Abstract
Lumbar disc herniation is one of the most common spinal degenerative disorders which may lead to low back pain (LBP) and radicular leg pain. However, it remains difficult to diagnose a degenerative herniated disc as the LBP generator in clinical practice. The purpose of this study is to explore the characteristic changes of a herniated disc causing LBP on MRI and to clarify the underlying role of inflammatory mediators and annulus fibrous (AF) tears in LBP generation associated with disc herniation. We prospectively collected intervertebral disc specimens and MRI from 57 single-segment disc herniation patients with radiculopathy. All subjects were grouped according to LBP occurrence or disc degeneration severity for the comparison of inflammatory mediators’ expression and AF tears occurrence (High Intensity Zone, HIZ, on MRI). LBP incidence under circumstances of different degeneration severity with or without HIZ was further analyzed. Both LBP incidence and Inflammatory mediators expression in moderately degenerated group was higher than mildly and severely degenerative groups. HIZ incidence was higher in moderately and severely degenerated groups. LBP incidence in the patients with both moderately degenerated discs and HIZ was 86.7%, much higher than the rest of the patient population. In conclusion, the high expression of inflammatory mediators with AF tears causes LBP associated with disc herniation. Moderately degenerative disc with HIZ is MRI morphological change of herniated disc causing LBP, which can be applied to diagnose LBP.
Keywords: Low back pain, lumbar disc herniation, annulus fibrous tear, inflammatory cytokine, high intensity zone
Introduction
Low back pain (LBP) has become one of the most serious public health problems, with a lifetime prevalence as high as 84% [1]. The total cost associated with LBP in the United States is estimated to exceed 100 billion dollars per year [2].
Spinal degenerative disorders, such as disc herniation, spinal stenosis, and degenerative spondylolisthesis may lead to LBP. According to a joint clinical practice guideline from the American College of Physicians and the American Pain Society, LBP was classified into three categories: nonspecific LBP, LBP potentially associated with radiculopathy or spinal stenosis, and LBP potentially associated with another specific spinal cause [3]. Of all, lumbar disc herniation is one of the most common spinal degenerative disorders leading to LBP associated with radiculopathy [3-6].
On the other hand, some studies found that disc herniation was actually common in asymptomatic people as well [5,7,8]. Boden et al performed MRI on asymptomatic subjects and found that herniated disc was demonstrated in 24% of the subjects overall, with 21% in the 20 to 39 age group, 22% in the 40 to 60 age group, and 36% in the over 60 age group [7]. Jensen et al conducted a similar study and showed that 27% of asymptomatic subjects had disc herniation [8].
Both disc inflammation and nerve root compression together are responsible for radicular pain [3-6]. However, what causes the difference in LBP occurrence among disc protrusion patients is still unclear. Great effort has been paid to find the answers. Inflammatory response has been acknowledged to be important in the process of disc degeneration [9-11] and may play an important role in pain generation [12-15]. However, controversial studies reporting its irrelevance with LBP makes it improbable to be the only answer [16,17]. Intervertebral disc Annual Fibrous (AF) tear is another important factor related to disc degeneration and pain generation [13,14,18-21]. Previous studies demonstrated that AF tear detected by histology and MRI in the patient with LBP could be considered a reliable marker for a painful disc [13,14,19,20]. However, controversial results were also reported [22,23], indicating that AF tear alone may not be sufficient to cause LBP arising from a degenerative disc. One comprehensively accepted explanation of AF tears causing LBP is that it could enhance the transportation of macromolecules from the NP to AF and ingrowth of nerve fibers into internal AF or NP [13,14,24,25]. Thus, we hypothesize that under the circumstances of disc degeneration, overexpressed inflammatory mediators acting as pain stimuli transport from the NP to external AF or even the periphery of AF, and interact with the nociceptive receptors that are usually located around the periphery of AF and have grown into the AF even NP through tears [13,14,24,25], consequently causing LBP.
The aims of the current study are (1) to explore the characteristic changes of a herniated disc causing LBP on MRI; (2) to clarify the underlying role of inflammatory mediators and AF tears in LBP generation associated with disc herniation.
Materials and methods
Subjects
Ethical approval from the local ethical committees and informed consent from patients were obtained.
A total of 57 disc protrusion patients (28 males and 29 females) undergoing lumbar intervertebral discectomy surgery from July 2011 to November 2013 in our department were included. All subjects were admitted to hospital because of lower extremity radiculopathy. The primary indication of discectomy is to manage radiculopathy, however, some of the patients had LBP whereas some did not. All subjects meet the following inclusion criteria: (1) a single-segment disc herniation on MRI; (2) lower extremity radiculopathy; (3) no response to conservative treatments, such as anti-inflammatory drugs and physical therapy. Patients with endplate Modic changes, facet joint arthritis, Schmorl’s nodes, spinal deformity, tumor, infection, spondylolisthesis or canal stenosis were excluded.
Clinical outcomes
All patients described the intensity of their LBP on a 0-10 (0, no pain; 10, worst pain) visual analogue scale (VAS) 1 week before surgery. Based on their preoperative VAS and the duration of LBP, all patients were divided into the LBP (+) group (VAS > 3, lasting for a minimum of 3 months) and the LBP (-) group, according to the rationale that a VAS of 4 is considered a disruption in quality of life [16].
MRI analysis
MR imaging was performed using a 3.0-T unit (Symphony Quantum, Siemens AG Medical Solutions). Spin echo T1-weighted (repetition time [TR] < 800) proton density (1,000 < TR < 2,000; echo time [TE] < 30) and T2-weighted (TR = 2000; 50 < TE < 100) sagittal and axial 4 mm thick MR images were generated. HIZ was evaluated based on the criteria set by Aprill [26]. Two experienced spine surgeons evaluated the MRI scans in a blinded fashion to determine HIZ by reviewing the sagittal and axial T-2 weighted images. Additionally, according to the degeneration degree (Pfirrmann grading system [27]) of the dissected level, all subjects were grouped into three categories: mildly degeneration (Grade II), moderately degeneration (Grade III), and severely degeneration (Grade IV and Grade V).
Sample tissue collection and preparation
Nucleus pulposus samples were harvested from the lumbar intervertebral discs of all 57 patients intraoperatively. Immediately upon collection, the samples were divided into two equal parts. One part was stored in liquid nitrogen for qRT-PCR. The second part was prepared by fixing in a 10% formalin solution for immunohistochemical analysis.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using Trizol reagent (Invitrogen), according to the manufacturer’s protocol. The complementary DNA was synthesized using a Reverse Transcription Kit (Toyobo FSQ-101), and the PCR reaction system was performed using the SYBR qPCR Mix Kit (Toyobo QPS-201), according to the manufacturer’s protocol. RT-PCR was carried out using a CFX 96 (BIO RAD) that detects SYBR Green fluorescent dye incorporated in double stranded DNA. Forty real-time PCR cycles were performed for denaturation (95°C for 15 s), annealing, and elongation (60°C for 45 s). The primers sequences were as follows: IL-1β: forward 5’-GACCTGAGCACCTTCTTTCC-3’; reverse: 5’-CTGGAGGTGGAGAGCTTTCA-3’; TNF-α: forward: 5’-TTCTGCCTGCTGCACTTTG-3’; reverse: 5’-TGGGCTACAGGCTTGTCACT-3’; iNOS: forward: 5’-ACCAGTACGTTTGGCAATGG-3’; reverse: 5’-GAACACGTTCTTGGCATGCA-3’; Substance P: forward: 5’-GAGCCCTTTGAGCATCTTCT-3’; reverse: 5’-TCTGGCCATGTCCATAAAGAG-3’; β-actin: forward: 5’-TGGATCAGCAAGCAGGAGTA-3’; reverse: 5’-TCGGCCACATTGTGAACTTT-3’. The mRNA levels of IL-1β, TNF-α, iNOS and Substance P were standardized versus β-actin. The mRNA expression of group LBP (+) was expressed as a ratio to group LBP (-). The mRNA expressions of moderately and severely degenerated patients were expressed as ratios to mildly degenerated patients.
Immunohistochemical analysis (IHC)
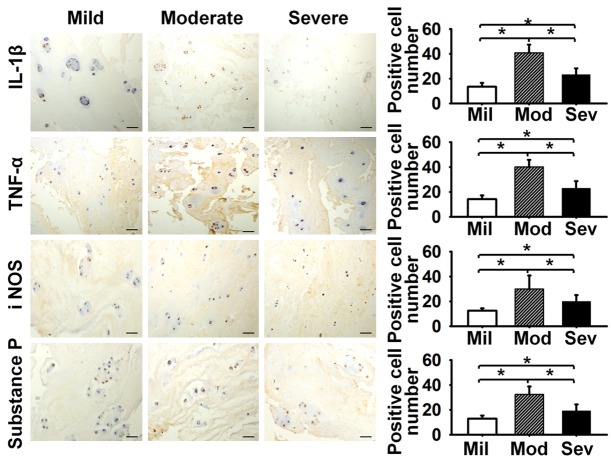
Disc samples were prepared for analysis by fixing in a 10% formalin solution, dehydrating in a graded alcohol series, treating with xylene for transparency, and embedding in paraffin. Thereafter, the paraffin blocks were sliced into 5 µm tissue sections. After antigen retrieval, quenching of endogenous peroxidase and blocking of non-specific binding, sections were incubated with mouse polyclonal antibody against TNF-α (Santa Cruz, sc-130349), rabbit polyclonal antibody against IL-1β (Santa Cruz, sc-7884), goat polyclonal antibody against substance P (Santa Cruz, sc-9758), and rabbit polyclonal antibody against iNOS (Santa Cruz, sc-651) at 1:250 dilution, followed by HRP-conjugated secondary antibody. Sections in which the primary antibodies had been omitted were used as negative controls. There were nine fields of view under microscopy, with 100 magnifications scanned to count the number of positive staining cells for IL-1β, TNF-α, iNOS and Substance P. Finally, the number of immunopositive cells was expressed as a percentage of the total number.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 (SPSS, Inc, Chicago, IL, USA). One-way analysis of variance (ANOVA) was performed to determine the significance of age distribution. The Mann-Whitney U test was performed to determine the significance of the percentage of immunopositive cells and mRNA expression between LBP (+) and LBP (-) groups. The Kruskal-Wallis H test was performed to determine the significance of the percentage of immunopositive cells and mRNA expression in mildly, moderately and severely degenerated discs. HIZ incidence and LBP occurrence were compared using a chi-square test. In most cases, numbers are presented as the mean ± standard deviation (SD). P < 0.05 was considered statistically significant.
Results
Subjects
The LBP (+) group consisted of 25 patients (12 males and 13 females), with a mean age of 30.2 ± 7.4 years. The LBP (-) group consisted of 32 patients (16 males and 16 females), with a mean age of 28.0 ± 6.0 years. There was no significant age distribution difference between the LBP (+) group and the LBP (-) group (P > 0.05).
LBP incidence and disc degeneration
LBP incidences were compared among groups according to Pfirrmann disc degeneration grading system. The data showed that 16.7% (2/12 patients) of patients with mildly degenerated discs and 28.6% (6/21) of patients with severely degenerated discs were with LBP respectively, while 62.5% (15/24) of patients with moderately degenerated discs were with LBP (P < 0.05) (Figure 1), suggesting that moderately degenerated discs are closely correlated to LBP.
Figure 1.
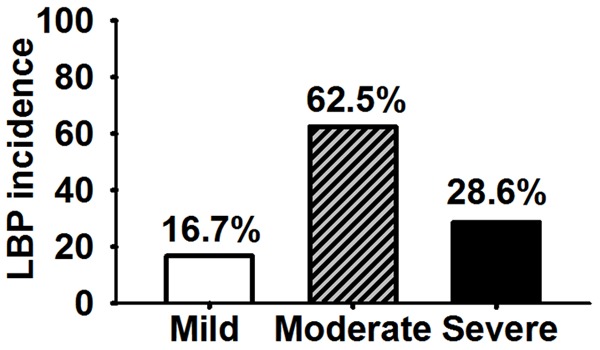
LBP incidence in moderately degenerated disc was higher than those in mildly and severely degenerated disc (P < 0.05).
Inflammatory mediators expression and disc degeneration
Immunoreactivity for the detected inflammatory mediators was observed in all categories of degenerated discs. In the mildly degenerated group, percentages of immunopositive cells were 13.5 ± 3.2% for IL-1β, 14.2 ± 3.0% for TNF-α, 12.6 ± 1.9% for iNOS and 13.0 ± 2.4% for Substance P. Compared to the mildly degenerated group, a significant increase of immunopositive cells percentages was observed in the moderately degenerated group (IL-1β 40.9 ± 6.6%, TNF-α 40.1 ± 5.7%, iNOS 29.9 ± 10.9%, Substance P 32.4 ± 6.3%, P < 0.05) and the severely degenerated group (IL-1β 23.2 ± 5.1%, TNF-α 22.9 ± 5.7%, iNOS 20.1 ± 4.9%, Substance P 19.2 ± 5.2%, P < 0.05). However, compared to the moderately degenerated group, a significant decrease of immunopositive cells percentages was observed in all four detected inflammatory mediators in the severely degenerated group (P < 0.05) (Figure 2).
Figure 2.
The inflammatory mediators expression in moderately degenerated disc was the highest for IL-1β, TNF-α, iNOS and Substance P (P < 0.05), bar = 100 um. Mil: Mild, Mod: Moderate, Sev: Severe.
Similar expression trends of the detected inflammatory mediators in mRNA levels were observed. Compared to the mildly degenerated group, a significant increase of mRNA level was observed in the moderately degenerated group (IL-1β 3.663-fold, TNF-α 3.693-fold, iNOS 3.801-fold, Substance P 3.625-fold, P < 0.05), and the severely degenerated group (IL-1β 1.893-fold, TNF-α 1.797-fold, iNOS 2.016-fold, Substance P 1.852-fold, P < 0.05). However, compared to the moderately degenerated group, a significant decrease of mRNA levels was observed in all four detected inflammatory mediators in the severely degenerated group (P < 0.05) (Figure 3).
Figure 3.
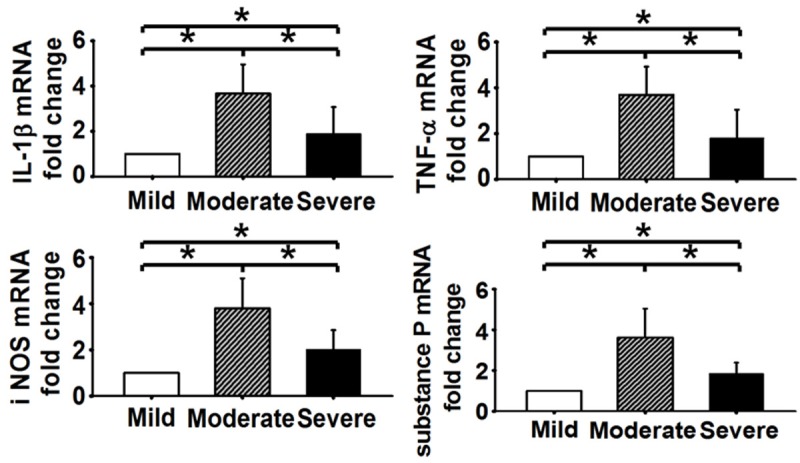
The mRNA level in moderately degenerated disc was the highest for IL-1β, TNF-α, iNOS and Substance P (P < 0.05).
These data suggest that inflammatory mediators’ expression depends on the severity of disc degeneration. Compared to mildly and severely degenerated discs, moderately degenerated discs demonstrate the highest expression of inflammatory mediators.
Inflammatory mediators’ expression and LBP
In the asymptomatic group, the immunopositive cells percentages of the detected inflammatory mediators were 28.2 ± 9.7% for IL-1β, 33.1 ± 5.9% for TNF-α, 22.5 ± 9.9% for iNOS and 22.3 ± 9.8% for Substance P (Figure 4). In the LBP group, the immunopositive cells percentages of the detected inflammatory mediators were 33.2 ± 11.4% for IL-1β, 37.9 ± 12.7% for TNF-α, 24.9 ± 9.3% for iNOS and 26.9 ± 9.7% for Substance P (Figure 4). Differences between the two groups did not reach statistical significance (P > 0.05). Compared to the asymptomatic group, the detected inflammatory mediators expression in mRNA level was IL-1β 1.484-fold, TNF-α 1.383-fold, iNOS 1.35-fold and Substance P 1.46-fold in LBP group. The differences between the two groups did not achieve statistical significance (P > 0.05) (Figure 5).
Figure 4.
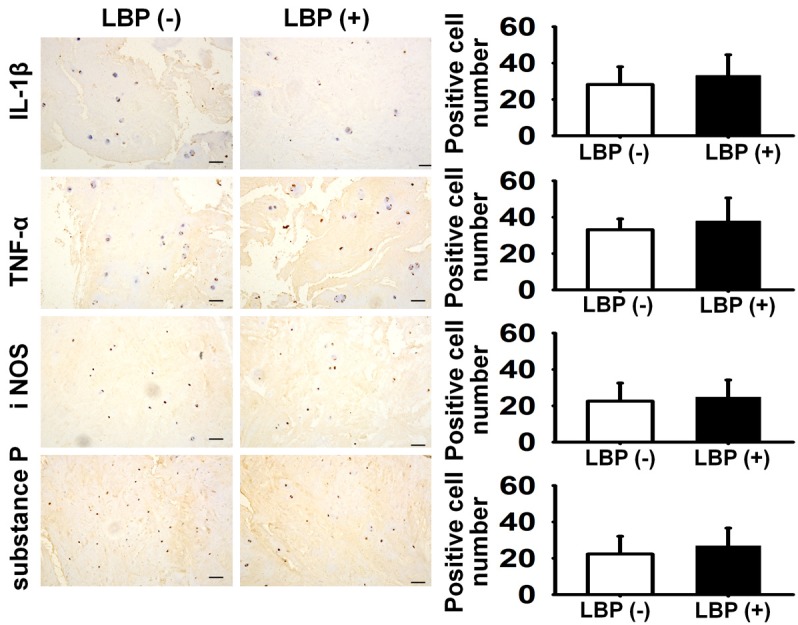
The inflammatory mediators expression difference between LBP (+) and LBP (-) for IL-1β, TNF-α, iNOS and Substance P did not reach significance (P > 0.05), bar = 100 um.
Figure 5.
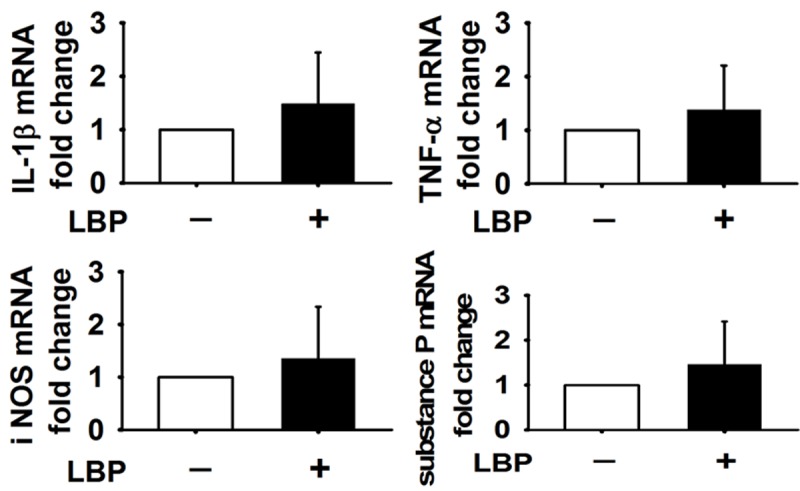
The mRNA level difference between LBP (+) and LBP (-) for IL-1β, TNF-α, iNOS and Substance P did not reach significance (P > 0.05).
HIZ incidence and disc degeneration
No HIZ (0/12) was found in mildly degenerated discs in the current study, while 62.5% (15/24) of moderately degenerated discs and 61.9% (13/21) of severely degenerated discs demonstrated HIZ (Figure 6).
Figure 6.
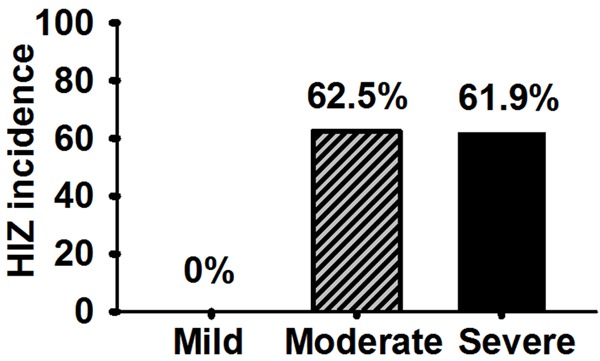
HIZ incidence in moderately and severely degenerated discs were higher than that in mildly degenerated disc (P < 0.05).
HIZ incidence and LBP incidence
LBP incidences were compared between the groups of HIZ (+) and HIZ (-). The data demonstrated that 60.7% (17/28 patients) of patients in HIZ (+) group were with LBP, while 20.7% (6/29 patients) of patients in HIZ (-) group were with LBP (P < 0.05) (Figure 7).
Figure 7.
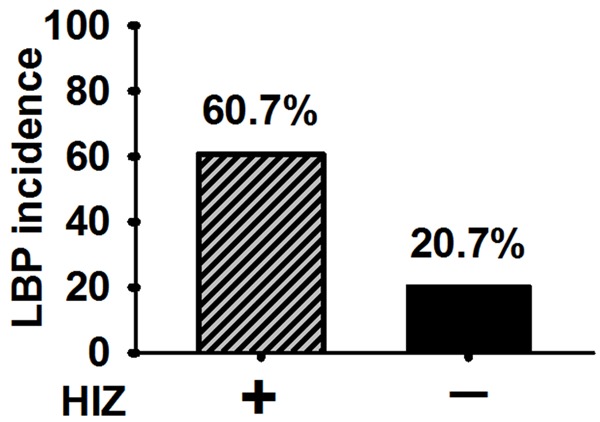
LBP incidence in HIZ (+) was higher than that in HIZ (-) (P < 0.05).
LBP incidence analysis with degeneration severity and HIZ incidence
To further elucidate the relationship between disc degeneration and LBP, all subjects were divided into groups according to the severity of disc degeneration and HIZ existence, which were mild degeneration + HIZ (-), mild degeneration + HIZ (+), moderate degeneration + HIZ (-), moderate degeneration + HIZ (+), severe degeneration + HIZ (-), severe degeneration + HIZ (+). LBP incidence of the above groups were 16.7% (2/12), 0% (0/0), 22.2% (2/9), 86.7% (13/15), 25% (2/8), and 30.8% (4/13), respectively. LBP incidence was significantly higher in the group demonstrating moderate degeneration + HIZ (+) than in any of the other groups (P < 0.05) (Figure 8). Magnetic resonance imaging effectively illustrated the role of moderate degeneration + HIZ (+) in LBP pathogenesis, and suggests that both are necessary (Figure 9).
Figure 8.
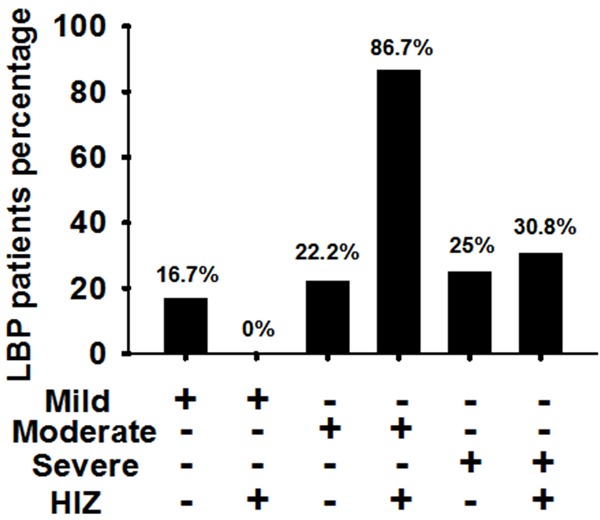
LBP incidence was significantly higher in the group with both moderately degenerated discs and HIZ than those in the other groups (P < 0.05).
Figure 9.
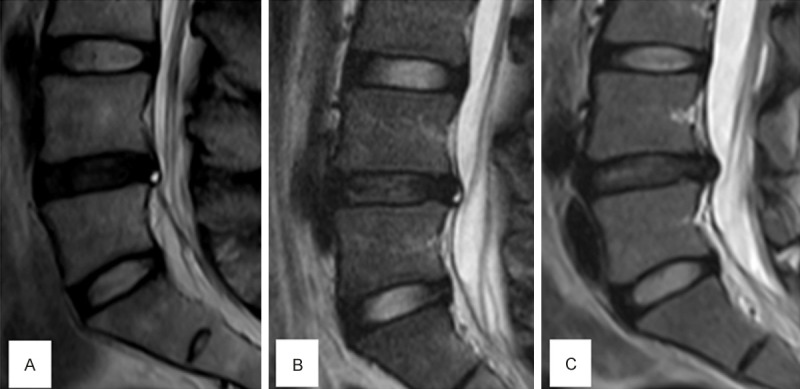
Three representative MRI scan imaging of intervertebral disc protrution patients. A: Severely degenerated disc with HIZ had no LBP. B: Moderately degenerated disc with HIZ had LBP. C: Moderately degenerated disc without HIZ had no LBP.
Discussion
Etiology of LBP is complicated and requires further elucidation. Many factors in the process of spinal degeneration may cause LBP [28,29]. Lumbar disc herniation is one of the most common spinal degenerative disorders. And it usually leads to a combination of discogenic lumbago and radicular leg pain [30,3-6], while there are still some of the disc protrusion patients only presenting radicular leg pain.
Magnetic Resonance Imaging (MRI) is the gold standard for evaluating the relationship of disc material to soft tissue and neural structures [27,31-33]. MRI often can identify morphological changes in low back structures; however, it cannot conclusively identify them as pain generators. So far there is no sensitive noninvasive diagnostic tool to help with identifying whether a degenerative herniated disc is a source of pain, and whether the treatments targeting a degenerative herniated disc would well relieve the LBP symptom. Our study prospectively studied disc herniation patients who had discectomy indicated for lower extremity radiculopathy. Patients with acknowledged LBP contributors like Modic changes, facet joint arthritis, Schmorl’s nodes, canal stenosis, spondylolesthesis were excluded. The purpose of the current study is to explore the characteristic changes of a herniated disc causing LBP on MRI and to clarify the underlying role of inflammatory mediators and AF tears in LBP generation associated with disc herniation.
In the current study, the correlation between disc degeneration severity and LBP incidence is not linear, and moderately degenerative disc group showed the highest (62.5%) LBP incidence, while mildly and severely degenerative disc groups were much lower (16.7% and 28.6%, respectively) (Figure 1). As we acknowledged, this is the first time to show the association of disc degeneration severity and LBP. This result may partially explain why disc degeneration was common in asymptomatic people. If the study subjects include a high percentage of patients with severely degenerative discs, the LBP incidence may be affected and overlooked.
Inflammation response has been acknowledged to be important in the process of disc degeneration [9-11]. The current study demonstrated that both severely and mildly degenerated discs showed a lower expression of inflammatory mediators, while moderately degenerated discs showed the highest expression level, which is a novel finding not found in previous reports (Figures 2, 3). The decrease in inflammatory mediators’ expression in severely degenerated disc may be attributed to the reduced cell number and the decreased metabolic activity of disc cells [28,34-39]. Importantly, this expression pattern of inflammatory mediators was corresponding to the LBP occurrence pattern, both highest in the moderately degenerative discs. It suggested that inflammatory mediators might play an important role in LBP.
Inflammation response has been acknowledged to be possibly related to LBP generation [12-15]. Our study directly compared inflammatory mediators’ expression between painful and asymptomatic group patients. Unexpectedly, no significant difference (Figures 4, 5) was found between the two groups. It suggests that if it is true that inflammation does correlate to LBP generation, inflammatory mediators expression by itself may be insufficient to induce LBP, Considering that the LBP incidence in the moderately degenerative group with a high level inflammatory mediators expression was only around 60% (Figure 1), even though higher than mildly or severely degenerative groups, it is reasonable to assume that some other factors may take effect and influence the LBP generation.
AF tear is a common phenomenon along with the process of disc degeneration [18,21,36]. The nerve fibers tend to extend along AF tears into the internal part of AF, even NP tissue [13,14]. Besides, with such tears, the transport of inflammation cytokines and nociceptive stimulators from within NP to nociceptive receptors in AF would be enhanced [25]. In this accepted theory, AF tear plays an important role in LBP generation. High intensity zone (HIZ) on MRI is considered to represent AF tears [19,20,26]. In the current study, HIZ was not observed in mildly degenerated discs, and the HIZ incidence of moderately and severely degenerated discs was around 60% (Figure 6), suggesting that AF tears are closely related to disc degeneration. Since the LBP incidence in HIZ (+) group was only around 60% (Figure 7), it is also reasonable to speculate that HIZ is not a single factor to induce LBP. Therefore, a combined analysis of disc degeneration severity and HIZ on LBP incidence was further conducted. Interestingly, the LBP incidence of the population having a moderately degenerated disc along with HIZ was 86.7%, while a much lower incidence was found in each of the other situations (Figure 8). It not only confirmed that AF tears exerted a crucial effect on pain generation, but also both moderate disc degeneration with a high level inflammatory mediator expression and AF tears are essential for LBP associated with disc herniation.
Figure 10 shows an example of the above theory in clinical practice. The patient suffered from chronic LBP for over one year. MRI showed a moderately degenerated disc with mild herniation and HIZ. After performing radiofrequency ablation, the LBP symptoms were relieved, but the follow-up MRI remained unchanged. The pain relief was probably caused by inflammatory mediators’ inactivation by the heat of radiofrequency ablation, which eliminated the stimulation for nociceptive receptors.
Figure 10.
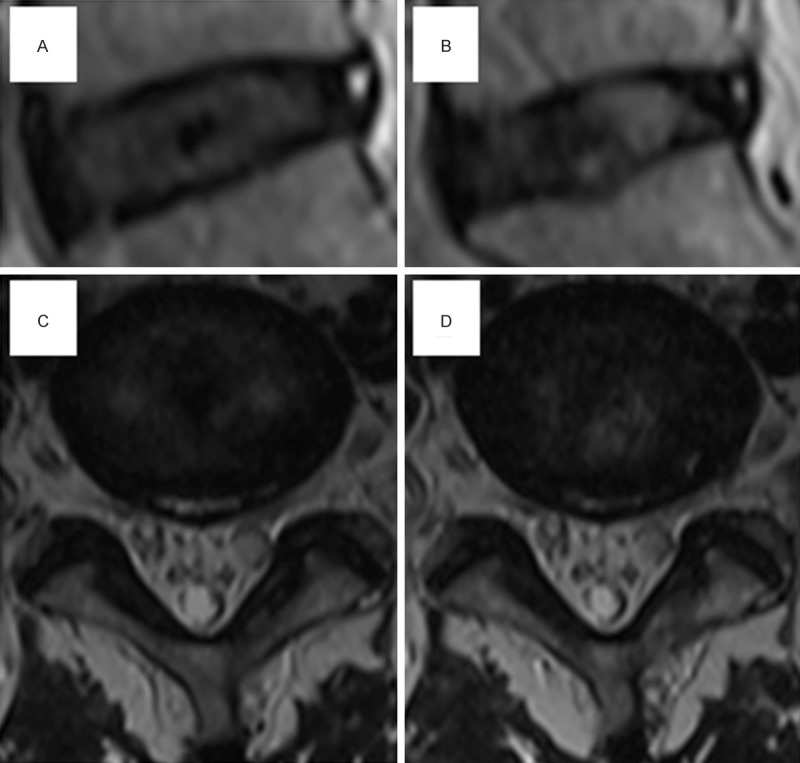
A typical disc protrusion case performing radiofrequency ablation. A and C: Preoperative MRI scan imaging showed moderate degeneration and HIZ. After radiofrequency ablation, the low back pain patient had pain relief. B and D: After half one year, MRI scan imaging had no obvious changes. But, the patient had no recurrence of LBP.
The current study has several limitations. Firstly, we only investigated a few kinds of inflammatory mediators which have been confirmed to be involved in degenerative disc and LBP. Secondly, to confirm our proposed mechanism of LBP pathogenesis, lumbar disc herniation animal studies will be necessary to identify the synergistic contribution of HIZ and high inflammatory mediator expression. Thirdly, a prospectively randomized controlled study is needed to evaluate diagnosis value of moderately degenerative disc with HIZ in clinical practice.
Conclusion
In summary, our results indicate that the high expression of inflammatory mediators with AF tears causes LBP associated with disc herniation. Moderately degenerative disc with HIZ is MRI morphological change of herniated disc causing LBP, which can be applied to diagnose LBP.
Acknowledgements
We gratefully acknowledge the funding from National Natural Science Foundation of China (81071511 and 81101384).
Disclosure of conflict of interest
None.
References
- 1.Balagué F, Mannion AF, Pellisé F, Cedraschi C. Non-specific low back pain. Lancet. 2012;379:482–491. doi: 10.1016/S0140-6736(11)60610-7. [DOI] [PubMed] [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain. socioeconomic factors and consequences. J Bone Joint Surg. 2006;88(Suppl 2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 3.Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr, Shekelle P, Owens DK Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478–491. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 4.Goupille P, Jayson MI, Valat JP, Freemont AJ. The role of inflammation in disk herniation-associated radiculopathy. Semin Arthritis Rheum. 1998;28:60–71. doi: 10.1016/s0049-0172(98)80029-2. [DOI] [PubMed] [Google Scholar]
- 5.McCall IW. Lumbar herniated disks. Radiol Clin North Am. 2000;38:1293–1309. doi: 10.1016/s0033-8389(08)70007-1. [DOI] [PubMed] [Google Scholar]
- 6.Van Boxem K, Cheng J, Patijn J, van Kleef M, Lataster A, Mekhail N, Van Zundert J. Lumbosacral radicular pain. Pain Pract. 2010;10:339–358. doi: 10.1111/j.1533-2500.2010.00370.x. [DOI] [PubMed] [Google Scholar]
- 7.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg. 1990;72:403–408. [PubMed] [Google Scholar]
- 8.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 9.Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF 3rd, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976) 1996;21:271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1β and TNFα expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JY, Kuh SU, Park HS, Kim KS. Comparative expression of matrix-associated genes and inflammatory cytokines-associated genes according to disc degeneration: analysis of living human nucleus pulposus. J Spinal Disord Tech. 2011;24:352–357. doi: 10.1097/BSD.0b013e3181fee4df. [DOI] [PubMed] [Google Scholar]
- 12.Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 13.Peng B, Hao J, Hou S, Wu W, Jiang D, Fu X, Yang Y. Possible pathogenesis of painful intervertebral disc degeneration. Spine (Phila Pa 1976) 2006;31:560–566. doi: 10.1097/01.brs.0000201324.45537.46. [DOI] [PubMed] [Google Scholar]
- 14.Peng B, Wu W, Hou S, Li P, Zhang C, Yang Y. The pathogenesis of discogenic low back pain. J Bone Joint Surg. 2005;87:62–67. [PubMed] [Google Scholar]
- 15.Schroeder M, Viezens L, Schaefer C, Friedrichs B, Algenstaedt P, Rüther W, Wiesner L, Hansen-Algenstaedt N. Chemokine profile of disc degeneration with acute or chronic pain. J Neurosurg Spine. 2013;18:496–503. doi: 10.3171/2013.1.SPINE12483. [DOI] [PubMed] [Google Scholar]
- 16.Andrade P, Hoogland G, Garcia MA, Steinbusch HW, Daemen MA, Visser-Vandewalle V. Elevated IL-1β and IL-6 levels in lumbar herniated discs in patients with sciatic pain. Eur Spine J. 2013;22:714–720. doi: 10.1007/s00586-012-2502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HJ, Suh BG, Lee DB, Lee GW, Kim DW, Kang KT, Chang BS, Lee CK, Yeom JS. The influence of pain sensitivity on the symptom severity in patients with lumbar spinal stenosis. Pain Physician. 2013;16:135–144. [PubMed] [Google Scholar]
- 18.Gallucci M, Anselmi M, Di Sibio A, Gregori LM. Annular tears, fissures or HIZ? Neuroradiology. 2011;53(Suppl 1):S161–S165. doi: 10.1007/s00234-011-0933-4. [DOI] [PubMed] [Google Scholar]
- 19.Kang CH, Kim YH, Lee SH, Derby R, Kim JH, Chung KB, Sung DJ. Can magnetic resonance imaging accurately predict concordant pain provocation during provocative disc injection? Skeletal Radiol. 2009;38:877–885. doi: 10.1007/s00256-009-0709-7. [DOI] [PubMed] [Google Scholar]
- 20.Peng B, Hou S, Wu W, Zhang C, Yang Y. The pathogenesis and clinical significance of a high-intensity zone (HIZ) of lumbar intervertebral disc on MR imaging in the patient with discogenic low back pain. Eur Spine J. 2006;15:583–587. doi: 10.1007/s00586-005-0892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith LJ, Nerurkar NL, Choi KS, Harfe BD, Elliott DM. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech. 2011;4:31–41. doi: 10.1242/dmm.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricketson R, Simmons JW, Hauser BO. The prolapsed intervertebral disc. The high-intensity zone with discography correlation. Spine Phila Pa 1976. 1996;21:2758–2762. doi: 10.1097/00007632-199612010-00010. [DOI] [PubMed] [Google Scholar]
- 23.Stadnik TW, Lee RR, Coen HL, Neirynck EC, Buisseret TS, Osteaux MJ. Annular tears and disk herniation: prevalence and contrast enhancement on MR images in the absence of low back pain or sciatica. Radiology. 1998;206:49–55. doi: 10.1148/radiology.206.1.9423651. [DOI] [PubMed] [Google Scholar]
- 24.Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O’Brien J, Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178–181. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 25.Ito K, Creemers L. Mechanisms of Intervertebral Disk Degeneration/Injury and Pain: A Review. Global Spine J. 2013;3:145–152. doi: 10.1055/s-0033-1347300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aprill C, Bogduk N. High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361–369. doi: 10.1259/0007-1285-65-773-361. [DOI] [PubMed] [Google Scholar]
- 27.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 28.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 29.Brisby H. Pathology and possible mechanisms of nervous system response to disc degeneration. J Bone Joint Surg. 2006;88(Suppl 2):68–71. doi: 10.2106/JBJS.E.01282. [DOI] [PubMed] [Google Scholar]
- 30.Kallewaard JW, Terheggen MA, Groen GJ, Sluijter ME, Derby R, Kapural L, Mekhail N, van Kleef M. 15. Discogenic low back pain. Pain Pract. 2010;10:560–579. doi: 10.1111/j.1533-2500.2010.00408.x. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Fredrickson V, Resnick DK. How Should We Grade Lumbar Disc Herniation and Nerve Root Compression? A Systematic Review. Clin Orthop Relat Res. 2014 doi: 10.1007/s11999-014-3674-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majumdar S, Link TM, Steinbach LS, Hu S, Kurhanewicz J. Diagnostic tools and imaging methods in intervertebral disk degeneration. Orthop Clin North Am. 2011;42:501–511. doi: 10.1016/j.ocl.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Sasiadek MJ, Bladowska J. Imaging of degenerative spine disease--the state of the art. Adv Clin Exp Med. 2012;21:133–142. [PubMed] [Google Scholar]
- 34.Abbott RD, Purmessur D, Monsey RD, Brigstock DR, Laudier DM, Iatridis JC. Degenerative Grade Affects the Responses of Human Nucleus Pulposus Cells to Link-N, CTGF, and TGFβ3. J Spinal Disord Tech. 2013;26:E86–E94. doi: 10.1097/BSD.0b013e31826e0ca4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan WC, Sze KL, Samartzis D, Leung VY, Chan D. Structure and biology of the intervertebral disk in health and disease. Orthop Clin North Am. 2011;42:447–464. doi: 10.1016/j.ocl.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 37.Larson JW 3rd, Levicoff EA, Gilbertson LG, Kang JD. Biologic modification of animal models of intervertebral disc degeneration. J Bone Joint Surg. 2006;88(Suppl 2):83–87. doi: 10.2106/JBJS.F.00043. [DOI] [PubMed] [Google Scholar]
- 38.Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9:R45. doi: 10.1186/ar2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Liu H, Zheng ZM, Zhang KB, Wang TP, Sribastav SS, Liu WS, Liu T. Role of death receptor, mitochondrial and endoplasmic reticulum pathways in different stages of degenerative human lumbar disc. Apoptosis. 2011;16:990–1003. doi: 10.1007/s10495-011-0644-7. [DOI] [PubMed] [Google Scholar]