Abstract
Background: Epidemiologic data suggest that low serum 25-hydroxyvitamin D [25(OH)D] increases insulin resistance and the risk of type 2 diabetes. Few interventional trials have assessed the effect of vitamin D on insulin metabolism, and published results are discordant.
Objective: The goal of this study was to perform a detailed assessment of the effect of ergocalciferol administration on glucose and insulin metabolism in healthy people with low total 25(OH)Dtotal.
Design: This was a 12-wk, double-blinded, randomized controlled trial. We enrolled 90 healthy volunteers aged 18–45 y with serum 25(OH)D ≤20 ng/mL (by immunoassay) and administered 50,000 IU ergocalciferol/wk or placebo for 12 wk. Primary endpoints were change in first-phase insulin response and insulin sensitivity as measured by intravenous glucose tolerance test. Secondary endpoints included change in homeostasis model assessment of insulin resistance; fasting glucose, insulin, and lipids; body mass index (BMI); and blood pressure.
Results: On-study 25(OH)Dtotal was assessed by liquid chromatography–tandem mass spectrometry. In the treated group, 25(OH)Dtotal rose from 18 ± 7 to 43 ± 12 ng/mL (P < 0.001) with no change in the placebo group. Despite this increase, at 12 wk, there were no between-group differences in either insulin response or insulin sensitivity; nor were there differences in any measured secondary endpoints. There was no evidence of effect modification by sex, race, glucose tolerance status, baseline 25(OH)Dtotal, or BMI.
Conclusion: In healthy persons with low 25(OH)Dtotal, ergocalciferol administration for 12 wk normalizes 25(OH)Dtotal but does not improve insulin secretion, insulin sensitivity, or other markers of metabolic health. This trial was registered at clinicaltrials.gov as NCT00491322.
Keywords: vitamin D, ergocalciferol, insulin, diabetes, glucose, insulin resistance
INTRODUCTION
The pathogenesis of type 2 diabetes is multifactorial, with genetics and environmental factors playing key roles. The prevalence of type 2 diabetes is increasing rapidly and is associated with substantial morbidity and mortality as well as an increase in health care costs (1). Public health interventions to decrease the risk of type 2 diabetes are urgently needed.
Type 2 diabetes arises in the setting of increased insulin resistance coupled with an inability of the pancreatic β cell to compensate by increasing insulin secretion (2). Vitamin D has been proposed to modify both insulin resistance and secretion. Substantial expression of the vitamin D receptor has been found in β cells, suggesting that vitamin D may play an important role in the regulation of β-cell function (3). In animal models of vitamin D deficiency, stimulated insulin secretion is impaired independent of circulating serum calcium and can be restored by repleting vitamin D (4, 5). Insulin resistance is likely also affected by vitamin D. Notably, polymorphisms in both the gene encoding the vitamin D receptor and the gene encoding the vitamin D binding protein are associated with insulin resistance (6, 7). In addition, vitamin D may play a role in modulating the inflammatory response to obesity, which contributes to insulin resistance (8).
Several longitudinal observational human studies have demonstrated an association between low dietary vitamin D intake or low serum 25-hydroxyvitamin D [25(OH)D]7 and incident type 2 diabetes (9–14). Recent meta-analyses have reported that the risk of developing type 2 diabetes is 40% lower in subjects with higher serum 25(OH)D than in those with lower concentrations (15, 16). Randomized controlled trials of vitamin D administration and glycemic outcomes have, however, had conflicting results (17–23). Most published trials have evaluated glycemic outcomes by using either fasting measures of glucose and insulin or indices derived from oral-glucose-tolerance tests (OGTTs) (15, 24). Intravenous-glucose-tolerance tests (IVGTTs), however, may assess altered β-cell function more accurately and reproducibly than data derived from OGTTs (25, 26). Both the Institute of Medicine and the Endocrine Society have called on the scientific community to rigorously study the potential beneficial nonskeletal effects of vitamin D (27, 28). In this randomized controlled trial, we administered high-dose ergocalciferol (vitamin D2) or placebo to healthy adults with low total serum 25(OH)D [25(OH)Dtotal] (≤20 ng/mL) and assessed the effects on fasting glucose homeostasis and IVGTT-derived measures of insulin sensitivity and secretion. Ergocalciferol was chosen because the high-dose form was readily available by prescription in the United States at the time the study was performed and thus has potential to be a public health intervention.
METHODS
Subjects
Healthy subjects aged 18–45 y were recruited via advertisements and mass mailings. Results of the effect of ergocalciferol on hormones of mineral metabolism from this cohort have been reported previously (29). Screening 25(OH)Dtotal was measured by chemiluminescent immunoassay (CLIA) (Diasorin), and subjects were eligible if 25(OH)Dtotal was ≤20 ng/mL. The CLIA assay is 100% cross-reactive to 25-hydroxyvitamin D2 [25(OH)D2] and 25-hydroxyvitamin D3 [25(OH)D3] as per the manufacturer. Subjects with a history of clinically significant cardiac, hepatic, gastrointestinal, oncologic, or thyroid disease or any disorder known to affect vitamin D metabolism were excluded. In addition, male subjects were required to have a serum testosterone in the reference range, and females were required to have regular menses. Use of oral contraceptives was allowed. Subjects who met these criteria then underwent a second screening visit at which a 2-h OGTT with 75 g dextrose was administered; subjects were classified as having either normal glucose metabolism (fasting glucose <100 mg/dL and 2-h glucose <140 mg/dL) or impaired glucose metabolism (fasting glucose 100–125 mg/dL and/or 2-h glucose 140–199 mg/dL). No subject had OGTT results consistent with diabetes (fasting glucose ≥126 mg/dL and/or 2-h glucose ≥200 mg/dL). Subjects self-identified race and ethnicity. This study was approved by the Partners Human Research Committee, and informed consent was obtained from all subjects. This trial was registered as NCT00491322 at clinicaltrials.gov.
Randomization
Subjects were randomly allocated in a 1:1 ratio to ergocalciferol treatment or to matching placebo by computer-generated assignment, in randomly varying blocks of 2, 4, or 6. The study statistician communicated the allocation sequence to a research pharmacist who dispensed study drug containers with identically appearing capsules. Before randomization, subjects were stratified by sex, by screening 25(OH)Dtotal [25(OH)Dtotal ≤10 ng/mL compared with 25(OH)Dtotal >10 ng/mL], and by glucose metabolism status (normal compared with impaired). Both subjects and investigators were blinded to treatment allocation.
Study design
In this 12-wk, double-blinded, randomized controlled trial, subjects were given either ergocalciferol 50,000 IU/wk or matching placebo to be taken orally for 12 wk. Study drug was dispensed by a research pharmacist in sequentially numbered containers and was given to research subjects by study staff. Both subjects and study staff were blinded to the assignment. Subjects in the placebo arm were given 50,000 IU/d for 7 d on completion of the study protocol. Calcium intake was maintained at 1000–1500 mg/d in both groups with use of calcium supplements, as necessary, based on dietary intake questionnaires. This study was conducted at a clinical research center of a tertiary care hospital. Subjects were recruited from June 2006 through November 2007, and study visits occurred from June 2006 through February 2008. As noted in Table 1, subjects were recruited year-round (baseline visits in spring, n = 15; summer, n = 20; fall, n = 33; and winter, n = 22).
TABLE 1.
Baseline demographic and clinical characteristics1
| Placebo (n = 50) | Ergocalciferol (n = 40) | P value | |
| Age, y | 29 ± 92 | 28 ± 7 | 0.46 |
| Female sex, n (%) | 31 (62) | 24 (60) | 0.85 |
| Race, n (%) | |||
| Asian | 6 (12) | 11 (28) | 0.15 |
| Black | 18 (36) | 8 (20) | |
| White | 20 (40) | 18 (45) | |
| Other | 6 (12) | 3 (7) | |
| Ethnicity, n (%) | |||
| Hispanic | 6 (12) | 2 (5) | 0.29 |
| Non-Hispanic | 44 (88) | 38 (95) | |
| Family history of type 2 diabetes, n (%) | 27 (54) | 24 (60) | 0.57 |
| Baseline visit season, n (%) | |||
| Winter (January–March) | 11 (22) | 11 (28) | 0.91 |
| Spring (April–June) | 9 (18) | 6 (15) | |
| Summer (July–September) | 12 (24) | 8 (20) | |
| Fall (October–December) | 18 (36) | 15 (37) | |
| BMI, kg/m2 | 26.1 ± 6.7 | 25.2 ± 4.5 | 0.46 |
| BMI ≥25, n (%) | 25 (50) | 16 (40) | 0.34 |
| Waist-to-hip ratio | 0.86 ± 0.08 | 0.84 ± 0.09 | 0.41 |
| Systolic blood pressure, mm Hg | 113 ± 13 | 114 ± 13 | 0.71 |
| Diastolic blood pressure, mm Hg | 71 ± 9 | 72 ± 10 | 0.77 |
| Screening 25(OH)D | |||
| CLIA, ng/mL | 15 ± 4 | 14 ± 3 | 0.54 |
| ≤10 ng/mL, n (%) | 8 (16) | 6 (15) | 0.90 |
| Week 0 (LC-MS/MS), ng/mL | |||
| 25(OH)Dtotal | 18 ± 7 | 18 ± 7 | 0.83 |
| 25(OH)D2 | 1 ± 2 | 1 ± 1 | 0.31 |
| 25(OH)D3 | 17 ± 7 | 17 ± 7 | 0.98 |
| Fasting glucose, mg/dL | 85 ± 8 | 87 ± 7 | 0.11 |
| Fasting insulin, μU/mL | 10.0 ± 6.6 | 9.3 ± 4.5 | 0.75 |
| HOMA-IR | 2.2 ± 1.7 | 2.0 ± 1.1 | 0.65 |
| Impaired glucose metabolism, n (%) | 8 (16) | 4 (10) | 0.54 |
| Total cholesterol, mg/dL | 161 ± 39 | 156 ± 32 | 0.59 |
| HDL cholesterol, mg/dL | 58 ± 15 | 56 ± 15 | 0.50 |
| LDL cholesterol, mg/dL | 86 ± 36 | 84 ± 29 | 0.77 |
| Triglycerides, mg/dL | 83 ± 47 | 84 ± 51 | 0.95 |
P values were calculated by t test for continuous variables and by χ2 or Fisher exact test as appropriate for categorical variables. CLIA, chemiluminescence immunoassay; LC-MS/MS, liquid chromatography–tandem mass spectrometry; 25(OH)Dtotal, total 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyvitamin D2; 25(OH)D3, 25-hydroxyvitamin D3.
Mean ± SD (all such values).
After the screening visits, subjects were seen at 0, 4, 8, and 12 wk. At each visit, fasting serum samples were obtained between 0700 and 0900. Blood pressure, height, weight, and waist measurements were obtained. At the 0- and 12-wk visits, subjects underwent a modified IVGTT as previously described (30). Briefly, an intravenous dextrose bolus of 0.3 g/kg was administered over 30 s. Blood samples for insulin and glucose measurements were obtained at 10 and 1 min before infusion and then at 2, 4, 6, 8, 10, 12, 14, 19, 25, 30, and 40 min. First-phase insulin response, a measure of insulin secretion, was determined by using values from 0 to 10 min after intravenous dextrose administration, and insulin sensitivity was determined by using values from 10 to 40 min after administration. These calculations correlate well with insulin sensitivity as determined by euglycemic-hyperinsulinemic clamp and by the minimal model in standard IVGTT (31). HOMA-IR was calculated at each visit as previously described (32).
Laboratory testing
Screening serum 25(OH)Dtotal was measured by CLIA (Diasorin), which was the available 25(OH)D assay at our institution at that time. On-study 25(OH)Dtotal [including 25(OH)D2 and 25(OH)D3] was measured by liquid chromatography–tandem mass spectrometry with a lower limit of detection of 6 ng/mL and an interassay CV of 6–9%. We have previously reported on the very good agreement between these assays in our study population (29). Serum insulin was measured by radioimmunoassay with an interassay CV of 3–6% (Linco). Parathyroid hormone was measured by using a 2-site immunoradiometric assay (Nichols Institute Diagnostics) with a sensitivity of 1 ng/L and intra- and interassay CVs of 2–3% and 6%, respectively. Serum samples for insulin and 25(OH)D via liquid chromatography–tandem mass spectrometry were frozen at −80°C, and all samples from each subject were measured in a single batch. Glucose concentrations were measured on plasma samples in real time via enzymatic assay (Abbot). All batched laboratory analyses were performed in 2008 and 2009.
Statistical analyses
On the basis of published means and variances of insulin resistance as measured by IVGTT (33), we designed the study to have 80% power to detect 30% improvement in insulin sensitivity with vitamin D repletion with an α level of 0.05 (thus 40 subjects were needed per group); given an anticipated 20% loss to follow-up rate, we planned to enroll 100 subjects.
This was a per-protocol analysis; data from the 7 subjects who did not complete the study were not included. Pearson correlations were used to examine univariate associations of baseline 25(OH)Dtotal with other variables. The primary endpoints were 12-wk change in first-phase insulin response and change in insulin sensitivity as measured by modified IVGTT. Change was analyzed by ANCOVA after control for baseline values of each outcome respectively. The effect of ergocalciferol compared with placebo on HOMA-IR was assessed by comparison of the AUC as defined by values at each of the 4 time points. In prespecified analyses, we examined the interactions of race and glucose homeostasis (normal compared with impaired) with first-phase insulin secretion and insulin sensitivity by ANCOVA. Post hoc analyses were also performed to examine the effect of sex and baseline BMI (in kg/m2) on these parameters. The effect of treatment on fasting insulin and fasting glucose was examined by using a mixed-model ANOVA with random slopes. Unpaired t tests were used to examine the effect of ergocalciferol on the 12-wk change in BMI, waist-to-hip ratio, serum lipids (total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides), and blood pressure. Analyses were conducted by using SAS version 9.2 (SAS Institute).
RESULTS
Enrollment and protocol adherence
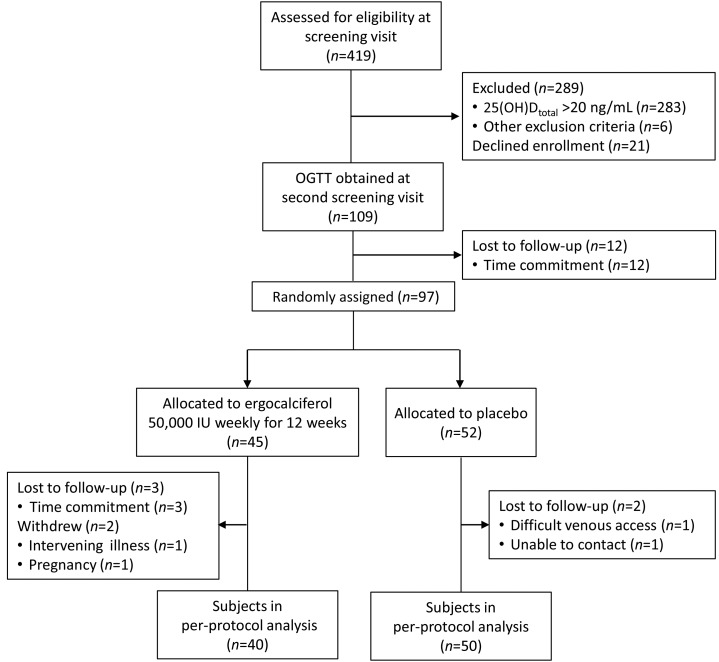
The study flow diagram is shown in Figure 1. Of the 97 subjects who were randomly allocated, 90 completed all 4 study visits. Compliance with the intervention was monitored by medication diaries and by pill counts of returned bottles. Of subjects randomly allocated to ergocalciferol who completed the study, 38 (95%) reported taking every pill, and 2 (5%) reported missing one dose each. Of subjects randomly allocated to placebo who completed the study, 47 (94%) reported taking every pill, 2 (4%) missed 1 dose, and 1 (2%) missed 2 doses.
FIGURE 1.
Flow diagram of study subjects. OGTT, oral-glucose-tolerance test; 25(OH)Dtotal, total 25-hydroxyvitamin D.
Subject characteristics
Baseline characteristics are described in Table 1. Subjects were well matched for demographic, clinical, and laboratory parameters. As previously reported, baseline 25(OH)Dtotal was negatively correlated with parathyroid hormone (r = −0.29, P = 0.006) (29). Baseline 25(OH)Dtotal was not significantly associated with fasting glucose, fasting insulin, HOMA-IR, lipids, BMI, or blood pressure with univariate testing (data not shown). Twelve subjects (13%) had impaired glucose metabolism: 2 had elevated fasting glucose, 9 had impaired glucose tolerance, and 1 had both elevated fasting glucose and impaired glucose tolerance.
Ergocalciferol administration and measures of glucose homeostasis
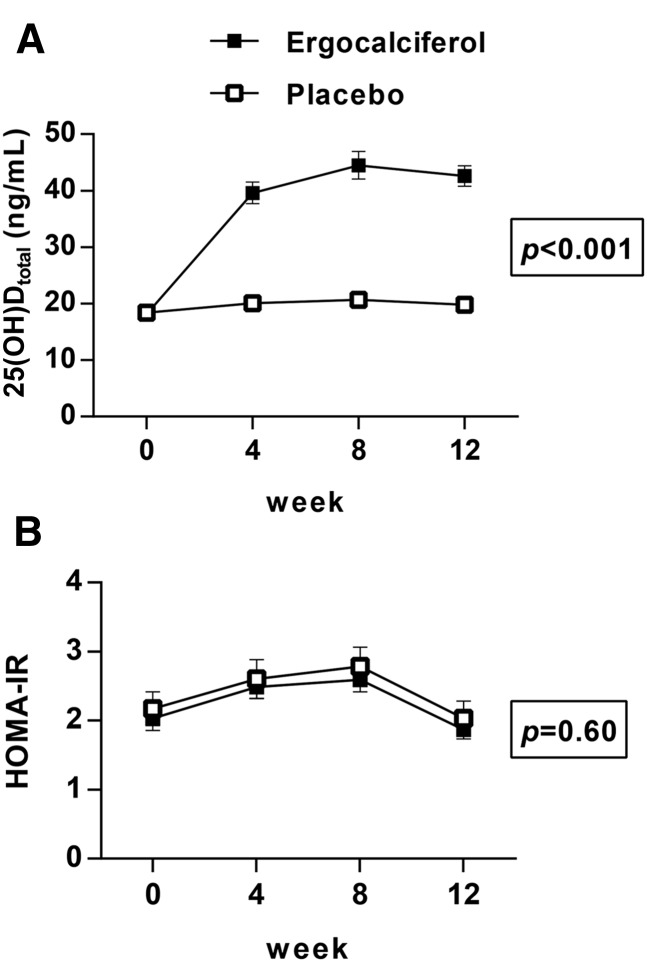
As we have previously reported, administration of high-dose ergocalciferol increased the mean 25(OH)Dtotal concentration significantly from 18 ± 7 to 43 ± 12 ng/mL (these and all subsequent data are presented as means ± SDs), whereas 25(OH)Dtotal did not change significantly in the placebo group (18 ± 7 to 20 ± 10 ng/mL; P < 0.001 for comparison between groups) (Figure 2A) (29). In the ergocalciferol group, 25(OH)D2 increased from 1 ± 1 to 35 ± 12 ng/mL, 25(OH)D3 decreased from 17 ± 7 to 8 ± 4 ng/mL (P < 0.001 for both), and neither 25(OH)D2 nor 25(OH)D3 changed significantly in the placebo group (1 ± 2 to 1 ± 1 ng/mL, P = 0.16, and 17 ± 7 to 19 ± 9 ng/mL, P = 0.13, respectively). There was no significant difference in the increment in 25(OH)Dtotal among subjects in the treatment arm by season (P = 0.34 by ANOVA). The change in HOMA-IR did not differ between the treatment and placebo groups as assessed by AUC comparison (P = 0.60) (Figure 2B). HOMA-IR did not change significantly within the treated group (within-group change of −0.2; 95% CI: −0.5, 0.2; P = 0.62) or the placebo group (within-group change of −0.1; 95% CI: −0.5, 0.2; P = 0.78). Additional analyses revealed no effect of ergocalciferol on fasting insulin or fasting glucose (P = 0.85 and P = 0.69 for between-group differences, respectively).
FIGURE 2.
Mean ± SEM changes in 25(OH)Dtotal (A) and HOMA-IR (B) with ergocalciferol administration. Open squares: placebo group (n = 50); filled squares: ergocalciferol group (n = 40). P values are for between-group comparisons by repeated-measures ANOVA (A) or by ANOVA of AUC (B). 25(OH)Dtotal, total 25-hydroxyvitamin D.
Among the group in the active arm, 25(OH)Dtotal concentrations after 12 wk of ergocalciferol treatment were negatively associated with HOMA-IR (r = −0.35, P = 0.03), as were 25(OH)D2 concentrations (r = −0.34, P = 0.03). We found no association of 25(OH)D3 concentration with HOMA-IR at week 12 (r = 0.02, P = 0.90). There was no association of 25(OH)Dtotal, 25(OH)D2, or 25(OH)D3 with HOMA-IR at 12 wk in the placebo group. These associations were not significant after Bonferroni correction for multiple comparisons. In post hoc within-group analyses, there were no associations of the AUC for HOMA-IR with 25(OH)Dtotal, 25(OH)D2, or 25(OH)D3 concentrations in the active arm by linear regression.
Effect of ergocalciferol on IVGTT measures
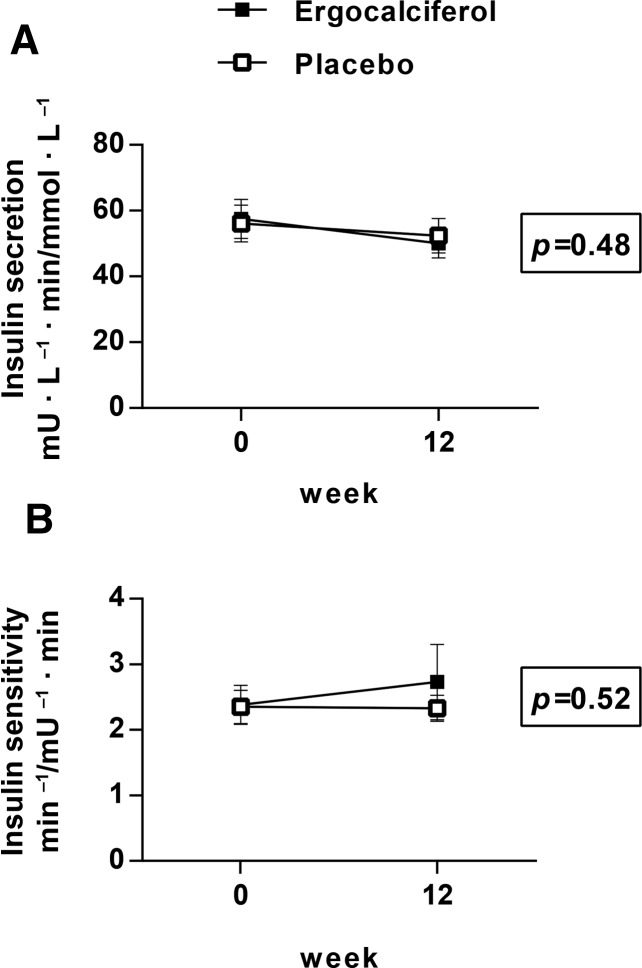
At baseline and at week 12, IVGTTs were performed and first-phase insulin secretory response and insulin sensitivity were calculated (30). As shown in Figure 3A, there was no significant difference in the 12-wk change in first-phase insulin secretion (decrease of 7.8 ± 31.8 mU · L−1 · min/mmol · L−1 in the treatment group and decrease of 3.7 ± 22.1 mU · L−1 · min/mmol · L−1 in the placebo group, P = 0.48). As shown in Figure 3B, there was no significant difference in the 12-wk change in insulin sensitivity (increase of 0.3 ± 3.0 min−1/mU−1 · min in the treatment group and change of 0.0 ± 1.9 min−1/mU−1 · min in the placebo group, P = 0.52, with higher values indicating increased insulin sensitivity). In a prespecified analysis, we found that the effect of ergocalciferol on insulin secretion and on insulin sensitivity was not modified by baseline measures of glucose homeostasis; in particular, we observed no difference between glucose-tolerant and glucose-intolerant subjects. In a second prespecified analysis, we found no evidence that race modified these same parameters. In post hoc analyses, we also found no significant interaction of sex, severity of baseline vitamin D deficiency (25(OH)Dtotal ≤10 ng/mL compared with 25(OH)Dtotal >10 ng/mL), or BMI (<25 compared with ≥25) with treatment for either of these outcomes. In within-group analyses, we found no association of 25(OH)Dtotal, 25(OH)D2, or 25(OH)D3 concentrations with change in insulin secretion or change in insulin sensitivity in the active arm.
FIGURE 3.
Mean ± SEM changes in first-phase insulin secretory response (mU · L−1 · min/mmol · L−1) (A) and insulin sensitivity (min−1/mU−1 · min) (B) as measured by IVGTT with ergocalciferol administration (30). Open squares: placebo group (n = 50); filled squares: ergocalciferol group (n = 40). P values are for between-group difference in change in insulin secretion (A) or insulin sensitivity (B) by ANCOVA with control for baseline value. IVGTT, intravenous-glucose-tolerance test.
Ergocalciferol administration and additional metabolic outcomes
We found no significant between-group differences in the 12-wk change in BMI, waist-to-hip ratio, systolic or diastolic blood pressure, or concentrations of total cholesterol, LDL cholesterol, HDL cholesterol, or triglycerides (Table 2).
TABLE 2.
Twelve-week change (Δ) in selected metabolic parameters1
| Placebo (n = 50) | Ergocalciferol (n = 40) | P value | |
| Δ BMI, kg/m2 | 0.0 ± 0.8 | 0.3 ± 0.6 | 0.08 |
| Δ Waist-to-hip ratio | −0.01 ± 0.03 | 0.01 ± 0.05 | 0.26 |
| Δ Systolic blood pressure, mm Hg | −1 ± 11 | −1 ± 7 | 0.87 |
| Δ Diastolic blood pressure, mm Hg | 0 ± 8 | −1 ± 8 | 0.38 |
| Δ Total cholesterol, mg/dL | 2 ± 25 | 6 ± 21 | 0.38 |
| Δ HDL cholesterol, mg/dL | −3 ± 10 | −1 ± 6 | 0.21 |
| Δ LDL cholesterol, mg/dL | 5 ± 20 | 8 ± 17 | 0.50 |
| Δ Triglycerides, mg/dL | 1 ± 35 | −3 ± 37 | 0.64 |
Values are means ± SDs of baseline values subtracted from week 12 values. P values were calculated by t test.
DISCUSSION
In this longitudinal trial of healthy adults with low 25(OH)Dtotal, we found no effect of ergocalciferol administration and consequent increase in 25(OH)Dtotal on measures of insulin secretion or resistance as assessed by IVGTT. In addition, we found no effect of ergocalciferol administration on other indices of glucose metabolism, including fasting glucose concentration, fasting insulin concentration, and HOMA-IR. Other markers of metabolic health, including blood pressure, serum lipids, and BMI, were similarly unaffected. HOMA-IR increased in both the placebo and treatment groups over the first 8 wk of the study and then declined to baseline at week 12. This variability was seen regardless of season of baseline visit. Although the reason for this fluctuation is not clear, the similarity between the treatment and placebo groups implies that this was not a result of ergocalciferol administration. Overall, these results imply that supplementation of unselected populations with ergocalciferol would not lead to decreased risk of the development of type 2 diabetes.
Published clinical trials of vitamin D administration on glycemic outcomes have had varied results. In the 2 largest clinical trials, supplementation with low daily doses (400–800 IU) of cholecalciferol (vitamin D3) did not affect laboratory measures of glucose homeostasis or rates of incident type 2 diabetes (18, 19). The mean achieved 25(OH)D concentrations in these studies were estimated to be 26 and 25 ng/mL, respectively. In contrast, a smaller study found that administration of 700 IU vitamin D3/d for 3 y attenuated the rise in fasting glucose and HOMA-IR in subjects with impaired fasting glucose at baseline (17). In this latter study, baseline 25(OH)Dtotal concentrations were higher (28–32 ng/mL), leading to a mean achieved 25(OH)Dtotal concentration of ∼40 ng/mL among subjects in the treatment arm.
A meta-analysis of studies investigating the effect of vitamin D administration on glycemic outcomes reported that the beneficial effect was limited to those with impaired glucose tolerance or diabetes (24). However, subsequent studies in this population have reported discordant results. In one recent study, supplementation with 2000 IU vitamin D3 for 16 wk led to a beneficial increase in the glucose disposition index, an integrated measure of insulin secretion and sensitivity that reflects risk of type 2 diabetes (23, 34). This increase appeared to be driven by an increase in insulin secretion as measured by the first-phase insulin response. Conversely, in a study of African American subjects with prediabetes or mild diabetes, administration of 4000 IU vitamin D3/d for 12 wk increased insulin secretion but decreased insulin sensitivity in response to OGTT, with no overall change in the glucose disposition index (35). Similarly, high-dose weekly vitamin D3 supplementation for 12 mo among primarily Latino and black subjects with prediabetes showed no effect on measures derived from OGTT or on incident diabetes, with a significant but small (0.2%) decrease in hemoglobin A1c (36). Mean achieved 25(OH)Dtotal in this study was ∼70 ng/mL. Although the number of subjects with glucose intolerance in our study was low, we saw no effect of ergocalciferol administration on glycemic outcomes in this subset of subjects. Interestingly, 2 recent studies reported that vitamin D administration, as either vitamin D2 or vitamin D3, improved glycemic outcomes in obese adolescents, suggesting that intervention at an earlier developmental stage may be more beneficial (37, 38).
One potential explanation for these discrepancies is the reliance on total 25(OH)D concentrations rather than free or bioavailable concentrations as measures of both deficiency and adequacy of therapy (39). Although renal reuptake of vitamin D occurs complexed to vitamin D–binding protein (40, 41), recent data suggest that bioavailable 25(OH)D may be a better marker of vitamin D sufficiency, at least for some outcomes (42–44). Similarly, genotypic variants of DBP, which encodes the vitamin D–binding protein, may predict the response of 25(OH)D concentrations to vitamin D supplementation (45). Our finding that the achieved 25(OH)Dtotal concentration at week 12 in the ergocalciferol arm correlated with the week 12 HOMA-IR may reflect a genotype-dependent effect.
Our study had several important strengths. Subjects were selected to have low serum 25(OH)Dtotal, which would tend to highlight an effect of increasing 25(OH)Dtotal concentration. Our inclusion serum 25(OH)Dtotal threshold was consistent with the Institute of Medicine definition of low vitamin D (27), and mean 25(OH)Dtotal concentrations in the ergocalciferol-treated subjects increased to >30 ng/mL, the concentration recommended in some recent practice guidelines, in 90% of the treated subjects (46). We used indexes derived from IVGTT that are more reproducible than fasting or OGTT measures, particularly in the setting of decreased β-cell reserve, and can separate effects on insulin secretion from effects on insulin resistance (25, 47). Our cohort was racially and ethnically diverse, distinct from prior studies of the effects of vitamin D administration in subjects with normal glucose tolerance (48). Finally, we intentionally recruited a healthy cohort and used a method, modified IVGTT, that would rigorously assess the effects of ergocalciferol repletion on insulin handling because of the public health implications of this research. Our negative findings are important, nonetheless, because they support the Institute of Medicine’s recommendation that appropriate caution be used when attributing beneficial effects to vitamin D and that randomized clinical trials to separate associations from causality as well as to define patient subtypes that may benefit from an intervention are essential.
Our study also had limitations. We had few subjects with impaired glucose tolerance and were thus not powered to detect effects in this subgroup. However, we did not observe even a trend toward improved insulin secretion or sensitivity with ergocalciferol administration among these subjects, suggesting that a larger sample size would not have led to statistically significant differences. In addition, although IVGTT measures are more reproducible, they are arguably less physiologic than an OGTT or mixed meal challenge in which carbohydrates are absorbed enterally (47). Although we enrolled subjects with screening 25(OH)Dtotal concentrations ≤20 ng/mL, because of the use of a more sensitive assay (liquid chromatography–tandem mass spectrometry compared with CLIA) for the on-protocol visits, some subjects had 25(OH)Dtotal >20 ng/mL at the first on-protocol visit. Some studies that have found a positive effect of vitamin D treatment on glycemic outcomes have enrolled subjects with lower baseline 25(OH)Dtotal concentrations (20, 21). However, given the lack of effect modification by baseline 25(OH)Dtotal concentration on insulin secretion or sensitivity, it is unlikely that this contributed to our negative result. Finally, it is also possible that an alternative replacement strategy, such as use of daily instead of weekly supplements or administration of cholecalciferol instead of ergocalciferol, may have altered our findings, particularly because many studies have suggested that cholecalciferol is more effective than ergocalciferol at raising 25(OH)Dtotal concentrations, in part because ergocalciferol supplementation may induce a decrease in 25(OH)D3 concentrations (49–51). However, the sustained elevations in 25(OH)Dtotal that we observed suggest that, as other authors have found, our treatment strategy was adequate (52, 53).
In summary, our data add to a growing body of literature that suggests that an increase in the concentration of 25(OH)Dtotal does not improve metabolic parameters predictive of the development of type 2 diabetes, at least in unselected populations. Broadly, these data have 3 potential interpretations. Vitamin D may play no physiologic role in the regulation of glucose homeostasis; the previously reported associations of higher 25(OH)Dtotal concentrations and decreased insulin resistance or incident type 2 diabetes may reflect confounding. Alternatively, appropriate glucose regulation may be vitamin D dependent but require relatively low concentrations; thus, impaired insulin secretion or sensitivity would be observed only at extremely low concentrations. Finally, vitamin D may influence glucose homeostasis over a longer time scale, the demonstration of which would require an extended duration of vitamin D administration. Thus, future studies are warranted to examine the role of vitamin D in particular high-risk populations such as those with pre-existing abnormalities in glucose homeostasis and those with extremely low 25(OH)D concentrations. In addition, an improved understanding of the genetic contributions to total and bioavailable 25(OH)D concentrations, as well as of the interaction of 25(OH)D concentrations with other genetic variants contributing to glucose and insulin metabolism, may identify a target population likely to respond to this intervention.
Acknowledgments
The authors’ responsibilities were as follows—BZL, EC, JSF, and S-AMB-B: designed the research; NM, MPH, and S-AMB-B: conducted the research; DMM, DLH, and S-AMB-B: analyzed data; DMM, BZL, DLH, JSF, and S-AMB-B: wrote the manuscript; and S-AMB-B: had primary responsibility for the final content. The authors reported no conflicts of interest related to this study.
Footnotes
7Abbreviations used: CLIA, chemiluminescent immunoassay; IVGTT, intravenous-glucose-tolerance test; OGTT, oral-glucose-tolerance test; 25(OH)D, 25-hydroxyvitamin D; 25(OH)Dtotal, total 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyvitamin D2; 25(OH)D3, 25-hydroxyvitamin D3.
REFERENCES
- 1.Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, Thompson TJ. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care 2001;24:1936–40. [DOI] [PubMed]
- 2.Weir GC, Bonner-Weir S. Islet beta cell mass in diabetes and how it relates to function, birth, and death. Ann N Y Acad Sci 2013;1281:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark SA, Stumpf WE, Sar M, DeLuca HF, Tanaka Y. Target cells for 1,25 dihydroxyvitamin D3 in the pancreas. Cell Tissue Res 1980;209:515–20. [DOI] [PubMed] [Google Scholar]
- 4.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980;209:823–5. [DOI] [PubMed] [Google Scholar]
- 5.Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest 1984;73:759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh JY, Barrett-Connor E. Association between vitamin D receptor polymorphism and type 2 diabetes or metabolic syndrome in community-dwelling older adults: the Rancho Bernardo Study. Metabolism 2002;51:356–9. [DOI] [PubMed] [Google Scholar]
- 7.Hirai M, Suzuki S, Hinokio Y, Hirai A, Chiba M, Akai H, Suzuki C, Toyota T. Variations in vitamin D–binding protein (group-specific component protein) are associated with fasting plasma insulin levels in Japanese with normal glucose tolerance. J Clin Endocrinol Metab 2000;85:1951–3. [DOI] [PubMed] [Google Scholar]
- 8.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care 2005;28:2926–32. [DOI] [PubMed] [Google Scholar]
- 10.Liu E, Meigs JB, Pittas AG, Economos CD, McKeown NM, Booth SL, Jacques PF. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. Am J Clin Nutr 2010;91:1627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care 2010;33:2021–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsur A, Feldman BS, Feldhammer I, Hoshen MB, Leibowitz G, Balicer RD. Decreased serum concentrations of 25-hydroxycholecalciferol are associated with increased risk of progression to impaired fasting glucose and diabetes. Diabetes Care 2013;36:1361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim S, Kim MJ, Choi SH, Shin CS, Park KS, Jang HC, Billings LK, Meigs JB. Association of vitamin D deficiency with incidence of type 2 diabetes in high-risk Asian subjects. Am J Clin Nutr 2013;97:524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, Sikaris K, Ebeling PR, Daly RM. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab). J Clin Endocrinol Metab 2012;97:1953–61. [DOI] [PubMed] [Google Scholar]
- 15.Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr 2011;65:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, Hu FB. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 2013;36:1422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007;30:980–6. [DOI] [PubMed] [Google Scholar]
- 18.de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, Larson JC, Manson JE, Margolis KL, Siscovick DS, Weiss NS; Women's Health Initiative. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women's Health Initiative. Diabetes Care 2008;31:701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avenell A, Cook JA, MacLennan GS, McPherson GC. Vitamin D supplementation and type 2 diabetes: a substudy of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing 2009;38:606–9. [DOI] [PubMed] [Google Scholar]
- 20.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med 2009;26:19–27. [DOI] [PubMed] [Google Scholar]
- 21.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—a randomised, placebo-controlled trial. Br J Nutr 2010;103:549–55. [DOI] [PubMed] [Google Scholar]
- 22.Nikooyeh B, Neyestani TR, Farvid M, Alavi-Majd H, Houshiarrad A, Kalayi A, Shariatzadeh N, Gharavi A, Heravifard S, Tayebinejad N, et al. Daily consumption of vitamin D− or vitamin D+ calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr 2011;93:764–71. [DOI] [PubMed] [Google Scholar]
- 23.Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic beta cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr 2011;94:486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med 2012;29:e142–50. [DOI] [PubMed] [Google Scholar]
- 25.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667–77. [DOI] [PubMed] [Google Scholar]
- 26.Ader M, Stefanovski D, Richey JM, Kim SP, Kolka CM, Ionut V, Kabir M, Bergman RN. Failure of homeostatic model assessment of insulin resistance to detect marked diet-induced insulin resistance in dogs. Diabetes 2014;63:1914–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev 2012;33:456–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burnett-Bowie SM, Leder BZ, Henao MP, Baldwin CM, Hayden DL, Finkelstein JS. Randomized trial assessing the effects of ergocalciferol administration on circulating FGF23. Clin J Am Soc Nephrol 2012;7:624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galvin P, Ward G, Walters J, Pestell R, Koschmann M, Vaag A, Martin I, Best JD, Alford F. A simple method for quantitation of insulin sensitivity and insulin release from an intravenous glucose tolerance test. Diabet Med 1992;9:921–8. [DOI] [PubMed] [Google Scholar]
- 31.Tura A, Sbrignadello S, Succurro E, Groop L, Sesti G, Pacini G. An empirical index of insulin sensitivity from short IVGTT: validation against the minimal model and glucose clamp indices in patients with different clinical characteristics. Diabetologia 2010;53:144–52. [DOI] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 33.Borissova AM, Tankova T, Kirilov G, Dakovska L, Kovacheva R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract 2003;57:258–61. [PubMed] [Google Scholar]
- 34.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 2002;51(Suppl 1):S212–20. [DOI] [PubMed] [Google Scholar]
- 35.Harris SS, Pittas AG, Palermo NJ. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes Metab 2012;14:789–94. [DOI] [PubMed] [Google Scholar]
- 36.Davidson MB, Duran P, Lee ML, Friedman TC. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care 2013;36:260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr 2013;97:774–81. [DOI] [PubMed] [Google Scholar]
- 38.Poomthavorn P, Nantarakchaikul P, Mahachoklertwattana P, Chailurkit LO, Khlairit P. Effects of correction of vitamin D insufficiency on serum osteocalcin and glucose metabolism in obese children. Clin Endocrinol (Oxf) 2014;80:516–23. [DOI] [PubMed] [Google Scholar]
- 39. Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol 2014;144:132–7. [DOI] [PMC free article] [PubMed]
- 40.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999;96:507–15. [DOI] [PubMed] [Google Scholar]
- 41.Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3). Proc Natl Acad Sci USA 2001;98:13895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, Thadhani RI. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int 2012;82:84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, et al. Vitamin D–binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013;369:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin D–binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res 2011;26:1609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Didriksen A, Grimnes G, Hutchinson MS, Kjaergaard M, Svartberg J, Joakimsen RM, Jorde R. The serum 25-hydroxyvitamin D response to vitamin D supplementation is related to genetic factors, BMI, and baseline levels. Eur J Endocrinol 2013;169:559–67. [DOI] [PubMed] [Google Scholar]
- 46.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin d deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 47.Borai A, Livingstone C, Ferns GA. The biochemical assessment of insulin resistance. Ann Clin Biochem 2007;44:324–42. [DOI] [PubMed] [Google Scholar]
- 48.Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, Hanley DA, Pittas AG, Tjosvold L, Johnson JA. Clinical review: effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab 2014;99:3551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab 2011;96:E447–52. [DOI] [PubMed] [Google Scholar]
- 50.Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D, Drezner MK. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab 2011;96:981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lehmann U, Hirche F, Stangl GI, Hinz K, Westphal S, Dierkes J. Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo-controlled trial. J Clin Endocrinol Metab 2013;98:4339–45. [DOI] [PubMed] [Google Scholar]
- 52.Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 2008;93:677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab 2013;98:973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]