, Huang W, Xu YY, Shen ZH, Wu CQ, Qiao SY, et al. Blocking of N-acetylglucosaminyltransferase V induces cellular endoplasmic reticulum stress in human hepatocarcinoma 7,721 cells. Cell Res. 2006;16:82–92. doi: 10.1038/sj.cr.7310011. [DOI] [PubMed] [Google Scholar]
- 195.Yanagisawa K, Konishi H, Arima C, Tomida S, Takeuchi T, Shimada Y, et al. Novel metastasis-related gene CIM functions in the regulation of multiple cellular stress-response pathways. Cancer research. 2010;70:9949–58. doi: 10.1158/0008-5472.CAN-10-1055. [DOI] [PubMed] [Google Scholar]
- 196.Sheshadri N, Catanzaro JM, Bott AJ, Sun Y, Ullman E, Chen EI, et al. SCCA1/SERPINB3 Promotes Oncogenesis and Epithelial-Mesenchymal Transition via the Unfolded Protein Response and IL6 Signaling. Cancer research. 2014;74:6318–29. doi: 10.1158/0008-5472.CAN-14-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gade P, Ramachandran G, Maachani UB, Rizzo MA, Okada T, Prywes R, et al. An IFN-gamma-stimulated ATF6-C/EBP-beta-signaling pathway critical for the expression of Death Associated Protein Kinase 1 and induction of autophagy. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10316–21. doi: 10.1073/pnas.1119273109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nature reviews Immunology. 2008;8:663–74. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 199.Kim JY, Hwang JH, Cha MR, Yoon MY, Son ES, Tomida A, et al. Arctigenin blocks the unfolded protein response and shows therapeutic antitumor activity. J Cell Physiol. 2010;224:33–40. doi: 10.1002/jcp.22085. [DOI] [PubMed] [Google Scholar]
- 200.Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E869–78. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–4. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ri M, Tashiro E, Oikawa D, Shinjo S, Tokuda M, Yokouchi Y, et al. Identification of Toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing. Blood cancer journal. 2012;2:e79. doi: 10.1038/bcj.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sanches M, Duffy NM, Talukdar M, Thevakumaran N, Chiovitti D, Canny MD, et al. Structure and mechanism of action of the hydroxy-aryl-aldehyde class of IRE1 endoribonuclease inhibitors. Nat Commun. 2014;5:4202. doi: 10.1038/ncomms5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen S, Zhao Y, Zhang Y, Zhang D. Fucoidan induces cancer cell apoptosis by modulating the endoplasmic reticulum stress cascades. PloS one. 2014;9:e108157. doi: 10.1371/journal.pone.0108157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjohn J, et al. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006;443:548–52. doi: 10.1038/nature05124. [DOI] [PubMed] [Google Scholar]
- 206.Backer JM, Krivoshein AV, Hamby CV, Pizzonia J, Gilbert KS, Ray YS, et al. Chaperone-targeting cytotoxin and endoplasmic reticulum stress-inducing drug synergize to kill cancer cells. Neoplasia. 2009;11:1165–73. doi: 10.1593/neo.09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Firczuk M, Gabrysiak M, Barankiewicz J, Domagala A, Nowis D, Kujawa M, et al. GRP78-targeting subtilase cytotoxin sensitizes cancer cells to photodynamic therapy. Cell Death Dis. 2013;4:e741. doi: 10.1038/cddis.2013.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Schonthal AH. Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem Pharmacol. 2013;85:653–66. doi: 10.1016/j.bcp.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 209.Fels DR, Ye J, Segan AT, Kridel SJ, Spiotto M, Olson M, et al. Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways. Cancer research. 2008;68:9323–30. doi: 10.1158/0008-5472.CAN-08-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–16. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li X, Zhang K, Li Z. Unfolded protein response in cancer: the physician’s perspective. J Hematol Oncol. 2011;4:8. doi: 10.1186/1756-8722-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Garcia-Gomez A, Quwaider D, Canavese M, Ocio EM, Tian Z, Blanco JF, et al. Preclinical activity of the oral proteasome inhibitor MLN9708 in Myeloma bone disease. Clin Cancer Res. 2014;20:1542–54. doi: 10.1158/1078-0432.CCR-13-1657. [DOI] [PubMed] [Google Scholar]
- 213.Crawford LJ, Walker B, Irvine AE. Proteasome inhibitors in cancer therapy. J Cell Commun Signal. 2011;5:101–10. doi: 10.1007/s12079-011-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Goldberg AL. Development of proteasome inhibitors as research tools and cancer drugs. The Journal of cell biology. 2012;199:583–8. doi: 10.1083/jcb.201210077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chaachouay H, Ohneseit P, Toulany M, Kehlbach R, Multhoff G, Rodemann HP. Autophagy contributes to resistance of tumor cells to ionizing radiation. Radiother Oncol. 2011;99:287–92. doi: 10.1016/j.radonc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 216.Ito H, Daido S, Kanzawa T, Kondo S, Kondo Y. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. Int J Oncol. 2005;26:1401–10. [PubMed] [Google Scholar]
- 217.Wilson EN, Bristol ML, Di X, Maltese WA, Koterba K, Beckman MJ, et al. A switch between cytoprotective and cytotoxic autophagy in the radiosensitization of breast tumor cells by chloroquine and vitamin D. Horm Cancer. 2011;2:272–85. doi: 10.1007/s12672-011-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tsukumo Y, Tsukahara S, Furuno A, Iemura S, Natsume T, Tomida A. TBL2 is a novel PERK-binding protein that modulates stress-signaling and cell survival during endoplasmic reticulum stress. PloS one. 2014;9:e112761. doi: 10.1371/journal.pone.0112761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mahadevan NR, Anufreichik V, Rodvold JJ, Chiu KT, Sepulveda H, Zanetti M. Cell-extrinsic effects of tumor ER stress imprint myeloid dendritic cells and impair CD8(+) T cell priming. PloS one. 2012;7:e51845. doi: 10.1371/journal.pone.0051845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Thevenot PT, Sierra RA, Raber PL, Al-Khami AA, Trillo-Tinoco J, Zarreii P, et al. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity. 2014;41:389–401. doi: 10.1016/j.immuni.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]