Abstract
Neurodegeneration is a devastating manifestation in the majority of >50 lysosomal storage disorders (LSDs). Neuronal ceroid lipofuscinoses (NCLs) are the most common childhood neurodegenerative LSDs. Mutations in 13 different genes (called CLNs) underlie various types of NCLs, of which the infantile NCL (INCL) and congenital NCL (CNCL) are the most lethal. Although inactivating mutations in the CLN1 gene encoding palmitoyl-protein thioesterase-1 (PPT1) cause INCL, those in the CLN10 gene encoding cathepsin D (CD) underlie CNCL. PPT1 is a lysosomal thioesterase that cleaves the thioester linkage in S-acylated proteins required for their degradation by lysosomal hydrolases like CD. Thus, PPT1 deficiency causes lysosomal accumulation of these lipidated proteins (major constituents of ceroid) leading to INCL. We sought to determine whether there is a common pathogenic link between INCL and CNCL. Using biochemical, histological and confocal microscopic analyses of brain tissues and cells from Cln1−/− mice that mimic INCL, we uncovered that Cln10/CD is overexpressed. Although synthesized in the endoplasmic reticulum, the CD-precursor protein (pro-CD) is transported through endosome to the lysosome where it is proteolytically processed to enzymatically active-CD. We found that despite Cln10 overexpression, the maturation of pro-CD to enzymatically active-CD in lysosome was disrupted. This defect impaired lysosomal degradative function causing accumulation of undegraded cargo in lysosome leading to INCL. Notably, treatment of intact Cln1−/− mice as well as cultured brain cells derived from these animals with a thioesterase-mimetic small molecule, N-tert-butyl-hydroxylamine, ameliorated the CD-processing defect. Our findings are significant in that they define a pathway in which Cln1 mutations disrupt the maturation of a major degradative enzyme in lysosome contributing to neuropathology in INCL and suggest that lysosomal CD deficiency is a common pathogenic link between INCL and CNCL.
Introduction
In multicellular organisms, lysosome is the major degradative organelle (1) responsible for disposing off the damaged macromolecules and organelles from the cell (2,3). It has been reported that impaired lysosomal degradative capability leads to pathogenesis of many neurodegenerative disorders including lysosomal storage disorders (LSDs) (4). In the majority of >50 LSDs, neurodegeneration is a devastating manifestation (5). Moreover, lysosomal degradative capability has been reported to be impaired in several late-onset neurodegenerative diseases such as Alzheimer's (3,6), Huntington's (7) and Parkinson's (8). Cathepsin D (CD) is a major lysosomal aspartic protease in lysosomes (9). Lysosomal CD activity catalyzes degradation and clearance of exogenous as well as endogenous macromolecules and damaged organelles delivered to the lysosomes (9). Intracellular accumulation of undegraded long-lived proteins and other macromolecules leads to pathogenesis of many neurodegenerative disorders (2,3). Paradoxically, both CD overexpression (10–12) and CD deficiency (13–15) have been reported to underlie neurodegenerative diseases. However, despite intense studies, this paradox until now remained unresolved.
Neuronal ceroid lipofuscinoses (NCLs), also known as Batten disease (16,17), are the most common (1 in 12 500 births) (18), autosomal recessive, neurodegenerative LSDs mostly affecting children. Mutations in 13 different genes (called CLNs) underlie various types of NCLs (19,20). Among all the NCLs, the infantile NCL (INCL) and congenital NCL (CNCL) are the most devastating diseases. Although the inactivating mutations in the CLN1 gene (21) encoding palmitoyl-protein thioesterase-1 (PPT1) (22) cause INCL, mutations in the CLN10/Ctsd gene encoding CD underlie CNCL (13–15). In the present study, we sought to determine whether there is a common pathogenic link between INCL and CNCL.
The synthesis of CD occurs in the endoplasmic reticulum (ER) as a pre-pro-peptide with a molecular mass of ∼50 kDa (9). The cleavage of the leader peptide in the ER generates the 48 kDa precursor of mature-CD (pro-CD). In the Golgi complex, attachment of mannose 6-phosphate to pro-CD facilitates its binding to endosomal/lysosomal sorting receptors. The receptor–ligand complexes then exit the trans-Golgi network in clathrin-coated intermediates and fuses with the endosomal system (23). The low pH of the late endosomal lumen facilitates dissociation of the receptor–ligand complexes and allows the ligand (i.e. pro-CD) to be delivered to lysosome (23). The pro-CD then undergoes further proteolytic cleavage by cathepsin B (CB) and cathepsin L (CL), respectively, which generates the 31 and 14 kDa fragments, non-covalent dimerization of which constitutes the mature, catalytically active-CD.
In the present study, we used Cln1−/−/Ppt1−/− mice (24), which recapitulate virtually all clinical and pathological features of INCL (25), to test a hypothesis that there is a common pathogenic link between INCL and CNCL. Our results show that despite Cln10/Ctsd overexpression, defective processing of pro-CD to mature-CD in lysosome leads to lysosomal CD deficiency causing neuropathology in INCL. Because CD deficiency underlies CNCL, we propose that CD deficiency in lysosome is a common pathogenic link between INCL and CNCL. Furthermore, our results suggest that N-tert-butyl-hydroxylamine (NtBuHA) may have therapeutic implications for patients with INCL.
Results
Cln1−/−/Ppt1−/− mice overexpress Cln10/Ctsd gene in the brain
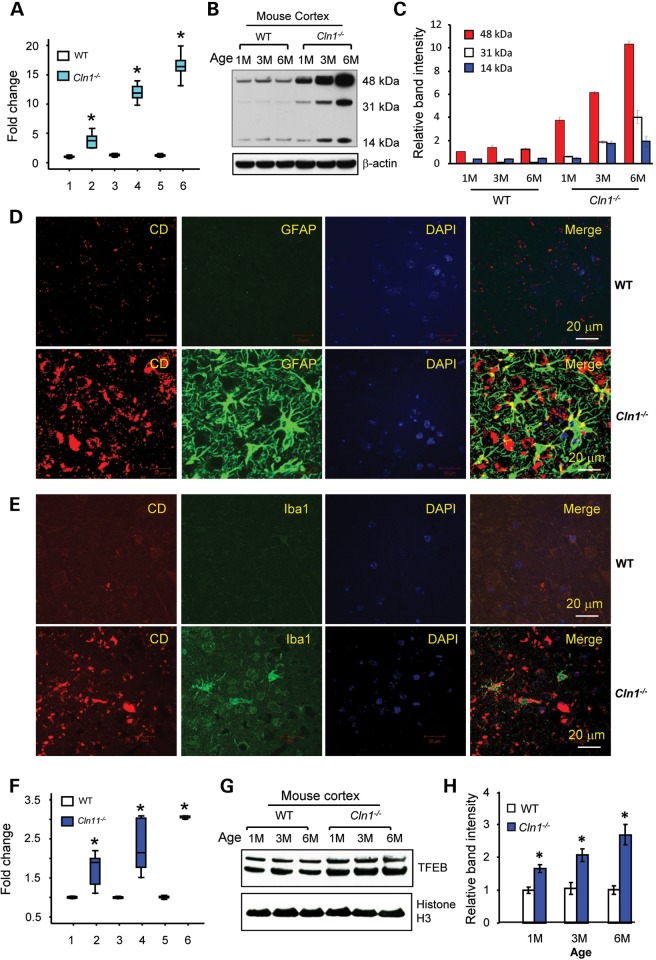
To evaluate the status of Cln10/Ctsd expression in Cln1−/− mice (24), we first evaluated the mRNA levels of several cathepsins (i.e. B, C, D, F, H, K, L, O, S and Z) by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) using total RNA from the brains of 1-, 3- and 6-month-old Cln1−/− mice and those of their Wild type (WT) littermates. The results showed that in the brain of Cln1−/− mice, the mRNA levels of several cathepsins (Supplementary Material, Fig. S1A) including CD (Fig. 1A) were substantially elevated. We used mice of three different age groups to determine whether CD overexpression was age dependent. It should be noted that Cln1−/− mice are phenotypically normal at birth but around 6 months of age, they begin to manifest signs of neurological deterioration attested by clasping behavior (24,25). Notably, CD-mRNA levels in the cortex of 6-month-old Cln1−/− mice were >15-fold higher than those of their WT littermates (Fig. 1A). These results showed that elevated CD-mRNA expression in the cerebral cortex of Cln1−/− mice occurs in an age-dependent manner and that this increase was appreciable long before they manifested the signs of overt neurologically impairment. Western blot analyses of total lysates of cortical tissues from Cln1−/− mice and those of their WT littermates showed that compared with the CD-protein levels in the brain of WT mice (Fig. 1B, left 3 lanes), those in Cln1−/− littermates were significantly higher (Fig. 1B, right 3 lanes and C). Moreover, we found that in the total lysates of cortical tissues from Cln1−/− mice, the levels of both CB and CL proteins (Supplementary Material, Fig. S1B), which are essential for processing pro-CD to mature-CD in lysosome, were also elevated.
Figure 1.
Overexpression of Cln10/Ctsd gene in the cerebral cortex of Cln1−/− mice. (A) CD-mRNA levels as assessed by qRT-PCR using total RNA from the cerebral cortices of 1-, 3- and 6-month-old Cln1−/− mice and those of their WT littermates. 1. WT 1 month; 2. Cln1−/− 1 month; 3. WT 3 months; 4. Cln1−/− 3 months; 5. WT 6 months; 6. Cln1−/− 6 months (n = 5 animals in each group, *P < 0.05). (B) Western blot analyses of CD using total homogenates of cortical tissues from WT mice and those of their Cln1−/− littermates. 1M, 1 month; 3M, 3 months; 6M, 6 months. (C) Quantitation of the CD-protein bands in the western blot. (D) Colocalization of CD with astrocyte marker, GFAP, in cortical tissue sections of the brain from 6-month-old WT and Cln1−/− mice (n = 3). (E) Colocalization of CD with microglial marker, Iba1, in cortical section brains from WT and Cln1−/− mice (n = 3 animals in each group). (F) Expression of Transcription factor EB (TFEB)-mRNA in cerebral cortices of WT and Cln1−/− mice determined by qRT-PCR. 1. 1-Month-old WT; 2. 1-month-old Cln1−/−; 3. 3-month-old WT; 4. 3-month-old Cln1−/−; 5. 6-month-old WT; 6. 6-month-old Cln1−/− (n = 5 per age group, *P < 0.05). (G) Western blot analysis of TFEB in cortical homogenates from WT and Cln1−/− mouse brain, respectively. (H) Densitometric quantitation of the TFEB-protein bands in western blots (n = 3 separate experiments, *P < 0.05).
To further confirm the results of western blot analyses, we performed immunohistochemical detection of CD. The results showed that compared with the levels of CD immunoreactivity in the brain of WT mice (Supplementary Material, Fig. S2A, upper panels), those in Cln1−/− littermates (Supplementary Material, Fig. S2A, lower panels) were substantially more intense. Moreover, the increased levels of CD immunoreactivity in Cln1−/− mouse brain clearly correlated with advancing age of the animals (Supplementary Material, Fig. S2A, lower panels). Immunohistochemical analyses also confirmed higher levels of CB (Supplementary Material, Fig. S2B) and CL (Supplementary Material, Fig. S2C) proteins in cortical tissues of Cln1−/− mice. These results showed that CD, CB and CL were overexpressed in the brain of Cln1−/− mice but the specific cell type(s) that overexpressed Cln10/Ctsd gene was unknown.
Cln10/Ctsd overexpression occurs predominantly in astrocytes and microglia
Previous studies have shown that increased apoptosis of the neurons in INCL patients' brain (26) as well as in those of Cln1−/− mice (27) lead to neurodegeneration. Moreover, the decline in the neuron population in the brain of Cln1−/− mice is followed by progressive infiltration of activated astrocytes and microglia (27,28) as assessed by increased levels of glial fibrillary acidic protein (GFAP) (Supplementary Material, Fig. S3A, upper panel) and ionized calcium binding adaptor-1(Iba1) (Supplementary Material, Fig. S3A, middle panel), respectively. The results of immunohistochemical studies (Supplementary Material, Fig. S3B) further confirmed these results. Thus, we sought to determine if astrocytes and microglia in the brain of Cln1−/− mice overexpressed CD. Accordingly, we performed double immunofluorescence confocal microscopy of brain tissue sections from Cln1−/− and WT mice using antibodies to CD as well as those of GFAP and Iba1. The results showed that compared with WT littermates, high levels of CD immunoreactivity colocalized with both GFAP-positive (Fig. 1D) and Iba1-positive cells in Cln1−/− mouse brain (Fig. 1E). In contrast, there were no appreciable differences in the colocalization of CD immunoreactivity with that of the neuronal marker, NeuN, in the brain tissues from WT (Supplementary Material, Fig. S3C, upper panel) mice and in those of their Cln1−/− littermates (Supplementary Material, Fig. S3C, lower panel). These results strongly suggested that CD overexpression in the brain of Cln1−/− mice occurs predominantly in astrocytes and microglia.
Overexpression of CD is mediated by upregulation of TFEB in the Cln1−/− mouse brain
Recently, it has been reported that a palindromic sequence, called Coordinated Lysosomal Expression and Regulation (CLEAR) element, which is highly enriched in the 5′-promoter region of many genes encoding lysosomal proteins (29). In aberrant lysosomal storage conditions of LSDs, Transcription factor EB (TFEB), a master regulator of lysosomal proteins, binds to the CLEAR element and stimulates the expression of its target genes (30). Consistent with these reports, we found that there are two CLEAR elements localized in the 5′-promoter region of the CD gene (Supplementary Material, Fig. S4A). Thus, we determined the level of TFEB-mRNA in cortical tissues of Cln1−/− mice and those of their WT littermates by real-time RT-PCR. The results showed that the cortical tissues of Cln1−/− mice expressed higher levels of TFEB-mRNA and this increase was age dependent (Fig. 1F). Consistent with these results, TFEB-protein levels were also higher in Cln1−/− mouse brain (Fig. 1G and H).
To identify the cell types, which expressed TFEB, we analyzed the level of TFEB-immunofluorescence in cortical tissues from WT and Cln1−/− mice. The results showed elevated levels of TFEB-immunoreactivity in both astrocytes (Supplementary Material, Fig. S4B) and microglia (Supplementary Material, Fig. S4C) in the cerebral cortex of Cln1−/− mice. Notably, although the astrocytes from Cln1−/− mice expressed higher levels of TFEB-mRNA, its expression albeit low level in cultured neurons from both WT and Cln1−/− mice was virtually identical (Supplementary Material, Fig. S4D). The results of immunocytochemical analysis further confirmed these results (Supplementary Material, Fig. S4E). Taken together, these results revealed that TFEB is overexpressed primarily by astrocytes and microglia in Cln1−/− mouse brain.
Oxidative stress in Cln1−/− mouse brain upregulates TFEB-mediating CD overexpression
Oxygen is essential for life, but paradoxically, as a by-product of metabolism, it generates reactive oxygen species, which is highly toxic to the cells, especially in the brain (31). Although a link between oxidative stress and neurodegeneration has long been suggested, the mechanism(s) by which it contributes to pathogenesis in specific neurodegenerative diseases has not been clearly elucidated (31). Previously, we reported that in the brain of Cln1−/− mice, high levels of ER and oxidative stress are readily detectable (27,32). To determine whether oxidative stress upregulated TFEB-mediating CD overexpression in Cln1−/− mouse brain, we used cultured astrocytes from WT mice and induced oxidative stress by treating the cells with H2O2. We found that compared with the untreated cells, those treated with H2O2 expressed markedly higher levels of both TFEB-mRNA (Fig. 2A) and TFEB-protein (Fig. 2B and C). Consistent with these results, oxidative stress also caused the expression of substantially higher levels of CD-mRNA (Fig. 2D) and CD protein (Fig. 2E and F).
Figure 2.
Oxidative stress upregulates TFEB-mediating CD overexpression. (A) The levels of TFEB-mRNA in WT astrocytes subjected to oxidative stress by H2O2 (400 µm for 4 h) treatment (n = 3 independent experiments, *P < 0.05). (B) TFEB-protein levels in WT astrocytes treated with H2O2. (C) Densitometric quantitation of the TFEB-protein blot (n = 3, *P < 0.05). (D) The levels of CD-mRNA in WT astrocytes subjected to oxidative stress as indicated above (n = 3 experiments, *P < 0.05). (E) CD-protein levels in WT astrocytes subjected to oxidative stress. (F) Densitometric quantitation of CD-protein bands in the immunoblot (n = 3 experiments, *P < 0.05). (G) TFEB immunoblot after the WT astrocytes were treated with control or TFEB-shRNA followed by induction of oxidative stress. (H) Quantitation of the TFEB immunoblot (n = 3 experiments, *P < 0.05 with respect to control; **P < 0.05 with respect to scrambled-shRNA + H2O2); (I) CD immunoblot and its densitometric quantifications after the WT astrocytes were treated with control. (J) TFEB-shRNA followed by induction of oxidative stress (n = 3 experiments, *P < 0.05 with respect to control, **P < 0.05 with respect to scrambled-shRNA + H2O2).
To further confirm that oxidative stress-induced CD overexpression was mediated by TFEB, we transfected WT astrocytes with either scrambled- or TFEB-shRNA and then treated the cells with H2O2. The results of western blot analysis showed that treatment of the cells with TFEB-shRNA, but not with scrambled-shRNA, caused significant reduction in oxidative stress-induced upregulation of TFEB (Fig. 2G and H). Consistent with these results, a robust decline in CD-protein levels in TFEB-shRNA-transfected astrocytes was found compared with those treated with scrambled-shRNA (Fig. 2I and J). These results demonstrated that oxidative stress-mediated Cln10/Ctsd gene overexpression via upregulation of TFEB. To our knowledge, this is the first report that oxidative stress upregulates TFEB-mediating CD overproduction.
Lysosomal processing of pro-CD to mature-CD is impaired in Cln1−/− mice
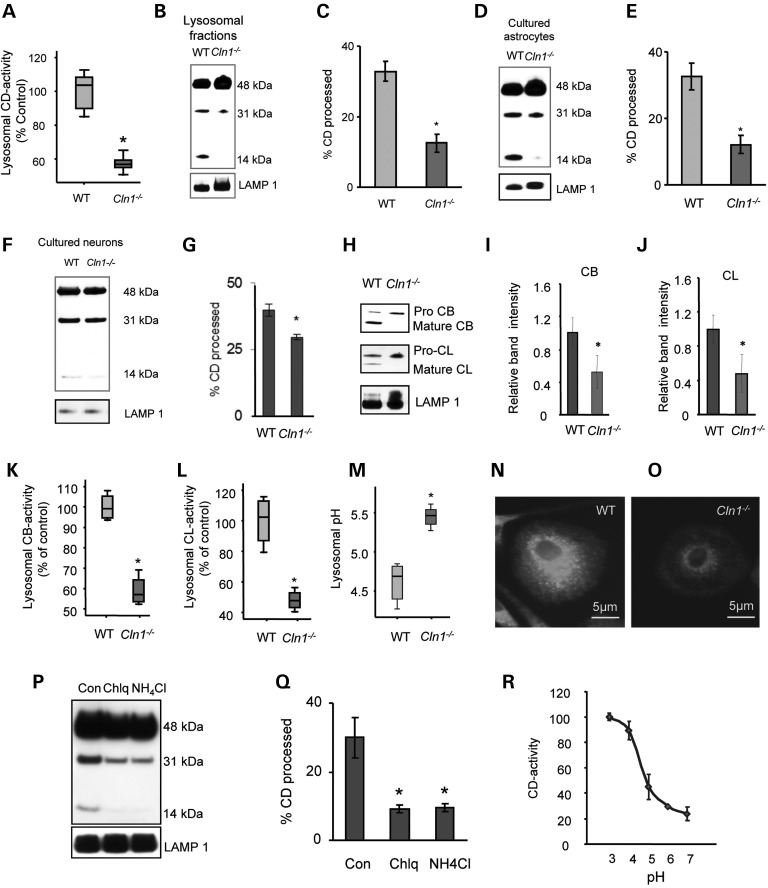
Elevated levels of Cln10/Ctsd gene expression have been reported in total lysates of cultured cells from patients with Gaucher disease and other sphingolipidoses (12) as well as in those of patients with Alzheimer's disease (10,11) and Niemann Pick C1 disease (33). However, in these studies, the CD activity in lysosome has not been evaluated. Thus, it remained unclear whether high levels of CD protein in total cell and tissue lysates reflected the levels of enzymatically active-CD in lysosomes. This is a very important and relevant question to be resolved because various cargos imported to the cell or endogenously generated by autophagy are delivered to lysosomes for degradation by lysosomal hydrolases (3). Thus, the levels of enzymatically active-CD in lysosome may be a determining factor of the degradative capability of this organelle. We reasoned that even though the levels of pro-CD and mature-CD in total lysates of cultured astrocytes and brain tissues from Cln1−/− mice were high, defective processing of pro-CD to mature-CD, which occurs in the lysosome, may lead to CD deficiency in this organelle, thereby causing accumulation of undegraded cargo, which leads to pathogenesis. This hypothesis, at least in part, is supported by the fact that inactivating mutations in the Cln10/CD gene also underlie pathogenesis of a devastating neurodegenerative LSD, CNCL (13–15). Consistent with this hypothesis, we found that CD enzymatic activity in purified lysosomal fractions from Cln1−/− mouse brain was disproportionately low compared with that of their WT littermates (Fig. 3A).
Figure 3.
Elevated pH disrupts CD maturation in lysosome suppressing CD activity. (A) Lysosomal fractions isolated from brain tissues of Cln1−/− mice and those of their WT littermates were analyzed for CD-enzyme activity (n = 4 animals in each group, *P < 0.05). (B) CD-protein levels in isolated lysosomal fractions from WT and Cln1−/− mice brain cortex. (C) Percentage CD processing as analyzed from the CD-protein blot as (density of 31 kDa band + 14 kDa band)/total CD band intensities (n = 4 animals, *P < 0.05). (D) The levels of CD protein in the lysosomal fractions from primary cultures of astrocytes isolated from cerebral cortices of WT and Cln1−/− pups. (E) Percentage CD processing as analyzed from the CD-protein blot (n = 3 independent experiments, *P < 0.05). (F) CD-protein levels in the lysosomal fractions from primary neuronal cells isolated from cerebral cortices of WT and Cln1−/− embryos. (G) Percentage CD processing in lysosomal fractions from primary neuronal cells (n = 3 independent experiments, *P < 0.05). (H) The levels of mature-CB and -CL in lysosomal fractions obtained from cortical tissues from WT and Cln1−/− mice. (I) Densitometric quantitation of mature-CB-protein bands in western blots (n = 4, *P < 0.05). (J) Densitometric quantitation of CL-protein bands in western blots (n = 4, *P < 0.05). (K) Enzymatic activities of CB in the lysosomal fractions isolated from Cln1−/− mouse cerebral cortex (n = 4 animals, *P < 0.05). (L) Enzymatic activity of CL in the lysosomal fractions isolated from Cln1−/− mouse cerebral cortex (n = 4 animals, *P < 0.05). (M) Lysosomal pH in WT and Cln1−/− astrocytes as measured by Oregon green-dextran and Tetramethylrhodamine (TMR)-dextran (n = 3 separate experiments, *P < 0.05). (N) Fluorescence images of WT astrocytes after 10 min of loading with Lysosensor green DND-189 (n = 10). (O) Fluorescence images of Cln1−/− astrocytes after 10 min of loading with Lysosensor green DND-189 (n = 10). (P) Levels of pro- and mature-CD proteins in lysosomal fractions isolated from WT astrocytes treated with Chlq or NH4Cl. (Q) Percentage CD processing as calculated from the CD immunoblot (n = 3 experiments, *P < 0.05). (R) Dependence of CD activity on pH (n = 3 experiments).
To delineate whether the lysosomal processing of pro-CD to mature-CD in Cln1−/− mouse brain was impaired, we first determined the levels of pro-CD (48 kDa) and mature-CD (31 + 14 kDa) proteins in purified lysosomal fractions from the brain of Cln1−/− mice and from those of their WT littermates. Notably, the results showed that the lysosomal fractions from Cln1−/− mouse brain contained a substantially lower level of the 31 kDa fragment and a virtually undetectable 14 kDa fragment of the CD protein (Fig. 3B and C). Because non-covalent dimerization of 31 and 14 kDa fragments constitutes the enzymatically active-CD, the markedly reduced levels of any one of these fragments are likely to cause decreased CD activity in lysosome.
Defective lysosomal maturation of pro-CD to active-CD in astrocytes from Cln1−/− mice
Because we found that CD overexpression occurred predominantly in astrocytes and microglia in the brain of Cln1−/− mice, we sought to determine whether the processing of pro-CD to mature, enzymatically active-CD in the lysosome was also defective. Accordingly, we isolated lysosomal fractions from cultured astrocytes derived from cerebral cortices of WT mice and those of their Cln1−/− littermates and performed western blot analysis using CD antibody. The results showed that although the lysosomal fractions from WT astrocytes contained both pro-CD and mature-CD proteins, those from the Cln1−/− astrocytes showed only the 31 kDa band albeit markedly lower density and a virtually undetectable 14 kDa CD-protein band (Fig. 3D and E). Although unlike the astrocytes, neurons do not overexpress CD, we sought to determine if the lysosomal processing defect was detectable in neurons from Cln1−/− mice. The results showed that even though CD was expressed at a much lower level in neurons compared with Cln1−/− astrocytes and microglia, the defective processing of pro-CD to mature-CD in lysosomal fractions was clearly appreciable (Fig. 3F and G). Taken together, these results strongly suggested that the pro-CD-processing defect is present in neurons, astrocytes and microglia in the brain of Cln1−/− mice.
Suppressed CB and CL activities in Cln1−/− mouse brain disrupt maturation of pro-CD
As stated earlier, the synthesis of CD occurs in the ER as a pre-pro-peptide with a molecular mass of ∼50 kDa (9). In the ER, the leader peptide is cleaved off generating the 48 kDa precursor of CD (pro-CD). The pro-CD then undergoes further proteolytic cleavage in the lysosome by CB and CL, respectively, generating the 31 and 14 kDa fragments, non-covalent dimerization of which generates the enzymatically active-CD (9). Thus, we first determined the levels of CB and CL proteins in the brain lysosomal fractions from Cln1−/− mice and from those of their WT littermates. The results showed that in Cln1−/− mice, the levels of mature-CB and -CL proteins in lysosomal fractions were markedly lower than their WT littermates (Fig. 3H–J). Consistent with these results, the enzymatic activities of both CB (Fig. 3K) and CL (Fig. 3L) in lysosomal fractions from Cln1−/− mouse brain were also reduced. Cumulatively, these results suggested that in the Cln1−/− mouse brain, the processing of pro-CD to mature-CD in lysosomes is disrupted at least in part due to reduced CB and CL activities.
Elevated lysosomal pH in Cln1−/− cells adversely affects CD maturation
Elevated lysosomal pH has been reported in virtually all LSDs and in many non-LSD adult-onset neurodegenerative diseases like Alzheimer's (10,11). Thus, we reasoned that defective maturation of pro-CD to enzymatically active-CD in this organelle may be due to altered lysosomal pH in cells from Cln1−/− mice. To test this hypothesis, we measured the pH in lysosomes of cultured astrocytes from Cln1−/− mice and those of their WT littermates (Supplementary Material, Fig. S5A). The results showed that astrocytes isolated from WT mice had a pH of 4.6, whereas those isolated from Cln1−/− mice was ∼5.4 (Fig. 3M). To confirm these results, we performed experiments in which the cells were labeled with Lysosensor Green DND-189 and continuously monitored the intracellular fluorescence for 10 min. The results showed that the fluorescence intensity in astrocytes from Cln1−/− mice was substantially lower compared with those of their WT littermates (Fig. 3N and O). Similar results were obtained when an identical experiment was conducted using cultured fibroblasts from patients with INCL and those from normal control subjects (Supplementary Material, Fig. S5B–D).
Next, we cultured WT astrocytes, which were either untreated (control) or treated with chloroquine or NH4Cl as both of these agents have been reported to increase lysosomal pH. We then measured lysosomal pH in untreated as well as in cells treated with these agents and evaluated lysosomal processing of pro-CD to mature-CD proteins by western blot analysis. As expected, the results showed that compared with untreated WT astrocytes, the lysosomal pH in both chloroquine- and NH4Cl-treated cells was significantly higher (Supplementary Material, Fig. S5E). The results of western blot analysis using CD antibody also showed that compared with the untreated control cells, the lysosomal processing of pro-CD to mature-CD in both chloroquine- and NH4Cl-treated WT astrocytes was substantially impaired (Fig. 3P and Q). We also measured the CD enzymatic activity using recombinant CD (R&D Systems, Cat # 1029-AS-010) in an assay in which the pH of the assay buffer was varied (ranging from pH 3.5 to 7.5). The results showed that increasing the pH above 5 caused a substantial reduction in CD activity (Fig. 3R). Taken together, these results suggested that elevated lysosomal pH in astrocytes from Cln1−/− mice not only caused reduced enzymatic activity of CD but also that of CB and CL, which are essential for processing pro-CD to mature-CD in the lysosome.
Impaired degradation of α-synuclein in lysosomal fractions of Cln1−/− mouse brain
A common pathological finding in many neurodegenerative disorders is the presence of inclusion bodies in the brain, which mainly consist of filamentous aggregates of α-synuclein (34). Because the degradation of α-synuclein requires catalytically active-CD in lysosome (35), we determined whether disrupted lysosomal processing of pro-CD to mature-CD in Cln1−/− mouse brain impaired the degradation of this protein. The results showed that α-synuclein levels were significantly higher in lysosomal fractions from Cln1−/− mouse brain compared with that of their WT littermates (Fig. 4A and B). These results provided further evidence that lysosomal proteolysis mediated by active-CD is impaired in Cln1−/− mouse brain causing accumulation of α-synuclein contributing to INCL pathogenesis.
Figure 4.
Impaired lysosomal proteolysis in cultured astrocytes from Cln1−/− mice. (A) Levels of α-synuclein, a specific substrate of CD, in the purified lysosomal fraction of brain tissues from WT and Cln1−/− littermates. (B) Quantitation of protein bands in α-synuclein immunoblot (n = 4 animals in each group, *P < 0.05). (C) [3H]-leucine incorporation into long-lived intracellular proteins in cultured astrocytes from WT and Cln1−/− mice (n = 4 experiments). (D) Measurement of radioactive long-lived protein degradation in WT and Cln1−/− astrocytes 24 h after [3H]-leucine labeling. During the chase period, cells were incubated in serum-containing or serum-free media. 1. WT astrocytes in serum-containing media; 2. WT astrocytes in serum-free media; 3. Cln1−/− astrocytes in serum-containing media and 4. Cln1−/− astrocytes in serum-free media (*P < 0.05, n = 4 experiments). (E) Quantitative evaluation of proteolysis in astrocytes from WT and Cln1−/− mice, respectively, following 24 h culture in serum-free or serum-containing media. *P < 0.05, n = 4 experiments. (F) Lysosomal protein accumulation in the brain of 6-month-old Cln1−/− mice compared with that of their WT littermates. (G) Densitometric quantitation of the numbered bands (designated 1–9) in F (western blot), n = 4 animals in each group, *P < 0.05. (H) Levels of pro- and mature-CD in total lysates from WT and Cln1−/− astrocytes. (I) Quantitation of the CD immunoblot (n = 3 experiments, *P < 0.05). (J) The levels of CD from conditioned media from culturing Cln1−/− astrocytes, prelabeled with [35S]-methionine. 1. WT (0 h); 2. Cln1−/− (0 h); 3. WT (1 h); 4. Cln1−/− (1 h); 5. WT (3 h); 6. Cln1−/− (3 h); 7. WT (6 h) and 8. Cln1−/− (6 h). (K) Densitometric analysis of the CD immunoblot (n = 3 independent experiment, *P < 0.05 compared with WT control at 1 h). (L) Cln1−/− astrocytes were treated with vehicle or with GM6001, labeled with [35S]-methionine and chased for 6 h with unconditioned media supplemented with non-radioactive cysteine and methionine. 1. WT; 2. Cln1−/− treated with vehicle (control) and 3. Cln1−/− mice treated with GM6001. (M) Percentage CD processing as analyzed from the CD immunoblot (n = 3 per treatment group, *P < 0.05). (N) Detection of CB in conditioned media from cultured WT and Cln1−/− astrocytes (n = 3).
Impaired degradation of intracellular long-lived proteins in cultured Cln1−/− astrocytes
Long-lived intracellular proteins as well as damaged organelles in the cytoplasm are degraded and cleared from cells by lysosomal proteases such as CD (2,3,6). However, despite the fact that in neurodegenerative LSDs lysosomal degradative ability is impaired, the molecular mechanism(s) of this defect until now remained poorly understood. We reasoned that defective processing of pro-CD to mature-CD in lysosome of Cln1−/− mouse brain may impair the degradation of long-lived proteins. We tested this hypothesis, using cultured astrocytes from Cln1−/− mice and from those of their WT littermates, which were labeled with [3H]-leucine for 48 h followed by 24 h chase with either complete or serum-free media with excess non-radioactive leucine. Because macroautophagy is a major degradative process in cells that also requires active lysosomal hydrolases, we induced macroautophagy in cultured astrocytes from WT and Cln1−/− mice by serum starvation and labeled the intracellular long-lived proteins in these cells with [3H]-leucine. The results showed that the levels of [3H]-leucine-labeled proteins in astrocytes from both WT and Cln1−/− mice were virtually identical (Fig. 4C). However, the clearance of [3H] leucine-labeled intracellular long-lived proteins in astrocytes from Cln1−/− mice was substantially reduced compared with that of their WT littermates under both serum-supplemented and serum-free conditions (Fig. 4D and E). These results demonstrated a significant impairment of long-lived protein degradation in Cln1−/− astrocytes.
To evaluate whether impaired protein degradation in lysosomal fractions of Cln1−/− astrocytes disrupted cellular clearance of these proteins causing accumulation in lysosomes, we resolved total proteins from purified lysosomal fractions of WT and Cln1−/− mouse brain by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), silver stained the protein bands and performed densitometric analysis. The results showed that compared with the levels of several protein bands in the lysosomal fractions of cortical tissues from WT mice, those in the lysosomal fractions from Cln1−/− littermates were appreciably higher (Fig. 4F and G). Because palmitoylated proteins are refractory to degradation by lysosomal hydrolases (36), we also determined the total palmitoylated protein load in the brain of WT and Cln1−/− mice. The results showed that the levels of total palmitoylated proteins (constituents of ceroid) in lysosomal fractions from Cln1−/− mouse brain were higher compared with those of the WT littermates (Supplementary Material, Fig. S5F and G). These results revealed that impaired lysosomal protein degradation due to the defective processing of pro-CD to mature-CD may at least in part be a contributing factor underlying neuropathology in Cln1−/− mice.
Presence of pro- and mature-CDs in conditioned media from cultured Cln1−/− astrocytes
Thus far, our results have shown that despite high levels of pro-CD protein in total lysates of brain tissues from Cln1−/− mice, there is a lack of mature-CD (31 and 14 kDa fragments) in lysosomes. This defect led to the reduced CD activity in lysosomal fractions from Cln1−/− mouse brain as well as in those of cultured astrocytes from these mice. However, as stated earlier, both pro-CD and mature-CD were detectable in total lysates of cortical tissues from Cln1−/− mice, but mature-CD was virtually undetectable in purified lysosomal fractions from the same tissues. To understand this paradox, we considered the possibility that the astrocytes from Cln1−/− mice may secrete pro-CD into the extracellular environment where it is processed to mature-CD by an as yet unknown mechanism. In order to exclude the possibility that pro-CD in Cln1−/− astrocytes were not processed to mature-CD in the cytoplasm and then secreted extracellularly, we first performed western blot analysis of total lysates of cultured astrocytes from WT and Cln1−/− mice after the cells were thoroughly washed. This was done to remove any potential contamination of mature-CD from the conditioned media in which the Cln1−/− astrocytes were cultured. The results showed that although both 31 and 14 kDa protein bands were detectable in total lysates of astrocytes from WT mice, those from Cln1−/− astrocytes showed only the 31 kDa CD-protein band with markedly reduced density and a virtual lack of the 14 kDa CD fragment (Fig. 4H and I). Because the heterodimer of the 31 and 14 kDa fragments constitutes the enzymatically active-CD, our results provided a reasonable explanation for decreased CD activity in lysosomes despite CD overexpression in Cln1−/− mouse brain.
To determine whether pro-CD was secreted into the extracellular environment by Cln1−/− astrocytes, we labeled cultured WT and Cln1−/− astrocytes with [35S]-methionine for 6 h followed by a chase with medium containing non-radioactive methionine for varying lengths of time (0–6 h) in the presence of cycloheximide (10 µg/ml) to inhibit new protein synthesis. Aliquots of the conditioned culture media were immunoprecipitated with CD antibody and the immunoprecipitates were resolved by SDS–PAGE. The radioactive CD-protein bands were detected by autoradiography. The results showed that the astrocytes from Cln1−/− mice secreted significantly higher levels of pro-CD into the culture media that was processed to mature-CD in a time-dependent manner compared with those of their WT littermates (Fig. 4J and K). These results demonstrated that the astrocytes from Cln1−/− mice secreted substantially higher levels of pro-CD into the culture media raising the possibility that secreted pro-CD processed extracellularly to mature-CD.
Extracellular processing of secreted pro-CD is catalyzed by matrix metalloproteinases
How might pro-CD secreted by Cln1−/− astrocytes be extracellularly processed to mature-CD? We hypothesized that extracellular CD processing may be catalyzed at least in part by matrix metalloproteinases (MMPs). This hypothesis is partially supported by our previous finding that several MMPs are overexpressed in the brain tissues of Cln1−/− mice compared with those of their WT littermates (37). Thus, we used conditioned media from culturing Cln1−/− astrocytes, which were either untreated or treated with a generalized MMP inhibitor, GM6001 (10 nm), and then labeled with [35S]-methionine (see Materials and Methods for detail). The conditioned media from the labeled cells were then immunoprecipitated using CD antibody and the immunoprecipitates were resolved by SDS–PAGE. The radioactive CD-protein bands were detected by autoradiography. The results showed that the culture media of GM6001-treated Cln1−/− astrocytes contained substantially reduced levels of radioactive mature-CD-protein bands (Fig. 4L and M). To determine the potential contribution of CB and CL in extracellular processing of pro-CD to mature-CD, we measured the levels of mature-CB and -CL proteins as well as their enzymatic activities. The results showed that pro-CB protein is readily detectable in western blot analysis, whereas mature-CB protein band was undetectable (Fig. 4N). Moreover, neither pro-CL nor mature-CL proteins were detectable (data not shown). Consistent with these findings, the CB and CL activities were also undetectable in conditioned media from Cln1−/− astrocyte cultures (data not shown). Taken together, these results suggested that Cln1−/− astrocytes secreted pro-CD, which was extracellularly processed to mature-CD, most likely by the MMPs.
Neurotoxicity of secreted CD in conditioned medium from Cln1−/− astrocyte cultures
Because increased neuronal death leads to neurodegeneration in INCL, we sought to determine whether extracellular CD from astrocytes and microglia from Cln1−/− mice was toxic to the neurons. To address this question, we first generated conditioned media by culturing astrocytes from WT and Cln1−/− mice. We then incubated cultured cortical neurons from WT mice with either non-conditioned or conditioned media from culturing astrocytes from WT mice or those from their Cln1−/− littermates (Fig. 5A) and determined the viability of the neurons after 12 h of incubation using MTT assay (38). The results showed that incubation of WT neurons with conditioned media from Cln1−/− astrocytes significantly reduced their viability (Fig. 5B), whereas incubation with non-conditioned media or media conditioned by culturing WT astrocytes showed virtually no reduction in viability (Fig. 5B). To confirm that the reduced viability of neurons incubated with conditioned media from Cln1−/− astrocytes was due to the toxic effects of CD, we first depleted CD from the conditioned media from culturing Cln1−/− astrocytes, by incubating with agarose beads conjugated to CD antibody and then incubating the WT neuron cultures with the CD-depleted conditioned media (Fig. 5C). The results showed that the viability of neurons incubated with CD-depleted conditioned media from Cln1−/− astrocytes remained unaltered, whereas the same conditioned media mixed with agarose beads without CD antibody (control) or with agarose beads conjugated to a non-specific IgG markedly reduced viability (Fig. 5D). Taken together, these results indicated that extracellular CD, secreted by Cln1−/− astrocytes, is toxic to neurons and may contribute to neuropathology in Cln1−/− mice.
Figure 5.
Secreted CD in conditioned media from Cln1−/− astrocyte culture is neurotoxic. (A) Schematic depiction of the experimental design. Cultured neurons from WT mice were either untreated or treated with unconditioned media only or with media conditioned by culturing WT or Cln1−/− astrocytes for 12 h. (B) Viability of the neurons was tested using MTT assay. 1. Untreated; 2. media only; 3. conditioned media from WT astrocytes; 4. conditioned media from Cln1−/− astrocytes (n = 3 experiments, *P < 0.05). (C) Conditioned culture media from growing Cln1−/− astrocytes were mixed with agarose beads alone or agarose beads conjugated with CD antibody or with non-specific IgG (control). (D) Neurons from WT mice were treated with the conditioned media for 12 h before performing the MTT assay. 1, Untreated; 2, conditioned media from Cln1−/− astrocytes; 3, conditioned media from Cln1−/− astrocytes + bead only; 4, conditioned media from Cln1−/− astrocytes + non-specific IgG; 5, conditioned media from Cln1−/− astrocytes + CD antibody (n = 3 experiments, *P < 0.05).
A thioesterase-mimetic antioxidant corrects the lysosomal CD-processing defect
As stated earlier, palmitoylation (S-acylation) is a posttranslational modification in which a 16-carbon fatty acid (predominantly palmitate) is attached to specific cysteine residues via thioester linkage (39). Cln1 gene product, PPT1, cleaves the thioester linkage in S-acylated proteins required for their degradation by lysosomal hydrolases. Because Cln1−/− mice as well as INCL patients manifest high levels of oxidative stress, we reasoned that compounds, which cleave the thioester linkage in S-acylated proteins and possess antioxidant property, may ameliorate the pro-CD-processing defect in Cln1−/− mice. Hydroxylamine, like Ppt1, specifically cleaves thioester linkage in palmitoylated proteins (39) although at high doses, it has toxic effects. However, it has been reported that a derivative of hydroxylamine, NtBuHA, is non-toxic, possesses antioxidant property and improves mitochondrial function in vitro and in vivo extending longevity in aging mice (40). Moreover, we previously reported that NtBuHA, cleaved thioester linkage in S-acylated proteins mediating depletion of ceroid, improved motor function and modestly increased lifespan in Cln1−/− mice (41), although we did not investigate the status of CD in these mice. We therefore sought to determine if treatment of Cln1−/− astrocytes with NtBuHA may correct the CD-processing defect. We also sought to determine whether NtBuHA treatment had any positive effect on lysosomal acidification, which is impaired in virtually all LSDs and many neurodegenerative disorders and may be responsible for adversely affecting the maturation of pro-CD to catalytically active-CD. Accordingly, we isolated lysosomes from untreated and NtBuHA-treated astrocytes derived from Cln1−/− mice and determined the pH and evaluated the processing of pro-CD to mature-CD. The results showed that NtBuHA treatment caused appreciable decline in lysosomal pH (Fig. 6A–C) and improved the processing of pro-CD to mature-CD in lysosomal fractions from Cln1−/− astrocytes (Fig. 6D and E).
Figure 6.
NtBuHA ameliorates lysosomal maturation of pro-CD in cellula and in vivo. (A) Lysosomal pH of untreated and NtBuHA-treated astrocytes from Cln1−/− mice measured using Oregon green-dextran and TMR-dextran (n = 3 independent experiment, *P < 0.05). (B) Fluorescence imaging of lysosomal pH of untreated astrocytes from Cln1−/− mice loaded with lysosensor DND-189. (C) Fluorescence imaging of lysosomal pH of NtBuHA-treated astrocytes from Cln1−/− mice loaded with lysosensor DND-189. (D) Levels of pro-CD as well as mature-CD in the lysosomal fractions of WT, untreated and NtBuHA-treated Cln1−/− astrocyte. (E) Percentage CD processing calculated from CD immunoblot (n = 3 experiments, *P < 0.05 with respect to WT control, **P < 0.05 with respect to untreated Cln1−/− mice). (F) Western blot analysis showing pro- and mature-CD-protein bands in lysosomal fraction from brain tissues of untreated Cln1−/− mice. (G) Western blot analysis showing pro- and mature-CD-protein bands in lysosomal fraction from brain tissues of Cln1−/− mice treated with NtBuHA (1 mm in drinking water) for 3 months (from 3 months of age until they reached 6 months of age) (n = 5 animals, *P < 0.05). (H–J) Enzyme activities of CD, CB and CL, respectively, in lysosomes of untreated and NtBuHA-treated Cln1−/− mice. 1. Untreated Cln1−/− and 2. NtBuHA-treated Cln1−/− (*P < 0.05; n = 4 animals).
To delineate whether these in vitro effects of NtBuHA are replicable in vivo, we used brain tissues from untreated and NtBuHA-treated Cln1−/− mice and performed western blot analysis of lysosomal fractions using CD antibody. The results showed that purified lysosomes from the brain tissues from NtBuHA-treated Cln1−/− mice contained both the 31 and 14 kDa protein bands (Fig. 6F and G) and also displayed an increase in lysosomal CD activity (Fig. 6H) compared with those of their untreated littermates. The enzyme activities of CB (Fig. 6I) as well as CL (Fig. 6J) in lysosomal fractions from brain tissues of NtBuHA-treated Cln1−/− mice were also increased. These results demonstrated that NtBuHA with its thioesterase-mimetic and antioxidant properties is capable of lowering the lysosomal pH in Cln1−/− mouse brain, which provided the optimal condition for activation of CB, CL and CD.
Depletion of intracellular long-lived proteins in Cln1−/− astrocytes treated with NtBuHA
To determine whether NtBuHA treatment-mediated depletion of intracellular long-lived proteins, we determined the level of α-synuclein, a specific CD substrate (35), in the brain of untreated and NtBuHA-treated Cln1−/− mice. We found that the levels of α-synuclein in the brain of untreated Cln1−/− mice were substantially higher than in those of their WT littermates. Importantly, the levels of α-synuclein in brain tissues from NtBuHA-treated Cln1−/− mice were substantially lower compared with those of untreated controls (Fig. 7A and B).
Figure 7.
Treatment with NtBuHA improves lysosomal proteolysis. (A and B) Levels of α-synuclein and its quantitation in lysosomes isolated from cortical tissues of WT mice and those of untreated- and NtBuHA-treated Cln1−/− mice (n = 3 experiments) (*P < 0.05 with respect to WT control, **P < 0.05 with respect to untreated Cln1−/− mice). (C) Evaluation of long-lived protein degradation 24 h after [3H]-leucine labeling of untreated or NtBuHA-treated astrocytes from Cln1−/− mice. Astrocytes were treated with 1 mm NtBuHA for 7 days. 1. Untreated Cln1−/− cells in serum-containing media; 2. untreated Cln1−/− cells in serum-free media; 3. NtBuHA-treated Cln1−/− cells in serum-containing media; 4. NtBuHA-treated Cln1−/− cells in serum-free media (*P < 0.05, n = 3 experiments). (D) Cln1−/− astrocytes were treated with NtBuHA (1 mm) and labeled with [3H]-leucine. The ratio of proteolysis 24 h after removal of serum compared with serum-replete condition in untreated or NtBuHA-treated Cln1−/− astrocytes are presented. 1. Untreated Cln1−/− and 2. NtBuHA-treated Cln1−/− (*P < 0.05, n = 3). (E) Levels of lysosomal proteins in the brains of 6-month-old WT, untreated or NtBuHA-treated Cln1−/− mice. Three-month-old Cln1−/− mice were continuously treated with NtBuHA from 3 months of age until they were 6 months old before sacrifice. (F) Densitometric quantification of the protein bands (numerically designated as 1–9) in panel (n = 4 experiments, *P < 0.05). (G) Model explaining the pathway of pro-CD to mature-CD maturation in lysosome of WT mice (left panel) and Cln1−/− littermates (right panel). Under normal circumstances, pro-CD is proteolytically processed by the catalytic action of CB and CL, whereas in Cln1−/− mice, suppression of CB and CL activities impaired processing of pro-CD to mature-CD causing accumulation of undegraded cargo in lysosome. Thus, despite overexpression of the CD gene in Cln1−/− mice, CD deficiency in lysosome contributes to pathogenesis.
To determine whether NtBuHA treatment increased CD activity and enhanced the degradation and clearance of long-lived intracellular proteins, we treated Cln1−/− astrocytes with NtBuHA (1 mm) for 7 days and then incubated them with [3H]-leucine to label the newly synthesized proteins. The results showed that the levels of incorporation of [3H]-leucine were similar in both untreated and NtBuHA-treated cells (data not shown). However, notably, NtBuHA treatment significantly increased the degradation of long-lived intracellular proteins in Cln1−/− astrocytes cultured in control as well as in serum-deficient medium (Fig. 7C and D). Moreover, Cln1−/− mice treated with NtBuHA for 3 months showed a substantial reduction in total protein levels in brain lysosomes compared with those from their untreated littermates (Fig. 7E and F). A model explaining the pathway of pro-CD to mature-CD in lysosomes of WT mice and in those of Cln1−/− littermates is presented (Fig. 7G). Cumulatively, our results strongly suggested that despite Cln10/Ctsd overexpression in the brain of Cln1−/− mice, a processing defect of pro-CD to mature-CD in lysosome caused relative deficiency of active-CD in lysosome contributing to neuropathology and NtBuHA with its thioesterase-mimetic and antioxidant properties ameliorated this defect.
Discussion
Although mutations in 13 distinctly different genes underlie various types of NCLs, their clinical and pathologic manifestations are very similar. These similarities include psychomotor retardation, myoclonus and seizures and intracellular accumulation of autofluorescent material (16,17). These features may indicate functional relationships among the genes mutations of which cause various types of NCLs. Despite intense studies, functional relationships among any of the 13 genes until now remained unexplored. The findings in our present study at least in part demonstrate that mutations in Cln1/Ppt1 and Cln10/Ctsd cause the same abnormality, which is CD deficiency.
Cathepsins are the major aspartic proteases that play important roles in lysosomal protein degradation. Our results showed elevated mRNA levels of several cathepsins in the brain of Cln1−/− mice. Increased levels and altered distribution of cathepsins have been reported in a number of neurodegenerative diseases including Alzheimer's disease, amyotrophic lateral sclerosis as well as several lysosomal storage diseases. In these pathological conditions, elevated levels of cathepsins have been shown to activate the apoptotic and necrotic pathways, which may adversely affect the neurons, thereby contributing to progressive neurodegeneration. Consonant with these findings, the results of our present study indicate reduced viability of cultured WT neurons when they were cultured in the conditioned media derived from culturing Cln1−/− astroglia, which contained elevated levels of CD. In contrast, exposure of the neurons to conditioned media from WT astroglia did not show this adverse effect. It remains to be determined whether other cathepsins have similar adverse effects on neurons.
Overexpression of Cln10/Ctsd has been reported in several neurodegenerative disorders although in these studies the CD enzymatic activity in lysosome was not determined. That CD deficiency may lead to a devastating neurodegenerative disease as attested by the fact that inactivating mutations in the Cln10/Ctsd gene underlie CNCL (13–15). Our results implicate that overexpression of the Cln10/Ctsd gene does not necessarily mean that pro-CD is processed to catalytically active-CD in the lysosome. Thus, the results of our study raise the possibility that in neurodegenerative diseases in which CD overexpression has been previously reported may have had CD deficiency in lysosome leading to pathogenesis and suggest that varying factors suppressing lysosomal CD activity may cause neuropathology even though CLN10/CTSD gene is overexpressed. Thus, our results at least in part explains the paradox how both CD overexpression and CD deficiency may be associated with neurodegenerative disorders.
As stated earlier, mutations in at least 13 different genes (CLNs) (42) underlie various types of NCLs (19,20). Among all the NCL types, two of the most devastating diseases are INCL and CNCL, caused by mutations in two distinctly different genes, CLN1/PPT1 and CLN10/CTSD, respectively. We also found that pervasive oxidative stress in the brain of these mice (27,32) via upregulation of TFEB-mediated Cln10/Ctsd overexpression. Moreover, our results showed that Cln10/Ctsd overexpression occurred predominantly in activated astrocytes and microglia. Although oxidative stress has long been a suggested cause of neurodegenerative diseases (43,44), its mechanistic link to pathogenesis of specific neurodegenerative diseases remained elusive (31). Previously, we reported that high level of oxidative stress is prevalent in cultured cells from INCL patients (32) as well as in the brain of Cln1−/− mice (27). The results of our present study demonstrated that oxidative stress caused upregulation of TFEB, a master regulator of genes encoding lysosomal proteins (29,30), mediated overexpression of Cln10/Ctsd. To our knowledge, this is the first report of TFEB upregulation by oxidative stress. Although these results suggest a relevant association between oxidative stress and TFEB upregulation, they warrant further investigations to confirm a causal association between increased oxidative stress and TFEB upregulation.
An unexpected finding in this study was that cultured astrocytes from Cln1−/− mice secreted high levels of pro-CD into the culture media. It is possible that elevated levels of lysosomal exocytosis (45) may account for increased pro-CD in the culture media of Cln1−/− astrocytes. One of the hallmarks of lysosomal exocytosis is the translocation of lysosomal membrane markers such as Lamp1 to the plasma membrane (46–48). Consistent with these reports, the results of our present study show that higher levels of Lamp1 are localized to the plasma membrane of cultured astrocytes from Cln1−/− mice compared with those from their WT littermates (Supplementary Material, Fig. S6). On the basis of the results of our present study, we conclude that even though Cln10/Ctsd gene is overexpressed in the brain of Cln1−/− mice, defective processing of pro-CD to mature-CD in lysosome led to a virtual deficiency of catalytically active-CD in this organelle similar to the effects of inactivating mutations in the CLN10/CTSD gene, which cause CNCL.
How might secreted pro-CD be processed extracellularly to mature-CD? This question is relevant because under normal physiological conditions, pro-CD is processed to mature-CD in the lysosomes primarily by two enzymes, CB and CL (9). Previously, we reported that in the brain tissues of Cln1−/− mice, MMPs are overexpressed (37). Thus, we investigated the possibility that MMPs may contribute to extracellular processing of pro-CD to mature-CD, even though CB and CL may not be fully active in that environment. Consistent with this hypothesis, the results of our present study showed that treatment of cultured astrocytes from Cln1−/− mice with a generalized inhibitor of MMPs, GM6001, substantially reduced the levels of mature-CD in the conditioned media in which these cells were cultured. These results suggested that the processing of extracellular pro-CD to mature-CD may at least in part be catalyzed by the MMPs. The possibility that lysosomal exocytosis may cause CB and CL to be present in the conditions media, which processed pro-CD to mature-CD, can be excluded because our results showed that the conditioned media from Cln1−/− astrocytes did not contain catalytically active CB or CL. Therefore, it appears that maturation of pro-CD in the conditioned media is most likely catalyzed by the MMPs. Consistent with this assumption, recently, it has been reported that a membrane-type 1-MMP is expressed at a high level in neurodegenerative and neuroinflammatory diseases (49).
The mechanism we propose here for the apparent defect in the lysosomal processing of pro-CD to mature-CD in the brain of Cln1−/− mice is summarized in a schematic diagram (Supplementary Material, Fig. S7). The Cln10/CD is overexpressed in the Cln1−/− mouse brain and pro-CD is transported via the mannose-6-phosphate receptors and sortilin through the endosomes to the lysosome. In WT mice, the acidic pH in lysosome and activated CB and CL proteolytically process pro-CD (48 kDa) to mature-CD (31 and 14 kDa), dimerization of which generates enzymatically active-CD. In contrast, the elevated lysosomal pH in Cln1−/− mouse brain suppresses the activation of CB and CL and as a result, the processing of pro-CD to mature-CD is suppressed. Thus, the lysosomes in Cln1−/− mice may be considered CD deficient similar to the ones in CNCL patients as well as in Cln10−/− mice. Therefore, for all practical purposes, the deficiency of enzymatically active-CD in lysosomes links the pathogenesis of these two devastating diseases.
Although in this study, we have shown that elevated lysosomal pH contributed to suppression of CB and CL activities, which adversely affected the maturation of pro-CD to enzymatically active-CD, we have not provided an explanation as to how endosomal/lysosomal pH might be elevated in Cln1−/− mice. In many neurodegenerative disorders, and in virtually all LSDs, lysosomal acidification is dysregulated (2–4) although a mechanism underlying this abnormality has not yet been uncovered. One possibility for lysosomal pH abnormality may be that the vacuolar proton pump (v-ATPase), a multi-subunit protein that regulates lysosomal acidic pH (50,51), is dysfunctional in Cln1−/− mice. Whether v-ATPase is dysfunctional in these mice and how Ppt1 deficiency may impair v-ATPase function is the focus of our current experiments.
Materials and Methods
Chemicals
Chloroquine (Cat # C6628), cycloheximide (Cat # C4859), ammonium chloride (Cat # A9434), N-acetyl cysteine (Cat # A-8199), NtBuHA acetate (Cat # 479 675) and cysteamine (Cat # M9768) were purchased from Sigma–Aldrich (St Louis, MO). The reagents, catalog numbers and the names of the suppliers are as follows: epidermal growth factor (Stem Cell Technologies, Cat # 02633), GM-CSF (R&D Systems, Cat # 415-ML/CF), B27 supplement (Invitrogen, Cat # 17504-044), glutaMAX (Invitrogen, Cat # 35050), GM6001 (Millipore, Cat # CC1010) and resveratrol (RSV, Cat # 501-36-0) were purchased from Orchid Pharmaceuticals (Chennai, India). The three TFEB-shRNAs (Cat # V3LMM_448818, Cat # V3LMM_448819 and Cat # V3LMM_521313) and a scrambled-shRNA (Cat # RHS4346) were purchased from GE Healthcare, Inc., Dharmacon (Lafayette, CO).
Animals and treatments
Cln1−/− mice (24), a reliable animal model of INCL (25), were a generous gift from Dr Sandra W. Hofmann, University of Texas Southwestern Medical Center. All animal procedures were carried out in accordance with institutional guidelines of the National Institute of Child Health and Human Development (NICHD), NIH, Animal Care and Use Committee (ACUC) after the approval of the study protocol (#10-012). Animals of both sexes were genotyped and randomized and used for the treatments. NtBuHA (1 mm) was dissolved in drinking water containing 1 mm NaCl. The treatment of the animals was started at 3 months of age and continued until they were 6 months old. The investigator performing the analyses of data was unaware of the genotype of the animals and of the treatment.
Astrocyte culture and treatment
Astrocytes and microglia were isolated from P2 to P8 mouse pups as previously reported (52). Briefly, cerebral cortices were dissected out from WT and Cln1−/− pups, stripped off meninges, minced in Hank's Balanced Salt Solution (HBSS) and dissociated by trituration in 40 µm mesh. The triturated tissue suspensions were centrifuged (300g) and 1 ml of 0.05% trypsin was added over the pellets. After incubation for 1 min at 37°C, 10 ml of HBSS was added. Cells were centrifuged (300g) and plated in 75 cm2 plastic tissue culture flasks (coated with poly D lysine) containing 10 ml complete Dulbecco's modified Eagle's medium (DMEM)/F12 medium with 10% fetal bovine serum at a density of 1 × 106 cells/ml. On the 11th day, confluent cultures were vigorously agitated on a rotary shaker for 2 h (37°C, at 240 rpm). GFAP-positive astrocytes remained adherent to the flasks. H2O2 (400 μm for 4 h) was added to the culture medium to induce oxidative stress. NtBuHA (1 mm) was added to astrocyte cultures from Cln1−/− mice for 7 days replacing fresh medium containing NtBuHA every 12 h. Chloroquine (10 μm) or NH4Cl (2 mm) was added to astrocyte cultures to increase the lysosomal pH. shRNA experiments were performed using Lipofectamine 2000 (Invitrogen) following manufacturer's protocol. Fibroblasts from normal and INCL patients (provided by Late Dr K.E. Wisniewski) were cultured as reported previously (26,32).
Neuron culture and treatments
Cortical neurons were isolated from E15/17 WT and Cln1−/− mouse pups, plated on poly-d-lysine coated 6-well plates and maintained in a humidified incubator with 5% CO2 at 37°C. Cultures were maintained in Neurobasal medium supplemented with B27 and glutamine (Invitrogen). Neuronal cells, cultured for 1 week, were treated with non-conditioned or conditioned media (generated from culturing WT or Cln1−/− astrocytes for 12 h). Astrocytes were isolated from the brain of P2–P8 WT and Cln1−/− pups and cultured in complete DMEM/F12 medium as described above. The fresh media was added to the astrocyte cultures and incubated for 12 h at 37°C with 5% CO2 in a humidified incubator. The conditioned media were centrifuged at 1000g for 5 min before adding to neuronal cultures. In some experiments, the conditioned media were pretreated with agarose beads alone or agarose beads conjugated with CD antibody or a non-specific IgG before adding to neuronal cultures.
MTT assay
Cell viability was determined according to a previously reported method (38). Briefly, following treatment of the neuronal cultures with unconditioned or conditioned media, they were incubated with MTT (Sigma) for 4 h at 37°C. The resulting formazan crystals were dissolved in acidified isopropanol and absorbance was measured at 570 nm. Viability results were presented as the percent of untreated control.
RNA isolation and quantitative RT-PCR analyses
Total RNA from the cerebral cortices of WT and Cln1−/− mice was isolated and mRNA levels were quantitated as described previously (27). The primers used for quantitative RT-PCR (qRT-PCR) are presented in Supplementary Material, Table S1.
Isolation of lysosomal and nuclear fractions
Nuclear fractions from flash frozen cells and tissues were isolated using ProteoExtract subcellular proteome extraction kit (Calbiochem; Cat # 539790) following the manufacturer's instructions. A lysosome isolation kit (Sigma, Cat # LYSISO1) was used for purification of the lysosomal fractions. The lysosome-enriched fractions were centrifuged at 1 00 000g for 1 h and were washed several times to remove the cytosolic contaminations.
Western blot analyses
Extracts of cortical tissues from WT and Cln1−/− mice were prepared in protein-extracting buffer (50 mm Tris–HCl, 150 mm NaCl, 0.25% SDS, 1 mm EDTA and 1% NP-40) containing protease-inhibitor cocktails (Pierce, Cat # 1861278). Cell lysates from cultured astrocytes were prepared in Phosphosafe extraction reagent (EMD Biosciences, Cat # 71296-3). Western blot was performed using standard protocol as described previously (27). The primary antibodies, their suppliers and dilutions are as follows: Santa Cruz Biotechnology: anti-CD (Cat # sc-6487, 1:200), anti-CL (Cat # sc-6498, 1:200), anti-LAMP-1 (Cat # sc-19992, 1:500); Cell Signaling: anti-CB (Cat # 3383, 1:1000), anti-GFAP (Cat # 3670, 1:1000), anti-Histone H3 (Cat # 2592, 1:10 000); Wako: anti-Iba1 (Cat # 016-20001, 1:500); Abcam: anti-TFEB (Cat # ab2636, 1:500) and US Biological: anti-β-actin (Cat # A0760-40, 1:10 000). Chemiluminescence was detected using SuperSignal west pico luminol/enhancer solution (Pierce, Cat # 34080) according to the manufacturer's specifications.
Immunohistochemistry/immunofluorescence
The brain tissues were fixed in 4% paraformaldehyde, and histological sections prepared. Following overnight incubation with primary antibody, the sections were washed three times with 1× PBS and were further processed and developed using biotinylated secondary antibodies (Vector Laboratories) and ABC complex (Vector Laboratories) as previously described (27). For immunofluorescence, Alexa Fluor-conjugated secondary antibodies (Invitrogen) were used. Nuclei were stained with 4', 6-Diamidino-2-Phenylindole. Fluorescence was visualized with the Zeiss LSM 510 Inverted Meta confocal microscope (Carl Zeiss), and images were processed using the LSM image software (Carl Zeiss). In each experiment, images were acquired using identical settings and the same threshold was used for all groups.
Enzyme activity assays for cathepsins
CB, CD and CL enzymatic activities were measured using a fluorometric assay kit from Abcam (Cat # ab65300, ab65302 and ab65306, respectively) according to the manufacturer's protocol in a 96-well assay format.
Pulse-chase experiments
WT and Cln1−/− astrocytes were metabolically labeled with [35S]-methionine/cysteine following a previous method (11) with minor modifications. Briefly, cells were grown to 70% confluency and were preincubated in serum-free DMEM depleted of methionine and cysteine for 1 h at 37°C. [35S]-methionine/cysteine mixture (TRANS 35S-LABEL from Perkin Elmer, Cat # 51006) was added to each culture (100 µCi/ml in a 3.5 ml Petri dish) and the cells were incubated at 20°C for 2 h. After washing three times with PBS to remove unincorporated labels, complete medium (supplemented with 0.06 mg/ml methionine and 0.1 mg/ml cysteine) was added and chased for 0, 1, 3 and 6 h at 37°C. The chased medium was immunoprecipitated with anti-CD antibody (Santa Cruz Biotechnology Inc.) conjugated to Protein A/G agarose beads (Santa Cruz Biotechnology Inc.) and analyzed by SDS–PAGE and fluorography.
Long-lived protein degradation assay
Long-lived protein degradation in cultured cells was determined by pulse-chase experiments described previously (11). Briefly, WT and Cln1−/− astrocytes were grown in 6-well plates. Selective labeling of long-lived proteins in confluent cultures were performed by first incubating the cells in medium containing [3H]-leucine (2 µCi/ml) for 48 h at 37°C. After 48 h, the cells were washed and further incubated either in complete DMEM medium with 10% fetal bovine serum or in serum-depleted medium, in which proteolysis is induced. To prevent [3H]-leucine reincorporation into newly synthesized proteins after labeling, the incubation medium was supplemented with excess (2.8 mm) unlabeled leucine. Aliquots of the medium were collected at 24 h after labeling and precipitated with 10% trichloroacetic acid and filtered using a membrane filter (pore size: 0.22 mm), and radioactivity in the flow-through was measured. Proteolysis is expressed as the percentage of the initial acid-precipitable radioactivity (protein), which was converted to acid-soluble radioactivity (amino acids and small peptides) after 24 h.
Determination of lysosomal pH
Lysosomal pH was measured using Oregon green-dextran and Tetramethylrhodamine (TMR)-dextran as described previously (53) with minor modifications. The ratio of pH sensitive Oregon green fluorescence to pH independent TMR fluorescence allows quantitation of lysosomal pH in cells. For actual pH measurement, the fluorescence ratio of cells loaded with the pH sensors resuspended in the same calibration buffer (pH ∼7.6) without nigericin and monencin were plotted and calculated using the calibration curve. For imaging primary astrocytes isolated from WT and Cln1−/−, mouse brain was incubated with pH-sensitive DND-189 for 10 min and washed with PBS (two to three times) and visualized under the microscope.
Statistical analysis
Data presented are the mean of at least three independent experiments ± standard deviation. Statistical analyses were performed by one-way analysis of variance post hoc t-test using Microsoft SPSS, and a P-value of <0.05 was considered statistically significant.
Supplementary Material
Funding
This study was supported in full by the intramural research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health.
Supplementary Material
Acknowledgements
Cln1−/− mice were a generous gift from Dr Sandra L. Hofmann. These mice were generated by Cln1 gene targeting in ES cells as previously reported (24). We also thank Drs Sondra W. Levin, Ida Owens, Jyoti K. Jaiswal and Janice Y. Chou for critical review of the manuscript and helpful suggestions. We are grateful to Dr Vincent Schran, Microscopy and Imaging Core, for his expert assistance in confocal microscopy.
Conflict of Interest statement. None declared.
References
- 1.de Duve C. (1983) Lysosomes revisited. Eur. J. Biochem., 137, 391–397. [DOI] [PubMed] [Google Scholar]
- 2.Harris H., Rubinzstein D.C. (2011) Control of autophagy as a therapy for neurodegenerative disease. Nat. Rev. Neurol., 8, 108–117. [DOI] [PubMed] [Google Scholar]
- 3.Nixon R.A. (2013) The role of autophagy in neurodegenerative disease. Nat. Med., 19, 983–997. [DOI] [PubMed] [Google Scholar]
- 4.Platt F.M., Boland B., van der Spoel A.C. (2012) The biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction . J. Cell Biol., 199, 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proia R.L., Wu Y.-P. (2004) Blood to brain to the rescue. J. Clin. Invest., 113, 1108–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Vincente M., Cuervo A.M. (2007) Autophagy and neurodegeneration: when the cleaning crew goes on strike. Lancet Neurol., 6, 352–361. [DOI] [PubMed] [Google Scholar]
- 7.Zuccato C., Valenza M., Cattaneo E. (2010) Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol. Rev., 90, 905–981. [DOI] [PubMed] [Google Scholar]
- 8.Schneider L., Zhang J. (2010) Lysosomal function in macromolecular homeostasis and bioenergetics in Parkinson's disease. Mol. Neurodegener., 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaidi N., Maurer A., Nieke S., Kalbacher H. (2008) Cathepsin D: a cellular roadmap. Biochem. Biophys. Res. Commun., 376, 5–9. [DOI] [PubMed] [Google Scholar]
- 10.Cataldo A.M., Barnett J.L., Berman S.A., Li J., Quarless S., Bursztajn S., Lippa C., Nixon R.A. (1995) Gene expression and cellular content of cathepsin D in Alzheimer's disease brain: evidence for early up-regulation of the endosomal-lysosomal system. Neuron, 14, 671–680. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.H., Yu W.H., Kumar A., Lee S., Mohan P.S., Peterhoff C.M., Wolfe D.M., Martinez-Vicente M., Massey A.C., Sovak G. et al. (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell, 141, 1146–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vitner E.B., Dekel H., Zigdon H., Shachar T., Farfel-Becker T., Eilam R., Karlsson S., Futerman A.H. (2010) Altered expression and distribution of cathepsins in neuropathic forms of Gaucher disease and in other sphingolipidoses. Hum. Mol. Genet., 19, 3583–3590. [DOI] [PubMed] [Google Scholar]
- 13.Shacka J.J., Roth K.A. (2007) Cathepsin D deficiency and NCL/Batten disease: there's more to death than apoptosis. Autophagy, 3, 474–476. [DOI] [PubMed] [Google Scholar]
- 14.Steinfeld R., Reinhardt K., Schreiber K., Hillebrand M., Kraetzner R., Bruck W., Saftig P., Gartner J. (2006) Cathepsin D deficiency is associated with a human neurodegenerative disorder. Am. J. Hum. Genet., 78, 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koike M., Nakanishi H., Saftig P., Ezaki J., Isahara K., Ohsawa Y., Schulz-Schaeffer W., Wantanabe T., Waguri S., Kametaka S. et al. (2000) Cathepsin D deficiency induces lysosomal storage with ceroid lipofuscin in mouse CNS neurons. J. Neurosci., 20, 6898–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haltia M. (2006) The neuronal ceroid lipofuscinoses: from past to present. Biochim. Biophys. Acta, 1762, 850–856. [DOI] [PubMed] [Google Scholar]
- 17.Anderson G.W., Goebel H.H., Simonati A. (2013) Human pathology in NCL. Biochim. Biophys. Acta, 1832, 1807–1826. [DOI] [PubMed] [Google Scholar]
- 18.Rider J.A., Rider D.L. (1988) Batten disease: past, present, and future. Am. J. Med. Genet., 5 (suppl.), 21–26. [DOI] [PubMed] [Google Scholar]
- 19.Kousi M., Lehesjoki A.E., Mole S.E. (2012) Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinosis. Hum. Mutat., 33, 42–63. [DOI] [PubMed] [Google Scholar]
- 20.Warrier V., Vieira M., Mole S.E. (2013) Genetic basis and phenotypic correlations of the neuronal ceroid lipofuscinoses. Biochim. Biophys. Acta, 1832, 1827–1830. [DOI] [PubMed] [Google Scholar]
- 21.Vesa J., Hellsten E., Verkruyse L.A., Camp L.A., Rapola J., Santavuori P., Hofmann S.L., Peltonen L. (1995) Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature, 376, 584–587. [DOI] [PubMed] [Google Scholar]
- 22.Camp L.A., Verkruyse L.A., Afendis S.J., Slaughter C.A., Hofmann S.L. (1994) Molecular cloning and expression of palmitoyl-protein thioesterase. J. Biol. Chem., 269, 23212–23219. [PubMed] [Google Scholar]
- 23.Bonifacino J.S., Traub L.M. (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem., 72, 395–447. [DOI] [PubMed] [Google Scholar]
- 24.Gupta P., Soyombo A.A., Atashband A., Wisniewski K.E., Shelton J.M., Richardson J.A., Hammer R.E., Hofmann S.L. (2001) Disruption of PPT1 or PPT2 causes neuronal ceroid lipofuscinosis in knockout mice. Proc. Natl Acad. Sci. USA, 98, 13566–13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bible E., Gupta P., Hofmann S., Cooper J.D. (2004) Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol. Dis., 16, 346–359. [DOI] [PubMed] [Google Scholar]
- 26.Riikonen R., Vanhanen S.L., Tyynela J., Santavuori P., Turpeinen U. (2000) CSF insulin-like growth factor-1 in infantile neuronal ceroid lipofuscinosis. Neurology, 54, 1828–1832. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z., Lee Y.C., Kim S.J., Choi M.S., Tsai P.C., Xu Y., Xiao Y.J., Zhang P., Heffer A., Mukherjee A.B. (2006) Palmitoyl-protein thioesterase-1 deficiency mediates the activation of the unfolded protein response and neuronal apoptosis in INCL. Hum. Mol. Genet., 15, 337–346. [DOI] [PubMed] [Google Scholar]
- 28.Macauley S.L., Pekny M., Sands M.S. (2011) The role of attenuated astrocyte activation in infantile neuronal ceroid lipofuscinosis. J. Neurosci., 31, 15575–15585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Settembre C., Fraldi A., Medina D.L., Ballabio A. (2013) Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol., 14, 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmieri M., Impey S., Kang H., di Ronza A., Pelz C., Sardiello M. (2011) Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet., 20, 3852–3866. [DOI] [PubMed] [Google Scholar]
- 31.Andersen J.K. (2004) Oxidative stress in neurodegeneration: cause or consequence? Nat. Med., 10 (suppl.), S18–S25. [DOI] [PubMed] [Google Scholar]
- 32.Wei H., Kim S.J., Zhang Z., Tsai P.C., Wisniewski K.E., Mukherjee A.B. (2008) ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum. Mol. Genet., 17, 469–477. [DOI] [PubMed] [Google Scholar]
- 33.Amritraj A., Peake K., Kodam A., Salio C., Merighi A., Vance J.E., Kar S. (2009) Increased activity and altered subcellular distribution of lysosomal enzymes determine neuronal vulnerability in Niemann-Pick type C1-deficient mice. Am. J. Pathol., 175, 2540–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. (1997) Alpha-synuclein in Lewy bodies. Nature, 388, 839–840. [DOI] [PubMed] [Google Scholar]
- 35.Sevlever D., Jiang P., Yen S.H. (2008) Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry, 47, 9678–9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J.Y., Verkruyse L.A., Hofmann S.L. (1996) Lipid thioesters derived from acylated proteins accumulate in infantile neuronal ceroid lipofuscinosis: correction of the defect in lymphoblasts by recombinant palmitoyl-protein thioesterase. Proc. Natl Acad. Sci. USA, 93, 10046–10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saha A., Sarkar C., Singh S.P., Zhang Z., Munasinghe J., Peng S., Chandra G., Kong E., Mukherjee A.B. (2012) The blood-brain barrier is disrupted in a mouse model of infantile neuronal ceroid lipofuscinosis: amelioration by resveratrol. Hum. Mol. Genet., 21, 2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosmann T. (1983) Rapid colorimetric assay for cellular growth and survival . J. Immunol. Methods, 65, 55–63. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt M.F.G. (1989) Fatty acylation of proteins. Biochim. Biophys. Acta, 988, 411–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atamna H., Robinson C., Ingersoll R., Elliott H., Ames B.N. (2001) N-t-butyl hydroxylamine is an antioxidant that reverses age-related changes in mitochondria in vivo and in vitro. FASEB J., 15, 2196–2204. [DOI] [PubMed] [Google Scholar]
- 41.Sarkar C., Chandra G., Peng S., Zhang Z., Liu A., Mukherjee A.B. (2013) Neuroprotection and lifespan extension in Ppt1 (-/-) mice by NtBuHA: therapeutic implications for INCL. Nat. Neurosci., 16, 1608–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams R.E., Mole S.E. (2012) New nomenclature and classification scheme for neuronal ceroid lipofuscinoses. Neurology, 79, 183–191. [DOI] [PubMed] [Google Scholar]
- 43.Keller J.N., Schmitt F.A., Scheff S.W., Ding Q., Chen Q. (2005) Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology, 64, 1152–1156. [DOI] [PubMed] [Google Scholar]
- 44.Gilgun-Sherki Y., Melamed E., Offen D. (2001) Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology, 40, 959–975. [DOI] [PubMed] [Google Scholar]
- 45.Andrews N.W., Almeida P.E., Corrotte M. (2014) Damage control: cellular mechanisms of plasma membrane repair. Trends Cell Biol., pii: S0962-8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrews N.W. (2000) Regulated secretion of conventional lysosomes. Trends Cell Biol., 10, 316–321. [DOI] [PubMed] [Google Scholar]
- 47.Reddy A., Caler E.V., Andrews N.W. (2001) Plasma membrane repair is mediated by Ca(2+) regulated exocytosis of lysosomes. Cell, 106, 157–169. [DOI] [PubMed] [Google Scholar]
- 48.Medina D.L., Fraldi A., Bouche V., Annunziata F., Mansueto G., Spampanato C. (2011) Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell, 21, 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langenfurth A., Rinnenthal J.L., Vinnakota K., Prinz V., Carlo A.S., Stadelmann C., Siffrin V., Peaschke S., Enders M., Heppner F. et al. (2014) Membrane-type 1 metalloproteinase is upregulated in microglia/brain macrophages in neurodegenerative and neuroinflammatory diseases. J. Neurosci. Res., 92, 275–286. [DOI] [PubMed] [Google Scholar]
- 50.Forgac M. (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol., 8, 917–929. [DOI] [PubMed] [Google Scholar]
- 51.Poëa-Guyon S., Ammar M.R., Erard M., Amar M., Moreau A.W., Fossier P., Gleize V., Vitale N., Morel N. (2013) The V-ATPase membrane domain is a sensor of granular pH that controls the exocytic machinery. J. Cell Biol., 203, 283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moussaud S., Draheim H.J. (2010) A new method to isolate microglia from adult mice and culture them for an extended period of time. J. Neurosci. Methods, 187, 243–253. [DOI] [PubMed] [Google Scholar]
- 53.Haggie P.M., Verkman A.S. (2009) Unimpaired lysosomal acidification in respiratory epithelial cells in cystic fibrosis. J. Biol. Chem., 284, 7681–7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.