Abstract
Previous studies have established that when a prey animal knows the identity of a particular predator, it can use this knowledge to make an ‘educated guess' about similar novel predators. Such generalization of predator recognition may be particularly beneficial when prey are exposed to introduced and invasive species of predators or hybrids. Here, we examined generalization of predator recognition for woodfrog tadpoles exposed to novel trout predators. Tadpoles conditioned to recognize tiger trout, a hybrid derived from brown trout and brook trout, showed generalization of recognition of several unknown trout odours. Interestingly, the tadpoles showed stronger responses to odours of brown trout than brook trout. In a second experiment, we found that tadpoles trained to recognize brown trout showed stronger responses to tiger trout than those tadpoles trained to recognize brook trout. Given that tiger trout always have a brown trout mother and a brook trout father, these results suggest a strong maternal signature in trout odours. Tadpoles that were trained to recognize both brown trout and brook trout showed stronger response to novel tiger trout than those trained to recognize only brown trout or only brook trout. This is consistent with a peak shift in recognition, whereby cues that are intermediate between two known cues evoke stronger responses than either known cue. Given that our woodfrog tadpoles have no evolutionary or individual experience with trout, they have no way of knowing whether or not brook trout, brown trout or tiger trout are more dangerous. The differential intensity of responses that we observed to hybrid trout cues and each of the parental species indicates that there is a likely mismatch between risk and anti-predator response intensity. Future work needs to address the critical role of prey naivety on responses to invasive and introduced hybrid predators.
Keywords: learning, predator recognition, risk assessment, generalization, hybrid predators, invasive species
1. Introduction
Predation is a pervasive selective force affecting many aspects of the lives of prey animals including their morphology, life history and behaviour [1,2]. We know that prey exhibit sophisticated avoidance of locations and times when predators are active [3,4]. A prerequisite for such responses is that prey animals recognize predators as threats, and consequently, there has been considerable effort to understand predator recognition dynamics [5,6]. The variable nature of risk through both space and time, combined with the cost of genetic fixation of traits, means that learned predator recognition is paramount in most systems [7–9].
After prey come to recognize a predator as a threat, they can use this learned information to generalize their responses to other prey [5,10]. In essence, the prey use their knowledge to make ‘educated guesses' about the likelihood that other similar looking or smelling animals will be also be a threat. In a pioneering paper, Griffin et al. [10] documented that tammar wallabies (Macropus eugenii) do not innately recognize stuffed feral cats (Felis catus) or red foxes (Vulpes vulpes) as dangerous. However, after being trained to recognize foxes, the wallabies showed anti-predator responses to both foxes and cats. The wallabies generalized their recognition of the foxes to the cats based on characteristics the cats and the foxes share in common. However, the generalization by the wallabies was not extended to a stuffed non-predatory juvenile goat (Capra hircus). Clearly, some characteristic(s), perhaps frontally placed eyes, are shared by foxes and cats, but not goats. Ferrari et al. [5] completed a similar generalization study using predator odours rather than predator models. In that case, fathead minnows (Pimephales promelas) that were trained to recognize lake trout (Salvelinus namaycush) odour as a threat, subsequently responded to lake trout odour, but they also generalized their recognition to congeneric brook trout (Salvelinus fontinalis) and confamilial rainbow trout (Oncorhynchus mykiss) but not distantly related predatory pike (Esox lucius) or non-predatory suckers (Catostomus commersoni).
The papers by Griffin et al. [10] and Ferrari et al. [5] sparked a number of similar studies examining generalization of predator recognition in a variety of taxa [11–16]. In cases of the generalization of odours, we typically see that the prey respond with a less intense response towards the animals to which they are generalizing, compared with the model that they have learned as dangerous. The animals to which the prey are generalizing are potential, but not certain, predators. Ferrari et al. [5,17] argue that phylogenetic distance between the learned predator and the other novel animals is a good predictor of the degree of generalization. Closely related animals tend to share similar foraging strategies, therefore the closer the unknown animal is to the species that the prey has learned as dangerous, the greater the probability is that the unknown species is actually a predator. This has intuitive appeal as it fits well within the behavioural ecology context that prey exhibit graded anti-predator responses based on level of perceived threat [18–20]. However, it is somewhat paradoxical that the reduction in intensity of responses that occurs when animals generalize their predator recognition between species can result in a mismatch between the intensity of response that the prey exhibit and their true vulnerability. Indeed, the predator to which prey are generalizing may be more dangerous than the predator that the prey already recognizes.
Over the past several decades, we have seen increasing concerns over the impact of invasive and introduced species on native prey communities [21–24]. Introduced species are often efficient predators because prey fail to recognize them as a threat. Indeed, prey naiveté is often invoked as a primary driver of the high impact of introduced predators [25,26]. With the introduction of non-native predators, we also frequently see anthropogenic hybridization [27,28]. Species that typically would not come into contact do so as a result of introduction programmes. One such example is the case of tiger trout, a hybrid from the unnatural sympatry of the North American brook trout and European brown trout (Salmo trutta). While the natural presence of tiger trout seems to be low in the wild, the hatchery-based hybridization between female brown and male brook trout is one of the few crosses which does not result in overwhelming mortalities [29–31]. Tiger trout have a fast growth rate but the hybrids are sterile [32]. Not surprisingly, tiger trout have been widely stocked for sport fishing in North America.
There are no published reports of responses of prey animals to introduced tiger trout. Our work here begins to address this issue, specifically examining recognition and generalization of hybrid trout odours by larval woodfrogs (Lithobates slyvaticus). We chose woodfrog tadpoles as a model ranid frog species to test for recognition and generalization because there is considerable evidence that several species of ranid frogs suffer considerable mortality as a result of introduced predators, including trout [33–36]. Woodfrogs are ideal for this work, as they have the ability to learn and generalize recognition of predators [14,17]. In our first experiment, we trained woodfrog tadpoles to recognize the odour of hybrid tiger trout and then ask how the prey responded to tiger trout as well as each of the parent species (brook trout and brown trout). We also assessed generalization of recognition to other salmonid fishes (rainbow trout) and distantly related goldfish (Carassius auratus). In our second experiment, we trained tadpoles to recognize either brown trout, brook trout or both parental species and then ask how the tadpoles generalize their response to the tiger trout or a goldfish control. These two experiments should help us understand how parental species mediate risk from hybrid species and vice versa.
2. Material and methods
(a). Tadpole collection and maintenance and preparation of fish odours
We used dipnets to capture wild tadpoles from a pond in Strathcona County in south-central Alberta, Canada. The pond contains no fish predators but has numerous species of invertebrate predators. Our previous work has established that tadpoles from this pond do not respond to fish odours with an anti-predator response [4,14]. After capture, the tadpoles were held outdoors in large pools in a shaded aspen forest for 2 days before the experiments began. Tadpoles fed on algae present in the pool and their diet was supplemented with alfalfa pellets and fish food (Tetramin flakes). The water used to house the tadpoles and used in the subsequent experiments was well water that had been aged outdoors in a 1900 l pool for three weeks prior to the experiment. The well water pool was seeded with plankton and aquatic plants ensuring that the water contained many natural odours but no predator odours. We prepared fish odours by placing individual fish in 15 l of water for 24 h. We used a minimum of four different fish of each species (tiger trout, brook trout, brown trout, rainbow trout and goldfish) to prepare each odour. The fish were size-matched (total length approx. 15 cm) to ensure consistency in concentration between species.
(b). Experiment 1
The goal of this experiment was to train tadpoles to recognize the odour of a hybrid tiger trout and test their behavioural response to tiger trout odour as well as their ability to generalize their recognition to both parent species (brown trout and brook trout), to closely related rainbow trout and to a distantly related fish (goldfish). Tadpoles were removed from the holding pool and placed individually in 500 ml round plastic containers completely filled with well water. After a 2 h acclimation period, the tadpoles were exposed to either 5 ml of tiger trout odour paired with 5 ml of alarm cue solution (conditioned group) or to 5 ml of tiger trout odour paired with 5 ml of well water (control group). Alarm cues were prepared following the methodology of Chivers & Ferrari [37]. This consisted of crushing a tadpole in a mortar and pestle and then adding 10 ml of pond water. The solution was filtered through cotton gauze prior to being used. Given that we added 5 ml of alarm cue solution to each cup during the conditioning, we added the equivalent of half a crushed tadpole to each container.
We did not want the tadpoles in the control group to associate exposure to predator odour with disturbance associated with moving them. Consequently, we waited 1 h after the conditioning and then moved tadpoles to a series of 20 l containers, where they were matched for treatment and housed in groups of 20. The following day, the tadpoles were placed individually in 0.5 l cups. After a 2 h acclimation period, they were tested for their response to 5 ml of tiger trout, brook trout, brown trout, rainbow trout or goldfish odour. We conducted between 12–16 replicates in the control treatments (tadpoles exposed to tiger trout odour paired with distilled water) and 27–30 replicates in the experimental treatments (tadpoles conditioned with tiger trout odour paired with alarm cues). We had fewer control tadpoles because we have conducted hundreds of control trials using tadpoles from this pond and are reasonably confident that the tadpoles will fail to respond to fish cues unless they are trained. Animal care concerns associated with the excessive use of animals dictates that we not needlessly replicate control treatments. Tadpoles were only used once and we tested a total of 215 tadpoles. The experimenter was blind to the treatments.
We used a well-established testing protocol to quantify the responses of tadpoles to predator odours [6,38]. We observed the behaviour of each tadpole for 4 min prior to injection of the stimulus and 4 min after injection. The stimulus was injected slowly on the side of the cup using a syringe. During both observation periods, we recorded the number of times the tadpole passed the medial line of the cup. A reduction in activity is a typical anti-predator response displayed by most animals and is a standard bioassay for tadpole anti-predator responses [4,39].
(c). Experiment 2
The goal of this experiment was to train tadpoles to recognize the odour of brown trout, brook trout or both brown and brook trout and subsequently test their behavioural response to tiger trout odour or the odour of a distantly related fish (goldfish).
Our conditioning protocol was identical to that used in experiment 1 except that we conditioned tadpoles in groups of 20 in 3 l pails (filled with 2 l of water) rather than individually. Moreover, we conditioned the tadpoles twice rather than once, to control for the double-conditioning necessary for tadpoles to recognize both brown and brook trout odours. We had three experimental groups: tadpoles conditioned twice to recognize brown trout as a predator, tadpoles conditioned twice to recognize brook trout as a predator, or tadpoles conditioned once to recognize brook trout and once to recognize brown trout as predators. For this group, half the tadpoles were taught brook trout first, while the other half were taught brown trout first. The water was changed 1 h after the first conditioning, and the second conditioning took place 3 h later. We injected 10 ml of injured tadpole cues paired with 20 ml of trout odour in each of the pails.
The testing procedure was identical to that used in experiment 1. We tested 163 tadpoles for a response to 5 ml of tiger trout odour or 5 ml of goldfish odour (n = 25–31 treatment−1). Each tadpole was only tested once, and the observer was blind to the treatments.
(d). Statistical analysis
Experiment 1: We computed a proportion change in activity from the pre-stimulus baseline ([post − pre]/pre) which was used as our response variable in the subsequent analysis. We conducted a two-way ANOVA, testing for the effect of training (naive versus experienced) and cue (tiger trout, brown trout, brook trout, rainbow trout and goldfish) on the response of tadpoles. Tukey post hoc tests were used to compare among groups.
Experiment 2: The statistical analysis was split to address two aspects of the design. First, we performed a three-way nested ANOVA on the proportion change in activity of the tadpoles to test for the effect of conditioning (brown, brook or both) and test cue (tiger versus goldfish). However, tadpoles conditioned in the same pail were not statistically independent, and hence, we introduced pail as a nested factor in our analysis, and also tested for any cue × pail interaction (Type I SS). Second, we tested whether the order of conditioning for the brown/brook group would affect the response of tadpoles to the cues. Using the tadpoles from the brook/brown group only, we performed a three-way nested ANOVA, testing for the effect of order (brown first versus brook first) and cue on the responses of tadpoles. Similar to the previous analysis, we introduced pail (and pail × cue) as a random factor in the analysis to ensure pail, not tadpole, was our unit of replication.
Preliminary analyses performed on pre-stimulus data (using the models described above) indicated no existing behavioural differences among tadpoles from different experimental groups prior to the injection of cues (all p > 0.2 for experimental 1, and p > 0.1 for experimental 2). All statistics were performed using SPSS v. 21.
3. Results
For experiment 1, the two-way ANOVA on the proportion change in activity revealed a significant interaction between experience and cue on the behavioural response of tadpoles (F4,205 = 9.6, p < 0.001). Tadpoles in the control treatment that underwent the false training did not differ in their response to any of the five cues (F4,65 = 0.4, p = 0.8). However, when tadpoles were trained to recognize a tiger trout, they differed in their response to the cues (F4,140 = 24.2, p < 0.001). The results of Tukey post hoc comparisons are presented in figure 1. In short, tadpoles generalized their anti-predator response from the tiger trout to all trout species, but not goldfish. Within the trout responses, tadpoles tended to show a stronger response to brown trout than tiger trout but a weaker response to brook trout than to tiger trout. This meant that while the tiger trout did not differ from either brook or brown trout, there was a significantly lower response to brook trout than to brown trout.
Figure 1.
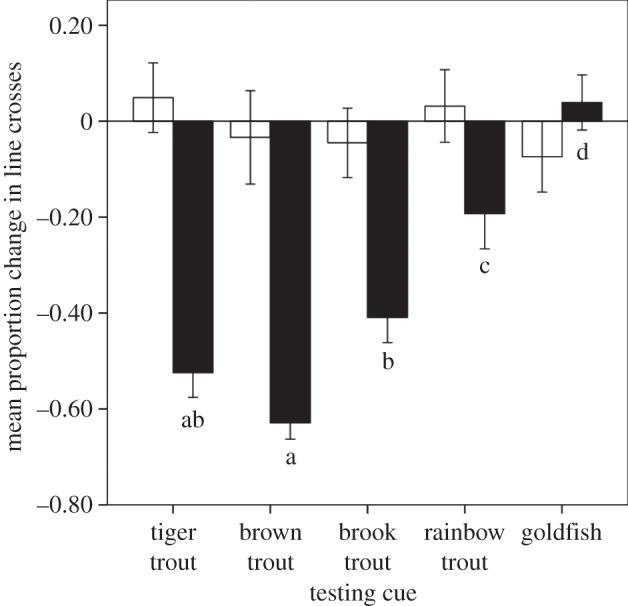
Mean (±s.e.) proportion change in line crosses for woodfrog tadpoles conditioned to tiger trout odour with alarm cue (black bars) or control water (white bars) and subsequently exposed to tiger trout, brown trout, brook trout, rainbow trout or goldfish odour. Different letters indicate significant differences at α = 0.05 based on Tukey post-hoc tests.
For experiment 2, we found a significant interaction between conditioning and cue on the responses of tadpoles (F2,15.3 = 14.9, p < 0.001; figure 2). Neither pail (F21,16.4 = 1.5, p = 0.2) nor pail × cue (F21,115 = 0.4, p = 0.9) were found to be significant. Tadpoles responded similarly to goldfish, regardless of their conditioning treatment (F2,15.1 = 1.9, p = 0.2; pail: F21,51 = 0.5, p = 0.9). Tadpoles from different conditioning groups, however, differed in their responses to tiger trout (F2,18.2 = 27.7, p < 0.001; pail: F21,64 = 0.5, p = 0.9). Tukey post hoc tests revealed that all groups differed from one another (all p < 0.031), with tadpoles conditioned to brook trout displaying the weakest response to the tiger trout, and those conditioned to both brook and brown trout displaying the strongest response to tiger trout.
Figure 2.

Mean (±s.e.) proportion change in line crosses for woodfrog tadpoles in response to tiger trout odour (black bars) or goldfish odour (white bars). Tadpoles were previously conditioned twice with tadpole alarm cues to recognize brown trout (2 × (TP + BnT)), twice to recognize brook trout (2 × (TP + BkT)) or once to recognize brown trout and once to recognize brook trout ((TP + BnT) + (TP + BkT)).
Tadpoles conditioned to recognize both trout species responded stronger to tiger trout than goldfish (F1,2.2 = 270, p = 0.003), but the responses were not affected by the order of conditioning (F1,4.2 = 4.6, p = 0.1) and we failed to find an interaction between those two factors (F1,2.5 = 0.2, p = 0.7). Again, there was no effect of pail on the responses of tadpoles (F6,3.2 = 0.9, p = 0.5), nor any pail × cue interaction (F6,36 = 0.2, p = 0.9).
4. Discussion
The results of our study demonstrate that woodfrog tadpoles can learn trout odours and use this information to generalize their recognition to other trout odours. The intensity of the behavioural responses that the tadpoles exhibit towards each of the predator odours indicates that odours may have a strong maternal signature. Generalized responses to hybrid predators may lead to a mismatch between risk and anti-predator responses.
In the first experiment, we observed that tadpoles which were conditioned to recognize the odour of tiger trout, generalized their response to the other trout, but not to distantly related goldfish. This matches that pattern of response we would expect based on the well-established generalization framework [5,12]. However, the intensity of the responses that we observed was somewhat unexpected if both parents contributed equally to the odour of their offspring. Woodfrogs have no evolutionary history with salmonid fishes and our tadpoles had never experienced fish odours prior to the experiment. This means that after the tadpoles learned the tiger trout was a predator, they should have generalized their recognition to other odours based on the similarity between the new odour and the tiger trout odour that they already recognized. Accordingly, we should have seen the strongest response to the hybrid tiger trout and less of a response to either parent species. Mechanistically, it is easy to imagine that generalization could result from the hybrid sharing, with the parent, a similar suite of chemicals that contributes to its odour. Differentiating the hybrid and the parent species indicates that not all of the chemicals in the odour are the same or that there are slight differences in the concentration of specific chemical(s). As predicted, in our experiment, we observed that tadpoles tended to show a weaker response to brook trout than to tiger trout. However, the tadpoles tended to show a stronger response to brown trout than to tiger trout. This indicates that the specific chemicals which constitute the predator odours are in greater quantity in brown trout. Given that tiger trout always have a brown trout mother and that females contribute mitochondrial DNA, perhaps the specific suite of chemicals that is recognized as ‘brown trout’ have a mitochondrial origin. Alternatively, the brown trout may contribute a higher proportion of dominant alleles which would explain why the tiger trout odour would be more similar to brown trout.
In our second experiment, when the tadpoles were trained to recognize a brown trout, we found that they responded to the tiger trout with a higher intensity response than those that were trained to recognize brook trout. This again indicates that tadpoles are responding more strongly to odours of maternal origin. What was most revealing is that when the tadpoles were taught that both brown and brook trout were predators, they responded more to tiger trout than if they were trained to recognize only brown trout or only brook trout. In all cases, the tadpoles went through two training sessions. The stronger response to tiger trout when both parental species are recognized as predators could simply reflect the high-risk value of the chemical make-up of tiger trout. Indeed, this ‘novel’ species has an odour matching two learned predators. By contrast, in the other group, the match between the learned predator (brown or brook only) and the ‘novel’ predator is only partial. An alternative explanation could be that the higher intensity response represents a peak shift in generalization. When animals are trained to respond to two similar stimuli and then exposed to an intermediate stimulus to which they are not trained, the animals often show the greatest intensity response to the intermediate stimulus [40]. Lynn et al. [41] argue that such a peak shift in the context of discrimination may be the outcome of the kind of decision processes that underlie signal detection theory. However, our data does not allow us to tease the two options apart, because we do not have a side-by-side comparison of the response to the known (brook or brown) and the hybrid predator. Thus, we cannot tell whether the response to the tiger trout is stronger or weaker than that to the maternal species. However, from a conservation point of view, one clear result is that prey species knowing both brook and brown trout will respond to the hybrid with a much stronger intensity of anti-predator response than those prey only familiar with one of the species. While naturally occurring tiger trout will be found in water bodies containing both brook and brown trout, recreational stocking of tiger trout implies that only one or none of the parental species may be present in the water bodies in which they are released. Our present work suggest that native prey might fare better with tiger trout if they are already familiar with one of the parental species, and might fare the best in water bodies where both parental species are present.
Previous work has established that when prey generalize their recognition of a known predator to an unknown animal, they show a lower intensity response to the odour to which they are generalizing than to the odour that the prey already recognizes. In one sense, this meshes well within the context of graded anti-predator responses based on level of threat [18–20]. However, the prey has no real information about the relative risk of the unknown predator and hence may pay a survival cost by under-responding. This would dictate that the prey should respond equally strong to the known odour and the odour to which they are generalizing. Only after gaining additional pieces of information should the prey adjust the intensity of their response to match the real risk posed by the predator [42]. In our first experiment, the response of the tadpoles to the hybrid was intermediate between the responses to each of the parent species. The prey showed a significantly lower response to brook trout than to brown trout. Given that the tadpoles have no evolutionary or individual experience with trout, they have no way of knowing whether or not brook trout or brown trout are more dangerous, hence responding differentially may be problematic. In experiment 2, the prey responded stronger to hybrid tiger trout when they knew both brown trout and brook trout compared to when they knew only one of the two predators. No studies, to our knowledge have been conducted comparing the vulnerability of tadpoles to our suite of predators but clearly differential responses of the prey to different predators in the absence of information means that some cues are over or underestimated compared with actual vulnerability. If peak shifts are common in recognition of similar predators, then we would predict that prey will always respond strongest to hybrid predators. Taken together, our results indicate that generalizing hybrid predators could lead to a mismatch between response intensity and risk. Future work should address how much individual experience is needed for prey to overcome this mismatch.
Supplementary Material
Acknowledgements
We thank Harvey Pollock for delivering the trout odours to our field site during the middle of fish stocking season.
Ethics
All work carried herein was in accordance with the University of Saskatchewan Animal Care protocol no. 20060014.
Data Accessibility
Data submitted as the electronic supplementary material.
Authors' Contributions
D.C. and M.F. designed the experiments; J.S. prepared the fish odours; D.C. and M.F. conducted experiment 1; D.C., M.F. and A.M. conducted experiment 2; M.F. analysed the data, D.C. and M.F. wrote the first draft of the paper, all authors contributed to the final version of the manuscript.
Competing Interests
We declare we have no competing interests.
Funding
Funding was provided to M.F. and D.C. from the Natural Sciences and Engineering Council of Canada.
References
- 1.Stankowich T, Blumstein DT. 2005. Fear in animals: a meta-analysis and review of risk assessment. Proc. R. Soc. B 272, 2627–2634. ( 10.1098/rspb.2005.3251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lima SL, Dill LM. 1990. Behavioral decision made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. ( 10.1139/z90-092) [DOI] [Google Scholar]
- 3.Sih A, Ziemba R, Harding KC. 2000. New insights on how temporal variation in predation risk shapes prey behavior. Trends Ecol. Evol. 15, 3–4. ( 10.1016/S0169-5347(99)01766-8) [DOI] [PubMed] [Google Scholar]
- 4.Ferrari MCO, Chivers DP. 2009. Temporal variability, threat sensitivity and conflicting information about the nature of risk: understanding the dynamics of tadpole antipredator behaviour. Anim. Behav. 78, 11–16. ( 10.1016/j.anbehav.2009.03.016) [DOI] [Google Scholar]
- 5.Ferrari MCO, Gonzalo A, Messier F, Chivers DP. 2007. Generalization of learned predator recognition: an experimental test and framework for future studies. Proc. R. Soc. B 274, 1853–1859. ( 10.1098/rspb.2007.0297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown GE, Ferrari MCO, Elvidge CK, Ramnarine I, Chivers DP. 2013. Phenotypically plastic neophobia: a response to variable predation risk. Proc. R. Soc. B 280, 20122712 ( 10.1098/rspb.2012.2712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown GE, Chivers DP. 2005. Learning as an adaptive response to predation. In Ecology of predator–prey interactions (eds Barbosa P, Castellanos I.), pp. 34–54. Oxford, UK: Oxford University Press. [Google Scholar]
- 8.Lima SL, Bednekoff PA. 1999. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153, 649–659. ( 10.1086/303202) [DOI] [PubMed] [Google Scholar]
- 9.Dall SRX, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW. 2005. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193. ( 10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]
- 10.Griffin AS, Evans CS, Blumstein DT. 2001. Learning specificity in acquired predator recognition. Anim. Behav. 62, 577–589. ( 10.1006/anbe.2001.1781) [DOI] [Google Scholar]
- 11.Stankowich T, Coss RG. 2007. The re-emergence of felid camouflage with the decay of predator recognition in deer under relaxed selection. Proc. R. Soc. B 274, 175–182. ( 10.1098/rspb.2006.3716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell MD, McCormick MI, Chivers DP, Ferrari MCO. 2013. Generalization of learned predator recognition in coral reef ecosystems: how cautious are damselfish? Funct. Ecol. 27, 299–304. ( 10.1111/1365-2435.12043) [DOI] [Google Scholar]
- 13.Ferrari MCO, Messier F, Chivers DP, Messier O. 2008. Can prey exhibit threat-sensitive generalization of predator recognition? Extending the predator recognition continuum hypothesis. Proc. R. Soc. B 275, 1811–1816. ( 10.1098/rspb.2008.0305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrari MCO, Chivers DP. 2009. Sophisticated early life lessons: threat-sensitive generalization of predator recognition by embryonic amphibians. Behav. Ecol. 20, 1295–1298. ( 10.1093/beheco/arp135) [DOI] [Google Scholar]
- 15.Polo-Cavia N, Gonzalo A, López P, Martín J. 2010. Predator recognition of native but not invasive turtle predators by naïve anuran tadpoles. Anim. Behav. 80, 461–466. ( 10.1016/j.anbehav.2010.06.004) [DOI] [Google Scholar]
- 16.Brown GE, Ferrari MCO, Malka PH, Russo S, Tressider M, Chivers DP. 2011. Generalization of predators and non-predators by juvenile rainbow trout: learning what is and what is not a threat. Anim. Behav. 81, 1249–1256. ( 10.1016/j.anbehav.2011.03.013) [DOI] [Google Scholar]
- 17.Ferrari MCO, Brown GE, Messier F, Chivers DP. 2009. Threat-sensitive generalization of predator recognition by larval amphibians. Behav. Ecol. Sociobiol. 63, 1369–1375. ( 10.1007/s00265-009-0779-5) [DOI] [Google Scholar]
- 18.Helfman GS. 1989. Threat-sensitive predator avoidance in damselfish-trumpetfish interactions. Behav. Ecol. Sociobiol. 24, 47–58. ( 10.1007/BF00300117) [DOI] [Google Scholar]
- 19.Chivers DP, Mirza RS, Bryer PJ, Kiesecker JM. 2001. Threat-sensitive predator avoidance by slimy sculpins: understanding the importance of visual versus chemical information. Can. J. Zool. 79, 867–873. ( 10.1139/z01-049) [DOI] [Google Scholar]
- 20.Puttlitz MH, Chivers DP, Kiesecker JM, Blaustein AR. 1999. Threat-sensitive predator avoidance by larval pacific treefrogs (Amphibia, Hylidae). Ethology 105, 449–456. ( 10.1046/j.1439-0310.1999.00416.x) [DOI] [Google Scholar]
- 21.Mallet J. 2005. Hybridization as an invasion of the genome. Trends Ecol. Evol. 20, 229–237. ( 10.1016/j.tree.2005.02.010) [DOI] [PubMed] [Google Scholar]
- 22.Wolf DE, Takebayashi N, Rieseberg LH. 2001. Predicting the risk of extinction through hybridization. Conserv. Biol. 15, 1039–1053. ( 10.1046/j.1523-1739.2001.0150041039.x) [DOI] [Google Scholar]
- 23.Parker IM, et al. 1999. Impact: toward a framework for understanding the ecological effects of invaders. Biol. Invasions 1, 3–19. ( 10.1023/a:1010034312781) [DOI] [Google Scholar]
- 24.Vitousek PM, D'antonio CM, Loope LL, Rejmanek M, Westbrooks R. 1997. Introduced species: a significant component of human-caused global change. NZ J. Ecol. 21, 1–16. [Google Scholar]
- 25.Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR. 2010. Predator-prey naivete, antipredator behavior, and the ecology of predator invasions. Oikos 119, 610–621. ( 10.1111/j.1600-0706.2009.18039.x) [DOI] [Google Scholar]
- 26.Cox JG, Lima SL. 2006. Naiveté and an aquatic–terrestrial dichotomy in the effects of introduced predators. Trends Ecol. Evol. 21, 674–680. ( 10.1016/j.tree.2006.07.011) [DOI] [PubMed] [Google Scholar]
- 27.Grosholz E. 2002. Ecological and evolutionary consequences of coastal invasions. Trends Ecol. Evol. 17, 22–27. ( 10.1016/S0169-5347(01)02358-8) [DOI] [Google Scholar]
- 28.Allendorf FW, Leary RF, Spruell P, Wenburg JK. 2001. The problems with hybrids: setting conservation guidelines. Trends Ecol. Evol. 16, 613–622. ( 10.1016/S0169-5347(01)02290-X) [DOI] [Google Scholar]
- 29.Blanc JM, Chevassus B. 1986. Survival, growth and sexual maturation of the tiger trout hybrid (Salmo trutta ♀×Salvelinus fontinalis ♂). Aquaculture 52, 59–69. ( 10.1016/0044-8486(86)90108-0) [DOI] [Google Scholar]
- 30.Blanc JM, Chevassus B. 1979. Interspecific hybridization of salmonid fish. Aquaculture 18, 21–34. ( 10.1016/0044-8486(79)90097-8) [DOI] [Google Scholar]
- 31.Blanc JM, Chevassus B. 1982. Interspecific hybridization of salmonid fish. II. Survival and growth up to the 4th month after hatching in F1 generation hybrids. Aquaculture 29, 383–387. ( 10.1016/0044-8486(82)90151-X) [DOI] [Google Scholar]
- 32.McKay LR, Ihssen PE, McMillan I. 1992. Growth and mortality of diploid and triploid tiger trout (Salmo trutta×Salvelinus fontinalis). Aquaculture 106, 239–251. ( 10.1016/0044-8486(92)90256-K) [DOI] [Google Scholar]
- 33.Vredenburg VT. 2004. Reversing introduced species effects: experimental removal of introduced fish leads to rapid recovery of a declining frog. Proc. Natl Acad. Sci. USA 101, 7646–7650. ( 10.1073/pnas.0402321101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kats LB, Ferrer RP. 2003. Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Divers. Distrib. 9, 99–110. ( 10.1046/j.1472-4642.2003.00013.x) [DOI] [Google Scholar]
- 35.Blaustein AR, Wake DB. 1995. The puzzle of declining amphibian populations. Sci. Am. 272, 52–57. ( 10.1038/scientificamerican0495-52) [DOI] [Google Scholar]
- 36.Kiesecker JM, Blaustein AR. 1998. Effects of introduced bullfrogs and smallmouth bass on microhabitat use, growth, and survival of native red-legged frogs (Rana aurora). Conserv. Biol. 12, 776–787. ( 10.1046/j.1523-1739.1998.97125.x) [DOI] [Google Scholar]
- 37.Chivers DP, Ferrari MCO. 2014. Social learning of predators by tadpoles: does food restriction alter the efficacy of tutors as information sources? Anim. Behav. 89, 93–97. ( 10.1016/j.anbehav.2013.12.018) [DOI] [Google Scholar]
- 38.Ferrari MCO, Chivers D. 2013. Temporal dynamics of information use in learning and retention of predator-related information in tadpoles. Anim. Cogn. 16, 667–676. ( 10.1007/s10071-013-0602-6) [DOI] [PubMed] [Google Scholar]
- 39.Chivers DP, Mirza RS. 2001. Importance of predator diet cues in responses of larval wood frogs to fish and invertebrate predators. J. Chem. Ecol. 27, 45–51. ( 10.1023/A:1005663815856) [DOI] [PubMed] [Google Scholar]
- 40.Blough DS. 1975. Steady state data and a quantitative model of operant generalization and discrimination. J. Exp. Psychol. Anim. Behav. Process. 1, 3–21. ( 10.1037/0097-7403.1.1.3) [DOI] [Google Scholar]
- 41.Lynn SK, Cnaani J, Papaj DR. 2005. Peak shift discrimination learning as a mechanism of signal evolution. Evolution 59, 1300–1305. ( 10.1111/j.0014-3820.2005.tb01780.x) [DOI] [PubMed] [Google Scholar]
- 42.Ferrari MCO, Chivers DP. 2006. Learning threat-sensitive predator avoidance: how do fathead minnows incorporate conflicting information? Anim. Behav. 71, 19–26. ( 10.1016/j.anbehav.2005.02.016) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data submitted as the electronic supplementary material.


