Abstract
Estrogen chemical carcinogenesis involves 4-hydroxylation of estrone/estradiol (E1/E2) by P450 1B1, generating catechol and quinone genotoxic metabolites that cause DNA mutations and initiate/promote breast cancer. Inflammation enhances this effect by up-regulating P450 1B1. The present study tested the three authenticated medicinal species of licorice, [Glycyrrhiza glabra (GG), G. uralensis (GU), and G. inflata (GI)], used by women as dietary supplements, for their anti-inflammatory activities and their ability to modulate estrogen metabolism. The pure compounds, liquiritigenin (LigF), its chalcone isomer isoliquiritigenin (LigC), and the GI specific licochalcone A (LicA) were also tested. The licorice extracts and compounds were evaluated for anti-inflammatory activity by measuring inhibition of iNOS activity in macrophage cells: GI > GG > GU and LigC ≅ LicA > LigF. The Michael acceptor chalcone LicA, is likely responsible for the anti-inflammatory activity of GI. A sensitive LC-MS/MS assay was employed to quantify estrogen metabolism by measuring 2-MeOE1 as non-toxic and 4-MeOE1 as genotoxic biomarkers in the non-tumorigenic human mammary epithelial cell line, MCF-10A. GG, GU, and LigC increased 4-MeOE1, whereas GI and LicA inhibited 2- and 4-MeOE1 levels. GG, GU (5 μg/mL), and LigC (1 μM) also enhanced P450 1B1 expression and activities, which was further increased by inflammatory cytokines (TNF-α and IFN-γ). LicA (1 μM, 10 μM) decreased cytokine- and TCDD-induced, P450 1B1 gene expression and TCDD-induced xenobiotic response element luciferase reporter (IC50=12.3 μM), suggesting an antagonistic effect on the aryl hydrocarbon receptor, which regulates P450 1B1. Similarly, GI (5 μg/mL) reduced cytokine- and TCDD-induced P450 1B1 gene expression. Collectively, these data suggest that of the three licorice species that are used in botanical supplements, GI represents the most promising chemopreventive licorice extract for women’s health. Additionally, the differential effects of the Glycyrrhiza species on estrogen metabolism emphasize the importance of standardization of botanical supplements to species-specific bioactive compounds.
Keywords: Aryl hydrocarbon receptor, cytochrome P450 1B1, estrogen carcinogenesis, estrogen metabolism, Glycyrrhiza glabra, inflata, uralensis, inflammation, iNOS, nuclear factor-kappaB, quinone, xenobiotic response element, licochalcone A, isoliquiritigenin
Introduction
Breast cancer is the second leading cause of cancer-related deaths among women in the United States,1 and the risk of developing the disease increases with cumulative exposure to estrogens including hormone therapy (HT).1,2 The chemical mechanism of estrogen carcinogenesis involves metabolism of estrone/estradiol (E1/E2) to 2-hydroxy (non-toxic) and 4-hydroxy estrogen (genotoxic) metabolites by P450 1A1 and P450 1B1 enzymes, respectively (Figure 1).2 These estrogen metabolizing enzymes are typically up-regulated through activation of the aryl hydrocarbon receptor (AhR), which also controls polycyclic aromatic hydrocarbon (PAH) metabolism.3 The genotoxic estrogen o-quinone formed from 4-hydroxy estrogen produces reactive oxygen species (ROS) and DNA adducts that induce malignant transformation of normal breast cells.4–9 Alternatively, the estrogen o-quinones are reduced by NAD(P)H:quinone oxidoreductase 1 (NQO1), which are up-regulated by antioxidant response element (ARE) activation (Figure 1).10
Figure 1. Hypothesis: effect of botanicals on inflammation-stimulated estrogen chemical carcinogenesis.
Inflammation potentiates estrogen chemical carcinogenesis through activation of transcription factor NF-κB which up-regulates estrogen metabolizing enzyme P450 1B1 that is classically regulated by AhR (figure has been simplified for clarity). P450 1B1 increases the estrogen metabolite 4-OHE1 which redox cycles with the genotoxic estrogen quinone 4-OHE1-Q to form ROS. E2 (not shown for clarity) is similarly metabolized to genotoxic metabolites. The hypothesis is that, to prevent estrogen carcinogenesis, several steps in the inflammation-stimulated estrogen carcinogenesis pathway could be modulated by chemopreventive botanicals, as indicated with green arrows.
It is becoming increasingly clear that inflammation plays a major role in breast cancer.11,12 However, the potential of inflammatory stimuli to enhance estrogen metabolism and increase breast cancer risk has unknown implications in women. Inflammation stimulates the NF-κB pathway, leading to induction of the genotoxic estrogen 4-hydroxylase, P450 1B1 (Figure 1).13,14 P450 1B1 is also upregulated by prostaglandin E2 (PGE2)15 and inflammatory cytokines in breast and endometrial cancer cells.15–17 Furthermore, NF-κB can cooperatively bind with AhR to up-regulate xenobiotic response element (XRE)-responsive genes such as P450 1B1.18 As inflammation is often correlated with poor prognoses of both ER(−) and ER(+) breast cancers,11,12,19 inflammation-stimulated estrogen carcinogenesis may take on a larger role in the initiation and progression of breast cancer than initially suspected.
Botanical dietary supplements are popularly used as natural alternatives to traditional HT and often contain a variety of chemopreventive constituents that have anti-inflammatory, antioxidant, chemopreventive, as well as weak estrogenic properties.20–26 The roots of licorice used medicinally according to worldwide pharmacopeias, including the United States Pharmacopeia and Chinese Materia Medica are Glycyrrhiza glabra (GG), G. uralensis (GU), and G. inflata (GI)27,28 with GG being the most popular species used in the United States.25,29 Traditionally, licorice has been used to treat bacterial and viral infections, ulcers, inflammation/swelling, and asthma.25,30 Today, licorice extracts are among some of the most popular botanicals for women’s health in the United States.25 As there is evidence that licorice extracts contain compounds that have anti-inflammatory and chemopreventive properties,22–24 licorice may also have the potential to protect women from estrogen carcinogenesis (Figure 1).
All three Glycyrrhiza species contain the chalcone, isoliquiritigenin (LigC, C for chalcone) either in aglycone form or as its glycosides (Figure 2, Table 1, Figure S1, S2), which induces detoxification enzymes, including NQO1.23,31,32 LigC is in equilibrium with the ERβ selective estrogenic flavanone, liquiritigenin (LigF, F for flavanone).25,33 GI also contains the Michael acceptor chalcone licochalcone A (LicA) as a major compound, which is not present in the other two species (Figure 2, Table 1, Figure S1).34 These differences in the chemical profiles (Table 1, Figure S1) of medicinal licorice extracts suggest that their safety and efficacy in women will also be variable. The three medicinally used licorice species can be identified botanically based on their aerial parts; however, the medical part of the plant, which is the root, has indistinguishable morphology which could lead to misidentification, and confusing biological data.35 In the current study, DNA fingerprinting and chemical profiling have been performed to unambiguously authenticate each species.36 The effect of the individual Glycyrrhiza species (GG, GU, GI) and their constituents (LigF, LigC, LicA) on estrogen oxidative metabolism was then compared. The hypothesis was that these licorice polyphenols might modulate inflammation (i.e. iNOS activity) and P450 1B1 expression, through manipulation of NF-κB and AhR activation, and might decrease the formation of estrogen quinones (Figure 1). The data showed that the three Glycyrrhiza species contain different chemical profiles, which differentially modulate estrogen metabolism further emphasizing the importance of standardization of botanical dietary supplements to species specific bioactive compounds.
Figure 2.
Key bioactive marker compounds in licorice.
Table 1.
Concentration of the Bioactive Markers in Three Licorice Extracts determined by UHPLC-UV.
| Species | Compounds in licorice (% w/w) | ||||
|---|---|---|---|---|---|
| LicAa | LigC | LigC equivalentsb | LigF | LigF equivalents | |
| GG | - | 0.06 ± 0.00 | 2.97 ± 0.01 | 0.24 ± 0.01 | 5.61 ± 0.02 |
| GU | - | 0.09 ± 0.01 | 0.81 ± 0.03 | 0.41 ± 0.01 | 2.96 ± 0.03 |
| GI | 5.42 ± 0.34 | 0.13 ± 0.01 | 2.47 ± 0.05 | 0.12 ± 0.04 | 0.82 ± 0.06 |
LicA was below limit of detection in GG and GU.
The term LigC equivalents is used to represent the total amount of LigC aglycone plus LigC glycosides (isoliquiritin, isoliquiritin apioside, licuraside (Figure 2S) in the crude extract. LigF equivalents is used to represent the total amount of LigF aglycone plus LigF glycosides (liquiritin, liquiritin apioside, liquiritigenin -7-O-apiosylglucoside (Figure 2S)) in the crude extract. The values (% weight compound/weight crude extract) are expressed as mean ± SD from three independent measures.
Materials and Methods
Chemicals and reagents
All chemicals and reagents were purchased from Fisher Scientific (Hanover Park, IL) or Sigma (St. Louis, MO), unless otherwise stated. Liquiritigenin and isoliquiritigenin were obtained from ChromaDex (Irvine, CA). IKK inhibitor VII was purchased from EMD Millipore (Billerica, MA), and 2-methoxyestrone (MeOE1)-1,4,16,16-d4 was obtained from CDN isotope (Pointe-Claire, Quebec). E2, 2- and 4-MeOE1 reference compounds were acquired from Steraloids Inc. (Newport, RI). TNF-α and IFN-γ were purchased from R&D systems (Minneapolis, MN). Cell media were obtained from Invitrogen (Carlsbad, CA).
Plant material, extraction, and characterization
Glycyrrhiza glabra was purchased from a local supplier in Chinatown (Chicago, IL) and Glycyrrhiza uralensis from Indiana Botanical Garden. Glycyrrhiza inflata was collected in Xinjiang province, China, in 2012. All plant materials were identified through a series of macroscopic and microscopic analyses, comparing the plants with voucher specimens from the Field Museum in Chicago, IL. DNA authentication27 confirmed the botanical identity of all Glycyrrhiza species. Standardized licorice extracts were prepared as previously reported.25 The amount of LigC/LigF equivalents was determined via UHPLC-UV. For the analysis, each licorice extract was prepared at 10 mg/mL in 100% MeOH HPLC grade. All samples were analyzed on a Kinetex XB-C18 column and eluted with a gradient composed of H2O 0.1% formic acid (A) and ACN 0.1% formic acid (B).33 Except for licochalcone A, all compounds were isolated from GU.33,37 A standard curve containing the following 11 reference standards was used for their quantitation in each extract. The area under the curve (AUC) was taken at 360 nm for all chalcones (isoliquiritin, isoliquiritin apioside, licuraside, LigC, licochalcone A), and at 275 nm for all flavanones (liquiritin, liquiritin apioside, liquiritigenin 7-O-apiosylglucoside, LigF) (Figure S2). Quantitative results obtained for each LigF glycoside were corrected by a factor corresponding to [molecular weight (MW) of LigF]/[MW of LigF glycoside], thereby leading to their concentration as LigF equivalents; LigC equivalents were likewise quantitated.
Purity determination of the isolated licorice constituents
The purity of each investigated isolated compound was determined by quantitative 1D 1H NMR using the 100% method38 and yielded the following purity percentages (in % w/w): LicA 96.1% (ratio trans/cis = 93/7), LigF 96.6%, and LigC 98.6%.
Cell culture conditions
MCF-10A, HepG2, and RAW 264.7 cells were obtained from American Type Culture Collection (Manassas, VA). MCF-10A cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM/F12) and supplemented with 1% penicillin-streptomycin, 5% horse serum, cholera toxin (0.1 μg/mL), epidermal growth factor (20 ng/mL), hydrocortisone (0.5 μg/mL), and insulin (10 μg/L). Additional media for MCF-10A cells were prepared with DMEM/F12 media without phenol red and supplemented with 5% charcoal-dextran treated horse serum. HepG2 cells were maintained in DMEM/F12 medium with 10% FBS and 1% penicillin-streptomycin. RAW 264.7 cells were maintained in DMEM medium supplemented with 10% FBS and 1% penicillin-streptomycin.
Griess assay for detection of NO2−
This assay to detect NO2− levels produced from cells was performed according to a previous method39,40 with some minor modifications. RAW 264.7 macrophage cells were plated at a density of 12×104 cells/mL in 96-well plates overnight. Cells were treated with the agent for 1 h, prior to addition of LPS (1 μg/mL) for 24 h. Griess reagent (0.5% sulfanilamide, 0.05% (N-1-naphthyl) ethylenediamine dihydrochloride) in 2.5% w/w H3PO4 was prepared, and media (50 μL) was collected from cells and incubated with the Griess reagent (150 μL) for 30 min in 96-well plates at RT. Absorbance was then measured at 530 nm, and concentrations were calculated based on a NaNO2 standard curve.
Analysis of estrogen methyl ethers as their dansylated derivatives by LC-MS/MS
The 2- and 4- MeOE1 metabolites were measured as biomarkers of the 2-OH and 4-OH metabolic pathways using modifications of literature methods (Figure 1).9,41 MCF-10A cells were plated 24 h before treatment, and cells were incubated with E2 (1 μM) in the presence and absence of the agent and vehicle control for 3 days. The internal standard, 2-methoxyestrone-d4, and ascorbic acid (2 mM) were added to the collected cell media which was extracted twice (x 4 mL) with CH2Cl2. Organic layers were combined and dried under a gentle stream of nitrogen. Dansylation reaction was then performed with NaHCO3 (100 μL) buffer (0.1 M, pH 9.5) and 100 μL dansyl chloride in acetone (1 mg/mL), in a 60 °C water bath with agitation for 5 min, and thereafter kept on ice. Samples were analyzed by positive ion electrospray tandem mass spectrometric method using an Agilent 1200 series nano flow LC system (Agilent Technologies, Aanta Clara, CA) coupled to a AB SCIEX QTRAP™ 5500 System (AB SCIEX, Framingham, MA). The liquid chromatography separation was carried out with a 50 mm × 3 mm i.d. Acquity Beh C-18 column packed with 1.7 μm particles (Waters, Milford, MA) and maintained at 40 °C. A total of 5 μL of each sample was injected into the column. The mobile phase (flow rate of 500 μL/min) consisted of 30% MeOH in water with 0.1% (v/v) formic acid as solvent A and 0.1% (v/v) formic acid in MeOH as solvent B. The initial conditions were set at 45% B. The liquid chromatographic gradient was held at 45% B for 1 min followed by a linear gradient to 80% B in 8 min and was held 1 min before equilibrating with initial conditions for 1 min. Mass spectrometer parameters were optimized as follows: the ion spray voltage was 5.5 kV; the source temperature was 500 °C; the curtain gas was 30 psi; the ion source gas 1 (nebulizer) was set to 40 psi and the ion source gas 2 (turbo gas) to 50 psi; the dwell time for each transition was 50 ms. Multiple reactions monitoring transitions were selected as follows: 534.4 – 171.2 for the detection of dansylated MeOE1 and 538.4 – 171.2 for dansylated MeOE1-d4. MS fragmentation pattern of dansylated 4-MeOE1 is shown in supporting information (Figure S3). Quantitation was performed using the Analyst software (Applied Biosystems, Forster City, CA) in SRM mode, and data were normalized to the E2 control treatment in each independent experiment.9
Quantification of P450 1B1 mRNA expression via qPCR
MCF-10A cells at a density of 25 × 104 cells/mL were plated in 6-well plates and incubated with treatments for 24 h. The total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. RNA was reverse transcribed according to Invitrogen’s SuperScript® III First-Strand Synthesis System for RT-PCR. The resulting cDNA (2 μL) was used for real-time PCR quantification using the StepOnePlus™ Real-Time PCR System (Applied Biosystems, Carlsbad, CA). Taqman gene expression master mix and P450 1B1 primer with FAM/MGB probe (Applied Biosystems, Carlsbad, CA) were added to a 96-well reaction plate with cDNA. Real-time quantitative PCR consisted of one cycle of 50°C for 2 min and 95 °C for 10 min and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Data were analyzed with the comparative CT (ΔΔCT) method, using HPRT1 as the endogenous control. The results are expressed as fold difference in reference to the HPRT1 control.
EROD assay for P450 1A/1B activity
Ethoxyresorufin-O-deethylation (EROD) activity was measured in both MCF-10A cells and with recombinant proteins as previously described for enzyme modulation and inhibition.42,43 For enzyme modulation, MCF-10A cells were plated in 96-well plates for 24 h. Compounds were added 1 h prior to TCDD (10 nM) for 48 h. Cells were washed twice with PBS and incubated with ethoxyresorufin (2.5 μM) and salicylamide (1.5 mM) at 37°C. For enzyme inhibition, MCF-10A cells pretreated with TCDD for two days were washed with PBS and pre-incubated with test compounds for 5 min. Subsequently, ethoxyresorufin (2.5 μM) and salicylamide (1.5 mM) were added at 37 °C. For the recombinant protein inhibition assay, 0.15 pmole of CYP1A1 or 0.8 pmole of CYP1B1 (BD Biosciences, Woburn, MA) per well was pre-incubated with test compounds or 2,3′,4,5′-tetramethoxystilbene (TMS) for 5 min at 37 °C in 50 mM potassium phosphate buffer (pH = 7.4) with 1 mM NADPH before adding ethoxyresorufin (2.5 μM). EROD activity was measured as resorufin formation by fluorescence with excitation at 530 nm and emission at 590 nm every minute for 20 min at 37 °C using BioTek’s Synergy H4 Multi-Mode reader (Winooski, VT). A resorufin standard curve was used to calculate the P450 1A/1B activity.
XRE-luciferase reporter assay
HepG2 cells were plated in 12-well plates overnight. Cells were transfected at 70% confluency with Promega’s (Madison, WI) XRE pGL4.43 luciferase plasmid (1 μg) and pRL-TK (500 ng) using Lipofectamine® 2000 reagent (Invitrogen, Grand Island, NY) for 6 h. After 6 h transfection, cells were incubated with treatments for 24 h and lysed with passive lysis buffer. Lysates were centrifuged and analyzed for luciferase activity according to Promega’s Dual-luciferase® Reporter Assay System protocol using the FLUOstar OPTIMA luminometer (BMG Labtechnologies, Germany).
Statistics
The data were expressed as mean ± SEM of three independent experiments. Significance was determined using one-way ANOVA with Dunnett’s post-test, comparing sample columns to the control. For statistical analysis of P450 1B1 mRNA expression, the student’s t-test was employed to compare samples to DMSO control and samples with cytokines to cytokine control.
Results
Bioactive compounds in Glycyrrhiza species
In order to directly characterize and compare the most commonly used medicinal licorice species, we authenticated each species by DNA fingerprinting and chemical profiling.36 Various glycosidated forms of LigF and LigC were present in the three Glycyrrhiza species (Figure S1 and S2). Their content was collectively expressed as % w/w of each extract as LigF equivalents (LigF, liquiritin, liquiritin apioside, and liquiritigenin 7-O-apiosylglucoside) and LigC equivalents (LigC, isoliquiritin, isoliquiritin apioside, and licuraside) (Table 1, Figure S1, S2).36 Chalcone and flavanone glycosides can be considered as bio-precursors of the corresponding aglycones, LigF and LigC, as they are enzymatically deglycosylated by the gut flora in vivo.44,45 LicA was only present in GI (5.42%), and not in glycosidated form. Due to the high content of LicA, the GI extract contained the highest total amount of chalcones (Table 1), which are known for their anti-inflammatory and detoxification enzyme inducing properties.24,32,46,47 In contrast, GU contained the lowest chalcone amount. These profiles showed that the three licorice extracts differed substantially in their composition of bioactive compounds.
Licorice extracts/compounds differentially modulated iNOS activity in macrophage cells
Since inflammation can increase oxidative estrogen metabolism (Figure 1),17 the anti-inflammatory activity of the licorice species and their major bioactive compounds was determined. Previous studies have measured the anti-inflammatory activities of some licorice species;28,48–50 however, the current studies represent the first direct comparison of authenticated extracts and pure compounds. Among the three Glycyrrhiza species, GI was the most anti-inflammatory extract in RAW 264.7 macrophage cells, reducing iNOS activity to 35% of the LPS control versus 67% of control for GG at 20 μg/mL (Figure 3A). No anti-inflammatory effect was detected up to 20 μg/mL for GU. To determine the effect of the compounds on iNOS activity, LigF, LigC, and LicA were tested in the Griess assay (Figure 3B). The chalcones, LicA and LigC, reduced iNOS activity in macrophage cells to 50% at 10 μM and to 25% and 20% (20 μM) of the LPS control, respectively. LigF did not reduce iNOS activity. These data showed that GI’s anti-inflammatory activity is mainly due to LicA, which is only present in GI.
Figure 3. Licorice extracts/compounds differentially modulated iNOS activity in macrophage cells.
Nitrite levels from macrophage RAW 264.7 cells were detected with Griess reagent after 24 h treatment with LPS (1 μg/mL) and A) GG, GU, and GI and B) licorice compounds LigF, LigC, and LicA. Results are the means ± SEM of three independent experiments analyzed by one-way ANOVA with Dunnett’s multiple comparison post-test, *p < 0.001.
Optimization of LC-MS/MS assay for detection of estrogen metabolites
Analysis of 2- and 4-MeOE1 from MCF-10A cells was performed as described previously9,41 with several modifications to optimize the method. The time of incubation was reduced from 6 days to 3 days, which also reduced the volume of media to be extracted. In addition, a more sensitive LC-MS/MS system (AB SCIEX QTRAP™ 5500 System) enhanced sensitivity and signal to noise ratio and the Agilent 1200 series LC system reduced the run time per sample from 32 min to 10 min, which increased through-put (Figure S4). In addition, the injection volume was reduced from 10 μL to 5 μL. These methodological improvements gave enhanced sensitivity (>100-fold relative to previous studies)9 and allowed for rapid detection of estrogen methyl ethers, to determine the effect of extracts/compounds on metabolism.
Licorice extracts/compounds differentially modulated estrogen metabolism
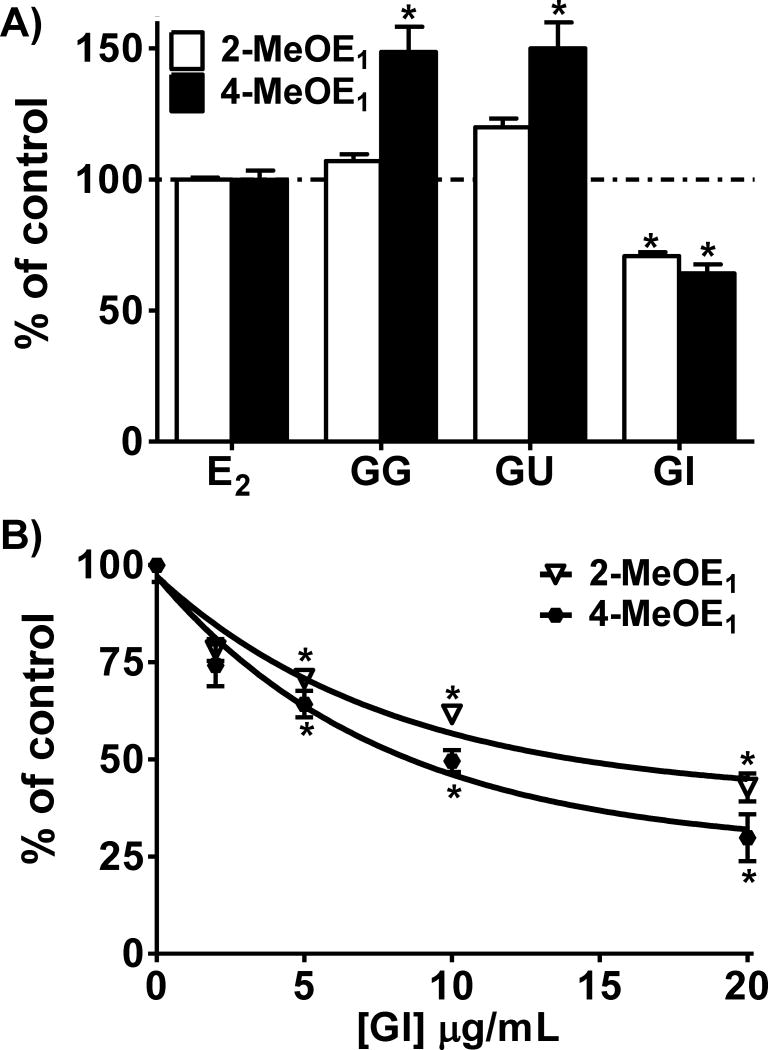
The improved LC-MS/MS assay was utilized to determine if the Glycyrrhiza species and their compounds alter estrogen oxidative metabolism. The amount of 2- and 4-MeOE1 produced in the control cells treated with E2 was set to 100%. GI (5 μg/mL) was the only Glycyrrhiza species tested which inhibited the formation of 2-MeOE1 and 4-MeOE1 metabolites in MCF-10A cells (Figure 4A, B). In contrast, both GG and GU increased 4-MeOE1 to 150% of control. Experiments with LigC showed that it significantly increased both 2-MeOE1 and 4-MeOE1 at 1 μM and gave a maximum enhancement up to 300% of control at 10 μM (Figure 4C, D). In contrast, LicA dose-dependently reduced both 2-MeOE1 and 4-MeOE1 to 37% and 12% of control, respectively, at 10 μM (Figure 4C, D). LigF very moderately increased both metabolites at 10 μM likely due to equilibration with its chalcone isomer LigC.37 These data showed that the three medicinal licorice extracts and their bioactive compounds have differential effects on estrogen metabolism.
Figure 4. Licorice extracts/compounds differentially modulated estrogen metabolism.
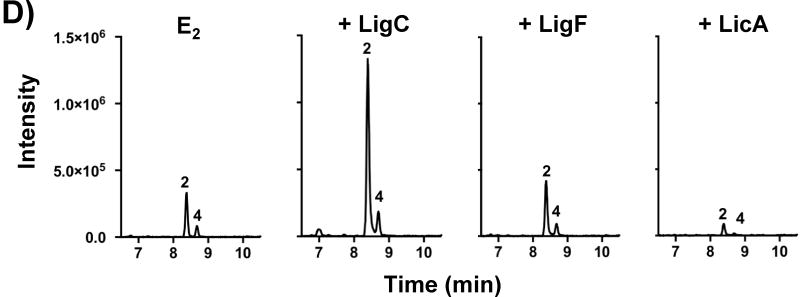
MCF-10A cells treated for 3 days with E2 (1 μM) and A) GG, GU, and GI (5 μg/mL) or B) various doses of GI. C) After 3 days treatment of MCF-10A cells with E2 (1 μM) and LigF, LigC, and LicA, media were collected and analyzed for 2-MeOE1 and 4-MeOE1 metabolites by LC-MS/MS. Results are the means ± SEM of three independent experiments analyzed by one-way ANOVA with Dunnett’s multiple comparison post-test, *p < 0.001. D) Representative SRM chromatograms with transition 534.4 – 171.2 showing 2-MeOE1 and 4-MeOE1 peaks after 3 days treatment of MCF-10A cells with E2 (1 μM) and LigF, LigC, and LicA (10 μM).
Licorice extracts/compounds differentially modulated inflammation-stimulated P450 1B1 gene expression in MCF-10A cells
It has been shown that the inflammatory cytokine TNF-α induces P450 1B1 gene expression in breast cancer cell lines (MCF-7).17 Similar experiments were performed using non-tumorigenic breast epithelial cells (MCF-10A). Treatment with TNF-α alone had little effect on P450 1B1, whereas co-treatment with TNF-α and IFN-γ elicited a 3-fold induction (Figure 5A, Figure S5). To determine if AhR or NF-κB inhibition could reduce cytokine-stimulated P450 1B1 mRNA, cells were treated with a combination of cytokines (TNF-α and IFN-γ) and AhR antagonist (CH-223191)51 or NF-κB pathway inhibitor (IKK inhibitor VII).52 Both inhibited cytokine-stimulated P450 1B1 to nearly the levels of the DMSO control. These data extended literature reports13,16,17,53 by showing that inflammatory cytokines could induce P450 1B1 in “normal” breast epithelial cells (MCF-10A) as well as in cancer cells.
Figure 5. Licorice extracts/compounds differentially modulated inflammation-stimulated P450 1B1 gene expression in MCF-10A cells.
P450 1B1 mRNA expression analyzed after 24 h via qPCR in MCF-10A cells with cytokines (TNF-α and IFN-γ, 10 ng/mL each) (black bars) and without cytokines (open bars). A) AhR antagonist CH-223191 (100 nM) and NF-κB pathway inhibitor IKK inhibitor VII (2 μM). MCF-10A cells treated with B) GG, GU, and GI (5 μg/mL) C) LigF, LigC, and LicA (1 μM). Results with extracts/compounds were analyzed by Student’s t-test, *p < 0.05 (GG, GU, LigC) and *p < 0.001 (GI and LicA) to compare treatment groups to DMSO and cytokine controls. D) Dose-response for LicA (open squares) and LicA + TNF-α and IFN-γ (black squares). Results are the means ± SEM of three independent experiments analyzed by one-way ANOVA with Dunnett’s multiple comparison post-test, *p < 0.001.
To determine the effect of licorice on inflammation-stimulated P450 1B1 mRNA expression in MCF-10A cells, extracts of GG, GU, and GI (5 μg/mL) were incubated with and without cytokines for 24 h (Figure 5B). Both GU and GG increased P450 1B1 mRNA expression, particularly in the presence of cytokines. In contrast, GI treatment led to a 10-fold reduction with or without cytokines. To explain this difference in activity between Glycyrrhiza species, LigF, LigC, and LicA were also evaluated (Figure 5C). LicA (1 μM) strongly inhibited P450 1B1 mRNA expression to 20% of DMSO control and 5% of cytokine controls (Figure 5C). LicA also dose-dependently reduced basal and cytokine-induced P450 1B1 mRNA levels (Figure 5D) significantly at 0.5 μM. In contrast, LigC (1 μM) increased P450 1B1 mRNA expression to approximately 200% of the DMSO control and potentiated cytokine-induced P450 1B1 mRNA expression by an additional 2-fold (Figure 5C). LigF did not affect P450 1B1 mRNA expression. These data showed that LicA and LigC and the three licorice species have differential effects on P450 1B1 expression, which mirror estrogen metabolism data.
Licorice compounds modulated TCDD-induced P450 1B1 gene expression, P450 1A/1B activity, and XRE-luciferase reporter activity
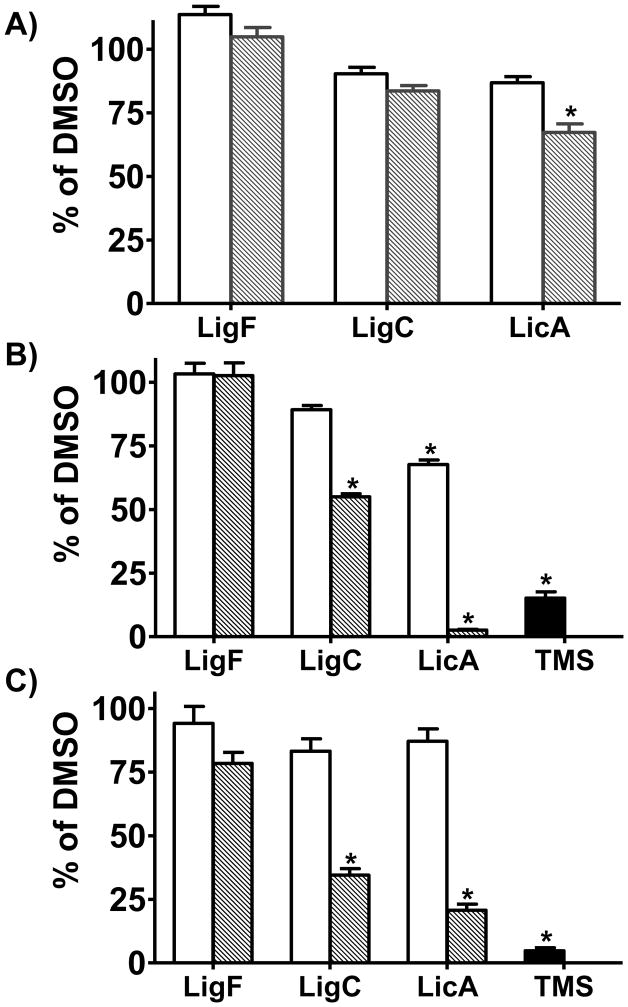
In order to determine the mechanism of licorice compound modulation of P450 1B1, MCF-10A cells were treated with the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD).54,55 TCDD strongly increased P450 1B1 induction 20-fold. LigC slightly increased TCDD-induced P450 1B1 mRNA expression, whereas LigF had no effect (Figure 6A). In contrast, LicA (10 μM) treatment dramatically decreased TCDD-induced P450 1B1 mRNA expression to 10% of the TCDD response (Figure 6A). Since licorice compounds modulated estrogen metabolism and P450 1B1 expression, P450 1A/1B activity was also determined in MCF-10A cells after treatment of cells with DMSO or TCDD and compounds for 48 h (Figure 6B). TCDD induced P450 1A/1B activity 30-fold. LicA (10 μM) completely inhibited both P450 1A/1B activities of DMSO and TCDD controls. In contrast, LigC (10 μM) enhanced P450 1A/1B activity to more than 5-fold and also increased TCDD-induced P450 1A/1B activity significantly. LigF had no effect on P450 1A/1B activity. These results mirrored the observations made with estrogen metabolism and P450 mRNA expression, in which LicA inhibited, LigC stimulated, and LigF had little effect.
Figure 6. LicA down-regulated TCDD-induced P450 1B1 mRNA expression, P450 1A/1B activity, and XRE-luciferase activity.
A) P450 1B1 mRNA expression was analyzed via qPCR after 24 h treatment of MCF-10A cells with TCDD (10 nM) and licorice compounds (10 μM). B) P450 1A/1B activity measured via EROD assay in MCF-10A cells after treatment with compounds (10 μM) 1 h prior to treatment with TCDD (10 nM) for an additional 48 h. C) HepG2 cells were incubated with TCDD (10 nM) and LicA for 24 h before analysis of XRE-luciferase reporter activity. Results are the means ± SEM of three independent experiments analyzed by one-way ANOVA with Dunnett’s multiple comparison post-test, *p < 0.001.
To investigate the mechanism by which LicA inhibited P450 1B1 gene expression and P450 1A/1B activities, XRE-luciferase reporter activity was analyzed in HepG2 cells. LicA dose-dependently inhibited TCDD-induced XRE-luciferase reporter activity showing significant inhibition (Figure 6C, IC50 = 12.3 μM). While LigC slightly increased XRE-luciferase reporter activity, LigF had no effect (Figure S6). These data suggest that LicA can antagonize AhR, resulting in downregulation of P450 1A/1B, leading to inhibition of estrogen metabolism. In contrast, LigC may only be a very weak AhR agonist and likely has other biological targets, which induce P450 1B1 expression and P450 1A/1B activity.
LicA only moderately inhibited P450 1A/1B activity in MCF-10A cells and did not show P450 1A1 or 1B1 selectivity
It is possible that the modulatory effects observed above could be due to direct effects on P450 1A/1B activity. We performed inhibition assays using recombinant proteins as well as testing the overall P450 1A/1B inhibitory effect in MCF-10A cells. After induction of P450 1A/1B with TCDD, cells were treated with compounds and substrate (EROD) for 20 min, to directly determine the effect of the compounds on P450 1A/1B enzyme activity. No inhibition of P450 1A/1B activity was observed in the MCF-10A cells except at the highest concentration of LicA (10 μM, Figure 7A). Inhibitory effects were only observed in experiments with the purified enzymes at the highest concentrations of chalcones (10 μM) and no selectivity of P450 1A/1B enzyme activity was detected (Figure 7B, C). These data suggest that modulation of P450 protein levels by compounds in licorice extracts rather than direct inhibitory effects are most likely responsible for changes in estrogen metabolism.
Figure 7. LicA only moderately inhibited P450 1A/1B activity in MCF-10A cells and did not show P450 1A1 or 1B1 inhibition selectivity.
A) MCF-10A cells were pretreated with TCDD (10 nM) for 48 h to induce P450 enzymes and thereafter pre-incubated with LigF/LigC/LicA (1 μM, open bars) and (10 μM, striped bars) for 5 min at 37°C before ethoxyresorufin and NADPH was added for 20 min. B) Human recombinant P450 1A1 and C) P450 1B1 protein with reductase were incubated with ethoxyresorufin, NADPH and LigF/LigC/LicA (1 μM, open bars) and (10 μM, striped bars) or 2,3′,4,5′-tetramethoxystilbene (TMS) (2 μM) at 37°C for 20 min. EROD activity was measured and results were analyzed by one-way ANOVA with Dunnett’s multiple comparison post-test, * p < 0.001.
Discussion
The women’s health initiative has shown that traditional HT can increase breast cancer risk. Estrogens are metabolized by P450 1A1/1B1 to 2- and 4-hydroxy metabolites, respectively. The 4-hydroxy metabolite forms genotoxic quinones that cause depurinating, mutagenic DNA adducts. P450 1A1 2-hydroxylation generates quinones that form stable adducts, which are not tumorigenic.56 It is also known that inflammation could enhance chemical estrogen carcinogenesis by up-regulating P450 1B1 (Figure 1),13–17, 51 while down-regulating P450 1A1.13 In this study, cytokines up-regulated P450 1B1 in non-tumorigenic breast epithelial cells (MCF-10A), similar to what was observed in MCF-7 breast cancer cells.16 When either AhR or NF-κB activation was inhibited with an antagonist and IKK inhibitor, cytokine-induced P450 1B1 expression was reduced to nearly DMSO control levels (Figure 5A). These data suggested that botanicals that target both NF-κB and AhR might inhibit estrogen oxidative metabolism.
Licorice is currently used in many women’s botanical dietary supplements because of its estrogenic properties, likely due to the weak ER ligand, LigF.25,57 Only three licorice species, GG, GU, and GI, are approved for use in licorice botanical dietary supplements, which usually consist of GG in the United States.25 The roots of the three licorice species have indistinguishable morphology and could be misidentified.35 Thus, DNA fingerprinting and chemical profiling must be performed to authenticate each species. In this study, the three Pharmacopeial licorice species have been carefully authenticated and chemically profiled and systematically analyzed for their effect on inflammation and estrogen metabolism.
The anti-inflammatory activities between these three Glycyrrhiza species were highly dependent on the amount of chalcones. GI was the most anti-inflammatory botanical compared to GU and GG (Figure 3A), probably because of the high quantity of the Michael acceptor chalcone, LicA (Figure 3B). In support of the importance of the α,β-unsaturated carbonyl in LicA, it has been shown that reducing the double bond eliminated its anti-inflammatory effects.58 GG also showed anti-inflammatory activity likely due to LigC. GU showed no anti-inflammatory effect, since the concentrations of LigC was much lower than in GG. Both LicA and LigC Michael acceptors could potentially modify cysteine residues on NF-κB covalently, leading to inhibition of NF-κB DNA binding and in turn iNOS expression/activity, similar to other alkylating agents that modify p50 or p65 NF-κB subunits.59–61 Alternativley, it has been reported that LigC might demonstrate anti-inflammatory activity through suppression of IKK, ERK1/2 and p38 phosphorylation.22 The flavanone LigF, which lacks the Michael acceptor moiety, did not have anti-inflammatory activity. These results were similar to what has been reported in other anti-inflammatory studies which showed that LigC > LigF.62,63 Anti-inflammatory activities have also been shown for different extracts of GG,28,49 GU,28,50 and GI.48 However, various experimental conditions were used making comparisons between the anti-inflammatory activities of the licorice species difficult. The present study gives a direct comparison of authenticated and chemically profiled licorice species and their bioactive compounds on reducing inflammation in a cell based system.
The LC-MS/MS methods previously described9,41 were optimized to monitor the effect of licorice extracts/compounds on P450 mediated estrogen metabolism by measuring 2-MeOE1 (non-toxic biomarker) and 4-MeOE1 (genotoxic biomarker) in MCF-10A cells. Only GI inhibited estrogen metabolism (Figure 4), as well as reducing basal and cytokine-induced P450 1B1 mRNA levels (Figure 5). LicA, the major compound in GI (Table 1), was also the only compound that inhibited estrogen metabolism (Figure 4C, D), P450 1B1 mRNA levels (Figure 5C, D, 6A), TCDD-induced P450 1A/1B activity (Figure 6B), and TCDD-induced XRE-luciferase reporter activity (Figure 6C), all at low μM concentrations. Collectively, these data suggested that LicA modulates estrogen metabolism through antagonizing AhR. However, since it is possible that LicA might directly inhibit P450 1A1/1B1 activity, inhibition assays were carried out with MCF-10A cells and recombinant proteins. While LicA and LigC led to a significant inhibition of recombinant P450 1A1/1B1 enzyme activity (Figure 7B, C), LicA (10 μM) only inhibited 25% of P450 1A/1B enzyme activity in MCF-10A cells (Figure 7A). No selective inhibition of P450 1A1 or 1B1 was detected. These data suggest that regulatory suppression of P450 1B1 mRNA/protein levels by LicA (0.5 μM, Figure 5D) rather than inhibition of enzyme activities (10 μM, Figure 7A) dominates its inhibitory effect on 2- and 4-MeOE1 formation.
Other natural products have been shown to inhibit estrogen oxidative metabolism. For instance, the chemopreventive compound, resveratrol, reduces estrogen oxidative metabolism.64,65 Resveratrol (20–25 μM) reduced TCDD-induced P450 1B1 expression, decreased oxidative estrogen metabolism, and DNA damage,64,65 resulting in reduced E2/TCDD-induced malignant transformation in MCF-10F cells.64 Resveratrol reduced P450 1A1 or 1B1 expression in several cell lines and decreased TCDD-induced AhR binding,66,67, 65 suggesting that resveratrol is a natural AhR antagonist in the low μmolar range.67–69 Another study showed that resveratrol might not only inhibit 4-OHE2-induced MCF-10A transformation through its ability to function as an AhR antagonist, but also through its inhibiting effects on NF-κB signaling.70 Similar to resveratrol, LicA inhibits oxidative estrogen metabolism in the low μM range, most likely through inhibiting AhR signaling and possibly through its anti-inflammatory effects. Further studies will determine if LicA can also reduce E2-induced malignant transformation in MCF-10A cells similar to resveratrol.
Induction of phase II enzymes by natural compounds is another mechanism that reduces genotoxic estrogen metabolites. Sulforaphane (10 μM), for example, is a potent chemopreventive compound that induces phase II enzymes, downregulated P450 1B1 protein levels by 50% and reduced estrogen DNA adducts by 60% in MCF-10A cells. Reduction of DNA adducts by sulforaphane was also observed in experiments with siKeap1, which suggests that inducers of the Keap1/Nrf2 pathway can significantly inhibit estrogen oxidative metabolism through up-regulating enzymes that detoxify genotoxic estrogen quinones.71 GI and LicA both activated the Keap1/Nrf2/ARE pathway in different cell lines, resulting in significant up-regulation of NQO1 in Hepa1c1c7 hepatoma cells and moderate induction in MCF-10A cells.72 These additional chemopreventive activities may increase LicA’s and GI’s overallchemopreventive potential.
In contrast to the inhibitory effects of GI, licorice extracts GG and GU stimulated estrogen metabolism (Figure 4A). Interestingly, in spite of LigC’s anti-inflammatory activity, it enhanced oxidative estrogen metabolism (Figure 4C) and P450 1B1 expression (Figure 5C). P450 1B1 induction by LigC is in line with Cuendet et al.’s results, which previously reported that LigC increases P450 1A1 in mammary tissues.23 XRE-luciferase reporter activity was measured to determine if LigC increased 2- and 4-MeOE1 through activation of AhR. LigC (1.5-fold induction) did not significantly activate XRE-luciferase reporter activity compared to a 70-fold induction with TCDD (Figure S6). In support of these data, Wong et al. showed that LigC did not activate XRE-luciferase reporter activity or affect the binding of AhR to XRE, as analyzed with EMSA in MCF-7 cells.73 LigC is possibly a very weak AhR agonist and/or acts via numerous other AhR-independent pathways that regulate P450 1A1/1B1 induction.74 Future mechanistic studies will explore the specific biological targets of LigC. The slight induction of estrogen metabolism seen with LigF (Figure 4C) may be the result of isomerization of LigF to LigC over the three day incubation,37 especially since LigF had no effect on P450 1B1 gene expression after 24 h (Figure 5C).
Interestingly, GG and GU increased both 4-MeOE1 and P450 1B1 mRNA levels (Figure 4A, Figure 5B). The concentration of LigC even if all of the glycosides were hydrolyzed (i.e. LigC equivalents) in GG and GU are still much lower than those of pure LigC tested (μM) suggesting that additional constituents in the licorice extracts that were not evaluated in these studies may also modulate P450 1B1-mediated estrogen oxidative metabolism, P450 1B1/1A1 levels, and activities. For example, dibenzoylmethane, which is known to be present in GG, increases P450 1A1 and 1B1 in HepG2 cells and in rat liver accompanied by AhR activation.75,76 Furthermore, glycyrrhetinic acid increases protein expression of P450 1A1 in mice.77 These data suggests that LigC may not solely contribute to the stimulating effect on estrogen oxidative metabolism seen with GG and GU.
In summary, this study compared the three medicinally used Glycyrrhiza species using well authenticated plant material and clearly showed the chemical and pharmacological differences between the Glycyrrhiza species. Therefore, this study highlights the importance of standardization of licorice extracts to species-specific bioactive compounds when used in botanical dietary supplements for women’s health. The varied effects of the Glycyrrhiza species on estrogen metabolism might be due in part to differences in the biological targets of the Michael acceptor chalcones, LicA and LigC. Both have similar chemopreventive72 and anti-inflammatory effects (this study); however, LicA is a strong AhR receptor antagonist and virtually shuts down genotoxic estrogen metabolism, whereas LigC stimulates oxidative estrogen metabolism through an unknown biological target. Because LicA and GI inhibit genotoxic estrogen metabolism in vitro, are anti-inflammatory, and activate detoxification enzymes72 our in vitro results suggest that GI could be a promising chemopreventive licorice extract for women’s health.
Supplementary Material
Acknowledgments
Funding information
This research was supported by NIH Grants P50 AT00155 and T32 AT007533 from the Office of Dietary Supplements (ODS), National Institutes of Health, and the National Center for Complementary & Integrative Health (NCCIH), formerly NCCAM.
We would like to sincerely thank Dr. Dejan Nikolić for his advice with the LC-MS/MS experiments. We are also very grateful to Dr. Liang Zhao from Lanzhou Institute of Chemical Physics, CAS, for providing a Glycyrrhiza inflata sample as a generous gift.
Abbreviations
- 2-MeOE1
2-methoxyestrone
- 4-MeOE1
4-methoxyestrone
- AhR
aryl hydrocarbon receptor
- ARE
antioxidant response element
- COMT
catechol O-methyltransferase
- E1
estrone
- E2
estradiol
- EROD
ethoxyresorufin-O-deethylation
- GG
Glycyrrhiza glabra
- GI
Glycyrrhiza inflata
- GU
Glycyrrhiza uralensis
- HT
hormone therapy
- Interferon gamma
IFN-γ
- Keap1
Kelch-like ECH-associated protein 1
- LicA
licochalcone A
- LigC
isoliquiritigenin
- LigF
liquiritigenin
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-kappaB
- NQO1, NAD(P)H
quinone oxidoreductase 1
- P450 1A1
cytochrome P450 1A1
- P450 1B1
cytochrome P450 1B1
- PAH
polycyclic aromatic hydrocarbon
- PGE2
prostaglandin E2
- ROS
reactive oxygen species
- TCDD
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- TNF-α
tumor necrosis factor-alpha
- WHI
women’s health initiative
- XRE
xenobiotic response element
Footnotes
The chemical profiles of GG, GU, and GI measured by UHPLC-UV, structures of LigC and LigF equivalents, MS fragmentation pattern of dansylated 4-MeOE1, representative SRM chromatograms with transition 534.4 – 171.2 analyzing 2-MeOE1 and 4-MeOE1 metabolites showing improved sensitivity, TNF-α and IFN-γ cooperative induction of P450 1B1 mRNA levels, XRE-luciferase, and inhibition of P450 1A1/1B1 activity are available in Supporting Information. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Cavalieri E, Rogan E. The molecular etiology and prevention of estrogen-initiated cancers: Ockham’s Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity. Mol Aspects Med. 2014;36:1–55. doi: 10.1016/j.mam.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorg O. AhR signalling and dioxin toxicity. Toxicol Lett. 2014;230:225–233. doi: 10.1016/j.toxlet.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez SV, Russo IH, Russo J. Estradiol and its metabolites 4-hydroxyestradiol and 2-hydroxyestradiol induce mutations in human breast epithelial cells. Int J Cancer. 2006;118:1862–1868. doi: 10.1002/ijc.21590. [DOI] [PubMed] [Google Scholar]
- 5.Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, Garber J, Russo IH. 17-Beta-estradiol induces transformation and tumorigenesis in human breast epithelial cells. FASEB J. 2006;20:1622–1634. doi: 10.1096/fj.05-5399com. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez SV, Russo J. Estrogen and xenoestrogens in breast cancer. Toxicol Pathol. 2010;38:110–122. doi: 10.1177/0192623309354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalieri EL, Rogan EG. Depurinating estrogen-DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 2010;6:75–91. doi: 10.2217/fon.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yager JD. Mechanisms of estrogen carcinogenesis: The role of E2/E1–quinone metabolites suggests new approaches to preventive intervention–A review. Steroids. 2014 doi: 10.1016/j.steroids.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemachandra L, Madhubhani P, Chandrasena R, Esala P, Chen S-N, Main M, Lankin DC, Scism RA, Dietz BM, Pauli GF. Hops (Humulus lupulus) inhibits oxidative estrogen metabolism and estrogen-induced malignant transformation in human mammary epithelial cells (MCF-10A) Cancer Prev Res. 2012;5:73–81. doi: 10.1158/1940-6207.CAPR-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaikwad NW, Rogan EG, Cavalieri EL. Evidence from ESI-MS for NQO1-catalyzed reduction of estrogen ortho-quinones. Free Radical Biol Med. 2007;43:1289–1298. doi: 10.1016/j.freeradbiomed.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinecke JL, Ridnour LA, Cheng RY, Switzer CH, Lizardo MM, Khanna C, Glynn SA, Hussain SP, Young HA, Ambs S. Tumor microenvironment-based feed-forward regulation of NOS2 in breast cancer progression. PNAS. 2014;111:6323–6328. doi: 10.1073/pnas.1401799111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumgarten SC, Frasor J. Minireview: inflammation: an instigator of more aggressive estrogen receptor (ER) positive breast cancers. Mol Endocrinol. 2012;26:360–371. doi: 10.1210/me.2011-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Šmerdová L, Svobodová J, Kabátková M, Kohoutek J, Blažek D, Machala M, Vondráček J. Up-regulation of CYP1B1 expression by inflammatory cytokines is mediated by the p38 MAP kinase signal transduction pathway. Carcinogenesis. 2014;35:2534–2543. doi: 10.1093/carcin/bgu190. [DOI] [PubMed] [Google Scholar]
- 14.Patel S, Bhambra U, Charalambous M, David R, Edwards R, Lightfoot T, Boobis A, Gooderham N. Interleukin-6 mediated upregulation of CYP1B1 and CYP2E1 in colorectal cancer involves DNA methylation, miR27b and STAT3. Br J Cancer. 2014;111:2287–2296. doi: 10.1038/bjc.2014.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han EH, Kim HG, Hwang YP, Song GY, Jeong HG. Prostaglandin E2 induces CYP1B1 expression via ligand-independent activation of the ERα pathway in human breast cancer cells. Toxicol Sci. 2010;114:204–216. doi: 10.1093/toxsci/kfq013. [DOI] [PubMed] [Google Scholar]
- 16.Kamel M, Shouman S, El-Merzebany M, Kilic G, Veenstra T, Saeed M, Wagih M, Diaz-Arrastia C, Patel D, Salama S. Effect of tumour necrosis factor-alpha on estrogen metabolic pathways in breast cancer cells. J Cancer. 2012;3:310–321. doi: 10.7150/jca.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salama SA, Kamel MW, Diaz-Arrastia CR, Xu X, Veenstra TD, Salih S, Botting SK, Kumar R. Effect of tumor necrosis factor-α on estrogen metabolism and endometrial cells: potential physiological and pathological relevance. J Clin Endocrinol Metab. 2009;94:285–293. doi: 10.1210/jc.2008-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel CF, Khan EM, Leung PS, Gershwin ME, Chang WW, Wu D, Haarmann-Stemmann T, Hoffmann A, Denison MS. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-κB. J Biol Chem. 2014;289:1866–1875. doi: 10.1074/jbc.M113.505578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ristimaki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 20.Yu L, Zhang F, Hu Z, Ding H, Tang H, Ma Z, Zhao X. Novel prenylated bichalcone and chalcone from Humulus lupulus and their quinone reductase induction activities. Fitoterapia. 2014;93:115–120. doi: 10.1016/j.fitote.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Yao J, Zhang B, Ge C, Peng S, Fang J. Xanthohumol, a polyphenol chalcone present in hops, activating Nrf2 enzymes to confer protection against oxidative damage in PC12 cells. J Agric Food Chem. 2015;63:1521–1531. doi: 10.1021/jf505075n. [DOI] [PubMed] [Google Scholar]
- 22.Kim J-Y, Park SJ, Yun K-J, Cho Y-W, Park H-J, Lee K-T. Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-κB in RAW 264.7 macrophages. Eur J Pharmacol. 2008;584:175–184. doi: 10.1016/j.ejphar.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 23.Cuendet M, Guo J, Luo Y, Chen S, Oteham CP, Moon RC, van Breemen RB, Marler LE, Pezzuto JM. Cancer chemopreventive activity and metabolism of isoliquiritigenin, a compound found in licorice. Cancer Prev Res. 2010;3:221–232. doi: 10.1158/1940-6207.CAPR-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kühnl J, Roggenkamp D, Gehrke SA, Stäb F, Wenck H, Kolbe L, Neufang G. Licochalcone A activates Nrf2 in vitro and contributes to licorice extract-induced lowered cutaneous oxidative stress in vivo. Exp Dermatol. 2015;24:42–47. doi: 10.1111/exd.12588. [DOI] [PubMed] [Google Scholar]
- 25.Hajirahimkhan A, Dietz BM, Bolton JL. Botanical modulation of menopausal symptoms: Mechanisms of action? Planta Med. 2013;79:538–553. doi: 10.1055/s-0032-1328187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajirahimkhan A, Simmler C, Yuan Y, Anderson JR, Chen SN, Nikolić D, Dietz BM, Pauli GF, van Breemen RB, Bolton JL. Evaluation of estrogenic activity of licorice species in comparison with hops used in botanicals for menopausal symptoms. PLoS One. 2013;8:e67947. doi: 10.1371/journal.pone.0067947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondo K, Shiba M, Nakamura R, Morota T, Shoyama Y. Constituent properties of licorices derived from Glycyrrhiza uralensis, G. glabra, or G. inflata identified by genetic information. Biol Pharm Bull. 2007;30:1271–1277. doi: 10.1248/bpb.30.1271. [DOI] [PubMed] [Google Scholar]
- 28.Park C-G, Lee AY, Lee JM, Lee S-H, Lee JH, Choi AJ, Park CB, Cho JE, Lee S. Comparison of biological activities of Glycyrrhiza glabra and G. uralensis. Arch Biol Sci. 2014;66:1431–1439. [Google Scholar]
- 29.Upton R, Graff A, Jolliffe G, Länger R, Williamson E. American Herbal Pharmacopoeia: Botanical Pharmacognosy-Microscopic Characterization of Botanical Medicines. CRC Press Taylor and Francis Group; Boca Raton, FL: 2010. Glycyrrhiza uralensis Fisch. ex. DC. Glycyrrhiza inflata Batalin, Glycyrrhiza glabra L; p. 412. [Google Scholar]
- 30.Kao T-C, Wu C-H, Yen G-C. Bioactivity and potential health benefits of licorice. J Agric Food Chem. 2014;62:542–553. doi: 10.1021/jf404939f. [DOI] [PubMed] [Google Scholar]
- 31.Cuendet M, Oteham CP, Moon RC, Pezzuto JM. Quinone reductase induction as a biomarker for cancer chemoprevention. J Nat Prod. 2006;69:460–463. doi: 10.1021/np050362q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong H, Zhang B-k, Yan M, Fang P-f, Hu C-p, Yang Y, Cao P, Jiang P, Fan X-r. A protective mechanism of licorice (Glycyrrhiza uralensis): Isoliquiritigenin stimulates detoxification system via Nrf2 activation. J Ethnopharmacol. 2014;162:134–139. doi: 10.1016/j.jep.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 33.Simmler C, Jones T, Anderson JR, Nikolić DC, Breemen RB, Soejarto DD, Chen SN, Pauli GF. Species-specific standardisation of licorice by metabolomic profiling of flavanones and chalcones. Phytochem Anal. 2013;25:378–388. doi: 10.1002/pca.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farag MA, Porzel A, Wessjohann LA. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC–MS, LC–MS and 1D NMR techniques. Phytochemistry. 2012;76:60–72. doi: 10.1016/j.phytochem.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Tao W, Duan J, Zhao R, Li X, Yan H, Li J, Guo S, Yang N, Tang Y. Comparison of three officinal Chinese pharmacopoeia species of Glycyrrhiza based on separation and quantification of triterpene saponins and chemometrics analysis. Food Chem. 2013;141:1681–1689. doi: 10.1016/j.foodchem.2013.05.073. [DOI] [PubMed] [Google Scholar]
- 36.Simmler C, Anderson JR, Gauthier L, Lankin DC, McAlpine JB, Chen S-N, Pauli GF. Metabolite Profiling and Classification of DNA Authenticated Licorice Botanicals. J Nat Prod. doi: 10.1021/acs.jnatprod.5b00342. unpublished results 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmler C, Hajirahimkhan A, Lankin DC, Bolton JL, Jones T, Soejarto DD, Chen S-N, Pauli GF. Dynamic residual complexity of the isoliquiritigenin–liquiritigenin interconversion during bioassay. J Agric Food Chem. 2013;61:2146–2157. doi: 10.1021/jf304445p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pauli GF, Jaki BU, Lankin DC. Quantitative 1H NMR: development and potential of a method for natural products analysis. J Nat Prod. 2005;68:133–149. doi: 10.1021/np0497301. [DOI] [PubMed] [Google Scholar]
- 39.Dirsch VM, Stuppner H, Vollmar AM. The Griess assay: suitable for a bio-guided fractionation of anti-inflammatory plant extracts? Planta Med. 1998;64:423–426. doi: 10.1055/s-2006-957473. [DOI] [PubMed] [Google Scholar]
- 40.Dunlap T, Abdul-Hay SO, Chandrasena REP, Hagos GK, Sinha V, Wang Z, Wang H, Thatcher GR. Nitrates and NO-NSAIDs in cancer chemoprevention and therapy: in vitro evidence querying the NO donor functionality. Nitric Oxide. 2008;19:115–124. doi: 10.1016/j.niox.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X, Keefer LK, Ziegler RG, Veenstra TD. A liquid chromatography-mass spectrometry method for the quantitative analysis of urinary endogenous estrogen metabolites. Nat Protoc. 2007;2:1350–1355. doi: 10.1038/nprot.2007.176. [DOI] [PubMed] [Google Scholar]
- 42.Trapani V, Patel V, Leong CO, Ciolino HP. DNA damage and cell cycle arrest induced by 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203, NSC 703786) is attenuated in aryl hydrocarbon receptor deficient MCF-7 cells. Br J Cancer. 2003;88:599–605. doi: 10.1038/sj.bjc.6600722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrieux L, Langouët S, Fautrel A, Ezan F, Krauser JA, Savouret JF, Guengerich FP, Baffet G, Guillouzo A. Aryl hydrocarbon receptor activation and cytochrome P450 1A induction by the mitogen-activated protein kinase inhibitor U0126 in hepatocytes. Mol Pharmacol. 2004;65:934–943. doi: 10.1124/mol.65.4.934. [DOI] [PubMed] [Google Scholar]
- 44.Asano T, Ishihara K, Morota T, Takeda S, Aburada M. Permeability of the flavonoids liquiritigenin and its glycosides in licorice roots and davidigenin, a hydrogenated metabolite of liquiritigenin, using human intestinal cell line Caco-2. J Ethnopharmacol. 2003;89:285–289. doi: 10.1016/j.jep.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Kamei J, Saitoh A, Asano T, Nakamura R, Ichiki H, Iiduka A, Kubo M. Pharmacokinetic and pharmacodynamic profiles of the antitussive principles of Glycyrrhizae radix (licorice), a main component of the Kampo preparation Bakumondo-to (Mai-men-dong-tang) Eur J Pharmacol. 2005;507:163–168. doi: 10.1016/j.ejphar.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 46.Chu X, Ci X, Wei M, Yang X, Cao Q, Guan M, Li H, Deng Y, Feng H, Deng X. Licochalcone A inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo. J Agric Food Chem. 2012;60:3947–3954. doi: 10.1021/jf2051587. [DOI] [PubMed] [Google Scholar]
- 47.Lau GT, Ye L, Leung LK. The licorice flavonoid isoliquiritigenin suppresses phorbol ester-induced cyclooxygenase-2 expression in the non-tumorigenic MCF-10A breast cell line. Planta Med. 2010;76:780–785. doi: 10.1055/s-0029-1240699. [DOI] [PubMed] [Google Scholar]
- 48.Kim J-K, Oh S-m, Kwon H-S, Oh Y-S, Lim SS, Shin H-K. Anti-inflammatory effect of roasted licorice extracts on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biochem Biophys Res Commun. 2006;345:1215–1223. doi: 10.1016/j.bbrc.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 49.Thiyagarajan P, Chandrasekaran C, Deepak H, Agarwal A. Modulation of lipopolysaccharide-induced pro-inflammatory mediators by an extract of Glycyrrhiza glabra and its phytoconstituents. Inflammopharmacology. 2011;19:235–241. doi: 10.1007/s10787-011-0080-x. [DOI] [PubMed] [Google Scholar]
- 50.Wu T-Y, Khor TO, Saw CLL, Loh SC, Chen AI, Lim SS, Park JHY, Cai L, Kong A-NT. Anti-inflammatory/Anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J. 2011;13:1–13. doi: 10.1208/s12248-010-9239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S-H, Henry EC, Kim D-K, Kim Y-H, Shin KJ, Han MS, Lee TG, Kang J-K, Gasiewicz TA, Ryu SH. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2, 3, 7, 8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol. 2006;69:1871–1878. doi: 10.1124/mol.105.021832. [DOI] [PubMed] [Google Scholar]
- 52.Waelchli R, Bollbuck B, Bruns C, Buhl T, Eder J, Feifel R, Hersperger R, Janser P, Revesz L, Zerwes H-G. Design and preparation of 2-benzamido-pyrimidines as inhibitors of IKK. Bioorg Med Chem Lett. 2006;16:108–112. doi: 10.1016/j.bmcl.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 53.Umannová L, Machala M, Topinka J, Nováková Z, Milcová A, Kozubík A, Vondráček J. Tumor necrosis factor-α potentiates genotoxic effects of benzo [a] pyrene in rat liver epithelial cells through upregulation of cytochrome P450 1B1 expression. Mutat Res-Fundam Mol Mech Mutag. 2008;640:162–169. doi: 10.1016/j.mrfmmm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 55.Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014;14:801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cavalieri E, Stack D, Devanesan P, Todorovic R, Dwivedy I, Higginbotham S, Johansson S, Patil K, Gross M, Gooden J. Molecular origin of cancer: catechol estrogen-3, 4-quinones as endogenous tumor initiators. PNAS. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snelten CS, Dietz B, Bolton JL. Modulation of estrogen chemical carcinogenesis by botanical supplements used for postmenopausal women’s health. Drug Discov Today Dis Mech. 2012;9:47–54. doi: 10.1016/j.ddmec.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Funakoshi-Tago M, Nakamura K, Tsuruya R, Hatanaka M, Mashino T, Sonoda Y, Kasahara T. The fixed structure of Licochalcone A by α, β-unsaturated ketone is necessary for anti-inflammatory activity through the inhibition of NF-κB activation. Int Immunopharmacol. 2010;10:562–571. doi: 10.1016/j.intimp.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Linnewiel-Hermoni K, Motro Y, Miller Y, Levy J, Sharoni Y. Carotenoid derivatives inhibit nuclear factor kappa B activity in bone and cancer cells by targeting key thiol groups. Free Radical Biol Med. 2014;75:105–120. doi: 10.1016/j.freeradbiomed.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 60.Switzer CH, Cheng RY-S, Ridnour LA, Murray MC, Tazzari V, Sparatore A, Del Soldato P, Hines HB, Glynn SA, Ambs S. Dithiolethiones inhibit NF-κB activity via covalent modification in human estrogen receptor–negative breast cancer. Cancer Res. 2012;72:2394–2404. doi: 10.1158/0008-5472.CAN-11-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xia Y-F, Ye B-Q, Li Y-D, Wang J-G, He X-J, Lin X, Yao X, Ma D, Slungaard A, Hebbel RP. Andrographolide attenuates inflammation by inhibition of NF-κB activation through covalent modification of reduced cysteine 62 of p50. J Immunol. 2004;173:4207–4217. doi: 10.4049/jimmunol.173.6.4207. [DOI] [PubMed] [Google Scholar]
- 62.Honda H, Nagai Y, Matsunaga T, Saitoh S-i, Akashi-Takamura S, Hayashi H, Fujii I, Miyake K, Muraguchi A, Takatsu K. Glycyrrhizin and isoliquiritigenin suppress the LPS sensor Toll-like receptor 4/MD-2 complex signaling in a different manner. J Leukocyte Biol. 2012;91:967–976. doi: 10.1189/jlb.0112038. [DOI] [PubMed] [Google Scholar]
- 63.Feldman M, Santos J, Grenier D. Comparative evaluation of two structurally related flavonoids, isoliquiritigenin and liquiritigenin, for their oral infection therapeutic potential. J Nat Prod. 2011;74:1862–1867. doi: 10.1021/np200174h. [DOI] [PubMed] [Google Scholar]
- 64.Lu F, Zahid M, Wang C, Saeed M, Cavalieri EL, Rogan EG. Resveratrol prevents estrogen-DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev Res. 2008;1:135–145. doi: 10.1158/1940-6207.CAPR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen ZH, Hurh YJ, Na HK, Kim JH, Chun YJ, Kim DH, Kang KS, Cho MH, Surh YJ. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis. 2004;25:2005–2013. doi: 10.1093/carcin/bgh183. [DOI] [PubMed] [Google Scholar]
- 66.Beedanagari SR, Bebenek I, Bui P, Hankinson O. Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicol Sci. 2009:kfp079. doi: 10.1093/toxsci/kfp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciolino HP, Yeh GC. Inhibition of aryl hydrocarbon-induced cytochrome P-450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol Pharmacol. 1999;56:760–767. [PubMed] [Google Scholar]
- 68.Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret J-F. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56:784–790. [PubMed] [Google Scholar]
- 69.Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998;58:5707–5712. [PubMed] [Google Scholar]
- 70.Park S-A, Na H-K, Surh Y-J. Resveratrol suppresses 4-hydroxyestradiol-induced transformation of human breast epithelial cells by blocking IκB kinaseβ-NF-κB signalling. Free Radical Res. 2012;46:1051–1057. doi: 10.3109/10715762.2012.671940. [DOI] [PubMed] [Google Scholar]
- 71.Yang L, Zahid M, Liao Y, Rogan EG, Cavalieri EL, Davidson NE, Yager JD, Visvanathan K, Groopman JD, Kensler TW. Reduced formation of depurinating estrogen–DNA adducts by sulforaphane or KEAP1 disruption in human mammary epithelial MCF-10A cells. Carcinogenesis. 2013;34:2587–2592. doi: 10.1093/carcin/bgt246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hajirahimkhan A, Simmler C, Dong H, Lantvit D, Li G, Chen S-N, Nikolic D, Pauli GF, van Breemen RB, Dietz BM, Bolton JL. Chemopreventive effects of licorice used in botanical dietary supplements for women’s health: in vitro and in vivo evaluation of NAD(P)H:quinone oxidoreductase 1 (NQO1) induction unpublished results 2015. [Google Scholar]
- 73.Wong TY, Lin S-m, Poon CH, Leung LK. The licorice flavonoid isoliquiritigenin reduces DNA-binding activity of AhR in MCF-7 cells. Chem-Biol Interact. 2014;221:70–76. doi: 10.1016/j.cbi.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 74.Sissung TM, Price DK, Sparreboom A, Figg WD. Pharmacogenetics and regulation of human cytochrome P450 1B1: implications in hormone-mediated tumor metabolism and a novel target for therapeutic intervention. Mol Cancer Res. 2006;4:135–150. doi: 10.1158/1541-7786.MCR-05-0101. [DOI] [PubMed] [Google Scholar]
- 75.MacDonald CJ, Ciolino HP, Yeh GC. Dibenzoylmethane modulates aryl hydrocarbon receptor function and expression of cytochromes P450 1A1, 1A2, and 1B1. Cancer Res. 2001;61:3919–3924. [PubMed] [Google Scholar]
- 76.Mancia MD, Reid ME, DuBose ES, Campbell JA, Jackson KM. Qualitative identification of dibenzoylmethane in licorice root (Glycyrrhiza glabra) using gas chromatography-triple quadrupole mass spectrometry. Nat Prod Commun. 2014;9:91–94. [PubMed] [Google Scholar]
- 77.Hu W, Li Y, Hou Y, He K, Chen J, But P, Zhu X. The induction of liver microsomal cytochrome P450 by Glycyrrhiza uralensis and glycyrrhetinic acid in mice. Biomed Environ Sci. 1999;12:10–14. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.