Abstract
The intestinal tissue notably responds to stressful, cholinergic and innate immune signals by microRNA (miRNA) changes, but whether and how those miRNA regulators modify the intestinal cholinergic and innate immune pathways remained unexplored. Here, we report changes in several miRNA regulators of cholinesterases (ChEs) and correspondingly modified ChE activities in intestine, splenocytes and the circulation of mice exposed to both stress and canonical or alternative Toll-Like Receptor 9 (TLR9) oligonucleotide (ODN) aptamer activators or blockers. Stressful intraperitoneal injection of saline, the anti-inflammatory TLR9 agonist mEN101 aptamer or the inflammation-activating TLR9 aptamer ODN 1826 all increased the expression of the acetylcholinesterase (AChE)-targeting miR-132. In comparison, mEN101 but neither ODN 1826 nor saline injections elevated intestinal miR-129-5p, miR-186 and miR-200c, all predicted to target both AChE and the homologous enzyme butyrylcholinesterase (BChE). In cultured immune cells, BL-7040, the human counterpart of mEN101, reduced AChE activity reflecting inflammatory reactions in a manner preventable by the TLR9 blocking ODN 2088. Furthermore, the anti-inflammatory BL-7040 TLR9 aptamer caused reduction in nitric oxide and AChE activity in both murine splenocytes and human mononuclear cells at molar concentrations four orders of magnitude lower than ODN 1826. Our findings demonstrate differential reaction of cholinesterase-targeting miRNAs to distinct TLR9 challenges, indicating upstream miRNA co-regulation of the intestinal alternative NFκB pathway and cholinergic signaling. TLR9 aptamers may hence potentiate miRNA regulation that enhances cholinergic signaling and the resolution of inflammation, which opens new venues for manipulating bowel diseases.
Keywords: Acetylcholinesterase, Butyrylcholinesterase, Intestinal inflammation, MicroRNA, Non-neuronal acetylcholine, Toll-Like Receptor 9
Highlights
-
•
Stress and TLR9 aptamers modulate intestinal cholinesterase-targeting microRNAs.
-
•
Cholinesterase-targeting microRNA increases associate with declined cholinesterase activity.
-
•
Acetylcholinesterase-targeting microRNA modifications reflect stress insults.
-
•
TLR9 aptamers, but not stress, modulate butyrylcholinesterase-targeting microRNAs.
-
•
TLR9 links with cholinergic signaling operate through microRNA regulators.
1. Introduction
Intestinal inflammation is a common physiological response to infection, tissue damage or stress. Homeostasis is restored when inflammation is restricted in time and space, but chronic inflammation can trigger autoimmunity diseases, tissue damage and cancer [1]. Specifically, inflammatory bowel disease (IBD) is a condition caused by chronic/persistent intestinal inflammation. The hallmarks of IBD include elevated levels of intestinal pro-inflammatory cytokines, disruption of the gut tissue and severe clinical symptoms. Recent expansion of immunological research describes the role of cholinergic signaling pathways [2] and of the pattern-recognition, innate immunity receptors, called toll-like receptors (TLRs), in intestinal inflammation [3]. Both of these pathways involve hierarchically high signaling regulators that communicate with each other to control inflammatory reactions. However, the underlying molecular mechanisms regulating this communication between the cholinergic and TLR pathways remained incompletely understood.
The different TLRs, expressed by macrophages, dendritic cells and B cells, are distinguished by their specific ligands. For example, TLR4, the most extensively studied TLR, is known to react to the gram-negative bacterial cell wall component lipopolysaccharide (LPS) and initiates the NFκB cascade [3]. TLR9, on the other hand, is a sensor of bacterial DNA with un-methylated CpG motifs [4] that plays a role in multiple autoimmune disorders [5] as well as in intestinal immune tolerance [6]. Unlike most TLRs, which are trans-membrane proteins, TLR9 is primarily expressed in endosomes. Two main types of CpG oligonucleotides (ODNs), type A and B can activate TLR9 by initiating distinct signaling cascades [7]. Briefly, CpG Type A activates the adapter protein MyD88, initiating the transcription factor interferon regulatory factor 7 (IRF7). Activated IRF7 translocates to the nucleus and induces the expression of interferon-α (INF-α) [8] through I kappa B kinase α (IKKα). CpG Type B also activates MyD88, which then phosphorylates the kinase IKKβ, within its complex with IKKα. Once phosphorylated, IKKβ phosphorylates the protein IkB, bound to the p65/p50 dimer of the NFκB family transcription factors, and prevents their nuclear translocation. Following IkB degradation, the dimer is free to translocate to the nucleus and induce expression of the pro-inflammatory cytokines interleukin (IL)-1β, IL-6 and TNF-α and the inducible nitric oxide synthase (iNOS), which produces nitric oxide (NO). This NFκB pathway is called the ‘canonic’ or pro-inflammatory NFκB pathway [3].
Over the last decade, a second, alternative pathway was discovered, in which MyD88 is not involved. Instead, NFκB-inducing kinase [9] is phosphorylated, which in turn phosphorylates IKKα alone [10]. Phosphorylated IKKα cleaves another dimer of the NFκB family protein, p100/RelB, into p52/RelB [11], [12], [13]. Then, p52/RelB can translocate to the nucleus and induce the expression of other proteins, like the acetylcholine hydrolyzing enzyme acetylcholinesterase [14], [15] and the immune regulatory enzyme indoleamine 2, 3-dioxygenase [15], [16]. The alternative pathway can be activated by several known ligands, such as cluster of differentiation 40 ligand (CD40L) [10]. Certain TLR9 ligands can also activate this anti-inflammatory pathway [17], suggesting that TLR9 may function in a MyD88-unrelated manner.
Importantly, NFκB also serves additional receptors, e.g. the nicotinic acetylcholine receptor (nAChR), suggesting intricate inter-related control over the canonical and anti-inflammatory TLR9 pathways. For example, α7 nAChR signaling can restrict intestinal inflammation by activating the JAK2–STAT3 cascade and promoting secretion of the anti-inflammatory cytokine IL-10 [2]. Further, acute activation of the canonical NFκB pathway is followed by rapid over-expression of AChE messenger RNA (mRNA) [18] via the NFκB recognition motif in the AChE promoter or due to epigenetic regulation [19], [20]. Correspondingly, the cholinergic–TLR9 interaction was recently shown to modulate circulation cytokines [21].
The brain restricts inflammation and prevents sepsis through ACh released from efferent vagus nerve fibers, and ACh binds α7 nAChR on macrophages and dendritic cells, blocking NFκB function [22]. Hydrolysis of ACh by AChE and BChE [23] can induce overproduction of pro-inflammatory cytokines [15]. Pattern recognition of bacterial CpG DNA motifs by TLR9 is instrumental both in maintaining the persistence and inducing the resolution of inflammation. Stimulating TLR9-expressing dendritic cells in the intestinal lamina propria (LpDC) with CpG DNA disrupts intestinal homeostasis by recruiting pro-inflammatory T helper cells (Th1 and Th17) [24].
At the post-transcriptional level, both TLR and cholinergic signaling processes are subjected to microRNA (miRNA) regulation. MiRNAs are small, non-coding RNAs that regulate mRNA post-transcriptionally by limiting their translation and inducing their degradation [25]. Each miRNA targets many different transcripts, which often participate in the same biological pathway. We have recently identified the subclass of CholinomiRs: miRNAs predicted to target the inflammation-suppressive cholinergic signaling pathway [26]. Those include the AChE-targeting miR-132, increases in which elevate cholinergic signaling and limit peripheral and intestinal cytokine production [18]. But the response of CholinomiRs to TLR9 manipulations remained unknown.
To approach this question, we used BL-7040, an AChE mRNA-targeting antisense oligonucleotide that suppresses AChE mRNA levels, thereby elevating ACh and alleviating inflammation [14], [27], [28]. BL-7040 reduced serum AChE activity in mice, which may potentiate the cholinergic anti-inflammatory effect [29]. Therefore, we predicted that CholinomiR suppression of circulation AChE could cause such anti-inflammatory consequences. However, the BL-7040 reaction was prevented by the TLR9 inhibiting oligonucleotide ODN 2088 and was not mimicked by the inflammation inducing ODN 1826 [29], suggesting that the mechanism involved anti-inflammatory activation of TLR9. To investigate the interaction between the intestinal cholinergic and innate immune pathways, and to explore the potential links between those pathways and TLR9, we tested CholinomiR modulations under different TLR9-modifying ODNs in intestinal inflammation.
2. Material & methods
2.1. Animal experiments
Animal experiments were performed in accordance with The Hebrew University's animal procedures (Ethics number of research: NS-10205-4).
2.2. Intestinal sections
We exposed male C57/B6J mice to predator scent and injected them intra-peritoneally on four consecutive days with different ODN's (mEN101, ODN 1826, ODN 2088, 500 μg/kg) or saline, essentially as in [21]. Oligonucleotides used were:
-
1.mEN101, murine anti-inflammatory TLR9 activator:
- 5′-CTG CAA TAT TTT CTT GCA metCmetCmet-3′
-
2.ODN 1826, a pro-inflammatory CpG type B TLR9 activator:
- 5′-TCC ATG ACsGs TTC CTG ACsGs TT-3′
-
3.ODN 2088, a TLR9 blocking aptamer
- 5′-TsCsCs TsGsGs CsGsGs AsGs Ts-3′
Non-injected mice served as controls. 7 days later, we removed intestinal sections from mice of all groups as in [18]. The mice were housed four per cage, at 21 ± 1 °C, in a 12-h light/dark cycle.
2.3. In vitro procedures
Murine mononuclear splenocytes were isolated using lymphocyte separation medium (Mediatech Inc.) and cultured in complete RPMI medium (supplemented with 10% fetal bovine serum, 200 mM l-glutamine, 10,000-U/ml penicillin and streptomycin, 50 μM 2-β-mercaptoethanol) in 48 well plates, 0.5 million cells per well. Cells were challenged with BL-7040 (0–10 μM) in the presence or absence of the TLR9 blocker ODN 2088 (0.1 μM). Additional oligonucleotides used:
-
4.BL-7040 (2′O-methyl), an anti-inflammatory TLR9 activator:
- 5′-CTG CCA CGT TCT CCT GCA metCmetCmet-3′
-
5.ODN 1585, a pro-inflammatory CpG type A TLR9 activator:
- 5′GsGsGGTCAACGTTGAGsGsGsGsGsGs-3'
24 h post-challenge, media and cells were collected.
2.4. Primary human blood mononuclear cells (PBMCs)
Primary human blood mononuclear cells (PBMCs) were obtained and cultured as previously described in [18]. AChE activity in human mononuclear cells was measured 24 h after treatment with 0.01 μM BL-7040 or 0.1 μM of CpG B (ODN 1826), CpG A (ODN 1585) or TLR9 Inhibitor (ODN 2088).
2.5. Nitric oxide measurements
Nitric oxide measurements involved the Griess method [30]. NO levels were determined in conditioned medium from the murine macrophage cell line RAW264.7 cultures. 100 μl of each sample + 100 μl of Griess reagent (1% sulfanilamide/0.1% naphthylethylenediamine dihydrochloride/2.5% H3PO4) were incubated for 10–20 min at room temperature. Absorbance was read at 546 nm in a micro-ELISA reader [31].
2.6. Cholinesterase catalytic activity measurements
AChE activity levels were assessed by measuring hydrolysis rates of 1 mM acetylthiocholine (ATCh, Sigma), as described previously [32], following 20 min incubation with and without 5 × 10− 5 M tetraisopropyl pyrophosphoramide (iso-OMPA, Sigma), a specific BChE inhibitor, to assay for AChE-specific or total cholinesterase activity. Each sample was assayed in n = 3 or more.
2.7. qRT-PCR
Isolation of small and total RNA from splenocytes or intestinal sections was carried out with the miRNeasy Mini Kit (QIAGEN). Expression and validation of specific miRNAs was assessed by quantitative real-time PCR using the PerfeCTa® microRNA Assay (Quanta). Expression levels were normalized to endogenous expression of U6 snRNA. Fold change values were calculated using the ΔΔCt method.
2.8. Bioinformatic analysis
Bioinformatic analysis of microRNA-target binding energies was conducted using the RNAhybrid algorithm, version 2.2 (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/).
2.9. Statistical analysis
Collected data was summarized and displayed as mean ± standard error of mean using the SPSS software (version 19.0, SPSS INC, Chicago, IL, USA). The statistical analysis was performed using Mann–Whitney's U-test or One-way ANOVA and Bonferroni post-hoc test, where α < 0.05 was considered significant.
3. Results
3.1. TLR9 ODNs induce CholinomiR changes in mouse intestinal sections
Our working hypothesis predicted that AChE overexpression enhances the inflammatory output, whereas AChE decreases reflect anti-inflammatory states [33]. To test this hypothesis, we prepared intestinal sections from untreated control mice and from mice subjected to stress following intra-peritoneal injections of saline or of mEN101, an activator of the anti-inflammatory TLR9 reaction, or ODN 1826, a pro-inflammatory Type B activator of NFκB. We extracted RNA and determined the levels of four CholinomiRs by qRT-PCR. All of the tested CholinomiRs were predicted to target the transcript encoding for the circulation soluble AChE-R [23], [26]. One CholinomiR, miR-132 was validated to also target the ‘synaptic’ AChE-S transcript [18]. The other three CholinomiRs, miR-129-5p, miR-186 and miR-200c are all predicted to target both the soluble alternative splicing variant AChE-R and BChE in the circulation (Fig. 1A). Interestingly, the calculated binding energies of these three miRNAs (miR-129-5p, miR-186 and miR-200c) towards their AChE-R and BChE targets showed preferred binding towards BChE; − 17.1 kcal/mol, − 16 kcal/mol, − 15.6 kcal/mol; in comparison to − 13.5 kcal/mol, − 11.6 kcal/mol or − 10.6 kcal/mol, − 11.8 kcal/mol towards AChE-R, respectively (Fig. 1B). Of note, the intestinal miR-132 levels were increased under both saline and ODN 1826 stresses. In comparison, none of the other CholinomiRs showed changes following saline injection. However, all of them presented increased intestinal levels under the anti-inflammatory mEN101 treatment, but not under the NFκB-stimulating ODN 1826 (Fig. 1B). The extent of the observed increases did not reflect the number of miRNA-binding elements on the targeted transcript, such that miR-186, with a total of 3 binding elements on AChE-R and BChE showed a similar change to that of miR-200c with only two sites (Fig. 1B).
Fig. 1.
TLR9 aptamers induce differential changes in intestinal miRNA controllers of Cholinesterases. A. To test the TLR9 effects on CholinomiRs targeting two cholinesterase variants, intestinal sections were prepared from mice exposed to 500 μg/kg of the NFκB stimulating ODN 1826 (red), to the anti-inflammatory TLR9 stimulating oligonucleotide mEN101 (blue) or saline (green), to control for the stress impact. The sequences of tested CholinomiRs [26] are shown; all of them are predicted to target AChE-R and either AChE-S or BChE. B. Seven days after exposure, qRT-PCR measurements quantified the intestinal levels of the AChE-S and AChE-R targeting CholinomiR-132 as well as miR-129-5p, − 186 or − 200c, all of which are predicted to target both AChE-R and BChE. The bar graphs show ΔΔCt values normalized to snRNA U6 (white: control; green: saline; blue: mEN101; red: ODN1826). Bonferroni corrected p-values are noted above. Note mEN101 induced increases in all of the AChE-R and BChE-targeting miRNAs, but not in miR-132. MiRNA-target binding structures (miRNA sequence: red, target mRNA sequence: green) binding energies in kcal/mol are shown. The binding energies of miR-132 towards the two AChE variants are equal, whereas the other three CholinomiRs show lower binding energies towards BChE.
3.2. Anti-inflammatory TLR9 activation reduces AChE activity in murine splenocytes
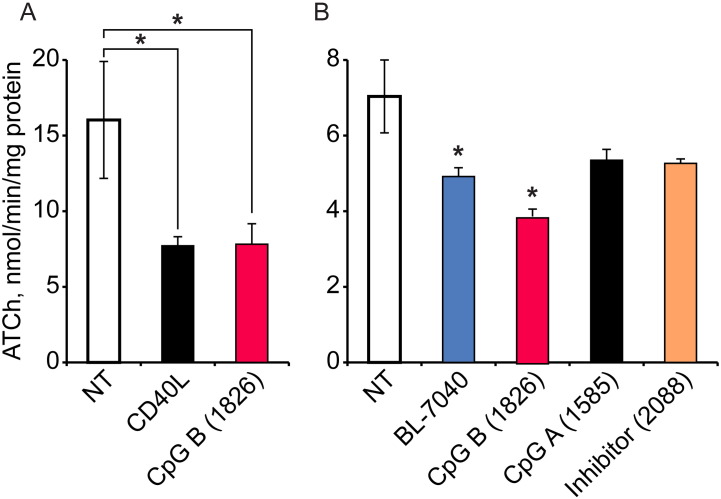
Pro-inflammatory NFκB activation leads to increases in circulation AChE levels under diverse inflammation and post-traumatic states [21], [29], [34]. In contrast, mild alternative activation of NFκB via anti-inflammatory TLR9 signaling leads to down-regulation of AChE activity, resolution of inflammation and restored homeostasis. Based on these considerations, the anti-inflammatory effects of the tested oligonucleotides could be mediated by TLR9 induction. However, the BL-7040 sequence was initially designed as an antisense to human AChE. This primarily implied that BL-7040 could potentially function in two pathways. Molecular modeling indicated that BL-7040 could not function as an antisense agent in mice due to 4 mismatches in its 20-nucleotides long sequence, which prevents interaction with its murine mRNA target (Fig. 2A). In spite of this difference, BL-7040 was able to reduce AChE activity levels by 50% in murine splenocytes (Fig. 2B), the same reduction seen following treatment with CD40L, an alternative NFκB pathway activator [29]. Moreover, this reduction was completely abolished under co-treatment with the TLR9 inhibitor ODN 2088, demonstrating that BL-7040 suppresses AChE through a TLR9-mediated, alternative NFκB mechanism. Competition assay using ODN 2088 as a TLR9 inhibitor in the murine macrophage cell line RAW264.7 showed that BL-7040 is a low affinity ligand of TLR9; ligand/inhibitor ratio of 100:1 was required to completely suppress TLR9 activation by BL-7040, whereas ligand/inhibitor ratio of 1:100 was sufficient to suppress activation by the pro-inflammatory ODN 1826 (Fig. 2C).
Fig. 2.
BL-7040 induces a decrease in murine AChE activity through low affinity TLR9 activation. A. Molecular modeling of complexes between the second exon of human (h) and mouse (m) AChE (orange) and BL-7040 (blue). Mismatches are portrayed in red. B. AChE activity in murine splenocytes 24 h after treatment with BL-7040 (0.01 μM) with or without ODN 2088 (0.1 μM). C. Nitrite production in RAW264.7 macrophages following exposure to 0.01 μM BL-7040 or 0.1 μM ODN 1826, concomitantly with increasing doses of ODN 2088 that block their effects (molar ratio of ODN 2088/agonist shown). * indicates p-value < 0.05 using Mann–Whitney's U-test.
3.3. TLR9 activation suppresses AChE activity in murine splenocytes and human mononuclear cells
Next, we tested the inflammation-regulating effects of the three types of immune-stimulatory CpG ODNs as sub-classified by their distinct effects on leukocytes: CpG type A ODNs promote a strong production of interferons (IFNs) in dendritic cells via the interferon responding factor (IRF) transcription complex; CpG type B ODNs induce both AP-1 and NFκB-mediated transcription, which promotes B cell survival and production of pro-inflammatory mediators; and CpG type C ODNs compromise both activities. At the structural level, CpG type A and B ODNs are typically monomeric, but CpG type C ODNs act as dimers [21], [29]. Murine splenocytes treated with low dose CpG-B ODN 1826 showed ca. 50% reduction in AChE activity, similar to that seen following treatment with CD40L (Fig. 3A). CpG-A ODN 1585 and the TLR9 antagonist ODN 2088 failed to induce such reductions. In comparison, primary human blood mononuclear cells (PBMCs) reacted to BL-7040 as well as to ODN 1826 by less pronounced, albeit significant reduction in AChE activity, whereas the CpG A ODN 1585 or the TLR9 inhibitor ODN treatment failed to change AChE activities in PBMCs (Fig. 3B). Taken together, these findings show that anti-inflammatory activation of TLR9, as opposed to its pro-inflammatory activation, may induce a delayed suppression of inflammatory response in circulation immune cells.
Fig. 3.
Anti-inflammatory TLR9-mediated response induces a decline of AChE activity in murine splenocytes and human mononuclear cells. A. Murine splenocytes 24 h after treatment with 1 μg/ml CD40L or 0.01 μM of ODN 1826 show ~ 50% reduction in AChE activity. B. AChE activity in human mononuclear cells, 24 h after treatment with 0.01 μM BL-7040 or 0.1 μM of CpG B (ODN 1826), CpG A (ODN 1585) or TLR9 Inhibitor (ODN 2088), n = 3. * indicates p-value < 0.05 using Mann–Whitney's U-test.
4. Discussion
TLR9-mediated signaling possesses a yet unknown duality with regard to the inflammatory process. Namely, it participates in both the breakdown and the maintenance of immune tolerance; and it can propagate or restrict inflammation. Our findings suggest that both upstream miRNA regulation and downstream signaling pathways to TLR9 determine pro- and anti-inflammatory intestinal activities. Specifically, we show that BL-7040, a synthetic, weak-binding TLR9-specific aptamer, triggers miRNA changes supporting the non-canonical pathway of NFκB activation, and that ex-vivo, BL-7040 proved effective in diminishing inflammation in murine splenocytes and PBMCs.
Our results suggest a miRNA-mediated mechanism initiating an anti-inflammatory, homeostatic TLR9 signaling that suppresses intestinal and immune cell inflammation via cholinergic blockade. Intriguingly, this response associates with increases in three dual targeting CholinomiRs that share sequence complementarity with the two soluble cholinesterases in the circulation, AChE-R and BChE: miR-129-5p, miR-186 and miR-200c. Of those, miR-200c is known to be increased in blood of Crohn's disease patients [9], which supports our results. In contrast, miR-132, which targets AChE-R and AChE-S, but not BChE, showed increased levels in intestinal sections following injection of the pro-inflammatory CpG A ODN 1826 as well as of saline, suggesting relevance for both psychological stress and inflammation. This is supported by increased levels of miR-132 in intestinal explants removed from human IBD patients during surgery [35]. The minimal binding energies with BChE of the three CholinomiRs targeting the soluble ChEs are lower than those towards AChE-R, indicating preferential binding of BChE. This might further explain the different changes in the levels of these miRNAs following ODN treatments in comparison to those of miR-132. Notably, miR-132 shows equal binding energies towards the soluble AChE-R and the synaptic AChE-S variant, but fails to target BChE. Differences between the reactions of these CholinomiRs to diverse TLR9 modulators could indicate distinct roles for the two circulating cholinesterases in inflammatory states. Supporting this notion, the amount of BChE in the circulation is much higher than that of AChE and mutation-induced impairments in the capacity of BChE to hydrolyze ACh associate with acute responses to anti-cholinesterase treatment or exposure [36], [37].
The ability of TLR9-expressing innate immune cells to respond to pathogenic DNA, while ignoring self-originated DNA or DNA from harmless bacteria requires tight regulation. Our findings indicate that upstream CholinomiR regulation modulates the cholinergic anti-inflammatory reflex, known to attenuate cytokine production in innate immunity cells. This mechanism might play a role in the response to weak binding of “benign” DNA motifs to TLR9. Such binding induces non-canonical activation of NFκB and therefore restricts an inflammatory response. The efficacy of BL-7040 as a prospective oral treatment for Myasthenia gravis (MG) has been described previously [38], [39]. BL-7040 was mainly accounted for as an AChE-R suppressing aptamer in vivo, but our current observations expand this concept towards miRNA regulation and non-canonical NFκB activation. MG is an autoimmune disorder involving production of antibodies against the α7 nAChR, which results in a reduced capacity of the α7 nAChR to block inflammation in pro-inflammatory signaling. In contrast, BL-7040 treatment of murine splenocytes reduced both NO and AChE, indicating a concerted anti-inflammatory effect. Given that ODN 2088 may block much of BL-7040's activity, these findings suggest that BL-7040 acts primarily as a weak TLR9 agonist, which might account for its notable lack of cholinergic side effects in therapy.
In conclusion, we propose that miRNA regulators suppress the production of AChE and BChE in peripheral blood leukocytes and the circulation, thus modifying ACh levels and regulating cholinergic anti-inflammatory blockade of the NFκB pathway through the α7 nAChR. Both CpG type B oligonucleotides and the BL-7040 TLR9 aptamer can activate inflammatory modifying responses, whereas the blocking aptamer ODN 2088 prevents TLR9 signaling through the NfκB pathway. Fig. 4 presents this concept schematically, with the pro-inflammatory and anti-inflammatory reactions color-coded (red and blue, respectively). The canonical activation mode is pro-inflammatory, whereas the alternative mode is anti-inflammatory and induces self-created immunosuppression, while simultaneously suppressing AChE activity. This increases ACh levels and potentiates the cholinergic anti-inflammatory response. Our findings hence demonstrate differential reaction of cholinesterase-targeting miRNAs to distinct TLR9 changes; indicating co-regulation of the intestinal alternative NFκB pathway and cholinergic signaling. We further show that ODN aptamers of TLR9 may potentiate miRNA regulation that enhances cholinergic signaling, leading to resolution of inflammation. This may open new ways for manipulating bowel diseases, especially IBD.
Fig. 4.
Oligonucleotide discriminators of the TLR9-cholinergic links. Left: miRNA regulators suppress the production of AChE and BChE, thus modifying ACh levels and regulating the cholinergic anti-inflammatory blockade of the NfκB pathway through the α7 nAChR. Right: Both CpG type B oligonucleotides and the BL-7040 TLR9 aptamer can activate inflammatory modifying responses, whereas the blocking aptamer ODN 2088 prevents TLR9 signaling to the NfκB pathway. Bottom: The canonical activation mode is pro-inflammatory (marked red), whereas the alternative mode is anti-inflammatory (marked blue) and induces immunosuppression, while at the same time suppressing AChE activity, increasing ACh levels and potentiating the cholinergic anti-inflammatory response.
Acknowledgments
The authors are grateful to Mr. Yochai Wolf and Dr. Estelle R. Bennett, Jerusalem for their helpful advice and discussions. We would like to acknowledge the European Research Council (Advanced Award 321501, to H.S.), The Israel Science Foundation grant No. 817/13 and the Legacy Heritage Science Initiative (LHSI) of The Israel Science Foundation Grant No. 378/11 (to H.S.). BN is a recipient of a PhD fellowship from The Hebrew University's Bioengineering Program.
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 2.Pavlov V.A., Tracey K.J. The vagus nerve and the inflammatory reflex–linking immunity and metabolism. Nat. Rev. Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vallabhapurapu S., Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 4.Barton G.M., Kagan J.C., Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat. Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 5.Krieg A.M., Vollmer J. Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunol. Rev. 2007;220:251–269. doi: 10.1111/j.1600-065X.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee J., Mo J.H., Katakura K., Alkalay I., Rucker A.N., Liu Y.T. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat. Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino K., Sugiyama T., Matsumoto M., Tanaka T., Saito M., Hemmi H. IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature. 2006;440:949–953. doi: 10.1038/nature04641. [DOI] [PubMed] [Google Scholar]
- 8.Krieg A.M. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 9.Paraskevi A., Theodoropoulos G., Papaconstantinou I., Mantzaris G., Nikiteas N., Gazouli M. Circulating microRNA in inflammatory bowel disease. J. Crohns Colitis. 2012;6:900–904. doi: 10.1016/j.crohns.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Bonizzi G., Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Vallabhapurapu S., Powolny-Budnicka I., Riemann M., Schmid R.M., Paxian S., Pfeffer K. Rel/NF-kappaB family member RelA regulates NK1.1 − to NK1.1 + transition as well as IL-15-induced expansion of NKT cells. Eur. J. Immunol. 2008;38:3508–3519. doi: 10.1002/eji.200737830. [DOI] [PubMed] [Google Scholar]
- 12.Zarnegar B., Yamazaki S., He J.Q., Cheng G. Control of canonical NF-kappaB activation through the NIK–IKK complex pathway. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3503–3508. doi: 10.1073/pnas.0707959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarnegar B.J., Wang Y., Mahoney D.J., Dempsey P.W., Cheung H.H., He J. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat. Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollak Y., Gilboa A., Ben-Menachem O., Ben-Hur T., Soreq H., Yirmiya R. Acetylcholinesterase inhibitors reduce brain and blood interleukin-1beta production. Ann. Neurol. 2005;57:741–745. doi: 10.1002/ana.20454. [DOI] [PubMed] [Google Scholar]
- 15.Pick M., Perry C., Lapidot T., Guimaraes-Sternberg C., Naparstek E., Deutsch V. Stress-induced cholinergic signaling promotes inflammation-associated thrombopoiesis. Blood. 2006;107:3397–3406. doi: 10.1182/blood-2005-08-3240. [DOI] [PubMed] [Google Scholar]
- 16.Tas S.W., Vervoordeldonk M.J., Hajji N., Schuitemaker J.H., van der Sluijs K.F., May M.J. Noncanonical NF-kappaB signaling in dendritic cells is required for indoleamine 2,3-dioxygenase (IDO) induction and immune regulation. Blood. 2007;110:1540–1549. doi: 10.1182/blood-2006-11-056010. [DOI] [PubMed] [Google Scholar]
- 17.Puccetti P., Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat. Rev. Immunol. 2007;7:817–823. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- 18.Shaked I., Meerson A., Wolf Y., Avni R., Greenberg D., Gilboa-Geffen A. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity. 2009;31:965–973. doi: 10.1016/j.immuni.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Meshorer E., Toiber D., Zurel D., Sahly I., Dori A., Cagnano E. Combinatorial complexity of 5′ alternative acetylcholinesterase transcripts and protein products. J. Biol. Chem. 2004;279:29740–29751. doi: 10.1074/jbc.M402752200. [DOI] [PubMed] [Google Scholar]
- 20.Sailaja B.S., Cohen-Carmon D., Zimmerman G., Soreq H., Meshorer E. Stress-induced epigenetic transcriptional memory of acetylcholinesterase by HDAC4. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E3687–E3695. doi: 10.1073/pnas.1209990110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerman G., Shaltiel G., Barbash S., Cohen J., Gasho C.J., Shenhar-Tsarfaty S. Post-traumatic anxiety associates with failure of the innate immune receptor TLR9 to evade the pro-inflammatory NFkappaB pathway. Transl. Psychiatry. 2012;2:e78. doi: 10.1038/tp.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tracey K.J. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 23.Soreq H., Seidman S. Acetylcholinesterase—new roles for an old actor. Nat. Rev. Neurosci. 2001;2:294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- 24.Hall J.A., Bouladoux N., Sun C.M., Wohlfert E.A., Blank R.B., Zhu Q. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadorp B., Soreq H. Predicted overlapping microRNA regulators of acetylcholine packaging and degradation in neuroinflammation-related disorders. Front. Mol. Neurosci. 2014;7:9. doi: 10.3389/fnmol.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evron T., Moyal-Segal L.B., Lamm N., Geffen A., Soreq H. RNA-targeted suppression of stress-induced allostasis in primate spinal cord neurons. Neurodegener. Dis. 2005;2:16–27. doi: 10.1159/000086427. [DOI] [PubMed] [Google Scholar]
- 28.Gilboa-Geffen A., Lacoste P.P., Soreq L., Cizeron-Clairac G., Le Panse R., Truffault F. The thymic theme of acetylcholinesterase splice variants in myasthenia gravis. Blood. 2007;109:4383–4391. doi: 10.1182/blood-2006-07-033373. [DOI] [PubMed] [Google Scholar]
- 29.Gilboa-Geffen A., Wolf Y., Hanin G., Melamed-Book N., Pick M., Bennett E.R. Activation of the alternative NFkappaB pathway improves disease symptoms in a model of Sjogren's syndrome. PLoS One. 2011;6:e28727. doi: 10.1371/journal.pone.0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 31.Guimaraes-Sternberg C., Meerson A., Shaked I., Soreq H. MicroRNA modulation of megakaryoblast fate involves cholinergic signaling. Leuk. Res. 2006;30:583–595. doi: 10.1016/j.leukres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Meshorer E., Erb C., Gazit R., Pavlovsky L., Kaufer D., Friedman A. Alternative splicing and neuritic mRNA translocation under long-term neuronal hypersensitivity. Science. 2002;295:508–512. doi: 10.1126/science.1066752. [DOI] [PubMed] [Google Scholar]
- 33.Waiskopf N., Ofek K., Gilboa-Geffen A., Bekenstein U., Bahat A., Bennett E.R. AChE and RACK1 promote the anti-inflammatory properties of fluoxetine. J. Mol. Neurosci. 2014;53:306–315. doi: 10.1007/s12031-013-0174-6. [DOI] [PubMed] [Google Scholar]
- 34.Grisaru D., Pick M., Perry C., Sklan E.H., Almog R., Goldberg I. Hydrolytic and nonenzymatic functions of acetylcholinesterase comodulate hemopoietic stress responses. J. Immunol. 2006;176:27–35. doi: 10.4049/jimmunol.176.1.27. [DOI] [PubMed] [Google Scholar]
- 35.Maharshak N., Shenhar-Tsarfaty S., Aroyo N., Orpaz N., Guberman I., Canaani J. MicroRNA-132 modulates cholinergic signaling and inflammation in human inflammatory bowel disease. Inflamm. Bowel Dis. 2013;19:1346–1353. doi: 10.1097/MIB.0b013e318281f47d. [DOI] [PubMed] [Google Scholar]
- 36.Browne R.O., Moyal-Segal L.B., Zumsteg D., David Y., Kofman O., Berger A. Coding region paraoxonase polymorphisms dictate accentuated neuronal reactions in chronic, sub-threshold pesticide exposure. FASEB J. 2006;20:1733–1735. doi: 10.1096/fj.05-5576fje. [DOI] [PubMed] [Google Scholar]
- 37.Loewenstein-Lichtenstein Y., Schwarz M., Glick D., Norgaard-Pedersen B., Zakut H., Soreq H. Genetic predisposition to adverse consequences of anti-cholinesterases in ‘atypical’ BCHE carriers. Nat. Med. 1995;1:1082–1085. doi: 10.1038/nm1095-1082. [DOI] [PubMed] [Google Scholar]
- 38.Dori A., Soreq H. Neuromuscular therapeutics by RNA-targeted suppression of ACHE gene expression. Ann. N. Y. Acad. Sci. 2006;1082:77–90. doi: 10.1196/annals.1348.004. [DOI] [PubMed] [Google Scholar]
- 39.Sussman J.D., Argov Z., McKee D., Hazum E., Brawer S., Soreq H. Antisense treatment for myasthenia gravis: experience with monarsen. Ann. N. Y. Acad. Sci. 2008;1132:283–290. doi: 10.1196/annals.1405.022. [DOI] [PubMed] [Google Scholar]