Abstract
Introduction
Plasmodium chabaudi AS-infection in pregnant A/J and C57BL/6J mice results in mid-gestational pregnancy loss. Although associated with increased systemic and placental pro-inflammatory responses and coagulopathy, the molecular mechanisms that underlie poor pregnancy outcomes in these mice are not yet fully understood. This study investigates the relationships between inflammation, apoptosis and malaria-induced pregnancy loss.
Methods
Infection with Plasmodium chabaudi AS in early murine pregnancy and term human placental tissues from an endemic setting were assessed by histology, immunohistochemistry, TUNEL staining, real-time PCR, flow cytometry, western blot, and ELISA.
Results
Quantitative PCR reveals accumulation of lymphocytes and monocytes and upregulation of chemokines that attract these cell types in malaria-exposed mid-gestational A/J conceptuses. Monocyte accumulation is confirmed by flow cytometry and placental immunohistochemistry. Concurrent with initiation of malaria-induced abortion, markers of apoptosis are evident in the junctional zone, but not the labyrinth, of A/J placentae. In contrast, mid-gestation conceptuses in infected C57BL/6J lack evidence for monocyte accumulation, exhibiting low or no in situ placental staining despite trophoblast immunoreactivity for the monokine, CCL2. Additionally, placental apoptosis is not consistently observed, and when evident, appears after malaria-induced abortion typically initiates. Similarly, trophoblast apoptosis in term human placental malaria is not observed. Of those studied, a sole common feature of malaria-induced abortion in A/J and C57BL/6J mice is elevation of plasma tumor necrosis factor.
Discussion
Consistent with our previous observations, tumor necrosis factor is likely to be a central driver of malaria-induced pregnancy loss in both strains, but likely operates through mechanisms distinct from placental apoptosis in C57BL/6J mice.
Keywords: placental malaria, apoptosis, inflammation, pregnancy, abortion, Plasmodium chabaudi AS
INTRODUCTION
More than 100 million pregnancies worldwide are at risk of malaria infection every year [1]. Intrauterine growth restriction and/or premature delivery, leading to low birth weight, are the main adverse pregnancy outcomes of malaria during pregnancy in endemic settings. Abortion and stillbirth are also associated with malaria in women with little or no pre-existent immunity to malaria [2].
While extensive epidemiological data clearly outline the adverse pregnancy outcomes related to malaria infection, the precipitating immunopathological mechanisms for the most part remain a mystery. Adherence of Plasmodium falciparum-infected red blood cells to chondroitin sulfate A is considered to be the initiator of placental malaria (PM) pathogenesis [3]. Sequestering parasites release bioactive molecules that stimulate both maternal cells [4] and fetal syncytiotrophoblast [5, 6] to produce proinflammatory cytokines and chemokines, which in turn recruit, activate and retain inflammatory cells in the maternal intervillous space [4, 6-12]. Indeed, accumulation of maternal monocyte/macrophages, and increased levels of inflammatory cytokines, especially tumor necrosis factor (TNF), are common in human PM [4, 7, 12-14], and directly or indirectly, via impaired materno-fetal exchange of nutrients and gases and/or high metabolic demand of infiltrated leukocytes [15], contribute to poor birth outcomes [16]. These important contributions notwithstanding, the precise molecular and cellular mechanisms that lead to placental failure and fetal compromise must still be identified.
Using a murine model of PM during late pregnancy, previous studies have demonstrated the ability of P. berghei ANKA to recapitulate the characteristic features of human PM including infected red blood cell (iRBC) adherence to placental tissue [17]. In pregnant BALB/c mice infected with P. berghei ANKA at gestation day 13, necrosis, maternal blood sinusoid constriction, syncytiotrophoblast hyperplasia, distension of perivascular space, and mononuclear cell infiltration are observed in the term placenta [18].
In this model, MyD88-dependent inflammatory response [19], oxidative stress, apoptosis [20, 21], angiogenic dysregulation, and complement component C5a [22] have been proposed as mediators of fetal compromise. Additionally, trophoblast phagocytosis of red blood cells is associated with pregnancy loss in mice infected with P. chabaudi AS [23] as well as P. berghei [24]. Like P. berghei ANKA, P. chabaudi AS-iRBCs have also been reported to accumulate in placental tissue [25]. P. chabaudi AS infection early in pregnancy leads to poor outcomes in C57BL/6J (B6) mice as well as in A/J mice, with characteristic features of human PM that lead to poor pregnancy outcomes being found in both strains [25-28]. Reduced thickness of the labyrinth, extensive hemorrhage, and coagulopathy are found in mid-gestation placentae of B6 mice infected with P. chabaudi AS during early pregnancy [26, 28]. Whereas TNF responses to malaria are observed in both strains, levels are quite high in A/J mice [25, 26]; ablation of this response with neutralizing antibodies significantly improves mid-gestational pregnancy success in B6 [26] but not in A/J mice [25], in which higher neutralizing activity may be required. Ultimately, B6 mice recover from this infection, but A/J mice die by gestation day 14 [23, 25].
Although ultrasound studies suggest that the negative impact of malaria is detectable during early pregnancy in humans [29], most studies in malaria during pregnancy are conducted at term when the placenta is expelled. Therefore, little is known about the impact of malaria in early pregnancy because the placenta is not accessible for direct assessment.
Given the amenable nature of the P. chabaudi AS model for studies of malaria pathogenesis during early pregnancy, and the unsuitability of the P. berghei model for such work (initiation of infection on gestation day 7 leads to maternal lethality [30]), the current study of placental pathogenic mechanisms in the context of P. chabaudi AS infection initiated at conception was undertaken.
This work reveals that P. chabaudi AS infection during pregnancy in A/J and B6 mice differentially induces accumulation of lymphocytes and monocytes and chemokine upregulation in conceptuses, with markedly elevated responses in A/J mice. A/J mice also exhibit enhanced markers of apoptosis in the placenta, with cell death appearing concurrently with systemic TNF release and initiation of abortion. In contrast, markers of apoptosis are evident in B6 placentae only after malaria-induced abortion has begun. The results indicate that apoptosis and local placental inflammation cannot be invoked as universally important initiators of fetoplacental damage promoted by malaria in murine pregnancy.
MATERIALS AND METHODS
Parasites and mice
Plasmodium chabaudi AS was obtained from Dr. Michael Waisberg, National Institutes of Health, Besthesda, MD, USA and was maintained by routine passage through female A/J mice as previously described [27]. C57BL/6J (B6) and A/J mice were originally purchased from The Jackson Laboratory and were used to generate breeding stock and experimental animals in the University of Georgia Coverdell Vivarium. Infection in experimental female mice, aged 8 to 12 weeks, was initiated on gestation day 0 (with evidence of a vaginal plug) and monitored as previously described [27]. In this manuscript, experiment day (ED) is directly comparable to gestation day because mice are infected the day on which a vaginal plug is observed. On ED 0 mice were infected intravenously with 1000 infected red blood cells per 20 g of body weight. The development of parasitemia was monitored by counting at least 1000 red blood cells in four high power fields on Giemsa-stained tail blood thin smears (data are shown elsewhere [25]). Sham-injected uninfected pregnant mice served as negative controls. All procedures described herein were performed in accordance with the Institutional Animal Care and Use Committee (IACUC) approval at the University of Georgia, Athens, GA.
Mouse tissue sample collection
Mice were serially sacrificed at EDs 9, 10, and 11. At sacrifice, the uterus was removed by cutting directly below the oviduct and above the cervix and separating from the mesometrium. In some animals, conceptuses were dissected from the uterine tissue and processed for flow cytometric evaluation of cells. Alternatively, part of the uterus was preserved in RNAlater (Ambion) and stored at −80°C until use, with the remainder being fixed in 4% paraformaldehyde and subsequently embedded in paraffin.
RNA extraction, cDNA synthesis and real time PCR
Primer sets for the following nine genes were designed using Primer Express (Applied Biosystems, Carlsbad CA) and oligonucleotides were synthetized by MWG Operon (Huntsville, AL): Ccl2 (sense, 5’-ATGCAGGTCCCTGTCATGCT-3’; antisense, 5’-GTCGAGCCAACACGTGGAT-3’), Ccl3 (sense, 5’-GCGCCATATGGAGCTGACA-3’ ; antisense, 5’-GATGAATTGGCGTGGAATCTTC-3’), Ccl4 (sense, 5’-TCTGCGTGTCTGCCCTCTCT-3’; antisense, 5’-AGCCCATTGGTGCTGAGAAC-3’ ), Csf-1 (sense, 5’-CATCTCCATTCCCTAAATCAAC-3’ ; antisense 5’- ACTTGCTGATCCTCCTTCC-3’), Mgl2 (sense, 5’-GGATCCCAAAATTCCCAGTT-3’; antisense, 5’-TCCCTCTTCTCCAGTGTGCT-3’), Cd3e (sense, 5’-TCTCGGAAGGTCGAGGACAGT-3’; antisense, 5’-ATCAGCAAGCCCAGAGTGAT-3’), Klrd1 (sense, 5’-TCACTCGGTGGAGACTGATG-3’; antisense, 5’-AGGCAAACACAGCATTCAGA-3’), CD22 (sense, 5’- GAAAATCCACCCGATACGTGC -3’; and antisense, 5’- TTGGAACGGTTTCTCCGAGAC -3’) [31, 32] and 18S Ribosomal RNA (18S) (sense 5’-AACGAACGAGACTCTGGCAT-3’ ; antisense, 5’-CGGACATCTAAGGGCATCACAG-3’). Total RNA was isolated from conceptuses preserved in RNAlater and reverse transcribed as previously described [26]. Real-time PCR analyses were performed using an ABI Prism 7500 (Applied Biosystems). The Brilliant II SYBR Green QPCR Master Mix (Agilent Technology, Inc, Santa Clara, CA) was used for all reactions and each sample was assayed in duplicate for all target and housekeeping genes. Average Ct values were normalized to the average of 18S RNA Ct values and relative expression was calculated for each target gene by the ΔΔCT method [33].
Flow cytometric analysis
Single cell suspensions were obtained via mincing and mechanical dispersion of whole viable ED 9 and 10 conceptuses as previously described [27]. Samples for flow cytometry were collected only at EDs 9 and 10 because malaria-induced abortion is a dynamic yet inevitable process in both A/J and B6 mice. At ED 11 conceptuses from infected pregnant (IP) animals are in various stages of progression to abortion and death, and thus are not a reliable source of the predominantly viable cells that are suitable for this technique. Staining of each sample with Trypan blue following red blood cell lysis with Tris-buffered ammonium chloride (0.14 M NH4Cl and 0.017 M Tris (pH 7.2)) demonstrated that cell viability at EDs 9 and 10 was routinely >90%. Cells were counted, washed, Fc-blocked with CD16/CD32 purchased from eBiosciences (San Diego, CA, USA) as per manufacturer specifications, and stained with the following monoclonal antibodies (eBiosciences) using standard methodologies: fluorescein isothiocyanate (FITC)-conjugated anti- CD4, FITC-conjugated anti-F4/80, phycoerythrin (PE)-conjugated anti-CD3e, PE-conjugated anti-CD115, PerCP-Cy5.5-conjugated anti-B220, allophycocyanin (APC)-conjugated anti-CD8, APC-conjugated anti-CD11b, APC-conjugated anti-NK1.1, and PE-Cy5.5 anti-GR1. Poor recovery of T and B cells disallowed analysis of these subpopulations. Data were acquired using a BD FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA), with a minimum of 10,000 cells being acquired per sample. The resultant data were analyzed with FlowJo 9.0 software (TreeStar, Inc., Ashland, OR, USA).
Immunohistochemistry (IHC)
Unstained, paraffin-embedded 5 μm mouse and human placental tissue sections (for whom the consent process and clinical and parasitological data were previously described [28]) were used for immunohistochemistry (IHC) according to previously reported methods [26] with a Polink-HRP kit for rat or rabbit antibodies (used according to manufacturer instructions; GBI, Inc. Mukilteo, WA, USA). Tissue sections were deparaffinized and rehydrated through xylene and graded alcohol series. Sections were then rinsed in tap water and antigen unmasked for 30 mn using 10 mM citrate buffer, pH 6 and a Pascal pressurized heating chamber (Dako; Code S2800; Carpinteria CA, USA). Detection of macrophages in mouse placental tissue sections was achieved with monoclonal rat anti-F4/80 (6A404; #SC-71087, Santa Cruz Biotechnology, Dallas, TX, USA) at 2.5 μg/mL for 1 h in a moist chamber. Total and cleaved caspase 3 were detected in mouse and human placenta by overnight staining with antibody (rabbit caspase 3 (CPP-32: #9662) and rabbit cleaved caspase 3 (ASP175; 5A1E: # 9664) from Cell Signaling (respectively used at 1/2000 and 1/800). Sections were counterstained with hematoxylin, dehydrated and mounted with Flo-Texx (Lerner Laboratories, Pittsburgh, PA, USA). For immunostaing of CCL2, we used a mouse monoclonal antibody (clone 2D8) (Millipore, Cat#MABN712; Bellerica, MA) and Vector M.O.M. Immunodection Kit according to manufacturer's instructions. Briefly, deparaffinized, hydrated, and antigen unmasked mouse placental tissue sections are incubated with endogenous enzyme activity blocker for 15 mn (3% Hydrogen Peroxyde in tap water). Tissue sections are avidin/biotin blocked and incubated for 1 hour in working solution of M.O.M. Mouse Ig Blocking Reagent. Sections are then successively incubated with primary antibody, biotinylated secondary antibody, and VECTASTAIN Elite ABC reagent prepared as recommended. DNA fragmentation was evaluated immunohistochemically using TUNEL (terminal uridine deoxynucleotidyl transferase dUTP nick-end labeling; Apoptag Plus Peroxidase In Situ Apoptosis Detection Kit, S7101; Chimicon International, Bellerica, MA, USA). The procedure was performed according to manufacturer's instructions.
Stained slides were viewed and photographed on a Nikon Eclipse E400 microscope with a Nikon DS-L1-5M imaging camera at X200 final magnification to monitor staining and assess results. Scoring for cleaved caspase 3 staining in murine tissues was done by examining all embryos on the slide and assigning a score on a zero to four scale. High resolution images for data presentation were taken with an Olympus BX41 microscope coupled with an Olympus Digital camera DP 25, saved as TIFF files, and cropped and resized using Adobe Photoshop CS6.
Measurement of systemic TNF by ELISA
Plasma levels of TNF and MCP1 (CCL2) were determined with sandwich assays as previously described [25] using commercially available ELISA kits from R&D Systems, Minneapolis, MN, according to manufacturer instructions.
Protein extraction and western blotting
Mouse samples were selected based on presence or absence of indicators of abortion (intrauterine hemorrhage, resorptions, and bloody, mucoid vaginal discharge with or without active expulsion). Regardless of abortion, three intact RNAlater-preserved conceptuses from each mouse were used. Proteins from these samples were isolated as described previously [28], separated by SDS-PAGE, blotted onto nitrocellulose membranes (GE Water and Process Technology, Trevose, PA, USA) and probed with monoclonal or polyclonal rabbit antibodies specific for PARP (poly (ADP-ribose) polymerase), BCL2, BAX and p17 and p19 cleavage products of caspase 3 (Cell Signaling, Danvers, MA, USA) with GAPDH (Cell Signaling Technologies) as a loading control. Overnight incubation with primary antibody at 4° C was followed by one hour incubation with anti-rabbit horseradish peroxydase secondary conjugates (Cell Signaling Technologies). Proteins were detected using an enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA) with blue autoradiography film (Genesee Scientific, San Diego, CA, USA) developed in a Konica SRX 101A developer (Konica Minolta Medical Imaging USA, Inc., Wayne, NJ).
Statistical analysis
All statistical analyses were performed using GraphPad Prism software package (version 5.01). The D'Agostino-Pearson normality test was used to verify normal distribution of data. In the case of normally distributed data, comparisons between two groups were made via t-tests. One-way ANOVA with Post-hoc Multiple Comparison Test was used for multiple group comparisons. Non-parametric, Mann-Whitney test for pairwise comparisons and Kruskal-Wallis test with Dunn's Multiple Comparison were used when data were not normally distributed for pairwise and multiple group comparisons, respectively. Differences with P<0.05 were considered significant.
RESULTS
Leukocyte-associated gene marker and flow cytometry analysis reveal monocyte accumulation exclusively in A/J mice
Because the most relevant clinical, parasitological, and pathological findings for A/J and B6 mice infected with P. chabaudi AS occur on EDs 9, 10, and 11 [25-28], these time points were the focus for this study.
The presence of T, B, and natural killer cells and activated macrophages in conceptuses of infected pregnant (IP) mice was identified by the expression of cell-specific gene markers Cd3e, Cd22, Klrd1, and Mgl2, respectively, relative to uninfected pregnant (UP) mice. These cells accumulate in conceptuses from P. chabaudi AS-infected A/J and B6 mice to varying degrees (Fig. 1).
Figure 1.
T cell (A), B cell (B), natural killer cell (C), and macrophage (D) gene markers are variously upregulated in infected A/J (open bars) and B6 (filled bars) conceptuses relative to uninfected conceptuses at experiment days (ED) 9, 10, and 11. Expression is relative to the mean expression value in uninfected pregnant (UP) conceptuses and normalized to mouse 18S ribosomal RNA values. Columns and bars represent mean ± SEM of the relative quantities (fold changes) for each gene from at least three conceptuses each from five mice per group. Comparative statistical tests were not done between A/J versus B6 mice for gene expression but the D'Agostino-Pearson normality test was used to verify normal distribution of the data.
In A/J mice, which begin to exhibit malaria-induced abortion on ED 9 [25] significant (>50-fold) T cell accumulation is observed at ED 10, declining to a 22-fold elevation relative to UP A/J mice on ED 11 (Fig. 1A). A similar robust pattern is observed for NK cells (Fig. 1C). B cells are increased in IP A/J mice, with highest levels at ED 11 (Fig. 1B). Strikingly, the macrophage marker Mgl2 is significantly upregulated by infection in A/J conceptuses (Fig. 1D), with >60-fold enhancement at ED 11.
In B6 mice, which begin to abort on ED 10 [25, 27, 28], increases in lymphocyte transcripts are transient with T cells being higher at ED 9 (Fig. 1A) than at EDs 10 and 11, and B cells conversely upregulated to the highest level at ED 11 (Fig. 1B). NK cells are variably elevated at ED 10 in B6 IP mice (Fig. 1C). Essentially no macrophage infiltration is observed in B6 mice at these time points (Fig. 1D).
Consistent with the gene expression analysis (Fig. 1), the number of monocytes (CD11b+/CD115+/Gr1-) detected by flow cytometric analysis of single cell suspensions of viable conceptuses is significantly higher at ED 10, but not ED 9, in IP A/J mice relative to uninfected mice (Fig. 2A). Moreover, monocytes with an inflammatory phenotype (CD11b+/CD115+/Gr1high) are simultaneously elevated in A/J IP mice (Fig. 2C). Elevations of these cells in some B6 IP conceptuses at ED 10 is evident (Figs. 2B and 2D), but levels overall are variable and do not differ statistically from uninfected mice.
Figure 2.
Number (X103) of CD11b+/CD115+/Gr1− monocytes (A, B) and CD11b+/CD115+/Gr1high inflammatory monocytes (C, D) per conceptus in A/J (open circles) and B6 (closed circles) mice at ED 9 (A, C) and ED 10 ( B, D) as indicated. Mice included in this experiment are uninfected pregnant (UP), and infected pregnant but not actively aborting (IP). Line at median is also indicated for each group and a Mann Whitney U test is used to compare total cell numbers between UP and IP for each strain. *P<0.05.
Midgestational placental macrophage infiltration is unique to placentae of infected A/J mice
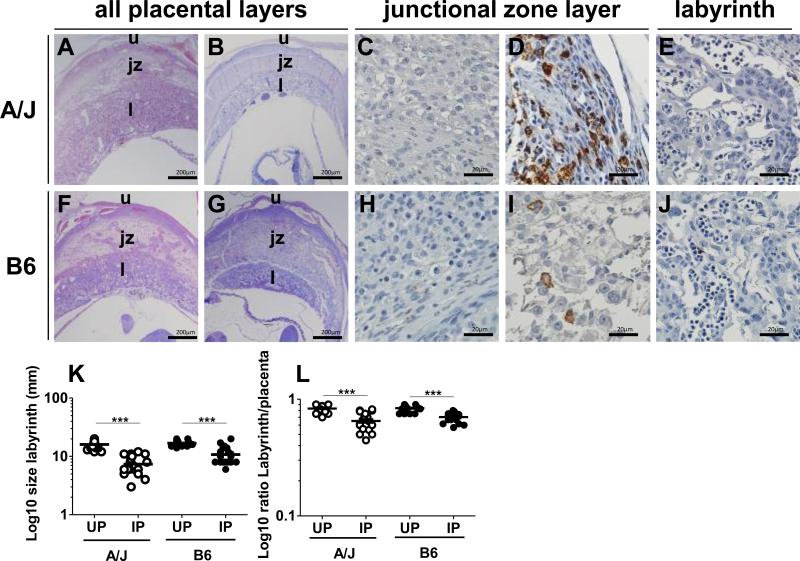
Significant thinning of the placental labyrinth is observed in P. chabaudi AS-infected B6 mice [26, 28]. IP A/J mice (Fig. 3B), like IP B6 mice (Fig. 3G), show this same pathological change relative to the UP counterparts (Figs. 3A, 3F, respectively and Fig. 3K, L). To confirm and further characterize observations of elevated Mgl2 transcripts and monocytes in A/J but not B6 conceptuses, placental macrophages were detected by immunohistochemistry. Staining for the macrophage marker F4/80 is rare in UP A/J mice (Fig. 3C). However, extensive infiltration of F4/80+ cells in IP A/J placentae, mainly within the junctional zone (Fig. 3D) rather than the labyrinth (Fig. 3E).
Figure 3.
Placental pathology and differential monocyte/macrophage accumulation in the placentae of infected pregnant (IP) A/J and B6 mice at ED 10. Panels A-E represent A/J mice and panels F-J represent B6 mice. Panels A, B and F, G show representative hematoxylin and eosin-stained full thickness placental tissue sections from uninfected (UP) (A, F) and infected pregnant (IP) (B, G) A/J and B6 mice at ED 10, with reduction in thickness evident in infected placenta (bar is 200 μm in these panels). Panels C-E (A/J mice) and H-J (B6 mice) show F4/80 staining by immunohistochemistry at ED 10. Extensive staining is seen in the junctional zone of IP A/J mice (D) relative to UP A/J mice (C). The labyrinth of IP A/J mice is free of F4/80+ staining (E). IP B6 mice also show infrequent F4/80 positive staining in the junctional zone (I) but there is no positive staining in UP B6 mice (H) and the labyrinth of the IP mouse placenta has minimal staining (J). Placental tissue sections from both strains were devoid of immunoreactivity with rat IgG2b control Ab (see inset, Supplemental Figure 5)). Panel K summarizes reduction in thickness of the labyrinth in IP relative to UP mice from both strains at ED 10, measured by grid under 100x magnification (median line is shown for each group). Panel L depicts the ratio of the size of the labyrinth to the total size of the placenta in the same animals shown in (K). Panels (C, D, E, H, I, and J) are representative of 3 mice per group; bars indicate 20 μm. u = uterine wall; j = junctional zone; l = labyrinth. A one-way ANOVA was used to compare infected vs uninfected, and infected vs infected in each and between strain respectively ***P<0.001.
F4/80 immunoreactivity is not evident in UP B6 mice (Fig. 3H). Staining is infrequent in IP B6 junctional zone (Fig. 3I), and is not observed in the labyrinthine layers (Fig. 3J).
Chemokine-associated genes are consistently elevated in malaria-exposed A/J conceptuses
To identify potential mechanisms by which IP A/J, but not IP B6, mice accumulate monocyte/macrophages in their placentae in response to malaria infection, transcripts of Ccl2, Ccl3, Ccl4, and Csf1 were assessed by quantitative PCR in conceptuses at EDs 9, 10, and 11. Expression of Ccl2, a chemokine that displays chemotactic activity for monocytes and basophils, increases over time in A/J conceptuses, reaching an 8-fold increase on ED 11 (Fig. 4A). Ccl3, a chemokine involved in the recruitment and activation of polymorphonuclear leukocytes, is also upregulated on EDs 9 and 11 in A/J mice (Fig. 4B). Ccl4, which is a chemoattractant for natural killer cells, monocytes, and a variety of other immune cells, is slightly increased at ED 9 and 10, with a dramatic 70-fold increase at ED 11 in A/J conceptuses (Fig. 4C). Csf1, a chemokine involved in the production, differentiation, and function of macrophages, shows significant enhancement in A/J conceptuses, with more than a 20-fold change on EDs 10 and 11 (Fig. 4D).
Figure 4.
Gene expression of Ccl2 (A), Ccl3 (B), Ccl4 (C), and Csf1 (D) in infected pregnant A/J (open bars) and infected pregnant B6 (black bars) conceptuses. Expression is relative to the mean expression value in uninfected pregnant conceptuses for each strain and normalized to mouse 18S RNA values obtained within each group. Columns and bars represent mean ± SEM of the relative quantities (fold changes) for each gene from at least three conceptuses each from six mice per group. Comparative statistical tests were not done between A/J versus B6 mice for gene expression but the D'Agostino-Pearson normality test was used to verify normal distribution of the data.
With the exception of Ccl3, which exhibits a transient 20-fold increase at ED 9 (Fig. 4B), chemokine upregulation in IP B6 conceptuses is minimal (Figs. 4A-D). Ccl2 expression increases over time, peaking at a 3-fold increase on ED 11 (Fig. 4A). Ccl4 transcripts are upregulated only at ED 9 in B6 conceptuses (Fig. 4C), and Csf1 is transiently and subtly upregulated on ED 10 (Fig. 4D).
In neither strain does abortion appear to influence expression of any of these genes, as within strain levels are similar between aborting and non-aborting animals (data not shown).
Plasma CCL2 is elevated with infection in A/J and B6 mice, and is expressed by trophoblast in the context of P. chabaudi AS infection
Because the pattern of Ccl2 expression (Fig. 4A) tends to parallel the timing and magnitude of monocyte accumulation in A/J and B6 IP mice (Figs. 2 and 3), production and localization of CCL2 was assessed in the mice. In both strains, CCL2 plasma levels are elevated from ED 9 to ED 11 in IP relative to UP mice (Fig. 5A). Immunohistochemical staining for CCL2 expression in placenta reveals diffuse junctional zone trophoblast staining in UP A/J mice (Fig. 5B, F, J). Immunoreactivity is significantly enhanced and is most intense at ED 9 in IP A/J mice (Fig. 5C), remaining prominent in junctional zone trophoblasts at ED 10 and 11 (Fig. 5G, K, respectively).
Figure 5.
Systemic CCL2 plasma levels in uninfected (UP) and infected pregnant (IP) A/J and B6 mice from ED 9 through ED 11 by ELISA (A). A single plasma CCL2 sample below the detection limit was assigned to 1 for the purpose of graphing and statistical analysis. Unpaired t test with Welch's correction was used for comparison between IP and UP for each strain. Immunostaining for CCL2 in placental tissue sections in A/J (B,C, F,G, J, and K) and B6 (D, E, H, I, L, and M) mice on EDs 9, 10, and 11. Sample size was 5 mice for each group at each time point.
UP B6 mice exhibit diffuse CCL2 immunoreactivity in junctional zone trophoblast (Fig. 5D, H, L). This expression is elevated with infection in B6 mice at ED 9 (Fig. 5E), increases in intensity at ED 10 (Fig. 5I) and declines at ED 11 (Fig. 5M).
Apoptosis is enhanced at the initiation of abortion in A/J mice, with a delay in aborting B6 mice
For both strains, IP mice with clear evidence of active abortion and those without were chosen for analysis of known markers of apoptosis by western blot. Because abortion begins on ED 9 for A/J mice [25], this time point was selected for analysis; correspondingly, ED 10 was chosen for B6 mice [26-28]. Low levels of cleaved caspase 3 (cCASP3) are evident in UP A/J conceptuses at ED 9 (Fig. 6A); cleaved PARP (cPARP) is undetectable (Fig. 6A). In contrast, cCASP3 and cPARP are significantly enhanced in aborting IP (IP-A) A/J mice (Fig. 6A). This pattern of cCASP3 and cPARP expression is also observed in IP-A A/J mice on EDs 10 and 11 (Supplemental Figure 1). Intermediate levels of these cleavage products are seen in conceptuses from non-aborting IP (IP-NA) A/J mice (Fig. 6A).
Figure 6.
Protein lysates from conceptuses of infected pregnant (IP) A/J at ED 9 (A) and B6 (B) mice at ED 10, undergoing active abortion (IP-A) or not (IP-NA), and uninfected (UP) were assessed by western blot for mouse cleaved PARP (cPARP) (89 kDa), and mouse cleaved Caspase 3 (cCASP3; 19 kDa, and 17 kDa), with GAPDH (37 kDa) as loading control. The immunoblots shown are representative of at least 3 individual experiments with 6 UP A/J, 11 IP A/J, 6 UP B6, and 10 IP B6 mice. Densitometry analysis for A and B is provided in Supplemental figure 3.
In B6 mice, cCASP3 and cPARP at ED 10 are evident in UP and IP conceptuses, the latter irrespective of abortion status (Fig. 6B). Thus, IP-A B6 conceptuses, relative to those derived from UP and IP-NA mice, show no evidence of enhanced apoptosis. Western blot testing for apoptotic markers at other time points (ED 9, n=2 UP, n=2 IP-NA, and ED 11, n=2 for each group) in B6 conceptuses similarly revealed absence of an enhanced pro-apoptotic response with malaria (Supplemental Figure 1).
To further assess apoptosis at the molecular level, BAX, a key promoter of apoptosis through mitochondrial stress [34], and pro-survival, anti-apoptotic BCL2, which inhibits mitochondrial cytochrome c release [35, 36], were also analysed by western blot. Overall levels of these factors are not influenced by infection in conceptuses of either strain (Supplemental Figure 1).
Spongiotrophoblast and inflammatory cells undergo apoptosis with malaria infection
To use an alternative approach for detection of apoptosis and simultaneous direct identification of placental compartments and cells undergoing apoptosis in P. chabaudi AS-infected mice, neutral formalin-fixed fixed placental tissue with associated uterine cells were immunochemically stained for CASP3 (total caspase 3) and cCASP3 (cleaved caspase 3). Prominent total (cleaved and uncleaved) CASP3 cytoplasmic staining is observed in the decidua and junctional zone of the mouse placenta irrespective to mouse strain and infection status (Figs. 7A-D). Interestingly, no CASP3 (Figs. 7A-D) or cCASP3 staining (Supplemental Figure 2) is evident in the labyrinth of infected (Fig. 7B, D) or uninfected (Fig. 7A, C) mice. Whereas little to no cCASP3 staining is present in UP A/J mice (Fig. 7E), strong cytoplasmic staining in maternal blood inflammatory cells (arrows) and junctional zone trophoblasts (arrowheads) is observed in IP A/J aborting mice at ED 10 (Fig. 7F), with evidence of substantial staining one day earlier as well (Fig. 7I). Overall, 75% of tested IP A/J mice show intense (score = 4) cCASP3 junctional zone staining (at ED 9, three of four mice; at ED 10, four of six; at ED 11, two of two). To further confirm these observations, TUNEL staining (which detects apoptosis-associated DNA fragmentation) was performed. Placentae from aborting A/J mice at ED 10 have clear evidence of TUNEL-positive apoptotic bodies in junctional zone trophoblast (arrowheads) and maternal blood vessels (arrows; Fig. 7J).
Figure 7.
Immunostaining for markers of apoptosis in placental tissue sections from IP (infected pregnant) and UP (uninfected pregnant) A/J and B6 mice. All panels are ED 10 unless otherwise labeled. Panels A-D depict the full thickness of the placental disk; panels E to L show the junctional zone. Total mouse caspase 3 (CASP3) immunostaining is depicted in panels (A-D) for UP and IP A/J (A, B, respectively) and B6 (C, D, respectively) mice. Cleaved caspase 3 (cCASP3) staining is depicted for UP ED 10 and IP A/J (E, F, respectively) and B6 (G, H, respectively) mice. Mouse cleaved caspase 3 (cCASP3) is also depicted for infected A/J at ED 9 (I) and B6 mice at ED 11 (K). Panels J and L show TUNEL staining in spongiotropholast for IP A/J and IP B6 mice at ED 10. Panel M summarizes cCASP3 immunostaining for ED 10 in both mouse strains. Images for ED 10 specimens are representative of 4 UP A/J, 7 IP A/J, 4 UP B6, and 8 IP B6 mice. Images for ED 11 IP B6 are representative of 2 out of 4 mice, and images for ED 9 IP A/J are representative of 3 out of 4 mice; other mice in these groups had relatively lower levels of immunostaining. Arrows: maternal inflammatory cells; arrowheads: trophoblast. u = uterus; jz = junctional zone; l = labyrinth; * = maternal blood sinusoid. Bars represent 200 μm in panels A-D and 20 μm in panels E-L. A Kruskal-Wallis test with Dunn's post test was used to compare different groups. P values are indicated as *P<0.05, **P<0.01 and ns for not significant.
Consistent with observations by western blot (Fig. 6), junctional zone trophoblast staining for cCASP3 in B6 placentae is similar between UP (Fig. 7G) and IP-A mice (Fig. 7H) at ED 10, in both cases being rare. At EDs 9 and 10, 63% of IP B6 mice (10/16) have no evidence of cCASP3 placental staining. In the remainder, the staining is minimal (score = 1), with a single sample having moderate staining (score = 2; Fig. 7H). However, analysis of ED 11 placental tissue sections from IP-A B6 mice shows strong staining (score = 3) in two of four animals tested (one representative is shown in Fig. 7K). Low frequency TUNEL-positive cells are seen in ED 10 IP B6 placenta (Fig. 7L). No TUNEL staining is observed in the placental labyrinth of either strain, with or without malaria infection, similar to the pattern for cCASP3 (Supplemental Figure 2). Quantification of junctional zone cCASP3-positive cells shows significant elevation in IP A/J placentae relative to UP mice, with no difference among B6 mice (Fig. 7M).
Plasma TNF levels in P. chabaudi AS-IP A/J mice are associated with abortion
IP A/J mice undergo abortion beginning on ED 9, with all embryos being nonviable by ED 11 [25]. To identify a potential association with fetal loss, plasma TNF levels were measured in IP mice aborting (IP-A) and non-aborting (IP-NA) as well as in UP mice on EDs 9, 10, and 11. TNF levels are elevated in IP A/J mice relative to the UP counterparts at each time point, with IP-A mice tending to have the highest levels (Figs. 8A, 8C, and 8E). In B6 mice, plasma levels of TNF are similarly elevated with infection, but do not differ between mice undergoing abortion compared to nonaborting mice (Figs. 8B, 8D, and 8F).
Figure 8.
Tumor necrosis factor (TNF) plasma levels in uninfected, infected non-aborting and infected, aborting mice. Plasma samples were assayed by ELISA. Data for ED 10 and ED 11 were previously presented as UP and IP [25], without segregation on the basis of abortion status. *P<0.05, **P<0.01, ***P<0.001. A Kruskal-Wallis test with Dunn's post test was used to compare systemic TNF levels between UP, IP-NA, and IP-A for each strain at each time point as indicated.
Apoptosis is confined to infiltrating maternal leucocytes in human PM at term
Intrigued by the apparent strain-dependence and strict junctional zone distribution of trophoblastic apoptosis in P. chabaudi AS-infected mice at mid-gestation, we assessed sections of term villous placenta from Kenyan women naturally exposed to P. falciparum for evidence of apoptosis . Despite intense interest in identification of molecular mechanisms underlying placental damage induced by malaria, molecular assessment of apoptosis in this context has not been studied. By immunohistochemical staining, full length (total) caspase 3 is evident in intervillous leukocytes and some villous stromal cells, with low level immunoreactivity seen in syncytiotrophoblast in malaria-uninfected (Fig. 9A) and malaria-infected human placenta (Fig. 9D). In uninfected placenta, cleaved human caspase 3 (cCASP3) staining appears in intervillous leukocytes; intense positive staining is restricted to intervillous maternal leukocytes in uninfected (Fig. 9B) and infected (Fig. 9E) placenta, with occasional positive staining in the villous stroma in the latter. TUNEL staining in these human placental tissue sections reveals the same pattern as for cCASP3 (Figs. 9C and 9F), suggesting that remarkable apoptosis is confined to leukocytes infiltrating the intervillous space during human PM at term (Suppemental Figure 4).
Figure 9.
Immunostaining for human total full length and cleaved caspase3 (CASP3 and cCASP3 respectively) and TUNEL staining of uninfected (A-C) and Plasmodium falciparum-infected (D-G) placental tissue sections from Kenyan women. CASP3 (A, D), cCASP3 (B, E), and TUNEL stained placental sections (C, F) are depicted. Images are representative of 6 uninfected and 11 infected tissue samples for human cCASP3, and 5 and 6, respectively, for TUNEL. Original magnification is 400x; bars indicate 20 μm. Panel G provides a summary for semi-quantitative scoring of human cCASP3 and TUNEL staining in uninfected (PM−) and placental malaria-infected (PM+) samples.
DISCUSSION
In general, very little is known about malaria pathogenesis in human pregnancy during the first and second trimesters, when susceptibility is greatest [2]. Emerging clues, however, indicate the impact of malaria is profound at this point in gestation [2, 29]. Advances in understanding of malarial pathogenesis in humans during the early, highly vulnerable phases of pregnancy are few because the affected placenta is unavailable for study. P. chabaudi AS infection in murine pregnancy, which can be easily employed to investigate infection throughout the course of pregnancy [23, 25-27], is therefore highly valuable in identifying the molecular mechanisms underlying the pathogenesis of PM early in gestation.
Nonpregnant A/J and B6 mice have been used extensively to explore immunoprotective and immunopathogenic responses to P. chabaudi AS infection [37-39]. While several studies have used P. berghei to investigate the relationship between malaria and pregnancy [17, 18, 20-22, 24, 40-42], the virulence of this parasite interferes with its utility to probe the impact of malaria infection in early pregnancy. Initiation of infection with the relatively low virulence subspecies P. berghei NK65 during early gestation results in maternal mortality in 7 to 8 days, making study of embryo outcome difficult [30].
Indeed, most current work with P. berghei uses the highly virulent subspecies ANKA, with infections initiated on gestation day 13 [18, 42]. Therefore, studies of malaria pathogenesis during early pregnancy must rely on a model that allows pregnancy to progress and avoids maternal mortality at early time points. To date, P. chabaudi AS infection in B6 and A/J mice has been the best characterized system for this purpose.
Both B6 [27, 28] and A/J mice [25] experience poor pregnancy outcomes as a result of P. chabaudi AS infection. As reflected by a higher rate of embryo resorption and abortion at ED 9, A/J mice experience accelerated parasite growth and pregnancy loss relative to B6 mice [25], which show more appreciable abortion beginning at ED 10 [27]. Midgestational pregnancy loss in both strains is associated with increased systemic and placental TNF expression together with high levels of soluble TNF receptors [23, 25, 26], with levels at midgestation, particularly EDs 9 and 10, being significantly higher in A/J mice (Fig. 8) [25]. While antibody neutralization of TNF rescues mid-gestational pregnancy in infected B6 mice, confirming a central role for this cytokine in malarial pathogenesis in pregnancy [26], the same treatment regimen is unable to overcome the high expression levels in A/J mice, and has no amelioratory impact [25]. A clear role for TNF in malaria pathogenesis in pregnancy is further borne out in other models: exogenous administration of TNF induces abortion in P. vinckei-infected mice [43], and systemic TNF is associated with fetal death in P. coatneyi-infected monkeys [44]. Moreover, high placental levels of TNF are associated with poor birth outcomes in human malaria [45, 46]. Nonetheless, similar links of poor birth outcomes to infiltration of maternal monocytes [13], a cell type known to secrete TNF [47], have contributed to the sense that these cells are critical to PM pathogenesis [16]. Considered all together, available evidence clearly indicates that TNF is pivotal to malaria-induced pregnancy compromise, yet the mechanism(s) by which it acts, and the important cellular players, remain unclear. In this context, it is possible that TNF, derived from either fetal trophoblast [23, 25, 26] or accumulating maternal inflammatory cells, induces apoptosis via interaction with its membrane receptors on target cells in the placenta. To explore this possibility, the present study investigated the relationships between pregnancy loss, inflammation, and apoptosis in P. chabaudi AS infection during pregnancy in A/J and B6 mice.
Accumulation of maternal monocyte/macrophages in the intervillous space is key feature of human PM at term [16] and is also observed in P. berghei ANKA infection in late murine pregnancy [18]. The extent to which cellular inflammatory responses occurs at mid-gestation in malaria-infected mice has to our knowledge heretofore not been addressed. Through application of several measurement methods (gene expression, flow cytometry and immunohistochemistry), the present study shows that a placental monocyte/macrophage response is observed in P. chabaudi AS-infected A/J, but not B6, mice, beginning at ED 10. The distribution of these cells in A/J placentae is consistent with a maternal origin of the cells, with accumulation in the junctional zone, the region closest to the uterine wall. An inflammatory infiltrate is not evident in the labyrinth, which is the anatomic and physiological counterpart of the intervillous space in the human placenta. This discrepancy may be due to differences in timing of infection and subsequent evaluation of the placenta: early to mid-gestation in this study versus late pregnancy in humans and the P. berghei model. No study has yet reported on malaria-induced placental histopathology in early human pregnancy; such work faces significant logistical and ethical challenges.
Why A/J but not B6 mice exhibit inflammatory cell infiltrate in the placenta is not clear, but is likely due to the disparate genetic backgrounds of these two strains [48], and clear differences in their immunologic responses to malaria in pregnancy [25]. As shown here, conceptus chemokine gene expression is clearly different between the two mouse strains, with A/J mice overall tending to have higher and/or more sustained expression of potentially pivotal chemoattractants (Ccl2 and Ccl4) for monocytes, cell types found to accumulate in the A/J conceptus. The potential sources for these chemokines are many; Ccl2 is secreted by monocytes and epithelia, including first trimester [49] and term human syncytiotrophoblast, albeit strictly in the context of cytomegalovirus infection in the latter [50]. Although CCL2 is not expressed by primary human syncytiotrophoblast exposed to cytoadherent P. falciparum [6], and is restricted to maternal macrophages and intravillous stromal cells in the P. falciparum-infected term human placenta [4], it is apparently produced by mid-gestation murine trophoblast exposed to P. chabaudi AS, irrespective to mouse strain. Although trophoblast CCL2 expression detected by immunohistochemistry is highest on days associated with initiation of malaria-induced abortion (ED 9 for A/J and ED 10 for B6), and plasma CCL2 is elevated with infection in both strains, absence of robust inflammatory cell infiltration in B6 placentae suggests that other chemoattractants may contribute to this response in A/J mice. Nonetheless, similar to human trophoblasts, which when stimulated with P. falciparum-iRBCs [6] or malarial hemozoin [51] chemoattract mononuclear cells, murine trophoblasts of at least some strains may respond to accumulation of P. chabaudi AS by secreting chemokines. This response may thereby precipitate a damaging maternal immune response at the placental level, a response further propagated by accumulating inflammatory cells.
Despite clear evidence of placental inflammatory infiltrate in A/J mice, this response is consistently observed one day following initiation of pregnancy loss in this strain, which is typically ED 9 [25]. Given that placental inflammatory infiltrate is largely absent in B6 mice, it is likely that P. chabaudi AS-induced placental inflammatory cell infiltrate is not a central initiator of mid-gestational abortion in this model. On the other hand, TNF plays a pivotal role in pregnancy loss in P. chabaudi AS-infected B6 mice [26] even with systemic expression that is low relative to A/J mice (Fig. 8) [25]. It is noteworthy that TNF levels are highest in IP A/J mice at ED 9 (Fig. 8), coincident with profound placental damage and embryo loss.
TNF is a pleiotropic cytokine that regulates both cell death and survival [52, 53]. In the context of pregnancy, this cytokine can be directly embryotoxic [54], inducing trophoblast apoptosis via TNF receptors [55]. While apoptosis and the factors involved in its regulation are active in almost all stages of placental development to facilitate normal tissue architecture and function [56], trophoblastic apoptosis is implicated in pathological conditions such as preeclampsia and intrauterine growth restriction [57, 58]. It was recently reported that P. berghei infection initiated at midgestation in BALB/c mice promotes placental apoptosis through the mitochondrial pathway [21]. However, in the absence of in situ assessment of markers of apoptosis, the identity of the cells undergoing apoptosis in the placentas of these mice was not confirmed. We have previously shown that IP A/J mice make significantly higher levels of systemic TNF than IP B6 mice [25]. Given the known embryotoxic effects of TNF, one might expect that IP A/J mice, especially those undergoing active abortion, will be more susceptible to placental apoptosis than IP B6 mice. Consistent with this expectation, in the present work IP A/J mice have clear evidence for significantly elevated levels of cCASP3, cleaved PARP, and DNA fragmentation as early as ED 9. Immunohistochemistry localizes the apoptotic cells to the placental junctional zone (spongiotrophoblast and trophoblastic glycogen cells), a pattern also observed in a mouse model of preterm labor [59]. Interestingly, this study reveals no evidence for trophoblast apoptosis as a prominent and consistent feature of malaria-induced abortion in B6 mice, with only a subset of animals showing some evidence of cCASP3 and TUNEL-positive cells in the placenta at ED 11, one day after malaria infection typically induces abortion. Although definitive evidence is pending, it is probable that an important TNF-induced, embryo-compromising event in B6 mice is tissue factor-mediated coagulopathy [26, 28]. Similar to B6 mice, there is no evidence that apoptosis is a key mechanism underlying syncytiotrophoblast damage in human PM at term (Fig. 9). Despite the obvious limitation in comparing the mid-gestational mouse model with malaria-exposed term human placenta, it is clear from the present study and work by others [60] that apoptosis may not be a universal initiator of placental damage and dysfunction in human or mouse malaria. In human placenta, syncytial knots have been proposed to be representative of apoptotic nuclei in the syncytiotrophoblast [61]. This is controversial, however, and, according to recent data [62, 63] only mononuclear cytophoblast, and not syncytiotrophoblast, undergo apoptosis[61, 64]. Indeed, assessment of condensed, apoptotic nuclei in syncytiotrophoblast and syncytial knots in malaria-exposed placenta shows no compelling statistical association between these features and PM [60].
In summary, P. chabaudi AS infection during early pregnancy in B6 and A/J mice results in pregnancy loss in both mouse strains [25, 26] with remarkably different cellular and placental histopathological patterns. Although the pathways galvanized remain to be fully elucidated in these genetically disparate mouse strains, apoptosis appears to be an important indicator of placental damage and embryo death in association with early pregnancy P. chabaudi AS infection only in A/J mice. In this context and in the absence of an adequate comparator (mid-gestational human pregnancy loss due to malaria infection), current evidence suggests that placental pathology in IP B6 mice at mid-gestation, with a dominant role for coagulopathy, more closely parallels that in the term malaria-exposed human placenta [28], which also lacks evidence for syncytiotrophoblast apoptosis. On the other hand, the cellular inflammatory response observed in A/J mice shares important features with the response observed in pathogenic human PM [16]. Importantly, a shared pathogenic feature in both mouse strains, as well as human PM [28] is elevated TNF levels [23, 25, 26]; continued work in these models is needed to definiteively identify the molecular mechainsms of TNF-associated placental damage and embryo loss in humans and mice. Ultimately, full characterization of the P. chabaudi AS model, including with initation of infection after implantation (work currently under way in our laboratory), will be required to establish the extent to which events observed in late pregnancy recapitulate human PM pathology, reflect pathology induced by infection early in pregnancy, and therefore, by extrapolation, predict the most critical pathogenic events that compromise human pregnancy complicated by malaria in the first and second trimesters.
Supplementary Material
Acknowledgments
We thank Dr. David Peterson, Department of Infectious Diseases at UGA for assistance in gene expression analysis and Julie Nelson at the Flow Cytometry Facility of the Center for Tropical and Emerging Global Diseases for flow cytometry services and technical assistance.
This work was supported by the National Institutes of Health grants R01 HD046860 and R01 AI050240 to J.M.M. The content is solely the responsibility of the authors and does not necessarily represent official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Allergy and Infectious Diseases, or the National Institutes of Health.
Abbreviations
- PM
placental malaria
- iRBC
infected red blood cell
- ED
Experiment day
- IP
Infected pregnant
- TUNEL
terminal uridine deoxynucleotidyl transferase dUTP nick-end labeling
- UP
Uninfected pregnant
- cPARP
cleaved poly (ADP-ribose) polymerase
- CASP3
total caspase 3
- cCASP3
cleaved caspase 3
- IP-A
Infected pregnant aborting
- IP-NA
Infected pregnant non-aborting
Footnotes
Author Contributions:
Conceived and designed the experiments: DS, JM. Performed the experiments: DS, TB, SO, GS and CC. Analyzed the data: DS, JM, and TN. Contributed reagents/materials/analysis tools: TN. Wrote the paper: DS and JM. DS, TB, SO, GS, CC, TN, and JM approved the paper.
Disclosures
The authors have no financial conflicts of interest and the manuscript has not been published alsewhere.
REFERENCES
- 1.Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med. 2010;7(1):e1000221. doi: 10.1371/journal.pmed.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai M, Ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7(2):93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 3.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272(5267):1502–4. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 4.Abrams ET, Brown H, Chensue SW, Turner GD, Tadesse E, Lema VM, Molyneux ME, Rochford R, Meshnick SR, Rogerson SJ. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated beta chemokine expression. Journal of immunology. 2003;170(5):2759–64. doi: 10.4049/jimmunol.170.5.2759. [DOI] [PubMed] [Google Scholar]
- 5.Lucchi NW, Koopman R, Peterson DS, Moore JM. Plasmodium falciparum-infected red blood cells selected for binding to cultured syncytiotrophoblast bind to chondroitin sulfate A and induce tyrosine phosphorylation in the syncytiotrophoblast. Placenta. 2006;27(4-5):384–94. doi: 10.1016/j.placenta.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Lucchi NW, Peterson DS, Moore JM. Immunologic activation of human syncytiotrophoblast by Plasmodium falciparum. Malaria journal. 2008;7:42. doi: 10.1186/1475-2875-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suguitan AL, Jr., Leke RG, Fouda G, Zhou A, Thuita L, Metenou S, Fogako J, Megnekou R, Taylor DW. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J Infect Dis. 2003;188(7):1074–82. doi: 10.1086/378500. [DOI] [PubMed] [Google Scholar]
- 8.Chaisavaneeyakorn S, Lucchi N, Abramowsky C, Othoro C, Chaiyaroj SC, Shi YP, Nahlen BL, Peterson DS, Moore JM, Udhayakumar V. Immunohistological characterization of macrophage migration inhibitory factor expression in Plasmodium falciparum-infected placentas. Infection and immunity. 2005;73(6):3287–93. doi: 10.1128/IAI.73.6.3287-3293.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leopardi O, Naughten W, Salvia L, Colecchia M, Matteelli A, Zucchi A, Shein A, Muchi JA, Carosi G, Ghione M. Malaric placentas. A quantitative study and clinico-pathological correlations. Pathol Res Pract. 1996;192(9):892–8. doi: 10.1016/S0344-0338(96)80068-9. discussion 9-900. [DOI] [PubMed] [Google Scholar]
- 10.Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, Kahigwa E, Hirt R, Cardesa A, Alonso PL. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31(1):85–93. doi: 10.1016/s0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 11.Ordi J, Ismail MR, Ventura PJ, Kahigwa E, Hirt R, Cardesa A, Alonso PL, Menendez C. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998;22(8):1006–11. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Ordi J, Menendez C, Ismail MR, Ventura PJ, Palacin A, Kahigwa E, Ferrer B, Cardesa A, Alonso PL. Placental malaria is associated with cell-mediated inflammatory responses with selective absence of natural killer cells. J Infect Dis. 2001;183(7):1100–7. doi: 10.1086/319295. [DOI] [PubMed] [Google Scholar]
- 13.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. The American journal of tropical medicine and hygiene. 2003;68(1):115–9. [PubMed] [Google Scholar]
- 14.Bayoumi NK, Bakhet KH, Mohmmed AA, Eltom AM, Elbashir MI, Mavoungou E, Adam I. Cytokine profiles in peripheral, placental and cord blood in an area of unstable malaria transmission in eastern Sudan. Journal of tropical pediatrics. 2009;55(4):233–7. doi: 10.1093/tropej/fmn062. [DOI] [PubMed] [Google Scholar]
- 15.Boeuf P, Aitken EH, Chandrasiri U, Chua CL, McInerney B, McQuade L, Duffy M, Molyneux M, Brown G, Glazier J, Rogerson SJ. Plasmodium falciparum malaria elicits inflammatory responses that dysregulate placental amino acid transport. PLoS pathogens. 2013;9(2):e1003153. doi: 10.1371/journal.ppat.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brabin BJ, Romagosa C, Abdelgalil S, Menendez C, Verhoeff FH, McGready R, Fletcher KA, Owens S, D'Alessandro U, Nosten F, Fischer PR, Ordi J. The sick placenta-the role of malaria. Placenta. 2004;25(5):359–78. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Hviid L, Marinho CR, Staalsoe T, Penha-Goncalves C. Of mice and women: rodent models of placental malaria. Trends in parasitology. 2010;26(8):412–9. doi: 10.1016/j.pt.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Neres R, Marinho CR, Goncalves LA, Catarino MB, Penha-Goncalves C. Pregnancy outcome and placenta pathology in Plasmodium berghei ANKA infected mice reproduce the pathogenesis of severe malaria in pregnant women. PLoS ONE. 2008;3(2):e1608. doi: 10.1371/journal.pone.0001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barboza R, Reis AS, da Silva LG, Hasenkamp L, Pereira KR, Camara NO, Costa FT, Lima MR, Alvarez JM, Boscardin SB, Epiphanio S, Marinho CR. MyD88 signaling is directly involved in the development of murine placental malaria. Infection and immunity. 2014;82(2):830–8. doi: 10.1128/IAI.01288-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma L, Kaur J, Rishi P, Shukla G. Plasmodium berghei: influence of infection on the oxidant and antioxidants levels in pregnant BALB/c mice. Experimental parasitology. 2012;131(2):215–22. doi: 10.1016/j.exppara.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Sharma L, Kaur J, Shukla G. Role of oxidative stress and apoptosis in the placental pathology of Plasmodium berghei infected mice. PLoS One. 2012;7(3):e32694. doi: 10.1371/journal.pone.0032694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conroy AL, Silver KL, Zhong K, Rennie M, Ward P, Sarma JV, Molyneux ME, Sled J, Fletcher JF, Rogerson S, Kain KC. Complement activation and the resulting placental vascular insufficiency drives fetal growth restriction associated with placental malaria. Cell Host Microbe. 2013;13(2):215–26. doi: 10.1016/j.chom.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Poovassery J, Moore JM. Association of malaria-induced murine pregnancy failure with robust peripheral and placental cytokine responses. Infection and immunity. 2009;77(11):4998–5006. doi: 10.1128/IAI.00617-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penha-Goncalves C, Gozzelino R, De Moraes LV. Iron overload in Plasmodium-infected placenta as a pathogenesis mechanism of fetal death. Front Pharmacol. 2014;5(155) doi: 10.3389/fphar.2014.00155. doi: 10.3389/fphar.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarr D, Smith GM, Poovassery JS, Nagy T, Moore JM. Plasmodium chabaudi AS induces pregnancy loss in association with systemic pro-inflammatory immune responses in A/J and C57BL/6 mice. Parasite Immunol. 2012;34(4):224–35. doi: 10.1111/j.1365-3024.2012.01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poovassery JS, Sarr D, Smith G, Nagy T, Moore JM. Malaria-induced murine pregnancy failure: distinct roles for IFN-gamma and TNF. Journal of immunology. 2009;183(8):5342–9. doi: 10.4049/jimmunol.0901669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poovassery J, Moore JM. Murine malaria infection induces fetal loss associated with accumulation of Plasmodium chabaudi AS-infected erythrocytes in the placenta. Infection and immunity. 2006;74(5):2839–48. doi: 10.1128/IAI.74.5.2839-2848.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avery JW, Smith GM, Owino SO, Sarr D, Nagy T, Mwalimu S, Matthias J, Kelly LF, Poovassery JS, Middii JD, Abramowsky C, Moore JM. Maternal malaria induces a procoagulant and antifibrinolytic state that is embryotoxic but responsive to anticoagulant therapy. PLoS One. 2012;7(2):e31090. doi: 10.1371/journal.pone.0031090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmiegelow C, Minja D, Oesterholt M, Pehrson C, Suhrs HE, Bostrom S, Lemnge M, Magistrado P, Rasch V, Nielsen BB, Lusingu J, Theander TG. Malaria and fetal growth alterations in the 3(rd) trimester of pregnancy: a longitudinal ultrasound study. PLoS One. 2013;8(1):e53794. doi: 10.1371/journal.pone.0053794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hioki A, Hioki Y, Ohtomo H. Influence of pregnancy on the course of malaria in mice infected with Plasmodium berghei. The Journal of protozoology. 1990;37(3):163–7. doi: 10.1111/j.1550-7408.1990.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 31.Epiphanio S, Mikolajczak SA, Goncalves LA, Pamplona A, Portugal S, Albuquerque S, Goldberg M, Rebelo S, Anderson DG, Akinc A, Vornlocher HP, Kappe SH, Soares MP, Mota MM. Heme oxygenase-1 is an anti-inflammatory host factor that promotes murine plasmodium liver infection. Cell Host Microbe. 2008;3(5):331–8. doi: 10.1016/j.chom.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Wen T, Mingler MK, Blanchard C, Wahl B, Pabst O, Rothenberg ME. The pan-B cell marker CD22 is expressed on gastrointestinal eosinophils and negatively regulates tissue eosinophilia. Journal of immunology. 2012;188(3):1075–82. doi: 10.4049/jimmunol.1102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3(6):1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 34.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy KM, Ranganathan V, Farnsworth ML, Kavallaris M, Lock RB. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ. 2000;7(1):102–11. doi: 10.1038/sj.cdd.4400597. [DOI] [PubMed] [Google Scholar]
- 36.Murphy KM, Streips UN, Lock RB. Bcl-2 inhibits a Fas-induced conformational change in the Bax N terminus and Bax mitochondrial translocation. J Biol Chem. 2000;275(23):17225–8. doi: 10.1074/jbc.C900590199. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson MM, Huang DY, Podoba JE, Nowotarski ME. Macrophage activation during Plasmodium chabaudi AS infection in resistant C57BL/6 and susceptible A/J mice. Infection & Immunity. 1992;60(3):1193–201. doi: 10.1128/iai.60.3.1193-1201.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fortin A, Stevenson MM, Gros P. Complex genetic control of susceptibility to malaria in mice. Genes Immun. 2002;3(4):177–86. doi: 10.1038/sj.gene.6363841. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Valladares M, Naessens J, Nagda S, Musoke AJ, Rihet P, Ole-Moiyoi OK, Iraqi FA. Comparison of pathology in susceptible A/J and resistant C57BL/6J mice after infection with different sub-strains of Plasmodium chabaudi. Experimental parasitology. 2004;108(3-4):134–41. doi: 10.1016/j.exppara.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Mota MM, Jarra W, Hirst E, Patnaik PK, Holder AA. Plasmodium chabaudi-infected erythrocytes adhere to CD36 and bind to microvascular endothelial cells in an organ-specific way. Infection and immunity. 2000;68(7):4135–44. doi: 10.1128/iai.68.7.4135-4144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oduola AM, Holbrook TW, Galbraith RM, Bank H, Spicer SS. Effects of malaria (Plasmodium berghei) on the maternal-fetal relationship in mice. The Journal of protozoology. 1982;29(1):77–81. doi: 10.1111/j.1550-7408.1982.tb02883.x. [DOI] [PubMed] [Google Scholar]
- 42.Rodrigues-Duarte L, de Moraes LV, Barboza R, Marinho CR, Franke-Fayard B, Janse CJ, Penha-Goncalves C. Distinct placental malaria pathology caused by different Plasmodium berghei lines that fail to induce cerebral malaria in the C57BL/6 mouse. Malaria journal. 2012;11:231. doi: 10.1186/1475-2875-11-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark IA, Chaudhri G. Tumor necrosis factor in malaria-induced abortion. The American journal of tropical medicine and hygiene. 1988;39(3):246–9. doi: 10.4269/ajtmh.1988.39.246. [DOI] [PubMed] [Google Scholar]
- 44.Davison BB, Kaack MB, Rogers LB, Rasmussen KK, Rasmussen TA, Henson EW, Henson MC, Parekh FK, Krogstad DJ. The role of soluble tumor necrosis factor receptor types I and II and tumor necrosis factor-alpha in malaria during pregnancy. J Infect Dis. 2006;194(1):123–32. doi: 10.1086/504694. [DOI] [PubMed] [Google Scholar]
- 45.Fried M, Muga RO, Misore AO, Duffy PE. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. Journal of immunology. 1998;160(5):2523–30. [PubMed] [Google Scholar]
- 46.Rogerson SJ, Brown HC, Pollina E, Abrams ET, Tadesse E, Lema VM, Molyneux ME. Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infection and immunity. 2003;71(1):267–70. doi: 10.1128/IAI.71.1.267-270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda M, Knowles RD, Kleinerman ES. Muramyl tripeptide phosphatidylethanolamine encapsulated in liposomes stimulates monocyte production of tumor necrosis factor and interleukin-1 in vitro. Cancer communications. 1991;3(10-11):313–21. doi: 10.3727/095535491820873740. [DOI] [PubMed] [Google Scholar]
- 48.Marquis JF, Gros P. Genetic analysis of resistance to infections in mice: A/J meets C57BL/6J. Current topics in microbiology and immunology. 2008;321:27–57. doi: 10.1007/978-3-540-75203-5_2. [DOI] [PubMed] [Google Scholar]
- 49.Chen SU, Chou CH, Chao KH, Lee H, Lin CW, Lu HF, Yang YS. Lysophosphatidic acid up-regulates expression of growth-regulated oncogene-alpha, interleukin-8, and monocyte chemoattractant protein-1 in human first-trimester trophoblasts: possible roles in angiogenesis and immune regulation. Endocrinology. 2010;151(1):369–79. doi: 10.1210/en.2009-0779. [DOI] [PubMed] [Google Scholar]
- 50.Hamilton ST, Scott G, Naing Z, Iwasenko J, Hall B, Graf N, Arbuckle S, Craig ME, Rawlinson WD. Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS One. 2012;7(12):e52899. doi: 10.1371/journal.pone.0052899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lucchi NW, Sarr D, Owino SO, Mwalimu SM, Peterson DS, Moore JM. Natural hemozoin stimulates syncytiotrophoblast to secrete chemokines and recruit peripheral blood mononuclear cells. Placenta. 2011;32(8):579–85. doi: 10.1016/j.placenta.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nature reviews Immunology. 2003;3(9):745–56. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 53.Gruss HJ, Dower SK. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood. 1995;85(12):3378–404. [PubMed] [Google Scholar]
- 54.Haimovici F, Hill JA, Anderson DJ. The effects of soluble products of activated lymphocytes and macrophages on blastocyst implantation events in vitro. Biol Reprod. 1991;44(1):69–75. doi: 10.1095/biolreprod44.1.69. [DOI] [PubMed] [Google Scholar]
- 55.Yui J, Hemmings D, Garcia-Lloret M, Guilbert LJ. Expression of the human p55 and p75 tumor necrosis factor receptors in primary villous trophoblasts and their role in cytotoxic signal transduction. Biology of Reproduction. 1996;55(2):400–9. doi: 10.1095/biolreprod55.2.400. [DOI] [PubMed] [Google Scholar]
- 56.Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocrine reviews. 2005;26(7):877–97. doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- 57.Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstetrics and gynecology. 2000;96(2):271–6. doi: 10.1016/s0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- 58.Crocker IP, Cooper S, Ong SC, Baker PN. Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am J Pathol. 2003;162(2):637–43. doi: 10.1016/S0002-9440(10)63857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jaiswal MK, Agrawal V, Mallers T, Gilman-Sachs A, Hirsch E, Beaman KD. Regulation of apoptosis and innate immune stimuli in inflammation-induced preterm labor. Journal of immunology. 2013;191(11):5702–13. doi: 10.4049/jimmunol.1301604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crocker IP, Tanner OM, Myers JE, Bulmer JN, Walraven G, Baker PN. Syncytiotrophoblast degradation and the pathophysiology of the malaria-infected placenta. Placenta. 2004;25(4):273–82. doi: 10.1016/j.placenta.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 61.Burton GJ, Jones CJ. Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwanese journal of obstetrics & gynecology. 2009;48(1):28–37. doi: 10.1016/S1028-4559(09)60032-2. [DOI] [PubMed] [Google Scholar]
- 62.Longtine MS, Chen B, Odibo AO, Zhong Y, Nelson DM. Caspase-mediated apoptosis of trophoblasts in term human placental villi is restricted to cytotrophoblasts and absent from the multinucleated syncytiotrophoblast. Reproduction. 2012;143(1):107–21. doi: 10.1530/REP-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Longtine MS, Chen B, Odibo AO, Zhong Y, Nelson DM. Villous trophoblast apoptosis is elevated and restricted to cytotrophoblasts in pregnancies complicated by preeclampsia, IUGR, or preeclampsia with IUGR. Placenta. 2012;33(5):352–9. doi: 10.1016/j.placenta.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burton GJ, Skepper JN, Hempstock J, Cindrova T, Jones CJ, Jauniaux E. A reappraisal of the contrasting morphological appearances of villous cytotrophoblast cells during early human pregnancy; evidence for both apoptosis and primary necrosis. Placenta. 2003;24(4):297–305. doi: 10.1053/plac.2002.0882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.