Abstract
Aspergillus fumigatus is an opportunistic fungal pathogen that typically infects the lungs of immunocompromised patients leading to a high mortality. H-Ficolin, an innate immune opsonin, is produced by type II alveolar epithelial cells and could participate in lung defences against infections. Here, we used the human type II alveolar epithelial cell line, A549, to determine the involvement of H-ficolin in fungal defence. Additionally, we investigated the presence of H-ficolin in bronchoalveolar lavage fluid from transplant patients during pneumonia. H-Ficolin exhibited demonstrable binding to A. fumigatus conidia via l-fucose, d-mannose and N-acetylglucosamine residues in a calcium- and pH-dependent manner. Moreover, recognition led to lectin complement pathway activation and enhanced fungal association with A549 cells. Following recognition, H-ficolin opsonization manifested an increase in interleukin-8 production from A549 cells, which involved activation of the intracellular signalling pathways mitogen-activated protein kinase MAPK kinase 1/2, p38 MAPK and c-Jun N-terminal kinase. Finally, H-ficolin concentrations were significantly higher in bronchoalveolar lavage fluid of patients with lung infections compared with control subjects (n = 16; P = 0·00726). Receiver operating characteristics curve analysis further highlighted the potential of H-ficolin as a diagnostic marker for lung infection (area under the curve = 0·77; P < 0·0001). Hence, H-ficolin participates in A. fumigatus defence through the activation of the lectin complement pathway, enhanced fungus–host interactions and modulated immune responses.
Keywords: Aspergillus, complement, ficolin, innate immunity
Introduction
Aspergillus fumigatus is the commonest mould pathogen in the developed world and is the primary causative species of the potentially fatal, invasive aspergillosis (IA) in immunocompromised patients. IA is an increasing problem because of the emergence of a number of antifungal-resistant strains and even if treated effectively, IA harbours an infection-associated mortality rate between 30 and 80%.1,2 Transplant patients are at particular risk and it is estimated that up to one in four lung or heart transplant patients will succumb to this infection.3 IA is propagated through the inhalation of small hydrophobic spores (conidia), which penetrate deep into the alveolar space where they germinate, invading the vasculature and disseminating to other organs such as the brain and skin.4,5 Aspergillus fumigatus can be found ubiquitously and is mostly omnipresent, making inhalation difficult to avoid. Fortunately, a functional innate immune system can provide robust and effective responses to aid the removal of spores. The initial defence to the inhalation of A. fumigatus is mucociliary clearance but if this is evaded then an innate immune response comprising type II pneumocytes, alveolar macrophages, neutrophils and serum pathogen-recognizing opsonins, can work synergistically to potentiate conidia recognition and removal.6 Members of one such family of opsonins are named the ficolins.
Ficolins are recently discovered serum opsonins with functions comparable to the widely studied collectins, mannose-binding lectin (MBL) and the surfactant proteins. They are composed of two key domains; an N-terminal collagen-like domain and a C-terminal fibrinogen-like domain with lectin activity highly specific for the acetylated carbohydrate, N-acetylglucosamine (GlcNAc), a key component of the A. fumigatus cell wall.7 Humans possess three types of ficolin; the membrane-bound M-ficolin and the serum types, L-ficolin and H-ficolin. Ficolins primarily function as opsonins whereby they can enhance the phagocytosis of pathogenic microorganisms but they are also capable of activating the lectin complement pathway through association with MBL-associated serine protease 2 (MASP-2).8,9 Novel immunomodulatory functions are also beginning to arise but mechanistic insights into these are in their early stages.10–13
Of the serum ficolins, H-ficolin is undoubtedly the most poorly understood, with the fewest pathogenic targets and a distinct lack of characterization in comparison to L-ficolin. We have recently indicated that the other serum ficolins (L-ficolin and its rodent orthologue, ficolin-A) are capable of enhancing host–pathogen interactions within the fungal airway immunity.10,14 Whereas L-ficolin is not produced in the lung, H-ficolin can be secreted directly by resident type II epithelial cells.15 In addition, previous observations have indicated that H-ficolin could recognize A. fumigatus conidia but this interaction was not characterized in any detail.16
Therefore, we investigated whether opsonization of A. fumigatus by H-ficolin could potentiate the functions of A549 cells, a cell line with characteristics of type II epithelial cells.17 Additionally, we investigated the role of H-ficolin in lectin complement pathway activation, cytokine modulation and we measured the H-ficolin concentrations in the bronchoalveolar lavage (BAL) of lung transplant patients with or without post-transplant infection.
Materials and methods
Ethical approval and patient consent
Sampling of BAL from lung transplant patients from the Royal Brompton and Harefield NHS Foundation Trust was performed under Biomedical Research Unit ethics approval (RBH/AS1).
Fungal pathogens
A clinical isolate of A. fumigatus, obtained from a patient with IA at the Norfolk and Norwich University NHS Foundation Trust (as used in refs 14,18), was sub-cultured on Sabouraud dextrose agar at 37° for 7 days, and conidia were harvested using sterile physiological saline (Oxoid, Basingstoke, UK). Resting live conidia were used immediately or fixed in 4% PBS–formaldehyde for 10 min at room temperature, washed, and resuspended in PBS. Fixed A. fumigatus conidia were stored at 4° for up to 1 month until further use.
Cells and reagents
All experiments were conducted using the A549 adenocarcinomic human alveolar basal epithelial cell line as a model for type II alveolar epithelial cells. A549 cells were maintained in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum and 50 IU/ml penicillin and 50 μg/ml streptomycin. Experiments were all performed in serum-free conditions. Recombinant H-ficolin was purchased from R&D Systems (Minneapolis, MN). FITC was purchased from Sigma-Aldrich (St Louis, MO). The mitogen-activated protein kinase (MAPK) kinase 1/2 (MEK 1/2) inhibitor (U0126), p38 MAPK inhibitor (SB202190) and the c-Jun N-terminal kinase (JNK) inhibitor (SP600125) were purchased from Tocris Biosciences (Bristol, UK).
Detection of infection and H-ficolin in BAL
Bronchoalveolar lavage fluid was collected from lung transplant recipients at Royal Brompton and Harefield NHS Foundation Trust by instilling 200 ml sterile saline into distal airway segments under flexible bronchoscopy. BAL return was centrifuged at 200 g for 10 min. Supernatant was subsequently analysed via the lateral-flow device for Aspergillus antigens, indicative of IA, as previously described19 and/or via detection of galactomannan (GM) using a Platelia™ Aspergillus antigen kit (Bio-Rad, Hercules, CA). For BAL samples, an index of < 0·5 was considered negative, an index of ≥ 0·5 was considered positive for GM.20 Samples were tested for a panel of respiratory viruses (multiplex PCR) and bacteria by culture (B57, UK standard for microbiology investigations).21 High-resolution computed tomography chest imaging was reviewed for evidence of findings consistent with fungal infection.21 The presence of H-ficolin in the BAL fluid of lung transplant patients was detected using a ficolin-3 human ELISA kit (Hycult Biotech, Uden, the Netherlands). Patients were categorized for possible, probable and proven invasive fungal infection according to revised European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) criteria.22
Fluorescent microscopy
To detect the presence of intracellular H-ficolin in A549 cells, the A549 cells were grown in eight-well chamber Permanox slides (Lab-Tek, Nunc, Roskilde, Denmark). Following growth, cells were permeabilized with PBS containing 0·1% Tween-20 before staining with primary monoclonal anti-H-ficolin antibodies (Hycult Biotech) and secondary Alexa Fluor 594 antibodies (Invitrogen, Carlsbad, CA). Cells were visualized using a Zeiss Axioplan II microscope at ×40 objective (Zeiss, Oberkochen, Germany).
Ficolin binding assays
Fixed A. fumigatus conidia were washed twice in ficolin-binding buffer (FBB) (5·0 mm CaCl2, 155·0 mm NaCl, 25·0 mm HEPES, 0·5% BSA, 0·1% Triton X-100). Aspergillus fumigatus was then incubated with 18·4 μg/ml H-ficolin for 1 hr at 37°. Fungi were washed as described above, before antibody-labelling of the Aspergillus–H-ficolin complex with monoclonal mouse anti-H-ficolin antibodies (1 : 100; R&D Systems) for 30 min at 4°. Following washing, the secondary antibody (goat anti-mouse immunoglobulin–FITC; Beckman Coulter, High Wycombe, UK] at a 1 : 200 dilution was incubated with the complex for 30 min at room temperature. Following washing, the Aspergillus–ficolin complex was fixed with 4% PBS–formaldehyde for 10 min at room temperature, washed and resuspended in sterile PBS.
Calcium-dependent binding was investigated in FBB without calcium and in the presence of 5 mm EGTA. For testing the influence of the pH on H-ficolin binding, the pH of FBB was adjusted with concentrated HCl or NaOH to generate a range of pH from pH 3·7 to 10·7.
The carbohydrate-recognition characteristics of H-ficolin were determined using a ligand inhibition assay before Aspergillus binding. Concentrations (0·75, 3·1, 12·5, 25, 50, 100 mm) of various carbohydrates (glucose, GlcNAc, galactose, N-acetylgalactosamine, l-fucose, d-mannose and N-acetylcysteine) were incubated with 18·4 μg/ml of H-ficolin for 2 hr at room temperature. Following the ligand inhibition, the ficolin–sugar complex was then added to the Aspergillus spp. and analysed for binding as described above.
H-Ficolin binding to conidia or hyphae was analysed by flow cytometry (Detection was on the FL1-A detector channel: laser excitation, 488 nm; emission detection, 533/30 nm) using a BD Accuri C6 flow cytometer with BD CFlow software (BD Accuri Cytometers, BD Biosciences, San Jose, CA).
Fungal association assays
Human A549 type II alveolar adenocarcinoma cells were seeded on 48-well plates (Nunc, Roskilde, Denmark, UK) in supplemented RPMI-1640 and grown to semi-confluence at 37° in a 5% CO2 atmosphere. FITC-labelled fixed A. fumigatus conidia were opsonized with 18·4 μg/ml BSA or H-ficolin for 1 hr at 37° in FBB lacking Triton X-100. The H-ficolin-opsonized A. fumigatus conidia (5 × 105) were incubated for 16 hr with adherent A549 cells (ratio of conidia to cells of 5 : 1) at pH 5·7 or pH 7·4 at 37° in a 5% CO2 atmosphere. Following incubation, non-adherent conidia and A549 cells were removed and the adherent cells were washed with warm supplemented RPMI-1640. Adherent cells were subsequently removed by the use of trypsin-EDTA and gentle trituration. Cells were pooled and fixed in 4% PBS–formaldehyde for 10 min at room temperature and analysed by flow cytometry using a BD Accuri C6 flow cytometer with BD CFlow software. The percentage of A549 cells associated with FITC-positive A. fumigatus conidia was determined by gating on the A549 cell population (forward scatter channel/side scatter channel) and calculating the percentage of A549 cells staining positive for fluorescence (FL1-A).
Cytokine determination
Supernatants were collected 24 hr following A549 challenge with live A. fumigatus conidia. Cytokine production from supernatants was quantified using a BD cytometric bead array Human Inflammatory Cytokines kit (BD Biosciences, San Jose, CA). Assays were conducted as outlined in the protocol. In brief, capture beads for the measurement of interleukin-8 (IL-8), IL-1β, IL-6, IL-10 and tumour necrosis factor-α were mixed together before their addition to the sample and standard tubes. Following the addition of capture beads to the samples, Human Inflammatory Cytokine PE Detection Reagent was added to all tubes and incubated for 3 hr in the absence of light. Following incubation, samples were washed in wash buffer for 5 min at 200 g before aspiration of the supernatant, re-suspension in wash buffer and flow cytometry (Exλ 488 nm, Emλ 585/40 nm) and (Exλ 633 nm, Emλ 780/30 nm) on a BD Accuri C6 flow cytometer with BD CFlow® software, collecting 1500 events as outlined in the protocol.
To investigate the role of MAPK signalling in IL-8 production, inhibitors of MEK 1/2 (U0126), p38 MAPK (SB202190) and JNK (SP600125) were added to cells at a concentration of 10 μm for 1 hr before and during A. fumigatus challenge.
Measurement of C3b deposition
Endogenous ficolins were removed from donor serum by passing 5 ml through a 1-ml N-acetyl-d-glucosamine column at 4° overnight. Removal of ficolins from the serum was confirmed using a ficolin-3 human ELISA kit (Hycult Biotech). Ficolin-depleted serum was stored at −80° until use.
To measure lectin-pathway C3 deposition, 96-well Maxisorb plates (Nunc) were coated with fixed A. fumigatus conidial suspensions (optical density at 550 nm of 0·5) or 5 μg/ml N-acetyl BSA in coating buffer (15 mm Na2CO3, 35 mm NaHCO3, pH 9·6) and incubated overnight at 4°. Non-specific proteins were then blocked with 1% (weight/volume) BSA-TBS for 2 hr at room temperature. Once blocked, plates were washed three times with wash buffer (TBS, 0·05% Tween-20, 5 mm CaCl2, pH 7·4). To quantify C3 deposition, 100 μl of ficolin-sufficient/deficient serum was added to the wells in barbital-buffered saline (4 mm barbital, 145 mm NaCl, 1 mm MgCl2, 2 mm CaCl2, pH 7·4). Ficolin-deficient serum was either reconstituted with recombinant H-ficolin (18·4 μg/ml) or left ficolin-deficient for 1·5 hr at 37°. Plates were washed again three times before the addition of primary antibody rabbit anti-human C3c (1 : 500; Dako, Glostrup, Denmark) and incubated for 1·5 hr at 37°. Following three washes, the secondary antibody anti-rabbit alkaline phosphatase (1 : 5000; Sigma-Aldrich) was added and incubated for 1 hr at room temperature. The assay was developed using p-nitrophenyl-phosphate (Sigma-Aldrich) and absorbance was measured at 405 nm. N-acetyl BSA was used as a positive control.
Statistical analysis
Results were expressed as mean ± SD. Descriptive and two-tailed Students t-test analyses were performed using GraphPad prism software version 5(GraphPad Software, Inc., La Jolla, CA, USA).. One-way analyses of variance were performed using SigmaStat software version 3.5(Systat Software GmbH, Erkrath, Germany). A value of P < 0·05 was considered statistically significant. Receiver operating characteristics (ROC) curve analysis was conducted using MedCalc version 13.1.1 (MedCalc Software bvba, Ostend, Belgium).
Results
H-Ficolin binds to A. fumigatus conidia
It has been acknowledged that H-ficolin is capable of binding A. fumigatus, albeit with low affinity.16 We further characterized this binding and showed that the recognition of A. fumigatus by H-ficolin occurred in a pH- and calcium-dependent manner and was inhibited by l-fucose, d-mannose and GlcNAc.
H-Ficolin is produced by type II alveolar epithelial cells.15 However, it was not established whether A549 cells; a widely used model for the type II alveolar epithelium, could also produce H-ficolin. We made the observation that H-ficolin could be found within A549 cells (data not shown).
We also demonstrated H-ficolin binding to A. fumigatus conidia. Following opsonization of fixed A. fumigatus conidia (5 × 105) with H-ficolin (18·4 μg/ml), we observed significant binding (median fluorescence intensity of 632·5 ± 21·0 versus 207·7 ± 1·5 and 215·0 ± 1·0 for H-ficolin-opsonized conidia versus un-opsonized and BSA-opsonized conidia, respectively; P = 4·01 × 10−6, Fig.1a, c).
Figure 1.

Binding characteristics of H-ficolin to Aspergillus fumigatus conidia. H-Ficolin (18·4 μg/ml) was incubated with 5 × 105 conidia in the presence or absence of calcium, in a range of pHs from pH 3·7 to 10·7, or following pre-incubation with a variety of carbohydrates. (a) Representative histogram of H-ficolin binding to A. fumigatus conidia in the presence of calcium. (b) Representative histogram of H-ficolin binding to A. fumigatus conidia in the absence of calcium. (c) Binding of H-ficolin to A. fumigatus conidia in the presence and absence of calcium indicating the median fluorescence intensity (MFI). (d) Binding of H-ficolin to A. fumigatus conidia in a range of pHs from pH 3·7 to pH 10·7. (e) Carbohydrate binding specificity of H-ficolin to A. fumigatus conidia. H-Ficolin was pre-incubated with a range of carbohydrates (glucose, galactose, l-fucose, d-mannose and N-acetylglucosamine) at various concentrations (0–100 mm) before conidial binding at pH 5·7. Results are representative of the average of all the data points gained from three independent experiments. Error bars represent the SD and significance was determined via two-tailed Students t-test. For carbohyrdrate inhibition, significance was determined via one-way analysis of variance and post hoc analysis was conducted using the Tukey test. *P < 0·05.
A characteristic of the related collectin family is the requirement of calcium to bind to their ligands.23 Therefore we investigated H-ficolin binding to conidia in the absence of extracellular calcium. When calcium was removed from the binding buffer and excess calcium was chelated by EGTA, H-ficolin binding was observed to significantly decrease, to the levels observed for the BSA control (median fluorescence intensity of 294·7 ± 13·8 versus 632·5 ± 21·0 and 206·3 ± 3·5 for H-ficolin-opsonized conidia versus un-opsonized and BSA-opsonized conidia, respectively; P = 2·02 × 10−5, Fig.1a and c).
The pH is an important aspect of infection because it decreases at the local site of infection to as low as pH 5·5.24 Therefore, it is vital that the functions of innate recognition proteins are enhanced during host infection. We had previously observed that binding of L-ficolin and its rodent orthologue, ficolin-A, to A. fumigatus was pH-dependent, occurring optimally at pH 5·7.10,14 Additionally, others had highlighted that pH could alter the confirmation of the fibrinogen-like domain24 therefore binding studies were conducted in a range of pH from pH 3·7 to pH 10·7.
Here, binding occurred in a pH-dependent manner, with maximal binding observed in acidic (pH 5·7) conditions. Binding at pH 5·7 was significantly greater than binding at pH 7·4 (median fluorescence intensity of 632·5 ± 21·0 versus 549·3 ± 12·0 for H-ficolin-opsonized conidia in pH 5·7 and pH 7·4 conditions, respectively; P = 0·00398, Fig.1d). However, binding at pH 7·4 was still significant (median fluorescence intensity of 549·3 ± 12·0 versus 207·6 ± 1·5 for H-ficolin-opsonized conidia versus un-opsonized conidia; P = 1·09 × 10−6; Fig.1d).
Ficolins recognize pathogens via carbohydrate moieties on their cell surface. Therefore, to investigate H-ficolin carbohydrate-binding specificity, H-ficolin was pre-incubated with a range of carbohydrates (glucose, galactose, GlcNAc, l-fucose and d-mannose) before incubation with A. fumigatus conidia. l-Fucose, GlcNAc and d-mannose exhibited the greatest binding inhibition (% total inhibitions of 70·0 ± 1·6%, 88·7 ± 7·0% and 93·4 ± 7·0% at maximal carbohydrate concentration of 100 mm and IC50 of < 0·75 mm, IC50 of < 3·25 mm and IC50 of < 3·25 mm for l-fucose, GlcNAc and d-mannose, respectively; Fig.1e). Conversely, pre-incubation with glucose and galactose led to significantly lower percentage inhibitions (% inhibitions of 15·5 ± 1·5% and 24·8 ± 10·5% at maximal carbohydrate concentration of 100 mm for glucose and galactose, respectively; Fig.1e).
H-Ficolin opsonization enhances association of A. fumigatus conidia with A549 cells
H-Ficolin is secreted from type II pneumocytes15 and can be found within A549 cells, which suggests it could be important in potentiating immune responses within the lung. Additionally, patients homozygous for a truncated form of H-ficolin exhibit a recurrence of respiratory infections.26 We were, therefore, interested in whether H-ficolin could modulate the association of A. fumigatus with the A549 airway epithelial cell line.
To investigate the interaction of H-ficolin-opsonized conidia with the alveolar epithelium, FITC-labelled, fixed A. fumigatus conidia were opsonized with H-ficolin before incubation with A549 cells for 16 hr in pH 5·7 or pH 7·4 conditions. A549 cells were gated (Fig.2a) and the percentages that were FITC-negative and FITC-positive were used to identify associated cells (Fig.2b). There was a uniform shift in the fluorescence of the A549 population meaning that the majority of A549 cells were associated with conidia. However, in the presence of H-ficolin, the number of conidia associated per A549 cell was significantly increased (based upon the median fluorescence intensity) but only in inflammatory (pH 5·7) conditions (median fluorescence intensity of 313 045 ± 77 970 versus 596 893 ± 18 846 for un-opsonized and H-ficolin-opsonized conidia, respectively; P = 0·00359, Fig.2c). Furthermore, A. fumigatus were still capable of germinating within A549 cells regardless of H-ficolin opsonization (data not shown).
Figure 2.
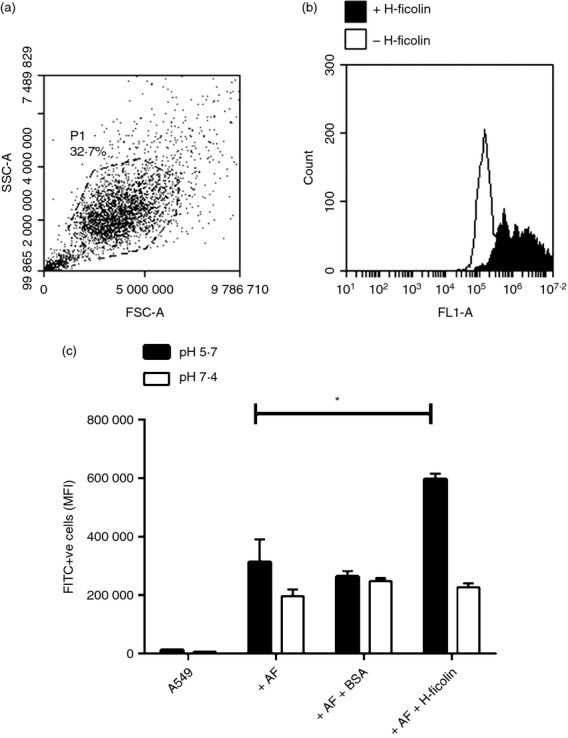
H-Ficolin opsonization enhances association of Aspergillus fumigatus conidia with A549 epithelial cells. FITC-labelled A. fumigatus conidia (5 × 105) were opsonized with 18·4 μg/ml H-ficolin before incubation with A549 cells (conidia : A549 ratio of 5 : 1) in pH 5·7 and pH 7·4 conditions for 16 hr. (a) P1 gate on the A549 population. (b) Representative histogram depicting the fluorescence (FL1-A) of the A549 population following challenge with non-opsonized or H-ficolin opsonized A. fumigatus conidia. (c) The relative number of associated FITC-labelled conidia (based upon the median fluorescence intensity (MFI) either non-opsonized (− H-ficolin), BSA-opsonized (+ BSA) or H-ficolin opsonized (+ H-ficolin). Results are representative of the average of all the data points gained from three independent experiments. Error bars represent the SD and significance was determined via two-tailed Students t-test. *P < 0·05.
H-Ficolin opsonization leads to lectin complement pathway C3b deposition on A. fumigatus conidia
Following the observation that H-ficolin could enhance host–fungus interactions, we explored the potential mechanisms to explain this augmentation. One way in which ficolins could enhance this interaction was through the activation of the lectin complement pathway and deposition of C3b on the pathogen surface. C3b itself can contribute to enhanced phagocytosis of Aspergillus by functioning as an opsonin.27 However, whether H-ficolin could activate the lectin complement pathway on A. fumigatus and contribute to C3 deposition was unknown.8,28
To investigate H-ficolin activation of the lectin pathway on A. fumigatus conidia, human serum was either run through a GlcNAc-sepharose column to remove endogenous ficolins or left ficolin-sufficient. Following the removal of endogenous ficolins, there was significantly diminished C3 deposition on A. fumigatus (P < 0·05) but ficolin-sufficient serum maintained maximal levels of C3 deposition (Fig.3). Here we observed an EC50 of 1·15% serum versus an EC50 of 0·32% serum for ficolin-deficient and ficolin-sufficient serum, respectively (not shown). Moreover, reconstitution of the ficolin-deficient serum with recombinant H-ficolin was capable of significantly restoring the level of C3 deposition to normal levels (EC50 of 0·34% serum; P < 0·05; Fig.3).
Figure 3.

H-Ficolin activates the lectin complement pathway on Aspergillus fumigatus conidia. The restoration of lectin complement pathway C3 deposition was observed on A. fumigatus following the reconstitution of ficolin-depleted human serum with recombinant H-ficolin. Serum dilutions of serum were incubated in Maxisorb microtitre 96-well coated plates and deposited C3 was detected by using rabbit anti-human C3c complement antibodies and goat anti-rabbit IgG-alkaline phosphatase conjugate. N-acetyl BSA was used as a positive control for C3 deposition (*P < 0·05). HS, human serum; HF, H-ficolin; AF, A. fumigatus; NBSA, N-acetyl BSA; rHF, recombinant H-ficolin. All data points are represented as mean absorbance measured at 405 nm in the presence of 1·25% serum and are representative of the data obtained from three independent experiments. Significance was determined via one-way analysis of variance and post hoc analysis was determined using the Tukey test.
Opsonization by H-ficolin increases IL-8 production from A549 cells after A. fumigatus challenge and involves the activation of the intracellular signalling pathways MEK 1/2, p38 MAPK and JNK
Previously, we have observed that another serum ficolin, ficolin-A, is capable of modulating IL-8 production.10 Therefore, we used cytometric bead arrays to measure the concentration of IL-8, IL-1β, IL-6, IL-10 and tumour necrosis factior-α following challenge by H-ficolin-opsonized live A. fumigatus conidia.
From the cytokine panel tested, H-ficolin opsonization induced a significant increase in the secretion of pro-inflammatory IL-8 compared with challenge with un-opsonized conidia after 24 hr (IL-8 concentrations of 611·5 pg/ml ± 56·1 versus 1893·3 pg/ml ± 263 for un-opsonized and H-ficolin-opsonized conidia, respectively; P = 0·00117, Fig.4a). H-Ficolin in the absence of conidia induced an increase in IL-8 but this increase was not statistically significant (P = 0·06725, Fig.4a). All other measured cytokines were not significantly affected by H-ficolin opsonization (data not shown).
Figure 4.

Interleukin-8 (IL-8) secretion from A549 epithelial cells is enhanced following challenge with H-ficolin opsonized Aspergillus fumigatus conidia and is reliant upon mitogen-activated protein kinase (MAPK) kinase 1/2 (MEK 1/2), p38 MAPK and c-Jun N-terminal kinase (JNK) activation. (a) Supernatants from A549 cells were collected 24 hr after challenge with H-ficolin (18·4 μg/ml) alone, conidia (1 × 106) alone or H-ficolin (18·4 μg/ml) -opsonized A. fumigatus (1 × 106 conidia), before cytometric bead array. (b) To investigate the cell signalling pathways involved, A549 cells were pre-incubated with 10 μm of the MEK 1/2 inhibitor (U0126), the p38 MAPK inhibitor (SB202190) or the JNK inhibitor (SP600125) before A. fumigatus challenge. Media from A549 cells without stimulus and lipopolysaccharide (LPS) (100 ng/ml; grey bar) were used as negative and positive controls, respectively. DMSO was used as a vehicle control for MAPK inhibitors (white bar). All data are represented as the average concentration of IL-8 produced (pg/ml) and are representative of the averages of all the data points gained from three independent experiments. Error bars represent the SD. Significance was determined via two-tailed Students t-test. *P < 0·05.
Based upon previous studies that showed that IL-8 secretion from respiratory cells following A. fumigatus challenge was governed by phosphoinositol 3-kinase, p38 MAPK and extracellular signal-regulated kinase 1/2 (ERK 1/2), we chose to inhibit key members within the MAPK signalling pathways.29 To investigate the intracellular signalling pathways involved in the increase of IL-8 secretion from A549 cells following challenge by H-ficolin opsonized A. fumigatus, we used the MEK 1/2 inhibitor U0126, the p38 MAPK inhibitor SB202190 and the JNK inhibitor SP600125 before the quantification of IL-8 by bead array. It was observed that MEK 1/2 inhibition completely abrogated IL-8 production following challenge with un-opsonized or H-ficolin-opsonized conidia (P < 0·001, Fig.4b). Inhibition of p38 MAPK and JNK also significantly reduced IL-8 production both in the presence and absence of H-ficolin opsonization (P < 0·001; Fig.4b).
The incremental increase of IL-8 from A549 cells that was induced by H-ficolin alone in the absence of A. fumigatus also appeared to be significantly reduced through inhibition of MEK 1/2 and JNK but less so by the p38 MAPK inhibitor SB202190 (P < 0·001, Fig.4b).
Raised H-ficolin concentrations in the BAL fluid of lung transplant recipients displaying signs of post-transplant pneumonia
H-Ficolin can be secreted into the BAL fluid from bronchial epithelial cells and alveolar type II epithelial cells; therefore, we chose to investigate the concentration of H-ficolin in the BAL fluid from transplant patients (a high-risk group for invasive aspergillosis) with or without signs of lung infection.
Here, we used an H-ficolin-specific ELISA to detect the presence of H-ficolin in the BAL samples of lung transplant recipients. H-Ficolin was observed to be present in both the lungs of healthy and infected transplant recipients. However, in patients who were diagnosed with invasive pulmonary fungal infection based on EORTC/MSG criteria and/or positive GM/lateral-flow, bacterial or viral infection, H-ficolin BAL concentration was significantly higher compared with healthy recipients (P = 0·00726; Fig.5a). Of the 16 infected lung transplant patients, one was infected with Penicillium spp., one with Staphylococcus aureus, one with rhinovirus, three with Pseudomonas aeruginosa, four with A. fumigatus and six were without detectable cultures but with radiology suggestive of fungal infection or positive GM and/or lateral flow test. Within these groups, patients infected with A. fumigatus had consistently higher concentrations of H-ficolin in the BAL fluid (average concentration of 19·4 ng/ml; Fig.5b). The one patient with rhinovirus had a higher BAL H-ficolin concentration (average concentration of 24·1 ng/ml) but this was only data from one patient (Fig.5b). An ROC curve analysis was conducted to investigate whether the detection of H-ficolin could be used as a potential biomarker/diagnostic tool for lung infection. The area under the curve was calculated to be 0·77, which suggested that there was a 77% chance that infected transplant patients would have H-ficolin present in their BAL fluid (P < 0·0001; Fig.5c).
Figure 5.

Bronchoalveolar lavage (BAL) H-ficolin concentrations from infected lung transplant patients are higher than those found in non-infected patients. The BAL fluid was collected following bronchoscopies on lung transplant patients before the measurement of H-ficolin concentrations by ELISA. (a) The concentration of H-ficolin (ng/ml) in BAL samples. BAL samples were considered positive (n = 16) or negative (n = 16) for infection dependent upon patients classification according to EORTC/MSG criteria, Aspergillus antigen detection, radiology and bacterial, viral or fungal cultures. (b) H-Ficolin concentration in the BAL fluid of patients testing culture positive for Penicillium (Pen; n = 1), Pseudomonas aeuriginosa (PsA; n = 3), A. fumigatus (AsF; n = 4), Staphylococcus aureus (StA; n = 1), rhinovirus (Rhi; n = 1) or culture negative (Nil; n = 6). (c) Receiver operating characteristics curve analysis for H-ficolin detection in infected transplant patients compared with non-infected transplant patients. Results are representative of the data points gained from three independent experiments (16 positive and 16 negative patients). Bars represent the median and significance was determined via two-tailed Students t-test. *P < 0·05.
Discussion
In this study, we focused on the role of H-ficolin in the innate immune recognition of A. fumigatus and its role in host–fungus interactions. This work led to a number of new observations. First, H-ficolin was capable of recognizing A. fumigatus conidia in a calcium-dependent and pH-dependent manner, in an interaction inhibited by d-mannose, l-fucose and GlcNAc. This recognition also led to enhanced host–microbe interactions. Second, H-ficolin opsonization led to activation of complement on A. fumigatus. Third, in the presence of H-ficolin, we observed an increase in the production of IL-8, which was abrogated following inhibition of MEK 1/2, p38 MAPK and JNK. Finally, H-ficolin concentration in the BAL fluid was greater in transplant recipients with post-transplant lung infections compared with uninfected control patients.
This work has, therefore, led us to postulate that as part of an anti-fungal inflammatory response, H-ficolin concentrations are increased in the lung environment. Moreover, H-ficolin recognizes conidia through its cell surface glycoproteins and enhances the immune function of lung epithelial cells in the defence against A. fumigatus.
To date, all of the serum ficolins had been recorded as able to bind to A. fumigatus conidia but of these serum ficolins, H-ficolin binding was least characterized.10,16,30 We therefore proceeded to characterize the manner in which H-ficolin recognized A. fumigatus conidia. H-Ficolin was observed to significantly bind to conidia, albeit much less avidly than serum L-ficolin or its rodent orthologue, ficolin-A. This could be explained by H-ficolin’s much lower affinity for GlcNAc compared with L-ficolin. Similarly to L-ficolin and the related collectins, MBL and the surfactant proteins, H-ficolin binding to A. fumigatus also required the presence of calcium.31,32
Ficolins are capable of distinguishing between self and non-self carbohydrates on pathogenic microorganisms via their fibrinogen-like domains33 and we can identify their specificity for carbohydrates in the pathogen cell wall via competitive inhibition of their fibrinogen-like domains. Few studies to date have highlighted the carbohydrate recognition properties of H-ficolin, with binding previously only observed to a polysaccharide of Aerococcus viridians, acetylated carbohydrates and very few pathogens.16,34,35 The cell wall of A. fumigatus is predominantly composed of glucan, chitin and galactomannan and it is these cell wall glycoproteins that constitute the Aspergillus virulence factors.36 When further deconstructed, the wall is composed of glucose, glucosamine, galactose and mannose.7,37 In the present study, we indicate novel binding targets for H-ficolin in the shape of d-mannose and l-fucose while re-establishing the preference of ficolin binding to GlcNAc. Interestingly, neither d-mannose nor l-fucose was able to inhibit binding of H-ficolin in previous studies but these inhibition studies were not conducted at varying pH.35 These data imply that H-ficolin recognizes its targets via multiple binding sites on the fibrinogen-like domain with additional sites for mannose and/or fucose as well as separate sites for GlcNAc. All in all, the binding of H-ficolin to a range of carbohydrate structures on the conidial surface is essential for efficient pathogen recognition. This could have the potential to influence Aspergillus infection by restricting fungal adhesion and masking the inflammation induced by surface virulence factors. However, those fungi evading immune recognition can provoke an inflammatory response and lead to tissue necrosis, acidifying the local pH.
Based upon our previous observations whereby ficolin-A binding to A. fumigatus was dependent upon the pH in addition to studies characterizing H-ficolin,10,35 we have shown that the affinity of H-ficolin for A. fumigatus is also significantly enhanced in an acidic pH. The pH is an important aspect of infection because it decreases at the local site of infection to as low as pH 5·5.24 Our findings support that the acidic conditions found in inflammatory sites could be capable of potentiating the opsonization of A. fumigatus by H-ficolin.
One of the key biological consequences of opsonization is the enhancement of uptake by host cells. Conidia are very small structures (2–4 μm in size) and because of this, they can penetrate deep into the alveolar space where they encounter the first line of defence in the shape of type II pneumocytes.38 The important role of ficolins in the enhancement of host–microbe interactions is well documented. We have previously shown that L-ficolin and ficolin-A can function as opsonins and enhance association of A. fumigatus and the clinically relevant yeast Cryptococcus neoformans with host cells.10,39 Our current study indicates that H-ficolin is also capable of significantly increasing the association of conidia with A549 cells in comparison to un-opsonized or BSA-opsonized controls, suggesting an important role for H-ficolin in immobilizing the fungus following inhalation.
One such immune mechanism that can lead to enhanced interactions is the activation of complement. Complement activation can lead to the deposition of C3b, which, like H-ficolin, also functions as an opsonin.27 Ficolins are able to activate the lectin complement pathway following the association with MASPs. Therefore, we investigated whether H-ficolin could contribute to complement activation on A. fumigatus. Following the depletion of ficolins from the serum we still observed some C3b deposition. This is probably the result of complement activation by the residual MBL and/or collectin-11 that was not removed by the depletion method. However, following reconstitution of ficolin-depleted human serum with recombinant H-ficolin, we observed a restoration of C3b deposition to normal levels. Previous observations by Ma et al.30 demonstrated a lack of H-ficolin binding to a clinical sample of A. fumigatus and, hence, no complement activation. However, recognition and lectin-pathway activation may be species and strain specific, as highlighted for Pseudomonas aeruginosa.40 Therefore, future studies with a range of established strains may be necessary to further illuminate the role of H-ficolin in lectin complement pathway activation.
Recent advances in the field have highlighted novel functions of ficolin-A, L-ficolin and M-ficolin in the immunomodulation of cytokines.10–12,14 Whether this could also be achieved following H-ficolin opsonization was previously unknown. Indeed, as identified previously for ficolin-A,10 we observed potentiation of IL-8 production following A549 cell challenge with H-ficolin-opsonized conidia. Interleukin-8 is crucial in the recruitment of neutrophils during aspergillosis, which play a key role in A. fumigatus infection.41 The A. fumigatus itself is not a strong stimulator of IL-8 production from the A549 cell line and dampening of the immune response could be a potential immune evasion mechanism.42 Therefore, we postulated that H-ficolin performs an important role in the enhancement of the immune response and could be important in immune responses, such as neutrophil recruitment. Unlike its serum counterparts, ficolin-A and L-ficolin, H-ficolin in the absence of A. fumigatus did not significantly enhance IL-8 production. As H-ficolin is produced directly in the lung, it would not be beneficial for it to increase inflammation in the absence of pathogen challenge; as excess inflammation is detrimental to normal lung function.10
The mechanisms underlying this increase were, and still are, poorly understood, as cell surface receptors for the serum ficolins and signalling pathways involved are unknown. Therefore, we aimed to delineate some of the signalling pathways that may be involved. Following inhibition of the ERK 1/2 activator, MEK 1/2, by the highly potent inhibitor U0126, inhibition of p38 MAPK by SB202190 and inhibition of JNK by SP600125, we observed almost complete abrogation of IL-8 production following A549 cell challenge with H-ficolin-opsonized conidia. Of these, MEK 1/2 inhibition appeared to lead to the greatest inhibition. The ERK 1/2 pathway has been demonstrated to be important for IL-8 synthesis following challenge by pathogens and elucidating how H-ficolin activates this pathway could prove important in the understanding of its immunomodulatory function.29,43,44 As a result of their importance in fungal immunity, potential cell surface receptor targets include Toll-like receptors and C-type lectin-like receptors of the dectin-1 cluster,45–49 but the signalling mechanisms involved require much greater investigation.
In addition to the in vitro studies, we were interested if in vivo observations could highlight an important role for H-ficolin during lung infections. To investigate this, we took lung transplant patients (a high risk group for IA) and measured the concentration of H-ficolin in their BAL fluid. H-Ficolin is normally secreted into the BAL fluid of healthy lungs regardless of infection but there were significantly higher concentrations of H-ficolin in the BAL of lung transplant recipients diagnosed with post-transplant pneumonia compared with healthy recipients. Interestingly, the average BAL H-ficolin concentration was highest in the patients who were infected with A. fumigatus but the absolute concentration of H-ficolin in the lung is difficult to quantify because of the variability of dilution factors during bronchoscopy. Recent in vivo observations have acknowledged that serum ficolins could play an important role in lung defence against pathogenic microorganisms, with serum L-ficolin present in the lung only during fungal infection14 and truncated forms of H-ficolin increasing susceptibility to respiratory infections.26 Although the current sample size is small (32 patients), ROC analysis has indicated that the presence of H-ficolin in the lungs of transplant patients could be a potential human diagnostic biomarker for infection and in combination with fungus-specific markers such as galactomannan, may be used in the diagnosis of fungal infection. However, this diagnostic potential will need to be further investigated in larger clinical trials.
In conclusion, H-ficolin may play a significant role in the defence against A. fumigatus via the activation of the lectin complement pathway, enhanced host–microbe interactions and modulation of the immune response. Yet, the use of the A549 cell line is a limitation to the current study and further investigation using primary airway epithelial cells could yield important observations.
Acknowledgments
The authors would like to acknowledge Professor Christopher Thornton (University of Exeter) for providing lateral-flow devices and Dr Chris Furze for providing complement reagents. S.B., A.A., M.Y., A.S and A.R. performed the experiments. S.B., D.W.S., R.W., D.A.J. and S.S designed the experiments. S.B., D.W.S. and S.S. wrote the manuscript. This work was supported by the Imperial College Healthcare Biomedical Research Centre, the Royal Brompton and Harefield Respiratory Biomedical Research Unit, the Medical Research Council (MR/K002708/1 to A.A., A.S., A.R. and D.A.J.) and the Faculty of Health, University of East Anglia (PhD studentship FMH 04.4.66 C4 to S.B., D.W.S. and S.S.).
Glossary
- BAL
bronchoalveolar lavage
- EORTC/MSG
European Organization for Research and Treatment of Cancer/Invasive Fungal Infection Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group
- ERK
extracellular signal-regulated kinase
- FBB
ficolin-binding buffer
- GalNAc
N-acetylgalactosamine
- GlcNAc
N-acetylglucosamine
- GM
galactomannan
- IA
invasive aspergillosis
- IL-8
interleukin-8
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MASP
MBL-associated serine protease
- MBL
mannose-binding lectin
- MEK
mitogen-activated protein kinase kinase
- ROC
receiver operating characteristics
Disclosures
All authors disclose no conflicts of interest.
References
- Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, Apewokin S, Horn DL. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect. 2012;65:453–64. doi: 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Taccone FS, Van den Abeele AM, et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19:7. doi: 10.1186/s13054-014-0722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS. [WWW document]. URL http://www.nhs.uk/conditions/Aspergillosis/Pages/Introduction.aspx. [accessed on 25 May 2015]
- Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2:403–11. doi: 10.1513/pats.200508-090JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelenz S, Goldsmith DJ. Aspergillus endophthalmitis: an unusual complication of disseminated infection in renal transplant patients. J Infect. 2003;47:336–43. doi: 10.1016/s0163-4453(03)00078-1. [DOI] [PubMed] [Google Scholar]
- Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11:275–88. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- Hearn VM, Sietsma JH. Chemical and immunological analysis of the Aspergillus fumigatus cell wall. Microbiology. 1994;140:789–95. doi: 10.1099/00221287-140-4-789. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Endo Y, Taira S, Sato Y, Fujita T, Ichikawa N, Nakata M, Mizuochi T. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–54. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Endo Y, Fujita T. Cutting edge: complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J Immunol. 2000;164:2281–4. doi: 10.4049/jimmunol.164.5.2281. [DOI] [PubMed] [Google Scholar]
- Bidula S, Kenawy H, Ali Y, Sexton D, Schwaeble W, Schelenz S. Role of ficolin-A and lectin complement pathway in the innate defense against pathogenic Aspergillus species. Infect Immun. 2013;81:1730–40. doi: 10.1128/IAI.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YJ, Kang HJ, Kim JY, Garred P, Lee MS, Lee BL. Mouse mannose-binding lectin-A and ficolin-A inhibit lipopolysaccharide-mediated pro-inflammatory responses on mast cells. BMB Rep. 2013;46:376–81. doi: 10.5483/BMBRep.2013.46.7.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YJ, Doni A, Romani L, Jurgensen HJ, Behrendt N, Mantovani A, Garred P. Ficolin-1-PTX3 complex formation promotes clearance of altered self-cells and modulates IL-8 production. J Immunol. 2013;191:1324–33. doi: 10.4049/jimmunol.1300382. [DOI] [PubMed] [Google Scholar]
- Luo F, Sun X, Wang Y, Wang Q, Wu Y, Pan Q, Fang C, Zhang XL. Ficolin-2 Defends against Virulent Mycobacteria Tuberculosis Infection In Vivo, and Its Insufficiency Is Associated with Infection in Humans. PLoS ONE. 2013;8:e73859. doi: 10.1371/journal.pone.0073859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidula S, Sexton DW, Abdolrasouli A, Shah A, Reed A, Armstrong-James D, Schelenz S. Serum opsonin, L-ficolin, is detected in human lungs of transplant patients following fungal infections and modulates inflammation and killing of A. fumigatus. J Infect Dis. 2015;212:234–46. doi: 10.1093/infdis/jiv027. [DOI] [PubMed] [Google Scholar]
- Akaiwa M, Yae Y, Sugimoto R, Suzuki SO, Iwaki T, Izuhara K, Hamasaki N. Hakata antigen, a new member of the ficolin/opsonin p35 family, is a novel human lectin secreted into bronchus/alveolus and bile. J Histochem Cytochem. 1999;47:777–86. doi: 10.1177/002215549904700607. [DOI] [PubMed] [Google Scholar]
- Hummelshøj T, Ma YJ, Munthe-Fog L, et al. The interaction pattern of murine serum ficolin-A with microorganisms. PLoS ONE. 2012;7:e38196. doi: 10.1371/journal.pone.0038196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KA, Oster CG, Mayer MM, Avery ML, Audus KL. Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp Cell Res. 1998;243:359–66. doi: 10.1006/excr.1998.4172. [DOI] [PubMed] [Google Scholar]
- Bidula S, Sexton DW, Abdolrasouli A, Shah A, Reed A, Armstrong-James D, Schelenz S. Serum opsonin L-ficolin, is detected in human lungs of transplant patients following fungal infections and modulates inflammation and killing of A. fumigatus. J Infect Dis. 2015;212:234–46. doi: 10.1093/infdis/jiv027. [DOI] [PubMed] [Google Scholar]
- Thornton CR. Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin Vaccine Immunol. 2008;15:1095–105. doi: 10.1128/CVI.00068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Haese J, Theunissen K, Vermeulen E, et al. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: analytical and clinical validity. J Clin Microbiol. 2012;50:1258–63. doi: 10.1128/JCM.06423-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigation of Bronchoalveolar Lavage. Sputum and Associated Specimens. UK Standards for Microbiology Investigations. 2014; B57: [WWW document]. URL http://www.hpa.org.uk/SMI/pdf [accessed on 25 March 2015]
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering JK, van Golde LM, Batenburg JJ. Collectins: players of the innate immune system. Eur J Biochem. 2004;271:1229–49. doi: 10.1111/j.1432-1033.2004.04040.x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Vermeulen M, Trevani A, et al. Extracellular acidosis induces neutrophil activation by a mechanism dependent on activation of phosphatidylinositol 3-kinase/Akt and ERK pathways. J Immunol. 2006;176:1163–71. doi: 10.4049/jimmunol.176.2.1163. [DOI] [PubMed] [Google Scholar]
- Garlatti V, Belloy N, Martin L, et al. Structural insights into the innate immune recognition specificities of L- and H-ficolins. EMBO J. 2007;26:623–33. doi: 10.1038/sj.emboj.7601500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthe-Fog L, Hummelshoj T, Honore C, Madsen HO, Permin H, Garred P. Immunodeficiency associated with FCN3 mutation and ficolin-3 deficiency. N Engl J Med. 2009;360:2637–44. doi: 10.1056/NEJMoa0900381. [DOI] [PubMed] [Google Scholar]
- Sturtevant JE, Latge JP. Interactions between conidia of Aspergillus fumigatus and human complement component C3. Infect Immun. 1992;60:1913–8. doi: 10.1128/iai.60.5.1913-1918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali YM, Lynch NJ, Haleem KS, et al. The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection. PLoS Pathog. 2012;8:e1002793. doi: 10.1371/journal.ppat.1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balloy V, Sallenave J, Wu Y, Touqui L, Latgé J, Si-Tahar M, et al. Aspergillus fumigatus -induced Interleukin-8 synthesis by respiratory epithelial cells is controlled by the phosphatidylinositol 3-kinase, p38 MAPK, and ERK 1/2 pathways and not by the toll-like receptor-MyD88 pathway. J Biol Chem. 2008;283:30513–21. doi: 10.1074/jbc.M803149200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YJ, Doni A, Hummelshøj T, et al. Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. J Biol Chem. 2009;284:28263–75. doi: 10.1074/jbc.M109.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, Turner MW. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun. 2000;68:688–93. doi: 10.1128/iai.68.2.688-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan T, Eggleton P, Kishore U, Strong P, Aggrawal SS, Sarma PU, Reid KP. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect Immun. 1997;65:3171–9. doi: 10.1128/iai.65.8.3171-3179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y, Lee S, Kon O, Lu J. Human L-ficolin: plasma levels, sugar specificty, and assignment of its lectin activity to the fibrinogen-like (FBG) domain. FEBS Lett. 1998;425:367–70. doi: 10.1016/s0014-5793(98)00267-1. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Kuraya M, Hamasaki N, Tsujimura M, Shirai H, Fujita T. Activation of the lectin complement pathway by H-ficolin (Hakata antigen) J Immunol. 2002;168:3502–6. doi: 10.4049/jimmunol.168.7.3502. [DOI] [PubMed] [Google Scholar]
- Zacho R, Jensen L, Terp R, Jensenius J, Thiel S. Studies of the pattern recognition molecule H-ficolin: specificity and purification. J Biol Chem. 2012;287:8071–81. doi: 10.1074/jbc.M111.301044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latge JP, Debeaupuis JP, Sarfati J, Diaquin M, Paris S. Cell wall antigens in Aspergillus fumigatus. Arch Med Res. 1993;24:269–74. [PubMed] [Google Scholar]
- Azuma I, Kimura H, Hirao F, Tsubura E, Yamamura Y. Biochemical and immunological studies on aspergillus. II. Immunological properties of protein and polysaccharide fractions obtained from Aspergillus fumigatus. Mycopathol Mycol Appl. 1969;37:289–303. doi: 10.1007/BF02051363. [DOI] [PubMed] [Google Scholar]
- DeHart DJ, Agwu DE, Julian NC, Washburn RG. Binding and germination of Aspergillus fumigatus conidia on cultured A549 pneumocytes. J Infect Dis. 1997;175:146–50. doi: 10.1093/infdis/175.1.146. [DOI] [PubMed] [Google Scholar]
- Schelenz S, Kirchhof N, Bidula S, Wallis R, Sexton DW. Opsonizing properties of rat ficolin-A in the defence against Cryptococcus neoformans. Immunobiology. 2013;218:477–83. doi: 10.1016/j.imbio.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Kenawy HI, Ali YM, Rajakumar K, Lynch NJ, Kadioglu A, Stover CM, Schwaeble WJ. Absence of the lectin activation pathway of complement does not increase susceptibility to Pseudomonas aeruginosa infections. Immunobiology. 2012;217:272–80. doi: 10.1016/j.imbio.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Hammond M, Laptoine G, Feucht P, et al. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J Immunol. 1995;155:1428–33. [PubMed] [Google Scholar]
- Zhang Z, Liu R, Noordhoek J, Kauffman H. Interaction of airway epithelial cells (A549) with spores and mycelium of Aspergillus fumigatus. J Infect. 2005;51:375–82. doi: 10.1016/j.jinf.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yokota K, Ayada K, Yamamoto Y, Okada T, Shen L, Oguma K. Helicobacter pylori heat-shock protein 60 induces interleukin-8 via a Toll-like receptor (TLR)2 and mitogen-activated protein (MAP) kinase pathway in human monocytes. J Med Microbiol. 2007;56:154–64. doi: 10.1099/jmm.0.46882-0. [DOI] [PubMed] [Google Scholar]
- Torok AM, Bouton AH, Goldberg JB. Helicobacter pylori induces interleukin-8 secretion by Toll-like receptor 2- and Toll-like receptor 5-dependent and -independent pathways. Infect Immun. 2005;73:1523–31. doi: 10.1128/IAI.73.3.1523-1531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier A, Kirschning CJ, Nikolaus T, Wagner H, Heesemann J, Ebel F. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell Microbiol. 2003;5:561–70. doi: 10.1046/j.1462-5822.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- Rubino I, Coste A, Le Roy D, et al. Species-specific recognition of Aspergillus fumigatus by Toll-like receptor 1 and Toll-like receptor 6. J Infect Dis. 2012;205:944–54. doi: 10.1093/infdis/jir882. [DOI] [PubMed] [Google Scholar]
- Mambula SS, Sau K, Henneke P, Golenbock DT, Levitz SM. Toll-like receptor (TLR) signaling in response to Aspergillus fumigatus. J Biol Chem. 2002;277:39320–6. doi: 10.1074/jbc.M201683200. [DOI] [PubMed] [Google Scholar]
- Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005;1:e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JL, Metz AE, Horn D, et al. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–46. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]


