Summary
As a consequence of conditioning visual cues with delayed reward, cue-evoked neural activity that predicts the time of expected future reward emerges in the primary visual cortex (V1). We hypothesized that this reward timing activity is engendered by a reinforcement signal conveying reward acquisition to V1. In lieu of behavioral conditioning, we assessed in vivo whether selective activation of either basal forebrain (BF) or cholinergic innervation is sufficient to condition cued interval timing activity. Substituting for actual reward, optogenetic activation of BF or cholinergic input within V1 at fixed delays following visual stimulation entrains neural responses that mimic behaviorally-conditioned reward timing activity. Optogenetically-conditioned neural responses express cue-evoked temporal intervals that correspond to the conditioning intervals, are bidirectionally modifiable, display experience-dependent refinement, and exhibit a scale invariance to the encoded delay. Our results demonstrate that the activation of BF or cholinergic input within V1is sufficient to encode cued interval timing activity, and indicate that V1 itself is a substrate for associative learning that may inform the timing of visually-cued behaviors.
Introduction
A fundamental task accomplished by the brain is the ability to predict the timing of behaviorally salient events. When environmental cues reliably precede future outcomes, neural mechanisms that learn the relationships and produce the interceding intervals offer a potential advantage by informing timed actions necessary to achieve expected outcomes. While numerous brain regions exhibit activity correlated with expected events [1–4], the neural mechanisms responsible for engendering temporal intervals elicited by behaviorally relevant cues remain largely unknown [5]. Exemplifying this issue and motivating the work presented here is the finding that pairing visual stimuli with delayed reward leads to the emergence of stimulus-evoked activity in the primary visual cortex (V1) that predicts the timing of expected future reward [6, 7].
The observation of reward timing activity in V1—the earliest stage of cortical processing of visual information—may be regarded as a reflection of event-anticipatory activity first established elsewhere in the brain. An unconventional yet plausible alternative is that this activity is present due to learning-induced changes local to V1. A computational model inspired by the report of reward timing in V1 provides a general solution as to how V1 could, in principle, learn and express arbitrary cue-reward intervals de novo [8]. This model describes the emergence of reward timing activity as resulting from a process of reinforcement learning [9], wherein a signal conveying behavioral outcome permits the modification of recently active synapses to encode the cue-reward delay. A critical component of this model, therefore, is the provision of this reinforcement signal. Should our model be applicable to the emergence of reward timing within V1, V1 itself must be a substrate of learning-induced changes controlled by such a signal.
A potential source providing this reward-related information is the basal forebrain (BF) as it projects directly and abundantly to V1 [10–14] and is responsive to the acquisition of reward [15–17]. Electrical stimulation of BF has been shown to enhance food and water intake [18], as well as self-administration behaviors [19]. In addition, pathological damage or experimental lesion of BF can cause substantive deficits in learning and memory [12, 20, 21]. Further, BF inputs in the neocortex are known to mediate cortical synaptic plasticity and have been implicated in various cognitive functions [6, 12, 15, 22–28]. Together, these observations give good cause to investigate whether BF inputs are sufficient to encode visually-cued reward timing activity.
We therefore tested the hypothesis that conditioning visually-evoked responses in vivo by optogenetically driving BF input within V1 at fixed temporal delays—mimicking the presumed effects of actual reward acquired behaviorally—results in reward timing-like activity. We demonstrate in mouse V1 that cue-evoked “reward” timing activity is indeed elicited by selective activation of BF input, as its defining features are recapitulated by this manipulation. Our data also demonstrate that optogenetically-entrained timing activity in V1 can be bidirectionally tuned to represent new conditioning intervals, is subject to experience-dependent refinement, and may serve as a neural correlate of the commonly reported “temporal scalar property” [29, 30]. We further examined the neuromodulatory nature of the putative reinforcement signal responsible for engendering cued interval timing activity by conditioning visually-evoked responses with selective cholinergic activation within V1. We found that activation of cholinergic innervation within V1 is indeed sufficient for cued interval-timing activity, in addition to it being necessary [6, 7]. These observations demonstrate the instructive role of basal forebrain and cholinergic innervation in educing visually-cued timing to expected outcomes of behavioral relevance.
Results
Behaviorally-conditioned reward timing in mouse V1
Behaviorally-conditioned reward timing activity within V1 emerges as a consequence of pairing visual stimulation with delayed reward [6, 7]. Such conditioning results in a large proportion of V1 neurons with extended post-stimulus spike modulation (in the seconds range) that report accurately the delay, as experienced in the past, from the cue to its associated reward. This reward timing activity is exhibited in three different response forms: those that express 1) a sustained increase in spiking until the expected reward time, 2) a sustained decrease in spiking until the expected reward time, and 3) a peak in spiking at the expected time of reward. As reward timing activity has thus far been reported only in rats, we first sought here to evidence its presence in mice to demonstrate its generality to another species and establish mouse V1 as a model system for its mechanistic investigation.
We implanted adult C57/BL6 mice with multi-electrode drives to record extracellular single-unit activity in a cued, delayed-reward task. Behavioral and visually-evoked responses to full-field retinal flash (100 ms) presented to the left or the right eye were conditioned by delivery of delayed water reward (Experimental Procedures and Figure S1A). Qualitatively, conditioned cues appeared to evoke V1 activity correlated with the expected reward times, as behaviorally experienced. Each of three previously reported reward timing response forms were observed (sustained increase, sustained decrease, peak; Figure 1A, left, middle, right, respectively). We algorithmically characterized and sorted evoked responses into these three classes (Experimental Procedures and Figure S2), resulting in 64% (131 of 206) of evoked responses being so identified. According to the classified response form, a moment of post-stimulus time was then ascribed as the neural report of reward time (NRT, Experimental Procedures and Figure S3), which we interpret as the temporal expectancy of reward conveyed by the neural response.
Figure 1.
If NRTs relate to the time of expected reward following visual stimulation as experienced in the past by the animal, then the central tendency of NRTs observed should accord with the central tendency of behaviorally-experienced reward times (BRT). Indeed, we found the median of the NRT distribution, observed per animal, to be indistinguishable (Figure 1B) from the median of the BRT distribution (Mouse #, median NRT vs BRT, Wilcoxon rank-sum test p-value: Mouse #1, 1.15 vs 1.22 s, p = 0.17; Mouse #2, 2.14 vs 2.16 s, p = 0.44; Mouse #3, 1.90 vs 2.20 s, p = 0.29). In contrast, NRT distributions do not concord with the overall envelop of licking (Figure S1B). Together, these observations confirm and extend our previous reports of reward timing activity from rat [6, 7] to mouse V1.
Selective activation of BF input within V1 conditions “reward” timing
Having established the ability of mouse V1 to express reward timing activity, we next tested the hypothesis [8, 31, 32] that the neural acquisition of visually-cued reward timing is instructed by the activation of basal forebrain (BF) innervation of V1. We targeted BF nuclei known to innervate V1 directly in rodents [10, 11, 13, 14], namely the diagonal band of Broca (DB, Figure S4B, D and E) and nucleus basalis/substantia innominata (nB/SI, Figure S4C, D and E), for viral-mediated expression of channelrhodopsin (ChR2, Figure 2A and B, and Experimental Procedures) to gain experimental control of the BF→V1 input. We found that in BF-infected animals receiving injection of a retrograde tracer in V1 (Experimental Procedures and Figure S4D), DB- and nB/SI-related structures were the only V1-projecting nuclei in the infected area (Figure S4E). Since in all identifiable V1-projecting nuclei the infection appeared to be restricted to BF (Figure S4E and F), we reasoned that BF→V1 input manipulation could be specifically achieved by adequate laser-light delivery [33, 34] through optical fibers implanted within V1 (Figure 2A and B, and Experimental Procedures). Infected animals were then subjected to an optogenetic conditioning protocol (Figure 2C) designed to mimic the temporal relationship between visual stimulation and reinforcement signaling within V1, as presumptively experienced by their behaviorally-conditioned counterparts.
Figure 2.
Whether this manipulation leads to the neural expression of the interval between the cue and BF→V1 activation was assessed by analyzing conditioned cue-evoked responses (Experimental Procedures) in two cohorts of animals, one with a 1 s (Opto-1s, n = 5) and the other with a 2 s (Opto-2s, n = 7) delay to ChR2 activation. Optogenetic conditioning appeared, qualitatively, to result in neuronal responses that exhibited each of the three identified response forms observed in V1 following behavioral conditioning, with post-stimulus spike modulation corresponding to the delay between cue and ChR2-activation (Figure 3A, top row = Opto-1s; bottom row = Opto-2s; left to right columns: sustained increase, sustained decrease, and peak response). By subjecting the optogenetic data to the same response-classification and NRT-scoring algorithms used for analysis of behaviorally-conditioned data, we found 35.7% (122 out of 342, Opto-1s) and 44% (146 out of 332, Opto-2s) of neural responses, respectively, to exhibit cue-evoked timing activity. The central tendency of NRTs for each experimental cohort (Figure 3B) accords with the corresponding optogenetically-conditioned interval (median value = 1.16 s for Opto-1s; 1.73 s for Opto-2s). As observed following behavioral conditioning, distinctively entrained intervals in the two cohorts resulted in significantly different NRT medians (Figure 3C; p < 10−7, Wilcoxon rank-sum test). This cue-evoked interval timing activity is a consequence of selective activation of basal forebrain input since optogenetic conditioning and recordings from a control cohort (with GFP-only expression in BF→V1 projections, n = 3) resulted in, in the preponderance of cases, responses relating only to the presentations of the visual stimulus (see examples in Figure S5; only 7 of 128, or 5.4% of responses, were identified by the same response-form classification scheme). Since basal forebrain is known to project widely across the cortical mantle [10, 14, 28], we further tested whether the optogenetic effects are specific to manipulations of BF→V1 inputs. In a separate cohort (n = 6) of animals receiving AAV-mediated ChR2 expression in BF, we paired visual stimulation with delayed (2 s) optogenetic activation of BF innervation of somatosensory cortex (S1, Figure 2D and E). Our analysis identified only 26/380 (6.8%) recorded neural responses in V1 with potential cued interval timing features, a fraction expected by chance alone. In contrast to the activation of BF→V1 input, in which a large fraction of neural responses exhibited acutely modulated changes in spike rates (Figure S6), V1 neurons did not respond to optogenetic activation of BF→S1 input. This observation provides functional support to anatomical reports of topographically segregated corticopetal projections from BF [10, 11, 24, 35]. Together, these data show that delayed activation of basal forebrain input within V1 following visual stimulation is sufficient to engender neural representation of the conditioning interval in V1.
Figure 3.
Cued interval timing activity is bidirectionally modifiable
We have previously shown that behaviorally-conditioned neural reports of time appropriately update to changes in the temporal relationships between cues and associated reward delays so as to conform with recent experience [6]. Therefore, we next investigated whether optogenetically-conditioned V1 responses can similarly adapt to reflect a subsequent increase or decrease in the conditioning interval. To examine this, a subset of Opto-1s (n = 3) and Opto-2s (n = 4) animals were further conditioned to novel intervals of 2 s and 1 s, respectively, forming the Opto-1to2s and Opto-2to1s cohorts (Figure 4A and B). The new conditioning contingencies elicit all three response forms reported previously (Figure 4C). We found that the median of the NRT distribution from the Opto-2to1s cohort (Figure 4D) accords with the new conditioning interval (median = 1.01 s). In comparison to the median observed following initial conditioning to 2 s, decreasing the conditioning interval caused a corresponding and significant leftward shift in NRTs (solid versus dotted red curve in Figure 4F; p < 10−7, Wilcoxon rank-sum test). Similarly, in the Opto-1to2s cohort (Figure 4E), the median of the NRT distribution accords with the new target interval (median = 1.80 s). The neural data from this group exhibited a significant rightward shift in the median compared to the initial conditioning to 1 s (solid versus dotted blue curve in Figure 4G; p < 10−7, Wilcoxon rank-sum test). The proximity of the medians of the NRT distributions to their respective conditioned intervals, along with the significant difference of NRT distributions between Opto-2to1s and Opto-1to2s cohorts (p < 10−7, Kolomogorov-Smirnov test), indicate that V1 activity can be bidirectionally modified, as observed following behavioral conditioning, to report newly imposed temporal contingencies between cue and BF→V1 input activation. Selective activation of BF→V1 projections therefore is sufficient for both the de novo synthesis and the bidirectional modification of cued interval timing activity.
Figure 4.
Experience-dependent refinement of the NRT distribution
Optogenetic conditioning to the new delays in the second contingency (Opto-1to2s and Opto-1to2s cohorts) appeared to decrease the error of encoding the target time in comparison to that obtained under the first contingency (Opto-1s and Opto-2s cohorts, Figure S7). To quantify the error in reporting the target time, the absolute difference between individual NRTs and their respective target time was scored (Difference from target, DT). The difference between the error in reporting the target in the first versus second contingency was then assessed by comparing the cumulative distributions of DT scores (Figure 5A), resulting in a significant leftward shift for the second contingency (p = 0.0081, Kolomogorov-Smirnov test). Best-fit gamma distributions for each of the cohorts that comprise the first and second contingencies (Figure S7) suggest that the precision of NRT distributions is increased under the second contingency (despite similar medians between cohorts encoding the same conditioning interval). To quantify the difference in precision, the absolute difference between individual NRTs and their respective cohorts’ median was scored (Difference from median, DM). The difference between the precision observed in the first versus second contingency was then assessed by comparing the cumulative distributions of DM scores (Figure 5B), resulting in a significant leftward shift for the second contingency (p = 0.0017, Kolomogorov-Smirnov test). Collectively, these comparisons indicate a refinement in the reporting of target time on account of training history.
Figure 5.

NRT distributions exhibit a scale invariance to the encoded interval
The error exhibited by human and animal subjects in interval estimation scales proportionally to the duration of time examined, a phenomenon termed the ‘scalar timing property’ [29, 30]. There have been numerous experimental reports in psychophysical and behavioral studies of the scalar timing property [29, 30], as well as recent experimental evidence of a neural correlate in higher-order cortex [36]. If reward timing activity observed within V1 were involved in timing behaviors that abide by the scalar property, it should similarly express a scale invariance to the interval encoded. To examine this, the NRT distribution observed following behavioral conditioning using the 7-lick requirement (Behav-7licks, solid red in Figure 6A; data taken from Mouse #1 in Figure 1B) was compared to that using the 10-lick requirement (Behav-10licks, solid blue in Figure 6A; data combined from Mouse #2 and #3 in Figure 1B) by a multiplicative transformation. After scaling the Behav-7licks distribution by the ratio of the medians between the two distributions, the “scaled up” distribution (Behav-7licks scaled, dotted red in Figure 6A) is indistinguishable to the Behav-10licks distribution (dotted red vs solid blue, Figure 6A; p = 0.66, Kolomogorov-Smirnov test). Therefore, behaviorally-conditioned NRT distributions exhibit a scale invariance to the encoded interval, analogous to the temporal scalar property commonly observed behaviorally.
Figure 6.
Since optogenetically-conditioned responses are demonstrated to mimic reward timing activity, we also assessed whether they exhibit this scale invariance. By multiplying the NRT distribution obtained from the Opto-1s cohort by the ratio of the medians between the Opto-1s and Opto-2s cohorts, we found that the scaled up data is superimposable with the Opto-2s NRT distribution (dotted blue vs solid red, Figure 6B; p = 0.82, Kolomogorov-Smirnov test). This scale invariance in NRT distributions also holds true when similarly comparing data obtained from Opto-2to1s and Opto-1to2s cohorts (Figure 6C, dotted red versus solid blue; p = 0.67, Kolomogorov-Smirnov test), despite the decrease in the error of reporting the new target intervals. This multiplicative scaling resulted in a better fit to the observed data than an additive shift, an effect that also held for the behaviorally conditioned distributions (Table S1). Finally, when comparisons are made between optogenetically- and behaviorally-conditioned NRTs, scaled optogenetic data are indistinguishable from behavioral NRT distributions (Figure 6D and E; p = 0.63, Opto-1s scaled vs Behav-7licks; p = 0.26, Opto-1s scaled vs Behav-10licks; p = 0.81, Opto-2s scaled vs Behav-7licks; p = 0.43, Opto-2s scaled vs Behav-10licks; Kolomogorov-Smirnov test). Therefore not only do optogenetically-conditioned NRTs exhibit a scale invariance, but they also express a distribution that is similarly shaped to that generated following behavioral conditioning.
Selective activation of cholinergic innervation within V1 conditions cued interval timing activity
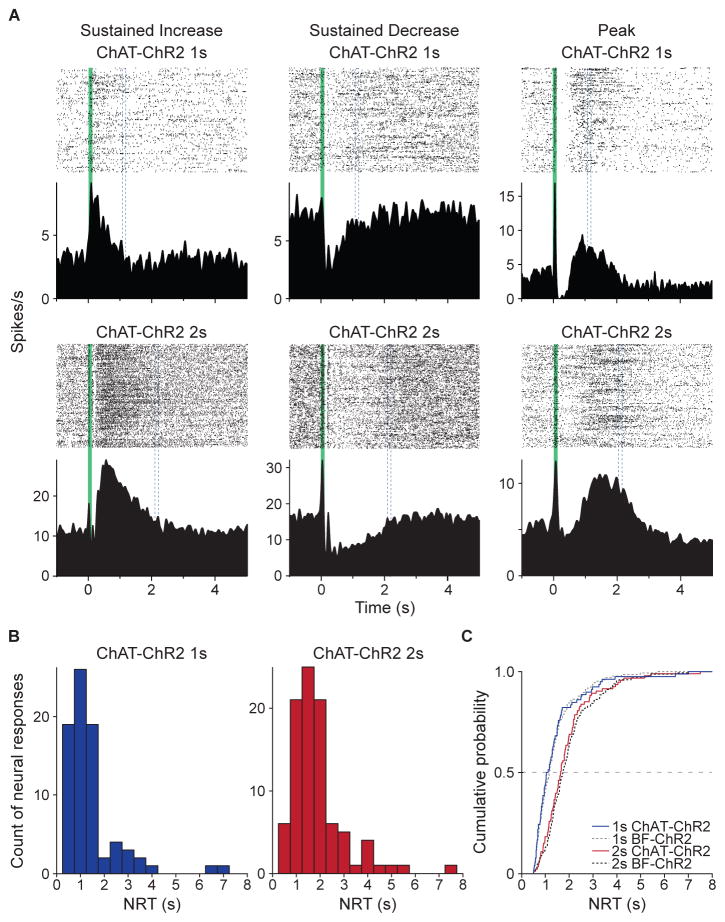
Given that we have previously shown that lesioning cholinergic basal forebrain innervation within V1 impairs the learning of reward timing [6, 7], might optogenetically commandeering cholinergic innervation of V1 be sufficient to educe cued interval timing activity? To test this, we employed a transgenic mouse line that expresses ChR2 under cholinergic-specific ChAT promoter control [37] to enable experimental manipulation of cholinergic innervation of V1. These animals (ChAT-ChR2) were then subjected to the same optogenetic conditioning protocol as before (Figure 2C), wherein visual stimulation is optogenetically-conditioned to either a one-second (ChAT-ChR2 1s, n = 3) or a two-second delay (ChAT-ChR2 2s, n = 4). Optogenetic conditioning appeared qualitatively to result in neuronal responses that exhibited each of the three identified response forms (Figure 7A) observed in V1 following either behavioral or BF→V1 conditioning. Further analysis revealed that 35.8 % (78 out of 218) of neural responses exhibited cued interval timing activity in the ChAT-ChR2 1s cohort, while 23.8% (93 out of 390) of neural responses were identified in the ChAT-ChR2 2s cohort. NRT distributions in these two cohorts (Figure 7B) of ChAT-ChR2 mice are significantly different (median = 1.08 s for ChAT-ChR2 1s, 1.65 s for ChAT-ChR2 2s; p = 0.00002, Wilcoxon rank-sum test). Optogenetic conditioning of cholinergic input resulted in NRT distributions that were indistinguishable, however, to their respective BF→V1 (i.e. BF-ChR2 in Figure 7C) conditioned counterparts (Figure 7C; ChAT-ChR2 vs BF-ChR2: p = 0.72 for 1s, 0.37 for 2s, Wilcoxon rank-sum test). In a control cohort of ChAT-ChR2 animals (n = 4) receiving only visual stimulation, we did not observe cued interval timing activity (only 11 of 194, or 5.7% of responses were identified by the response-form classification scheme). Therefore, cholinergic activation within V1 is sufficient to encode visually-cued timing activity to the conditioned intervals.
Figure 7.
Discussion
How brains learn and express the temporal interval between cues and expected outcomes is fundamental to the formation of adaptive behaviors, yet its physiological embodiment and mechanistic underpinnings are not well understood. Reward timing activity within V1 exemplifies the neural expression of learned temporal intervals of behavioral import associated with external cues. Its observation proffers the questions of where, how, and why this activity is generated. To facilitate a mechanistic investigation, we began by first establishing the presence of reward timing activity in mouse visual cortex, corroborating our prior findings in rat [6, 7] and affording a means of bringing putative reinforcement signals under experimental control using transgenic mice. Behavioral conditioning of visual cues with delayed reward induces in V1 post-stimulus activity—exhibiting three response forms—that correlates with the time of expected reward. We next demonstrated that selective activation of basal forebrain input within visual cortex is sufficient to condition stimulus-evoked neural responses that mimic behaviorally-conditioned reward timing activity. Optogenetically-conditioned V1 activity not only accords with conditioned intervals, but also is bidirectionally modifiable, updating to changes in cue-“reward” delay, and exhibits experience-dependent refinement. Further, similar to behaviorally-conditioned reward timing activity, we found that optogenetically-conditioned “reward” timing activity is scale-invariant to the interval encoded, a presumptive neuronal hallmark of the temporal scalar property [29, 30]. Finally, optogenetically commandeering cholinergic innervation within V1 is sufficient in and of itself to instill cued interval timing activity in vivo. Collectively, these observations advance an understanding of the neural mechanisms and properties of interval timing elicited by cues that are predictive of upcoming, behaviorally-relevant outcomes.
So where and how is this cued interval timing activity generated? Persistent or ramping activity recorded in higher-order brain regions (e.g. the frontal [38] and parietal [39] areas) bears resemblance to reward timing activity observed in V1 and has also been interpreted as relating behaviorally-relevant timing information [1, 3, 38, 39]. Therefore, it may reasonably be presumed that reward timing could be present in V1 simply as a consequence of V1 being privy, via feedback connections, to learning induced changes elicited elsewhere. Alternatively, rather than simply being the recipient of “top-down” information, V1 may itself actively transform input regarding visual cues into temporally extensive responses predictive of upcoming reward. Here we demonstrate that V1 is likely the site of learning the observed reward timing activity by selectively activating corticopetal innervation in V1 following visual stimulation, thereby engendering visually-cued interval timing that mimics reward timing activity. Therefore, as we have previously shown that lesioning of BF input impairs the ability of V1 to bidirectionally modify reward timing activity [6], our new results indicate that BF input is not only necessary but also sufficient for the bidirectional modification of cued interval timing. More fundamentally, selective activation of BF or cholinergic input is sufficient for the de novo synthesis of cued interval timing activity. That V1 is the site of learning this activity is consonant with BF and cholinergic-dependent cortical plasticity reported in primary sensory areas [6, 23–25, 40–42]. As it has recently been shown that both appetitive and aversive conditioning activates specific types of cortical neurons in primary sensory areas through cholinergic pathways [43–45], further investigation of the contribution of these neurons will provide a deeper understanding of the neural circuit involved in learning cued interval timing activity.
There is mounting evidence indicating behavioral state- and context-dependent modulation [35, 36, 46–48] of stimulus-evoked V1 responses. As BF projections are implicated in these processes [24, 35, 46, 49], a reasonable assumption is that they contribute to reward timing activity through modulating cortical states during task performance. However, as optogenetically-conditioned cued interval timing activity is entrained in the absence of a behavioral task, an accounting of observed interval timing activity based on behavioral state- and/or context-dependent modulation seems unlikely. In addition to the effects of gain modulation of visual responses by electrical stimulation of BF input [46] or optogenetic activation of cholinergic innervations in V1 [35], the results here indicate another role for BF and cholinergic projections in conveying the outcome associated with predictive visual cues, thereby instructing learning of the cue-reward interval.
To what end is this activity generated? We propose that cued interval timing in V1 signifies an internal model of the world is constructed that conveys temporal expectations of future, behaviorally-relevant events. In addition, two novel properties of cued interval timing in V1 revealed in this study—its experience-dependent refinement and scalar invariance—suggest that it is also possible that V1 participates in interval timing. Indeed, in contemporaneous work, we demonstrate that cued interval timing activity within V1 may inform the timing of visually cued actions by the animal in its effort to maximize reward [50]. These observations lend behavioral relevance to investigating the mechanism by which cued interval timing activity comes about in V1.
In summary, V1 provides a model system in which the neural genesis of temporal intervals of behavioral import may be investigated. We demonstrate that basal forebrain or cholinergic innervation of V1 is sufficient for conditioning cued interval timing activity, and that V1 itself is a substrate for learning and expressing cue-reward intervals. These findings corroborate a formal framework [8, 31, 32] for learning reward timing, a domain commonly considered the province of “higher” cortical regions, and advance a mechanistic understanding of reinforcement [5] and associative learning.
Experimental Procedures
Animals
All animal procedures were conducted in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by The Johns Hopkins University Institutional Animal Care and Use Committee. Adult male C57Bl/6 mice (≥ P30; Jackson Laboratory) were used for all behavioral and optogenetic experiments. ChAT-ChR2 mice for optogenetic experiments were derived from Dr. Guoping Feng at the Massachusetts Institute of Technology [37]. Animals were housed in a satellite facility with 12-h dark/12-h light cycle control, in which lights were provided from 07:00 to 19:00.
Surgical procedures
Animal surgeries were performed in accordance to the guidelines from The Johns Hopkins University Institutional Animal Care and Use Committee. All surgeries were conducted under sterile conditions with ketamine/xylazine anesthesia (50/10 mgkg−1, intraperitoneal). For animals used in behavioral conditioning experiments, custom-made 16-channel microdrives for single-unit activity recordings were implanted in the binocular segment of V1 [51]. In optogenetic conditioning experiments, virally infected animals (see below) and ChAT-ChR2 transgenic mice were implanted with multichannel recording arrays coupled to optical fibers (200 μm in core diameter, NA = 0.22, A.R.T. Photonics, Germany) for blue laser-light delivery in V1. The effects of BF→V1input-activation on cued interval timing were compared to a control cohort of infected animals presented with the same visual stimuli but conditioned by optogenetic activation of BF input within primary somatosensory cortex (S1, see below). Implanting and recording sites were confirmed histologically.
To achieve channelrhodopsin (ChR2) expression, adeno-associated virus (AAV2, serotype 8) carrying the ChR2:Venus cassette (ChR2:Venus is from Addgenge plasmid 15753, pCAGG-ChR2:Venus; DNA cloned by Rachael Neve at the MIT Viral Vector Core into Virovek’s plasmid backbone pFB-AAV-CMV-SV40 and packaged by Virovek, titer ~ 1e12 viral particles/ml) was injected into basal forebrain (BF) nuclei using a Nanoject II (Drummond Scientific). The stereotaxic coordinates (from bregma) for targeted areas [52] are as follows: 1) nucleus basalis/substantia innominata (NB/SI), 0.55 mm posterior; 1.75 mm lateral; 4.2 mm and 3.8 mm ventral; 2) ventral limb of the diagonal band, 0.75 mm anterior; 0.18 mm lateral; 4.55 mm and 4.05 mm ventral; 3) horizontal limb of the diagonal band, 0.5 mm anterior; 0.9 mm lateral; 5.2 mm and 5 mm ventral. At each penetration depth, 276 nl of viral solution was injected. In a control group of ChR2-infected animals, visual stimulation was conditioned with delayed (2 s) activation of BF input in S1 by optical fibers implanted in stereotaxic coordinates corresponding to S1 (from bregma: 1.94 mm posterior; 3.2 mm lateral; 0.3 mm ventral). Another group of animals were injected with AAV2/8-CMV-GFP and used as controls for optogenetic experiments. ChR2:Venus and GFP expression in BF, BF→V1, and BF→S1 projections were histologically verified.
To verify the infection specificity and efficiency of V1-projecting neurons in BF, a cohort of animals receiving AAV (n = 3 with ChR2:Venus and n = 1 with GFP) infection using the same protocol were injected with the retrograde tracer fluorogold (FG, Sigma-Aldrich; 2.0% in PBS, 200 nl) in V1 at sites corresponding to the location of electrode implants (Figure S4D). Serial sections containing BF nuclei (Figure S4D and E) were selected for calculating the percentage of retrogradely-labeled V1 neurons with ChR2:Venus or GFP expression. We found that 54 ± 5 % of FG-labeled neurons in BF (50 ± 8 % in NB/SI, 61 ± 6 % in VDB and HDB) were infected by the protocol. Conversely, we were unable to detect AAV-mediated protein expression in areas exhibiting strong FG-labeling outside of BF (Figure S4F).
Electrophysiology
Data from extracellularly recorded neural signals were digitally sampled at 33 kHz and stored by the Digital Lynx (Neuralynx Inc.) system. Single-unit activity from band-pass filtered (1–10 kHz) signals was isolated using commercial software (OfflineSorter, Plexon). Experimental events during behavioral (visual stimulations and reward deliveries) and optogenetic (visual stimulations and laser illuminations) conditioning were output from a custom-assembled module and controlled by user-defined MATLAB (MathWorks) programs. Timestamps for these events were collected by Digital Lynx, and peri-stimulus time histograms of spiking activity were compiled and displayed using the NeuroExplorer (Pelxon) software. Data analysis was accomplished by programs written in MATLAB.
Behavioral conditioning
Implanted animals were allowed 5–7 days to recover post-surgery before being subjected to water restriction in their home cages. A schedule for water restriction was carefully designed and closely monitored so that the weights of deprived animals were maintained at > 90% of their initial body weights. Behavioral conditioning for the reward timing task was performed similarly to the protocol previously described [7]. In brief, water-deprived animals received full-retinal illuminations (100 ms) to either the left- or right-eye through a pair of head-mounted goggles as the conditioning cues when accessing the nose-poke in the training apparatus. Solenoid-controlled delivery of water was achieved by licking the reward port for the designated number of times. The required number of licks for reward administration is the same for both left- and right-eye stimulation in each animal. Equal numbers of rewarded and unrewarded (catch) trials were pseudo-randomly presented to the animal. While mice initially exhibited excessive licking behavior in interleaved unrewarded trials, they learned to reduce the number of licks emitted following the required number to receive reward (Figure S1A). We used only data recorded from unrewarded trials for response-form classification and NRT scoring (see Data analysis).
Optogenetic conditioning
The timeline for optogenetic conditioning experiments is schematized in Figure 2A. Animals were allowed food and water access ad libitum in their home cages post-infection. We found that a minimal of 3 weeks post-infection was required for detectable ChR2:Venus expression in V1. ChR2 was activated by blue laser (473 nm, 500–650 μW, 100 ms) delivered through implanted optical fiber (350 ± 50 μm below pia). When optogenetically conditioned, animals were placed in the same training apparatus used for behavioral conditioning and allowed to explore freely. Comparable numbers of four different trial types (Figure 2C) were pseudo-randomly presented, and separated by randomized inter-trial intervals ranging between 5 to 7 seconds. To activate ChR2 expressed on BF→V1 input, brief (100 ms) illumination of blue laser light (473 nm) was delivered through locally implanted optical fiber in V1. Laser stimulation appeared to transiently modulate firing rates in ChR2-infected animals (Figure S6), but not in GFP-infected controls. Scored by visual inspection, approximately half (368/704) of neurons recorded from ChR2-infected animals displayed laser-modulated activity changes, indicating efficacious optogenetic control of BF→V1 input. To calculate optogenetically-conditioned neural reports of time (see below), we scored data from the trials in which laser-stimulation was withheld following visual stimulation (the goggle-only, “catch” trials). The same laser stimulation protocol was used for the control cohort with S1 optical fiber-implantation.
Data analysis: Detection, classification, and the determination of neural reports of time (NRT)
In order to identify, classify, and score cue-evoked timing activity, we first selected a subset of neural responses characteristic of the three previously described [7] reward timing response forms (n = 95 for sustained increase, 117 for sustained decrease, and 35 for peak responses; Figure S2A) to constitute a template for objective response form identification and classification. The post-stimulus (> 500 ms following visual stimulation) activity of these 247 neural responses was subjected to principal component (PC) analysis. Projecting the template data in a space defined by the first four PCs (which accounted for > 70% of the total variance, Figure S2B) resulted in clusters populated, predominantly, by responses that had been manually classified as sustained increase, sustained decrease and peak responses (as illustrated by the projection pattern in the first three PCs, Figure S2C). In order to achieve objective data classification with a Bayesian decision method, we deduced a template corresponding to the individual clusters from the mean and covariance matrices by assuming within-cluster multivariate Gaussian distributions of data [53]. Overall, this template categorized 87.4% (216/247) of the selected data into classes that agreed with response forms as manually assigned (Figure S2D).
This template was subsequently used to detect and classify putative reward timing responses from all of the data collected. Objective membership assignment of response form was achieved by determining the relative probabilities of a neural response being associated with the three individual clusters in the template. Setting a threshold of 75% relative probability from template clusters resulted in categorization that accorded well with subjective classification achieved through visual inspection while separating neural responses with putative timing features from those with no discernible cue-evoked activity or with only transient stimulus-evoked responses (as examples in Figure S5).
Following response-form classification, we determined the neural report of time (NRT) for each classified response. This was done by first determining the spontaneous activity level, characterized as a bounded range (defined by the upper and lower threshold in Figure S3A) that encompassed the pre-stimulus firing-rate fluctuations. The bounded range was set to capture 95% of the distribution of pre-stimulus firing-rates in optogenetic experiments. As animals performed fewer trials during behavioral conditioning, this range was set to contain 85% of the distribution of pre-stimulus firing-rates in behavioral experiments. This bounded range served to determine moments in time in which the post-stimulus activity was comparable to its pre-stimulus firing rate. In order to exclude from the analysis the contribution of spike modulation driven directly by the stimulus itself, we limited NRT analysis to data collected > 500 ms from stimulus onset. For the sustained increase response form, the NRT algorithm identified the first post-stimulus moment in which the firing rate crossed below the upper threshold of pre-stimulus activity and remained so for at least 100 ms (Figure S3A1). Similarly, for the sustained decrease response form (Figure S3A2), we assessed the first post-stimulus moment in which the firing rate crossed above the lower threshold and remained so for at least 100 ms. For the peak response form, the NRT algorithm determined the first post-stimulus time window (continuous for ≥ 200 ms) during which firing rates were outside of the bounded range, and set the NRT as the moment of maximal deviation from spontaneous firing within this window (Figure S3A3). We tested the reliability of the results determined by this algorithm, and found that NRTs are robust to changes in the Gaussian filtering of the neural responses (Figure S3B).
Supplementary Material
Highlights.
Reward timing activity can be behaviorally conditioned in mouse V1
Activating BF or cholinergic innervation conditions cued interval timing
Cued interval timing in V1 is bidirectionally modifiable and is scale invariant
V1 is a substrate for reinforcement learning and expression of temporal intervals
Acknowledgments
This work was supported by NIH (R01MH084911 & R01MH093665) and a grant from the Brain Science Institute at Johns Hopkins. We thank R. Gujarati, D. Reyes-Capo, D. Sundermann, M. Szaro, and H. Zhang for assisting in experimental setup and data collection, and M. Bear, R. Cudmore, E. Hughes, D. Nguyen, and H. Shouval for discussions and comments on the manuscript. We also thank H. Zhang for assistance on histology, and G. Feng for providing ChAT-ChR2 mouse line.
Footnotes
Supplemental Information includes seven figures and one table can be found with this article online.
Author Contributions
The experiments were conceived and designed by M.G.H.S and C.-H.L. C.-H.L performed the experiments, collected and analyzed the data. H.D and K.Z contributed to data analysis and software programming. J.E.C provided reagents for optogenetic experiments, and assisted in anatomical and histological analyses. M.G.H.S and C.-H.L wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leon MI, Shadlen MN. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron. 2003;38:317–327. doi: 10.1016/s0896-6273(03)00185-5. [DOI] [PubMed] [Google Scholar]
- 2.Mauk MD, Steinmetz JE, Thompson RF. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:5349–5353. doi: 10.1073/pnas.83.14.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mita A, Mushiake H, Shima K, Matsuzaka Y, Tanji J. Interval time coding by neurons in the presupplementary and supplementary motor areas. Nature neuroscience. 2009;12:502–507. doi: 10.1038/nn.2272. [DOI] [PubMed] [Google Scholar]
- 4.Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dayan P, Niv Y. Reinforcement learning: the good, the bad and the ugly. Curr Opin Neurobiol. 2008;18:185–196. doi: 10.1016/j.conb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Chubykin AA, Roach EB, Bear MF, Shuler MG. A cholinergic mechanism for reward timing within primary visual cortex. Neuron. 2013;77:723–735. doi: 10.1016/j.neuron.2012.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuler MG, Bear MF. Reward timing in the primary visual cortex. Science. 2006;311:1606–1609. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- 8.Gavornik JP, Shuler MG, Loewenstein Y, Bear MF, Shouval HZ. Learning reward timing in cortex through reward dependent expression of synaptic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6826–6831. doi: 10.1073/pnas.0901835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton RS, Barto AG. Reinforcement learning. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- 10.Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull. 1982;8:727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- 11.Carey RG, Rieck RW. Topographic projections to the visual cortex from the basal forebrain in the rat. Brain Res. 1987;424:205–215. doi: 10.1016/0006-8993(87)91463-6. [DOI] [PubMed] [Google Scholar]
- 12.Moreau PH, Cosquer B, Jeltsch H, Cassel JC, Mathis C. Neuroanatomical and behavioral effects of a novel version of the cholinergic immunotoxin mu p75-saporin in mice. Hippocampus. 2008;18:610–622. doi: 10.1002/hipo.20422. [DOI] [PubMed] [Google Scholar]
- 13.Rieck R, Carey RG. Evidence for a laminar organization of basal forebrain afferents to the visual cortex. Brain Res. 1984;297:374–380. doi: 10.1016/0006-8993(84)90579-1. [DOI] [PubMed] [Google Scholar]
- 14.Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13:627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 15.Masuda R, Fukuda M, Ono T, Endo S. Neuronal responses at the sight of objects in monkey basal forebrain subregions during operant visual tasks. Neurobiol Learn Mem. 1997;67:181–196. doi: 10.1006/nlme.1996.3756. [DOI] [PubMed] [Google Scholar]
- 16.Richardson RT, DeLong MR. Electrophysiological studies of the functions of the nucleus basalis in primates. Adv Exp Med Biol. 1991;295:233–252. doi: 10.1007/978-1-4757-0145-6_12. [DOI] [PubMed] [Google Scholar]
- 17.Wilson FA, Ma YY. Reinforcement-related neurons in the primate basal forebrain respond to the learned significance of task events rather than to the hedonic attributes of reward. Brain research. Cognitive brain research. 2004;19:74–81. doi: 10.1016/j.cogbrainres.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Robinson BW, Mishkin M. Alimentary responses to forebrain stimulation in monkeys. Exp Brain Res. 1968;4:330–366. doi: 10.1007/BF00235700. [DOI] [PubMed] [Google Scholar]
- 19.Fekete M, Bohus B, Van Wolfswinkel L, Van Ree JM, De Wied D. Comparative effects of the ACTH 4-9 analogue (ORG 2766), ACTH 4-10 and [D-Phe7] ACTH 4-10 on medial septal self-stimulation behaviour in rats. Neuropharmacology. 1982;21:909–916. doi: 10.1016/0028-3908(82)90083-1. [DOI] [PubMed] [Google Scholar]
- 20.Coyle JT, Price DL, DeLong MR. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 21.Dekker AJ, Connor DJ, Thal LJ. The role of cholinergic projections from the nucleus basalis in memory. Neurosci Biobehav Rev. 1991;15:299–317. doi: 10.1016/s0149-7634(05)80008-9. [DOI] [PubMed] [Google Scholar]
- 22.Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- 24.Kang JI, Vaucher E. Cholinergic pairing with visual activation results in long-term enhancement of visual evoked potentials. PLoS One. 2009;4:e5995. doi: 10.1371/journal.pone.0005995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 26.Lin SC, Nicolelis MA. Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron. 2008;59:138–149. doi: 10.1016/j.neuron.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manns ID, Alonso A, Jones BE. Rhythmically discharging basal forebrain units comprise cholinergic, GABAergic, and putative glutamatergic cells. Journal of neurophysiology. 2003;89:1057–1066. doi: 10.1152/jn.00938.2002. [DOI] [PubMed] [Google Scholar]
- 28.Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Ann N Y Acad Sci. 1999;877:339–367. doi: 10.1111/j.1749-6632.1999.tb09276.x. [DOI] [PubMed] [Google Scholar]
- 29.Gallistel CR, Gibbon J. Time, rate, and conditioning. Psychological review. 2000;107:289–344. doi: 10.1037/0033-295x.107.2.289. [DOI] [PubMed] [Google Scholar]
- 30.Gibbon J, Malapani C, Dale CL, Gallistel C. Toward a neurobiology of temporal cognition: advances and challenges. Curr Opin Neurobiol. 1997;7:170–184. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- 31.Gavornik JP, Shouval HZ. A network of spiking neurons that can represent interval timing: mean field analysis. J Comput Neurosci. 2011;30:501–513. doi: 10.1007/s10827-010-0275-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shouval HZ, Gavornik JP. A single spiking neuron that can represent interval timing: analysis, plasticity and multi-stability. J Comput Neurosci. 2011;30:489–499. doi: 10.1007/s10827-010-0273-0. [DOI] [PubMed] [Google Scholar]
- 33.Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- 34.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nature neuroscience. 2013 doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu M, Zhang SY, Dan Y, Poo MM. Representation of interval timing by temporally scalable firing patterns in rat prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:480–485. doi: 10.1073/pnas.1321314111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, et al. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nature methods. 2011;8:745–752. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 39.Janssen P, Shadlen MN. A representation of the hazard rate of elapsed time in macaque area LIP. Nature neuroscience. 2005;8:234–241. doi: 10.1038/nn1386. [DOI] [PubMed] [Google Scholar]
- 40.Miasnikov AA, Chen JC, Weinberger NM. Specific auditory memory induced by nucleus basalis stimulation depends on intrinsic acetylcholine. Neurobiol Learn Mem. 2008;90:443–454. doi: 10.1016/j.nlm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sachdev RN, Lu SM, Wiley RG, Ebner FF. Role of the basal forebrain cholinergic projection in somatosensory cortical plasticity. Journal of neurophysiology. 1998;79:3216–3228. doi: 10.1152/jn.1998.79.6.3216. [DOI] [PubMed] [Google Scholar]
- 42.Shulz DE, Ego-Stengel V, Ahissar E. Acetylcholine-dependent potentiation of temporal frequency representation in the barrel cortex does not depend on response magnitude during conditioning. Journal of physiology, Paris. 2003;97:431–439. doi: 10.1016/j.jphysparis.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Alitto HJ, Dan Y. Cell-type-specific modulation of neocortical activity by basal forebrain input. Frontiers in systems neuroscience. 2012;6:79. doi: 10.3389/fnsys.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- 45.Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nature neuroscience. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szuts TA, Fadeyev V, Kachiguine S, Sher A, Grivich MV, Agrochao M, Hottowy P, Dabrowski W, Lubenov EV, Siapas AG, et al. A wireless multi-channel neural amplifier for freely moving animals. Nature neuroscience. 2011;14:263–269. doi: 10.1038/nn.2730. [DOI] [PubMed] [Google Scholar]
- 49.Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Namboodiri VMK, Huertas M, Monk KJ, Shouval HZ, Hussain Shuler MG. Visually-cued action timing in the primary visual cortex. Neuron. 2015 doi: 10.1016/j.neuron.2015.02.043. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu CH, Heynen AJ, Shuler MG, Bear MF. Cannabinoid receptor blockade reveals parallel plasticity mechanisms in different layers of mouse visual cortex. Neuron. 2008;58:340–345. doi: 10.1016/j.neuron.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 52.Franklin KBJPG. The Mouse brain in stereotaxic coordinates. 3 2008. [Google Scholar]
- 53.Duda RE, Hart PE, Stork DG. Pattern Classification. 2. New York: Wiley; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.