Abstract
General use and popularity of over-the-counter supplemental antioxidants have rapidly spread all over the world and are believed to promote cardiovascular health and wellbeing. However, there is a paucity of information and lack of proof that physiological and above-physiological levels of oxidants do harm at the cellular and organismal levels. Instead, several reports demonstrated that reduction in Reactive Oxygen Species (ROS) did not improve vascular function. Interestingly, recent studies show that increased ROS levels play protective role in vascular endothelium and may improve coronary endothelial function. In the current review, we introduce the concept that increased ROS levels, often seen in association with cardiovascular disease, probably is an endothelial-way or ‘oxidative response’ to cope with vascular pathology.
Keywords: Antioxidants, Coronary artery disease, Oxidative stress, Endothelial health, Oxidative response
Introduction
Vascular health depends on both structural and functional well-being of the blood vessels. Pathological changes can take place at the level of vascular endothelium, vascular smooth muscle cells (VSMC) and connective tissue surrounding the blood vessels [1–4]. Decreased bioavailability of nitric oxide (NO), resulted from decreased synthesis of NO, reduced activation of endothelial nitric oxide synthase (eNOS) or increased quenching of NO by reactive oxygen species (ROS) [5], is believed to be one of the major determinants of microvascular endothelial dysfunction in aging, hypertension, diabetes, hyperlipidemia, and smoking [6–9]. Other pathological changes that follow endothelial dysfunction may include increase in microvascular tone, neointimal thickening, myogenic hypertrophic remodeling of resistance arterioles and small arteries [2,10].
Oxidants and vascular health
The notion that increased levels of reactive oxygen species (ROS) are detrimental to cardiovascular health has come into being for several reasons (Figure 1). Higher levels of ROS are often observed with microvascular pathology in Cardiovascular Diseases (CVD) including Coronary Artery Disease (CAD) and Ischemic Heart Disease (IHD) [11–15]. At first, these observations helped establish the paradigms that reduction in ROS levels in the vessel walls should improve cardiovascular functions [16]. However, several clinical trials using antioxidants, e.g. Alpha-Tocopherol Beta-Carotene (ATBC), Heart Outcomes Prevention Evaluation (HOPE) [17–22], have produced negative results in reducing primary endpoints of cardiovascular mortality and morbidity [17,23–26]. Other studies using animal models and/or antioxidants in Endothelial Cells (ECs) demonstrated that reduction in ROS levels failed to improve vascular functions [27–29]. Recent reports from several groups showed that ROS reduction resulted in the disruption of signal transduction leading to reduced Nitric Oxide (NO) generation in vascular endothelium [30–32] and decreased vasodilatation [30,31]. Surprisingly, a recent report demonstrated that increase in EC-specific ROS induces AMPK-eNOS-mediated endothelium-dependent coronary vasodilatation, and AMPK-mTOR-mediated protective autophagy [33].
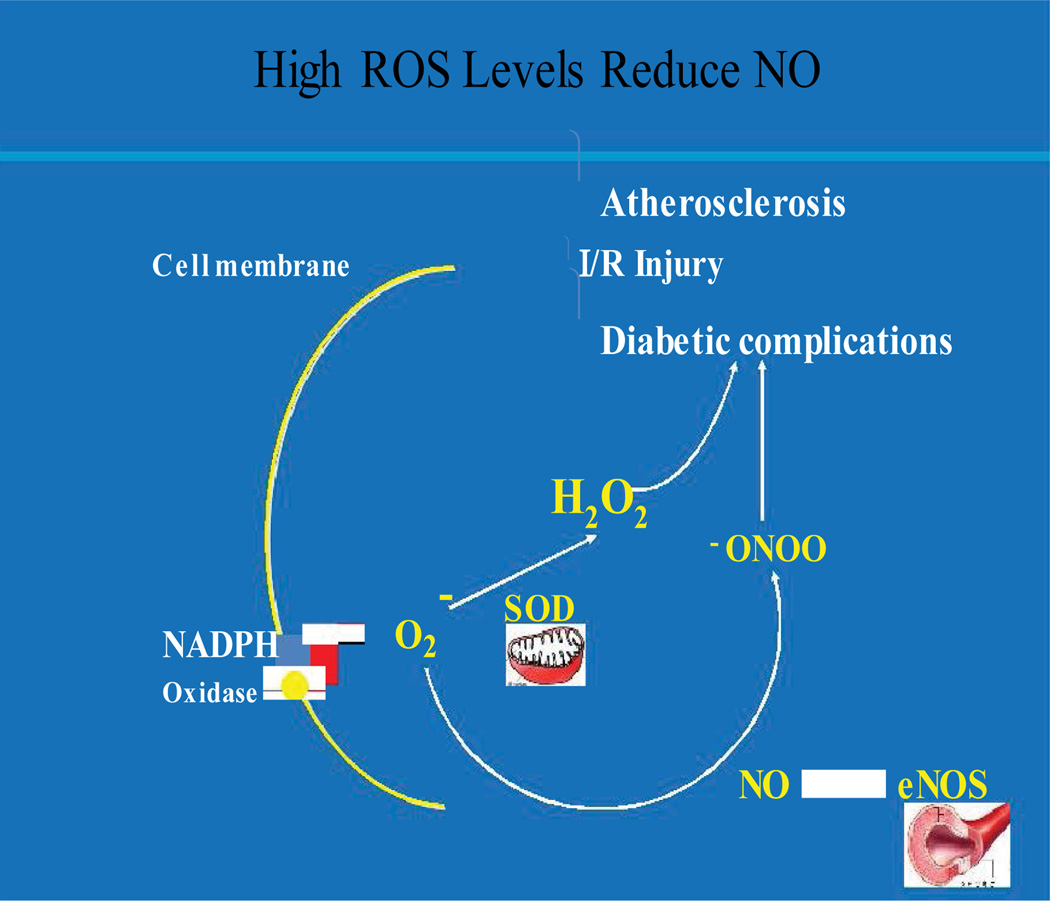
Figure 1. Sources of Endothelial ROS and NO.
It also demonstrates how increased ROS may tip the balance of NO vs. ONOO in vascular pathological conditions.
The redox paradox
So, why there is still a notion that ROS are harmful for cardiovascular health? There is no simple answer to this question. One reason would be that (i) ROS are found at increased levels in pathological condition involving cardiovascular system including CAD, myocardial ischemia, and myocardial infarct; (ii) another may be that our initial understanding of ROS is associated with their bactericidal effects in phagocytes, and (iii) the fact that there are many in vitro studies using ROS-inducing chemical agents that demonstrated apoptosis and other damages in vascular cells including ECs and VSMCs. If we look carefully and systematically, we will find that the first ‘reason’ is simple association, the second one does not take cell type and phenotypic differences into consideration, and the last one can simply be nonspecific effects of the so-called ROS-inducing chemicals that may have several other ‘indirect’ effects on vascular cell signal transduction, cell cycle and/or metabolism. It is thus critical to examine the sources and functions of vascular ROS. We will mostly focus on EC-specific ROS in the current review. There are several sources for intracellular ROS in EC including NADPH oxidases, mitochondria, cytochrome P450 and xanthine oxidase. The multi-subunit NADPH oxidase, which contains membrane-bound gp91phox (Nox2) and p22phox subunits, and cytosolic p47phox, p67phox and Rac1, is a major source of endothelial ROS [34–36]. NADPH oxidase is present in different subcellular compartments in ECs including cell and perinuclear membrane, and endoplasmic reticulum (ER) [36,37]. Several other NADPH oxidases e.g. nox4, nox1, nox5 are also present in ECs [38–42].
Recent work from others and our labs has shown a critical role for NADPH oxidase-derived ROS in the activation of downstream eNOS to synthesize NO [28,42–47]. Taken together, above findings suggest that NADPH oxidase-derived ROS play an important role in survival, health and growth of vascular ECs. We are yet to understand the precise mechanisms involving ROS-mediated signal transduction in ECs. Several reports showed that oxidants play crucial roles by activating signaling intermediates including PI3K-Akt-eNOS, PLCγ1, PKC and ERK1/2 in ECs [27,43,48]. Recently, pro-survival kinase AMPK that becomes activated during cellular stress including starvation and reduction in AMP/ATP ratio, has been shown to be regulated by ROS produced during hypoxia and fluid shear stress in ECs [49–51]. AMPK is involved in regulating a number of signaling intermediates and transcription factors including FOXO1, HIF-1α and PGC-1α [52–58], resulting in increased EC survival and proliferation (Figure 2). The protective role of AMPK is carried out through its regulatory role in autophagy, a process crucial for cell survival [51,59–62]. Autophagy helps recycle and re-utilize damaged macromolecules and organelles using lysosomal degradation pathway [59,63]. In a recent study from our lab using increase in EC-specific endogenous ROS in adult animals (a novel binary Tet-ON/OFF transgenic mouse) that induces 1.8 ± 0.42-fold increase in Nox2/gp91phox (NADPH oxidase 2)-derived ROS, we demonstrated that EC-ROS induced AMPK-eNOS-mediated endothelium-dependent coronary vasodilatation and AMPK-mTOR-mediated protective autophagy in EC [33]. However, several studies were performed using cultured ECs in vitro, global knockdown animal models of NADPH oxidase (p47phox−/− or gp91phox−/−), or constitutive overexpression of NADPH oxidases (e.g. Nox4) in ECs [28,35,43,44,64–66] that resulted in a wide variety of conclusions ranging from essential to harmful roles for ROS. These approaches, although yielding important information, may have precluded precise determination of endothelial contribution for redox-sensitive modulation of vascular functions. In brief, a reductionist view or an all-or-none theory may not apply to the roles for ROS in vascular function. One has to take the source, subcellular localization, intensity and temporal state of the redox molecule into consideration while studying the roles/effects of ROS in/on vascular function.
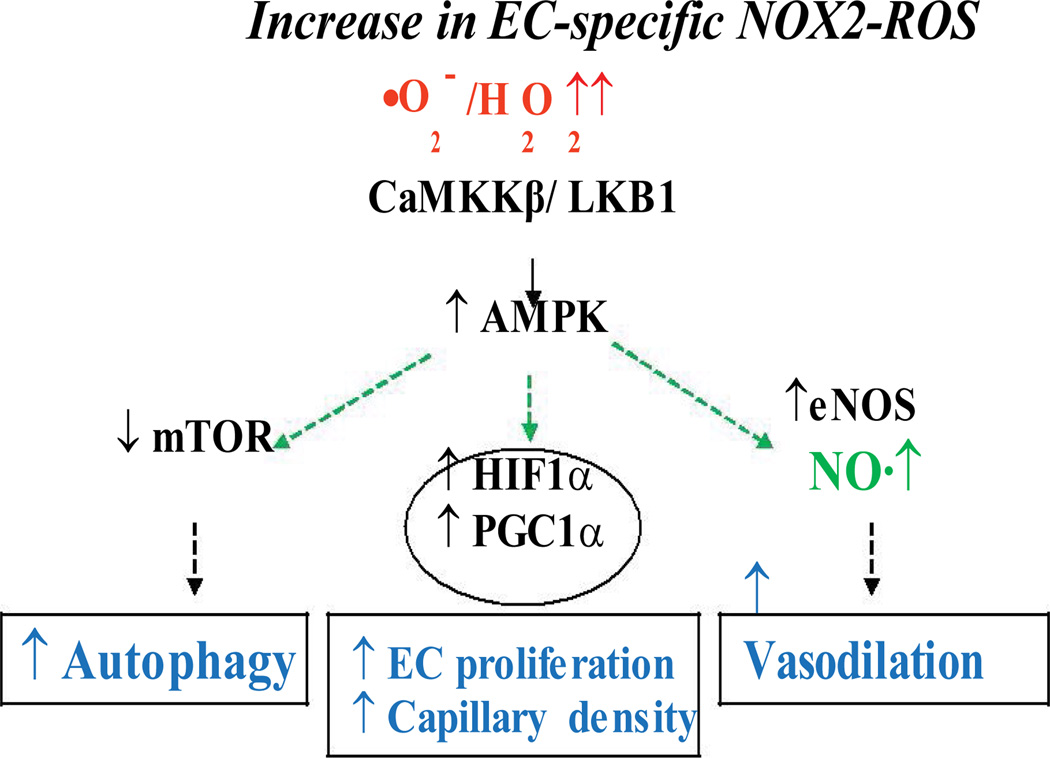
Figure 2. Model for EC-specific ‘Oxidative Response’ to improve endothelial function.
NADPH oxidase-derived ROS activates CaMKKβ-AMPK, which in turn, activates eNOS to induce NO-mediated vasodilatation and inhibits mTOR resulting in protective autophagy in vascular endothelium.
Oxidative stress or oxidative compensation?
One may ask why does the prevailing dogma still support a negative role for ROS? To address this question, we need to go back to the origin of the oxidative stress ‘theory’ in cardiovascular system. We must remember that the notion that increased levels ROS are detrimental to cardiovascular health has come into being for ROS’ association with several different cardiovascular pathology including CAD, myocardial ischemia-reperfusion, and myocardial infarct. We know that the most potent way myocardium employs to defend itself from ischemic insults is by preserving the existing capillary endothelial cells (EC) and/or by inducing growth of coronary blood vessels in the ischemic area [67]. Once the ischemic insult has occurred, survival of the affected cardiac tissue depends on the speed with which coronary vessels can increase blood flow through alternate means, namely vasodilatation, increase in vessel density, and/or preservation of the existing microvessels. Given the recent findings by others and our lab (as mentioned above), we propose a novel concept that vascular endothelium addresses this critical issue by attempting to ‘pre-condition’ the coronary vessels for better vasodilatory and angiogenic response by increasing oxidant levels in the vascular cells. This concept, a ‘compensatory’ role for ROS, is in contrast to the prevailing dogma and suggests that redox positively regulates endothelial signaling and selective vascular functions [30,31,68–72] in health and disease. This novel ‘oxidative compensation’ concept also derives its support from the findings that NADPH oxidase-derived ROS, a major source of EC-ROS, have also been shown to play crucial role in vascular endothelial growth factor (VEGF) signaling and coronary vascular functions [30,31,70]. As a molecular mechanism, it was shown that c-Src activation and its interaction with VEGFR-2 were dependent on the redox-mediated thiol oxidation of c-Src (sulphenic acid modification) in human coronary artery ECs [27]. Recent findings that increase in endogenous EC-ROS enhance protective autophagy by activating AMPK further supports a pre -conditioning or compensatory role for ROS to lead ECs towards a pro-survival mode (Figure 2). AMPK is also known to be activated in a state of calorie restriction. Caloric restriction or nutrition deprivation slows down energy-consuming processes and induces autophagy to provide amino acids for the synthesis of essential proteins, which in turn improves cell survival and inhibits apoptosis under stressful conditions including high oxidant state in many cell types. Autophagy is essential for cellular survival, homeostasis, differentiation, and tissue remodeling in pathophysiological condition. Depending on the pathophysiological settings, autophagy may play a protective role or contribute to cell damage. Thus, our recent findings demonstrating increased ROS levels elicit a ‘caloric restriction’-like response (AMPK-mediated mTOR inhibition and induction of autophagy) in endothelium may also have important clinical implication [33] and may further support the notion that ROS play a compensatory role during vascular insult. Activation of AMPK and induction of autophagy may well be a mechanism by which endothelium copes with higher redox state by slowing down endothelial metabolism and/or by recycling the oxidant-damaged cellular organelles. This compensatory mechanism may more appropriately be termed as ‘oxidative response’ of vascular endothelium. Future studies are required to address the tissue- and vascular bed-specific response to oxidants, and the outcomes of these studies are likely to bring a major shift in our attitude towards the high ROS levels that are found in many microvascular diseases and may generate enthusiasm to examine whether high oxidant level in the microvascular wall in IHD/CAD and other microvascular pathophysiology is an undesirable by-product (resulting in oxidative stress) or a homeostatic response (oxidative response) to an inflammatory environment. The delicate balance between the beneficial (oxidative response) and detrimental (oxidative stress) roles of ROS may be compartmentalized within the cell (subcellular localization). In addition, different tissues and vascular beds may respond to oxidants differently depending on the types of oxidants (O2, H2O2, HO2, ONOO), their subcellular source/localization, intensity and duration of the exposure. Outcomes of the studies addressing these critical issues will help guide investigators whether or not to interfere with redox levels (e.g. by antioxidants) in coronary and other microvascular disease, which if used inadvertently, may paradoxically affect redox-dependent signaling and may thus predispose patients to further ischemia. In conclusion, one should not consider increased ROS level as an “all or none” phenomenon or as oxidative stress. Each condition with increased oxidant levels should be assessed independently before initiating any antioxidant therapy, because interference with oxidative response may do more harm than good.
Acknowledgement
This work was supported by the NIH/NIGMS grant 1P20GM103652 (Project-3) and the AHA grant 14GRNT20460291 to MRA, and NIH grant HL46716 to FWS.
References
- 1.Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation. 1995;91:1432–1443. doi: 10.1161/01.cir.91.5.1432. [DOI] [PubMed] [Google Scholar]
- 2.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 3.Pritchard KA, Ackerman AW, Ou J, Curtis M, Smalley DM, et al. Native low-density lipoprotein induces endothelial nitric oxide synthase dysfunction: role of heat shock protein 90 and caveolin-1. Free radical biology & medicine. 2002;33:52–62. doi: 10.1016/s0891-5849(02)00851-1. [DOI] [PubMed] [Google Scholar]
- 4.Rudic RD, Sessa WC. Nitric oxide in endothelial dysfunction and vascular remodeling: clinical correlates and experimental links. American journal of human genetics. 1999;64:673–677. doi: 10.1086/302304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MY, Griendling KK. Redox signaling, vascular function, and hypertension. Antioxidants & redox signaling. 2008;10:1045–1059. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orr WC, Arnold LA, Sohal RS. Relationship between catalase activity, life span and some parameters associated with antioxidant defenses in Drosophila melanogaster. Mechanisms of ageing and development. 1992;63:287–296. doi: 10.1016/0047-6374(92)90006-y. [DOI] [PubMed] [Google Scholar]
- 7.Belik J, Jerkic M, McIntyre BA, Pan J, Leen J, et al. Age-dependent endothelial nitric oxide synthase uncoupling in pulmonary arteries of endoglin heterozygous mice. Am J Physiol Lung Cell Mol Physiol. 2009;297:1170–1178. doi: 10.1152/ajplung.00168.2009. [DOI] [PubMed] [Google Scholar]
- 8.Bauersachs J, Bouloumie A, Mulsch A, Wiemer G, Fleming I, et al. Vasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO synthase III and soluble guanylyl cyclase expression, and in superoxide anion production. Cardiovascular research. 1998;37:772–779. doi: 10.1016/s0008-6363(97)00250-2. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton SJ, Watts GF. Endothelial dysfunction in diabetes: pathogenesis, significance, and treatment. Rev Diabet Stud. 2013;10:133–156. doi: 10.1900/RDS.2013.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feihl F, Liaudet L, Levy BI, Waeber B. Hypertension and microvascular remodelling. Cardiovasc Res. 2008;78:274–285. doi: 10.1093/cvr/cvn022. [DOI] [PubMed] [Google Scholar]
- 11.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datla SR, Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension. 2010;56:325–330. doi: 10.1161/HYPERTENSIONAHA.109.142422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo Z, Teerlink T, Griendling K, Aslam S, Welch WJ, et al. Angiotensin II and NADPH oxidase increase ADMA in vascular smooth muscle cells. Hypertension. 2010;56:498–504. doi: 10.1161/HYPERTENSIONAHA.110.152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willcox BJ, Curb JD, Rodriguez BL. Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am J Cardiol. 2008;101:75D–86D. doi: 10.1016/j.amjcard.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 19.Virtamo J, Rapola JM, Ripatti S, Heinonen OP, Taylor PR, et al. Effect of vitamin E and beta carotene on the incidence of primary nonfatal myocardial infarction and fatal coronary heart disease. Arch Intern Med. 1998;158:668–675. doi: 10.1001/archinte.158.6.668. [DOI] [PubMed] [Google Scholar]
- 20.Rapola JM, Virtamo J, Haukka JK, Heinonen OP, Albanes D, et al. Effect of vitamin E and beta carotene on the incidence of angina pectoris. A randomized, double-blind, controlled trial. JAMA. 1996;275:693–698. doi: 10.1001/jama.1996.03530330037026. [DOI] [PubMed] [Google Scholar]
- 21.Rapola JM, Virtamo J, Ripatti S, Haukka JK, Huttunen JK, et al. Effects of alpha tocopherol and beta carotene supplements on symptoms, progression, and prognosis of angina pectoris. Heart. 1998;79:454–458. doi: 10.1136/hrt.79.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegele RA. Angiotensin-converting enzyme (ACE) inhibition in the secondary prevention of vascular disease: the Heart Outcomes Prevention Evaluation (HOPE) Trial and its substudies. Curr Atheroscler Rep. 2000;2:361–362. doi: 10.1007/s11883-000-0073-5. [DOI] [PubMed] [Google Scholar]
- 23.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 24.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- 25.Frei B. Cardiovascular disease and nutrient antioxidants: role of low-density lipoprotein oxidation. Crit Rev Food Sci Nutr. 1995;35:83–98. doi: 10.1080/10408399509527689. [DOI] [PubMed] [Google Scholar]
- 26.Bagi Z, Feher A, Beleznai T. Preserved coronary arteriolar dilatation in patients with type 2 diabetes mellitus: implications for reactive oxygen species. Pharmacol Rep. 2009;61:99–104. doi: 10.1016/s1734-1140(09)70011-8. [DOI] [PubMed] [Google Scholar]
- 27.Lee M, Choy WC, Abid MR. Direct sensing of endothelial oxidants by vascular endothelial growth factor receptor-2 and c-Src. PloS one. 2011;6:1. doi: 10.1371/journal.pone.0028454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng J, Damrauer SM, Lee M, Sellke FW, Ferran C, et al. Endothelium-dependent coronary vasodilatation requires NADPH oxidase-derived reactive oxygen species. Arterioscler Thromb Vasc Biol. 2010;30:1703–1710. doi: 10.1161/ATVBAHA.110.209726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abid MR, Kachra Z, Spokes KC, Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS lett. 2000;486:252–256. doi: 10.1016/s0014-5793(00)02305-x. [DOI] [PubMed] [Google Scholar]
- 30.Feng J, Damrauer SM, Lee M, Sellke FW, Ferran C, et al. Endothelium-dependent coronary vasodilatation requires NADPH oxidase-derived reactive oxygen species. Arterioscler Thromb Vasc Biol. 30:1703–1710. doi: 10.1161/ATVBAHA.110.209726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, et al. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol. 2008;28:1627–1633. doi: 10.1161/ATVBAHA.108.168278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawada N, Salomone S, Kim HH, Kwiatkowski DJ, Liao JK. Regulation of endothelial nitric oxide synthase and postnatal angiogenesis by Rac1. Circ Res. 2008;103:360–368. doi: 10.1161/CIRCRESAHA.108.178897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shafique E, Choy WC, Liu Y, Feng J, Cordeiro B, et al. Oxidative stress improves coronary endothelial function through activation of the pro-survival kinase AMPK. Aging. 2013;5:515–530. doi: 10.18632/aging.100569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abid MR, Tsai JC, Spokes KC, Deshpande SS, Irani K, et al. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. FASEB journal. 2001;15:2548–2550. doi: 10.1096/fj.01-0338fje. [DOI] [PubMed] [Google Scholar]
- 35.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, et al. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 36.Bayraktutan U, Blayney L, Shah AM. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:1903–1911. doi: 10.1161/01.atv.20.8.1903. [DOI] [PubMed] [Google Scholar]
- 37.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 38.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, et al. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bengtsson SH, Gulluyan LM, Dusting GJ, Drummond GR. Novel isoforms of NADPH oxidase in vascular physiology and pathophysiology. Clin Exp Pharmacol Physiol. 2003;30:849–854. doi: 10.1046/j.1440-1681.2003.03929.x. [DOI] [PubMed] [Google Scholar]
- 40.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, et al. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 41.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, et al. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, et al. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol. 2008;28:1627–1633. doi: 10.1161/ATVBAHA.108.168278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem. 2007;282:35373–35385. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- 44.Craige SM, Chen K, Pei Y, Li C, Huang X, et al. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai H, Li Z, Dikalov S, Holland SM, Hwang J, et al. NAD(P)H oxidase-derived hydrogen peroxide mediates endothelial nitric oxide production in response to angiotensin II. J Biol Chem. 2002;277:48311–48317. doi: 10.1074/jbc.M208884200. [DOI] [PubMed] [Google Scholar]
- 46.Thomas SR, Chen K, Keaney JF., Jr Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem. 2002;277:6017–6024. doi: 10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- 47.Cai H, Davis ME, Drummond GR, Harrison DG. Induction of endothelial NO synthase by hydrogen peroxide via a Ca(2+)/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler Thromb Vasc Biol. 2001;21:1571–1576. doi: 10.1161/hq1001.097028. [DOI] [PubMed] [Google Scholar]
- 48.Krotz F, Engelbrecht B, Buerkle MA, Bassermann F, Bridell H, et al. The tyrosine phosphatase, SHP-1, is a negative regulator of endothelial superoxide formation. J Am Coll Cardiol. 2005;45:1700–1706. doi: 10.1016/j.jacc.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 49.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, et al. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011;31:3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackenzie RM, Salt IP, Miller WH, Logan A, Ibrahim HA, et al. Mitochondrial reactive oxygen species enhance AMP-activated protein kinase activation in the endothelium of patients with coronary artery disease and diabetes. Clin Sci (Lond) 2013;124:403–411. doi: 10.1042/CS20120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Q, Liang B, Shirwany NA, Zou MH. 2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PloS one. 2011;6:e17234. doi: 10.1371/journal.pone.0017234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee M, Hwang JT, Lee HJ, Jung SN, Kang I, et al. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem. 2003;278:39653–39661. doi: 10.1074/jbc.M306104200. [DOI] [PubMed] [Google Scholar]
- 53.Dixit M, Bess E, Fisslthaler B, Hartel FV, Noll T, et al. Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc Res. 2008;77:160–168. doi: 10.1093/cvr/cvm017. [DOI] [PubMed] [Google Scholar]
- 54.Awad H, Nolette N, Hinton M, Dakshinamurti S. AMPK and FoxO1 regulate catalase expression in hypoxic pulmonary arterial smooth muscle. Pediatr Pulmonol. 2014;49:885–897. doi: 10.1002/ppul.22919. [DOI] [PubMed] [Google Scholar]
- 55.Yun H, Park S, Kim MJ, Yang WK, Im DU, et al. AMP-activated protein kinase mediates the antioxidant effects of resveratrol through regulation of the transcription factor FoxO1. FEBS J. 2014;281:4421–4438. doi: 10.1111/febs.12949. [DOI] [PubMed] [Google Scholar]
- 56.Hwang AB, Ryu EA, Artan M, Chang HW, Kabir MH, et al. Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2014;111:E4458–E4467. doi: 10.1073/pnas.1411199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aatsinki SM, Buler M, Salomaki H, Koulu M, Pavek P, et al. Metformin induces PGC-1alpha expression and selectively affects hepatic PGC-1alpha functions. Br J Pharmacol. 2014;171:2351–2363. doi: 10.1111/bph.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan Z, Root-McCaig J, Castellani L, Kemp BE, Steinberg GR, et al. Evidence for the role of AMPK in regulating PGC-1 alpha expression and mitochondrial proteins in mouse epididymal adipose tissue. Obesity (Silver Spring) 2014;22:730–738. doi: 10.1002/oby.20605. [DOI] [PubMed] [Google Scholar]
- 59.Cardaci S, Filomeni G, Ciriolo MR. Redox implications of AMPK-mediated signal transduction beyond energetic clues. J Cell Sci. 2012;125:2115–2125. doi: 10.1242/jcs.095216. [DOI] [PubMed] [Google Scholar]
- 60.Weerasekara VK, Panek DJ, Broadbent DG, Mortenson JB, Mathis AD, et al. Metabolic-Stress-Induced Rearrangement of the 14-3-3zeta Interactome Promotes Autophagy via a ULK1- and AMPK-Regulated 14-3-3zeta Interaction with Phosphorylated Atg9. Mol Cell Biol. 2014;34:4379–4388. doi: 10.1128/MCB.00740-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Chen C, Yao F, Su Q, Liu D, et al. AMPK inhibits cardiac hypertrophy by promoting autophagy via mTORC1. Arch Biochem Biophys. 2014;558:79–86. doi: 10.1016/j.abb.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 62.She C, Zhu LQ, Zhen YF, Wang XD, Dong QR. Activation of AMPK protects against hydrogen peroxide-induced osteoblast apoptosis through autophagy induction and NADPH maintenance: new implications for osteonecrosis treatment? Cell Signal. 2014;26:1–8. doi: 10.1016/j.cellsig.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 63.Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 64.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, et al. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 65.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 66.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, et al. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res. 2007;100:1016–1025. doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- 67.Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abid MR, Kachra Z, Spokes KC, Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000;486:252–256. doi: 10.1016/s0014-5793(00)02305-x. [DOI] [PubMed] [Google Scholar]
- 69.Abid MR, Tsai JC, Spokes KC, Deshpande SS, Irani K, et al. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. Faseb J. 2001;15:2548–2550. doi: 10.1096/fj.01-0338fje. [DOI] [PubMed] [Google Scholar]
- 70.Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem. 2007;282:35373–35385. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- 71.Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, et al. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- 72.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, et al. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]