Abstract
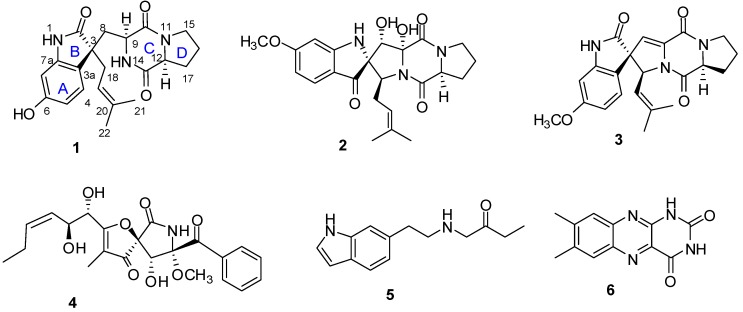
A new diketopiperazine alkaloid named spirotryprostatin K (1), and five known alkaloids, spiro[5H,10H-dipyrrolo[1,2-a:1′,2′-d]pyrazine-2(3H),2′-[2H]-indole]-3′,5,10(1′H)trione (2), 6-methoxyspirotryprostatin B (3), pseurotin A (4), N-β-acetyltryptamine (5), and lumichrome (6) were isolated from the endophytic fungus Aspergillus fumigatus. The structure and the absolute configuration of spirotryprostatin K were established by extensive spectroscopic analyses, acid hydrolysis and ECD calculations. Pseurotin A exhibited indirect anti-inflammatory activity by suppressing the lipopolysaccharide-induced proinflammatory factors in BV2 microglial cells, with an IC50 of 5.20 µM.
Keywords: endophytic fungus, Aspergillus fumigatus, Erythrophloeum fordii Oliv.
1. Introduction
Interest in endophytic fungi as sources of novel bioactive compounds is increasing because bioactive natural products from endophytic microbes have shown enormous potential as sources of new medicinal and agricultural products [1,2]. Considering that the relationship between endophytic fungi and host plants may range from latent phytopathogenesis to mutualistic symbiosis [1], endophytic fungi residing in poisonous plants may produce structurally diverse secondary metabolites as a consequence of their biological and biochemical evolution. We therefore initiated a research program focusing exclusively on the discovery of secondary metabolites from endophytic fungi associated with poisonous plants.
Erythrophloeum fordii Oliv. (Leguminosae) is widely distributed in the south of China and its bark and seed have been used in folk medicine to facilitate blood circulation and dispersing blood stasis [3]. We have previously discovered and reported a series of bioactive constituents from this plant [4]. Our efforts have now been directed at investigating the alkaloids found among the secondary metabolites of the endophytic fungus Aspergillus fumigatus isolated from the plant stem. In our study, a new diketopiperazine alkaloid, spirotryprostatin K (1), was isolated from the EtOAc extract of a culture of A. fumigatus, together with the five known alkaloids: spiro[5H,10H-dipyrrolo-[1,2-a:1′,2′-d]pyrazine-2(3H),2′-[2H]-indole]-3′,5,10(1′H)trione (2) [5], 6-methoxyspirotryprostatin B (3) [6], pseurotin A (4) [7], N-β-acetyltryptamine (5) [8], and lumichrome (6) [9]. In this paper, we describe the isolation, structural elucidation, and biological activity of the isolated compounds (Figure 1).
Figure 1.
Structures of compounds 1–6.
2. Results and Discussion
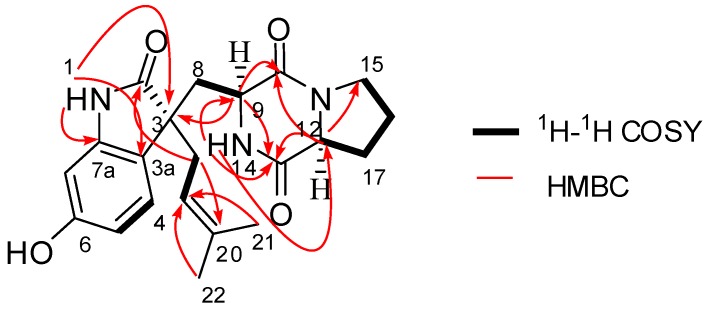
Compound 1 was obtained as a yellow amorphous powder and was assigned a molecular formula of C21H25N3O4 based on HRESIMS (m/z 406.1751 [M + Na]+; calcd 406.1737) and NMR data (Table 1). The UV spectrum showed maxima ascribable to a substituted benzene ring (213 and 270 nm), and the IR spectrum showed the presence of amide carbonyl (1692 and 1636 cm−1) and hydroxyl (3212 cm−1) groups. The 1H-NMR data (Table 1) revealed a 1,2,4-trisubstituted benzene ring [δH 7.41 (d, J = 8.1 Hz, H-4), 6.94 (dd, J = 8.1 and 2.2 Hz, H-5), and 6.91 (d, J = 2.2 Hz, H-7)], an olefinic proton, δH 5.27 (dd, J = 7.1 and 7.1 Hz, H-19), and two methyl signals, δH 1.51 (3H, s, Me-21) and 1.56 (3H, s, Me-22), along with signals due to several methine and methylene groups. The 13C-NMR data (Table 1) revealed twenty-one carbon resonances, including three amide carbonyls at δC 184.0 (C-2), 170.6 (C-10), and 166.1 (C-13), one oxygen-bearing sp2 carbon at δC 159.8. According to the above features, the NMR spectroscopic data of 1 were very similar to those of spirotryprostatin A [10], the major difference between 1 and spirotryprostatin A being that the methine carbon (δC 60.2, C-18) in spirotryprostatin A was replaced by a methylene carbon (δC 38.2, C-18) in 1. The chemical shift changes and analyses of the degrees of unsaturation of 1 and spirotryprostatin A showed that the bond between C-18 and N-14 in spirotryprostatin A was broken in 1, which was confirmed by the key HMBC correlations from H2-18 to C-2/C-20 and from H-14 to C-9/C-12/C-13 (Figure 2). The other difference between 1 and spirotryprostatin A was that the methoxy (δC 55.5, δH 3.80) in spirotryprostatin A was replaced by a hydroxyl in 1.
Table 1.
1H-NMR and 13C-NMR data of 1 (800 MHz for 1H, 200 MHz for 13C in pyridine-d5).
| Position | δC | δH (J in Hz) | HMBC |
|---|---|---|---|
| 1 (NH) | 11.96 s | C-2, C-3, C-3a, C-7a | |
| 2 | 184.0 | ||
| 3 | 52.8 | ||
| 3a | 122.3 | ||
| 4 | 125.6 | 7.41 d (8.1) | C-3, C-5, C-6, C-3a, C-7a |
| 5 | 109.8 | 6.94 dd (8.1, 2.2) | C-4, C-6, C-7, C-3a |
| 6 | 159.8 | ||
| 7 | 99.2 | 6.91 d (2.2) | C-5, C-6, C-3a, C-7a |
| 7a | 144.7 | ||
| 8 | 36.4 | 3.62 dd (15.0, 1.9) 2.50 dd (15.0, 8.4) |
C-2, C-3, C-18, C-9, C-10 |
| 9 | 53.3 | 4.06 dd (8.4, 1.9) | C-3, C-8, C-10, C-13 |
| 10 | 170.6 | ||
| 12 | 59.2 | 3.87 t (8.2) | C-10, C-13, C-15, C-16, C-17 |
| 13 | 166.1 | ||
| 14 (NH) | 7.81 s | C-9, C-10, C-12, C-13 | |
| 15 | 45.9 | 3.50 dt (11.3, 8.1) 3.37 m |
C-10, C-12, C-17 |
| 16 | 23.2 | 1.63 m 1.53 m |
C-12, C-15, C-17 |
| 17 | 28.4 | 2.10 m | C-12, C-14, C-15, C-16 |
| 18 | 38.2 | 2.84 dd (14.1, 7.1) 2.78 dd (14.1, 7.1) |
C-2, C-3, C-4, C-8, C-20 |
| 19 | 118.9 | 5.27 dd (7.1, 7.1) | C-3, C-18, C-20, C-21, C-22 |
| 20 | 135.9 | ||
| 21 | 26.1 | 1.51 s | C-19, C-20, C-22 |
| 22 | 18.3 | 1.56 s | C-19, C-21, C-22 |
Figure 2.
Key 1H-1H COSY and HMBC correlations of 1.
The relative configurations of C-9 and C-12 were deduced from the NOESY and NOE spectra (Figures S8 and S9 in Supporting Materials). NOESY correlation of H-9 with H-12 indicated that H-9 and H-12 were on the same face of ring C. A strong NOE was observed for H-12 after irradiation of H-9, which also indicated that H-9 and H-12 were on the same face of ring C. Marfey’s method [10] was applied to assign the absolute configuration of the proline residue resulting from acid hydrolysis of 1. HPLC analysis of the 1-fluoro-2,4-dinitrophenyl-5-L-alanine amide (FDAA) derivatives of the acid hydrolysate of 1 gave the same retention time as that prepared from sample of authentic L-proline (Figures S13–S15 in Supplementary Materials). Therefore, the proline residue in 1 was assigned the L-configuration. Considering the relative configurations established between C-9 and C-12 by NOESY and NOE data, the absolute configuration of C-9 was determined to be S. According to the above analyses, the possible absolute configurations of 1 were proposed to be (3S, 9S, 12S) (1a) or (3R, 9S, 12S) (1b). The calculated ECD spectrum of 1a displayed a CD curve similar to the experimental spectrum of 1 (Figure 3). Thus, the structure of 1 was determined as shown in Figure 1, and named spirotryprostatin K.
Figure 3.
Experimental and calculated ECD spectra of 1.
The known compounds, spiro[5H,10H-dipyrrolo-[1,2-a:1′,2′-d]pyrazine-2(3H),2′-[2H]-indole]- 3′,5,10(1′H)trione (2), 6-methoxyspirotryprostatin B (3), pseurotin A (4), N-β-acetyltryptamine (5), and lumichrome (6) were isolated and identified by comparing their experimental physical and spectroscopic data with literature values [5,6,7,8,9].
The isolated compounds 4 indirectly exhibited anti-inflammatory activity by suppressing lipopolysaccharide-induced NO production in mouse macrophages with IC50 values of 5.20 μM, Dexamethasone was used as the positive control, with IC50 = 2.5 × 10−2 μM. The other isolated compounds 2–6 were inactive (IC50 > 10 μM) for the inhibition of NO production.
3. Experimental Section
3.1. General Information
UV spectra were measured on a JASCO V650 spectrophotometer (Jasco, Tokyo, Japan). CD spectra were recorded on a JASCO J-815 spectropolarimeter (Jasco, Easton, MD, USA). IR spectra were recorded on a 5700 FT-IR spectrometer (Nicolet, Madison, WI, USA). HRESIMS data was recorded on a 6250 Accurate-Mass Q-TOF LC/MS spectrometer (Agilent, Santa Clara, CA, USA). NMR spectra were recorded on a Bruker 800 spectrometer (Bruker, Ettlingcn, Germany), for 1D and 2D NMR. Optical rotation was measured on a Jasco P-2000 automatic digital polarimeter (Jasco, Tokyo, Japan). Preparative HPLC was performed on an LC-6AD instrument (Shimadzu, Kyoto, Japan) equipped with an SPD-10A detector (Shimadzu, Kyoto, Japan) using an YMC-Pack ODS-A column (250 × 20 mm, 5 µm). Column chromatography (CC) was performed on silica gel (200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China).
3.2. Fungal Material
The fungus Aspergillus fumigatus was separated from the stem of Erythrophloeum fordii Oliv. by Jun-Gui Dai of the Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, and identified by Xian-Zhi Jiang of the Institute of Microbiology, Chinese Academy of Sciences (Beijing, China).
3.3. Extraction and Isolation
The liquid fermentation was applied to a D101 macroporous resin (eluted with H2O and 95% EtOH), and then the 95% EtOH fraction (1050 g) was extracted with EtOAc and n-butanol. The EtOAc extract (100 g) was subjected to silica gel column chromatography, eluting with CH3OH/CH2Cl2, to yield six fractions (FrA-F). Fraction E (7.6 g) was subjected to Sephadex LH-20 column chromatography eluting with MeOH to give ten fractions FrE1–E10. Fraction E2 (1.3 g) was separated by MPLC with MeOH/H2O (10%−95% MeOH in H2O), then further purified by preparative HPLC with CH3CN/H2O (20:80) to obtain compound 1 (10 mg), 2 (12 mg), 3 (10 mg), and with CH3CN/H2O (30:70) to obtain 4 (9 mg) and 5 (6 mg). Fraction E6 (1.6 g) subjected to Sephadex LH-20 colunm chromatography eluting with MeOH to give 6 (10 mg).
3.4. Product Characterization
Spirotryprostatin K (1): white, amorphous power; −50.1 (c 0.1, CH3OH); IR νmax 3212, 2922, 1692, 1636, 1434, 1203, 1028 cm−1; UV (MeOH) λmax (log ε): 202 (4.19), 213 (4.09), 270 (2.10); CD (MeOH) nm (Δε): 219 (−1.1), 236 (+1.28), 264 (−0.91); 1H-NMR and 13C-NMR see Table 1; HRESIMS m/z 406.1751 [M + Na]+ (calcd for 406.1737, C21H25 N3O4Na).
3.5. Absolute Configuration of 1
An amino acid (l-proline or d-proline standard, 0.2 mg, Sigma, St. Louis, MO, USA) was dissolved in H2O (30 μL) and treated with 1% 1-fluoro-2,4-dinitrophenyl-5-l-alanine amide (FDAA) in acetone (60 μL) and 6% Et3N in 30 μL of H2O at 40 °C for 1 h. After cooling to room temperature, the derivative was analyzed by reversed-phase HPLC with detection by UV absorption at 340 nm. The column [Agilent C18, 5 μm, 4.6 × 150 mm] was eluted with 35% CH3CN in H2O (0.1% trifluoroacetic acid) for 30 min. The standards gave the following retention times: 20.17 min for l-proline, and 23.90 min for d-proline. Compound 1 (1 mg) was hydrolyzed in 6 HCl (0.5 mL) and heated at 150 °C in a sealed vial for 1.5 h to yield the corresponding amino acids. The cooled reaction mixture was evaporated to dryness under reduced pressure, and HCl was removed from the residual acid hydrolysate by repeated evaporation from frozen H2O (1 mL). The amino acid mixture was then treated in the same manner as the standards above (1% FDAA and 6% Et3N). The mixture of FDAA derivatives was filtered, and the filtrate was diluted with H2O and analyzed by HPLC. The FDAA derivative of the amino acid liberated from 1 showed the peak at 20.14 min, matching the retention time of l-proline [11].
3.6. In Vitro Anti-Inflammatory Activity Assays
C57BL6/J mouse macrophages were cultured in 48-well plates in RPMI1640 medium at 37 °C for 24 h. Then, the cells were divided into four groups: the blank control (RPMI1640 medium only), the LPS control (1 μg/mL LPS in RPMI1640 medium), the experimental control (1 μg/mL LPS and the candidate compounds in RPMI1640 medium), and the positive control (10−6 M dexamethasone in RPMI1640 medium). The cells were then incubated at 37 °C for an additional 24 h. From each well, a total of 100 μL of the supernatant was mixed with the same amount of Griess reagent, and the absorbance value was measured at 570 nm using a microplate reader. Sodium nitrite was used as the standard to calculate the NO2− concentration [12,13].
4. Conclusions
A new diketopiperazine alkaloid, spirotryprostatin K (1), was isolated from the endophytic fungus Aspergillus fumigatus. Its structure and absolute configuration were determined by a combination of extensive spectroscopic methods, acid hydrolysis, and ECD calculations. A known alkaloid, pseurotin A (4) exhibited indirect anti-inflammatory activity by suppressing the lipopolysaccharide-induced proinflammatory factors in BV2 microglial cells, with an IC50 of 5.20 μM.
Acknowledgments
We are grateful to Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College for isolating the fungus Aspergillus fumigatus, and Institute of Microbiology, Chinese Academy of Sciences for identifying the fungus Aspergillus fumigatus.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/06/10793/s1.
Author Contributions
Yun-Bao Liu organized the study. Yu-Sheng Shi and Yan Zhang carried out the extraction and isolation. Xiao-Zhong Chen, Ning Zhang interpreted the results and helped write the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1−6 are available from the authors.
References
- 1.Zhang H.W., Song Y.C., Tan R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006;23:753–771. doi: 10.1039/b609472b. [DOI] [PubMed] [Google Scholar]
- 2.Schueffler A., Anke T. Fungal natural products in research and development. Nat. Prod. Rep. 2014;31:1425–1448. doi: 10.1039/C4NP00060A. [DOI] [PubMed] [Google Scholar]
- 3.China Flora Editing Group of China Science Academy . Leguminosae sp., Flora Reipublicae Popularis Sinicae. Volume 39. Science Press; Beijing, China: 1997. Leguminosae sp. pp. 117–119. [Google Scholar]
- 4.Qu J., Wang Y.H., Li J.B., Yu S.S., Liu Y.B. Rapid structural determination of new trace cassaine-type diterpenoid amides in fractions Erythrophleum fordii by liquid chromatography-diode-array detection-electrospray ionization tandem mass spectrometry and liquid chromatography-nuclear magnetic resonance. Rapid Commun. Mass Spectrom. 2007;21:2109–2119. doi: 10.1002/rcm.3069. [DOI] [PubMed] [Google Scholar]
- 5.Li X.J., Zhang Q., Zhang A.L., Gao J.M. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with melia azedarach, and their antifungal, antifeedant, and toxic activities. J. Agric. Food Chem. 2012;60:3424–3431. doi: 10.1021/jf300146n. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M., Wang W.L., Fang Y.C., Zhu T.J., Gu Q.Q., Zhu W.M. Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine-derived fungus Aspergillus sydowi. J. Nat. Prod. 2008;71:985–989. doi: 10.1021/np700737g. [DOI] [PubMed] [Google Scholar]
- 7.Bloch P., Tamm C., Bollinger P., Petcher T.J., Weber H.P. Pseurotin, A new metabolite of Pseudeurotium ovalis STOLKH having an unusual hetero-spirocyclic system. Helv. Chim. Acta. 1976;59:133–137. doi: 10.1002/hlca.19760590114. [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Li X.F., Kim D.S., Choi H.D., Son B.W. Indolyl alkaloid derivatives, Nb-acetyltryptamine and oxaline from a marine-derived fungus. Arch. Pharm. Res. 2003;26:21–23. doi: 10.1007/BF03179925. [DOI] [PubMed] [Google Scholar]
- 9.Grande H.J., van Schagen C., Jarbandhan G.T., Jarbandhan T., Müller F. An 1H-NMR spectroscopic study of alloxazines and isoalloxazines. Helv. Chim. Acta. 1977;60:348–366. doi: 10.1002/hlca.19770600207. [DOI] [Google Scholar]
- 10.Cui C.B., Kakeya H., Osada H. Novel mammalian cell cycle inhibitor, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M Phase. Tetrahedron. 1996;52:12651–12666. doi: 10.1016/0040-4020(96)00737-5. [DOI] [Google Scholar]
- 11.Marfey P. Determination of d-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res. Commun. 1984;49:591–596. doi: 10.1007/BF02908688. [DOI] [Google Scholar]
- 12.Sacco R.E., Waters W.R., Rudolph K.M., Drew M.L. Comparative nitric oxide production by LPS-stimulated monocyte-derived macrophages from Ovis canadensis and Ovis aries. Comp. Immunol. Microbiol. Infect. Dis. 2006;29:1–11. doi: 10.1016/j.cimid.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Mencherini T., Cau A., Bianco G., Della Loggia R., Aquino R.P., Autore G. An extract of Apium graveolens var. dulce leaves: Structure of the major constituent, apiin, and its anti-inflammatory properties. Pharm. Pharmacol. 2006;59:891–897. doi: 10.1211/jpp.59.6.0016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.